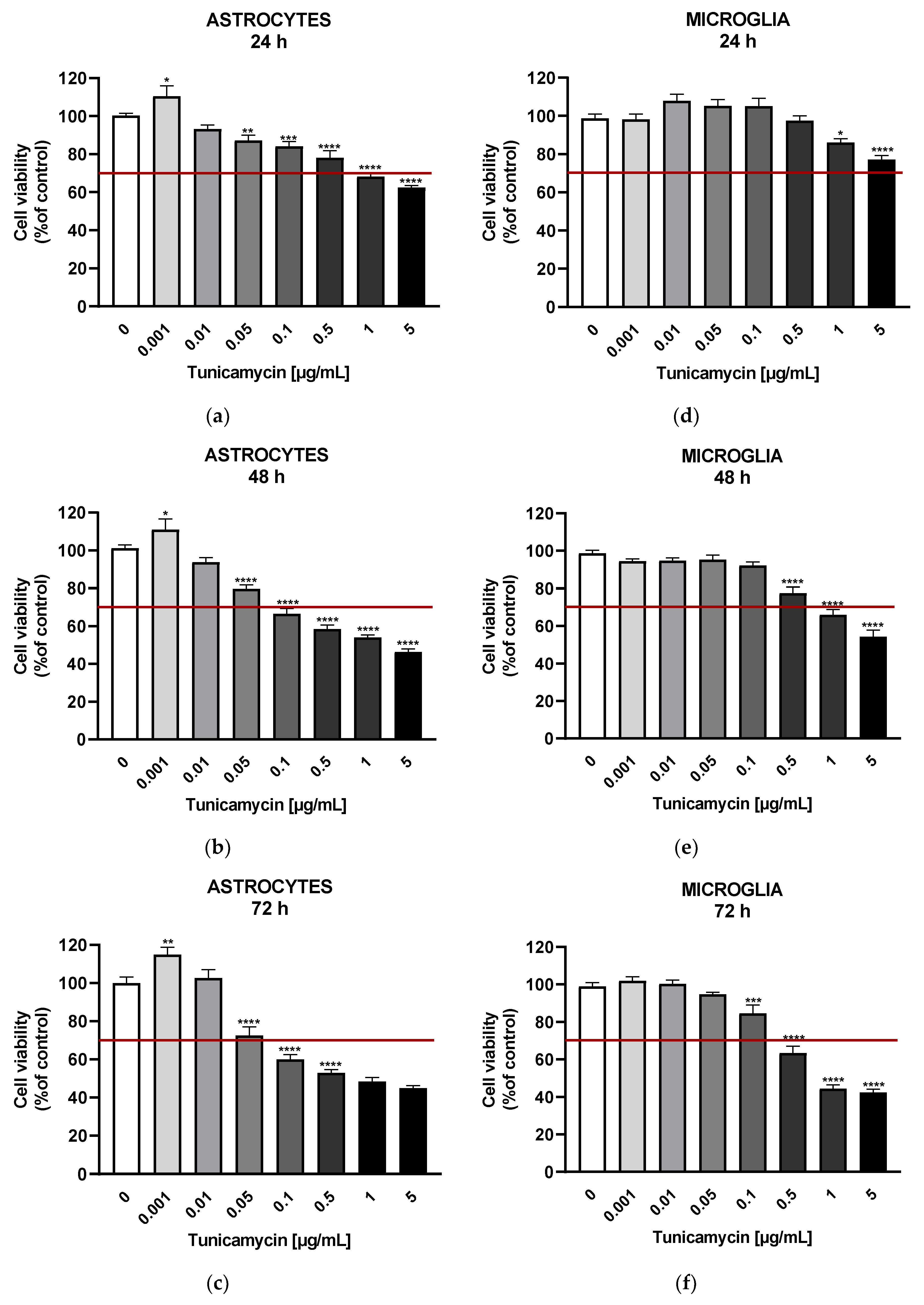1. Introduction
The endoplasmic reticulum (ER) plays a pivotal role in regulating numerous biological functions, among others, the synthesis, folding, maturation, and transport of proteins. Disruption of ER function leads to the buildup of unfolded and misfolded proteins in the ER, a cellular state known as ER stress [
1,
2]. To restore ER homeostasis, the unfolded protein response (UPR) pathway is activated, initially resulting in adaptive programmes leading to the cessation of protein translation, degradation of misfolded proteins, enhanced protein folding, and synthesis of molecular chaperones crucial for proper protein folding. When the damage becomes irreversible, the UPR pathway leads to cell death [
3]. These divergent outcomes depend on the duration and intensity of the stimuli, leading to conditions of mild, moderate, or severe ER stress [
4,
5]. During prolonged or severe ER stress, the objective of the UPR shifts from promoting cellular survival to triggering abnormal inflammatory signalling and apoptotic pathways [
5,
6,
7,
8]. In the central nervous system (CNS), persistent activation of ER stress has been associated with various neurodegenerative diseases (e.g., Creutzfeldt–Jakob disease, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease) [
9,
10,
11,
12], mood disorders, such as bipolar disorder [
13] and major depressive disorder (MDD) [
14]. In conditions such as stroke, ischemia, spinal cord injury, and amyotrophic lateral sclerosis, prolonged activation of ER stress is considered as a primary mechanism leading to neuronal disorders [
9].
It is important to note that activation of ER stress in neurons is a significant factor participating in their dysfunction. Stress reactions may be further affected by adjacent glial cells through glia-neuron crosstalk [
11] Among glial cells, astrocytes represent the most abundant population, playing an essential supportive role in brain function. Astrocytes secrete factors crucial for neuronal survival, neurite outgrowth, neurotransmission, and metabolic regulation. However, in response to ER stress, astrocytes generate inflammatory mediators, diminish trophic support, and have the capability to transmit ER stress to neighbouring cells. Reactive astrocytes also contribute to inflammatory responses by stimulating microglial activation [
