1. Introduction
Humans cannot live without air, water, food, and sleep. However, equally important are immunity, which protects against diseases, and the ability to fully digest food. For these processes, we need bacteria. Bacteria are single-celled free-living organisms, among the first forms of life to appear on Earth. They form a large domain of prokaryotic microorganisms. Bacteria, usually a few micrometers in length, have been and continue to be present in most habitats, making it virtually impossible for us to live without them. In our bodies, bacteria help break down the food we eat, provide us with nutrients, and neutralize toxins, to name just a few examples. They also play a crucial role in defending against infections by protecting colonized surfaces from pathogen invasion. Many bacteria are found both inside and outside organisms, including humans. Bacteria are also present in water, soil, and food, making them key players in Earth's ecosystems [
1,
2,
3,
4].
The majority of beneficial bacteria in the human body are located in the digestive system or the gut microbiome. These bacteria help break down food and keep us healthy. Some people regularly take probiotics or during antibiotic treatment to support gut health. These supplements contain strains of beneficial bacteria, such as Bifidobacteria and Lactobacillus. Probiotics are also used in food production to make yogurts and fermented products like sauerkraut, kimchi, and kombucha [
2,
3]
Bacteria are also present in water, soil, and food, making them key players in Earth's ecosystems. The proper functioning of the environmental ecosystem also depends on bacteria. For example, bacteria decompose dead matter in the environment, such as dead leaves, releasing carbon dioxide and nutrients. Without the release of carbon dioxide, plants cannot grow [
2].
Most people associate bacteria with disease, making it easy to think of the harm they cause. Therefore, it is challenging to rethink all the ways they help us because it is usually a more complex, multi-step process. This is only possible because Mother Nature has provided billions of bacteria that swarm over our skin and through our bodies, enough to give anyone a creepy-crawly feeling [
5].
2. Materials and Methods
Literature Review: The Indispensable Role of Bacteria in Human Life and Environmental Health. PubMed, Google Scholar, Web of Science and Scopus databases were searched using the keywords: "gut microbiota", "pathogen inhibition", "competitive exclusion", "antimicrobial compounds", "short-chain fatty acids", "lactic acid bacteria ", "immune modulation", "biofilm formation", "dietary fiber", "probiotics", "prebiotics". Publications in English or Polish, published over the last 10 years, including experimental studies, literature reviews, meta-analyses and reviews on the mechanisms of action of intestinal microflora were included. Articles not directly related to protection against pathogens and studies of low methodological quality were excluded.
Search Procedure: Databases were searched using defined keywords, articles were selected based on title and abstract, and then full-text analysis of selected articles was made for compliance with the inclusion criteria.
Data Analysis: The collected data was analyzed qualitatively and quantitatively. The main mechanisms of action of the intestinal microflora were identified and dietary and supplementation strategies supporting the microflora were compared. Software was used for reference management (EndNote, Zotero) and for qualitative data analysis (NVivo).
Evaluation of Research Quality: Assessment of the methodological quality of experimental studies and literature reviews was performed using tools such as the Cochrane Risk of Bias Tool and ROBINS-I. Two independent reviewers assessed the quality of each study, resolving discrepancies with the involvement of a third party when necessary.
Data Synthesis: Results were synthesized narratively and, if the data were sufficiently homogeneous, a meta-analysis was performed. The results were presented in the form of tables and charts illustrating the main mechanisms of action of intestinal microflora and descriptions of practical cases.
Revision and Validation: The manuscript was reviewed by specialists in the field of microbiology, immunology and dietetics. Regular literature reviews were conducted to update the review in light of the latest research.
Ethics: The review did not include studies involving humans or animals and did not require approval from an ethics committee. The authors declare no conflict of interest.
Limitations: There are possible limitations related to the selection of literature, which relate to the incompleteness of databases and restrictions to publications in specific languages. Methodological variability in the source studies could also affect the overall interpretation of the results.
The adoption of the above methodology provides a reliable and comprehensive review of the literature on the role of intestinal microflora in protection against pathogens, taking into account the latest scientific data.
3. Outside the Body: Small Recyclers
The proper functioning of the environmental ecosystem also depends on bacteria. There are more bacteria living on Earth than people, but this helps illustrate the significant role these organisms play. In soil and oceans, bacteria play a major role in breaking down organic matter and cycling chemical elements, such as carbon and nitrogen, essential for human life. Bacteria decompose dead matter in the environment, such as dead leaves, releasing carbon dioxide and nutrients. Without the release of carbon dioxide, plants cannot grow [
5].
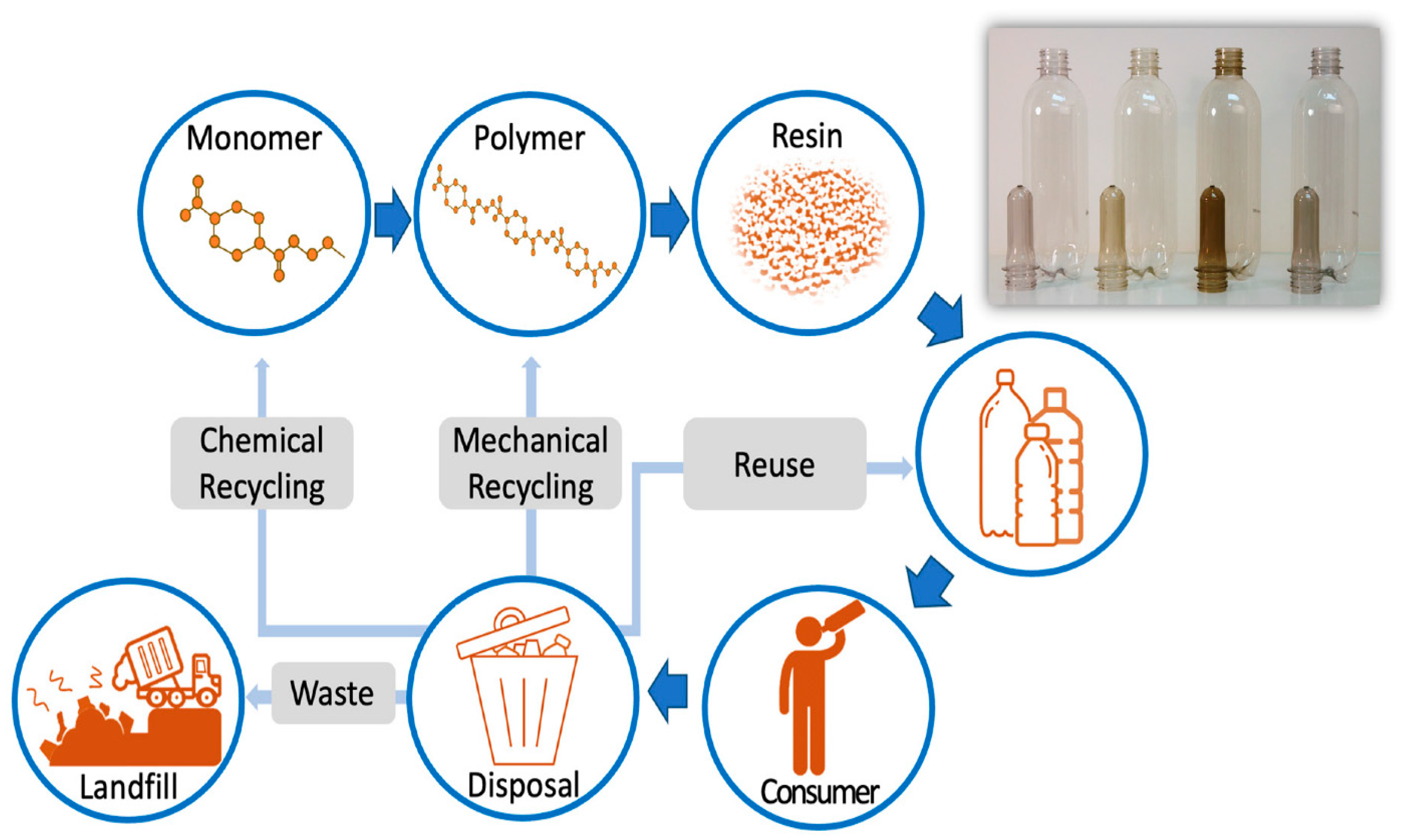
Disposing of plastic waste has become a widely debated issue due to the potential environmental impact of improper waste disposal. As much as approximately 45% was polyethylene terephthalate (PET) packaging in 2021, which represented 12% of global solid waste. Thanks to modern advances in decontamination processes for recycling post-consumer recycled PET (rPET or PCR), it has become a safe material to reuse as beverage packaging. By focusing on efficient recycling processes and leveraging the properties of PET, small recyclers can play a significant role in reducing plastic waste and promoting environmental sustainability (
Figure 1) [
6].
