Submitted:
21 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
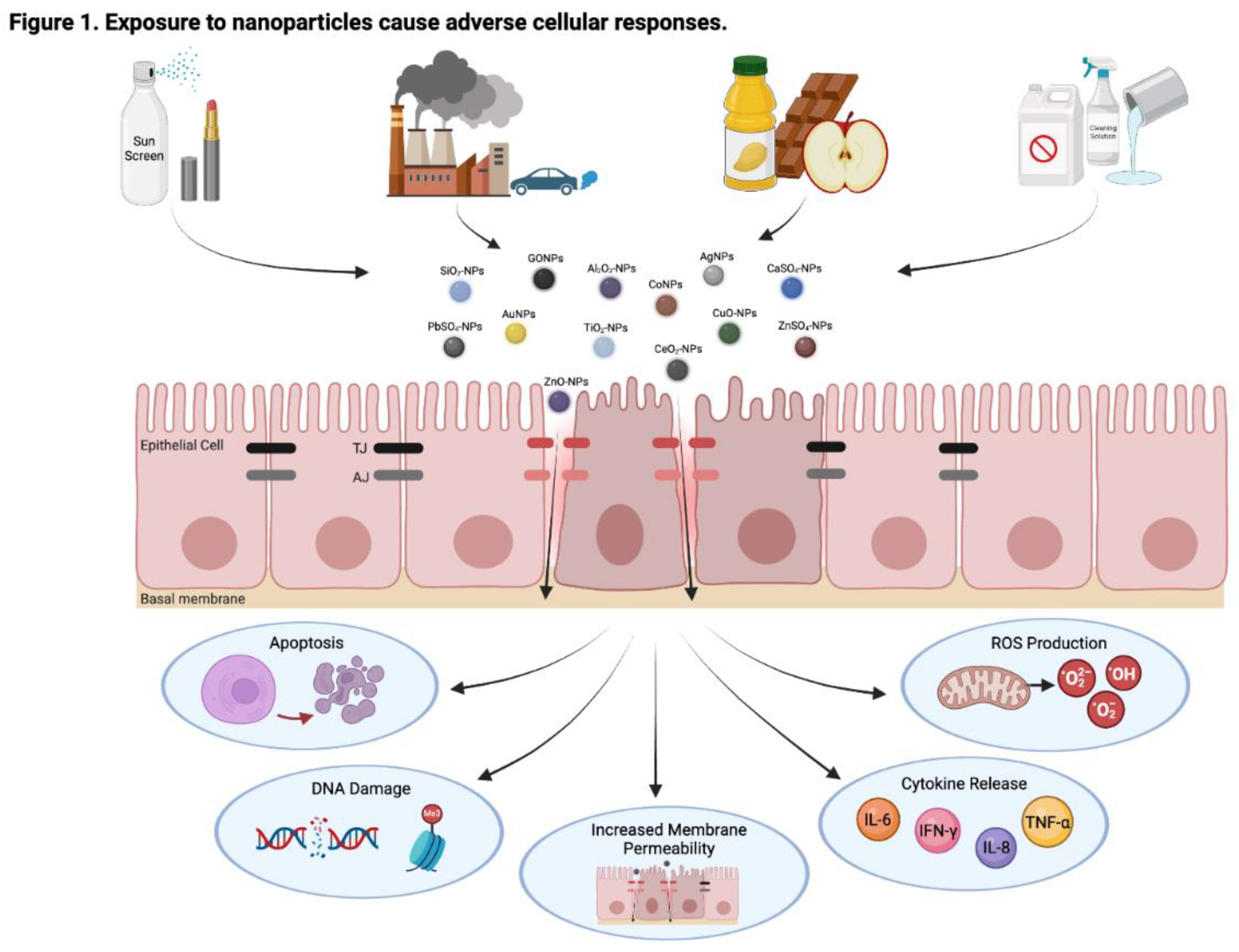
1. Introduction
2. Cell Culture Studies on Airway Cells:
2.1. 16. HBE14o- (16HBE) Human Bronchial Epithelial Cells
2.2. A549 Adenocarcinoma (A549) Cells
2.3. Cultured Human Airway Epithelial (Calu-3) Cells
2.4. Human Bronchial Epithelial (NHBE) Cells
3. Animal Models of Nanoparticle Toxicity on the Airway:
3.1. Silver Nanoparticles (AgNPs)
3.2. Zinc Oxide Nanoparticles (ZnO-NPs)
3.3. Titanium Dioxide Nanoparticles (TiO2-NPs)
3.4. Cerium Oxide Nanoparticles (CeO2-NPs)
3.5. Aluminum Oxide Nanoparticles (Al2O3-NPs)
3.6. Cobalt Nanoparticles (CoNPs)
3.7. Copper Oxide Nanoparticles (CuO-NPs)
4. Conclusion and Future Direction
Author Contributions
Funding
Data Availability Statement:
Conflicts of Interest
Abbreviations:
References
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front Microbiol 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.; et al. Evaluating the coating process of titanium dioxide nanoparticles and sodium tripolyphosphate on cucumbers under chilling condition to extend the shelf-life. Sci Rep 2021, 11, 20312. [Google Scholar] [CrossRef] [PubMed]
- Rokayya, S.; et al. Application of nano-titanum dioxide coating on fresh Highbush blueberries shelf life stored under ambient temperature. LWT 2021, 137, 110422. [Google Scholar] [CrossRef]
- Li, Q.; et al. Impact of food additive titanium dioxide on the polyphenol content and antioxidant activity of the apple juice. LWT 2022, 154, 112574. [Google Scholar] [CrossRef]
- Ibrahim, K.; Khalid, S.; Idrees, K. Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry 2019, 12, 908–931. [Google Scholar]
- Pujalté, I.; et al. Characterization of Aerosols of Titanium Dioxide Nanoparticles Following Three Generation Methods Using an Optimized Aerosolization System Designed for Experimental Inhalation Studies. Toxics 2017, 5. [Google Scholar] [CrossRef]
- Rajasekar, M.; et al. Recent developments in sunscreens based on chromophore compounds and nanoparticles. RSC Adv 2024, 14, 2529–2563. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. Journal of Nanobiotechnology 2022, 20, 262. [Google Scholar] [CrossRef]
- Chetan, P.; et al. Biological agents for synthesis of nanoparticles and their applications. Journal of King Saud University - Science 2022, 34, 101869. [Google Scholar]
- Moreno Ruiz, Y.P.; et al. Advanced Hydrogels Combined with Silver and Gold Nanoparticles against Antimicrobial Resistance. Antibiotics 2023, 12, 104. [Google Scholar] [CrossRef]
- Ali, H.; et al. Biosynthesis and characterization of cobalt nanoparticles using combination of different plants and their antimicrobial activity. Biosci Rep 2023, 43. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; et al. Size-Dependent Electrochemical Properties of Pure Metallic Nanoparticles. The Journal of Physical Chemistry C 2020, 124, 3403–3409. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; et al. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Frontiers in Chemistry 2020, 8. [Google Scholar] [CrossRef]
- Amna, R.; et al. 26 - Virus detection using nanobiosensors, in Nanosensors for Smart Agriculture, D. Adil; et al., Editors. 2022, Elsevier. p. 547-572.
- Serenella, M.; et al. An updated overview on metal nanoparticles toxicity. Seminars in Cancer Biology 2021, 76, 17–26. [Google Scholar]
- Pacurari, M.; et al. A Review on the Respiratory System Toxicity of Carbon Nanoparticles. International Journal of Environmental Research and Public Health 2016, 13, 325. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; et al. Progress and future of in vitro models to study translocation of nanoparticles. Arch Toxicol 2015, 89, 1469–1495. [Google Scholar] [CrossRef]
