Submitted:
04 July 2024
Posted:
04 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
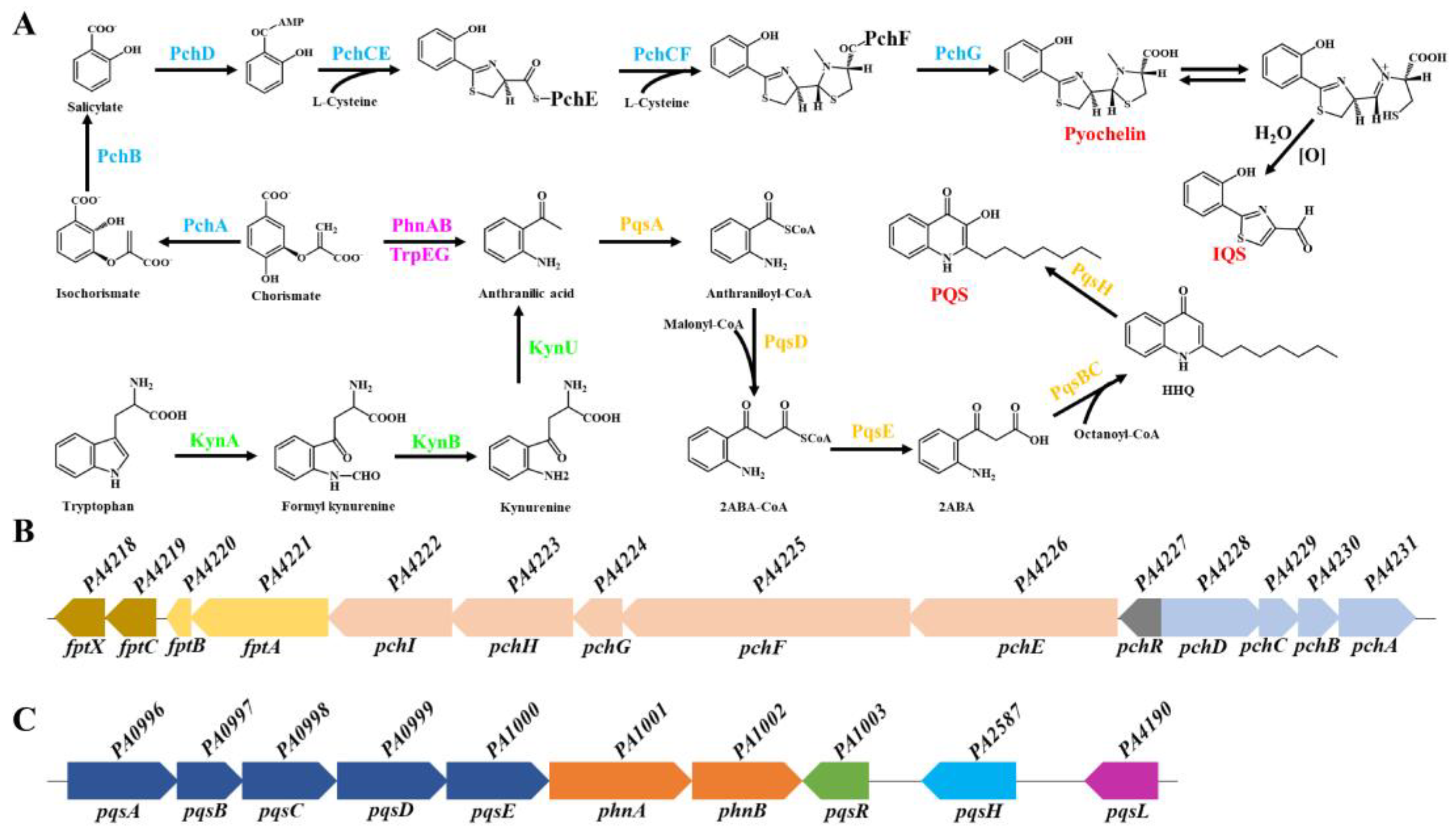
2. Biosynthesis of PCH and PQS
2.1. PCH Biosynthesis
2.2. Biosynthesis of PQS
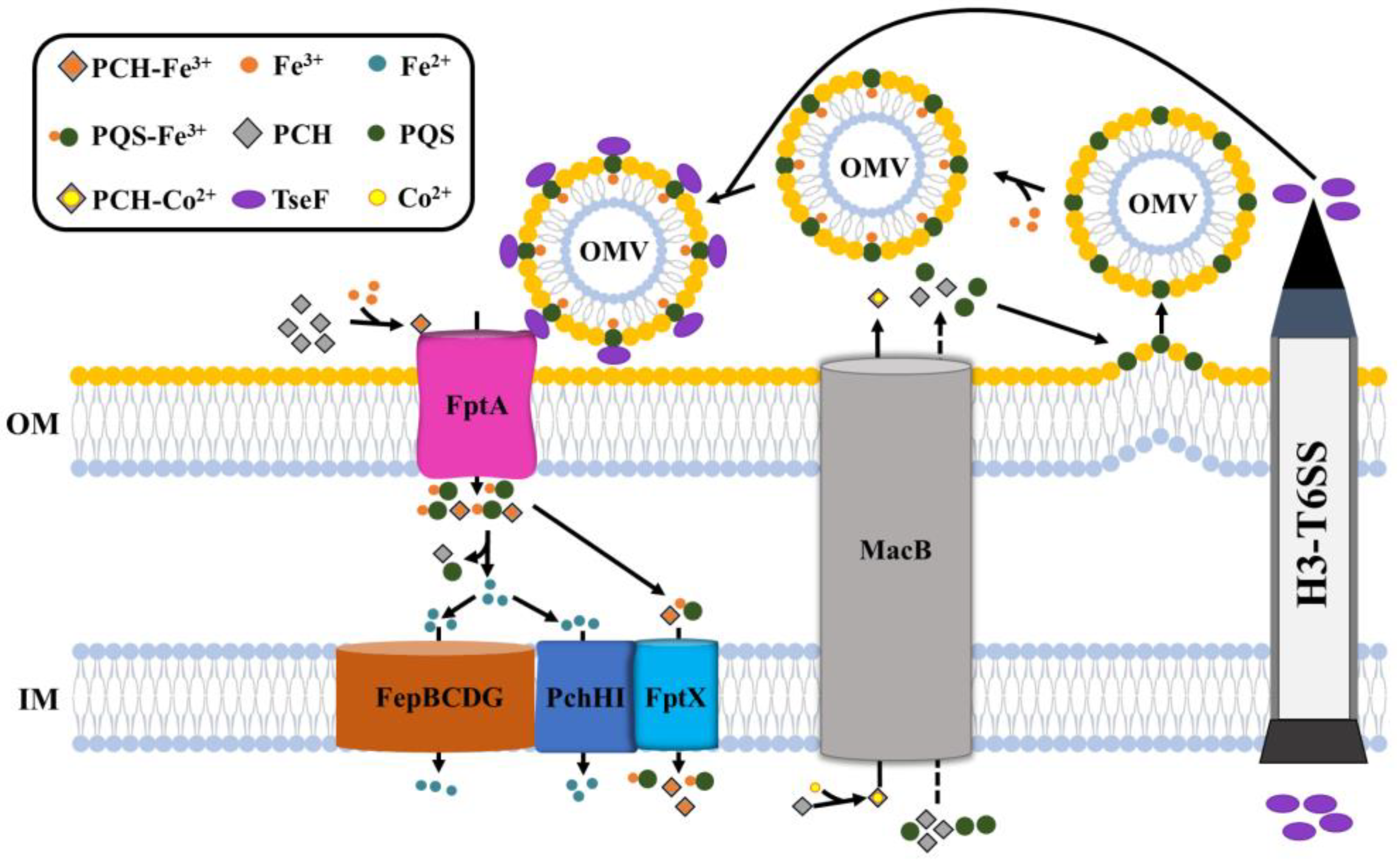
3. Shared Transport System
3.1. PCH and PQS Share Outer Membrane Transporter FptA to Mediate Iron Uptake
3.2. PCH and PQS Share Inner Membrane Transporters FptX, PchHI, and FepBCDG to Mediate Iron Uptake
3.3. Do PCH and PQS Share the Same Secretory Pathways?
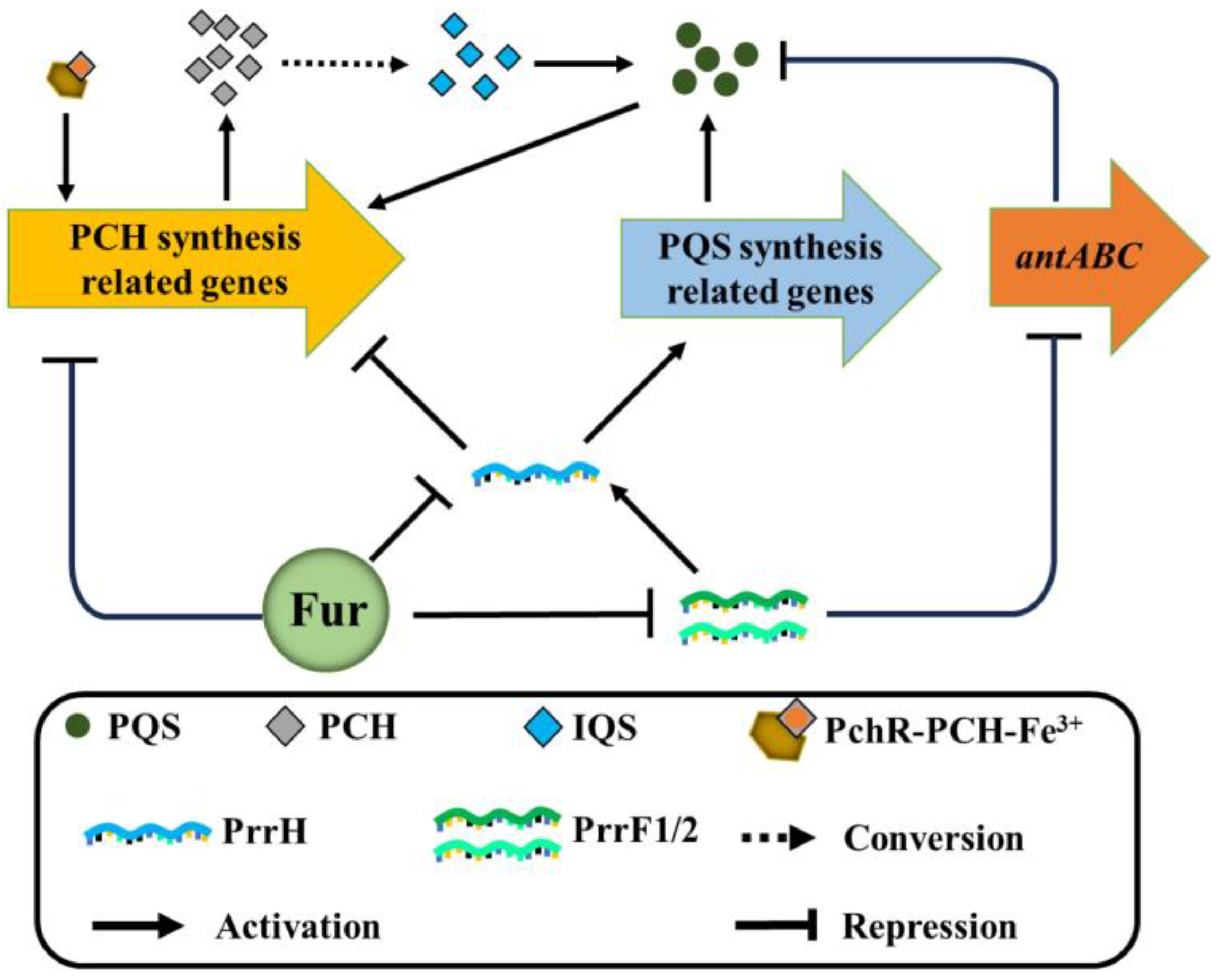
4. Regulatory Correlation
4.1. Regulation of PCH and PQS Biosynthesis by Fur
4.2. Mutual Regulation between PCH and PQS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, X.; Li, W.; Xiao, W.; Cheng, J.; Lin, J. Construction and phenotypic characterization of fur-deleted mutant of Pseudomonas aeruginosa. Acta Microbiologica Sinica. 2023, 64, 917–917. [Google Scholar]
- Drakesmith, H.; Prentice, A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008, 6, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jiménez, A.; Marcos-Torres, F.J.; Llamas, M.A. Mechanisms of iron homeostasis in Pseudomonas aeruginosa and emerging therapeutics directed to disrupt this vital process. Microb Biotechnol. 2023, 16, 1475–1491. [Google Scholar] [CrossRef]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS MICROBIOL REV. 2003, 27, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Ghssein, G.; Ezzeddine, Z. A Review of Pseudomonas aeruginosa Metallophores: Pyoverdine, Pyochelin and Pseudopaline. Biology. 2022, 11, 1711. [Google Scholar] [CrossRef]
- Llamas, M.A.; Sanchez-Jimenez, A. Iron Homeostasis in Pseudomonas aeruginosa: Targeting Iron Acquisition and Storage as an Antimicrobial Strategy. Adv Exp Med Biol. 2022, 1386, 29–68. [Google Scholar]
- Cornelis, P.; Dingemans, J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front Cell Infect Microbiol. 2013, 3, 75. [Google Scholar] [CrossRef]
- Hills, O.J.; Noble, I.O.K.; Heyam, A.; Scott, A.J.; Smith, J.; Chappell, H.F. Atomistic modelling and NMR studies reveal that gallium can target the ferric PQS uptake system in P. aeruginosa biofilms. Microbiology. 2023, 169, 001422. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, W.; Cheng, J.; Yang, X.; Zhu, K.; Wang, Y.; Wei, G.; Qian, P.Y.; Luo, Z.Q.; Shen, X. A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat Commun. 2017, 8, 14888. [Google Scholar] [CrossRef] [PubMed]
