Submitted:
27 June 2024
Posted:
29 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
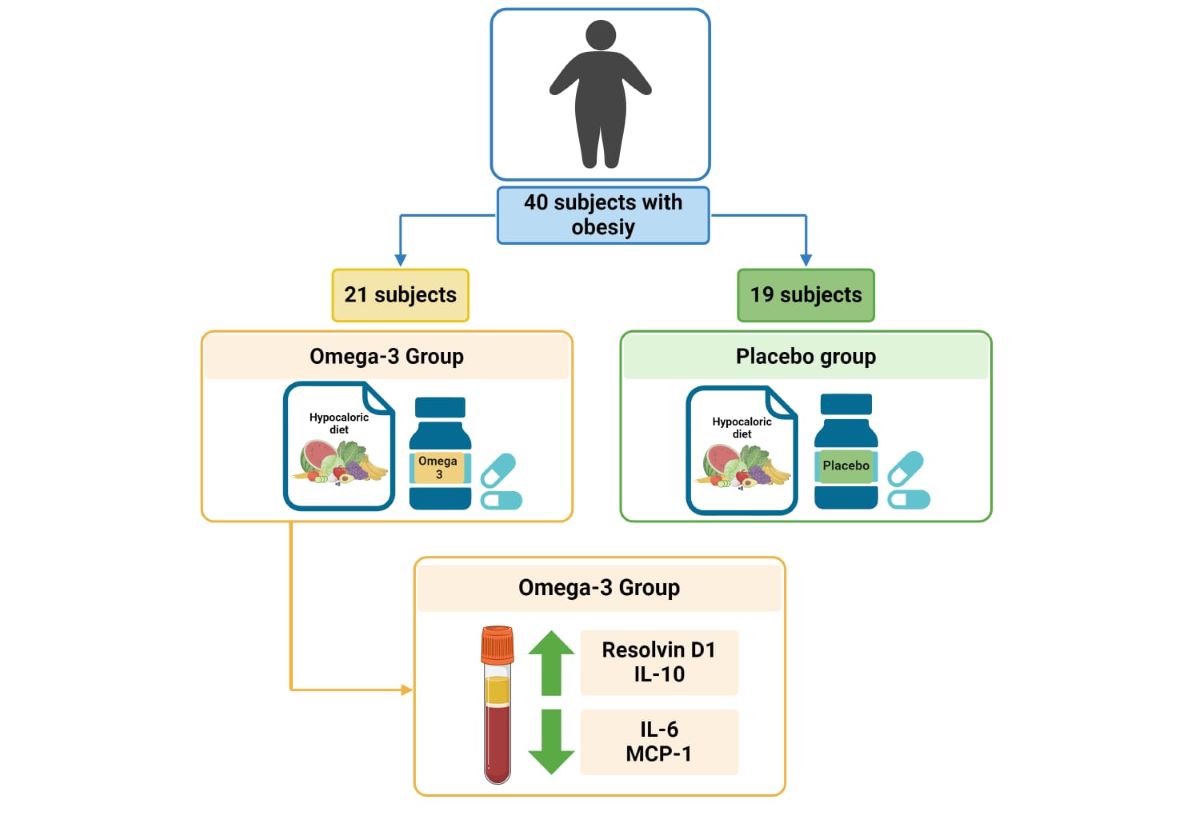
2.1. Study Design
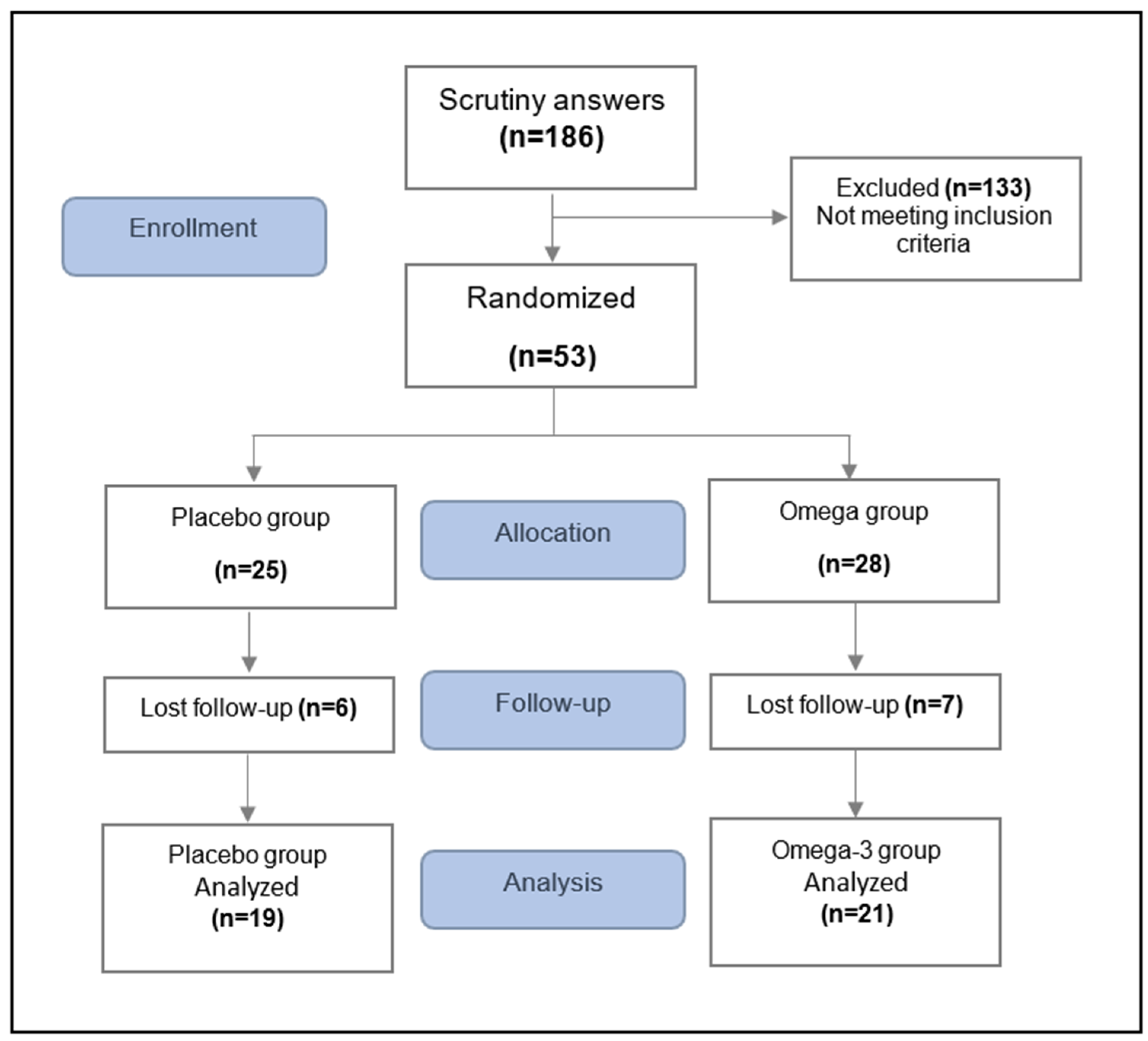
2.2. Study Participants
2.3. Randomization
2.4. Intervention
2.4.1. Nutritional Intervention
2.4.2. Omega 3 and Placebo Intervention Group
2.5. Outcome Measurements
2.5.1. Dietetic Analyses
2.5.2. Adherence Analyses
2.5.3. Anthropometric Measurements
2.5.4. Biochemical Measurements
2.5.5. Inflammatory Parameters
2.6. Statistical Analysis
3. Results
Characteristics of Diet and Adherence
Anthropometric and Biochemical Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primer 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; DiNicolantonio, J.J. The Importance of a Balanced ω-6 to ω-3 Ratio in the Prevention and Management of Obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef]
- Torres-Castillo, N.; Silva-Gómez, J.A.; Campos-Perez, W.; Barron-Cabrera, E.; Hernandez-Cañaveral, I.; Garcia-Cazarin, M.; Marquez-Sandoval, Y.; Gonzalez-Becerra, K.; Barron-Gallardo, C.; Martinez-Lopez, E. High Dietary ω-6:ω-3 PUFA Ratio Is Positively Associated with Excessive Adiposity and Waist Circumference. Obes. Facts 2018, 11, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Becerra, K.; Barron-Cabrera, E.; Muñoz-Valle, J.F.; Torres-Castillo, N.; Rivera-Valdes, J.J.; Rodriguez-Echevarria, R.; Martinez-Lopez, E. A Balanced Dietary Ratio of N-6:N-3 Polyunsaturated Fatty Acids Exerts an Effect on Total Fatty Acid Profile in RBCs and Inflammatory Markers in Subjects with Obesity. Healthcare 2023, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Hannoodee, S.; Nasuruddin, D.N. Acute Inflammatory Response. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.J.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of Inflammation: State of the Art, Definitions and Terms. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 325–332. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N. Specialized Pro-Resolving Mediator Network: An Update on Production and Actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [CrossRef]
- WHO Obesidad y Sobrepeso. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 July 2019).