- Crystal, R.G.; et al. Airway epithelial cells: Current concepts and challenges. Proc Am Thorac Soc 2008, 5, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier function of airway tract epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef]
- Gao, N.; Rezaee, F. Airway Epithelial Cell Junctions as Targets for Pathogens and Antimicrobial Therapy. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Niessen, C.M. Tight junctions/adherens junctions: Basic structure and function. J Invest Dermatol 2007, 127, 2525–2532. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; McCray, P.B., Jr.; Bals, R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J 2015, 45, 1150–1162. [Google Scholar] [CrossRef]
- Yuan, J.; et al. Effects of metal nanoparticles on tight junction-associated proteins via HIF-1α/miR-29b/MMPs pathway in human epidermal keratinocytes. Particle and Fibre Toxicology 2021, 18, 13. [Google Scholar] [CrossRef]
- Rotoli, B.M.; et al. Non-functionalized multi-walled carbon nanotubes alter the paracellular permeability of human airway epithelial cells. Toxicol Lett 2008, 178, 95–102. [Google Scholar] [CrossRef]
- Byrne, J.D.; Baugh, J.A. The significance of nanoparticles in particle-induced pulmonary fibrosis. Mcgill J Med 2008, 11, 43–50. [Google Scholar] [CrossRef]
- Lu, X.; et al. Right or Left: The Role of Nanoparticles in Pulmonary Diseases. International Journal of Molecular Sciences 2014, 15, 17577–17600. [Google Scholar] [CrossRef]
- Shah, D.D.; et al. Harnessing three-dimensional (3D) cell culture models for pulmonary infections: State of the art and future directions. Naunyn Schmiedebergs Arch Pharmacol 2023, 396, 2861–2880. [Google Scholar] [CrossRef]
- Frohlich, E.; Salar-Behzadi, S. Toxicological assessment of inhaled nanoparticles: Role of in vivo, ex vivo, in vitro, and in silico studies. Int J Mol Sci 2014, 15, 4795–4822. [Google Scholar] [CrossRef]
- Woodrow, J.S.; et al. Asthma: The Use of Animal Models and Their Translational Utility. Cells 2023, 12, 1091. [Google Scholar] [CrossRef]
- Altschuler, S.J.; Wu, L.F. Cellular heterogeneity: Do differences make a difference? Cell 2010, 141, 559–563. [Google Scholar] [CrossRef]
- Irvin, C.G.; Bates, J.H.T. Measuring the lung function in the mouse: The challenge of size. Respiratory Research 2003, 4, 1. [Google Scholar] [CrossRef]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. Methods Mol Biol 2019, 1894, 1–29. [Google Scholar]
- Callaghan, P.J.; et al. Epithelial barrier function properties of the 16HBE14o- human bronchial epithelial cell culture model. Biosci Rep 2020, 40. [Google Scholar] [CrossRef]
- Cozens, A.L.; et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol 1994, 10, 38–47. [Google Scholar] [CrossRef]
- Wan, H.; et al. Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o. Eur Respir J 2000, 15, 1058–1068. [Google Scholar] [CrossRef]
- Kerschner, J.L.; Paranjapye, A.; Harris, A. Cellular heterogeneity in the 16HBE14o(-) airway epithelial line impacts biological readouts. Physiol Rep 2023, 11, e15700. [Google Scholar] [CrossRef]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb Perspect Biol 2018, 10. [Google Scholar] [CrossRef]
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch 2017, 469, 135–147. [Google Scholar] [CrossRef]