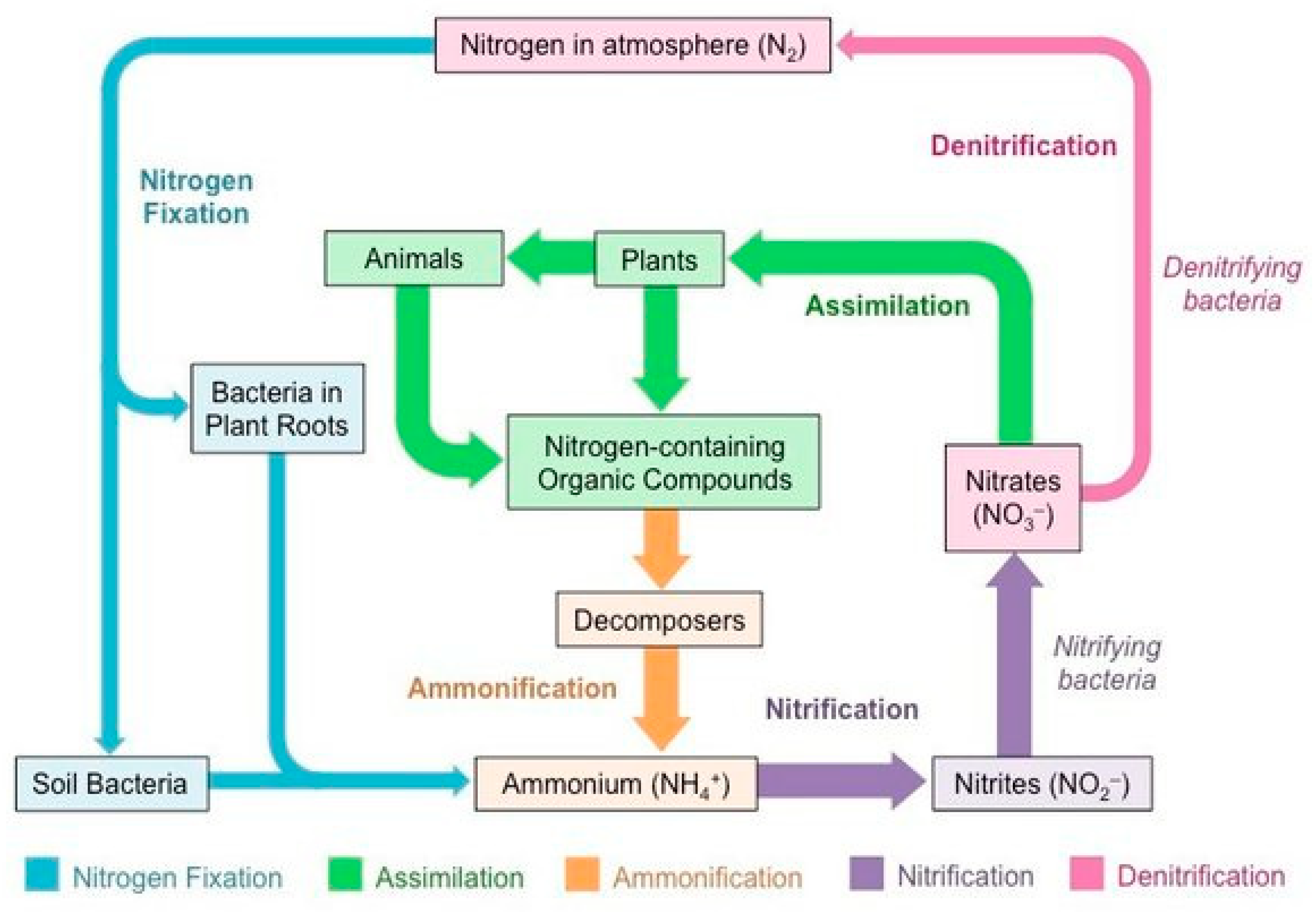
Since plants and animals cannot produce certain nitrogen molecules essential for life, soil bacteria and cyanobacteria (blue-green algae) play an absolutely irreplaceable role in converting atmospheric nitrogen into ammonia or nitrates, forms of nitrogen that plants can absorb to create amino acids and nucleic acids, the building blocks of DNA. We eat plants and benefit (
Figure 2) [
3,
6].
The nitrogen cycle is a biogeochemical cycle in which nitrogen is converted into various chemical forms, circulating through the atmosphere, land, and marine ecosystems via biological and physical processes. Key processes include fixation, ammonification, nitrification, and denitrification. Although 78% of Earth's atmosphere is nitrogen, it is largely unavailable for biological use, leading to nitrogen scarcity in ecosystems. This cycle is crucial for ecologists as nitrogen availability impacts primary production and decomposition. Human activities like fossil fuel combustion, artificial nitrogen fertilizers, and wastewater release have significantly disrupted the nitrogen cycle, harming the environment and human health [
8].