- Dandela, R.; Mantin, D.; Cravatt, B.F.; Rayo, J.; Meijler, M.M. Proteome-wide mapping of PQS-interacting proteins in Pseudomonas aeruginosa. Chem Sci. 2018, 9, 2290–2294. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, J.; Wang, Y.; Shen, X. The Pseudomonas Quinolone Signal (PQS): Not Just for Quorum Sensing Anymore. Front Cell Infect Microbiol. 2018, 8, 230. [Google Scholar] [CrossRef]
- Hodgkinson, J.T.; Gross, J.; Baker, Y.R.; Spring, D.R.; Welch, M. A new Pseudomonas quinolone signal (PQS) binding partner: MexG. Chem Sci. 2016, 7, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Baker, Y.R.; Hodgkinson, J.T.; Florea, B.I.; Alza, E.; Galloway, W.; Grimm, L.; Geddis, S.M.; Overkleeft, H.S.; Welch, M.; Spring, D.R. Identification of new quorum sensing autoinducer binding partners in Pseudomonas aeruginosa using photoaffinity probes. Chem Sci. 2017, 8, 7403–7411. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cheng, J.; Zhang, H.; Lin, J. Research progress in functional diversity of quorum sensing signaling molecule PQS in Pseudomonas aeruginosa. Acta Microbiologica Sinica. 2023, 63, 3500–3519. [Google Scholar]
- Zhang, H.; Yang, J.; Cheng, J.; Zeng, J.; Ma, X.; Lin, J. PQS and pyochelin in Pseudomonas aeruginosa share inner membrane transporters to mediate iron uptake. Microbiol Spectr. 2024, 12, e0325623. [Google Scholar] [CrossRef] [PubMed]
- Schalk, I.J.; Rigouin, C.; Godet, J. An overview of siderophore biosynthesis among fluorescent Pseudomonads and new insights into their complex cellular organization. Environ Microbiol. 2020, 22, 1447–1466. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Miyanaga, A. Recent advances in the structural biology of modular polyketide synthases and nonribosomal peptide synthetases. Curr Opin Chem Biol. 2022, 71, 102223. [Google Scholar] [CrossRef]
- Gaille, C.; Reimmann, C.; Haas, D. Isochorismate Synthase (PchA), the First and Rate-limiting Enzyme in Salicylate Biosynthesis of Pseudomonas aeruginosa. J Biol Chem. 2003, 278, 16893–16898. [Google Scholar] [CrossRef]
- Meneely, K.M.; Luo, Q.; Dhar, P.; Lamb, A.L. Lysine221 is the general base residue of the isochorismate synthase from Pseudomonas aeruginosa (PchA) in a reaction that is diffusion limited. Arch Biochem. 2013, 538, 49–56. [Google Scholar] [CrossRef]
- Reimmann, C.; Patel, H.M.; Walsh, C.T.; Haas, D. PchC thioesterase optimizes nonribosomal biosynthesis of the peptide siderophore pyochelin in Pseudomonas aeruginosa. J Bacteriol. 2004, 186, 6367–6373. [Google Scholar] [CrossRef]