15]. Microglia, serving as resident macrophages in the CNS, actively monitor the brain’s microenvironment, play key roles in synaptic refinement, injury recovery, homeostasis regulation, phagocytic activity, and intercellular communication. In response to injuries, inflammatory stimuli, ER stress or other insults microglia are crucial mediators of neuroinflammation due to their ability to initiate or influence a wide range of cellular reactions. Changes in microglial function are associated with both brain development and aging, as well as various neurodegenerative conditions [
16]. Dysregulated astrocytes and microglial cells promote events that lead to a neurotoxic microenvironment [
17,
18]. Understanding the complex interplay between astrocytes, microglia, and neuronal populations is crucial for unravelling the mechanisms underlying CNS disorders and developing targeted therapeutic interventions
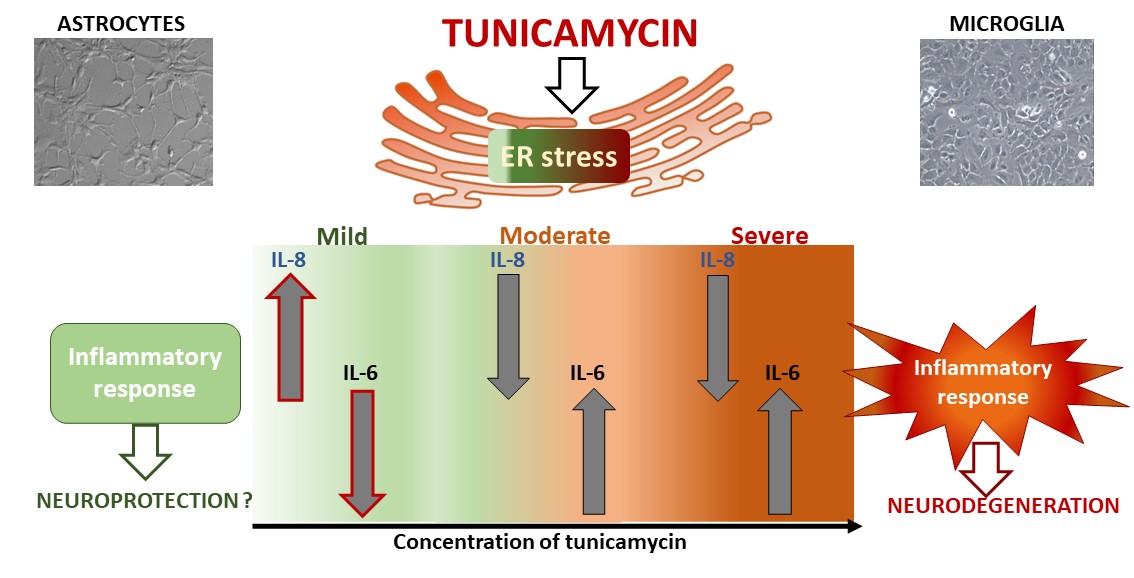
While many studies have highlighted the direct link between ER stress and inflammatory pathways, deeper exploration into the specific cellular mechanisms through which ER stress exacerbates neuroinflammation is still necessary. Defining the nature of the ER stress in different cell types in the brain, particularly with respect to induction or suppression of inflammatory signals, may contribute to fill the gap between experimental and clinical results. Targeting ER stress as therapeutic approach for neurodegenerative diseases or psychiatric disorders requires markers for drug development or monitoring disease progression. Therefore, the aim of the study was to provide comprehensive analysis of the effects of different forms of ER stress (mild, moderate, and severe) on the release of proinflammatory cytokines IL-6 and IL-8 from astrocytes and microglial cells. Tunicamycin was used as an inducer of ER stress due to its ability to hinder the formation of protein N-glycosidic linkages by blocking the transfer of N-acetylglucosamine 1-phosphate to dolichol monophosphate. [
19]. Furthermore, the effect of tunicamycin on cytokine release was compared to well-known immunostimulants – lipopolysaccharide (LPS) and tumor necrosis factor alpha (TNF-α) to investigate differences in cytokine secretion under the influence of inflammatory factors and ER stress inducer.
3. Discussion
