Submitted:
26 June 2024
Posted:
26 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
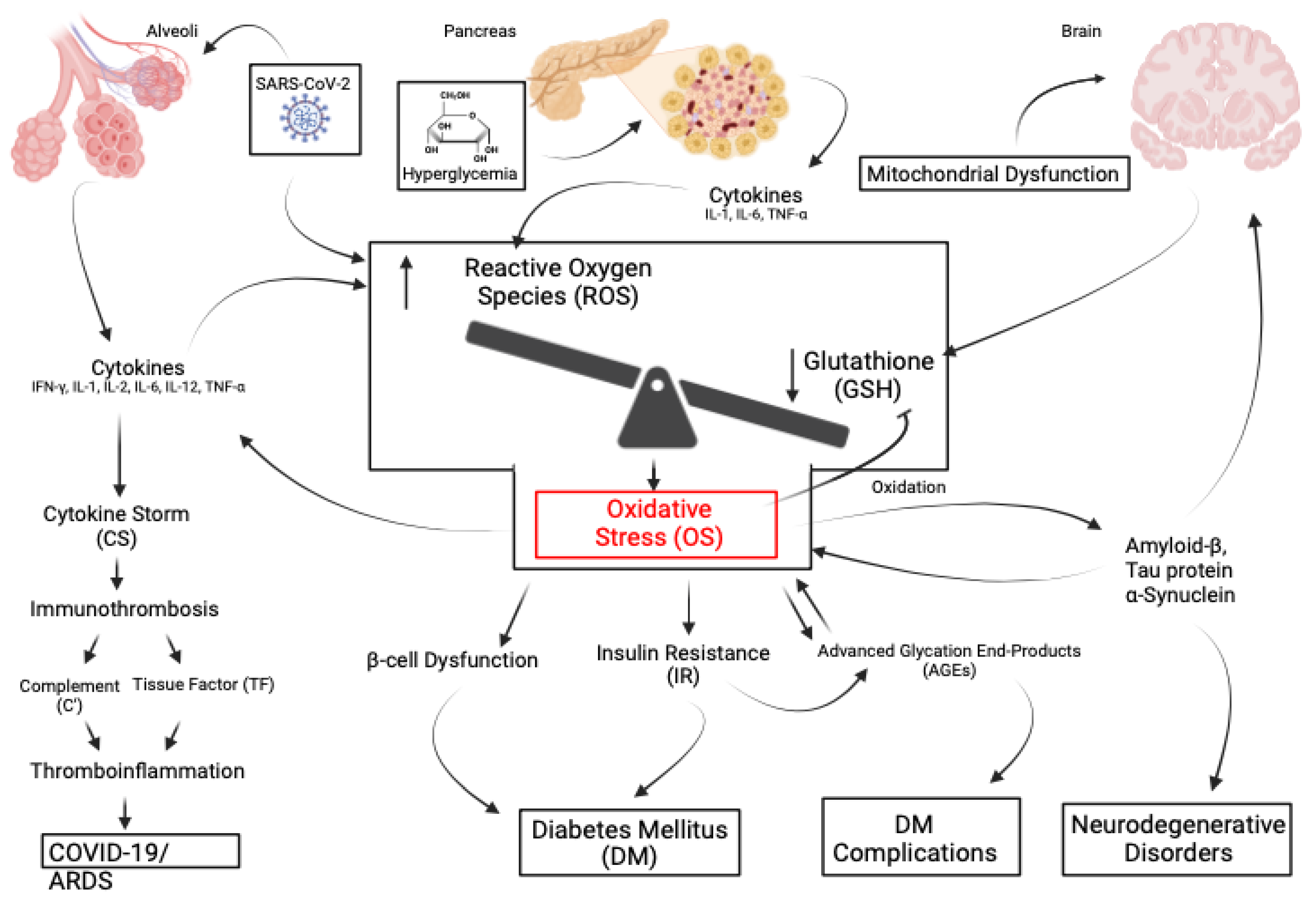
2. Oxidative Stress (OS)
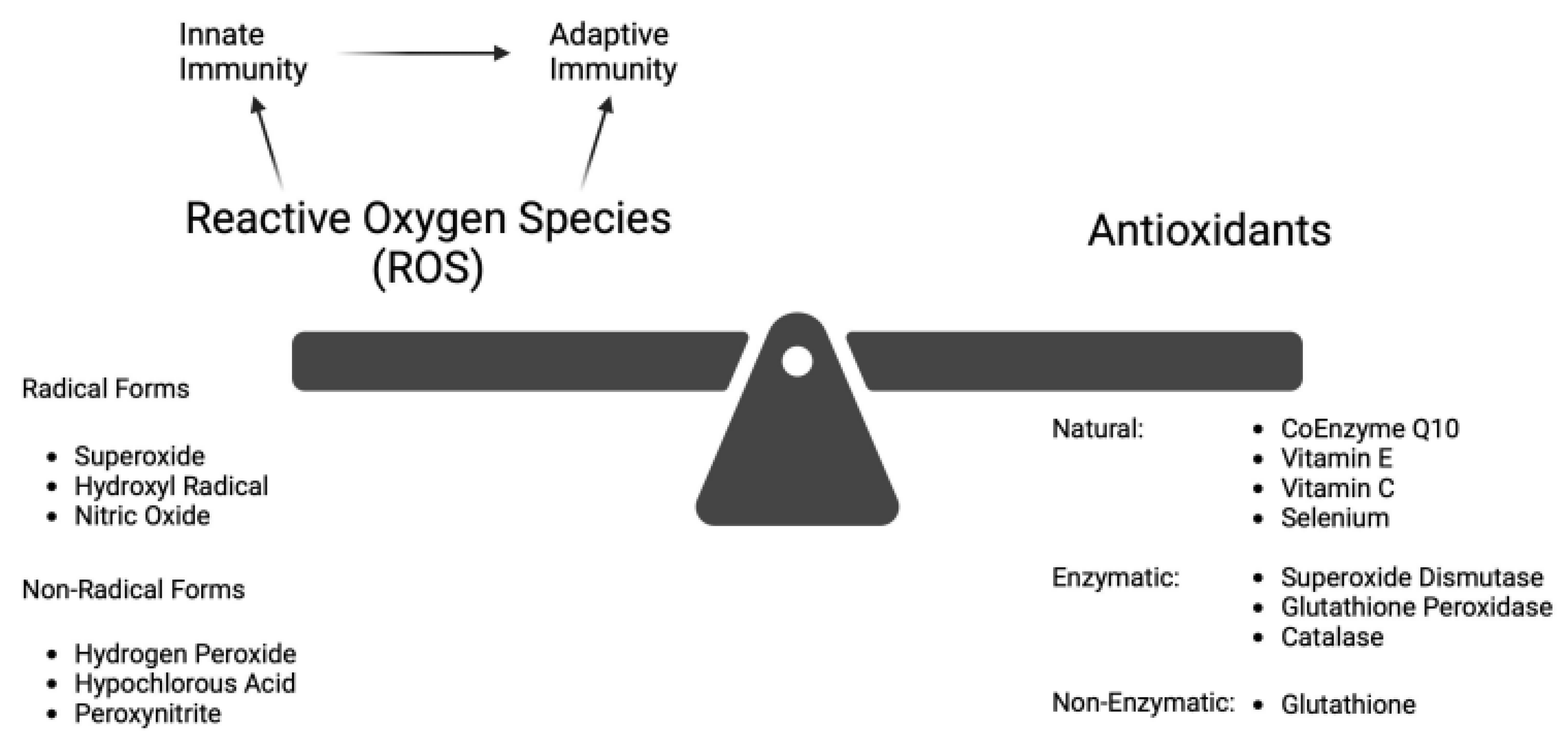
2.1. Reactive Oxygen Species and OS
2.2. Immunothrombosis and Thromboinflammation and OS
2.3. The Complement System and OS
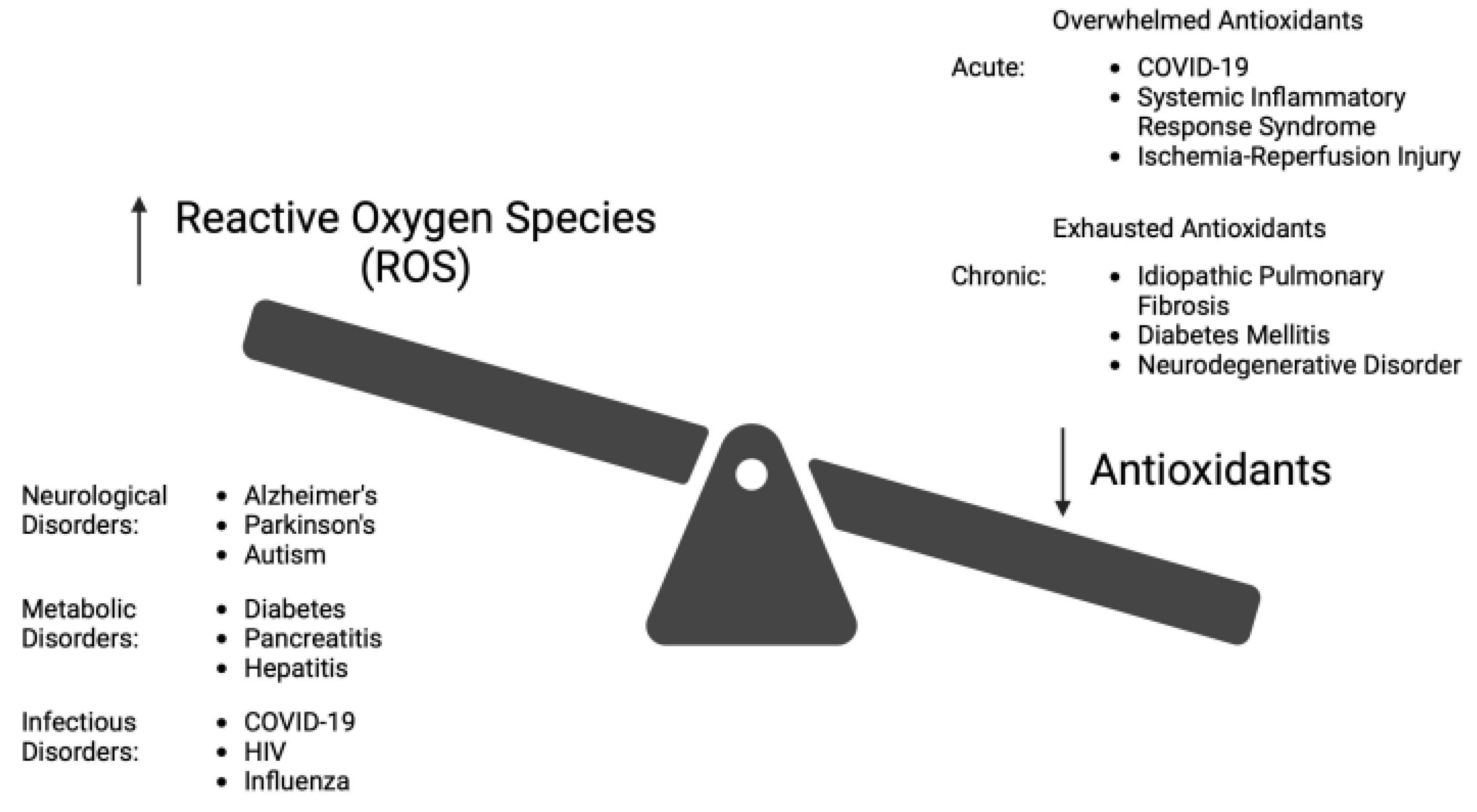
3. OS-Associated Diseases
3.1. COVID-19
3.2. Acute COVID-19
3.3. Long COVID
4. Diabetes and Its Complications
4.1. Diabetes Management
4.2. Diabetes Complications
4.3. Neurologic/Neurodegenerative Disorders
4.4. Alzheimer’s Disease
4.5. Parkinson’s Disease
4.6. Autism Spectrum Disorder
5. Encephalopathy
6. Fibrosis
7. OS-Associated Diseases
7.1. Pulmonary Fibrosis
7.2. Cystic Fibrosis
7.3. Hepatic Cirrhosis
7.4. Cardiovascular Disease
7.5. Viral Illnesses
7.5.1. Encephalitis/Thrombosis
7.5.2. Myocarditis
7.5.3. Hemorrhagic Fever
7.5.4. Respiratory Dysfunction
7.6. ISCHEMIA-Reperfusion Injury
7.6.1. Myocardial Ischemia
7.6.2. Central Nervous System Injuries
7.6.3. Organ Transplantation
7.6.4. Sickle Cell Disease
7.7. Pathologic Aging
7.8. Toxicities
7.8.1. Chemotherapy
7.8.2. Antiretroviral Therapy
7.8.3. Contrast-Induced Nephropathy
7.8.4. Acetaminophen Hepatotoxicity
7.8.5. Isoniazid and Rifampicin Hepatotoxicity
7.8.6. Amanitin Hepatotoxicity
7.9. Occular Diseases
7.9.1. Diabetic Retinopathy
7.9.2. Retinitis Pigmentosum
7.9.3. Age-Related Macular Degeneration
7.10. Systemic Inflammatory Response Syndrome/Sepsis
7.11. Pancreatitis
7.11.1. Acute Pancreatitis
7.11.2. Chronic Pancreatitis
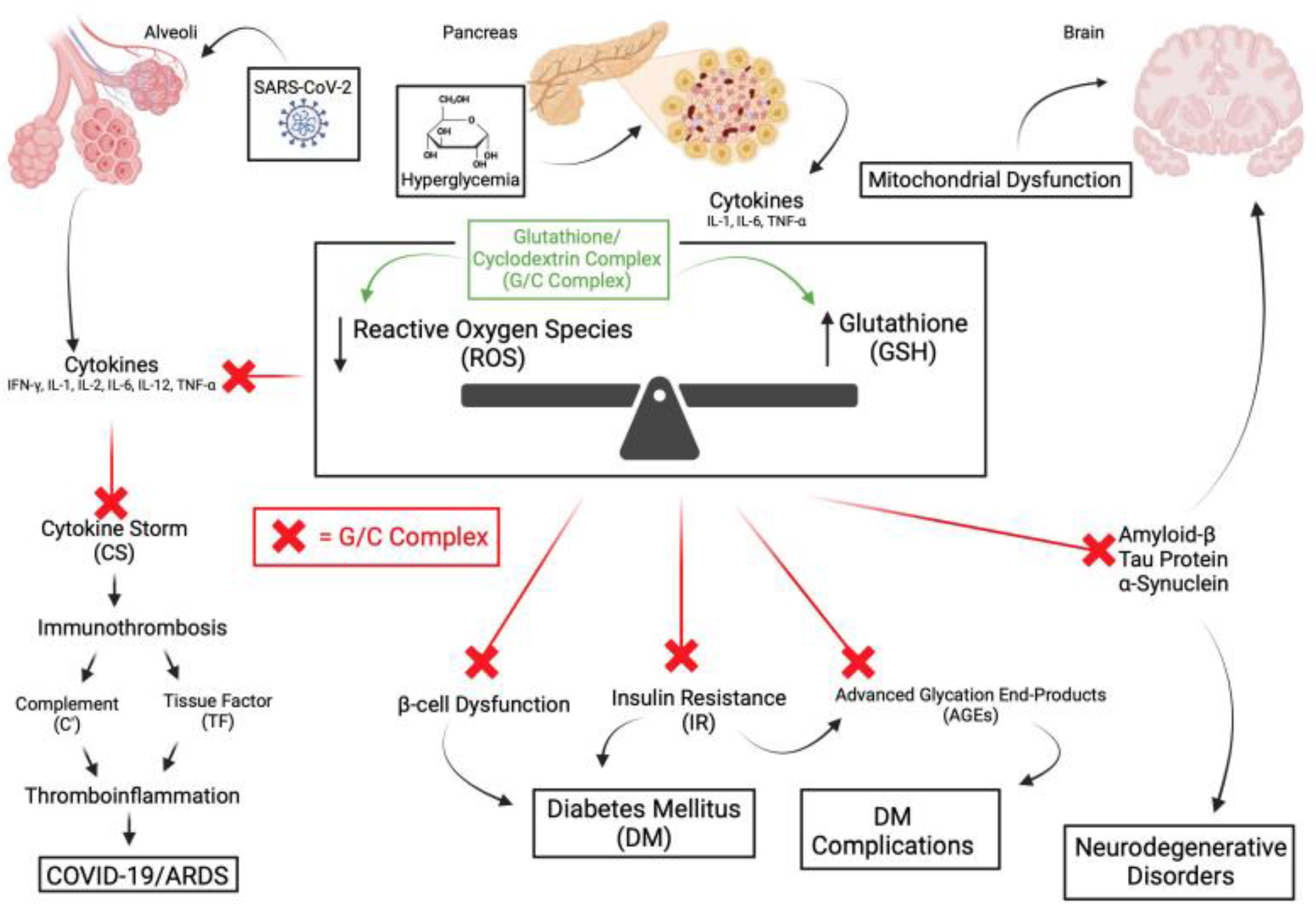
8. Discussion
9. Conclusions
Conflicts of Interest
References
- Sasaninia K, Kelley M, Abnousian A, et al. Topical Absorption of Glutathione-Cyclodextrin Nanoparticle Complex in Healthy Human Subjects Improves Immune Response against Mycobacterium avium Infection. Antioxidants (Basel). Jul 2 2023;12(7). [CrossRef]
- Patel N. The glutathione revolution : fight disease, slow aging, and increase energy with the master antioxidant. First edition. ed. Hachette Go, an imprint of Hachette Books; 2020:xix, 266 pages.
- Bardaweel SK, Gul M, Alzweiri M, Ishaqat A, HA AL, Bashatwah RM. Reactive Oxygen Species: the Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J Med. Oct 2018;50(3):193-201. [CrossRef]
- Pizzino G, Irrera N, Cucinotta M, et al. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:8416763. [CrossRef]
- Bassoy EY, Walch M, Martinvalet D. Reactive Oxygen Species: Do They Play a Role in Adaptive Immunity? Front Immunol. 2021;12:755856. [CrossRef]
- Tan BL, Norhaizan ME, Liew WP, Sulaiman Rahman H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front Pharmacol. 2018;9:1162. [CrossRef]
- Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757-772. [CrossRef]
- Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem. Apr 2020;467(1-2):1-12. [CrossRef]
- Hecker L. Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace. Am J Physiol Lung Cell Mol Physiol. Apr 1 2018;314(4):L642-L653. [CrossRef]
- Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. Mar 2009;390(3):191-214. [CrossRef]
- Garcia-Sanchez A, Miranda-Diaz AG, Cardona-Munoz EG. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid Med Cell Longev. 2020;2020:2082145. [CrossRef]
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. May-Jun 2003;57(3-4):145-55. [CrossRef]
- Yang J, Xu J, Xu S, et al. Oxidative stress in acute pulmonary embolism: emerging roles and therapeutic implications. Thromb J. Jan 12 2024;22(1):9. [CrossRef]
- Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. Sep 2021;20(9):689-709. [CrossRef]
- Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid Med Cell Longev. 2016;2016:1245049. [CrossRef]