- Quadri, L.E.; Keating, T.A.; Patel, H.M.; Walsh, C.T. Assembly of the Pseudomonas aeruginosa Nonribosomal Peptide Siderophore Pyochelin: In Vitro Reconstitution of Aryl-4,2-bisthiazoline Synthetase Activity from PchD, PchE, and PchF. Biochemistry. 1999, 38, 14941–51494. [Google Scholar] [CrossRef] [PubMed]
- Gaille, C.; Kast, P.; Haas, D. Salicylate biosynthesis in Pseudomonas aeruginosa. Purification and characterization of PchB, a novel bifunctional enzyme displaying isochorismate pyruvate-lyase and chorismate mutase activities. J Biol Chem. 2002, 277, 21768–21775. [Google Scholar] [CrossRef] [PubMed]
- Gasser, V.; Guillon, L.; Cunrath, O.; Schalk, I.J. Cellular organization of siderophore biosynthesis in Pseudomonas aeruginosa: Evidence for siderosomes. J Inorg Biochem. 2015, 148, 27–34. [Google Scholar] [CrossRef]
- Patel, H.M.; Tao, J.; Walsh, C.T. Epimerization of an l-Cysteinyl to a d-Cysteinyl Residue during Thiazoline Ring Formation in Siderophore Chain Elongation by Pyochelin Synthetase from Pseudomonas aeruginosa. Biochemistry. 2003, 42, 10514–10527. [Google Scholar] [CrossRef]
- Shelton, C.L.; Meneely, K.M.; Ronnebaum, T.A.; Chilton, A.S.; Riley, A.P.; Prisinzano, T.E.; Lamb, A.L. Rational inhibitor design for Pseudomonas aeruginosa salicylate adenylation enzyme PchD. J Biol Inorg Chem. 2022, 27, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Cunrath, O.; Gasser, V.; Hoegy, F.; Reimmann, C.; Guillon, L.; Schalk, I.J. A cell biological view of the siderophore pyochelin iron uptake pathway in Pseudomonas aeruginosa. Environ Microbiol. 2015, 17, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Ronnebaum, T.A.; McFarlane, J.S.; Prisinzano, T.E.; Booker, S.J.; Lamb, A.L. Stuffed Methyltransferase Catalyzes the Penultimate Step of Pyochelin Biosynthesis. Biochemistry. 2019, 58, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Keating, T.A.; Ehmann, D.E.; Kohli, R.M.; Marshall, C.G.; Trauger, J.W.; Walsh, C.T. Chain termination steps in nonribosomal peptide synthetase assembly lines: directed acyl-S-enzyme breakdown in antibiotic and siderophore biosynthesis. Chembiochem. 2001, 2, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Farrow, J.M., 3rd; Pesci, E.C. Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. J Bacteriol. 2007, 189, 3425–3433. [Google Scholar] [CrossRef]
- Palmer, G.C.; Jorth, P.A.; Whiteley, M. The role of two Pseudomonas aeruginosa anthranilate synthases in tryptophan and quorum signal production. Microbiology. 2013, 159, 959–969. [Google Scholar] [CrossRef]