The balance between ER stress and the folding capacity of the ER is crucial in managing the transition from an adaptive to a dysfunctional response. Erguler and co-workers proposed a combined mechanistic model encompassing the three signalling pathways of the UPR cascade (i.e., IRE1α, PERK and ATF6) showing the UPR response in three distinct states of behaviour: low, intermediate and high activity states [
21]. The states were linked to stress adaptation, tolerance, and the onset of apoptosis. The analysis demonstrated that the intermediate state may display fluctuations in translation inhibition and apoptotic signals therefore preconditioning that involves application of specific ER stress inducers to promote an adaptive response may prevent the harmful effects of cell death [
21,
22]. These results gave evidence that ER stress is a dynamic process that can be regulated to mitigate its adverse effects or to promote adaptation processes. In the context of these studies, it is worth emphasizing that mild ER stress can have beneficial effects in the CNS. Mild ER stress induced by tunicamycin was protective in the Parkinson’s 6-OHDA mouse model, indicating that keeping the UPR response at a moderate level could help prevent the disease [
23]. Similarly, Wang et al. showed that low doses of tunicamycin resulted in a mild ER-stress response without inducing cytotoxicity and tissue toxicity. Mild ER stress preconditioning reduced microglia and astrocyte activation and neuronal death as well as improved LPS-induced BBB impairment and cognitive ability dysfunction in rats [
5,
24].
For the above reasons, our goal was to determine concentrations of tunicamycin responsible for inducing a specific severity of ER stress. Analysis of expression of genes belonging to three UPR signalling pathways confirmed the role of tunicamycin as ER stress inducer in microglial cells. The same concentration of tunicamycin (i.e., 0.5 µg/mL) was used as in our previous studies to compare its effects in different cell types. We selected genes that are considered markers of ER stress i.e., HSPA5 encoding GRP78 protein or DDIT3 translated into CHOP. The results showed up-regulation of most genes selected in both glial cells with the highest expressions of marker genes, confirming the activation of the UPR pathway under the influence of the applied tunicamycin concentration (
Table 1).
