Submitted:
19 June 2024
Posted:
20 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
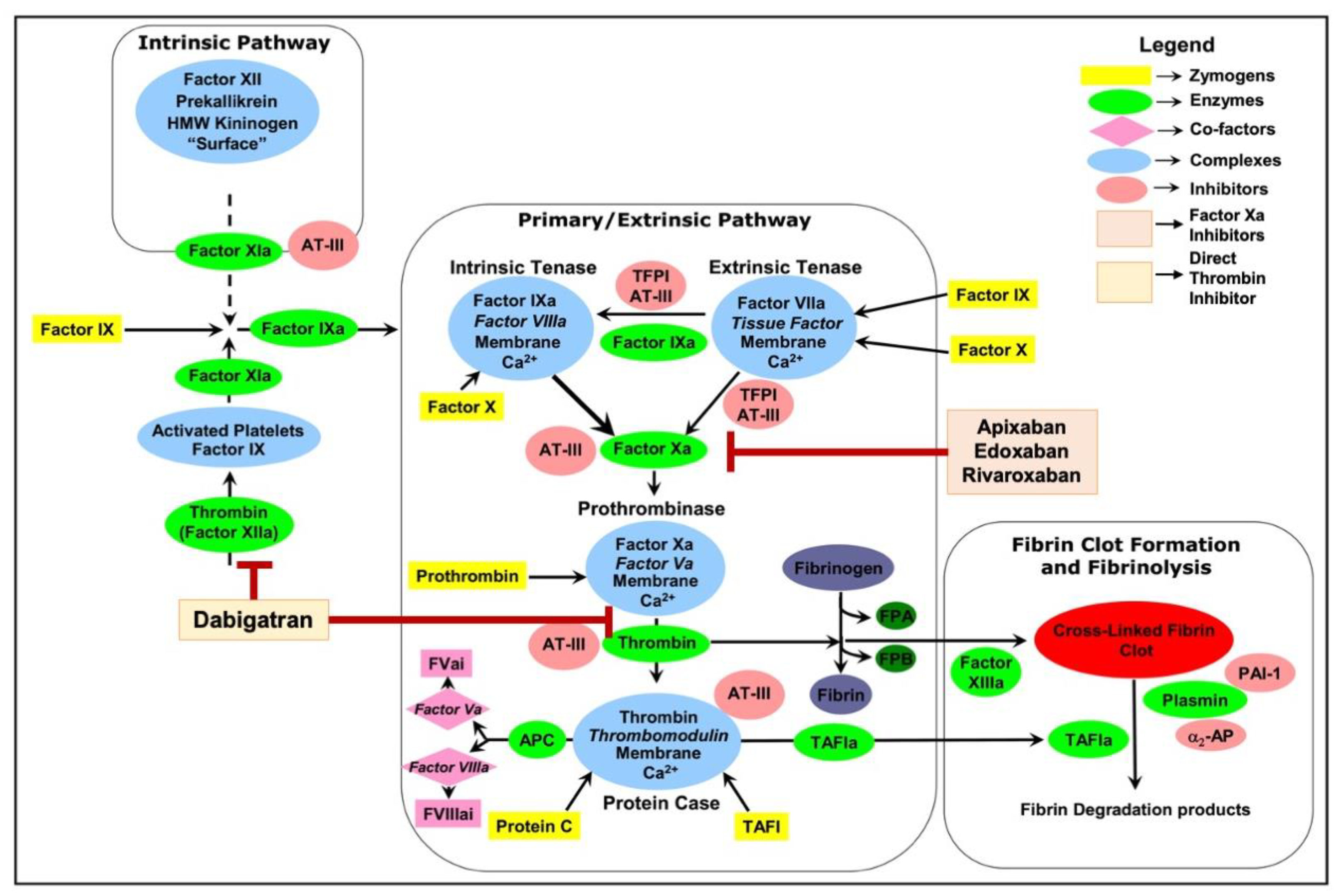
2. Exploring NOACs: From Pharmacokinetics to Clinical Efficacy and Pivotal Trials
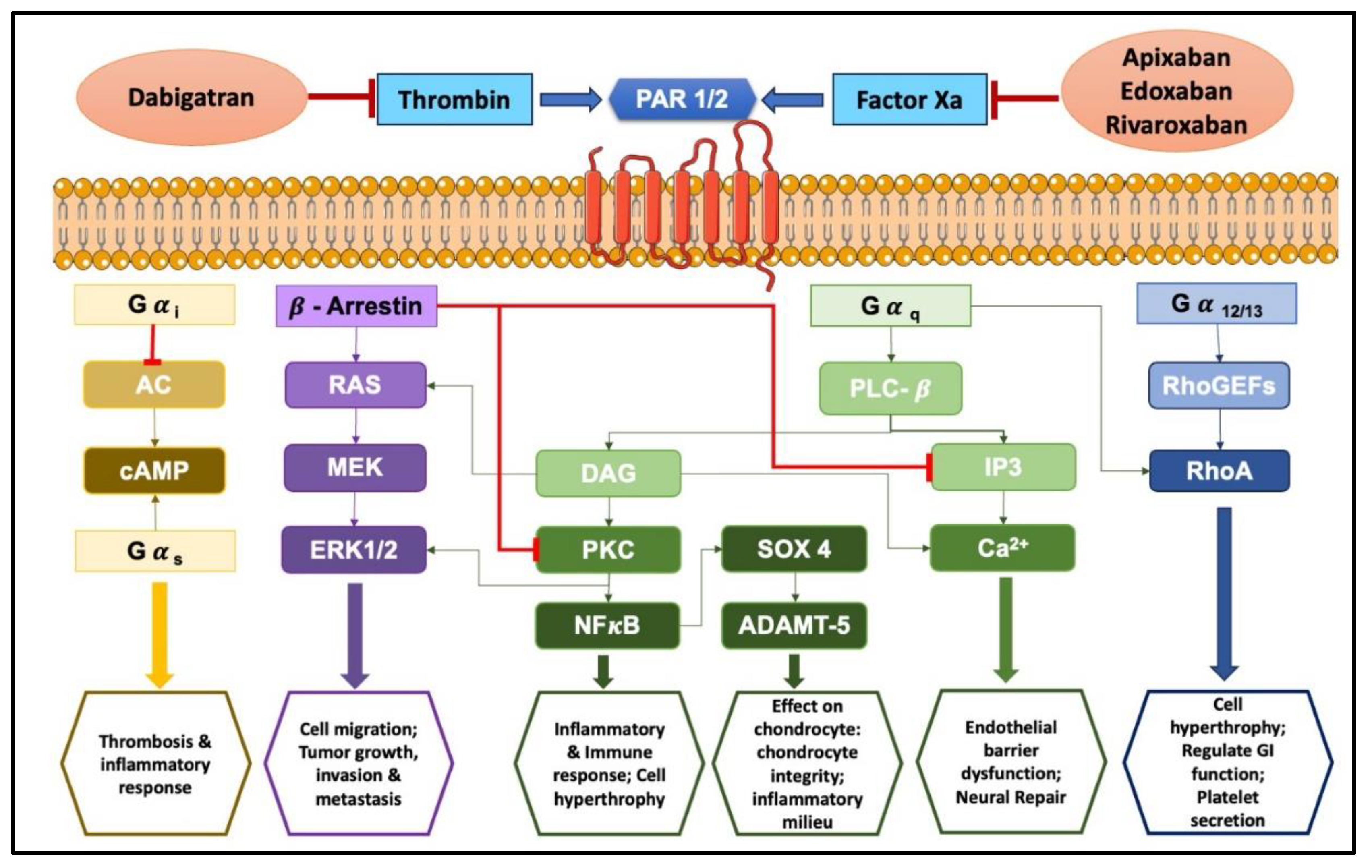
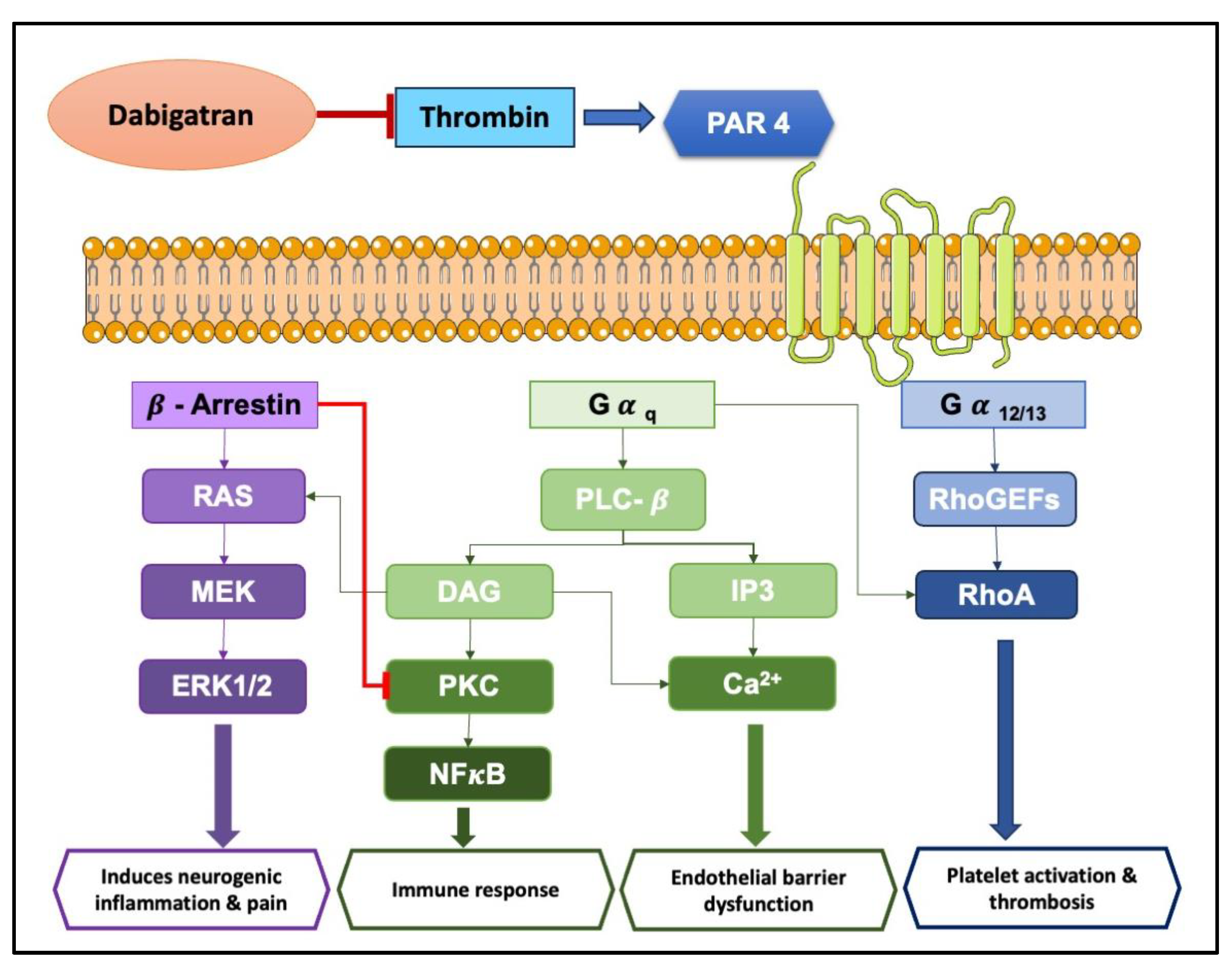
3. Exploring Protease-Activated Receptors (PARs): Unveiling Their Role in Inflammation and Disease
3.1. Exploring the Role of PARs in Neuroinflammation and Hyperalgesia
3.2. Chronic Gastrointestinal Inflammation
3.3. Inflammatory Disorders of Respiratory System
3.4. Cardiovascular Inflammatory Disorders
3.5. Inflammatory Disorders of Urinary System
3.6. Rheumatoid Arthritis and Osteoarthritis
4. Anti-Inflammatory Potentials of NOACs
4.1. Apixaban’s Anti-Inflammatory Profile
4.2. Edoxaban’s Anti-Inflammatory Profile
4.3. Rivaroxaban’s Anti-Inflammatory Profile
4.4. Dabigatran’s Anti-Inflammatory Profile
4.5. Comparative Anti-Inflammatory Potential of NOACs
5. Novel Revelations of Apixaban in Osteoarthritis
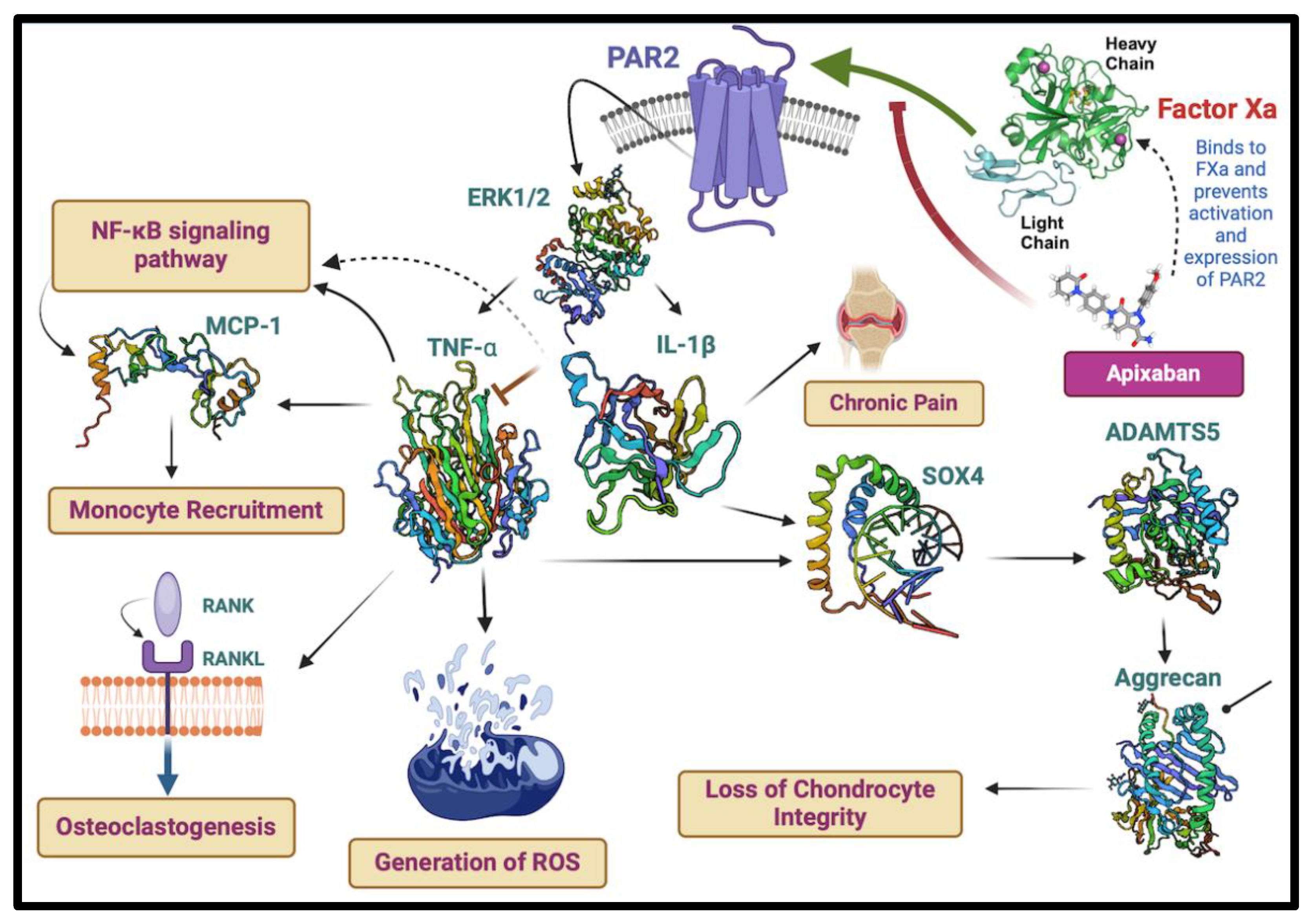
- 1.
- AR2 Inhibition: The blockage of PAR2 activation by apixaban may ameliorate the downstream signalling events mediated by ERK1/2 that led to the production of pro-inflammatory cytokines, such as TNF-α and IL-1β, potentially alleviating chronic pain associated with OA.
- 2.
- Cytokine Modulation: The expected reduction in TNF-α and IL-1β due to apixaban’s action on FXa mitigates the upregulation of molecules like MCP-1, which are involved in monocyte recruitment and the NF-κBsignaling pathway, both key contributors to inflammation and osteoclastogenesis.
- 3.
- Protein Expression: The illustration also indicates the potential effects of apixaban on the expression of regulatory proteins, including SOX4 and ADAMTS5, and their impact on critical components like aggrecan, which is essential for cartilage integrity.
- 4.
- Chondrocyte Integrity and Bone Health: By modulating these inflammatory and catabolic pathways, apixaban may help preserve chondrocyte integrity, mitigate the generation of reactive oxygen species (ROS), and contribute to maintaining joint health by potentially impacting the RANK/RANKL pathway, which is crucial for osteoclast activity and bone resorption
6. Unexplored Aspects of NOACs in Inflammation
6.1. Identified Gaps in Current Research
6.1.1. Limited Understanding of Molecular Mechanisms
6.1.2. Inconsistencies in Anti-Inflammatory Effects Across Models
6.1.3. Lack of Longitudinal Human Studies
6.1.4. Population Diversity in Research
6.1.5. Comparative Effectiveness of Different NOACs
6.2. Proposed Methodologies and Future Directions
6.2.1. Advanced Molecular and Cellular Studies
6.2.2. Standardized Experimental Models
6.2.3. Longitudinal Clinical Studies
