Submitted:
26 June 2024
Posted:
26 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Characteristics of Caffeine
2.1. General Information
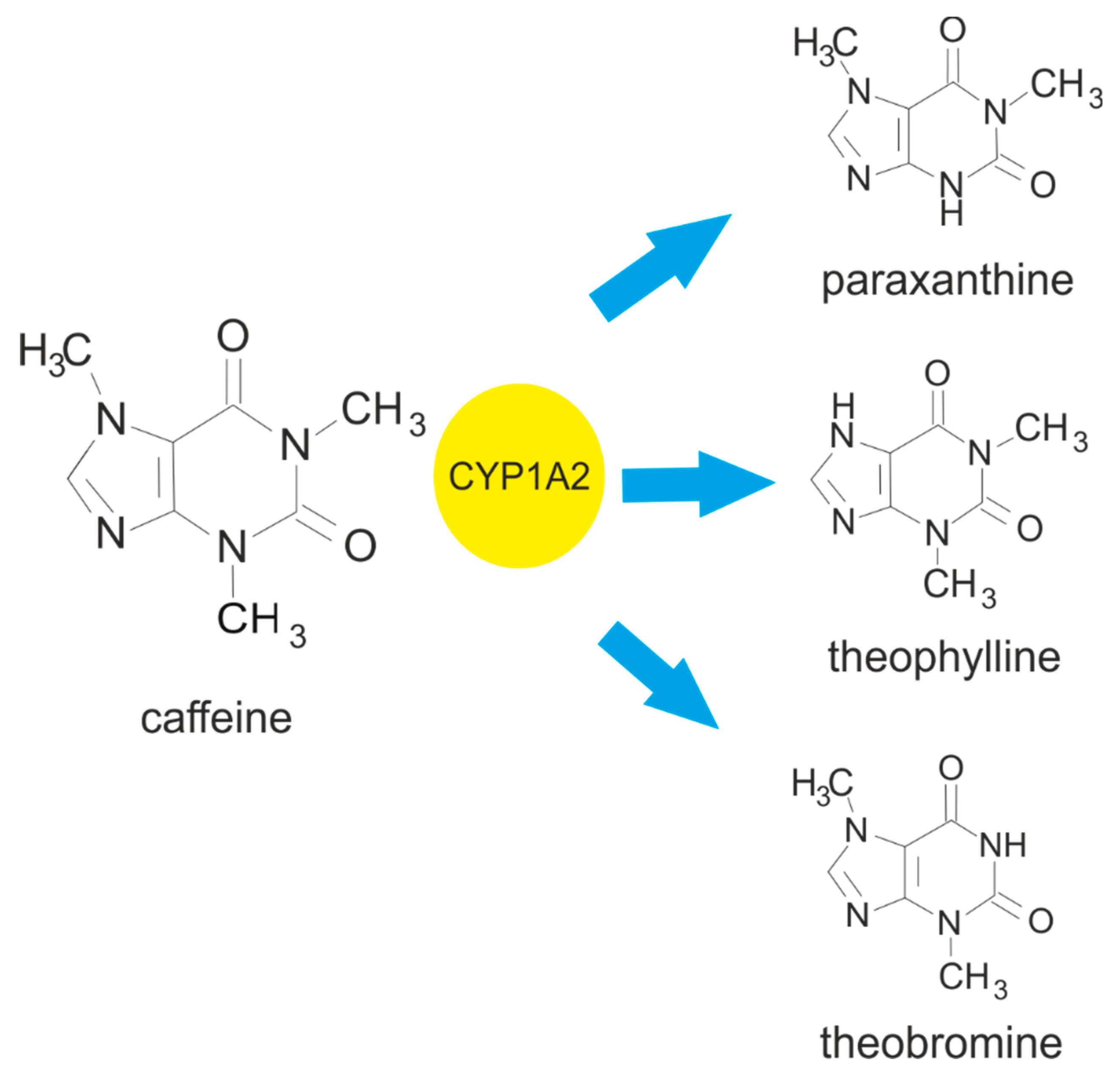
2.2. Metabolism of Caffeine in Humans
2.3. Biological Effects of Caffeine
2.4. Adverse Effects and Toxicity of Caffeine
3. Adenosine Receptor Characteristics
3.1. Overview
3.2. Function of Adenosine Receptors
| Subtype of adenosine receptos | Role in cardiovascular system | Citation |
|---|---|---|
| A1 | Vessel tone regulation Heart rate reduction New vessels formation Cardioprotecion |
[62] [50] [50] [63] |
| A2A | Vasodilation Wound healing Angiogenesis Vasculogenesis Blood pressure regulation Blood flow regulation Cardioprotection Inhibition of platelet function |
[50] [50] [64] [64] [65] [66] [63] [67] |
| A2B | Vasodilation Blood pressure regulation Blood flow regulation Angiogenesis Vasculogenesis |