- Silvagno F, Vernone A, Pescarmona GP. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants (Basel). Jul 16 2020;9(7). [CrossRef]
- Glassman I, Le N, Mirhosseini M, et al. The Role of Glutathione in Prevention of COVID-19 Immunothrombosis: A Review. Front Biosci (Landmark Ed). Mar 20 2023;28(3):59. [CrossRef]
- Almeida B, Domingues C, Mascarenhas-Melo F, et al. The Role of Cyclodextrins in COVID-19 Therapy-A Literature Review. Int J Mol Sci. Feb 3 2023;24(3). [CrossRef]
- Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. Feb 2014;141(2):150-9. [CrossRef]
- Hristov BD. The Role of Glutathione Metabolism in Chronic Illness Development and Its Potential Use as a Novel Therapeutic Target. Cureus. Sep 2022;14(9):e29696. [CrossRef]
- Alleman RJ, Katunga LA, Nelson MA, Brown DA, Anderson EJ. The “Goldilocks Zone” from a redox perspective-Adaptive vs. deleterious responses to oxidative stress in striated muscle. Front Physiol. 2014;5:358. [CrossRef]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. Mar 2004;134(3):489-92. [CrossRef]
- Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. Sep 2021;18(9):666-682. [CrossRef]
- Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. Sep 2018;15(9):505-522. [CrossRef]
- Perricone C, De Carolis C, Perricone R. Glutathione: a key player in autoimmunity. Autoimmun Rev. Jul 2009;8(8):697-701. [CrossRef]
- Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. Feb 28 2019;133(9):906-918. [CrossRef]
- Bai Y, Li K, Li X, et al. Effects of oxidative stress on hepatic encephalopathy pathogenesis in mice. Nat Commun. Jul 24 2023;14(1):4456. [CrossRef]
- Wang W, Zhao F, Ma X, Perry G, Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol Neurodegener. May 29 2020;15(1):30. [CrossRef]
- Subramaniam S, Jurk K, Hobohm L, et al. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood. Apr 20 2017;129(16):2291-2302. [CrossRef]
- Rawish E, Sauter M, Sauter R, Nording H, Langer HF. Complement, inflammation and thrombosis. Br J Pharmacol. Jul 2021;178(14):2892-2904. [CrossRef]
- Bettiol A, Galora S, Argento FR, et al. Erythrocyte oxidative stress and thrombosis. Expert Rev Mol Med. Aug 26 2022;24:e31. [CrossRef]
- Sagcan G, Konukoglu D, Uzun H, Arseven O, Okumus G, Cuhadaroglu C. Importance of oxidative stress in the evaluation of acute pulmonary embolism severity. BMC Pulm Med. Oct 17 2022;22(1):382. [CrossRef]
- Tran S, Ksajikian A, Overbey J, Li P, Li Y. Pathophysiology of Pulmonary Fibrosis in the Context of COVID-19 and Implications for Treatment: A Narrative Review. Cells. Aug 11 2022;11(16). [CrossRef]
- Patrucco F, Solidoro P, Gavelli F, Apostolo D, Bellan M. Idiopathic Pulmonary Fibrosis and Post-COVID-19 Lung Fibrosis: Links and Risks. Microorganisms. Mar 30 2023;11(4). [CrossRef]
- Cheresh P, Kim SJ, Tulasiram S, Kamp DW. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta. Jul 2013;1832(7):1028-40. [CrossRef]
- Estornut C, Milara J, Bayarri MA, Belhadj N, Cortijo J. Targeting Oxidative Stress as a Therapeutic Approach for Idiopathic Pulmonary Fibrosis. Front Pharmacol. 2021;12:794997. [CrossRef]
- Causer AJ, Shute JK, Cummings MH, et al. Circulating biomarkers of antioxidant status and oxidative stress in people with cystic fibrosis: A systematic review and meta-analysis. Redox Biol. May 2020;32:101436. [CrossRef]
- Moliteo E, Sciacca M, Palmeri A, et al. Cystic Fibrosis and Oxidative Stress: The Role of CFTR. Molecules. Aug 21 2022;27(16). [CrossRef]
- Honda Y, Kessoku T, Sumida Y, et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: an open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. Aug 8 2017;17(1):96. [CrossRef]