6.2.4. Population Specific Research
6.2.5. Comparative Effectiveness of Research
6.2.6. Integration of Multi-Omics Approaches
6.2.7. Innovative Experimental Models
6.2.8. Designing Better Thrombin and Factor Xa Using AI
6.2.9. Designing NOAC-Antibody Conjugates for Enhanced Anti-Inflammatory Activity
6.2.10. Design of Aptameric NOAC Conjugates to Attenuate PAR-Mediated Inflammation
7. Addressing Specific Research Gaps
7.1. Molecular Mechanism of NOACs
7.2. Comparative Studies in Diverse Models
7.3. Long-Term Impacts of NOACs
7.3. Broadening Research Populations
7.4. Integration of Multi-Omics Data
8. Materials and Methods
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(5):515-23. [CrossRef]
- Bernardi F, Mariani G. Biochemical, molecular and clinical aspects of coagulation factor VII and its role in hemostasis and thrombosis. Haematologica. 2021;106(2):351-62. [CrossRef]
- Owens AP, 3rd, Mackman N. Tissue factor and thrombosis: The clot starts here. Thromb Haemost. 2010;104(3):432-9.
- Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29(1):17-24. [CrossRef]
- England NHSN. Community pharmacy oral anticoagulant safety audit 2021/22: NHS England; 2023 [Available from: https://www.england.nhs.uk/long-read/community-pharmacy-oral-anticoagulant-safety-audit-2021-22/#about-this-report.
- Navar AM, Kolkailah AA, Overton R, Shah NP, Rousseau JF, Flaker GC, et al. Trends in Oral Anticoagulant Use Among 436 864 Patients With Atrial Fibrillation in Community Practice, 2011 to 2020. J Am Heart Assoc. 2022;11(22):e026723. [CrossRef]
- Arepally GM, Cines DB. Pathogenesis of heparin-induced thrombocytopenia. Transl Res. 2020;225:131-40. [CrossRef]
- Kimmel SE. Warfarin therapy: in need of improvement after all these years. Expert Opin Pharmacother. 2008;9(5):677-86. [CrossRef]
- Shields LBE, Fowler P, Siemens DM, Lorenz DJ, Wilson KC, Hester ST, et al. Standardized warfarin monitoring decreases adverse drug reactions. BMC Fam Pract. 2019;20(1):151. [CrossRef]
- Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165(10):1095-106.
- Tan CSS, Lee SWH. Warfarin and food, herbal or dietary supplement interactions: A systematic review. Br J Clin Pharmacol. 2021;87(2):352-74. [CrossRef]
- Chilbert MR, Zammit K, Ahmed U, Devlin A, Radparvar S, Schuler A, et al. A systematic review of therapeutic enoxaparin dosing in obesity. J Thromb Thrombolysis. 2024;57(4):587-97. [CrossRef]
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-51.
- Al Said S, Ellscheid M, Beltsios ET, Frey N. Non-Vitamin K Antagonist Oral Anticoagulants in Coronary Artery Disease. Hamostaseologie. 2022;42(3):201-9. [CrossRef]
- Kemkes-Matthes, B. [Anticoagulation-direct oral anticoagulants]. Internist (Berl). 2017;58(6):585-97.