Cell viability measurement revealed a time- and concentration-dependent relationship with tunicamycin, allowing us to determine the degree of ER stress activation. The adopted classification into mild, moderate, and severe ER stress based on cell viability was in line with the work of Wang and colleagues, who also used cell viability or cell apoptosis measurements to identify the concentration corresponding to mild, non-cytotoxic ER stress and more severe ER stress resulting in cell death. Furthermore, the authors of the work showed variable expression of proteins of the UPR pathway depending on the intensity of ER stress, e.g., the hippocampal expression levels of ATF4 and CHOP were increased only at the highest dose of TM (30 μg), which induced cell death. At lower tunicamycin doses i.e., 0.3 and 3 μg no altered expressions of these proteins were observed [
5]. Similarly, in cultured rat astrocytes lower doses of tunicamycin (0.1 and 1 ng/ml), in contrast to the concentration of 10 ng/mL, had no effect on ATF4 and CHOP expression levels [
24]. These results indicate that the analysis of expression of UPR pathway components may represent a way to identify the extent of ER stress severity in the CNS.
A possibility of developing new therapies supporting mild ER stress or preventing the harmful effects of chronic ER stress appears probable. Development of new therapies involves the search for specific markers that enable monitoring of therapeutic or toxic effects. One possibility is to measure levels of specific proteins involved in the UPR response to different severities of ER stress. However, due to a direct link between ER stress and inflammation manifested in ability of ER stress to regulate inflammatory pathways resulting in cytokine production [
8], we applied a different research approach and investigated whether the level of IL-6 and IL-8 secreted by glial cells under ER stress can change depending on ER stress severity.
IL-6 is a 26-kDa proinflammatory cytokine produced by a variety of cells. It is an activator of acute phase responses and the overproduction of IL-6 was seen in a variety of chronic autoimmune and inflammatory diseases [
25]. IL-6 is also a major cytokine in the CNS playing role in regulating neuronal development, survival, function but it also is involved in neuroinflammation accompanying CNS diseases [
26,
27]. The crosstalk between ER stress and IL-6 production in the brain was widely described. For example, in primary murine astrocytes the PERK mediated arm of the UPR was the most linked to the induction of inflammation and IL-6 release through interaction with the Janus kinase 1 (JAK1)/signal transducer and activator of transcription 3 (STAT3) signalling pathway [
28] Additionally, free IL-6 could bind to its cell membrane receptor and further activate JAK1/STAT3, amplifying inflammation [
29]. In microglia, both PERK/CHOP and IRE1a/XBP1s pathways were involved in modulating IL-6 secretion [
30].
IL-8 is primarily known for its pro-inflammatory properties, as it acts as a chemoattractant for neutrophils and other immune cells, promoting inflammation and tissue damage in response to infection or injury [
31]. The role of IL-8 in ER stress in the CNS has not been well understood so far, despite its involvement in neuroinflammation associated with schizophrenia [
29], major depressive disorder [
32] Alzheimer’s disease [
33] or Parkinson’s disease [
34]. IL-8 is synthesized and released by macrophages and brain microglia and astrocytes [
35], and may serve either pro- or anti-inflammatory role mainly depending on the concentration [
36]. IL-8 is produced early in the inflammatory response, possibly persisting for days or weeks, unlike most other inflammatory cytokines that are produced and cleared within a few hours. Thus, IL-8 might be specific for more chronic inflammatory changes in neurodegenerative and neuropsychological alterations in the brain [
37]. Some links between ER stress marker genes and cytokines were described. For example, Krupkova et al. observed a positive correlation between the expression of the GRP78 and IL-6 and IL-8 genes, whereas no such correlation was noted for IL-1β or TNF-α in the human intervertebral disc [
38].