- Masarone M, Rosato V, Dallio M, et al. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid Med Cell Longev. 2018;2018:9547613. [CrossRef]
- Al-Hakeim HK, Al-Rubaye HT, Al-Hadrawi DS, Almulla AF, Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol Psychiatry. Feb 2023;28(2):564-578. [CrossRef]
- Alrajhi NN. Post-COVID-19 pulmonary fibrosis: An ongoing concern. Ann Thorac Med. Oct-Dec 2023;18(4):173-181. [CrossRef]
- Chen Q, Wang Q, Zhu J, Xiao Q, Zhang L. Reactive oxygen species: key regulators in vascular health and diseases. Br J Pharmacol. Apr 2018;175(8):1279-1292. [CrossRef]
- Li L, Stegner D. Immunothrombosis versus thrombo-inflammation: platelets in cerebrovascular complications. Res Pract Thromb Haemost. Jan 2024;8(1):102344. [CrossRef]
- Trakkides TO, Schafer N, Reichenthaler M, et al. Oxidative Stress Increases Endogenous Complement-Dependent Inflammatory and Angiogenic Responses in Retinal Pigment Epithelial Cells Independently of Exogenous Complement Sources. Antioxidants (Basel). Nov 13 2019;8(11). [CrossRef]
- Labarrere CA, Kassab GS. Glutathione: A Samsonian life-sustaining small molecule that protects against oxidative stress, ageing and damaging inflammation. Front Nutr. 2022;9:1007816. [CrossRef]
- Cervia-Hasler C, Bruningk SC, Hoch T, et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science. Jan 19 2024;383(6680):eadg7942. [CrossRef]
- Labarrere CA, Kassab GS. Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease. Front Microbiol. 2022;13:979719. [CrossRef]
- Bhaskar S, Sinha A, Banach M, et al. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front Immunol. 2020;11:1648. [CrossRef]
- Gain C, Song S, Angtuaco T, Satta S, Kelesidis T. The role of oxidative stress in the pathogenesis of infections with coronaviruses. Front Microbiol. 2022;13:1111930. [CrossRef]
- Yiang GT, Wu YK, Tsai KW, et al. Immunothrombosis biomarkers as potential predictive factors of acute respiratory distress syndrome in moderate-to-critical COVID-19: A single-center, retrospective cohort study. Immunol Lett. Feb 2023;254:30-38. [CrossRef]
- Li H, Wu Q, Qin Z, et al. Serum levels of laminin and von Willebrand factor in COVID-19 survivors 6 months after discharge. Int J Infect Dis. Feb 2022;115:134-141. [CrossRef]
- Polonikov A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect Dis. Jul 10 2020;6(7):1558-1562. [CrossRef]
- Liu X, Wang X, Chang J, Zhang H, Cao P. Landscape analysis and overview of the literature on oxidative stress and pulmonary diseases. Front Pharmacol. 2023;14:1190817. [CrossRef]
- Wong KH, Xie Y, Huang X, et al. Delivering Crocetin across the Blood-Brain Barrier by Using gamma-Cyclodextrin to Treat Alzheimer’s Disease. Sci Rep. Feb 27 2020;10(1):3654. [CrossRef]
- Wang Q, Zennadi R. Oxidative Stress and Thrombosis during Aging: The Roles of Oxidative Stress in RBCs in Venous Thrombosis. Int J Mol Sci. Jun 15 2020;21(12). [CrossRef]
- Karkhanei B, Talebi Ghane E, Mehri F. Evaluation of oxidative stress level: total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19. New Microbes New Infect. Jul 2021;42:100897. [CrossRef]
- Roessler C, de Oliveira KCS, de Oliveira Portella AX, et al. Evaluation of oxidative stress level: reactive oxygen species, reduced glutathione, and D-dimer in patients hospitalized due to COVID-19. Redox Rep. Dec 2023;28(1):1-6. [CrossRef]
- Marseglia L, Manti S, D’Angelo G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. Dec 26 2014;16(1):378-400. [CrossRef]
- Meftahi GH, Jangravi Z, Sahraei H, Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of “inflame-aging”. Inflamm Res. Sep 2020;69(9):825-839. [CrossRef]
- Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. Dec 3 2020;383(23):2255-2273. [CrossRef]