- Mueck W, Schwers S, Stampfuss J. Rivaroxaban and other novel oral anticoagulants: pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring. Thromb J. 2013;11(1):10. [CrossRef]
- Eek AK, Oie E, Granas AG. Prescribing of NOACs has outnumbered warfarin: exploring how physicians choose anticoagulant treatments. Eur J Clin Pharmacol. 2018;74(3):323-30. [CrossRef]
- Di Minno A, Frigerio B, Spadarella G, Ravani A, Sansaro D, Amato M, et al. Old and new oral anticoagulants: Food, herbal medicines and drug interactions. Blood Rev. 2017;31(4):193-203. [CrossRef]
- Udayachalerm S, Rattanasiri S, Angkananard T, Attia J, Sansanayudh N, Thakkinstian A. The Reversal of Bleeding Caused by New Oral Anticoagulants (NOACs): A Systematic Review and Meta-Analysis. Clin Appl Thromb Hemost. 2018;24(9_suppl):117S-26S. [CrossRef]
- Hoffman M, Monroe DM. Impact of Non-Vitamin K Antagonist Oral Anticoagulants From a Basic Science Perspective. Arterioscler Thromb Vasc Biol. 2017;37(10):1812-8. [CrossRef]
- Ruff CT, Giugliano RP, Antman EM. Management of Bleeding With Non-Vitamin K Antagonist Oral Anticoagulants in the Era of Specific Reversal Agents. Circulation. 2016;134(3):248-61. [CrossRef]
- Coughlin SR, Camerer E. PARticipation in inflammation. J Clin Invest. 2003;111(1):25-7.
- van Gorp RH, Dijkgraaf I, Broker V, Bauwens M, Leenders P, Jennen D, et al. Off-target effects of oral anticoagulants - vascular effects of vitamin K antagonist and non-vitamin K antagonist oral anticoagulant dabigatran etexilate. J Thromb Haemost. 2021;19(5):1348-63. [CrossRef]
- Ito Y, Maejima Y, Nakagama S, Shiheido-Watanabe Y, Tamura N, Sasano T. Rivaroxaban, a Direct Oral Factor Xa Inhibitor, Attenuates Atherosclerosis by Alleviating Factor Xa-PAR2-Mediated Autophagy Suppression. JACC Basic Transl Sci. 2021;6(12):964-80.
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-62. [CrossRef]
- Eriksson BI, Dahl OE, Rosencher N, Clemens A, Hantel S, Feuring M, et al. Oral dabigatran etexilate versus enoxaparin for venous thromboembolism prevention after total hip arthroplasty: pooled analysis of two phase 3 randomized trials. Thromb J. 2015;13:36. [CrossRef]
- Goldhaber SZ, Schulman S, Eriksson H, Feuring M, Fraessdorf M, Kreuzer J, et al. Dabigatran versus Warfarin for Acute Venous Thromboembolism in Elderly or Impaired Renal Function Patients: Pooled Analysis of RE-COVER and RE-COVER II. Thromb Haemost. 2017;117(11):2045-52. [CrossRef]
- Diez-Villanueva P, Cosin-Sales J, Roldan-Schilling V, Barrios V, Riba-Artes D, Gavin-Sebastian O, et al. Use of Direct Acting Oral Anticoagulants in Elderly Patients with Atrial Fibrillation: A Multicenter, Cross-Sectional Study in Spain. J Clin Med. 2023;12(3). [CrossRef]
- Yu YB, Liu J, Fu GH, Fang RY, Gao F, Chu HM. Comparison of dabigatran and warfarin used in patients with non-valvular atrial fibrillation: Meta-analysis of random control trial. Medicine (Baltimore). 2018;97(46):e12841. [CrossRef]
- Grymonprez M, De Backer TL, Bertels X, Steurbaut S, Lahousse L. Long-term comparative effectiveness and safety of dabigatran, rivaroxaban, apixaban and edoxaban in patients with atrial fibrillation: A nationwide cohort study. Front Pharmacol. 2023;14:1125576. [CrossRef]
- Achilles A, Mohring A, Dannenberg L, Grandoch M, Hohlfeld T, Fischer JW, et al. Dabigatran enhances platelet reactivity and platelet thrombin receptor expression in patients with atrial fibrillation. J Thromb Haemost. 2017;15(3):473-6. [CrossRef]
- Russell RD, Hotchkiss WR, Knight JR, Huo MH. The efficacy and safety of rivaroxaban for venous thromboembolism prophylaxis after total hip and total knee arthroplasty. Thrombosis. 2013;2013:762310. [CrossRef]
- Prins MH, Lensing AW, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11(1):21. [CrossRef]
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-91. [CrossRef]
- Piazza G, Spyropoulos AC, Hsia J, Goldin M, Towner WJ, Go AS, et al. Rivaroxaban for Prevention of Thrombotic Events, Hospitalization, and Death in Outpatients With COVID-19: A Randomized Clinical Trial. Circulation. 2023;147(25):1891-901. [CrossRef]
- Ray WA, Chung CP, Stein CM, Smalley W, Zimmerman E, Dupont WD, et al. Association of Rivaroxaban vs Apixaban With Major Ischemic or Hemorrhagic Events in Patients With Atrial Fibrillation. JAMA. 2021;326(23):2395-404. [CrossRef]
- Ageno W, Bertu L, Bucherini E, Camporese G, Dentali F, Iotti M, et al. Rivaroxaban treatment for six weeks versus three months in patients with symptomatic isolated distal deep vein thrombosis: randomised controlled trial. BMJ. 2022;379:e072623. [CrossRef]
- Ajmal M, Friedman J, Sipra Q, Lassar T. Rivaroxaban: Expanded Role in Cardiovascular Disease Management-A Literature Review. Cardiovasc Ther. 2021;2021:8886210. [CrossRef]
- Young G, Lensing AWA, Monagle P, Male C, Thelen K, Willmann S, et al. Rivaroxaban for treatment of pediatric venous thromboembolism. An Einstein-Jr phase 3 dose-exposure-response evaluation. J Thromb Haemost. 2020;18(7):1672-85. [CrossRef]
- Cohen AT, Wallenhorst C, Rivera M, Ay C, Schaefer B, Abdelgawwad K, et al. Comparison of Clinical Outcomes in Patients with Active Cancer Receiving Rivaroxaban or Low-Molecular-Weight Heparin: The OSCAR-UK Study. Thromb Haemost. 2024. [CrossRef]
- Adelkhanova A, Oli PR, Shrestha DB, Shtembari J, Jha V, Shantha G, et al. Safety and efficacy of direct oral anticoagulants in comparison to warfarin in obese patients with atrial fibrillation: A systematic review and meta-analysis. Health Sci Rep. 2024;7(4):e2044. [CrossRef]
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807-15. [CrossRef]
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. [CrossRef]
- Liu X, Johnson M, Mardekian J, Phatak H, Thompson J, Cohen AT. Apixaban Reduces Hospitalizations in Patients With Venous Thromboembolism: An Analysis of the Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy (AMPLIFY) Trial. J Am Heart Assoc. 2015;4(12). [CrossRef]
- Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806-17.
- O’Donnell MJ, Eikelboom JW, Yusuf S, Diener HC, Hart RG, Smith EE, et al. Effect of apixaban on brain infarction and microbleeds: AVERROES-MRI assessment study. Am Heart J. 2016;178:145-50. [CrossRef]
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-92. [CrossRef]
- Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med. 2019;380(8):711-9. [CrossRef]
- Agnelli G, Becattini C, Meyer G, Munoz A, Huisman MV, Connors JM, et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N Engl J Med. 2020;382(17):1599-607. [CrossRef]
- Guntupalli SR, Brennecke A, Behbakht K, Tayebnejad A, Breed CA, Babayan LM, et al. Safety and Efficacy of Apixaban vs Enoxaparin for Preventing Postoperative Venous Thromboembolism in Women Undergoing Surgery for Gynecologic Malignant Neoplasm: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(6):e207410.
- Reinecke H, Engelbertz C, Bauersachs R, Breithardt G, Echterhoff HH, Gerss J, et al. A Randomized Controlled Trial Comparing Apixaban With the Vitamin K Antagonist Phenprocoumon in Patients on Chronic Hemodialysis: The AXADIA-AFNET 8 Study. Circulation. 2023;147(4):296-309. [CrossRef]
- Payne RM, Burns KM, Glatz AC, Male C, Donti A, Brandao LR, et al. Apixaban for Prevention of Thromboembolism in Pediatric Heart Disease. J Am Coll Cardiol. 2023;82(24):2296-309. [CrossRef]
- O’Brien SH, Rodriguez V, Lew G, Newburger JW, Schultz CL, Orgel E, et al. Apixaban versus no anticoagulation for the prevention of venous thromboembolism in children with newly diagnosed acute lymphoblastic leukaemia or lymphoma (PREVAPIX-ALL): a phase 3, open-label, randomised, controlled trial. Lancet Haematol. 2024;11(1):e27-e37. [CrossRef]
- Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-104. [CrossRef]
- Hokusai VTEI, Buller HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406-15.
- Raskob GE, van Es N, Segers A, Angchaisuksiri P, Oh D, Boda Z, et al. Edoxaban for venous thromboembolism in patients with cancer: results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematol. 2016;3(8):e379-87.
- Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018;378(7):615-24.
- Fuji T, Fujita S, Kawai Y, Nakamura M, Kimura T, Kiuchi Y, et al. Safety and efficacy of edoxaban in patients undergoing hip fracture surgery. Thromb Res. 2014;133(6):1016-22. [CrossRef]
- Fuji T, Wang CJ, Fujita S, Kawai Y, Nakamura M, Kimura T, et al. Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial. Thromb Res. 2014;134(6):1198-204. [CrossRef]
- Koretsune Y, Yamashita T, Kimura T, Fukuzawa M, Abe K, Yasaka M. Short-Term Safety and Plasma Concentrations of Edoxaban in Japanese Patients With Non-Valvular Atrial Fibrillation and Severe Renal Impairment. Circ J. 2015;79(7):1486-95. [CrossRef]
- Goette A, Merino JL, Ezekowitz MD, Zamoryakhin D, Melino M, Jin J, et al. Edoxaban versus enoxaparin-warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet. 2016;388(10055):1995-2003.
- Portman MA, Jacobs JP, Newburger JW, Berger F, Grosso MA, Duggal A, et al. Edoxaban for Thromboembolism Prevention in Pediatric Patients With Cardiac Disease. J Am Coll Cardiol. 2022;80(24):2301-10. [CrossRef]
- Shim CY, Seo J, Kim YJ, Lee SH, De Caterina R, Lee S, et al. Efficacy and safety of edoxaban in patients early after surgical bioprosthetic valve implantation or valve repair: A randomized clinical trial. J Thorac Cardiovasc Surg. 2023;165(1):58-67 e4.
- Hosokawa K, Watanabe H, Taniguchi Y, Ikeda N, Inami T, Yasuda S, et al. A Multicenter, Single-Blind, Randomized, Warfarin-Controlled Trial of Edoxaban in Patients With Chronic Thromboembolic Pulmonary Hypertension: KABUKI Trial. Circulation. 2024;149(5):406-9. [CrossRef]
- Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153 Suppl 1(Suppl 1):S263-82. [CrossRef]
- Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, et al. A dual thrombin receptor system for platelet activation. Nature. 1998;394(6694):690-4. [CrossRef]
- Lin H, Liu AP, Smith TH, Trejo J. Cofactoring and dimerization of proteinase-activated receptors. Pharmacol Rev. 2013;65(4):1198-213. [CrossRef]
- Ruf, W. Roles of factor Xa beyond coagulation. J Thromb Thrombolysis. 2021;52(2):391-6. [CrossRef]
- Bukowska A, Zacharias I, Weinert S, Skopp K, Hartmann C, Huth C, et al. Coagulation factor Xa induces an inflammatory signalling by activation of protease-activated receptors in human atrial tissue. Eur J Pharmacol. 2013;718(1-3):114-23. [CrossRef]
- Ohba T, Takase Y, Ohhara M, Kasukawa R. Thrombin in the synovial fluid of patients with rheumatoid arthritis mediates proliferation of synovial fibroblast-like cells by induction of platelet derived growth factor. J Rheumatol. 1996;23(9):1505-11.