- Essar, D.W.; Eberly, L.; Hadero, A.; Crawford, I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990, 172, 884–900. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yin, W.; Sun, X.; Cui, B.; Huang, L.; Li, P.; Yang, L.; Zhou, J.; Deng, Y. Anthranilic acid from Ralstonia solanacearum plays dual roles in intraspecies signalling and inter-kingdom communication. ISME J. 2020, 14, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Essar, D.W.; Eberly, L.; Han, C.Y.; Crawford, I.P. DNA sequences and characterization of four early genes of the tryptophan pathway in Pseudomonas aeruginosa. J Bacteriol. 1990, 172, 853–866. [Google Scholar] [CrossRef]
- Shaw, C.; Hess, M.; Weimer, B.C. Microbial-Derived Tryptophan Metabolites and Their Role in Neurological Disease: Anthranilic Acid and Anthranilic Acid Derivatives. Microorganisms. 2023, 11, 1825. [Google Scholar] [CrossRef] [PubMed]
- Knoten, C.A.; Wells, G.; Coleman, J.P.; Pesci, E.C. A conserved suppressor mutation in a tryptophan auxotroph results in dysregulation of Pseudomonas quinolone signal synthesis. J Bacteriol. 2014, 196, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Cámara, M. Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reyes, S.; Soberon-Chavez, G.; Cocotl-Yanez, M. The third quorum-sensing system of Pseudomonas aeruginosa: Pseudomonas quinolone signal and the enigmatic PqsE protein. J Med Microbiol. 2020, 69, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.P.; Hudson, L.L.; McKnight, S.L.; Farrow, J.M., 3rd; Calfee, M.W.; Lindsey, C.A.; Pesci, E.C. Pseudomonas aeruginosa PqsA is an anthranilate-coenzyme A ligase. J Bacteriol. 2008, 190, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Frank, M.W.; Zhu, K.; Mayasundari, A.; Rock, C.O. PqsD Is Responsible for the Synthesis of 2,4-Dihydroxyquinoline, an Extracellular Metabolite Produced by Pseudomonas aeruginosa. J Biol Chem. 2008, 283, 28788–28794. [Google Scholar] [CrossRef]
- Drees, S.L.; Fetzner, S. PqsE of Pseudomonas aeruginosa Acts as Pathway-Specific Thioesterase in the Biosynthesis of Alkylquinolone Signaling Molecules. Chem Biol. 2015, 22, 611–618. [Google Scholar] [CrossRef]
- Drees, S.L.; Li, C.; Prasetya, F.; Saleem, M.; Dreveny, I.; Williams, P.; Hennecke, U.; Emsley, J.; Fetzner, S. PqsBC, a Condensing Enzyme in the Biosynthesis of the Pseudomonas aeruginosa Quinolone Signal: CRYSTAL STRUCTURE, INHIBITION, AND REACTION MECHANISM. J Biol Chem. 2016, 291, 6610–6624. [Google Scholar] [CrossRef] [PubMed]