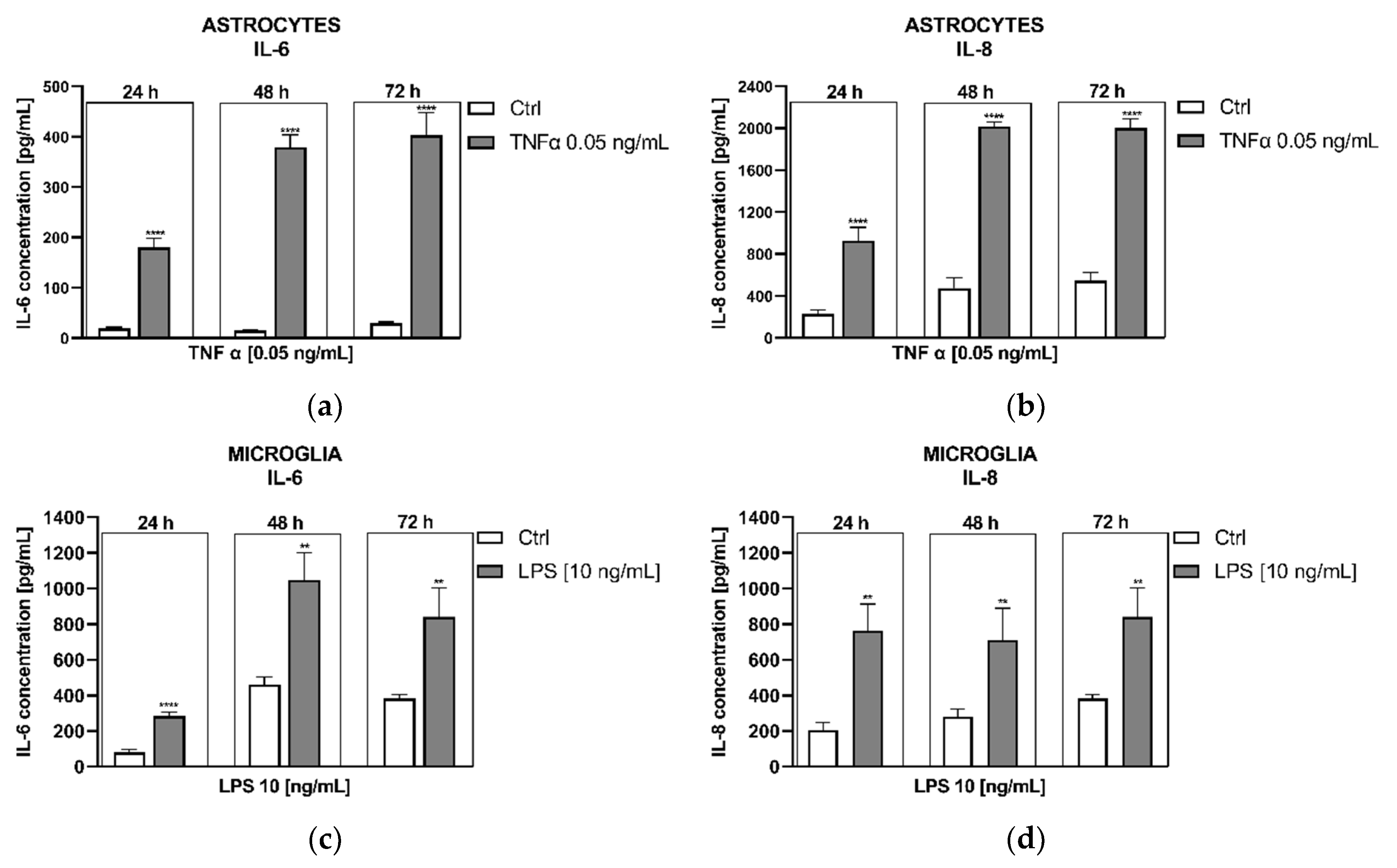
As positive controls we used TNF-α and LPS for astrocytes and microglia, respectively, due to cell type-dependent response to different inflammatory inducers. According to the study of Ehrlich et al. fetal and adult human microglia exhibit the highest response to IL-8 secretion when stimulated by LPS, followed by IL-1β, and least to TNF-α, conversely, fetal astrocytes show the strongest secretion of IL-8 following IL-1β administration, followed by TNF-α, with no effect observed under LPS stimulation [
35]. As expected, both inflammatory factors elicited a significant increase in IL-6 and IL-8 release from both types of glial cells (
Figure 3).