- Smallcombe, C.C.; et al. Titanium dioxide nanoparticles exaggerate respiratory syncytial virus-induced airway epithelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2020, 319, L481–L496. [Google Scholar] [CrossRef]
- Lee, C.E.; et al. 8-Bromo-cAMP attenuates human airway epithelial barrier disruption caused by titanium dioxide fine and nanoparticles. Tissue Barriers 2024, 2300579. [Google Scholar] [CrossRef]
- Ma, Y.; et al. Titanium dioxide nanoparticles induce size-dependent cytotoxicity and genomic DNA hypomethylation in human respiratory cells. RSC Advances 2017, 7, 23560–23572. [Google Scholar] [CrossRef]
- Guadagnini, R.; et al. Toxicity evaluation of engineered nanoparticles for medical applications using pulmonary epithelial cells. Nanotoxicology 2015, 9 Suppl 1:, 25–32. [Google Scholar] [CrossRef]
- Bao, X.; Shi, M. Toxic effect of silica nanoparticles on bronchial epithelial cells. Materials Express 2022, 12, 255–262. [Google Scholar] [CrossRef]
- Fu, J.; et al. Regulation of c-Myc and Bcl-2 induced apoptosis of human bronchial epithelial cells by zinc oxide nanoparticles. J Biomed Nanotechnol 2012, 8, 669–675. [Google Scholar] [CrossRef]
- Huo, L.; et al. High-Content Screening for Assessing Nanomaterial Toxicity. J Nanosci Nanotechnol 2015, 15, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Farcal, L.; et al. Comprehensive In Vitro Toxicity Testing of a Panel of Representative Oxide Nanomaterials: First Steps towards an Intelligent Testing Strategy. PLoS ONE 2015, 10, e0127174. [Google Scholar]
- Giard, D.J.; et al. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst 1973, 51, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Meindl, C.; et al. Screening for Effects of Inhaled Nanoparticles in Cell Culture Models for Prolonged Exposure. Nanomaterials (Basel) 2021, 11. [Google Scholar] [CrossRef]
- Srivastava, R.K.; et al. Nano-titanium dioxide induces genotoxicity and apoptosis in human lung cancer cell line, A549. Hum Exp Toxicol 2013, 32, 153–166. [Google Scholar] [CrossRef]
- Wang, Y.; et al. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ Sci Pollut Res Int 2015, 22, 5519–5530. [Google Scholar] [CrossRef]
- Chairuangkitti, P.; et al. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol In Vitro 2013, 27, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.B.; et al. Zinc oxide nanoparticles induce acute lung injury via oxidative stress-mediated mitochondrial damage and NLRP3 inflammasome activation: In vitro and in vivo studies. Environ Pollut 2024, 341, 122950. [Google Scholar] [CrossRef] [PubMed]
- Rafieepour, A.; et al. The Effect of Particle Size on the Cytotoxicity of Amorphous Silicon Dioxide: An in Vitro Toxicological Study. Asian Pac J Cancer Prev 2021, 22, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Birch, N.P.; Suresh, V. An Optimised Human Cell Culture Model for Alveolar Epithelial Transport. PLoS ONE 2016, 11, e0165225. [Google Scholar] [CrossRef] [PubMed]
- Karakocak, B.B.; et al. Rethinking of TEER measurement reporting for epithelial cells grown on permeable inserts. Eur J Pharm Sci 2023, 188, 106511. [Google Scholar] [CrossRef] [PubMed]
- Peter, Y.; et al. Epidermal growth factor receptor and claudin-2 participate in A549 permeability and remodeling: Implications for non-small cell lung cancer tumor colonization. Mol Carcinog 2009, 48, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Braun, N.J.; et al. Implementation of a Dynamic Co-Culture Model Abated Silver Nanoparticle Interactions and Nanotoxicological Outcomes In Vitro. Nanomaterials (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Konczol, M.; et al. Cellular uptake and toxic effects of fine and ultrafine metal-sulfate particles in human A549 lung epithelial cells. Chem Res Toxicol 2012, 25, 2687–2703. [Google Scholar] [CrossRef] [PubMed]