- Schertzer, J.W.; Brown, S.A.; Whiteley, M. Oxygen levels rapidly modulate Pseudomonas aeruginosa social behaviours via substrate limitation of PqsH. Mol Microbiol. 2010, 77, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Schalk, I.J.; Perraud, Q. Pseudomonas aeruginosa and its multiple strategies to access iron. Environ Microbiol. 2023, 25, 811–831. [Google Scholar] [CrossRef] [PubMed]
- Schalk, I.J.; Cunrath, O. An overview of the biological metal uptake pathways in Pseudomonas aeruginosa. Environ Microbiol. 2016, 18, 3227–3246. [Google Scholar] [CrossRef] [PubMed]
- Reimmann, C. Inner-membrane transporters for the siderophores pyochelin in Pseudomonas aeruginosa and enantio-pyochelin in Pseudomonas fluorescens display different enantioselectivities. Microbiology. 2012, 158, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Cobessi, D.; Celia, H.; Pattus, F. Crystal Structure at High Resolution of Ferric-pyochelin and its Membrane Receptor FptA from Pseudomonas aeruginosa. J Mol Biol. 2005, 352, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Mislin, G.L.A.; Hoegy, F.; Cobessi, D.; Poole, K.; Rognan, D.; Schalk, I.J. Binding Properties of Pyochelin and Structurally Related Molecules to FptA of Pseudomonas aeruginosa. J Mol Biol. 2006, 357, 1437–1448. [Google Scholar] [CrossRef]
- Hoegy, F.; Celia, H.; Mislin, G.L.; Vincent, M.; Gallay, J.; Schalk, I.J. Binding of Iron-free Siderophore, a Common Feature of Siderophore Outer Membrane Transporters of Escherichia coli and Pseudomonas aeruginosa. J Biol Chem. 2005, 280, 20222–20230. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Ahmed, A.; Moin, S.T. Iron coordination to pyochelin siderophore influences dynamics of FptA receptor from Pseudomonas aeruginosa: a molecular dynamics simulation study. BioMetals. 2021, 34, 1099–1119. [Google Scholar] [CrossRef]
- Braud, A.; Hannauer, M.l.; Mislin, G.t.L.A.; Schalk, I.J. The Pseudomonas aeruginosa Pyochelin-Iron Uptake Pathway and Its Metal Specificity. J Bacteriol. 2009, 191, 3517–3525. [Google Scholar] [CrossRef]
- Elfarash, A.; Dingemans, J.; Ye, L.; Hassan, A.A.; Craggs, M.; Reimmann, C.; Thomas, M.S.; Cornelis, P. Pore-forming pyocin S5 utilizes the FptA ferripyochelin receptor to kill Pseudomonas aeruginosa. Microbiology. 2014, 160, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Bachelard, A.; Reimmann, C. Ferripyochelin uptake genes are involved in pyochelin-mediated signalling in Pseudomonas aeruginosa. Microbiology. 2007, 153, 1508–1518. [Google Scholar] [CrossRef]