[50] [65] [66] [64] [64] |
| A3 | Cardioprotection Blood pressure regulation |
[63] [65] |
4. The Role of Platelets in Cardiovascular System Function
5. Adenosine Receptors-Mediated Effects of Caffeine on Platelets
5.1. Can Caffeine Modulate Effects of Inhibitors of Adenosine Receptors on Platelets?
6. Adenosine Receptor-Mediated Effects of Caffeine on the Cardiovascular System
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Biaggioni, I.; Paul, S.; Puckett, A.; Arzubiaga, C. Caffeine and theophylline as adenosine receptor antagonists in humans. J Pharmacol Exp Ther 1991, 258, 588–593. [Google Scholar] [PubMed]
- Chen, Y.; Zhang, Y.; Zhang, M.; Yang, H.; Wang, Y. Consumption of coffee and tea with all-cause and cause-specific mortality: a prospective cohort study. BMC Med 2022, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, S.; Sun, J.; Li, Y.; Zhang, D. Association of Coffee, Decaffeinated Coffee and Caffeine Intake from Coffee with Cognitive Performance in Older Adults: National Health and Nutrition Examination Survey (NHANES) 2011-2014. Nutrients 2020, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Goto, A.; Noma, H.; Iso, H.; Hayashi, K.; Noda, M. Effects of Coffee and Tea Consumption on Glucose Metabolism: A Systematic Review and Network Meta-Analysis. Nutrients 2018, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Coffee Report and Outlook, 2023, International Coffee Organization. Available online: https://icocoffee.org/documents/cy2023-24/Coffee_Report_and_Outlook_December_2023_ICO.pdf (accessed on 19 June 2024).
- National Coffee Data Trends 2022. n.d., National Coffee Association. Available online: https://www.ncausa.org/Newsroom/Coffee-consumption-hits-two-decade-high-2022-NCDT (accessed on 19 June 2024).
- Thiriet, M. Cardiovascular Disease: An Introduction. Vasculopathies. 2018, 1–90. [Google Scholar]
- World Heart Report 2023: Confronting the World’s Number One Killer; World Heart Federation: Geneva, Switzerland, 2023; Available online: https://world-heart-federation.org/wp-content/uploads/World-Heart-Report-2023.pdf (accessed on 19 June 2024).
- Coronado, F.; Melvin, S.C.; Bell, R.A.; Zhao, G. Global Responses to Prevent, Manage, and Control Cardiovascular Diseases. Prev Chronic Dis 2022, 19, E84. [Google Scholar] [CrossRef] [PubMed]
- Rodak, K.; Kokot, I.; Kratz, E.M. Caffeine as a Factor Influencing the Functioning of the Human Body-Friend or Foe? Nutrients 2021, 13, 3088. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Xu, C.; Xu, J.; Jiang, Z.; Liu, Q.; Liang, J.; Gu, A. Association of urinary caffeine and caffeine metabolites with cardiovascular disease risk in adults. Nutrition 2021, 84, 111121. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Xu, Y.M.; Lau, A.T.Y. The Epigenetic Effects of Coffee. Molecules 2023, 28, 1770. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E. ; Wlaź; P Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int J Mol Sci 2020, 22, 107. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sebastiao, A.M. Caffeine and Adenosine. J Alzheimers Dis. 2010, Suppl 1, S3–15. [Google Scholar] [CrossRef]
- Evans, J.; Richards, J.R.; Battisti, A.S. Caffeine in StatPearls. 2023, Treasure Island (FL): StatPearls Publishing. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519490/ (accessed on 19 June 2024).