- Stearns, R.C.; Paulauskis, J.D.; Godleski, J.J. Endocytosis of ultrafine particles by A549 cells. Am J Respir Cell Mol Biol 2001, 24, 108–115. [Google Scholar] [CrossRef]
- Shen, B.Q.; et al. Calu-3: A human airway epithelial cell line that shows cAMP-dependent Cl- secretion. Am J Physiol 1994, 266 5 Pt 1, L493–L501. [Google Scholar] [CrossRef]
- Lee, D.F.; Lethem, M.I.; Lansley, A.B. A comparison of three mucus-secreting airway cell lines (Calu-3, SPOC1 and UNCN3T) for use as biopharmaceutical models of the nose and lung. Eur J Pharm Biopharm 2021, 167, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, H.M.; et al. An Air-liquid Interface Bronchial Epithelial Model for Realistic, Repeated Inhalation Exposure to Airborne Particles for Toxicity Testing. J Vis Exp 2020. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; et al. Transferability and reproducibility of exposed air-liquid interface co-culture lung models. NanoImpact 2023, 31, 100466. [Google Scholar] [CrossRef] [PubMed]
- Stuetz, H.; et al. The Cultivation Modality and Barrier Maturity Modulate the Toxicity of Industrial Zinc Oxide and Titanium Dioxide Nanoparticles on Nasal, Buccal, Bronchial, and Alveolar Mucosa Cell-Derived Barrier Models. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- George, I.; et al. Metallic oxide nanoparticle translocation across the human bronchial epithelial barrier. Nanoscale 2015, 7, 4529–4544. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.; et al. Mechanisms of toxicity of amorphous silica nanoparticles on human lung submucosal cells in vitro: Protective effects of fisetin. Chem Res Toxicol 2012, 25, 2227–2235. [Google Scholar] [CrossRef]
- Forbes, I.I. Human airway epithelial cell lines for in vitro drug transport and metabolism studies. Pharm Sci Technol Today 2000, 3, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.A.; et al. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp Cell Res 1998, 243, 359–366. [Google Scholar] [CrossRef]
- Min, K.A.; Rosania, G.R.; Shin, M.C. Human Airway Primary Epithelial Cells Show Distinct Architectures on Membrane Supports Under Different Culture Conditions. Cell Biochem Biophys 2016, 74, 191–203. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; Tetley, T.D.; Janes, S.M. Airway and alveolar epithelial cells in culture. European Respiratory Journal 2019, 54, 1900742. [Google Scholar] [CrossRef]
- Zaidman, N.A.; Panoskaltsis-Mortari, A. ; S. M. O'Grady, Differentiation of human bronchial epithelial cells: Role of hydrocortisone in development of ion transport pathways involved in mucociliary clearance. Am J Physiol Cell Physiol 2016, 311, C225–C236. [Google Scholar]
- Gard, A.L.; et al. High-throughput human primary cell-based airway model for evaluating influenza, coronavirus, or other respiratory viruses in vitro. Sci Rep 2021, 11, 14961. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, F.; et al. cAMP-dependent activation of protein kinase A attenuates respiratory syncytial virus-induced human airway epithelial barrier disruption. PLoS ONE 2017, 12, e0181876. [Google Scholar] [CrossRef]
- Gao, N.; Raduka, A.; Rezaee, F. Respiratory syncytial virus disrupts the airway epithelial barrier by decreasing cortactin and destabilizing F-actin. J Cell Sci 2022, 135. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; et al. Comparative Study on Nanotoxicity in Human Primary and Cancer Cells. Nanomaterials (Basel) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Frontinan-Rubio, J.; et al. Rapid and efficient testing of the toxicity of graphene-related materials in primary human lung cells. Sci Rep 2022, 12, 7664. [Google Scholar] [CrossRef]