- COVID-19 updates: NIH outpatient treatment guidelines. Med Lett Drugs Ther. Feb 21 2022;64(1644):32.
- Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. Sep 2020;57(6):389-399. [CrossRef]
- Cappanera S, Palumbo M, Kwan SH, et al. When Does the Cytokine Storm Begin in COVID-19 Patients? A Quick Score to Recognize It. J Clin Med. Jan 15 2021;10(2). [CrossRef]
- Neves FF, Pott-Junior H, Yamashita KMC, et al. Do the oxidative stress biomarkers predict COVID-19 outcome? An in-hospital cohort study. Free Radic Biol Med. Oct 2023;207:194-199. [CrossRef]
- Rubin R. Paxlovid Is Effective but Underused-Here’s What the Latest Research Says About Rebound and More. JAMA. Jan 31 2024. [CrossRef]
- Yutani R, Venketaraman V. The COVID-19 Illness: Addressing the Current Treatment Limitations and Care Gaps with a Novel Alternative and Complementary Agent-the Glutathione-Cyclodextrin Complex. Altern Ther Health Med. May 2023;29(4):28-35.
- Haunhorst S, Bloch W, Wagner H, et al. Long COVID: a narrative review of the clinical aftermaths of COVID-19 with a focus on the putative pathophysiology and aspects of physical activity. Oxf Open Immunol. 2022;3(1):iqac006. [CrossRef]
- Jason LA, Dorri JA. ME/CFS and Post-Exertional Malaise among Patients with Long COVID. Neurol Int. Dec 20 2022;15(1):1-11. [CrossRef]
- Hama Amin BJ, Kakamad FH, Ahmed GS, et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann Med Surg (Lond). May 2022;77:103590. [CrossRef]
- Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. Nov 2022;28(11):2406-2415. [CrossRef]
- Sharma C, Bayry J. High risk of autoimmune diseases after COVID-19. Nat Rev Rheumatol. Jul 2023;19(7):399-400. [CrossRef]
- Vollbracht C, Kraft K. Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C. Front Pharmacol. 2022;13:899198. [CrossRef]
- Bonner C, Ghouralal SL. Long COVID and Chronic Conditions in the US Workforce: Prevalence, Productivity Loss, and Disability. J Occup Environ Med. Mar 1 2024;66(3):e80-e86. [CrossRef]
- Komaroff AL, Lipkin WI. ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med (Lausanne). 2023;10:1187163. [CrossRef]
- Bai NA, Richardson CS. Posttreatment Lyme disease syndrome and myalgic encephalomyelitis/chronic fatigue syndrome: A systematic review and comparison of pathogenesis. Chronic Dis Transl Med. Sep 2023;9(3):183-190. [CrossRef]
- Santopaolo M, Gregorova M, Hamilton F, et al. Prolonged T-cell activation and long COVID symptoms independently associate with severe COVID-19 at 3 months. Elife. Jun 13 2023;12. [CrossRef]
- Proal AD, VanElzakker MB, Aleman S, et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat Immunol. Oct 2023;24(10):1616-1627. [CrossRef]
- Ruiz-Pablos M, Paiva B, Zabaleta A. Epstein-Barr virus-acquired immunodeficiency in myalgic encephalomyelitis-Is it present in long COVID? J Transl Med. Sep 17 2023;21(1):633. [CrossRef]
- Zhang Z, Zhang X, Fang X, et al. Glutathione inhibits antibody and complement-mediated immunologic cell injury via multiple mechanisms. Redox Biol. Aug 2017;12:571-581. [CrossRef]
- Oggianu L, Lancellotti S, Pitocco D, et al. The oxidative modification of von Willebrand factor is associated with thrombotic angiopathies in diabetes mellitus. PLoS One. 2013;8(1):e55396. [CrossRef]
- Domingueti CP, Dusse LM, Carvalho M, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. May-Jun 2016;30(4):738-45. [CrossRef]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. Oct 29 2010;107(9):1058-70. [CrossRef]
- Li Y, Liu Y, Liu S, et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. Apr 10 2023;8(1):152. [CrossRef]
- Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. Aug 2008;19(8):491-504. [CrossRef]
- Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45-63.