- Roche, B.; Garcia-Rivera, M.A.; Normant, V.; Kuhn, L.; Hammann, P.; Bronstrup, M.; Mislin, G.L.A.; Schalk, I.J. A role for PchHI as the ABC transporter in iron acquisition by the siderophore pyochelin in Pseudomonas aeruginosa. Environ Microbiol. 2022, 24, 866–877. [Google Scholar] [CrossRef]
- Groleau, M.-C.; de Oliveira Pereira, T.; Dekimpe, V.; Déziel, E.; Shank, E.A.; Jorth, P. PqsE Is Essential for RhlR-Dependent Quorum Sensing Regulation in Pseudomonas aeruginosa. mSystems. 2020, 5, e00194–e00220. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.P.; Diggle, S.P.; Cámara, M.; Williams, P. Biosensor-based assays for PQS, HHQ and related 2-alkyl-4-quinolone quorum sensing signal molecules. Nat Protoc. 2007, 2, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology. 2023, 169, 001333. [Google Scholar] [CrossRef]
- Alcalde-Rico, M.; Olivares-Pacheco, J.; Alvarez-Ortega, C.; Cámara, M.; Martínez, J.L. Role of the Multidrug Resistance Efflux Pump MexCD-OprJ in the Pseudomonas aeruginosa Quorum Sensing Response. Front Microbiol. 2018, 9, 2752. [Google Scholar] [CrossRef]
- Otto, M.; Lamarche, M.G.; Déziel, E. MexEF-OprN Efflux Pump Exports the Pseudomonas Quinolone Signal (PQS) Precursor HHQ (4-hydroxy-2-heptylquinoline). PLoS ONE. 2011, 6. [Google Scholar]
- Secli, V.; Michetti, E.; Pacello, F.; Iacovelli, F.; Falconi, M.; Astolfi, M.L.; Visaggio, D.; Visca, P.; Ammendola, S.; Battistoni, A. Investigation of Zur-regulated metal transport systems reveals an unexpected role of pyochelin in zinc homeostasis. BioRxiv[Preprint] 2024. [Google Scholar] [CrossRef]
- Nosran, A.; Kaur, P.; Randhawa, V.; Chhibber, S.; Singh, V.; Harjai, K. Design, synthesis, molecular docking, anti-quorum sensing, and anti-biofilm activity of pyochelin-zingerone conjugate. Drug Dev Res. 2021, 82, 605–615. [Google Scholar] [CrossRef]
- Wilderman, P.J.; Sowa, N.A.; FitzGerald, D.J.; FitzGerald, P.C.; Gottesman, S.; Ochsner, U.A.; Vasil, M.L. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc Natl Acad Sci USA. 2004, 101, 9792–9797. [Google Scholar] [CrossRef] [PubMed]