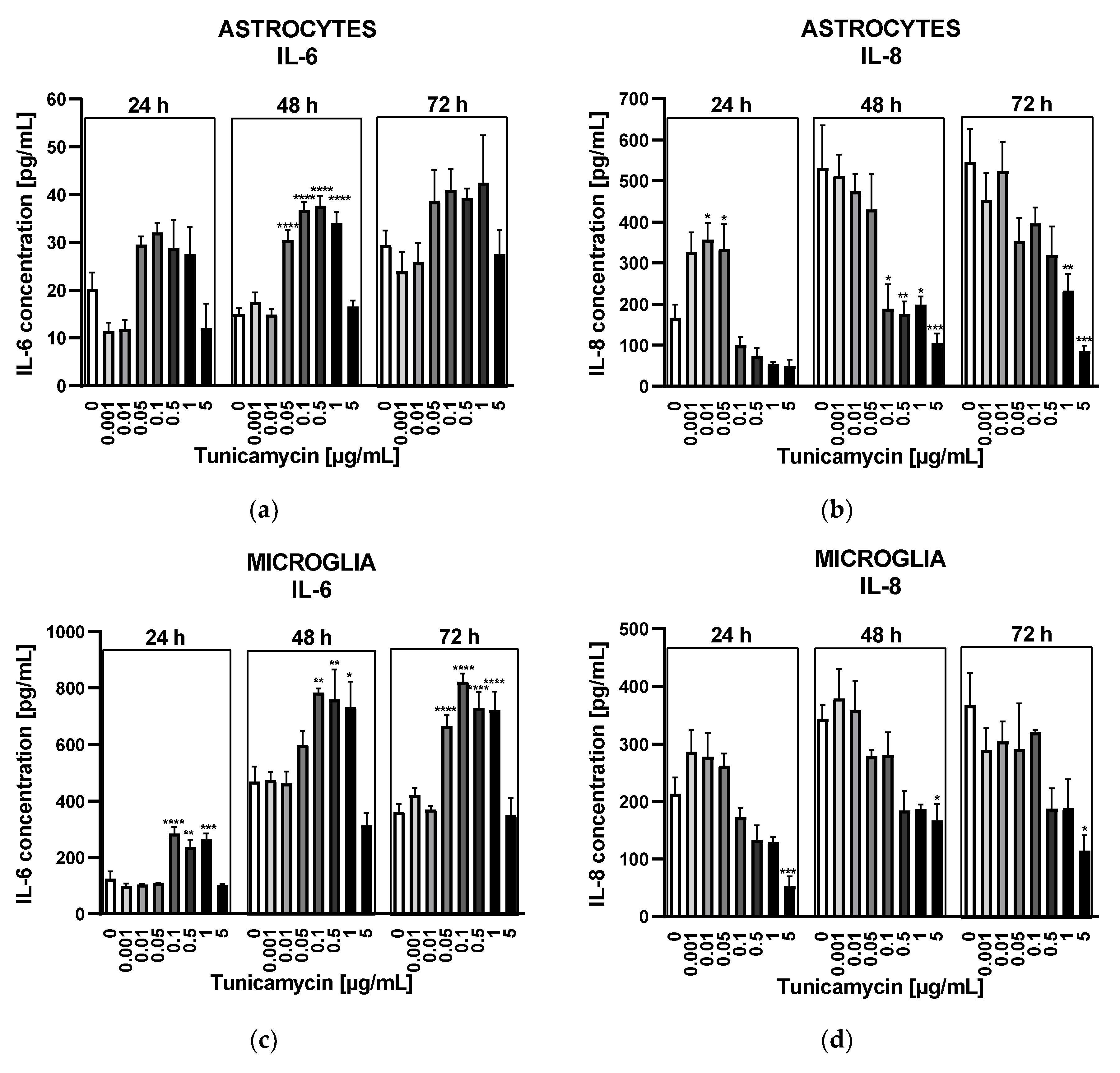
Dose-and time-dependent analysis of tunicamycin-induced release of IL-6 and IL-8 demonstrated interesting results. Mild ER stress (tunicamycin at concentrations of 0.001 – 0.05 µg/mL) reduced IL-6 release and significantly increased IL-8 production in human astrocytes after 24 h but this effect was transient and was not observed with longer exposure times i.e., 48 and 72 h (
Figure 2). In contrast, at the lowest tunicamycin concentration (0.001 µg/mL), astrocyte viability increased at all time points, indicating that mild ER stress might have long-term beneficial effect (
Figure 1). In other study, mild ER stress diminished LPS-induced astrocytic inflammatory responses and overactivation confirming its protective role in the CNS [
24]. In microglial cells, IL-8 production was only slightly increased after 24 hours of exposure to mild ER stress, without reaching statistical significance, and there was no change in IL-6 levels compared to the control. Given that ER stress is a dynamic process, further incubation of astrocytes and microglia with higher concentrations of tunicamycin led to a significant increase in IL-6 secretion and, interestingly, a decrease in IL-8 levels in both types of glial cells. (
Figure 2). This phenomenon was observed even at cytotoxic concentrations of ER stress inducer with cell viability below 70% indicating that severe and prolonged ER stress triggers inflammatory response in glial cells. Thus, the inflammatory response induced by tunicamycin at higher concentrations differs from that induced by LPS or TNF-α with regard to IL-8 but significance of this results needs further research.