- Bagnato G, Bitto A, Pizzino G, et al. Propylthiouracil modulates aortic vasculopathy in the oxidative stress model of systemic sclerosis. Vascul Pharmacol. Aug 2015;71:79-83. [CrossRef]
- Ottum MS, Mistry AM. Advanced glycation end-products: modifiable environmental factors profoundly mediate insulin resistance. J Clin Biochem Nutr. Jul 2015;57(1):1-12. [CrossRef]
- Das AK, Kalra S, Punyani H, Deshmukh S, Taur S. ‘Oxidative stress’-A new target in the management of diabetes mellitus. J Family Med Prim Care. Nov 2023;12(11):2552-2557. [CrossRef]
- Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. May 2008;57(5):1349-54. [CrossRef]
- Mengstie MA, Chekol Abebe E, Behaile Teklemariam A, et al. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front Mol Biosci. 2022;9:1002710. [CrossRef]
- Nowotny K, Jung T, Hohn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. Mar 16 2015;5(1):194-222. [CrossRef]
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. Jan 2006;21(1):1-6. [CrossRef]
- Sifuentes-Franco S, Pacheco-Moises FP, Rodriguez-Carrizalez AD, Miranda-Diaz AG. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J Diabetes Res. 2017;2017:1673081. [CrossRef]
- Sifuentes-Franco S, Padilla-Tejeda DE, Carrillo-Ibarra S, Miranda-Diaz AG. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int J Endocrinol. 2018;2018:1875870. [CrossRef]
- Santiago AR, Boia R, Aires ID, Ambrosio AF, Fernandes R. Sweet Stress: Coping With Vascular Dysfunction in Diabetic Retinopathy. Front Physiol. 2018;9:820. [CrossRef]
- Bokhary K, Aljaser F, Abudawood M, et al. Role of Oxidative Stress and Severity of Diabetic Retinopathy in Type 1 and Type 2 Diabetes. Ophthalmic Res. 2021;64(4):613-621. [CrossRef]
- Pang L, Lian X, Liu H, et al. Understanding Diabetic Neuropathy: Focus on Oxidative Stress. Oxid Med Cell Longev. 2020;2020:9524635. [CrossRef]
- Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. [CrossRef]
- Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. Apr 29 2005;4:5. [CrossRef]
- Ceriello A, Testa R, Genovese S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr Metab Cardiovasc Dis. Apr 2016;26(4):285-92. [CrossRef]
- Nandi A, Counts N, Broker J, et al. Cost of care for Alzheimer’s disease and related dementias in the United States: 2016 to 2060. NPJ Aging. Feb 8 2024;10(1):13. [CrossRef]
- Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. May 2018;15:490-503. [CrossRef]
- Salim S. Oxidative Stress and the Central Nervous System. J Pharmacol Exp Ther. Jan 2017;360(1):201-205. [CrossRef]
- Aoyama K. Glutathione in the Brain. Int J Mol Sci. May 9 2021;22(9). [CrossRef]
- Sidorova Y, Domanskyi A. Detecting Oxidative Stress Biomarkers in Neurodegenerative Disease Models and Patients. Methods Protoc. Sep 24 2020;3(4). [CrossRef]
- Cenini G, Lloret A, Cascella R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid Med Cell Longev. 2019;2019:2105607. [CrossRef]
- Wei Z, Li X, Li X, Liu Q, Cheng Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front Mol Neurosci. 2018;11:236. [CrossRef]
- Kumar A, Ratan RR. Oxidative Stress and Huntington’s Disease: The Good, The Bad, and The Ugly. J Huntingtons Dis. Oct 1 2016;5(3):217-237. [CrossRef]
- Cioffi F, Adam RHI, Bansal R, Broersen K. A Review of Oxidative Stress Products and Related Genes in Early Alzheimer’s Disease. J Alzheimers Dis. 2021;83(3):977-1001. [CrossRef]
- Sanabria-Castro A, Alape-Giron A, Flores-Diaz M, Echeverri-McCandless A, Parajeles-Vindas A. Oxidative stress involvement in the molecular pathogenesis and progression of multiple sclerosis: a literature review. Rev Neurosci. Jan 2 2024;doi:10.1515/revneuro-2023-0091.