- Cappelletti, S.; Piacentino, D.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr Neuropharmacol 2015, 13, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H.; Crozier, A. Caffeine: a well known but little mentioned compound in plant science. Trends Plant Sci 2001, 6, 407–413. [Google Scholar] [CrossRef]
- Pharmacology of Caffeine. Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations; National Academies Press (US): Washington, DC, USA, 2001; Available online: https://www.ncbi.nlm.nih.gov/books/NBK223808/ (accessed on 19 June 2024).
- Stefanello, N.; Spanevello, R.M.; Passamonti, S.; Porciúncula, L.; Bonan, C.D.; Olabiyi, A.A.; Teixeira da Rocha, J.B.; Assmann, C.E.; Morsch, V.M.; Schetinger, M.R.C. Coffee, caffeine, chlorogenic acid, and the purinergic system. Food Chem Toxicol 2019, 123, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol Rev 2018, 70, 384–411. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzewski, J.; Bartsch, F.; Köller, A.; König, M. Pharmacokinetics of Caffeine: A Systematic Analysis of Reported Data for Application in Metabolic Phenotyping and Liver Function Testing. Front Pharmacol 2021, 12, 752826. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Kacprowski, T.; Menni, C.; Gustafsson, S.; Pivin, E.; Adamski, J.; Artati, A.; Eap, C.B.; Ehret, G.; Friedrich, N.; et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet 2016, 25, 5472–5482. [Google Scholar] [CrossRef] [PubMed]
- Urry, E.; Jetter, A.; Landolt, H.P. Assessment of CYP1A2 enzyme activity in relation to type-2 diabetes and habitual caffeine intake. Nutr Metab (Lond) 2016, 13, 66. [Google Scholar] [CrossRef]
- Bjørngaard, J.H.; Nordestgaard, A.T.; Taylor, A.E.; Treur, J.L.; Gabrielsen, M.E.; Munafò, M.R.; Nordestgaard, B.G.; Åsvold, B.O.; Romundstad, P.; Davey Smith, G. Heavier smoking increases coffee consumption: findings from a Mendelian randomization analysis. Int J Epidemiol 2017, 46, 1958–1967. [Google Scholar] [CrossRef]
- Djordjevic, N.; Ghotbi, R.; Bertilsson, L.; Jankovic, S.; Aklillu, E. Induction of CYP1A2 by heavy coffee consumption is associated with the CYP1A2 -163C>A polymorphism. Eur J Clin Pharmacol 2010, 66, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Carswell, A.T.; Howland, K.; Martinez-Gonzalez, B.; Baron, P.; Davison, G. The effect of caffeine on cognitive performance is influenced by CYP1A2 but not ADORA2A genotype, yet neither genotype affects exercise performance in healthy adults. Eur J Appl Physiol 2020, 120, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Kiely, J. Are the Current Guidelines on Caffeine Use in Sport Optimal for Everyone? Inter-individual Variation in Caffeine Ergogenicity, and a Move Towards Personalised Sports Nutrition. Sports Med 2018, 48, 7–16. [Google Scholar] [CrossRef]
- dePaula, J.; Farah, A. Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Graham, T.E.; Sathasivam, P.; Rowland, M.; Marko, N.; Greer, F.; Battram, D. Caffeine ingestion elevates plasma insulin response in humans during an oral glucose tolerance test. Can J Physiol Pharmacol 2001, 79, 559–565. [Google Scholar] [CrossRef]
- Salomone, F.; Galvano, F.; Li Volti, G. Molecular Bases Underlying the Hepatoprotective Effects of Coffee. Nutrients 2017, 9, 85. [Google Scholar] [CrossRef]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr Res 2020, 80, 1–17. [Google Scholar] [CrossRef]
- Shushtari, N.; Abtahi Froushani, S.M. Caffeine Augments The Instruction of Anti-Inflammatory Macrophages by The Conditioned Medium of Mesenchymal Stem Cells. Cell J 2017, 19, 415–424. [Google Scholar]
- Kong, H.; Jones, P.P.; Koop, A.; Zhang, L.; Duff, H.J.; Chen, S.R. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J 2008, 414, 441–452. [Google Scholar] [CrossRef]
- Rosser, J.I.; Walsh, B.; Hogan, M.C. Effect of physiological levels of caffeine on Ca2+ handling and fatigue development in Xenopus isolated single myofibers. Am J Physiol Regul Integr Comp Physiol 2009, 296, R1512–R1517. [Google Scholar]
- Scientific Opinion on the safety of caffeine. EFSA Journal 2015, 13. Available online: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.4102 (accessed on 19 June 2024).