Table 1. provides a clear and organized summary of the main points related to the nitrogen cycle, its disruption, environmental and health impacts, recent findings, and proposed solutions.
Bacteria also play an important role in the water cycle, especially in precipitation processes. For example, scientists from Louisiana State University found that bacteria often form nuclei around which cloud droplets form, leading to precipitation. This suggests that bacteria can significantly influence the weather and water cycle (UNEP - United Nations Environment Programme) [
9].
Recent research highlights the urgent need to address nitrogen pollution. The latest UN Environment Frontiers report highlights nitrogen pollution as a major environmental problem, calling for global action to restore the nitrogen cycle through sustainable practices and better management of nitrogen sources (UNEP). Pollution and climate change have also been shown to worsen water scarcity, with nitrogen runoff contributing to deteriorating water quality and exacerbating problems such as algal blooms and waterborne diseases (Mongabay) (ScienceDaily).
To address these challenges, it is therefore necessary to implement measures such as improving wastewater treatment plants, reducing meat consumption to increase nutrient use efficiency, and adopting sustainable agricultural practices. These strategies can mitigate the adverse effects of nitrogen pollution and support a sustainable ecosystem (Mongabay) (UNEP - United Nations Environment Programme) (ScienceDaily).
4. Bacteria and Humans: An Intricate Relationship
Bacteria have played and continue to play a key role in the evolution of life on Earth, occupying every niche in every environment. Although bacteria are often viewed negatively due to their association with disease, they are an integral part of both human health and the environment. Their importance goes well beyond their pathogenic abilities and includes their importance and role in various biological, microbiological and industrial processes [
1,
3,
11].
4.1. Bacteria and the Human Body
Bacteria have played a key role in the evolution of life on Earth, occupying a variety of environments. The human body is home to a huge number of species of bacteria, collectively called the microbiome. These microorganisms colonize areas such as the skin, mouth and intestines. Consisting of trillions of bacteria, the gut microbiota is essential for a variety of body functions, including digestion, immune regulation, and even mental health. Research has shown that a balanced microbiome is crucial to maintaining health, while dysbiosis (an imbalance in the microbial community) can lead to conditions such as inflammatory bowel disease, obesity and allergies [
2,
6]. Although they are often associated with disease, they also play fundamental roles in human health and various industrial processes.
4.2. Human Microbiome
The human body is home to a huge number of bacterial species, called the microbiome, that colonize the skin, mouth and gut. The gut microflora, consisting of trillions of bacteria, is essential for digestion, immune regulation and mental health. A balanced microbiome is therefore crucial to health, while dysbiosis can lead to conditions such as inflammatory bowel disease, obesity and allergies [
10,
11,
12].
Impact of microbiota on intestinal health: Intestinal microbiota play a key role in maintaining intestinal health by fermenting dietary fibre and proteins, which allows the absorption of nutrients that would otherwise be unavailable. Bacteroides and Firmicutes are the main bacterial groups involved in these processes [
13].
Regulation of metabolism by short-chain fatty acids: Short-chain fatty acids (SCFAs) such as acetate, propionate and butyrate, produced by the microflora, play a key role as an energy source for colon cells. Additionally, they regulate metabolism by influencing hormonal signals and lipid metabolism [
4,
14]. The digestive tract is home to trillions of microorganisms, making up the gut microbiota, which break down undigested food such as dietary fibre. Fermentation produces short-chain fatty acids (SCFA) - acetate, propionate and butyrate - which provide numerous health benefits. The production and absorption of SCFA depend on the type of fibre and microorganisms in the intestines. SCFA supplementation, especially butyrate, is well documented in the treatment of gastrointestinal, metabolic, cardiovascular, and enter cerebrovascular disorders. The result is the absorption of SCFAs and their role in gastrointestinal health and metabolism, as well as their therapeutic use [
14].
4.3. Immune Support
Commensal gut bacteria support the immune system by modulating the immune response. Thanks to this interaction, the immune system can more effectively recognize and fight pathogens while preventing autoimmune reactions [
4].
Protection against pathogens: A strong gut microbiota provides protection against pathogens by competing for space and nutrients, producing antimicrobial compounds, and maintaining an acidic environment that is hostile to pathogen growth [
4,
6].
4.4. Bacterial Symbiosis
Digestive health: Bacteria like
Bacteroides and
Firmicutes help break down complex carbohydrates and fibre, converting them into short-chain fatty acids that nourish colon cells and regulate metabolism. Certain bacteria help break down complex carbohydrates, fibre and proteins, allowing the absorption of nutrients that would otherwise be unavailable. For example
, Bacteroides and
Firmicutes play a key role in fermenting dietary fibre into short-chain fatty acids, which serve as an energy source for colon cells and help regulate metabolism [
5].
4.5. Immune System Support
Here, commensal bacteria modulate the immune system, helping it distinguish pathogens from harmless microorganisms, which prevents autoimmune diseases and infections. This interaction helps prevent autoimmune diseases and infections [
15,
16,
17,
18].
4.5.1. Protection against Pathogens
A strong microflora inhibits the growth of pathogenic bacteria through competition, production of antimicrobial compounds and maintaining an acidic environment. The presence of a strong microflora can inhibit the colonization of pathogenic bacteria through competitive exclusion, production of antimicrobial compounds and maintaining an acidic environment hostile to pathogens [
4].
Recent research has provided additional insights into these protective mechanisms.
Recent Findings
Competitive Exclusion: Nutrient Competition: Commensal bacteria compete with pathogens for essential nutrients, depriving them of the resources needed for growth. This competition can significantly reduce pathogen colonization.
Adhesion Sites: Beneficial bacteria occupy binding sites on the intestinal lining, preventing pathogens from adhering and establishing an infection [
1,
3,
6].
Antimicrobial Compounds:
Bacteriocins: Certain commensal bacteria produce bacteriocins, proteinaceous toxins that inhibit the growth of closely related bacterial strains. These bacteriocins can directly target and kill pathogenic bacteria [
6,
19].
SCFAs: Short-chain fatty acids (SCFAs) like butyrate, produced by gut bacteria during fiber fermentation, have been shown to possess antimicrobial properties and can inhibit the growth of pathogenic bacteria such as
Salmonella and
E. coli [
14].
Maintaining an Acidic Environment:
Lactic Acid Production: Lactic acid bacteria (LAB) produce lactic acid, lowering the pH of the gut environment. An acidic pH inhibits the growth of many pathogens, as they typically prefer neutral or slightly alkaline conditions [
6,
11].
pH Modulation: By maintaining an acidic pH, commensal bacteria can suppress the proliferation of pathogens and promote the growth of beneficial bacteria that thrive in such conditions [
6].
Immune System Modulation: Enhanced Mucosal Immunity: Commensal bacteria stimulate the production of mucus and antimicrobial peptides in the gut lining, creating a physical and chemical barrier against pathogens [
3].