- Itsekson-Hayosh Z, Shavit-Stein E, Last D, Goez D, Daniels D, Bushi D, et al. Thrombin Activity and Thrombin Receptor in Rat Glioblastoma Model: Possible Markers and Targets for Intervention? J Mol Neurosci. 2015;56(3):644-51.
- Ofosu, FA. Protease activated receptors 1 and 4 govern the responses of human platelets to thrombin. Transfus Apher Sci. 2003;28(3):265-8. [CrossRef]
- Coughlin, SR. How the protease thrombin talks to cells. Proc Natl Acad Sci U S A. 1999;96(20):11023-7.
- Garcia PS, Ciavatta VT, Fidler JA, Woodbury A, Levy JH, Tyor WR. Concentration-Dependent Dual Role of Thrombin in Protection of Cultured Rat Cortical Neurons. Neurochem Res. 2015;40(11):2220-9. [CrossRef]
- Lee PR, Johnson TP, Gnanapavan S, Giovannoni G, Wang T, Steiner JP, et al. Protease-activated receptor-1 activation by granzyme B causes neurotoxicity that is augmented by interleukin-1beta. J Neuroinflammation. 2017;14(1):131.
- Kempuraj D, Selvakumar GP, Thangavel R, Ahmed ME, Zaheer S, Kumar KK, et al. Glia Maturation Factor and Mast Cell-Dependent Expression of Inflammatory Mediators and Proteinase Activated Receptor-2 in Neuroinflammation. J Alzheimers Dis. 2018;66(3):1117-29. [CrossRef]
- Takayama Y, Derouiche S, Maruyama K, Tominaga M. Emerging Perspectives on Pain Management by Modulation of TRP Channels and ANO1. Int J Mol Sci. 2019;20(14). [CrossRef]
- Poole DP, Amadesi S, Veldhuis NA, Abogadie FC, Lieu T, Darby W, et al. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J Biol Chem. 2013;288(8):5790-802. [CrossRef]
- Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117(3):636-47.
- Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, et al. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med. 2001;7(7):821-6. [CrossRef]
- Asfaha S, Brussee V, Chapman K, Zochodne DW, Vergnolle N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br J Pharmacol. 2002;135(5):1101-6. [CrossRef]
- Saeed MA, Ng GZ, Dabritz J, Wagner J, Judd L, Han JX, et al. Protease-activated Receptor 1 Plays a Proinflammatory Role in Colitis by Promoting Th17-related Immunity. Inflamm Bowel Dis. 2017;23(4):593-602. [CrossRef]
- Cenac N, Cellars L, Steinhoff M, Andrade-Gordon P, Hollenberg MD, Wallace JL, et al. Proteinase-activated receptor-1 is an anti-inflammatory signal for colitis mediated by a type 2 immune response. Inflamm Bowel Dis. 2005;11(9):792-8. [CrossRef]
- Boucher AA, Rosenfeldt L, Mureb D, Shafer J, Sharma BK, Lane A, et al. Cell type-specific mechanisms coupling protease-activated receptor-1 to infectious colitis pathogenesis. J Thromb Haemost. 2020;18(1):91-103.
- Lau C, Lytle C, Straus DS, DeFea KA. Apical and basolateral pools of proteinase-activated receptor-2 direct distinct signaling events in the intestinal epithelium. Am J Physiol Cell Physiol. 2011;300(1):C113-23. [CrossRef]
- Hansen KK, Sherman PM, Cellars L, Andrade-Gordon P, Pan Z, Baruch A, et al. A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc Natl Acad Sci U S A. 2005;102(23):8363-8. [CrossRef]
- Liu B, Yang MQ, Yu TY, Yin YY, Liu Y, Wang XD, et al. Mast Cell Tryptase Promotes Inflammatory Bowel Disease-Induced Intestinal Fibrosis. Inflamm Bowel Dis. 2021;27(2):242-55. [CrossRef]
- Her JY, Lee Y, Kim SJ, Heo G, Choo J, Kim Y, et al. Blockage of protease-activated receptor 2 exacerbates inflammation in high-fat environment partly through autophagy inhibition. Am J Physiol Gastrointest Liver Physiol. 2021;320(1):G30-G42. [CrossRef]
- Majewski S, Zhou X, Milkowska-Dymanowska J, Bialas AJ, Piotrowski WJ, Malinovschi A. Proteomic profiling of peripheral blood and bronchoalveolar lavage fluid in interstitial lung diseases: an explorative study. ERJ Open Res. 2021;7(1). [CrossRef]
- Bhargava M, Viken K, Wang Q, Jagtap P, Bitterman P, Ingbar D, et al. Bronchoalveolar Lavage Fluid Protein Expression in Acute Respiratory Distress Syndrome Provides Insights into Pathways Activated in Subjects with Different Outcomes. Sci Rep. 2017;7(1):7464. [CrossRef]
- Lambrecht BN, Hammad H. Asthma and coagulation. N Engl J Med. 2013;369(20):1964-6. [CrossRef]
- de Boer JD, Majoor CJ, van ’t Veer C, Bel EH, van der Poll T. Asthma and coagulation. Blood. 2012;119(14):3236-44.
- Deng Z, Xie H, Cheng W, Zhang M, Liu J, Huo Y, et al. Dabigatran ameliorates airway smooth muscle remodeling in asthma by modulating Yes-associated protein. J Cell Mol Med. 2020;24(14):8179-93.
- Yue M, Hu M, Fu F, Ruan H, Wu C. Emerging Roles of Platelets in Allergic Asthma. Front Immunol. 2022;13:846055. [CrossRef]
- Gandhi VD, Shrestha Palikhe N, Vliagoftis H. Protease-activated receptor-2: Role in asthma pathogenesis and utility as a biomarker of disease severity. Front Med (Lausanne). 2022;9:954990. [CrossRef]
- Ouyang X, Reihill JA, Douglas LEJ, Martin SL. Airborne indoor allergen serine proteases and their contribution to sensitisation and activation of innate immunity in allergic airway disease. Eur Respir Rev. 2024;33(172). [CrossRef]
- Rivas CM, Yee MC, Addison KJ, Lovett M, Pal K, Ledford JG, et al. Proteinase-activated receptor-2 antagonist C391 inhibits Alternaria-induced airway epithelial signalling and asthma indicators in acute exposure mouse models. Br J Pharmacol. 2022;179(10):2208-22. [CrossRef]
- Ricciardolo FL, Steinhoff M, Amadesi S, Guerrini R, Tognetto M, Trevisani M, et al. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am J Respir Crit Care Med. 2000;161(5):1672-80. [CrossRef]
- Carr MJ, Schechter NM, Undem BJ. Trypsin-induced, neurokinin-mediated contraction of guinea pig bronchus. Am J Respir Crit Care Med. 2000;162(5):1662-7. [CrossRef]
- Schmidlin F, Amadesi S, Vidil R, Trevisani M, Martinet N, Caughey G, et al. Expression and function of proteinase-activated receptor 2 in human bronchial smooth muscle. Am J Respir Crit Care Med. 2001;164(7):1276-81. [CrossRef]
- Hauck RW, Schulz C, Schomig A, Hoffman RK, Panettieri RA, Jr. alpha-Thrombin stimulates contraction of human bronchial rings by activation of protease-activated receptors. Am J Physiol. 1999;277(1):L22-9. [CrossRef]
- Lin C, von der Thusen J, Daalhuisen J, ten Brink M, Crestani B, van der Poll T, et al. Protease-activated receptor (PAR)-2 is required for PAR-1 signalling in pulmonary fibrosis. J Cell Mol Med. 2015;19(6):1346-56.
- Howell DC, Laurent GJ, Chambers RC. Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis. Biochem Soc Trans. 2002;30(2):211-6. [CrossRef]
- Lin C, von der Thusen J, Daalhuisen J, ten Brink M, Crestani B, van der Poll T, et al. Pharmacological Targeting of Protease-Activated Receptor 2 Affords Protection from Bleomycin-Induced Pulmonary Fibrosis. Mol Med. 2015;21(1):576-83. [CrossRef]
- Miyake Y, D’Alessandro-Gabazza CN, Takagi T, Naito M, Hataji O, Nakahara H, et al. Dose-dependent differential effects of thrombin in allergic bronchial asthma. J Thromb Haemost. 2013;11(10):1903-15. [CrossRef]
- Jose RJ, Williams AE, Mercer PF, Sulikowski MG, Brown JS, Chambers RC. Regulation of neutrophilic inflammation by proteinase-activated receptor 1 during bacterial pulmonary infection. J Immunol. 2015;194(12):6024-34. [CrossRef]
- Jose R, Williams A, Sulikowski M, Brealey D, Brown J, Chambers R. Regulation of neutrophilic inflammation in lung injury induced by community-acquired pneumonia. Lancet. 2015;385 Suppl 1:S52. [CrossRef]
- van den Boogaard FE, Brands X, Duitman J, de Stoppelaar SF, Borensztajn KS, Roelofs J, et al. Protease-Activated Receptor 2 Facilitates Bacterial Dissemination in Pneumococcal Pneumonia. J Infect Dis. 2018;217(9):1462-71. [CrossRef]
- Rayees S, Joshi JC, Tauseef M, Anwar M, Baweja S, Rochford I, et al. PAR2-Mediated cAMP Generation Suppresses TRPV4-Dependent Ca(2+) Signaling in Alveolar Macrophages to Resolve TLR4-Induced Inflammation. Cell Rep. 2019;27(3):793-805 e4. [CrossRef]
- de Stoppelaar SF, Van’t Veer C, van den Boogaard FE, Nieuwland R, Hoogendijk AJ, de Boer OJ, et al. Protease activated receptor 4 limits bacterial growth and lung pathology during late stage Streptococcus pneumoniae induced pneumonia in mice. Thromb Haemost. 2013;110(3):582-92. [CrossRef]
- Sabri A, Muske G, Zhang H, Pak E, Darrow A, Andrade-Gordon P, et al. Signaling properties and functions of two distinct cardiomyocyte protease-activated receptors. Circ Res. 2000;86(10):1054-61. [CrossRef]
- Ide J, Aoki T, Ishivata S, Glusa E, Strukova SM. Proteinase-activated receptor agonists stimulate the increase in intracellular Ca2+ in cardiomyocytes and proliferation of cardiac fibroblasts from chick embryos. Bull Exp Biol Med. 2007;144(6):760-3. [CrossRef]
- Pawlinski R, Tencati M, Hampton CR, Shishido T, Bullard TA, Casey LM, et al. Protease-activated receptor-1 contributes to cardiac remodeling and hypertrophy. Circulation. 2007;116(20):2298-306. [CrossRef]
- Weithauser A, Witkowski M, Rauch U. The Role of Protease-Activated Receptors for the Development of Myocarditis: Possible Therapeutic Implications. Curr Pharm Des. 2016;22(4):472-84. [CrossRef]
- Antoniak S, Owens AP, 3rd, Baunacke M, Williams JC, Lee RD, Weithauser A, et al. PAR-1 contributes to the innate immune response during viral infection. J Clin Invest. 2013;123(3):1310-22.