- Pina Cabral, J.; Sousa, D.L.; Carvalho, C.; Girao, A.; Pacheco Mendes, A.; Pina, R. Caffeine Intoxication: Unregulated, Over-the-Counter Sale of Potentially Deadly Supplements. Cureus 2022, 14, e21045. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit Contam 2003, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Budney, A.J.; Brown, P.C.; Griffiths, R.R.; Hughes, J.R.; Juliano, L.M. Caffeine Withdrawal and Dependence: A Convenience Survey Among Addiction Professionals. J Caffeine Res 2013, 3, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Meredit, S.E.; Juliano, L.M.; Hughes, J.R.; Griffiths, R.R. Caffeine Use Disorder: A Comprehensive Review and Research Agenda. J Caffeine Res 2013, 3, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and Classification of Adenosine Receptors. Pharmacol Rev. 2001, 53, 527–552. [Google Scholar] [PubMed]
- Vincenzi, F.; Pasquini, S.; Contri, C.; Cappello, M.; Nigro, M.; Travagli, A.; Merighi, S.; Gessi, S.; Borea, P.A.; Varani, K. Pharmacology of Adenosine Receptors: Recent Advancements. Biomolecules 2023, 13, 1387. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol Rev 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Ravid, K. Biology of Platelet Purinergic Receptors and Implications for Platelet Heterogeneity. Front Pharmacol 2018, 9, 37. [Google Scholar] [CrossRef]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine receptors: expression, function and regulation. Int J Mol Sci 2014, 15, 2024–2052. [Google Scholar] [CrossRef]
- Vecchio, E.A.; Baltos, J.A.; Nguyen, A.T.N.; Christopoulos, A.; White, P.J.; May, L.T. New paradigms in adenosine receptor pharmacology: allostery, oligomerization and biased agonism. Br J Pharmacol 2018, 175, 4036–4046. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Fuxe, K. Oligomeric Receptor Complexes and Their Allosteric Receptor-Receptor Interactions in the Plasma Membrane Represent a New Biological Principle for Integration of Signals in the CNS. Front Mol Neurosci 2019, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Sarasola, L.I.; Quiroz, C.; Ciruela, F. Presynaptic adenosine receptor heteromers as key modulators of glutamatergic and dopaminergic neurotransmission in the striatum. Neuropharmacology 2023, 223, 109329. [Google Scholar] [CrossRef] [PubMed]
- Peleli, M.; Fredholm, B.B.; Sobrevia, L.; Carlström, M. Pharmacological targeting of adenosine receptor signaling. Mol Aspects Med 2017, 55, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Guieu, R.; Deharo, J.C.; Maille, B.; Crotti, L.; Torresani, E.; Brignole, M.; Parati, G. Adenosine and the Cardiovascular System: The Good and the Bad. J Clin Med 2020, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Headrick, J.P.; Ashton, K.J.; Rose'meyer, R.B.; Peart, J.N. Cardiovascular adenosine receptors: Expression, actions and interactions. Pharmacology & Therapeutics 2013, 140, 92–111. [Google Scholar]
- Hussain, T.; Mustafa, S.J. Binding of Ax Adenosine Receptor Ligand [3H] 8-Cyclopentyl-l,3-Dipropylxanthine in Coronary Smooth Muscle. Circulation Research 1995, 77, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Klaasse, E.C.; Ijzerman, A.P.; de Grip, W.J.; Beukers, M.W. Internalization and desensitization of adenosine receptors. Purinergic Signal 2008, 4, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef]
- Zhao, Z.; Makaritsis, K.; Francis, C.E.; Gavras, H.; Ravid, K. A role for the A3 adenosine receptor in determining tissue levels of cAMP and blood pressure: studies in knock-out mice. Biochim Biophys Acta 2000, 1500, 280–290. [Google Scholar] [CrossRef]