Immune Cell Activation: Gut bacteria can modulate the activity of immune cells, such as macrophages and dendritic cells, enhancing their ability to recognize and respond to pathogenic threats [
22,
23].
Biofilm Formation: Protective Biofilms: Some beneficial bacteria form biofilms on the gut mucosa, which act as a physical barrier to pathogen invasion. These biofilms can also harbor antimicrobial substances, further protecting against infection [
26].
Practical Implications: Dietary Fiber: Increasing dietary fiber intake can promote the growth of SCFA-producing bacteria, enhancing gut health and pathogen resistance [
16].
Probiotic Supplements: Using probiotics containing lactic acid bacteria and bacteriocin-producing strains can help bolster the gut's defense mechanisms against pathogens [
11,
12,
17].
Prebiotics: Consuming prebiotics like inulin and fructooligosaccharides (FOS) can stimulate the growth of beneficial bacteria that contribute to pathogen inhibition [
23].
4.5.2. Pathogenic Bacteria
Bacteria such as
Staphylococcus aureus, Escherichia coli and
Mycobacterium tuberculosis can cause serious infections. Although antibiotics have revolutionized the treatment of bacterial infections, increasing antibiotic resistance poses a serious threat to public health. Advances in medical science, especially in the development of antibiotics, have played a key role in combating bacterial infections. However, the rise of antibiotic-resistant bacteria poses a serious public health challenge. Bacteria are divided into intracellular and extracellular. Most are beneficial, but some pathogens can cause mild to severe infections by using various mechanisms to evade host immunity. Susceptibility to infections depends on the immune system, health status, genetic factors, malnutrition, chronic diseases and age. Bacterial pathogens significantly impact public health, particularly through transmission in health care settings, which increases morbidity and mortality. Understanding the global burden of common bacterial pathogens and their pathogenicity is key to improving vaccination rates, vaccine effectiveness, and assessing the impact of antimicrobial resistance. Many bacteria have developed drug resistance, which complicates the treatment of infectious diseases and promotes the survival of resistant strains. [
17,
18].
4.5.3. Bacteria in Biotechnology and Industry
In agriculture: Nitrogen-fixing bacteria such as
Rhizobium increase soil fertility. They are also used in the production of biofertilizers and biopesticides, promoting sustainable agricultural practices. Nitrogen-fixing bacteria such as Rhizobium increase soil fertility by converting atmospheric nitrogen into forms usable by plants [
6]. Food production: Lactic acid bacteria are key in the fermentation of dairy products such as yogurt and cheese, which increases their nutritional value and digestibility, such as yogurt and cheese. Fermentation not only preserves food but also increases its nutritional value and digestibility [
6,
11]. Microbial biotechnology, focusing on the use of bacteria, has become a dynamic field revolutionizing industrial processes and contributing to environmental reclamation. Diverse applications of microbial biotechnology were explored, highlighting the potential of bacteria to drive sustainable innovation. The versatility of bacteria in terms of metabolic capacity and adaptability makes them indispensable tools in industry, mainly in biofuel production, enzyme synthesis, environmental purification, wastewater treatment, heavy metal removal and medical applications. Mon [
6] highlights the key role of bacterial processes in these areas, emphasizing sustainability, interdisciplinary collaboration and innovative technologies. From biofuel production, where the performance of bacteria is closely related to the selection of microbial strains, fermentation conditions, metabolic engineering and further processing, to enzyme synthesis and environmental remediation, where the abilities of microorganisms are used to create sustainable solutions, bacteria are showing their potential to solve many problems environmental. Microbiological consortiums that deal with wastewater treatment and the role of bacteria in the removal of heavy metals are examples of sustainable and cost-effective solutions. The interdisciplinary nature of microbial biotechnology, spanning microbiology, biochemistry, environmental sciences and engineering, highlights the need for collaboration to realize its full potential.
4.6. Pharmaceuticals and Medicine
Bacteria produce antibiotics, insulin and vaccines. Genetic engineering and synthetic biology have expanded the capabilities of bacterial production systems, leading to more efficient and cost-effective pharmaceutical production. Recently, the transformative role of bacteria in medicine and healthcare has been highlighted, from probiotics to bacteria engineered for drug delivery, bacterial biofilms in medical devices, applications in synthetic biology, and bacteriophages as therapeutic agents. The evolving role of bacteria in healthcare represents a paradigm shift from viewing bacteria solely as pathogens to recognizing their potential to promote human health [
6,
19].
4.6.1. Environmental Uses
Bacteria play a key role in bioremediation, a process that uses microbial metabolism to clean up polluted environments. This includes the degradation of oil spills, pollutants and wastewater treatment. Recent advances in this field have highlighted the versatility and effectiveness of bacteria in environmental management [
6].
Oil Spill Cleanup: Bacteria such as
Alcanivorax borkumensis and
Pseudomonas aeruginosa have the ability to break down hydrocarbons in oil spills. They use hydrocarbons as a source of carbon, breaking them down into less harmful substances. Recent research has demonstrated the potential of genetically modified bacteria to increase oil degradation efficiency. For example, bioengineered strains can be adapted to have a greater ability to degrade hydrocarbons or to better survive harsh marine conditions [
6].
Degradation of pollutants: Bacteria are also used to degrade various pollutants, including heavy metals, pesticides and industrial chemicals. For example, bacteria such as Geobacter sulfureducens can reduce and precipitate heavy metals such as uranium, chromium and iron, and thus removing them from contaminated groundwater. Recent research has focused on using consortiums of bacteria to degrade complex mixtures of pollutants more effectively than single strains. Additionally, advances in synthetic biology have enabled the creation of bacteria with improved degradation pathways for specific pollutants [
11].
Wastewater treatment: In sewage treatment plants, bacteria are necessary to break down organic matter, nitrogen compounds and other pollutants. Activated sludge systems rely on a diverse community of microbes to effectively treat wastewater. Recent innovations include the development of microbial fuel cells, which use bacteria to generate electricity while treating wastewater, thus providing the dual benefits of waste treatment and energy production. Anaerobic bacteria are also used to produce biogas, transforming organic waste into methane, which can later be used as a renewable energy source [
9].
Microbial electrochemical technologies: Microbial electrochemical technologies (MET), where bacteria are used in bioelectrochemical systems to treat wastewater, generate electricity and produce valuable chemicals. Bacteria such as
Shewanella oneidensis and
Geobacter spp. can transfer electrons to the electrodes, facilitating the breakdown of organic pollutants and generating electric current. Recent research focuses on optimizing systems for higher performance and scalability [
8].
Bioremediation of plastic waste: Certain bacteria, such as polyethylene terephthalate (PET), have shown promise in degrading plastics. For example, the bacterium
Ideonella sakaiensis can break down PET into monomers, which can then be reused to produce new plastics. Research is also being carried out to increase the ability of such bacteria to degrade plastics through genetic engineering and the development of scalable bioremediation strategies [
7,
11].