- Cunrath, O.; Graulier, G.; Carballido-Lopez, A.; Pérard, J.; Forster, A.; Geoffroy, V.A.; Saint Auguste, P.; Bumann, D.; Mislin, G.L.A.; Michaud-Soret, I.; Schalk, I.J.; Fechter, P. The pathogen Pseudomonas aeruginosa optimizes the production of the siderophore pyochelin upon environmental challenges. Metallomics. 2020, 12, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.-M.; Huang, W.; Gans, J.; Weiner, J.; Nowak, E.; Barbier, M.; Wilks, A.; Kane, M.A.; Oglesby, A.G. The heme-responsive PrrH sRNA regulates Pseudomonas aeruginosa pyochelin gene expression. mSphere. 2023, 8, e0039223. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Mourino, S.; Wilks, A. The heme-binding protein PhuS transcriptionally regulates the Pseudomonas aeruginosa tandem sRNA prrF1, F2 locus. J Biol Chem. 2021, 296, 100275. [Google Scholar] [CrossRef] [PubMed]
- Oglesby-Sherrouse, A.G.; Vasil, M.L. Characterization of a heme-regulated non-coding RNA encoded by the prrF locus of Pseudomonas aeruginosa. PLoS One. 2010, 5, e9930. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Shi, Q.; Liu, Y.; Li, M.; Lin, D.; Zhang, S.; Li, Q.; Pu, J.; Shen, C.; Huang, B.; Chen, C.; Zeng, J. The small RNA PrrH of Pseudomonas aeruginosa regulates hemolysis and oxidative resistance in bloodstream infection. Microb Pathog. 2023, 180, 106124. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R.; Bains, M.; Smith, M.L.; Spicer, V.; Lao, Y.; Taylor, P.K.; Mookherjee, N.; Hancock, R.E.W.; Kivisaar, M. The Small RNAs PA2952.1 and PrrH as Regulators of Virulence, Motility, and Iron Metabolism in Pseudomonas aeruginosa. Appl Environ Microbiol. 2021, 87, e02182–e02220. [Google Scholar] [CrossRef] [PubMed]
- Oglesby, A.G.; Farrow, J.M., 3rd; Lee, J.H.; Tomaras, A.P.; Greenberg, E.P.; Pesci, E.C.; Vasil, M.L. The influence of iron on Pseudomonas aeruginosa physiology: a regulatory link between iron and quorum sensing. J Biol Chem. 2008, 283, 15558–15567. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int J Mol Sci. 2020, 21. [Google Scholar] [CrossRef]
- Rather, M.A.; Saha, D.; Bhuyan, S.; Jha, A.N.; Mandal, M. Quorum Quenching: A Drug Discovery Approach Against Pseudomonas aeruginosa. Microbiol Res. 2022, 264, 127173. [Google Scholar] [CrossRef]
- Vadakkan, K.; Ngangbam, A.K.; Sathishkumar, K.; Rumjit, N.P.; Cheruvathur, M.K. A review of chemical signaling pathways in the quorum sensing circuit of Pseudomonas aeruginosa. Int J Biol Macromol. 2024, 254, 127861. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cheng, J. Quorum Sensing in Pseudomonas aeruginosa and Its Relationship to Biofilm Development; American Chemical Society: Washington, 2019; pp 1-16.