- Tatsumi K, Schmedes CM, Houston ER, Butler E, Mackman N, Antoniak S. Protease-activated receptor 4 protects mice from Coxsackievirus B3 and H1N1 influenza A virus infection. Cell Immunol. 2019;344:103949. [CrossRef]
- Naldini A, Carney DH. Thrombin modulation of natural killer activity in human peripheral lymphocytes. Cell Immunol. 1996;172(1):35-42. [CrossRef]
- Mackman N, Antoniak S. Roles of PAR1 and PAR2 in viral myocarditis. Thromb Res. 2014;133 Suppl 1(0 1):S18-20. [CrossRef]
- Pan HY, Yano M, Kido H. Effects of inhibitors of Toll-like receptors, protease-activated receptor-2 signalings and trypsin on influenza A virus replication and upregulation of cellular factors in cardiomyocytes. J Med Invest. 2011;58(1-2):19-28. [CrossRef]
- Kouzoukas DE, Meyer-Siegler KL, Ma F, Westlund KN, Hunt DE, Vera PL. Macrophage Migration Inhibitory Factor Mediates PAR-Induced Bladder Pain. PLoS One. 2015;10(5):e0127628. [CrossRef]
- Ma F, Hunt DE, Leng L, Bucala R, Meyer-Siegler KL, Vera PL. Protease activated-receptor 4 activation as a model of persistent bladder pain: Essential role of macrophage migration inhibitory factor and high mobility group box 1. Int J Urol. 2018;25(10):887-93. [CrossRef]
- Saban R, D’Andrea MR, Andrade-Gordon P, Derian CK, Dozmorov I, Ihnat MA, et al. Mandatory role of proteinase-activated receptor 1 in experimental bladder inflammation. BMC Physiol. 2007;7:4. [CrossRef]
- Monjotin N, Gillespie J, Farrie M, Le Grand B, Junquero D, Vergnolle N. F16357, a novel protease-activated receptor 1 antagonist, improves urodynamic parameters in a rat model of interstitial cystitis. Br J Pharmacol. 2016;173(14):2224-36. [CrossRef]
- Wang ZY, Wang P, Bjorling DE. Role of mast cells and protease-activated receptor-2 in cyclooxygenase-2 expression in urothelial cells. Am J Physiol Regul Integr Comp Physiol. 2009;297(4):R1127-35. [CrossRef]
- Madhusudhan T, Kerlin BA, Isermann B. The emerging role of coagulation proteases in kidney disease. Nat Rev Nephrol. 2016;12(2):94-109. [CrossRef]
- Blanc-Brude OP, Archer F, Leoni P, Derian C, Bolsover S, Laurent GJ, et al. Factor Xa stimulates fibroblast procollagen production, proliferation, and calcium signaling via PAR1 activation. Exp Cell Res. 2005;304(1):16-27. [CrossRef]
- Horinouchi Y, Ikeda Y, Fukushima K, Imanishi M, Hamano H, Izawa-Ishizawa Y, et al. Renoprotective effects of a factor Xa inhibitor: fusion of basic research and a database analysis. Sci Rep. 2018;8(1):10858. [CrossRef]
- Han Y, Tian L, Ma F, Tesch G, Vesey DA, Gobe GC, et al. Pharmacological inhibition of protease-activated receptor-2 reduces crescent formation in rat nephrotoxic serum nephritis. Clin Exp Pharmacol Physiol. 2019;46(5):456-64. [CrossRef]
- Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111(1):35-41.
- Kelso EB, Ferrell WR, Lockhart JC, Elias-Jones I, Hembrough T, Dunning L, et al. Expression and proinflammatory role of proteinase-activated receptor 2 in rheumatoid synovium: ex vivo studies using a novel proteinase-activated receptor 2 antagonist. Arthritis Rheum. 2007;56(3):765-71. [CrossRef]
- Russell FA, Schuelert N, Veldhoen VE, Hollenberg MD, McDougall JJ. Activation of PAR(2) receptors sensitizes primary afferents and causes leukocyte rolling and adherence in the rat knee joint. Br J Pharmacol. 2012;167(8):1665-78. [CrossRef]
- Nakano S, Mishiro T, Takahara S, Yokoi H, Hamada D, Yukata K, et al. Distinct expression of mast cell tryptase and protease activated receptor-2 in synovia of rheumatoid arthritis and osteoarthritis. Clin Rheumatol. 2007;26(8):1284-92. [CrossRef]
- Palmer HS, Kelso EB, Lockhart JC, Sommerhoff CP, Plevin R, Goh FG, et al. Protease-activated receptor 2 mediates the proinflammatory effects of synovial mast cells. Arthritis Rheum. 2007;56(11):3532-40. [CrossRef]
- Crilly A, Burns E, Nickdel MB, Lockhart JC, Perry ME, Ferrell PW, et al. PAR(2) expression in peripheral blood monocytes of patients with rheumatoid arthritis. Ann Rheum Dis. 2012;71(6):1049-54. [CrossRef]
- Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, Hollenberg MD, et al. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther. 2006;316(3):1017-24. [CrossRef]
- Kanno Y, Ishisaki A, Kawashita E, Kuretake H, Ikeda K, Matsuo O. uPA Attenuated LPS-induced Inflammatory Osteoclastogenesis through the Plasmin/PAR-1/Ca(2+)/CaMKK/AMPK Axis. Int J Biol Sci. 2016;12(1):63-71.
- Huang CY, Chen SY, Tsai HC, Hsu HC, Tang CH. Thrombin induces epidermal growth factor receptor transactivation and CCL2 expression in human osteoblasts. Arthritis Rheum. 2012;64(10):3344-54. [CrossRef]
- Hirano F, Kobayashi A, Hirano Y, Nomura Y, Fukawa E, Makino I. Thrombin-induced expression of RANTES mRNA through protease activated receptor-1 in human synovial fibroblasts. Ann Rheum Dis. 2002;61(9):834-7. [CrossRef]
- Furuhashi I, Abe K, Sato T, Inoue H. Thrombin-stimulated proliferation of cultured human synovial fibroblasts through proteolytic activation of proteinase-activated receptor-1. J Pharmacol Sci. 2008;108(1):104-11. [CrossRef]
- Xue M, Chan YK, Shen K, Dervish S, March L, Sambrook PN, et al. Protease-activated receptor 2, rather than protease-activated receptor 1, contributes to the aggressive properties of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 2012;64(1):88-98. [CrossRef]
- Patnaik R, Riaz S, Sivani BM, Faisal S, Naidoo N, Rizzo M, et al. Evaluating the potential of Vitamin D and curcumin to alleviate inflammation and mitigate the progression of osteoarthritis through their effects on human chondrocytes: A proof-of-concept investigation. PLoS One. 2023;18(12):e0290739. [CrossRef]
- Hijazi Z, Aulin J, Andersson U, Alexander JH, Gersh B, Granger CB, et al. Biomarkers of inflammation and risk of cardiovascular events in anticoagulated patients with atrial fibrillation. Heart. 2016;102(7):508-17. [CrossRef]
- Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130(21):1847-58.
- Sandhu RK, Ezekowitz JA, Hijazi Z, Westerbergh J, Aulin J, Alexander JH, et al. Obesity paradox on outcome in atrial fibrillation maintained even considering the prognostic influence of biomarkers: insights from the ARISTOTLE trial. Open Heart. 2018;5(2):e000908. [CrossRef]
- Golderman V, Gofrit SG, Maggio N, Gera O, Gerasimov A, Laks D, et al. A Novel Highly Sensitive Method for Measuring Inflammatory Neural-Derived APC Activity in Glial Cell Lines, Mouse Brain and Human CSF. Int J Mol Sci. 2020;21(7). [CrossRef]
- Granja T, Hohenstein K, Schussel P, Fischer C, Prufer T, Schibilsky D, et al. Multi-Modal Characterization of the Coagulopathy Associated With Extracorporeal Membrane Oxygenation. Crit Care Med. 2020;48(5):e400-e8. [CrossRef]
- Torramade-Moix S, Palomo M, Vera M, Jerez D, Moreno-Castano AB, Zafar MU, et al. Apixaban Downregulates Endothelial Inflammatory and Prothrombotic Phenotype in an In Vitro Model of Endothelial Dysfunction in Uremia. Cardiovasc Drugs Ther. 2021;35(3):521-32. [CrossRef]
- Muthupalani S, Annamalai D, Feng Y, Ganesan SM, Ge Z, Whary MT, et al. IL-1beta transgenic mouse model of inflammation driven esophageal and oral squamous cell carcinoma. Sci Rep. 2023;13(1):12732.