Thus, the use of bacteria in environmental management continues to grow with advances in biotechnology, synthetic biology, and genetic engineering. These developments hold great promise for solving some of our most challenging environmental problems, including pollution, waste management and resource recovery.
4.7. Cutting-Edge Research
Metagenomics and microbiome research have also revolutionized our understanding of the role of bacteria. The therapeutic possibilities of probiotics and fecal microflora transplants are being investigated. CRISPR-Cas technology, derived from the immune systems of bacteria, is used for gene editing and offers increasingly new possibilities for the treatment of genetic diseases and infections. In addition, CRISPR-Cas technology, derived from bacterial immune systems, is used to edit genes, offering potential drugs for genetic diseases and methods of treating infections [
20]. CRISPR-Cas technology, derived from the immune system of bacteria, has become a breakthrough gene editing tool. This technology offers new possibilities for the treatment of genetic diseases and infections, enabling precise DNA modifications that were previously unimaginable [
3,
22,
23]. Additionally, CRISPR-Cas systems are being investigated for their potential to develop new antimicrobials and gene therapies, representing significant progress in the treatment of genetic and infectious diseases [
3,
23]. Continued progress in metagenomics and CRISPR-Cas technology highlights the importance of bacteria beyond their traditional roles. These innovations open up new possibilities for personalized medicine, sustainable agriculture and environmental stewardship, demonstrating the irreplaceable role of bacteria in future scientific and medical breakthroughs.
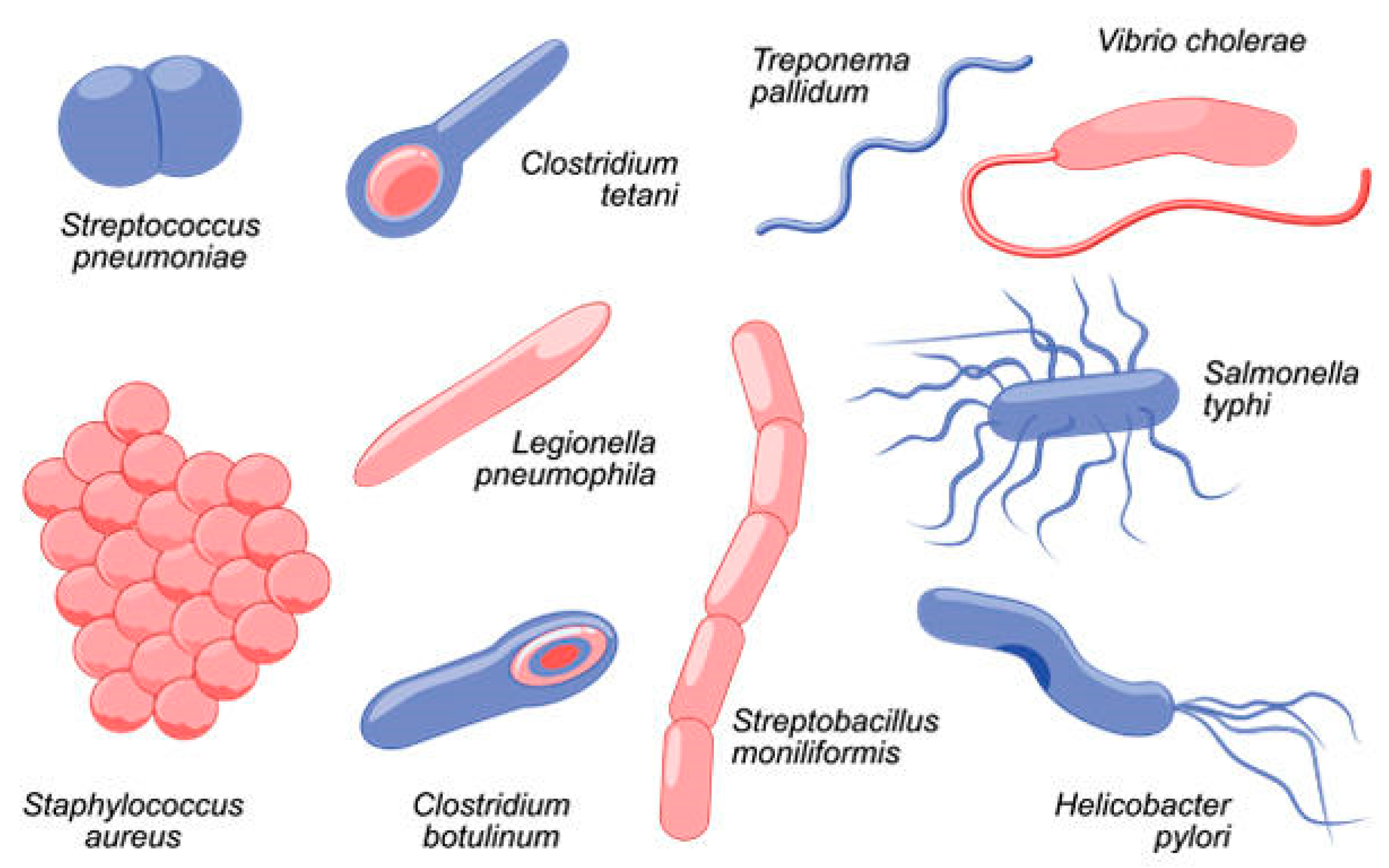
In contact with the body: On and inside the human body, bacteria offer many benefits. Outside the body, a forest of bacteria on the skin (according to scientists from New York University, almost 200 distinct species on a normal human) dominates the skin environment and its resources, preventing other bacteria from settling in. Both inside and outside, exposure to bacteria is an important part of the development of our immune system [
15].
Exposure to bacteria (
Figure 3), both mild and harmful, stimulates the immune system to respond to pathogenic invaders later in life. This does not mean that beneficial bacteria cannot also be dangerous. Typically, beneficial bacteria and harmful bacteria mutually exclude each other. However, these processes overlap, especially with bacteria inhabiting the body. Staphylococcus bacteria are a good example because they are found all over our skin. A colony of Staphylococcus aureus living on the arm can clog, displacing intruders without damaging the body, but if they enter the bloodstream, the immune system is weakened, and the bacteria can go on a rampage, causing infection [
19].
Inside the body: Most beneficial bacteria in the human body are located in the digestive system as the gut microbiome. Skin (especially the moist areas, such as the groin and between the toes), Conjunctiva respiratory tract (particularly the nose), Gastrointestinal tract (primarily the mouth and the loon), Urethra and bladder (urinary tract), Vagina, Placenta, Uterus, Oral cavity/ mucosa, Lung, Mammary glands, Ovarian follicles, Placenta [
2,
16]. These bacteria help break down food and keep us healthy. In the digestive system, they help us break down food, such as plant fibers, which we cannot handle well on our own. Thanks to bacteria, we get more nutrients from our food. Bacteria in the digestive system also provide us with essential vitamins such as biotin and vitamin K and are our main source of some of these nutrients [
18]
. Experiments conducted on guinea pigs have shown that animals raised in a sterile, bacteria-free environment are malnourished and die young.