- Rubio, M.A.; Moreno, C.; Cabrerizo, L. Guías para el tratamiento de las dislipemias en el adulto: Adult Treatment Panel III (ATP-III). Endocrinol. Nutr. 2004, 51, 254–265. [Google Scholar] [CrossRef]
- Lazcano-Ponce, E.; Salazar-Martínez, E.; Gutiérrez-Castrellón, P.; Angeles-Llerenas, A.; Hernández-Garduño, A.; Viramontes, J.L. Ensayos clínicos aleatorizados: variantes, métodos de aleatorización, análisis, consideraciones éticas y regulación. Salud Pública México 2004, 46, 559–584. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lizaur, A.B.; Gonzalez-Palacios, B.; Castro-Becerra, A. Sistema Mexicano de Alimentos Equivalentes; Cuaeta edición.; Fomento de Nutrición y Salud, A.C.: México, 2014; ISBN 978-607-00-7928-3. [Google Scholar]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A New Predictive Equation for Resting Energy Expenditure in Healthy Individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Norma Oficial Mexicana NOM-043-SSA2-2012, Servicios básicos de salud. Promoción y educación para la salud en materia alimentaria. Criterios para brindar orientación. 2013, 35.
- Cano-Pérez, E.; Meoño-Morales, E.; Mendoza-Salazar, L. Prevención, Diagnóstico y tratamiento del sobre peso y obesidad exógena 2012.
- Morales, P.A.K. Norma Oficial Mexicana NOM-037-SSA2-2012, Para la prevención, tratamiento y control de las dislipidemias. 2012.
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Homeostasis Model Assessment Insulin Resistance and β Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man.Pdf.
- Polus, A.; Zapala, B.; Razny, U.; Gielicz, A.; Kiec-Wilk, B.; Malczewska-Malec, M.; Sanak, M.; Childs, C.E.; Calder, P.C.; Dembinska-Kiec, A. Omega-3 Fatty Acid Supplementation Influences the Whole Blood Transcriptome in Women with Obesity, Associated with pro-Resolving Lipid Mediator Production. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 2016, 1861, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Kavyani, Z.; Musazadeh, V.; Fathi, S.; Hossein Faghfouri, A.; Dehghan, P.; Sarmadi, B. Efficacy of the Omega-3 Fatty Acids Supplementation on Inflammatory Biomarkers: An Umbrella Meta-Analysis. Int. Immunopharmacol. 2022, 111, 109104. [Google Scholar] [CrossRef]
- Organization, W.H. Adherence to Long-Term Therapies : Evidence for Action; World Health Organization, 2003; ISBN 978-92-4-154599-0.
- Lopez-Pentecost, M.; Hallmark, B.; Thomson, C.A.; Chilton, F.; Garcia, D.O. Association between Dietary Fatty Acid Intake and Liver Steatosis and Fibrosis in a Sample of Mexican-Origin Hispanic Adults with Overweight or Obesity. Int. J. Environ. Res. Public. Health 2023, 20, 3103. [Google Scholar] [CrossRef] [PubMed]
- Torres-Valadez, R.; Ramos-Lopez, O.; Frías Delgadillo, K.J.; Flores-García, A.; Rojas Carrillo, E.; Aguiar-García, P.; Bernal Pérez, J.A.; Martinez-Lopez, E.; Martínez, J.A.; Zepeda-Carrillo, E.A. Impact of APOE Alleles-by-Diet Interactions on Glycemic and Lipid Features– A Cross-Sectional Study of a Cohort of Type 2 Diabetes Patients from Western Mexico: Implications for Personalized Medicine. Pharmacogenomics Pers. Med. 2020, 13, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Liu, W.; Zhao, T.Y.; Tian, H.M. EFFICACY OF OMEGA-3 POLYUNSATURATED FATTY ACIDS SUPPLEMENTATION IN MANAGING OVERWEIGHT AND OBESITY: A META-ANALYSIS OF RANDOMIZED CLINICAL TRIALS. J Nutr Health Aging.