- Belardinelli, L.; Shryock, J.C.; Song, Y.; Wang, D.; Srinivas, M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. The FASEB Journal 1995, 9, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Umemura, S.; Toya, Y.; Uchibori, T.; Kogi, K.; Takagi, N.; Ishii, M. Identification of Adenosine A2 Receptor-cAMP System in Human Aortic Endothelial Cells. Biochem Biophys Res Commun 1994, 199, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhao, J.; Xu, X.; Zhang, D.; Shen, H.; Wang, S. Role of adenosine A2a receptor in cancers and autoimmune diseases. Immun Inflamm Dis 2023, 11, e826. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.G., Jr.; Fenton, R.A. Adenosine A receptor function in rat ventricular myocytes. Cardiovasc Res 1996, 34, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.R.; Talukder, M.A.; Ledent, C.; Mustafa, S.J. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am J Physiol Heart Circ Physiol 2002, 282, H437–H444. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, A.; Patterson, S.; Ravid, K. The Many Faces of the A2b Adenosine Receptor in Cardiovascular and Metabolic Diseases. J Cell Physiol 2015, 230, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, H.E.; Schnermann, J.; Oldenburg, P.J.; Mustafa, S.J. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol 2005, 288, H1411–H1416. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, V.J.; Lasley, R.D. Adenosine receptor-mediated cardioprotection: are all 4 subtypes required or redundant? J Cardiovasc Pharmacol Ther 2012, 17, 21–33. [Google Scholar] [CrossRef]
- Feoktistov, I.; Biaggioni, I.; Cronstein, B.N. Adenosine receptors in wound healing, fibrosis and angiogenesis. Handb Exp Pharmacol 2009, 193, 383–397. [Google Scholar]
- Surma, S.; Oparil, S. Coffee and Arterial Hypertension. Curr Hypertens Rep 2021, 23, 38. [Google Scholar] [CrossRef]
- Mustafa, S.J.; Morrison, R.R.; Teng, B.; Pelleg, A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol 2009, 161–188. [Google Scholar]
- Wolska, N.; Rozalski, M. Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy. Int J Mol Sci 2019, 20, 5475. [Google Scholar] [CrossRef] [PubMed]
- Repsold, L.; Joubert, A.M. Platelet Function, Role in Thrombosis, Inflammation, and Consequences in Chronic Myeloproliferative Disorders. Cells 2021, 10, 3034. [Google Scholar] [CrossRef] [PubMed]
- Smyth, S.S.; McEver, R.P.; Weyrich, A.S.; Morrell, C.N.; Hoffman, M.R.; Arepally, G.M.; French, P.A.; Dauerman, H.L.; Becker, R.C. 2009 Platelet Colloquium Participants.Platelet functions beyond hemostasis. J Thromb Haemost 2009, 7, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev 2017, 36, 195–198. [Google Scholar] [CrossRef]
- Ho-Tin-Noe, B.; Demers, M.; Wagner, D.D. How platelets safeguard vascular integrity. J Thromb Haemost 2011, 9, 56–65. [Google Scholar] [CrossRef]
- Halucha, K.; Rak-Pasikowska, A.; Bil-Lula, I. Protective Role of Platelets in Myocardial Infarction and Ischemia/Reperfusion Injury. Cardiol Res Pract 2021, 2021, 5545416. [Google Scholar] [CrossRef] [PubMed]
- Ledent, C.; Vaugeois, J.M.; Schiffmann, S.N.; Pedrazzini, T.; El Yacoubi, M.; Vanderhaeghen, J.J.; Costentin, J.; Heath, J.K.; Vassart, G.; Parmentier, M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 1997, 388, 674–678. [Google Scholar] [CrossRef]
- Yang, D.; Chen, H.; Koupenova, M.; Carroll, S.H.; Eliades, A.; Freedman, J.E.; Toselli, P.; Ravid, K. A new role for the A2b adenosine receptor in regulating platelet function. J Thromb Haemost 2010, 8, 817–827. [Google Scholar] [CrossRef]
- Johnston-Cox, H.A.; Ravid, K. Adenosine and blood platelets. Purinergic Signal 2011, 7, 357–365. [Google Scholar] [CrossRef]