Some people regularly take probiotics or during antibiotic treatment to support gut health. These supplements contain strains of beneficial bacteria, such as Bifidobacteria and
Lactobacillus. Probiotics are also used in food production to make yogurts and fermented products like sauerkraut, kimchi, and kombucha [
24]. The probiotics help in maintain the balance between useful and harmful bacteria. If balance between them is disturb it may cause many disorders sometimes even cancer [
25].
The gut microbiome is linked to an individual's genetics and diet, which is crucial to gut health and overall health. In health conditions, commensal microorganisms maintain homeostasis with the body, protecting it from pathogen invasion and supporting the metabolism of macromolecules in the intestines. Specific genetic variants, especially in immune system genes, may lead to intestinal dysbiosis, which in turn increases the risk of developing autoimmune diseases such as inflammatory bowel disease or type 1 diabetes [
26,
27,
28,
29].
Diet plays an important role in shaping the composition of the intestinal microflora by promoting the growth of specific microorganisms that metabolize dietary components. The metabolites produced by these bacteria may have both beneficial and potentially harmful effects, for example they may influence the risk of cancer development [
16,
17,
30,
31,
32].
Recently, there has been a lot of interest in the complex relationship between the human genome and the gut microbiome. This has led to multi-omics approaches in research. Research has sought to identify relationships between the entire genome and the microbiome in order to better understand this complex relationship [
33].
Summary: Bacteria are essential to human health, industry and the environment, and their benefits far outweigh their potential risks. Continued research and innovation in microbiology and biotechnology is crucial to further development and understanding of their role.
5. Healthy Microbiota as a Therapeutic Agent
Advances in our understanding of the crucial role that microflora play in health and disease are extremely interesting, and there is overwhelming experimental scientific evidence that microorganisms can play a significant role in neurological, cardiovascular, gastrointestinal, immune, and other bodily functions. Metagenomic analysis involves isolating DNA from microorganisms colonizing the small intestine, sequencing specific marker genes, and then performing taxonomic and functional analysis of the data with bioinformatics support. This provides a very clear picture of the families and strains with known positive or negative functions that are present in the small intestine, including their relative abundance and those that are lacking or present in excessive amounts [
3,
7,
10,
11].
Table 2 summarizes the role of microflora, including: In:
- −
health and disease: Microorganisms influence neurological, cardiovascular, gastrointestinal, immune and other functions, emphasizing the importance of a balanced microflora.
- −
Metagenomics: as this involves the analysis of the DNA of microorganisms in the small intestine using sequencing and bioinformatics in order to understand their structure and function especially in ecology.
- −
Challenges of metagenomics: include selecting appropriate sequencing methods and understanding gene roles.
- −
Intestinal microflora dysbiosis, or imbalance, is associated with obesity, diabetes, thyroid disease and inflammation.
- −
Fermented pineapple whey protein (PWF) restores intestinal flora in mice with cefixime-induced dysbiosis.
- −
PWF mechanism of action: Increases the number of beneficial bacteria (Weissella, Lactococcus, Faecalibaculum, Bacteroides acidophilus) and reduces the number of harmful bacteria (Akkermansia, Escherichia-Shigella).
- −
Microbial Metabolites: PWF modulates metabolites such as L-glutamate and L-threonine and improves amino acid metabolic pathways.
- −
Therapeutic use of PWF: raises potential dietary strategies for the treatment of antibiotic-induced diarrhea.
- −
Fecal microflora transplant (FMT): there are proven benefits in the treatment of gastrointestinal, metabolic, neurological and autoimmune diseases
Metagenomics is a new field that identifies all the genetic material of organisms, which allows discovering the genetic variability of microorganisms in the environment. This technology provides greater flexibility for microbiologists by enabling immediate understanding of the genetic diversity of a microbial community. However, identifying appropriate methods for sequencing specific genes is a challenge that limits the ability to precisely understand their metabolic and ecological roles. The 'targeted metagenomics' approach is key to gaining comprehensive knowledge of gene sequences relevant to ecosystems, including humans, animals and microorganisms. This approach allows for a better understanding of their function and evolution, although sequence-based analysis has its limitations [
7,
29,
30,
31,
34].
It has been confirmed that an excess of proteolytic bacteria affects health and generally reduces the number of species present or causes a deficit of beneficial gut bacteria. A healthy microflora and its changes over time during illness are well-documented. Comprehensive changes in microflora (dysbiosis) are common in obesity and are caused by an improper diet, medication, or a pro-inflammatory lifestyle. There are many studies linking type 1 and type 2 diabetes to microflora different from that found in the general population, and fewer studies on the thyroid [
30].
Luo et al. [
34] investigated the effects of fermented pineapple whey protein (PWF) in a cefixime-induced dysbiosis model in mice using 16S sequencing and untargeted metabolomics. The results showed that PWF had a positive effect on the restoration of intestinal flora. Cefixime lowered the overall abundance of intestinal flora and reduced the relative abundance of probiotics while inhibiting amino acid metabolism. PWF normalized the intestinal flora by increasing the populations of
Weissella, Lactococcus, Faecalibaculum and
Bacteroides acidophilus and reducing the numbers of
Akkermansia and
Escherichia-Shigella. Additionally, PWF modulates microbial metabolites such as L-glutamate and L-threonine and increases the activity of amino acid-related metabolic pathways. PWF may alleviate gut flora dysbiosis and antibiotic-induced metabolic disorders, suggesting its potential use as a dietary strategy for patients with antibiotic-induced diarrhea.
The key message is to maintain the balance of millions of microorganisms to stay healthy. We can achieve this with fecal microbiota transplantation (FMT) technology. The benefits of FMT treatment are confirmed in many diseases, especially gastrointestinal, metabolic, neurological, and autoimmune diseases, in cases of incurable diseases. However, there is still a gap between our understanding and translating it into practice [
29,
30,
32,
33].
5.1. Latest Achievements
Advanced metagenomic tools: Recent developments in metagenomic tools have improved the resolution and accuracy of microbial community analysis, enabling a better understanding of the role of the gut microbiome in health and disease [
35,
36].
- −
Personalized microbiome therapies: This research focuses on personalized microbiome therapies tailored to individual microbiome profiles, showing great promise in the treatment of complex diseases [
34,
35,
37,
38].
- −
Synthetic biology and microbiome engineering: Innovations in this field have led to the development of microbial consortiums with improved therapeutic properties, providing new opportunities for microbiome-based treatments [
39].