- Bender, N.; Portmann, M.; Heg, Z.; Hofmann, K.; Zwahlen, M.; Egger, M. Fish or N3-PUFA Intake and Body Composition: A Systematic Review and Meta-Analysis: Fish and Body Composition. Obes. Rev. 2014, 15, 657–665. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Andreoletti, P.; Surapureddi, S.; Nasser, B.; Cherkaoui-Malki, M.; Latruffe, N. Mechanisms Mediating the Regulation of Peroxisomal Fatty Acid Beta-Oxidation by PPARα. Int. J. Mol. Sci. 2021, 22, 8969. [Google Scholar] [CrossRef]
- Bays, H.E.; Tighe, A.P.; Sadovsky, R.; Davidson, M.H. Prescription Omega-3 Fatty Acids and Their Lipid Effects: Physiologic Mechanisms of Action and Clinical Implications. Expert Rev. Cardiovasc. Ther. 2008, 6, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The Role of Peroxisome Proliferator-Activated Receptors (PPAR) in Immune Responses. Metabolism. 2021, 114, 154338. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. N-3 Fatty Acids and Serum Lipoproteins: Human Studies. Am. J. Clin. Nutr. 1997, 65, 1645S–1654S. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Ferrari, F.; Scolari, F. Genetics, Dyslipidemia, and Cardiovascular Disease: New Insights. Curr. Cardiol. Rep. 2019, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, K.I.; Johnson, C.T.; Mezger, A.; Rice, S.L.; Norris, A.M.; Demeter, J.; Greenleaf, W.J.; Reiter, J.F.; Kopinke, D.; Jackson, P.K. Omega-3 Fatty Acids Activate Ciliary FFAR4 to Control Adipogenesis. Cell 2019, 179, 1289–1305.e21. [Google Scholar] [CrossRef]
- Cheshmehkani, A.; Senatorov, I.S.; Kandi, P.; Singh, M.; Britt, A.; Hayslett, R.; Moniri, N.H. Fish Oil and Flax Seed Oil Supplemented Diets Increase FFAR4 Expression in the Rat Colon. Inflamm. Res. 2015, 64, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Akinkuolie, A.O.; Ngwa, J.S.; Meigs, J.B.; Djoussé, L. Omega-3 Polyunsaturated Fatty Acid and Insulin Sensitivity: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. Edinb. Scotl. 2011, 30, 702–707. [Google Scholar] [CrossRef]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L. Omega-3, Omega-6, and Total Dietary Polyunsaturated Fat for Prevention and Treatment of Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMJ 2019, l4697. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of Adipose Tissue Inflammation by Bioactive Food Compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef]
- Spencer, M.; Yao-Borengasser, A.; Unal, R.; Rasouli, N.; Gurley, C.M.; Zhu, B.; Peterson, C.A.; Kern, P.A. Adipose Tissue Macrophages in Insulin-Resistant Subjects Are Associated with Collagen VI and Fibrosis and Demonstrate Alternative Activation. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E1016–E1027. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Finlin, B.S.; Unal, R.; Zhu, B.; Morris, A.J.; Shipp, L.R.; Lee, J.; Walton, R.G.; Adu, A.; Erfani, R.; et al. Omega-3 Fatty Acids Reduce Adipose Tissue Macrophages in Human Subjects With Insulin Resistance. Diabetes 2013, 62, 1709–1717. [Google Scholar] [CrossRef]