The limitation of this study is that it shows the effects of varying levels of ER stress on cytokine secretion only in cell cultures. Further research is needed in more complex animal models to check whether cytokine levels in the blood can also fluctuate under ER stress depending on its severity. It could help in understanding the potential significance of the findings presented in this study. For example, such confirmation at the organism level would enable the use of cytokine level measurements as predictive markers for monitoring ER stress severity and could aid in the development of new therapeutic strategies in research. Furthermore, maintaining a balanced level of cytokines appears to be crucial in the management of disorders induced by inflammation, which in turn, can impact ER stress. Therefore, therapeutic interventions aimed at restoring ER stress-disrupted equilibrium between IL-6 and IL-8 could hold promise in alleviating ER stress-related complications.
4. Materials and Methods
4.1. Reagents
Reagents for astrocyte culture i.e., medium, Fetal Bovine Serum (FBS), growth supplement, penicillin/streptomycin solution and poly-L-Lysine were from ScienCell Research Laboratories (Carlsbad, CA, USA). Reagents for microglial cell culture: Eagle’s Minimum Essential Medium (EMEM), FBS, Dulbecco’s Phosphate Buffered Saline (D-PBS) and Trypsin-EDTA solution were obtained from ATCC (Manassas, VA, USA). Tunicamycin, LPS, Trypsin-EDTA solution and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) were from Sigma-Aldrich (Saint Louis, MO, USA). The RNeasy Mini Kit was obtained from Qiagen (Germantown, WI, USA) and other reagents for Real-Time PCR, i.e., Custom PrimePCR™ Real-Time PCR Plates, iScript™ cDNA Synthesis Kit, 2xSsoAdvanced Universal SYBR Green Supermix, Prime PCR RT Control and Prime PCR Control Assay were from Bio-Rad (Berkeley, CA, USA). Human IL-6 DuoSet ELISA and Human IL-8 DuoSet ELISA kits, Recombinant Human TNF-alpha Protein were from R&D Systems (Minneapolis, MN, USA).