- Hussain, S.; et al. Carbon black and titanium dioxide nanoparticles elicit distinct apoptotic pathways in bronchial epithelial cells. Part Fibre Toxicol 2010, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand-Hammarström, B.; et al. Human primary bronchial epithelial cells respond differently to titanium dioxide nanoparticles than the lung epithelial cell lines A549 and BEAS-2B. Nanotoxicology 2012, 6, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Schlinkert, P.; et al. The oxidative potential of differently charged silver and gold nanoparticles on three human lung epithelial cell types. J Nanobiotechnology 2015, 13, 1. [Google Scholar] [CrossRef]
- Oberdorster, G.; et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A 2002, 65, 1531–1543. [Google Scholar] [CrossRef]
- Rossi, E.M.; et al. Inhalation exposure to nanosized and fine TiO2 particles inhibits features of allergic asthma in a murine model. Part Fibre Toxicol 2010, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Wahle, T.; et al. Evaluation of neurological effects of cerium dioxide nanoparticles doped with different amounts of zirconium following inhalation exposure in mouse models of Alzheimer's and vascular disease. Neurochem Int 2020, 138, 104755. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; et al. Exacerbation of Nanoparticle-Induced Acute Pulmonary Inflammation in a Mouse Model of Metabolic Syndrome. Front Immunol 2020, 11, 818. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; et al. Identification of the appropriate dose metric for pulmonary inflammation of silver nanoparticles in an inhalation toxicity study. Nanotoxicology 2016, 10, 63–73. [Google Scholar] [PubMed]
- Morimoto, Y.; et al. Evaluation of Pulmonary Toxicity of Zinc Oxide Nanoparticles Following Inhalation and Intratracheal Instillation. Int J Mol Sci 2016, 17. [Google Scholar] [CrossRef]
- Adamcakova-Dodd, A.; et al. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol 2014, 11, 15. [Google Scholar] [CrossRef]
- Grassian, V.H.; et al. Inflammatory response of mice to manufactured titanium dioxide nanoparticles: Comparison of size effects through different exposure routes. Nanotoxicology 2007, 1, 211–226. [Google Scholar] [CrossRef]
- Leung, Y.H.; et al. Toxicity of CeO2 nanoparticles – The effect of nanoparticle properties. Journal of Photochemistry and Photobiology B: Biology 2015, 145, 48–59. [Google Scholar] [CrossRef]
- Aalapati, S.; et al. Toxicity and bio-accumulation of inhaled cerium oxide nanoparticles in CD1 mice. Nanotoxicology 2014, 8, 786–798. [Google Scholar] [CrossRef]
- Nemmar, A.; et al. The acute pulmonary and thrombotic effects of cerium oxide nanoparticles after intratracheal instillation in mice. Int J Nanomedicine 2017, 12, 2913–2922. [Google Scholar] [CrossRef]
- Srinivas, A.; et al. Acute inhalation toxicity of cerium oxide nanoparticles in rats. Toxicol Lett 2011, 205, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Demokritou, P.; et al. An in vivo and in vitro toxicological characterisation of realistic nanoscale CeO(2) inhalation exposures. Nanotoxicology 2013, 7, 1338–1350. [Google Scholar] [CrossRef]