- Alqahtani T, Deore SL, Kide AA, et al. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and Amyotrophic Lateral Sclerosis -An updated review. Mitochondrion. Jul 2023;71:83-92. [CrossRef]
- Dubois B, von Arnim CAF, Burnie N, Bozeat S, Cummings J. Biomarkers in Alzheimer’s disease: role in early and differential diagnosis and recognition of atypical variants. Alzheimers Res Ther. Oct 13 2023;15(1):175. [CrossRef]
- Moreira PI. Sweet Mitochondria: A Shortcut to Alzheimer’s Disease. J Alzheimers Dis. 2018;62(3):1391-1401. [CrossRef]
- Tonnies E, Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J Alzheimers Dis. 2017;57(4):1105-1121. [CrossRef]
- Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev. 2013;2013:316523. [CrossRef]
- Yiannopoulou KG, Anastasiou AI, Zachariou V, Pelidou SH. Reasons for Failed Trials of Disease-Modifying Treatments for Alzheimer Disease and Their Contribution in Recent Research. Biomedicines. Dec 9 2019;7(4). [CrossRef]
- Haass C, Selkoe D. If amyloid drives Alzheimer disease, why have anti-amyloid therapies not yet slowed cognitive decline? PLoS Biol. Jul 2022;20(7):e3001694. [CrossRef]
- Kametani F, Hasegawa M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Front Neurosci. 2018;12:25. [CrossRef]
- Yiannopoulou KG, Papageorgiou SG. Current and Future Treatments in Alzheimer Disease: An Update. J Cent Nerv Syst Dis. 2020;12:1179573520907397. [CrossRef]
- Cheong SL, Tiew JK, Fong YH, et al. Current Pharmacotherapy and Multi-Target Approaches for Alzheimer’s Disease. Pharmaceuticals (Basel). Dec 14 2022;15(12). [CrossRef]
- Wright AL, Zinn R, Hohensinn B, et al. Neuroinflammation and neuronal loss precede Abeta plaque deposition in the hAPP-J20 mouse model of Alzheimer’s disease. PLoS One. 2013;8(4):e59586. [CrossRef]
- Nunomura A, Perry G. RNA and Oxidative Stress in Alzheimer’s Disease: Focus on microRNAs. Oxid Med Cell Longev. 2020;2020:2638130. [CrossRef]
- Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. Aug 2001;60(8):759-67. [CrossRef]
- Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. Aug 2014;1842(8):1240-7. [CrossRef]
- Suppiah S, Didier MA, Vinjamuri S. The Who, When, Why, and How of PET Amyloid Imaging in Management of Alzheimer’s Disease-Review of Literature and Interesting Images. Diagnostics (Basel). Jun 25 2019;9(2). [CrossRef]
- Sultana MA, Hia RA, Akinsiku O, Hegde V. Peripheral Mitochondrial Dysfunction: A Potential Contributor to the Development of Metabolic Disorders and Alzheimer’s Disease. Biology (Basel). Jul 19 2023;12(7). [CrossRef]
- Majeed A, Marwick B, Yu H, Fadavi H, Tavakoli M. Ophthalmic Biomarkers for Alzheimer’s Disease: A Review. Front Aging Neurosci. 2021;13:720167. [CrossRef]
- Klyucherev TO, Olszewski P, Shalimova AA, et al. Advances in the development of new biomarkers for Alzheimer’s disease. Transl Neurodegener. Apr 21 2022;11(1):25. [CrossRef]
- Auso E, Gomez-Vicente V, Esquiva G. Biomarkers for Alzheimer’s Disease Early Diagnosis. J Pers Med. Sep 4 2020;10(3). [CrossRef]
- Collin F, Cheignon C, Hureau C. Oxidative stress as a biomarker for Alzheimer’s disease. Biomark Med. Mar 2018;12(3):201-203. [CrossRef]
- Li W, Zhang M, Huang R, et al. Topographic metabolism-function relationships in Alzheimer’s disease: A simultaneous PET/MRI study. Hum Brain Mapp. Feb 1 2024;45(2):e26604. [CrossRef]
- Fracassi A, Marcatti M, Zolochevska O, et al. Oxidative Damage and Antioxidant Response in Frontal Cortex of Demented and Nondemented Individuals with Alzheimer’s Neuropathology. J Neurosci. Jan 20 2021;41(3):538-554. [CrossRef]
- Grari O, Elmoujtahide D, Sebbar E, Choukri M. The Biochemistry Behind Cognitive Decline: Biomarkers of Alzheimer’s Disease. EJIFCC. Dec 2023;34(4):276-283.
- Koronyo Y, Rentsendorj A, Mirzaei N, et al. Retinal pathological features and proteome signatures of Alzheimer’s disease. Acta Neuropathol. Apr 2023;145(4):409-438. [CrossRef]
- Tamagno E, Guglielmotto M, Vasciaveo V, Tabaton M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants (Basel). Sep 16 2021;10(9). [CrossRef]
- Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. Apr 2015;14(4):388-405. [CrossRef]
- Pardo-Moreno T, Gonzalez-Acedo A, Rivas-Dominguez A, et al. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics. May 24 2022;14(6). [CrossRef]
- Plascencia-Villa G, Perry G. Lessons from antiamyloid-beta immunotherapies in Alzheimer’s disease. Handb Clin Neurol. 2023;193:267-292. [CrossRef]
- Plascencia-Villa G, Perry G. Roles of Oxidative Stress in Synaptic Dysfunction and Neuronal Cell Death in Alzheimer’s Disease. Antioxidants (Basel). Aug 17 2023;12(8). [CrossRef]
- Gribkoff VK, Kaczmarek LK. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology. Jul 1 2017;120:11-19. [CrossRef]
- Yao W, Yang H, Yang J. Small-molecule drugs development for Alzheimer’s disease. Front Aging Neurosci. 2022;14:1019412. [CrossRef]
- Mehta RI, Mehta RI. The Vascular-Immune Hypothesis of Alzheimer’s Disease. Biomedicines. Jan 30 2023;11(2). [CrossRef]
- Walker KA, Le Page LM, Terrando N, Duggan MR, Heneka MT, Bettcher BM. The role of peripheral inflammatory insults in Alzheimer’s disease: a review and research roadmap. Mol Neurodegener. Jun 5 2023;18(1):37. [CrossRef]
- Rentz DM, Wessels AM, Annapragada AV, et al. Building clinically relevant outcomes across the Alzheimer’s disease spectrum. Alzheimers Dement (N Y). 2021;7(1):e12181. [CrossRef]
- Si ZZ, Zou CJ, Mei X, et al. Targeting neuroinflammation in Alzheimer’s disease: from mechanisms to clinical applications. Neural Regen Res. Apr 2023;18(4):708-715. [CrossRef]
- Novoa C, Salazar P, Cisternas P, et al. Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol Res. Dec 23 2022;55(1):39. [CrossRef]
- Puspita L, Chung SY, Shim JW. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol Brain. Nov 28 2017;10(1):53. [CrossRef]
- Aborode AT, Pustake M, Awuah WA, et al. Targeting Oxidative Stress Mechanisms to Treat Alzheimer’s and Parkinson’s Disease: A Critical Review. Oxid Med Cell Longev. 2022;2022:7934442. [CrossRef]
- Watanabe H, Dijkstra JM, Nagatsu T. Parkinson’s Disease: Cells Succumbing to Lifelong Dopamine-Related Oxidative Stress and Other Bioenergetic Challenges. Int J Mol Sci. Feb 7 2024;25(4). [CrossRef]
- Tavassolifar MJ, Vodjgani M, Salehi Z, Izad M. The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Dis. 2020;2020:5793817. [CrossRef]
- Motataianu A, Serban G, Barcutean L, Balasa R. Oxidative Stress in Amyotrophic Lateral Sclerosis: Synergy of Genetic and Environmental Factors. Int J Mol Sci. Aug 19 2022;23(16). [CrossRef]
- Ortiz GG, Pacheco-Moises FP, Bitzer-Quintero OK, et al. Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Dev Immunol. 2013;2013:708659. [CrossRef]
- Chauhan A, Audhya T, Chauhan V. Brain region-specific glutathione redox imbalance in autism. Neurochem Res. Aug 2012;37(8):1681-9. [CrossRef]