4.2. Cell Culture
Astrocyte cell line from human cerebral cortex was purchased from ScienCell Research Laboratories (San Diego, CA, USA) and maintained according to the protocol recommended by the company. Microglial HMC3 cell line from human brain (Cat. No (CRL 3304) was obtained from ATCC (Manassas, VA, USA) and cultured according to the recommended protocol.
4.3. Analysis of Gene Expression Related to ER Stress
Analysis of gene expression was performed according to the method described in our previous work [
20] to compare the results with those obtained for astrocyte cell culture. Briefly, microglial cells were seeded onto culture flasks at a density of 1- 1.5 × 106 cells/flask and cultured for 24 h. Following this, tunicamycin (0.5 µg/mL) was added to the culture and cells were further incubated for 24 hours. After the incubation period, cells were harvested, and the cell pellets were resuspended in RLT lysis buffer for RNA isolation and purification using the RNeasy Mini Kit from Qiagen. Reverse transcription was performed using the iScript™ cDNA Synthesis Kit in a CFX96 thermal cycler (Bio-Rad, Berkeley, CA, USA). The resulting cDNA and the 2xSsoAdvanced Universal SYBR Green Supermix reagent were added to appropriate wells on a 96-well Custom PrimePCR™ Real-Time PCR Plate according to the manufacturer’s protocol (Bio-Rad, Berkeley, CA, USA). The real-time PCR reaction was conducted in a CFX96 thermal cycler (Bio-Rad, Berkeley, CA, USA), and data analysis was performed using the 2−ΔΔCq method. GAPDH and TBP were chosen as reference genes for normalization, and statistical significance was determined by one-way ANOVA with a significance level of p < 0.05. A fold change value above 1.5 was considered significant.
4.4. Cell Viability
The colorimetric MTT assay was used for evaluation of cell viability under conditions of ER stress. Both types of glial cells were seeded onto 96-well plates at a final density of 7 × 103 cells/well. Following 24h of culture, the cells were treated with tunicamycin (0.001 – 5 μg/mL) for 24, 48 or 72h. After the respective incubation periods, MTT solution was added to the cell culture for another four hours. Absorbance was measured at 570 nm using a BioTek EL ×800 microplate reader (BioTek, Winooski, VT, USA), with the value being directly proportional to the number of viable cells. Cell viability was calculated using the formula: Viability [%] = (A/AC) × 100%, where A represents the absorbance of the investigated sample and AC represents the absorbance of the control (untreated cells).
4.5. ELISA Tests
Astrocytes and microglia were seeded onto 12-well plates at a density of 1.5× 105 cells/well. After 24h, the cells were exposed to tunicamycin (0.001 – 5 μg/mL) or LPS (10 ng/mL) or TNFα (0.05 ng/mL) for 24, 48 or 72h. Following the incubation periods, supernatants of the cells were collected and levels of IL-6 or IL-8 were measured using Human IL-6 DuoSet ELISA or Human IL-8 DuoSet ELISA kits. Absorbance was measured at 450 nm using a BioTek EL ×800 microplate reader (BioTek, Winooski, VT, USA).
4.6. Data Analysis
Data are expressed as mean ± standard error of the mean (SEM). The results were tested by one-way ANOVA followed by post hoc Tukey’s multiple comparisons test or unpaired t-test. For the calculations GraphPad InStat version 9.3.0 (GraphPad, San Diego, CA, USA) was used.
Author Contributions
Conceptualization, P.S.; methodology, P.S., A.W.O.; validation, P.S., A.W.O.; formal analysis, P.S.; investigation, P.S., A.W.O., M.J.B., J.T., S.G.S; resources, A.W.O.; data curation, P.S., A.W.O.; writing—original draft preparation, P.S.; writing—review and editing, P.S., A.W.O., M.J.B., J.T., S.G.S., E.K.; visualization, P.S.; supervision, P.S., A.W.O.; funding acquisition, E.K., M.J.B., A.W.O.; All authors have read and agreed to the published version of the manuscript.