- Fernández-Dueñas, V.; Gómez-Soler, M.; López-Cano, M.; Taura, J.J.; Ledent, C.; Watanabe, M.; Jacobson, K.A.; Vilardaga, J.P.; Ciruela, F. Uncovering caffeine's adenosine A2A receptor inverse agonism in experimental parkinsonism. ACS Chem Biol 2014, 9, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Ammaturo, V.; Perricone, C.; Canazio, A.; Ripaldi, M.; Ruggiano, A.; Zuccarelli, B.; Monti, M. Caffeine stimulates in vivo platelet reactivity. Acta Med Scand 1988, 224, 245–247. [Google Scholar] [CrossRef]
- Ardlie, N.G.; Glew, G.; Schultz, B.G.; Schwartz, C.J. Inhibition and reversal of platelet aggregation by methyl xanthines. Thrombosis and Haemostasis 1967, 18, 670–673. [Google Scholar] [CrossRef]
- Bhaskar, S.; Rauf, A.A. Modulatory effect of coffee on platelet function. Indian J Physiol Pharmacol 2010, 54, 141–148. [Google Scholar] [PubMed]
- Bydlowski, S.P.; Yunker, R.L.; Rymaszewski, Z.; Subbiah, M.T. Coffee extracts inhibit platelet aggregation in vivo and in vitro. Int J Vitam Nutr Res 1987, 57, 217–223. [Google Scholar] [PubMed]
- Bygdeman, S.; Johnsen, O. Methyl xantines in the inhibition of platelet aggregation. Acta Med Scand Suppl 1971, 525, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.W.; Santos PRJr Menezes, M.G.; Marques, H.O.; Cavalcante, L.P.; Pacheco, W.S. Influence of caffeine on blood pressure and platelet aggregation. Arq Bras Cardiol 2000, 75, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Colli, S.; Gianfranceschi, G.; Maderna, P.; Petroni, A.; Tremoli, E.; Marinovich, M.; Sirtori, C.R. Acute effects of ethanol, caffeine, or both on platelet aggregation, thromboxane formation, and plasma-free fatty acids in normal subjects. Drug Nutr Interact 1984, 3, 61–67. [Google Scholar] [PubMed]
- Monti, M.; Edvinsson, L.; Ranklev, E.; Fletcher, R. Methylxanthines reduce in vitro human overall platelet metabolism as measured by microcalorimetry. Acta Med Scand 1986, 220, 185–188. [Google Scholar] [CrossRef]
- Naito, S.; Yatagai, C.; Maruyama, M.; Sumi, H. Effect of coffee extracts on plasma fibrinolysis and platelet aggregation. Nihon Arukoru Yakubutsu Igakkai Zasshi 2011, 46, 260–269. [Google Scholar]
- Natella, F.; Nardini, M.; Belelli, F.; Pignatelli, P.; Di Santo, S.; Ghiselli, A.; Violi, F.; Scaccini, C. Effect of coffee drinking on platelets: inhibition of aggregation and phenols incorporation. Br J Nutr 2008, 100, 100,1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W. Influence of caffeine on the responsiveness of human platelet to agonists. Thromb Res 2003, 110, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Montoya, G.A.; Bakuradze, T.; Eirich, M.; Erk, T.; Baum, M.; Habermeyer, M.; Eisenbrand, G.; Richling, E. Modulation of 3',5'-cyclic AMP homeostasis in human platelets by coffee and individual coffee constituents. Br J Nutr 2014, 112, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Biaggioni, I.; Paul, S.; Puckett, A.; Arzubiaga, C. Caffeine and theophylline as adenosine receptor antagonists in humans. J Pharmacol Exp Ther 1991, 258, 588–593. [Google Scholar] [PubMed]
- Varani, K.; Portaluppi, F.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Caffeine alters A2A adenosine receptors and their function in human platelets. Circulation 1999, 99, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Portaluppi, F.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors : functional and biochemical aspects. Circulation 2000, 102, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Boncler, M.; Wzorek, J.; Wolska, N.; Polak, D.; Watala, C.; Rozalski, M. Adenosine receptor agonists deepen the inhibition of platelet aggregation by P2Y12 antagonists. Vascul Pharmacol 2019, 113, 47–56. [Google Scholar] [CrossRef]