Microbiome biomarkers in Parkinson's disease: The gelatinous microbiome is a biologically active substance in the treatment of Parkinson's disease (PD), which can protect against disease. Biomarkers identified based on the microbiome to help select patients for clinically targeted microbiome studies. To study the metabolites of Parkinson's disease clearly identify those with Parkinson's disease and identify dysbiosis’s, to classify patients according to their characteristics and dysbiotics, and to define the types of Parkinson's disease that affect the microbiome. Not all patients with Parkinson's disease have a dysbiotic microbiome, and the dysbiotic microbiome in Parkinson's disease develops under the influence of sodium. I propose a method of identifying ideal candidates for clinical trials on microbiome and personalized therapies based on dysbiosis profiles [
38].
The gelatinous microbiome is responsible for the treatment of Parkinson's disease (PD), which can prevent disease. To study the metabolites of Parkinson's disease in humans, as well as its dysbiotic counterparts, to classify patients as follows: Not all patients with Parkinson's disease have a dysbiotic microbiome, and the dysbiotic microbiome in Parkinson's disease develops under the influence of sodium. The best method for identifying ideal candidates for clinical trials on microbiome and personalized therapies is to determine the level of dysbiosis and its profile [
39].
Over the last decade, there has been increasing interest in engineering bacteria for therapeutically relevant applications. Previous work has focused on repurposing genetically susceptible model strains such as Escherichia coli. Engineering gut commensals is gaining popularity due to their innate ability to survive and reproduce well in the gut for extended periods of time. However, the main challenge is their limited genetic capabilities. Recent advances in systems and synthetic biology have unlocked the effective use of native gut commensals for therapeutic and diagnostic purposes. These advances include the design of synthetic microbial consortiums and the construction of synthetic cells that perform sense-and-response logic, enabling real-time detection and delivery of therapeutic payload in response to specific gut signals. Recent updates in microbiome therapies, with particular emphasis on intestinal commensal engineering based on synthetic biology and a systemic understanding of their molecular phenotypes [
38]. Taken together, these updates reflect the rapidly evolving field of microbiome research and its potential to revolutionize healthcare.
6. Future Directions
Our knowledge of the role of bacteria in human health and ecosystems is constantly evolving. The future of research on intestinal microflora and its impact on human health is promising, opening new possibilities for diagnosing and treating diseases. Faecal microbiota transplantation (FMT) technology shows potential in the treatment of a wide range of conditions, including gastrointestinal, metabolic and neurological disorders. The use of metagenomics and bioinformatics allows for precise analysis of the composition of the microbiome, which can lead to more personalized and effective interventions [
21,
28,
32].
As our understanding of the role of bacteria in human health and ecosystems continues to advance, several promising directions for research and application are emerging:
- −
However, many challenges remain. Further research is needed to understand the complex interactions between bacteria and their hosts, as well as the impact of various environmental factors and lifestyle choices on the microbiome. Furthermore, the development of new microbiota manipulation techniques and tools, such as prebiotics, probiotics and symbiotic, can significantly improve public health [
3,
11,
12,
17,
18,
21,
35].
- −
Advanced Therapeutic Applications: Fecal microbiota transplantation (FMT) holds significant promise in treating a wide array of conditions, including gastrointestinal disorders like Clostridium difficile infection, metabolic syndromes, and even neurological disorders. The ability to manipulate the microbiome through FMT opens new avenues for therapeutic interventions [
21,
28,
32].
- −
Precision Medicine and Personalized Interventions: Metagenomics and bioinformatics are enabling precise analyses of microbiome compositions. This capability is crucial for developing personalized interventions that can target specific microbial imbalances associated with various diseases, potentially leading to more effective treatments [
6,
9,
36,
37].
- −
Challenges and Areas for Further Research: Despite the progress, challenges remain in fully understanding the complex interactions between bacteria and their human hosts. Research efforts must continue to explore how environmental factors, diet, and lifestyle choices influence microbiome composition and function [
38].
- −
Innovative Microbiota Manipulation: The development of new tools and techniques such as prebiotics, probiotics, and symbiotic offers promising avenues to modulate the microbiome for improved public health outcomes [
23].
- −
Interdisciplinary Collaboration: Collaboration among microbiologists, medical researchers, ecologists, and computer scientists will be essential to harnessing the full potential of bacteria. This interdisciplinary approach will facilitate comprehensive studies and innovative solutions that integrate insights from diverse fields [
19,
22,
23].
- −
Revolutionizing Health and Environmental Protection: Recognizing bacteria as essential partners in human health and environmental sustainability has transformative potential. Understanding and leveraging their roles could lead to revolutionary changes in healthcare practices, nutritional strategies, and environmental conservation efforts [
11,
37,
38,
39].
- −
Growing interest in biochar, particularly due to its ability to mediate and facilitate the breakdown of microbial contaminants, as well as its potential for carbon sequestration. The economic and environmental benefits and future prospects of biochar can be assessed. The use of chemical fertilizers and pesticides can be managed sustainably through the use of engineered biochar, which promotes the creation of sustainable engineering infrastructure and induces a circular bioeconomy [
11,
39].
In the future, cooperation between scientists from various fields - microbiology, medicine, ecology and computer science - will be crucial to fully exploit the potential of bacteria to improve human health and protect the environment. Understanding the role of bacteria as irreplaceable partners in our lives can lead to revolutionary changes in the approach to health, nutrition and environmental protection [
24,
37,
38].
7. Conclusions
Our lives are closely linked to the presence of bacteria, both inside and outside our bodies. Bacteria play a key role in digestion, nutrient absorption and protection against pathogens, and are essential components of Earth's ecosystems:
Role in human health: Bacteria in the human microbiome, especially in the gut, are crucial for the proper functioning of the digestive system, vitamin synthesis and protection against infections. A healthy microbiota is essential to maintaining overall health and is linked to many aspects of health, from the immune system to neurological function.
Ecological importance: Bacteria are key players in the cycling of elements such as carbon and nitrogen, which are essential for plant growth and the overall functioning of ecosystems. Processes such as the decomposition of dead matter and the transformation of atmospheric nitrogen are made possible by bacteria, which highlights their invaluable role in the environment.
New therapeutic possibilities: Advances in research on the intestinal microbiota, including FMT technology, open new possibilities for the diagnosis and therapy of gastrointestinal, metabolic, neurological and other diseases. The use of metagenomics and bioinformatics enables precise analysis of the microbiome, which can lead to more personalized and effective interventions.
Recent research has demonstrated that the gut microbiome's role in Parkinson's disease is highly individualized, with significant variability in dysbiotic features among patients. This suggests that personalized microbiome-based therapies could be more effective than one-size-fits-all approaches, emphasizing the need for tailored treatments based on specific microbiome profiles.
Challenges and the future: Despite promising progress, many challenges remain. Further research is needed to understand the complex interactions between bacteria and their hosts and the impact of various environmental and lifestyle factors on the microbiome. Collaboration between scientists from different fields will be crucial to fully realize the potential of bacteria.