- Kim, I.S.; Baek, M.; Choi, S.J. Comparative cytotoxicity of Al2O3, CeO2, TiO2 and ZnO nanoparticles to human lung cells. J Nanosci Nanotechnol 2010, 10, 3453–3458. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; et al. Twenty-Eight-Day Repeated Inhalation Toxicity Study of Aluminum Oxide Nanoparticles in Male Sprague-Dawley Rats. Toxicol Res 2018, 34, 343–354. [Google Scholar] [CrossRef]
- Yousef, M.I.; et al. Aluminum oxide and zinc oxide induced nanotoxicity in rat brain, heart, and lung. Physiol Res 2022, 71, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.T.; et al. Airway irritation, inflammation, and toxicity in mice following inhalation of metal oxide nanoparticles. Nanotoxicology 2016, 10, 1254–1262. [Google Scholar] [CrossRef]
- Wan, R.; et al. Cobalt nanoparticles induce lung injury, DNA damage and mutations in mice. Part Fibre Toxicol 2017, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; et al. Biological tolerance of different materials in bulk and nanoparticulate form in a rat model: Sarcoma development by nanoparticles. J R Soc Interface 2006, 3, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; et al. Response-metrics for acute lung inflammation pattern by cobalt-based nanoparticles. Part Fibre Toxicol 2015, 12, 13. [Google Scholar] [CrossRef]
- Lai, X.; et al. Intranasal Delivery of Copper Oxide Nanoparticles Induces Pulmonary Toxicity and Fibrosis in C57BL/6 mice. Sci Rep 2018, 8, 4499. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; et al. Copper Oxide Nanoparticle-Induced Acute Inflammatory Response and Injury in Murine Lung Is Ameliorated by Synthetic Secoisolariciresinol Diglucoside (LGM2605). International Journal of Molecular Sciences 2021, 22, 9477. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-T.; et al. Pulmonary Toxicity and Proteomic Analysis in Bronchoalveolar Lavage Fluids and Lungs of Rats Exposed to Copper Oxide Nanoparticles. International Journal of Molecular Sciences 2022, 23, 13265. [Google Scholar] [CrossRef] [PubMed]
- Linfield, D.T.; et al. Airway tight junctions as targets of viral infections. Tissue Barriers 2021, 9, 1883965. [Google Scholar] [CrossRef] [PubMed]
| Cell Culture Model | Nanoparticle type | Nanoparticle Concentration |
Impact on Barrier Function | Cellular Responses | Reference |
|---|---|---|---|---|---|
|
16HBE14o- human bronchial epithelial cells (16HBE) |
10, 25, 50, 75, and 100 µg/mL |
↓ TEER ↑ Permeability |
↑ ROS production ↑ Viral infection ↑ Interleukins, TNF-α, IFN-γ, CCL-3, GM-CSF release |
[40] | |
|
100 µg/mL |
↓ TEER ↑ Permeability |
- |
[41] | ||
| TiO2-NPs |
0.1, 1, 10 and 100 µg/mL |
- |
↑ ROS production ↑ Cytotoxicity ↓ Cell viability ↓ DNA methylation |
[42] | |
|
3, 15 and 75 µg/cm2 |
- |
↑ ROS production ↑ GM-CSF, IL-6 and IL-8 release ↑ Cytotoxicity |
[43] | ||
|
20 µg/cm2 |
- |
↑ ROS production ↑ Apoptosis ↑ DNA fragmentation ↓ Lysosome stability |
[78] | ||
|
SiO2-NPs |
40 µg/mL |
- |
↓ Cell viability ↓ Cell migration ↑ Apoptosis ↑ Oxidative stress ↑ IL-6, IL-8, TNF-α release |
[44] | |
|
3, 15 and 75 µg/cm2 |
- |
↑ ROS production ↑ GM-CSF, IL6 and IL8 release ↑ Cytotoxicity |
[43] | ||
|
ZnO-NPs |
1, 5, 25 and 50 µg/mL |
- |
↑ ROS production ↓ Mitochondrial membrane potential ↑ Apoptosis |
[45] | |
|
Human A549 adenocarcinoma cells (A549) |
|
40 µg/mL |
- |
↑ Endocytosis of aggregates |
[60] |
|
|
1, 10 and 50 µg/mL |
- |
↑ ROS production ↓ Cell viability ↑ Cytotoxicity ↑ Apoptosis |
[50] | |
|
TiO2-NPs |
25, 50, 100, and 200 µg/mL |
- |
↑ Cytotoxicity ↑ Apoptosis ↑ DNA strand breaks ↓ Cell viability ↓ Mitochondrial membrane potential |
[51] | |
|
0.1, 1, 10 and 100 µg/mL |
- |
↑ ROS production ↑ Cytotoxicity ↓ Cell viability ↓ DNA methylation |
[42] | ||
|
3, 15 and 75 µg/cm2 |
- |