- Wolska, N.; Kassassir, H.; Luzak, B.; Watala, C.; Rozalski, M. Adenosine Receptor Agonists Increase the Inhibition of Platelet Function by P2Y(12) Antagonists in a cAMP- and Calcium-Dependent Manner. Pharmaceuticals (Basel) 2020, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Wolska, N.; Boncler, M.; Polak, D.; Wzorek, J.; Przygodzki, T.; Gapinska, M.; Watala, C.; Rozalski, M. Adenosine Receptor Agonists Exhibit Anti-Platelet Effects and the Potential to Overcome Resistance to P2Y(12) Receptor Antagonists. Molecules 2019, 25, 130. [Google Scholar] [CrossRef]
- Polak, D.; Talar, M.; Wolska, N.; Wojkowska, D.W.; Karolczak, K.; Kramkowski, K.; Bonda, T.A.; Watala, C.; Przygodzki, T. Adenosine Receptor Agonist HE-NECA Enhances Antithrombotic Activities of Cangrelor and Prasugrel in vivo by Decreasing of Fibrinogen Density in Thrombus. Int J Mol Sci 2021, 22, 3074. [Google Scholar] [CrossRef]
- Albino, L.; Sordi, R.; de Oliveira, G., Jr.; Daniel, F. Dose and Time-Dependent Effects of Caffeine on Cardiovascular Changes Induced by Adenosine. Brazilian Archives of Biology and Technology 2023, 66. [Google Scholar] [CrossRef]
- Alencar, A.K.N.; Montes, G.C.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. Adenosine Receptors As Drug Targets for Treatment of Pulmonary Arterial Hypertension. Front Pharmacol 2017, 8, 858. [Google Scholar] [CrossRef] [PubMed]
- Mcguire, M. Caffeine in Food and Dietary Supplements: Examining Safety: Workshop Summary. In Advances in nutrition (Bethesda, Md.); The National Academies Press: Washington, DC, USA, 2014; Volume 5, pp. 585–586. [Google Scholar]
- Ponnoth, D.S.; Sanjani, M.S.; Ledent, C.; Roush, K.; Krahn, T.; Mustafa, S.J. Absence of adenosine-mediated aortic relaxation in A(2A) adenosine receptor knockout mice. Am J Physiol Heart Circ Physiol 2009, 297, H1655–H1660. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M. Habitual coffee consumption and blood pressure: An epidemiological perspective. Vasc Health Risk Manag 2008, 4, 963–970. [Google Scholar] [CrossRef]
- Mesas, A.E.; Leon-Muñoz, L.M.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. Am J Clin Nutr 2011, 94, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Geethavani, G.; Rameswarudu, M.; Rameshwari, R.R. Effect of Caffeine on Heart Rate and Blood Pressure. International Journal of Scientific and Research Publications 2014, 4, 1–2. [Google Scholar]
- Mahmud, A.; Feely, J. Acute Effect of Caffeine on Arterial Stiffness and Aortic Pressure Waveform. Hypertension 2001, 8, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine's Vascular Mechanisms of Action. Int J Vasc Med 2010, 2010, 834060. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Panagiotakos, D.; Ioakeimidis, N.; Dima, I.; Stefanadis, C. Chronic coffee consumption has a detrimental effect on aortic stiffness and wave reflections. Am J Clin Nutr 2005, 81, 1307–1312. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Karatzi, K.; Karatzis, E.; Papamichael, C.; Lekakis, J.P. Acute effects of caffeine on arterial stiffness, wave reflections, and central aortic pressures. Am J Hypertens 2005, 18, 129–136. [Google Scholar] [CrossRef]
- Echeverri, D.; Pizano, A.; Montes, F.R.; Forcada, P. Acute effect of coffee consumption on arterial stiffness, evaluated using an oscillometric method. Artery Research 2017, 17, 16–32. [Google Scholar] [CrossRef]
- Li, P.; Mandilaras, G.; Jakob, A.; Dalla-Pozza, R.; Haas, N.A.; Oberhoffer, F.S. Energy Drinks and Their Acute Effects on Arterial Stiffness in Healthy Children and Teenagers: A Randomized Trial. J Clin Med 2022, 11, 2087. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.F.; Sulague, R.M.; Posas-Mendoza, T.; Lavie, C.J. Impact of Coffee Consumption on Cardiovascular Health. Ochsner J 2023, 23, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul Toxicol Pharmacol 2017, 89, 165–185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