- Liu X, Lin J, Zhang H, et al. Oxidative Stress in Autism Spectrum Disorder-Current Progress of Mechanisms and Biomarkers. Front Psychiatry. 2022;13:813304. [CrossRef]
- Pangrazzi L, Balasco L, Bozzi Y. Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders. Int J Mol Sci. May 6 2020;21(9). [CrossRef]
- Morimoto M, Hashimoto T, Tsuda Y, Suenaga M, Nakamura T, Katoh S. Study on oxidative stress and inflammatory/antioxidant substance levels in autism spectrum disorder. J Chin Med Assoc. May 1 2023;86(5):489-493. [CrossRef]
- Delhey LM, Tippett M, Rose S, et al. Comparison of Treatment for Metabolic Disorders Associated with Autism:Reanalysis of Three Clinical Trials. Front Neurosci. 2018;12:19. [CrossRef]
- Rose S, Melnyk S, Pavliv O, et al. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. Jul 10 2012;2(7):e134. [CrossRef]
- Bjorklund G, Dosa MD, Maes M, et al. The impact of glutathione metabolism in autism spectrum disorder. Pharmacol Res. Apr 2021;166:105437. [CrossRef]
- Wang M, Xu D, Zhang L, Jiang H. Application of Multimodal MRI in the Early Diagnosis of Autism Spectrum Disorders: A Review. Diagnostics (Basel). Sep 22 2023;13(19). [CrossRef]
- Lin X, Wang G, Shen S, Zhan J. Advances in the Diagnosis and Treatment of Autism Spectrum Disorders in Children. Altern Ther Health Med. Oct 27 2023;
- Wei JD, Xu X. Oxidative stress in Wernicke’s encephalopathy. Front Aging Neurosci. 2023;15:1150878. [CrossRef]
- Zhao M, Zhu P, Fujino M, et al. Oxidative Stress in Hypoxic-Ischemic Encephalopathy: Molecular Mechanisms and Therapeutic Strategies. Int J Mol Sci. Dec 10 2016;17(12). [CrossRef]
- Antar SA, Ashour NA, Marawan ME, Al-Karmalawy AA. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int J Mol Sci. Feb 16 2023;24(4). [CrossRef]
- Huang E, Peng N, Xiao F, Hu D, Wang X, Lu L. The Roles of Immune Cells in the Pathogenesis of Fibrosis. Int J Mol Sci. Jul 22 2020;21(15). [CrossRef]
- Pinzaru AD, Mihai CM, Chisnoiu T, et al. Oxidative Stress Biomarkers in Cystic Fibrosis and Cystic Fibrosis-Related Diabetes in Children: A Literature Review. Biomedicines. Sep 29 2023;11(10). [CrossRef]
- Grasemann H, Ratjen F. Cystic Fibrosis. N Engl J Med. Nov 2 2023;389(18):1693-1707. [CrossRef]
- Li B, Zhang C, Zhan YT. Nonalcoholic Fatty Liver Disease Cirrhosis: A Review of Its Epidemiology, Risk Factors, Clinical Presentation, Diagnosis, Management, and Prognosis. Can J Gastroenterol Hepatol. 2018;2018:2784537. [CrossRef]
- Delli Bovi AP, Marciano F, Mandato C, Siano MA, Savoia M, Vajro P. Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review. Front Med (Lausanne). 2021;8:595371. [CrossRef]
- Stickel F, Osterreicher CH, Datz C, et al. Prediction of progression to cirrhosis by a glutathione S-transferase P1 polymorphism in subjects with hereditary hemochromatosis. Arch Intern Med. Sep 12 2005;165(16):1835-40. [CrossRef]
- Grattagliano I, Calamita G, Cocco T, Wang DQ, Portincasa P. Pathogenic role of oxidative and nitrosative stress in primary biliary cirrhosis. World J Gastroenterol. May 21 2014;20(19):5746-59. [CrossRef]
- Doroszko A, Dobrowolski P, Radziwon-Balicka A, Skomro R. New Insights into the Role of Oxidative Stress in Onset of Cardiovascular Disease. Oxid Med Cell Longev. 2018;2018:9563831. [CrossRef]
- Frak W, Wojtasinska A, Lisinska W, Mlynarska E, Franczyk B, Rysz J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines. Aug 10 2022;10(8). [CrossRef]
- Pignatelli P, Menichelli D, Pastori D, Violi F. Oxidative stress and cardiovascular disease: new insights. Kardiol Pol. 2018;76(4):713-722. [CrossRef]
- Eberhardt N, Noval MG, Kaur R, et al. SARS-CoV-2 infection triggers pro-atherogenic inflammatory responses in human coronary vessels. Nat Cardiovasc Res. Oct 2023;2(10):899-916. [CrossRef]
- Zhang Z, Rong L, Li YP. Flaviviridae Viruses and Oxidative Stress: Implications for Viral Pathogenesis. Oxid Med Cell Longev. 2019;2019:1409582. [CrossRef]
- Khaiboullina SF, Levis S, Morzunov SP, et al. Serum Cytokine Profiles Differentiating Hemorrhagic Fever with Renal Syndrome and Hantavirus Pulmonary Syndrome. Front Immunol. 2017;8:567. [CrossRef]
- Tschope C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. Mar 2021;18(3):169-193. [CrossRef]
- Messaoudi I, Basler CF. Immunological features underlying viral hemorrhagic fevers. Curr Opin Immunol. Oct 2015;36:38-46. [CrossRef]
- Choi JH, Croyle MA. Emerging targets and novel approaches to Ebola virus prophylaxis and treatment. BioDrugs. Dec 2013;27(6):565-83. [CrossRef]
- van Leur SW, Heunis T, Munnur D, Sanyal S. Pathogenesis and virulence of flavivirus infections. Virulence. Dec 2021;12(1):2814-2838. [CrossRef]
- Hosakote YM, Jantzi PD, Esham DL, et al. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. Jun 1 2011;183(11):1550-60. [CrossRef]
- Castro SM, Guerrero-Plata A, Suarez-Real G, et al. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med. Dec 15 2006;174(12):1361-9. [CrossRef]
- Guler MC, Tanyeli A, Ekinci Akdemir FN, et al. An Overview of Ischemia-Reperfusion Injury: Review on Oxidative Stress and Inflammatory Response. Eurasian J Med. Dec 2022;54(Suppl1):62-65. [CrossRef]
- Choi EK, Lim DG. Hepatic ischemia-reperfusion injury with respect to oxidative stress and inflammatory response: a narrative review. J Yeungnam Med Sci. Apr 2023;40(2):115-122. [CrossRef]
- Chazelas P, Steichen C, Favreau F, et al. Oxidative Stress Evaluation in Ischemia Reperfusion Models: Characteristics, Limits and Perspectives. Int J Mol Sci. Feb 27 2021;22(5). [CrossRef]
- Xiang M, Lu Y, Xin L, et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid Med Cell Longev. 2021;2021:6614009. [CrossRef]
- He J, Liu D, Zhao L, et al. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (Review). Exp Ther Med. Jun 2022;23(6):430. [CrossRef]
- Kurian GA, Rajagopal R, Vedantham S, Rajesh M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid Med Cell Longev. 2016;2016:1656450. [CrossRef]
- Zhao C, Liu T, Wei H, Li J. Serum oxidative stress factors predict myocardial ischemia reperfusion injury after percutaneous coronary intervention in patients with acute myocardial infarction and type 2 diabetes mellitus. Postepy Kardiol Interwencyjnej. Dec 2023;19(4):333-342. [CrossRef]
- Wu L, Xiong X, Wu X, et al. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front Mol Neurosci. 2020;13:28. [CrossRef]
- Jurcau A, Ardelean AI. Oxidative Stress in Ischemia/Reperfusion Injuries following Acute Ischemic Stroke. Biomedicines. Mar 1 2022;10(3). [CrossRef]
- Li Z, Bi R, Sun S, et al. The Role of Oxidative Stress in Acute Ischemic Stroke-Related Thrombosis. Oxid Med Cell Longev. 2022;2022:8418820. [CrossRef]
- Zhang Y, Khan S, Liu Y, Wu G, Yong VW, Xue M. Oxidative Stress Following Intracerebral Hemorrhage: From Molecular Mechanisms to Therapeutic Targets. Front Immunol. 2022;13:847246. [CrossRef]