↑ ROS production ↑ IL-6 release ↑ Cytotoxicity |
[43] | ||
|
5–500 µg/mL |
- |
↑ ROS production ↑ IL-8 release ↓ Cell viability |
[79] | ||
|
AgNPs |
25, 50, 100 or 200 µg/mL |
- |
↑ ROS production ↓ Cell viability ↓ Mitochondrial membrane potential ↑ Apoptosis |
[52] | |
|
5 µg/mL *Co-culture with U937 |
- |
↑ IL-1β, IL-6, IL-8, TNF-α release ↑ ROS production (less than A549 alone) |
[58] | ||
|
0.05-0.8 µg/cm2 |
No change |
↑ ROS production |
[80] | ||
|
AuNPs |
0.05-0.8 µg/cm2 |
No change |
↑ ROS production |
[80] | |
|
SiO2-NPs |
10, 50, 100, and 250 µg/mL |
- |
↓ Cell viability ↑ ROS production ↓ Mitochondrial membrane potential |
[54] | |
|
3, 15 and 75 µg/cm2 |
- |
↑ ROS production ↑ GM-CSF, IL-6, IL-1β, IL-8 release ↑ Cytotoxicity |
[43] | ||
|
10, 50, 100, 200, 300, 400 µg/mL |
- |
↓ Cell viability ↑ Necrosis ↑ NP accumulation and retention |
[76] | ||
|
ZnO-NPs |
5, 10, 15, 20 µg/mL |
- |
↓ Cell viability ↑ ROS production ↓ Mitochondrial membrane potential |
[53] | |
|
GONPs |
0.05, 0.5, 5, 50, 100 µg/mL |
- |
↑ Necrosis ↑ Apoptosis ↓ Cell viability |
[77] | |
|
CaSO4-NPs |
10-320 µg/mL |
- |
↑ ROS production |
[59] | |
|
ZnSO4-NPs |
10-320 µg/mL |
- |
↑ ROS production ↑ DNA damage ↑ Cytotoxicity ↑ JNK regulation |
||
|
PbSO4-NPs |
10-320 µg/mL |
- |
↑ ROS production |
||
|
Cultured human airway epithelial cells (Calu-3) |
|
1 µg/cm2 |
No change |
No changes in cell viability, barrier integrity, or cytokine release | [64] |
|
TiO2-NPs |
0.25-2 mM |
No change |
No changes in cell viability or cytotoxicity | [65] | |
|
|
5 and 10 µg/cm2 |
↑ TEER |
↑ NP internalization |
[66] | |
|
SiO2-NPs |
5 and 10 µg/cm2 |
↑ TEER |
↑ NP internalization |
[66] | |
|
100 µg/mL |
- |
↑ ROS production ↑ Cytotoxicity ↑ Apoptosis ↑ IL-6, IL-8 release |
[67] | ||
|
ZnO-NPs |
0.25-2 mM |
↓ TEER |
↓ Cell viability |
[65] | |
|
Normal human bronchial epithelial cells (NHBE) |
|
20 µg/cm2 |
- |
↑ ROS production ↑ Apoptosis ↑ DNA fragmentation ↓ Lysosome stability |
[78] |
|
TiO2-NPs |
10, 25, 50, 75, and 100 µg/mL |
↓ TEER ↑ Permeability |
- |
[40] | |
|
|
5–500 µg/mL |
- |
↑ ROS production (weak) ↑ IL-6, IL-1β, G-CSF, VEGF release ↓ Cell viability |
[79] | |
|
SiO2-NPs |
10, 50, 100, 200, 300, 400 µg/mL |
- |
↓ Cell viability ↑ Necrosis ↑ NP accumulation and retention |
[76] | |
|
GONPs |
0.05, 0.5, 5, 50, 100 µg/mL |
- |
↑ Necrosis ↑ Apoptosis ↓ Cell viability ↑ Oxidative stress |
[77] | |
|
AgNPs |
0.05-0.8 µg/cm2 |
- |
↑ LDH release ↓ Cell viability ↑ ROS production (weak) |
[80] | |
|
AuNPs |
0.05-0.8 µg/cm2 |
- |
↑ LDH release ↓ Cell viability ↑ ROS production (weak) |
[80] |
| Nanoparticle Type | Murine Model | Concentration & Exposure Route | Inflammatory Response | BALF Analysis and Other Cellular Responses | Reference |
|---|---|---|---|---|---|
|
AgNPs |
Male C57BL/6J mice | 1 mg/mL, Oropharyngeal aspiration | ↑ Expression of MIP-2, MCP-1, IL-6, IL-1β, CXCL1 | ↑ Total cell count ↑ Neutrophil count |
[84] |
|
Male F344/DuCrl rats |
41-1105 µg/m3, Nose-only inhalation |
↑ Expression of MCP-1, IL-1β |
↑ Total cell count ↑ Neutrophil count ↑ LDH release ↑ Concentration-dependent lung deposition |
[85] | |
|
ZnO-NPs |
Male F344 rats |
0.8 and 4 mg/kg, Intratracheal instillation |
↑ CINC-1 and CINC-2 *(Only seen at highest concentration) |
↑ Total cell count ↑ Neutrophil count ↑ LDH release ↑ Oxidative stress ↑ Macrophage count *(Only seen at highest concentration) |
[86] |
|
2 and 10 mg/m3, Inhalation* | |||||
|
Male C57BL/6 mice |
3.5 mg/m3, Inhalation, 2 weeks |
↑ Expression of IL-12p(40) and MIP-1α |