- Lee, J.; Wu, J.; Deng, Y.; Wang, J.; Wang, C.; Wang, J.; Chang, C.; Dong, Y.; Williams, P.; Zhang, L.-H. A cell-cell communication signal integrates quorum sensing and stress response. Nat Chem Biol. 2013, 9, 339–343. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, P. Putting an end to the Pseudomonas aeruginosa IQS controversy. Microbiologyopen. 2020, 9, e962. [Google Scholar] [CrossRef]
- Ye, L.; Cornelis, P.; Guillemyn, K.; Ballet, S.; Hammerich, O. Structure revision of N-mercapto-4-formylcarbostyril produced by Pseudomonas fluorescens G308 to 2-(2-hydroxyphenyl)thiazole-4-carbaldehyde [aeruginaldehyde]. Nat Prod Commun. 2014, 9, 789–794. [Google Scholar] [CrossRef]
- Trottmann, F.; Franke, J.; Ishida, K.; Garcia-Altares, M.; Hertweck, C. A Pair of Bacterial Siderophores Releases and Traps an Intercellular Signal Molecule: An Unusual Case of Natural Nitrone Bioconjugation. Angew Chem Int Ed Engl. 2019, 58, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Bennett, R.C.; Lin, J.; Hao, Y.; Zhu, L.; Akinbi, H.T.; Lau, G.W. Surfactant phospholipids act as molecular switches for premature induction of quorum sensing-dependent virulence in Pseudomonas aeruginosa. Virulence. 2020, 11, 1090–1107. [Google Scholar] [CrossRef]
- Chatterjee, P.; Sass, G.; Swietnicki, W.; Stevens, D.A. Review of Potential Pseudomonas Weaponry, Relevant to the Pseudomonas–Aspergillus Interplay, for the Mycology Community. J Fungi. 2020, 6. [Google Scholar] [CrossRef]
- Bredenbruch, F.; Geffers, R.; Nimtz, M.; Buer, J.; Häussler, S. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ Microbiol. 2006, 8, 1318–1329. [Google Scholar] [CrossRef]
- Rampioni, G.; Pustelny, C.; Fletcher, M.P.; Wright, V.J.; Bruce, M.; Rumbaugh, K.P.; Heeb, S.; Cámara, M.; Williams, P. Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. Environ Microbiol. 2010, 12, 1659–1673. [Google Scholar] [CrossRef]
- Diggle, S.P.; Matthijs, S.; Wright, V.J.; Fletcher, M.P.; Chhabra, S.R.; Lamont, I.L.; Kong, X.; Hider, R.C.; Cornelis, P.; Cámara, M.; Williams, P. The Pseudomonas aeruginosa 4-Quinolone Signal Molecules HHQ and PQS Play Multifunctional Roles in Quorum Sensing and Iron Entrapment. Chem Biol. 2007, 14, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.M.P.D.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin Microbiol Rev. 2020, 33, e00181–e00219. [Google Scholar] [CrossRef] [PubMed]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000, 2, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