- Rasic-Milutinovic, Z.; Perunicic, G.; Pljesa, S.; Gluvic, Z.; Sobajic, S.; Djuric, I.; Ristic, D. Effects of N-3 PUFAs Supplementation on Insulin Resistance and Inflammatory Biomarkers in Hemodialysis Patients. Ren. Fail. 2007, 29, 321–329. [Google Scholar] [CrossRef]
- Salsinha, A.S.; Socodato, R.; Rodrigues, A.; Vale-Silva, R.; Relvas, J.B.; Pintado, M.; Rodríguez-Alcalá, L.M. Potential of Omega-3 and Conjugated Fatty Acids to Control Microglia Inflammatory Imbalance Elicited by Obesogenic Nutrients. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids 2023, 1868, 159331. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The Effects of Low-Ratio n-6/n-3 PUFA on Biomarkers of Inflammation: A Systematic Review and Meta-Analysis. Food Funct. 2021, 12, 30–40. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2–Nonsteroidal Antiinflammatory Drugs and Transcellular Processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- López-Vicario, C.; Rius, B.; Quiles, J.; Alonso, V.; Lopategi, A.; Titos, E.; Claria, J. Pro-Resolving Mediators Produced from EPA and DHA: Overview of the Pathways Involved and Their Mechanisms in Metabolic Syndrome and Related Liver Diseases. Eur. J. Pharmacol. 2015, 785. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-J.; Zhang, J.-T.; Zhao, F.-L.; Xu, D.-L.; Pan, J.; Liu, T. Resolvin D1/N-Formyl Peptide Receptor 2 Ameliorates Paclitaxel-Induced Neuropathic Pain through the Activation of IL-10/Nrf2/HO-1 Pathway in Mice. Front. Immunol. 2023, 14, 1091753. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. The Resolution Code of Acute Inflammation: Novel Pro-Resolving Lipid Mediators in Resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef]
- Clària, J.; López-Vicario, C.; Rius, B.; Titos, E. Pro-Resolving Actions of SPM in Adipose Tissue Biology. Mol. Aspects Med. 2017, 58, 83–92. [Google Scholar] [CrossRef] [PubMed]
| Placebo group (n=19) | Omega-3 group (n=21) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Baseline | Final | ∆ | Baseline | Final | ∆ | p1 | p2 | p∆ |
| Energy (kcal) | 2138 (1822–2324) | 1616 (1271–1838) | -548.2 (-822.8– -149) | 1928 (1706-3075) | 1271 (1046-1713) | -358.41 (-1311 - 42) | 0.038 | 0.060 | 0.964 |
| n-3 PUFAs (g) | 1 (0.7–1.6) | 2.9 (1.4–4.8) | 2.0 (0.62-3.31) | 1.2 (0.8–1.9) | 1.8 (1.1–4.4) | 2.0 (0.03-3.41) | 0.012 | 0.023 | 0.649 |
| n-6 PUFAs (g) | 10.9 (6.4–17.4) | 8.9 (7.7–11.9) | -1.9 (-10.5 – 2.9) | 11.4 (8.9–17.1) | 6.6 (5.3–7.7) | -3.3 (-5.9- -1.41) | 0.307 | 0.012 | 0.698 |
| n-6:n-3 ratio | 9.9 (7.5–13.1) | 3.4 (2.3–5.2) | -7.0 (-11.0- -3.1) | 9 (7.6–13.3) | 2.8 (1.7–5.2) | -5.6 (-10.9- -4.12) | 0.019 | 0.003 | 0.677 |
| Placebo group (n=19) | Omega 3 group (n=21) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Baseline | 4 weeks | Final | ∆ | Baseline | 4 weeks | Final | ∆ | p1 | p2 | p∆ |