- Patnaik R, Jannati S, Sivani BM, Rizzo M, Naidoo N, Banerjee Y. Efficient Generation of Chondrocytes From Bone Marrow-Derived Mesenchymal Stem Cells in a 3D Culture System: Protocol for a Practical Model for Assessing Anti-Inflammatory Therapies. JMIR Res Protoc. 2023;12:e42964. [CrossRef]
- Suarez-Martinez E, Suazo-Sanchez I, Celis-Romero M, Carnero A. 3D and organoid culture in research: physiology, hereditary genetic diseases and cancer. Cell Biosci. 2022;12(1):39. [CrossRef]
- Nzou G, Wicks RT, VanOstrand NR, Mekky GA, Seale SA, El-Taibany A, et al. Author Correction: Multicellular 3D Neurovascular Unit Model for Assessing Hypoxia and Neuroinflammation Induced Blood-Brain Barrier Dysfunction. Sci Rep. 2020;10(1):20384. [CrossRef]
- Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, et al. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J Immunol Res. 2018;2018:2180373. [CrossRef]
- Duan L, Rao X, Sigdel KR. Regulation of Inflammation in Autoimmune Disease. J Immunol Res. 2019;2019:7403796. [CrossRef]
- Zhu Q, Wu Y, Mai J, Guo G, Meng J, Fang X, et al. Comprehensive Metabolic Profiling of Inflammation Indicated Key Roles of Glycerophospholipid and Arginine Metabolism in Coronary Artery Disease. Front Immunol. 2022;13:829425. [CrossRef]
- Oe Y, Hayashi S, Fushima T, Sato E, Kisu K, Sato H, et al. Coagulation Factor Xa and Protease-Activated Receptor 2 as Novel Therapeutic Targets for Diabetic Nephropathy. Arterioscler Thromb Vasc Biol. 2016;36(8):1525-33. [CrossRef]
- Bieber M, Foerster KI, Haefeli WE, Pham M, Schuhmann MK, Kraft P. Treatment with Edoxaban Attenuates Acute Stroke Severity in Mice by Reducing Blood-Brain Barrier Damage and Inflammation. Int J Mol Sci. 2021;22(18). [CrossRef]
- Millenaar D, Bachmann P, Bohm M, Custodis F, Schirmer SH. Effects of edoxaban and warfarin on vascular remodeling: Atherosclerotic plaque progression and collateral artery growth. Vascul Pharmacol. 2020;127:106661. [CrossRef]
- Morishima Y, Shibutani T, Noguchi K, Ito Y, Honda Y. Edoxaban, a direct oral factor Xa inhibitor, ameliorates coagulation, microvascular thrombus formation, and acute liver injury in a lipopolysaccharide-induced coagulopathy model in rats. J Thromb Thrombolysis. 2021;52(1):9-17. [CrossRef]
- Baker JV, Wolfson J, Peterson T, Mooberry M, Gissel M, Mystakelis H, et al. Factor Xa Inhibition Reduces Coagulation Activity but Not Inflammation Among People With HIV: A Randomized Clinical Trial. Open Forum Infect Dis. 2020;7(2):ofaa026. [CrossRef]
- Goette A, Mollenhauer M, Rudolph V, Lamparter M, Meier M, Bohm M. Pleiotropic effects of NOACs with focus on edoxaban: scientific findings and potential clinical implications. Herzschrittmacherther Elektrophysiol. 2023;34(2):142-52.
- Narita Y, Hamamura K, Kashiyama M, Utsumi S, Kakizoe Y, Kondo Y, et al. Edoxaban Exerts Antioxidant Effects Through FXa Inhibition and Direct Radical-Scavenging Activity. Int J Mol Sci. 2019;20(17). [CrossRef]
- Romero-Guevara R, Ioannides A, Xinaris C. Kidney Organoids as Disease Models: Strengths, Weaknesses and Perspectives. Front Physiol. 2020;11:563981. [CrossRef]
- Fagundes A, Jr., Ruff CT, Morrow DA, Murphy SA, Palazzolo MG, Chen CZ, et al. Neutrophil-lymphocyte ratio and clinical outcomes in 19,697 patients with atrial fibrillation: Analyses from ENGAGE AF- TIMI 48 trial. Int J Cardiol. 2023;386:118-24. [CrossRef]
- Chan MY, Lin M, Lucas J, Moseley A, Thompson JW, Cyr D, et al. Plasma proteomics of patients with non-valvular atrial fibrillation on chronic anti-coagulation with warfarin or a direct factor Xa inhibitor. Thromb Haemost. 2012;108(6):1180-91. [CrossRef]
- Monux G, Zamorano-Leon JJ, Marques P, Sopena B, Garcia-Garcia JM, Laich de Koller G, et al. FXa inhibition by rivaroxaban modifies mechanisms associated with the pathogenesis of human abdominal aortic aneurysms. Br J Clin Pharmacol. 2017;83(12):2661-70. [CrossRef]
- Miyazawa K, Pastori D, Hammerstingl C, Cappato R, Meng IL, Kramer F, et al. Left atrial thrombus resolution in non-valvular atrial fibrillation or flutter: biomarker substudy results from a prospective study with rivaroxaban (X-TRA). Ann Med. 2018;50(6):511-8. [CrossRef]
- Martins GL, Duarte RCF, Vieira ELM, Rocha NP, Figueiredo EL, Silveira FR, et al. Comparison of Inflammatory Mediators in Patients With Atrial Fibrillation Using Warfarin or Rivaroxaban. Front Cardiovasc Med. 2020;7:114. [CrossRef]
- Mrozik D, Jackiewicz A, Krzeminski M. Dabigatran vs. low molecular weight heparin in preventing venous thromboembolism after elective hip and knee arthroplasty: evaluation of selected clinical parameters. Pol Orthop Traumatol. 2012;77:111-4.
- Paar V, Jirak P, Gruber S, Prodinger C, Cadamuro J, Wernly B, et al. Influence of dabigatran on pro-inflammatory cytokines, growth factors and chemokines - Slowing the vicious circle of coagulation and inflammation. Life Sci. 2020;262:118474. [CrossRef]
- Bogatkevich GS, Ludwicka-Bradley A, Nietert PJ, Akter T, van Ryn J, Silver RM. Antiinflammatory and antifibrotic effects of the oral direct thrombin inhibitor dabigatran etexilate in a murine model of interstitial lung disease. Arthritis Rheum. 2011;63(5):1416-25. [CrossRef]
- Kopec AK, Joshi N, Towery KL, Kassel KM, Sullivan BP, Flick MJ, et al. Thrombin inhibition with dabigatran protects against high-fat diet-induced fatty liver disease in mice. J Pharmacol Exp Ther. 2014;351(2):288-97. [CrossRef]
- Sparkenbaugh EM, Chantrathammachart P, Mickelson J, van Ryn J, Hebbel RP, Monroe DM, et al. Differential contribution of FXa and thrombin to vascular inflammation in a mouse model of sickle cell disease. Blood. 2014;123(11):1747-56. [CrossRef]
- Ellinghaus P, Perzborn E, Hauenschild P, Gerdes C, Heitmeier S, Visser M, et al. Expression of pro-inflammatory genes in human endothelial cells: Comparison of rivaroxaban and dabigatran. Thromb Res. 2016;142:44-51. [CrossRef]
- Nakase T, Moroi J, Ishikawa T. Anti-inflammatory and antiplatelet effects of non-vitamin K antagonist oral anticoagulants in acute phase of ischemic stroke patients. Clin Transl Med. 2018;7(1):2. [CrossRef]
- Durmaz S, Kurtoglu T, Rahman OF, Tataroglu C, Yilmaz M, Barbarus E, et al. Direct oral anticoagulant agents attenuate temporary aortic occlusion-induced renal oxidative and inflammatory responses in rats. Turk Gogus Kalp Damar Cerrahisi Derg. 2022;30(2):184-91. [CrossRef]
- Hiramoto K, Akita N, Nishioka J, Suzuki K. Edoxaban, a Factor Xa-Specific Direct Oral Anticoagulant, Significantly Suppresses Tumor Growth in Colorectal Cancer Colon26-Inoculated BALB/c Mice. TH Open. 2023;7(1):e1-e13. [CrossRef]
- Christopoulou EC, Filippatos TD, Elisaf MS. Non-hemorrhage-related adverse effects of rivaroxaban. Arch Med Sci Atheroscler Dis. 2017;2:e108-e12. [CrossRef]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583-9. [CrossRef]
- Lin Z, Akin H, Rao R, Hie B, Zhu Z, Lu W, et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science. 2023;379(6637):1123-30. [CrossRef]
- Dauparas J, Anishchenko I, Bennett N, Bai H, Ragotte RJ, Milles LF, et al. Robust deep learning-based protein sequence design using ProteinMPNN. Science. 2022;378(6615):49-56. [CrossRef]
- Johnson SR, Fu X, Viknander S, Goldin C, Monaco S, Zelezniak A, et al. Computational scoring and experimental evaluation of enzymes generated by neural networks. Nat Biotechnol. 2024.
- Martin L, Stricher F, Misse D, Sironi F, Pugniere M, Barthe P, et al. Rational design of a CD4 mimic that inhibits HIV-1 entry and exposes cryptic neutralization epitopes. Nat Biotechnol. 2003;21(1):71-6. [CrossRef]
- Petruzzella A, Bruand M, Santamaria-Martinez A, Katanayeva N, Reymond L, Wehrle S, et al. Antibody-peptide conjugates deliver covalent inhibitors blocking oncogenic cathepsins. Nat Chem Biol. 2024. [CrossRef]
- Nimjee SM, White RR, Becker RC, Sullenger BA. Aptamers as Therapeutics. Annu Rev Pharmacol Toxicol. 2017;57:61-79.