To sum up, bacteria are an integral part of life on Earth, and their understanding and use can lead to revolutionary changes in the approach to health, nutrition and environmental protection.
This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
Author Contributions
Conceptualization, PDG. and B.S.; methodology, PDG, D.S, B S.; software, D.S.; validation, B.S., D.S.; formal analysis, D.S.; investigation, B.S.; resources, D.S.PDG.; data curation, D.S.; writing—original draft preparation, PDG, D.S.; writing—review and editing, B.S., PDG.; visualization, D.S.; supervision, B.S, PDG; project administration, PDG.; funding acquisition, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
This research did not generate any new data.
Acknowledgments
The authors would like to thank the University of Life Sciences in Lublin, Poland, for administrative support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gupta, P.D. Bacteria: The Powerful Creatures: A Mini Review. J. Cell Tissue Res. 2018, 18, 6555–6558. [Google Scholar]
- Gupta, P.D. Human vaginal microbiota: Boon or bane. J. Obstet. Gynaecol. Reprod. Sci. 2021, 5. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; et al. From dietary fibre to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Hyde, E.R.; Haarmann, D.P.; Lynne, A.M.; Bucheli, S.R.; Petrosino, J.F. The living dead: Bacterial community structure of a cadaver at the onset and end of the bloat stage of decomposition. PLoS ONE 2013, 30, e77733. [Google Scholar] [CrossRef]
- Mfon, E.I. Microbial Biotechnology: Application of Bacteria in Various Industrial Processes and Environment Remediation. Microbial Biotechnology. Int. J. Dev. Sustain. Environ. Manag. 2024, 4, 16–24. [Google Scholar]
- Awasthi, M.K.; Ravindran, B.; Sarsaiya, S.; Chen, H.; Wainaina, S.; Singh, E.; Liu, T.; Kumar, S.; Pandey, A.; Singh, L.; et al. Metagenomics for taxonomy profiling: Tools and approaches. Bioengineered 2020, 11, 356–374. [Google Scholar] [CrossRef] [PubMed]
- Monib, A.W.; Niazi, P.; Barai, S.M.; Sawicka, B.; Baseer, A.Q.; Nikpay, A.; Fahmawi SM, S.; Singh, D.; Alikhail, M.; Thea, B. Nitrogen Cycling Dynamics: Investigating Volatilization and its Interplay with N2 Fixation. J. Res. Appl. Sci. Biotechnol. 2024, 3, 17–31. [Google Scholar] [CrossRef]
- Hooper, R. Bacteria make snow and rain fall. New Sci. 2008, 197, 2646. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Liu, H.; Kumar, V.; Yadav, V.; Guo, S.; Sarsaiya, S.; Binod, P.; Sindhu, R.; Xu, P.; Zhang, Z.; Pandey, A.; et al. Bioengineered biochar as smart candidate for resource recovery toward circular bio-economy: A review. Bioengineered 2021, 12, 10269–10301. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Rinne, M.; Gueimonde, M.; Kalliomäki, M.; et al. Gut microbiota composition and butyrate production in children affected by non-IgE-mediated cow's milk allergy. Gut Microbes 2020, 11, 1321–1331. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.D.; Tyagi, S. Development of the immune system from newborn to adult: A new look. J. Cell Tissue Res. 2020, 20, 6853–6860. [Google Scholar]
- Gupta, P.D.; Gupta, A. Immunopathology of the human placenta. J. Obstetrics Gynaecology, and Reproductive Sciences 2021, 5. [Google Scholar]
- Pushkala, K.; Gupta, P.D. Faecal microbiota transplant technology in the management of Huntington's disease. J. Infect. Dis. Treat. 2023, 1, 1–3. [Google Scholar] [CrossRef]
- Soni, J.; Sinha, S.; Pandey, R. Understanding bacterial pathogenicity: A closer look at the journey of harmful microbes. Front Microbiol. 2024, 20, 1370818. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Lynch, S.V. Community ecology as a framework for human microbiome research. Nat. Med. 2019, 25, 884–889. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Mullish, B.H.; Kelly, C.; Fischer, M. The evolution of the use of fecal microbiota transplantation and emerging therapeutic indications. Lancet 2019, 394, 420–431. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. The role of faecal microbiota transplantation (FMT) in gastrointestinal disorders. Neurogastroenterol. Motil. 2020, 32, e13757. [Google Scholar]
- Barrangou, R.; Doudna, J.A. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 2016, 34, 933–941. [Google Scholar] [CrossRef]
- Komor, A.C.; Badran, A.H.; Liu, D.R. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell 2017, 168, 20–36. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Gorbach, S.L. Bacterial Infections: Overview. Int. Encycl. Public Health 2008, 273–282. [Google Scholar]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit Rev Food Sci Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, B.; Johar, K.; Sood, P.P.; Gupta, P.D. Imbalance of gut microbiota induces cancer. J Cell Tissue Res. 2017, 17, 6073–6084. [Google Scholar]
- Pushkala, K.; Gupta, P.D. Fecal microbiota transplant (FMT): An effective therapeutic agent in Parkinson's disease. New Medical Innovations and Research 2023, 4. [Google Scholar] [CrossRef]
- Pushkala, K.; Gupta, P.D. Polycystic ovary syndrome treated with fecal transplant technology. J. Gynaecol. Obstet. Mother Health 2023, 1, 1–4. [Google Scholar]
- Muhammad, A.Y.; Amonov, M.; Baig, A.A.; Alvi, J.F. Advanced Gut & Microbiome Research [in] Gut Microbiome: An Intersection between Human Genome, Diet, and Epigenetics 2024. [CrossRef]
- Gupta, P.D.; Pushkala, K. Faecal microbiota transplant technology: An effective therapeutic method for many diseases. J. of Clinical and Medical Case Reports and Reviews, 2023, V(2)I(2).
- Gupta, P.D.; Pushkala, K. FMT as an effective therapeutic agent in the treatment of endometriosis. J. of Clinical and Medical Case Reports and Reviews, 2023, V(2)I(2).
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. New Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xiao, S.; Ma, D.; Xiang, J.; Wang, B.; Cai, Y.; Wang, J. Investigating the Impact of Pineapple–Whey Protein Fermentation Products on Cefixime-Induced Intestinal Flora Dysbiosis in Mice Using 16S Sequencing and Untargeted Metabolomics Techniques. Foods 2024, 13, 1927. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; et al. Immune interactions of the microbiome and gut-brain axis: Relevance to development and neurological disorders. Front. Immunol. 2020, 11, 1125. [Google Scholar] [CrossRef]
- Qin, J.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Vilela, C.; Araújo, B.; Guedes, C.; Show GTeixeira, F.; et al. From the Gut to the Brain: Is Microbiome a New Paradigm in Parkinson’s Disease Treatment? 2024. [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polym. 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).