↑ Zn2+ ions ↑ Total cell count ↑ Neutrophil count ↑ Macrophage count ↑ LDH release ↑ Hematocrit |
[87] | |
|
3.5 mg/m3, Inhalation, 13 weeks |
- |
↑ Zn2+ ions ↑ Total cell count ↑ Macrophage count ↑ Hematocrit |
|||
| Male Wistar rats | 100 mg/kg, Oral gavage |
↑ TNF-α and IL-6 levels | ↑ 8-OHdG levels ↓ Glutathione (GSH) |
[96] | |
| Female BALB/cJ mice |
4-271 mg/m3, Inhalation |
- |
↑ Neutrophil count ↑ Lymphocyte count ↑ Break time |
[97] |
|
|
TiO2-NPs |
Female C57BL/6 mice |
0.5-5 mg/kg, Intranasal instillation |
↑ Leukocyte count ↑ Expression of IL-1α, IL-6, IL-10, TNFα, IFNγ, MCP-1, RANTES, LIF, IP-10 |
↑ Total protein ↑ Barrier permeability |
[40] |
|
Female BALB/c/Sca mice |
10 ± 2 mg/m3, Inhalation, Asthmatic mice |
↓ Expression of IL-1β, TNF-α, IL-4, IL-13, IL-10, Foxp3 ↓ Expression of CCL-3, CXCL5, CXCL2 ↓ IgE levels |
↓ Eosinophil count ↓ Lymphocyte count ↓ PAS+ goblet cells |
[82] |
|
| 10 ± 2 mg/m3, Inhalation, Healthy mice |
↓ Expression of IL-1β ↑ Expression of CXCL5 |
↑ Neutrophil count |
|||
|
Male C57BL/6 mice |
0.77-7.03 mg/m3, Inhalation |
↑ Expression of IL-1β and IL-6 |
↑ Total cell count ↑ Macrophage count |
[88] | |
| 0.1-3.0 mg/mL, Intranasal instillation | ↑ Total cell count ↑ Neutrophil count |
||||
| Female BALB/cJ mice | 4-271 mg/m3, Inhalation |
- |
↑ DNA damage ↑ Break time |
[97] | |
|
CeO2-NPs |
Male CD1 mice |
2 mg/m3, Inhalation |
↑ Expression of TNF-α, IL-1β, and IL-6 |
↓ Cell viability ↑ Neutrophil count ↑ Total protein ↓ Glutathione (GSH) |
[90] |
| Male and female BALB/c mice | 0.1 or 0.5 mg/kg, Intratracheal instillation | ↑ Expression of TNF-α ↓ Catalase |
↑ Total cell count ↑ Neutrophil count |
[91] | |
|
Male and female Wistar rats |
641 mg/m3, Inhalation |
↑ Leukocyte count ↑ Expression of TNF-α, IL-1β, and IL-6 |
↓ Cell viability ↑ Total cell count ↑ LDH release ↑ Neutrophil count |
[92] | |
| Male Sprague-Dawley rats | 2.7 mg/m3, Inhalation |
- |
↑ LDH release ↑ PMNs |
[93] | |
| Female BALB/cJ mice | 4-271 mg/m3, Inhalation |
- |
↑ Neutrophil count ↑ Lymphocyte count |
[97] | |
|
Al2O3-NPs |
Male Sprague-Dawley rats |
0.2, 1, and 5 mg/m3, Nasal inhalation |
↑ Expression of TNF-α and IL-6 |
↑ Total cell count ↑ LDH release |
[95] |
| Male Wistar rats | 70 mg/kg, Oral gavage |
↑ TNF-α and IL-6 levels | ↑ 8-OHdG levels ↓ Glutathione (GSH) |
[96] | |
|
CoNPs |
Male and female gpt delta transgenic mice |
50 μg, Intratracheal instillation |
↑ CXCL1/KC levels |
↑ LDH release ↑ Neutrophil count ↑ Macrophage count ↑ Total protein ↑ DNA damage |
[98] |
|
Sprague-Dawley rats |
100 mg, Bilateral implantation |
- |
↑ Nuclei and mitotic rates ↑ Expression of PCNA |
[99] | |
|
CoO-NPs |
Female rats |
40, 100, and 400 μg/rat, Intratracheal instillation |
↑ IL-6, eotaxin, and IL-13 levels ↑ Eosinophilic inflammation |
↑ LDH release ↑ Total protein ↑ Solubility in ALF |
[100] |
|
Co3O4-NPs |
↑ CINC-3 levels ↑ Neutrophilic inflammation |
↑ LDH release (Only seen at highest concentration) ↓ Solubility in ALF |
|||
|
CuO-NPs |
C57BL/6 mice |
2.5, 5, 10 mg/kg, Intranasal instillation | ↑ Expression of CCL-2, IL-4, TNF-α, α-SMA and collagen-I |
↑ Apoptosis |
[101] |
|
Female C57BL/6 mice |
15 µg/bolus, Intranasal instillation |
↑ Leukocyte count ↑ HMGB1, IL-1β, and TNF-α levels |
↑ Neutrophil count ↑ Protein chlorination ↑ Total protein |
[102] | |
|
Male Sprague-Dawley rats |
0.15 and 1.5 mg/kg, Intratracheal instillation |
↑ MIP-2 and TNF-α levels |
↑ Total cell count ↑ PMN count ↑ Total protein ↑ LDH release ↓ Expression of catalase, Gpx-1, and Prx-2 |
[103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