- Yu H, Kumar S, Frederiksen JW, Kolyadko VN, Pitoc G, Layzer J, et al. Aptameric hirudins as selective and reversible EXosite-ACTive site (EXACT) inhibitors. Nat Commun. 2024;15(1):3977. [CrossRef]

| ANTICOAGULANT | APIXABAN | EDOXABAN | RIVAROXABAN | DABIGATRAN |
| Mechanism | Direct FXa Inhibitor | Direct FXa Inhibitor | Direct FXa Inhibitor | Direct Thrombin Inhibitor |
| Prodrug/absorption | No/3-4h | No/Rapid | No/Rapid | Yes/Rapid |
| Bioavailability/half-life | 50%/12h | 62%/9-11h | 66% w/o food up to 100% with food/5-9h (young) 11-13h (elderly) |
6%/12-17h |
| Vd | 21L | 107L | 50L |
50-70L |
| Time to reach max. Plasma conc./protein binding | 1-4h/87% |
1-2h/55% | 2-4h/92-95% | 0.5-2h/35% |
| Liver metabolism | Yes | Minimal | Yes |
No |
| Renal excretion | 25% | 50% | 35% |
80% |
| Effect of diet | No effect on exposure | No effect on exposure | Peak levels reached at 3h on fasting and 4h with food. |
Delayed absorption |
| Effect of age | Exposure is 32% greater in patients above 65 years | Exposure is 32% greater in patients above 65 years | Bioavailability greater in elderly with no difference in concentration |
2 times higher Bioavailability in elders |
| Effect of body weight | Weight < 50 kg have 20-30% increased exposure & weight >120 kg has 20-30% reduced exposure | Weight < 50 kg have 20-30% increased exposure & weight >120 kg has 20-30% reduced exposure | Weight < 50 kg have 20-30% increased exposure & weight >120 kg has 20-30% reduced exposure |
None |
| Effect of renal impairment | Peak concentration unaffected. However, there is a rise in exposure by 16%, 29%, and 44% corresponding to creatinine clearances of 51–80, 30–50, and 15–29 ml/min, respectively. | Peak concentration unaffected. However, there is a rise in exposure by 16%, 29%, and 44% corresponding to creatinine clearances of 51–80, 30–50, and 15–29 ml/min, respectively. |
Rise in exposure with moderate or severe renal impairment | 6 times higher exposure with severe renal impairment, half-life extended to 28h |
| Effect of hepatic impairment | No change in exposure with Child-Pugh classification A/B | No change in exposure with Child-Pugh classification A/B |
Increased on exposure with Child-Pugh classification B | No change in exposure with Child-Pugh classification B |
| Doses | 2.5 mg, 5 mg | 15 mg, 30 mg, 60mg | 2.5 mg, 10 mg, 15 mg, 20 mg |
75 mg, 110 mg, 150 mg |
| Dosing/dosing form | Two times a day/Tablet | One time a day/Tablet |
One time a day/Tablet | Two time a day/Capsule |
| ADR | >10% haematologic and oncologic haemorrhage; <10% haematuria, epistaxis; <1% hyper-sensitivity reactions | >10% haematologic and oncologic haemorrhage; <10% skin rash, anaemia; <1% intra cranial haemorrhage, interstitial pulmonary disease |
>10% haematologic and oncologic haemorrhage; <10% pruritus, abdominal pain; <1% angioedema cholestasis | >10% gastro-intestinal symptoms; <10% gastritis, esophagitis; <1% allergic oedema, thrombocytopenia |
| Pre- & post-operative care for minor surgical procedures | Suspend treatment for 2 days before the surgical procedure (meaning, skip 1 dose), and recommence 24 hours after the surgery | Suspend treatment for 2 days before the surgical procedure (meaning, skip 1 dose), and recommence 24 hours after the surgery |
Suspend treatment for 2 days before the surgical procedure (meaning, skip 1 dose), and recommence 24 hours after the surgery |
Creatine clearance greater than 50 ml/min suspend treatment for 2 days before the surgical procedure (meaning, skip 1 dose), and recommence 24 hours after the surgery |
| Pre- & post-operative care for major surgical procedures | Suspend treatment for 3 days before the surgical procedure (meaning, skip 4 doses), and recommence 48 hours after the surgery |
Suspend treatment for 3 days before the surgical procedure (meaning, skip 4 doses), and recommence 48 hours after the surgery |
Suspend treatment for 3 days before the surgical procedure (meaning, skip 2 doses), and recommence 48 hours after the surgery |
Creatine clearance greater than 50 ml/min suspend treatment for 3 days before the surgical procedure (meaning, skip 4 doses), and recommence 48 hours after the surgery |
| Laboratory monitoring (optimal method) | Anti-FXa assay | Anti-FXa assay | Anti-FXa assay | Ecarin clotting time; Dilute thrombin time |
| Laboratory monitoring (emergency) | Dilute Prothrombin Time | Limited substantial data | Prothrombin Time (preferably with specific calibrated reagents) | Activated Partial Thromboplastin Time (preferably with specific calibrated reagents) |
| ARISTOTLE | ENGAGE AF-TIMI 48 |
ROCKET-AF |
RE-LY | |||||||
| Apixaban | Warfarin | Edoxaban 60 mg | Edoxaban 30 mg | Warfarin | Rivaroxaban | Warfarin | Dabigatran 150 mg | Dabigatran 110 mg | Warfarin | |
| Population | 9120 | 9081 | 7035 | 7034 | 7036 | 7131 | 7133 | 6076 | 6015 | 6022 |
|
Age (years) >75 years |
70 31% |
70 31% |
72 41% |
72 40% |
72 40% |
73 43% |
73 43% |
71.5 40% |
71.4 38% |
71.6 39% |
| Women | 36% | 35% | 39% | 39% | 38% | 40% | 40% | 37% | 38% | 39% |
| Persistent AF | 85% | 84% | 75% | 74% | 75% | 81% | 81% | 67% | 68% | 66% |
| Previous stroke/TIA | 19% | 18% | 28% | 29% | 28% | 55% | 55% | 20% | 20% | 20% |
| Previous VKA use | 57% | 57% | 59% | 59% | 59% | 62% | 63% | 50% | 50% | 49% |
| Previous Aspirin use | 31% | 31% | 29% | 29% | 30% | 36% | 37% | 39% | 40% | 41% |
| Median follow-up (years) | 1.8 | 1.8 | 2.8 | 2.8 | 2.8 | 1.9 | 1.9 | 2.0 | 2.0 | 2.0 |
| Receptor | Amino acids | Tethered ligands | Cleavage type | Classical proteases | Cleavage site | Activating synthetic ligands |
| PAR1 | 425 | h: SFFLR m:SFLLR |
Canonical | Thrombin | ||
| Factor Xa | 38 LDPR*SFLL 45 | SFLLRN-NH2 | ||||
| Plasmin | ||||||
| MMP1 | 36 ATLD*PRSF 43 | PRSFLLRN-NH2 | ||||
| MMP13 | 39DPRS*FLLR46 | |||||
| Non-canonical | Elastase | 42SFLL*RNPN49 | RNPNDKYEPF-NH2 | |||
| Proteinase-3 | 33 ATNA*TLDp40 | TLDPRSF-NH2 | ||||
| Activated Protein C | 43FLLR*NPND50 | NPNDKYEPF-NH2 | ||||
| PAR2 | 395 | h: SLIGKV m: SLIGRL |
Canonical | Trypsin | Isox-Cha-Chg-AR-NH2 | |
| Mast cell Tryptase | 33SKGR*SLIG40 | SLIGKV-NH2 | ||||
| Factor Xa | ||||||
| Thrombin | SLIGRL-NH2 | |||||
| Elastase | 64FSAS*VLTG71 | |||||
| Non-Canonical | Proteinase-3 | 57VFSV*DEFS64 | ||||
| Cathepsin G | 61VDEF*SASV68 | |||||
| Cathepsin S | 53VTVE*TVFS60 | TVFSVDEFSA-NH2 | ||||
| PAR3 | 483 | h: TFRGAP m: SFNGGP |
Canonical | Thrombin | 35LPIK*TFRG42 | TFRGAP-NH2 |
| Non-Canonical | Activated protein C | 38 KTFR*GAPp45 | SFNGGP-NH2 |
|||
| PAR4 | 385 | h: GYPGQV m: GYPGKF |
Canonical | Thrombin | ||
| Trypsin | 44 PAPRIGYPG51 | GYPGQV-NH2 | ||||
| Cathepsin G |
| Inflammatory condition | Anti-inflammatory properties | Pro-inflammatory properties |
| Neuroinflammation |
|
|
| Pruritus and pain |
|
|
| Inflammatory bowel disease (IBS) |
|
|
| Asthma |
|
|
| Pneumonia |
|
|
| Myocarditis |
|
|
| Cystitis |
|
|
| Nephritis |
|
|
| Arthritis |
|
|
| Model or Population | Mono/Combination therapy | Condition Investigated | Inflammatory Markers Examined | Effect observed | Reference |
|---|---|---|---|---|---|
| Inflammatory marker was measured at study entry, and at 2 months in 4830 patients in the ARISTOTLE trial with 1.8 years median follow-up. | Monotherapy | Atrial fibrillation | IL-6 | Repeated measurements of IL-6 suggest that persistent systemic inflammation is independently associated with increased mortality in apixaban treated AF patients, even after considering established clinical risk factors and other strong cardiovascular biomarkers. | Circulation. 2014 Nov 18;130(21):1847-58. |
| 18,201 patients with AF from the ARISTOTLE trial, out of which biomarkers were measured for 14,798 patients. | Monotherapy | Atrial fibrillation | GDF-15 | The effects of apixaban in reducing stroke, mortality, and bleeding were consistent irrespective of GDF-15 levels, but no data is shown if apixaban had any influence on the levels of GDF-15 to mitigate inflammation. | Heart. 2016 Apr;102(7):508-17.(***) |
| 18,201 patients with AF who were randomized to receive either apixaban or warfarin in the ARISTOTLE trial. | Monotherapy | Atrial fibrillation | hs-CRP and IL-6 | No Significant interactions were observed with respect to apixaban and IL-6 or CRP levels and their outcomes. | Heart. 2016 Apr;102(7):508-17. (***) |
| 14,753 patients from the Apixaban for Reduction In STroke and Other ThromboemboLic Events in Atrial Fibrillation (ARISTOTLE) trial. | Monotherapy | Atrial fibrillation | Hs-CRP and IL-6 | Obesity was found to be linked with better survival outcomes in apixaban treated patients. Given that obesity is often associated with chronic inflammation, it is possible that apixaban may have a modulatory role in dampening inflammation in obese, anticoagulated patients with AF. | Open Heart. 2018 Nov 1;5(2):e000908. |
| In vitro model: Glia cell lines (specifically microglial and astrocytic cell lines).In vivo animal model: Adult male mice, of similar genetic lineage as C57BL/6, developed by Institute of Cancer Research.Human model: Human plasma and cerebrospinal fluid (CSF), specifically, the CSF taken from viral meningoencephalitis patients and controls was analysed. | Combination therapy where apixaban individually and in combination with alpha-naphthylsulphonylglycyl-4-amidinophenylalanine piperidine (NAPAP) was used. | Measurement of activated protein C (APC) activity in the context of neural inflammation:
|
Conventional inflammatory markers were not assessed but APC titres were measured. APC modulates inflammation by:
|
Apixaban increases the specificity of APC activity, which in turn alludes to the possibility that APC’s anti-inflammatory effects are advantageously affected in the presence of apixaban. | Int J Mol Sci. 2020 Mar 31;21(7):2422. |
| Venovenous extracorporeal membrane oxygenation (n = 10) due to acute respiratory distress syndrome; and patients treated with venoarterial extracorporeal membrane oxygenation (n = 8) due to cardiocirculatory failure in the ICU of a university hospital. | Monotherapy | Coagulopathy associated with extracorporeal membrane oxygenation (ECMO) in patients with severe cardiocirculatory and/or respiratory failure. | Plasmatic coagulation and platelet aggregation. | Plasmatic coagulation and platelet aggregation were impaired before ECMO due to apixaban. | Crit Care Med. 2020 May;48(5):e400-e408. |
| Macrovascular endothelial cells: HUVEC (Human Umbilical Vein Endothelial Cells)Microvascular endothelial cells: HMEC (Human Microvascular Endothelial Cells) | Monotherapy | Protective role of apixaban in uremia caused by chronic kidney disease (CKD). | VCAM-1, ICAM-1, p38MAPK and p42/44 (also known as ERK1/2) | Apixaban reduced VCAM-1, ICAM-1, and VWF expression, normalized ROS levels and eNOS, and inhibited p38MAPK and p42/44 activation in endothelial cells exposed to uremic serum. | Cardiovasc Drugs Ther. 2021 Jun;35(3):521-532. |