| Anthropometric | |||||||||||
| Weight (kg) | 103.0 ± 20.0 | 101.5 ± 19.5 | 100.8 ± 19.2 | -2.24 ± 3.4 | 96.7 ± 16.1 | 93.4 ± 15.4 | 92.3 ± 15.2 | -3.76 ± 3.5 | 0.004 | 0.001 | 0.157† |
| BMI (kg/m2) | 36.0 ± 4.7 | 35.7 ± 5.1 | 35.5 ± 4.9 | -0.50 ± 1.3 | 33.6 ± 3.4 | 32.9 ± 3.1 | 32.5 ± 3.0 | -1.24 ± 1.2 | 0.049* | 0.001* | 0.111† |
| Body fat (%) | 44.9 ± 5.8 | 44.7 ±6.1 | 43.6 ± 7.2 | -1.32 ± 2.4 | 41.7 ± 5.1 | 41.7 ± 5.2 | 41.1 ± 5.5 | -1.10 ±1.8 | 0.043 | 0.010 | 0.752 |
| WC (cm) | 108.6 ± 14.1 | 107.4 ± 14.6 | 104.6 ± 13.3 | -3.63 ± 2.4 | 104.2 ± 11.8 | 100.9 ± 10.5 | 98.7 ± 11 | -5.03 ± 3.3 | 0.001 | 0.001 | 0.158 |
| Biochemical | |||||||||||
| TC (mg/mL) | 160.4 ± 32.0 | 155.1 ± 35.3 | 160.2 ± 34.9 | -1.05 ± 12.4 | 171.1 ± 26.6 | 163.0 ± 23.9 | 166.4 ± 31.5 | -3.63 ± 18.8 | 0.213 | 0.230 | 0.629 |
| TGs (mg/mL)* | 167.3 ± 73.3 | 139.8 ± 62.1 | 146.5 ± 65.4 | -17.94 ± 47.3 | 195.3 ± 77.7 | 155.5 ± 63.8 | 155.0 ± 99.5 | -42.15 ± 85.3 | 0.019* | 0.003* | 0.494† |
| HDL-c (mg/mL) | 35.3 ± 7.1 | 34.1 ± 7.2 | 33.4 ± 6.5 | -2.61 ± 4.71 | 35.3 ± 6.4 | 33.5 ± 7.4 | 33.1 ± 8.0 | -2.52 ± 4.0 | 0.090 | 0.050 | 0.953 |
| LDL-c (mg/mL) | 91.7 ± 24.7 | 93.1 ± 25.8 | 97.9 ± 25.2 | 5.38 ± 16.0 | 97.3 ± 21.0 | 98.2 ± 21.4 | 102.2 ± 27.0 | 7.15 ± 17.8 | 0.346 | 0.216 | 0.754 |
| VLDL-c(mg/mL)* | 33.5 ± 14.6 | 27.8 ± 12.5 | 29.3 ± 13.0 | -3.55 ± 9.4 | 39.0 ± 15.5 | 31.1 ± 12.7 | 30.8 ± 19.9 | -8.57 ± 17.0 | 0.014* | 0.005* | 0.369† |
| Glucose (mg/mL) | 94.0 ± 9.4 | 95.1 ± 7.2 | 98.3 ± 9.6 | 4.27 ± 9.27 | 96.4 ± 10.8 | 95.0 ± 9.1 | 97.8 ± 10.7 | 1.89 ± 13.3 | 0.154 | 0.272 | 0.534 |
| Baseline (%) | Final (%) | p value | ||
|---|---|---|---|---|
|
Placebo group (n=19) |
88.9 | 83.3 | 0.978 | |
|
Omega-3 group (n=21) |
95 | 60 | 0.016 | |
| Placebo group (n=19) | Omega-3 group (n=21) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Baseline | Final | ∆ | Baseline | Final | ∆ | p1 | p2 | p∆ |
| Inflammatory | |||||||||
| TNF-α(pg/mL) | 0.52 (0.4-0.8) | 0.07 (0.05-0.11) | -1.85 (-4.52- -0.71) | 0.61 (0.4-1.6) | 0.07 (0.04-0.17) | -1.82 (-5.74- -0.59) | 0.002 | 0.001 | 0.745 |
| IL-6 (pg/mL) | 2.9 (0.9-7.8) | 4.7 (0.6-7.7) | 0.03 (-0.59-1.84) | 2.3 (0.7-6.4) | 2.0 (0.3-4.4) | -0.67 (-2.72- -0.01) | 0.407 | 0.010 | 0.015 |
| hs-CRP (mg/L) | 3.4 (2.7-5.7) | 2.7 (2.7-6.6) | -0.7 (-1.30-0.30) | 4.5 (2.0-10.1) | 2.4 (1.5-8.2) | -0.5 (-3.07-0.87) | 0.296 | 0.271 | 0.603 |
| IL-10 (pg/mL) | 5.9 (3.8-8.0) | 4.58 (3.3-5.1) | -2.0 (-5.0-0.05) | 5.3 (4.5-7.2) | 7.02 (4.3-12.1) | 1.4 (-0.7-4.6) | 0.081 | 0.035 | 0.001 |
| MCP-1 (pg/mL) | 233 (204-337) | 277 (234-313) | 18.3 (-97.3-66.35) | 266 (215-295) | 235 (174-274) | -29.6(-94.9-5.50) | 0.758 | 0.040 | 0.064 |
| RvD1 (pg/mL) | 443.3 (358-545) | 403 (264-567) | -16.8 (-237.8-92.50) | 466 (392-563) | 562 (473-779) | 129.3 (-90.1-193.5) | 0.586 | 0.048 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