- Shao L, Chen S, Ma L. Secondary Brain Injury by Oxidative Stress After Cerebral Hemorrhage: Recent Advances. Front Cell Neurosci. 2022;16:853589. [CrossRef]
- Gross BA, Jankowitz BT, Friedlander RM. Cerebral Intraparenchymal Hemorrhage: A Review. JAMA. Apr 2 2019;321(13):1295-1303. [CrossRef]
- Simona MS, Alessandra V, Emanuela C, et al. Evaluation of Oxidative Stress and Metabolic Profile in a Preclinical Kidney Transplantation Model According to Different Preservation Modalities. Int J Mol Sci. Jan 5 2023;24(2). [CrossRef]
- Hofmann J, Puhringer M, Steinkellner S, et al. Novel, Innovative Models to Study Ischemia/Reperfusion-Related Redox Damage in Organ Transplantation. Antioxidants (Basel). Dec 24 2022;12(1). [CrossRef]
- Sundd P, Gladwin MT, Novelli EM. Pathophysiology of Sickle Cell Disease. Annu Rev Pathol. Jan 24 2019;14:263-292. [CrossRef]
- Antwi-Boasiako C, Dankwah GB, Aryee R, Hayfron-Benjamin C, Donkor ES, Campbell AD. Oxidative Profile of Patients with Sickle Cell Disease. Med Sci (Basel). Jan 25 2019;7(2). [CrossRef]
- Wang Q, Zennadi R. The Role of RBC Oxidative Stress in Sickle Cell Disease: From the Molecular Basis to Pathologic Implications. Antioxidants (Basel). Oct 13 2021;10(10). [CrossRef]
- Vona R, Sposi NM, Mattia L, Gambardella L, Straface E, Pietraforte D. Sickle Cell Disease: Role of Oxidative Stress and Antioxidant Therapy. Antioxidants (Basel). Feb 16 2021;10(2). [CrossRef]
- Chiang KC, Gupta A, Sundd P, Krishnamurti L. Thrombo-Inflammation in COVID-19 and Sickle Cell Disease: Two Faces of the Same Coin. Biomedicines. Jan 25 2023;11(2). [CrossRef]
- Carrero D, Soria-Valles C, Lopez-Otin C. Hallmarks of progeroid syndromes: lessons from mice and reprogrammed cells. Dis Model Mech. Jul 1 2016;9(7):719-35. [CrossRef]
- Villa-Bellosta R. Redox theory in progeria. Aging (Albany NY). Oct 31 2020;12(21):20934-20935. [CrossRef]
- Trigueros-Motos L, Gonzalez JM, Rivera J, Andres V. Hutchinson-Gilford progeria syndrome, cardiovascular disease and oxidative stress. Front Biosci (Schol Ed). Jun 1 2011;3(4):1285-97. [CrossRef]
- Seco-Cervera M, Spis M, Garcia-Gimenez JL, et al. Oxidative stress and antioxidant response in fibroblasts from Werner and atypical Werner syndromes. Aging (Albany NY). Mar 2014;6(3):231-45. [CrossRef]
- Iskusnykh IY, Zakharova AA, Pathak D. Glutathione in Brain Disorders and Aging. Molecules. Jan 5 2022;27(1). [CrossRef]
- Ben-Shachar R, Chen Y, Luo S, Hartman C, Reed M, Nijhout HF. The biochemistry of acetaminophen hepatotoxicity and rescue: a mathematical model. Theor Biol Med Model. Dec 19 2012;9:55. [CrossRef]
- Deavall DG, Martin EA, Horner JM, Roberts R. Drug-induced oxidative stress and toxicity. J Toxicol. 2012;2012:645460. [CrossRef]
- Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. Dec 2004;3(4):294-300. [CrossRef]
- Kusirisin P, Chattipakorn SC, Chattipakorn N. Contrast-induced nephropathy and oxidative stress: mechanistic insights for better interventional approaches. J Transl Med. Oct 20 2020;18(1):400. [CrossRef]
- Oh GS, Kim HJ, Shen A, et al. New Therapeutic Concept of NAD Redox Balance for Cisplatin Nephrotoxicity. Biomed Res Int. 2016;2016:4048390. [CrossRef]
- Volarevic V, Djokovic B, Jankovic MG, et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. Mar 13 2019;26(1):25. [CrossRef]
- Narezkina A, Nasim K. Anthracycline Cardiotoxicity. Circ Heart Fail. Mar 2019;12(3):e005910. [CrossRef]
- Carrasco R, Castillo RL, Gormaz JG, Carrillo M, Thavendiranathan P. Role of Oxidative Stress in the Mechanisms of Anthracycline-Induced Cardiotoxicity: Effects of Preventive Strategies. Oxid Med Cell Longev. 2021;2021:8863789. [CrossRef]
- Jiang H, Zuo J, Li B, et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox Biol. Jul 2023;63:102754. [CrossRef]
- Fabiani I, Aimo A, Grigoratos C, et al. Oxidative stress and inflammation: determinants of anthracycline cardiotoxicity and possible therapeutic targets. Heart Fail Rev. Jul 2021;26(4):881-890. [CrossRef]
- Ramachandran A, Jaeschke H. Mitochondria in Acetaminophen-Induced Liver Injury and Recovery: A Concise Review. Livers. Jun 2023;3(2):219-231. [CrossRef]
- Biswas A, Santra S, Bishnu D, Dhali GK, Chowdhury A, Santra A. Isoniazid and Rifampicin Produce Hepatic Fibrosis through an Oxidative Stress-Dependent Mechanism. Int J Hepatol. 2020;2020:6987295. [CrossRef]
- Le Dare B, Ferron PJ, Gicquel T. Toxic Effects of Amanitins: Repurposing Toxicities toward New Therapeutics. Toxins (Basel). Jun 11 2021;13(6). [CrossRef]
- Nebbioso M, Franzone F, Lambiase A, et al. Oxidative Stress Implication in Retinal Diseases-A Review. Antioxidants (Basel). Sep 10 2022;11(9). [CrossRef]
- Kusuhara S, Fukushima Y, Ogura S, Inoue N, Uemura A. Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes Metab J. Oct 2018;42(5):364-376. [CrossRef]
- Cecilia OM, Jose Alberto CG, Jose NP, et al. Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J Diabetes Res. 2019;2019:8562408. [CrossRef]
- Vingolo EM, Casillo L, Contento L, Toja F, Florido A. Retinitis Pigmentosa (RP): The Role of Oxidative Stress in the Degenerative Process Progression. Biomedicines. Mar 2 2022;10(3). [CrossRef]
- Abokyi S, To CH, Lam TT, Tse DY. Central Role of Oxidative Stress in Age-Related Macular Degeneration: Evidence from a Review of the Molecular Mechanisms and Animal Models. Oxid Med Cell Longev. 2020;2020:7901270. [CrossRef]
- Ruan Y, Jiang S, Gericke A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int J Mol Sci. Jan 28 2021;22(3). [CrossRef]
- Toma C, De Cilla S, Palumbo A, Garhwal DP, Grossini E. Oxidative and Nitrosative Stress in Age-Related Macular Degeneration: A Review of Their Role in Different Stages of Disease. Antioxidants (Basel). Apr 23 2021;10(5). [CrossRef]
- Bar-Or D, Carrick MM, Mains CW, Rael LT, Slone D, Brody EN. Sepsis, oxidative stress, and hypoxia: Are there clues to better treatment? Redox Rep. Sep 2015;20(5):193-7. [CrossRef]
- Hsiao SY, Kung CT, Su CM, et al. Impact of oxidative stress on treatment outcomes in adult patients with sepsis: A prospective study. Medicine (Baltimore). Jun 26 2020;99(26):e20872. [CrossRef]
- Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9(4):401-10. [CrossRef]
- Robles L, Vaziri ND, Ichii H. Role of Oxidative Stress in the Pathogenesis of Pancreatitis: Effect of Antioxidant Therapy. Pancreat Disord Ther. Apr 1 2013;3(1):112. [CrossRef]
- Padureanu V, Florescu DN, Padureanu R, Ghenea AE, Gheonea DI, Oancea CN. Role of antioxidants and oxidative stress in the evolution of acute pancreatitis (Review). Exp Ther Med. Mar 2022;23(3):197. [CrossRef]
- Verlaan M, Roelofs HM, van-Schaik A, et al. Assessment of oxidative stress in chronic pancreatitis patients. World J Gastroenterol. Sep 21 2006;12(35):5705-10. [CrossRef]
- Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. Feb 2005;3(2):254-67. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