| Model or Population | Mono/Combination Therapy | Condition Investigated | Inflammatory Markers Examined | Effect Observed | Reference |
|---|---|---|---|---|---|
| Male diabetic mice models, specifically eNOS+/+DM, eNOS+/-DM, and eNOS-/-DM, to investigate the effects of FXa inhibition. Effects of PAR2 absence on DN were assessed using F2rl1-/-; Ins2Akita/+; eNOS+/- mice and compared to F2rl1+/+; Ins2Akita/+; eNOS+/- mice. |
Monotherapy | Diabetic nephropathy | TGFbeta, PAI-1, Collagen Type I, Collagen Type IV, TNF-alpha, MCP-1, IL- 8 and Prostaglandin-endoperoxide Synthase 2 or COX-2 | Edoxaban ameliorated diabetic nephropathy by reducing the expression of key proinflammatory and profibrotic genes, as well as the expression of PAR1 and PAR2. | Arterioscler Thromb Vasc Biol. 2016;36(8):1525-1533. |
| Unilateral ureteral obstruction (UUO) mice (a renal tubulointerstitial fibrosis model) and data from the Food and Drug Administration Adverse Events Reporting System (FAERS) database | Monotherapy | Renal tubulointerstitial injury, which is associated with inflammation and is a major cause of chronic kidney disease (CKD). | PAR1, PAR2, Collagen type 1 and 3, Fibronectin, F4/80 (macrophage marker), TNF-alpha, IL-10, MCP1, IL1beta, TGFbeta, Alpha-smooth muscle actin. | Edoxaban suppressed the elevated levels of FX, PAR-1, and PAR-2, reduced fibrosis and extracellular matrix expression, and attenuated the upregulation of inflammatory molecules and macrophage infiltration in the UUO mouse model of renal tubulointerstitial injury. | Sci Rep. 2018;8(1):10858. |
| Immortalized proximal tubule epithelial cell line (HK-2) derived from normal adult human kidneys | Monotherapy | CKD | The study investigated markers of oxidative stress which is closely interrelated with inflammation. (Intracellular ROS, superoxide anion, peroxynitrite, radical scavenging activity for hydroxyl radicals and hydrogen peroxide. | Edoxaban through its FXa inhibitory and direct radical scavenging activity alleviates oxidative stress induced by FXa, thereby breaking the cycle between oxidative stress and inflammation. | Int J Mol Sci. 2019;20(17):4140. |
| Apolipoprotein E knockout (ApoE -/-) mice fed with cholesterol-rich diet categorized into three groups: a control (Co) group on the diet alone, a Warf group treated with warfarin and vitamin K1, and an Edo group treated with edoxaban. | Monotherapy and combination therapy approaches. Co group received cholesterol-rich diet only, Warf group received warfarin and vitamin K1, and the Edo group received edoxaban. |
Vascular remodelling, atherosclerosis and arteriogenesis. | Histological assessment of smooth muscle cells per collateral artery in both hind limbs; Frequency of perivascular macrophages in the ligated and non-ligated hind limb; Expression of inflammatory cytokines – IL6, MCP-1, IL1b and TNF-alpha. | Edoxaban did not exhibit significant effect on inflammation, specifically the mRNA expression of IL6, MCP-1, IL1b and TNF-alpha in the murine hindlimbs or the spleen remained unaltered. | Vascul Pharmacol. 2020;127:106661. (***) |
| Individuals aged ≥18 years, on continuous ART with maintained HIV RNA <200 copies/mL for 2+ years, having plasma D-dimer level ≥100 ng/mL, creatinine clearance ≥50 mL/min, and weight ≥60 kg, excluding those with recent VTE, contraindications to anticoagulant therapy, certain health conditions like prior stroke, and invasive cancer within the past year, among other criteria. | Monotherapy | Effects of edoxaban in relation to elevated risk of thrombotic events and pro-inflammatory state in the HIV-positive population. | Systemic inflammation – IL6 (high-sensitivity), IL1b and TNFr-1; Monocyte activation – sCD14 and sCD163; Vascular injury – sVCAM; Thrombin generation – D-dimer, TAT; Circulating microparticle procoagulant activity – MPTF, WBTF, functional phospholipid surface assay; immunophenotyping of cryopreserved PBMCs | Edoxaban did not significantly impact most inflammatory or immune activation markers, but it reduced specific coagulation markers like D-dimer and TAT and was associated with a decrease in effector memory T cells. | Open Forum Infect Dis. 2020;7(2):ofaa026. (***) |
| Male C57Bl/6 N mice aged 6-8 weeks subjected to a transient middle cerebral artery occlusion (tMCAO) procedure. Edoxaban was administered in two different doses across three dosing regimens, with a specific dose of 3.3 mg/kg used for tMCAO experiments. Additionally, the vitamin K antagonist (VKA) phenprocoumon was given at 0.3 mg/kg, three times prior to tMCAO, aiming to achieve INR between 2 and 3 during the tMCAO procedure. | Monotherapy where both drugs (edoxaban and phenprocoumon) were given via oral gavage using a gastric tube and were dissolved in 0.5% (w/v) methyl cellulose. | Effect of edoxaban on thrombin mediated inflammatory processes in ischemic stroke. | The inflammatory markers and pathways assessed included IL-1b, IL-6, TNF-alpha gene expression, invasion of immune cells (T cells, neutrophils, macrophages/microglia), and the stabilization of the blood-brain barrier (BBB) through tight junction protein expression and reduced Evans Blue extravasation. | Edoxaban reduced infarct volumes, improved neurological outcomes, enhanced blood-brain barrier function, and attenuated brain tissue inflammation, suggesting its potential protective effects in ischemic stroke. | Int J Mol Sci. 2021;22(18):9893 |
| Male Slc:Wistar rats were used in an LPS-induced microvascular thrombosis model. | Monotherapy | Effect of edoxaban on risk of thrombosis during infection, specifically focusing on lipopolysaccharide (LPS)-induced coagulopathy in rats, where the effect focused on microvascular thrombus formation in the context of this coagulopathy. | The study investigated microvascular thrombus formation in the liver and kidneys, focusing on inflammatory markers IL-6, TNF-alpha and MCP-1. | Edoxaban did not affect the levels of inflammatory cytokines, | J Thromb Thrombolysis. 2021;52(1):9-17. (***) |
| Patients with atrial fibrillation (AF) from the ENGAGE AF-TIMI 48 trial. This randomized trial compared edoxaban versus warfarin in these patients and followed them for a median of 2.8 years. | Monotherapy with either edoxaban or warfarin | Relationship between neutrophil-to-lymphocyte ratio (NLR) and clinical outcomes in AF patients. | NLR | Systemic inflammation (as reflected by NLR) is associated with adverse outcomes in AF patients, and that edoxaban offers protection against some of these outcomes regardless of the inflammatory state | Int J Cardiol. 2023;386:118-124. |
| Human Model or Population | Mono/Combination Therapy | Condition Investigated | Inflammatory Markers Examined | Effect Observed | Reference |
| Japanese subjects with non-valvular chronic AF undergoing anti-coagulation therapy, analysed using unbiased liquid chromatography/tandem mass spectroscopy and candidate multiplexed protein immunoassays. | Monotherapy either with rivaroxaban or warfarin. | Modulation of biologically-relevant plasma proteins in AF |
|
Compared with warfarin, rivaroxaban was associated with a greater increase in thrombomodulin and a trend towards a reduction in MMP-9 over 24 weeks. | Thromb Haemost. 2012;108(6):1180-1191. |
| Ex vivo samples of abdominal aortic aneurysms (AAA) with intraluminal thrombus from six patients. These samples were treated with and without rivaroxaban to assess its effects on inflammation and oxidative stress. Abdominal aortic samples from six organ donors were used as controls. | Monotherapy | AAA with intraluminal thrombus | IL-6, IL-10, MMP-9, nitric oxide synthase 2 and NADPH oxidase subunits (gp67-phox, gp91-phox, and gp47-phox) | Rivaroxaban reduced key inflammatory and oxidative stress markers in human AAA sites. | Br J Clin Pharmacol. 2017;83(12):2661-2670. |
| Ancillary analysis of the X-TRA study with AF patients having left atrial/left atrial appendage (LA/LAA) thrombus. | Monotherapy | Relationship between plasma biomarkers and left atrial thrombus resolution in AF patients using rivaroxaban. |
|
|
Ann Med. 2018 Sep;50(6):511-518 |
| 127 patients with non-valvular atrial fibrillation (NVAF) | Monotherapy (in comparison with warfarin) | AF and its association with inflammation | IL-2, IL-4, IL-10, TNF, IFN-γ, CCL5 (also known as RANTES), CXCL9 (also known as MIG), CCL2 (also known as MCP-1), CXCL10 (also known as IP-10), TGF-β1, ADAMTS13 GDF-15, sICAM-1, p-selectin lipocalin-2 (also known as NGAL), sVCAM-1, SAA |
|
Front Cardiovasc Med. 2020;7:114. |
| Male Wistar rats | Combination therapy of Sunitinib and Rivaroxaban. | Cardiotoxicity induced by Sunitinib. | Serum levels of Ca2+, Mg2+, Fe3+/Fe2+, lipid profiles, and cardiac enzymes. Additionally, measurements of oxidant/antioxidant balance gene and protein expressions in cardiac tissues. |
|
Cardiovasc Toxicol. 2020;20(3):281-290. |
| Patients with atrial fibrillation (AF) scheduled for cardioversion without adequate anticoagulation at baseline. | Rivaroxaban (monotherapy) vs. Vitamin-K antagonist (VKA) (monotherapy). | Effects of anticoagulation in patients with atrial fibrillation (AF) scheduled for cardioversion. |
|
|
TH Open. 2020 Jan 23;4(1):e20-e32. |
| Multi-centre, prospective, randomized, open-label trial involving 179 participants with type 2 diabetes and subclinical inflammation. | Monotherapy | Type 2 diabetes mellitus patients who had subclinical inflammation and were exhibiting stimulated coagulation, activated platelets, and endothelial dysfunction | hsCRP, IL-6, MCP-1, MMP-9 and sCD40L | Rivaroxaban displayed anti-inflammatory effect and improvement of endothelial function. | Diabetologia. 2021;64(12):2701-2712. |
| Observational, multicenter, prospective study involving newly diagnosed AF patients with CKD stages 3b - 4. | Monotherapy comparison of Rivaroxaban and Warfarin. | Effects on inflammation, progression of heart valve calcification, and kidney function in AF patients with CKD stages 3b - 4. | Plasma inflammatory mediators (measured via ELISA) and cytokine (IL-1b, IL-6, TGF-b, TNF-a) levels. |
|
Int J Cardiol. 2021 Dec 15;345:90-97. |
| Non-valvular atrial fibrillation (NVAF) patients treated with rivaroxaban or warfarin. | Monotherapy (rivaroxaban vs. warfarin). | Inflammation and endothelial activation in patients with AF. | Circulating pro-inflammatory extracellular vesicles (EVs) profiles, proteomics of enriched plasma EVs, and levels of soluble P-selectin. |
|
J Thromb Haemost. 2021;19(10):2583-2595. |
| Prospective, randomized study on 228 patients with venous thromboembolism (VTE) |
Combination therapy. Control group received conventional treatment (warfarin or rivaroxaban), whereas the rosuvastatin-intervention group received rosuvastatin 10 mg daily in addition to their conventional treatment. |
The impact of rosuvastatin on D-dimer and other inflammatory serum markers in VTE patients. | D-dimer, mean platelet volume (MPV), neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio. |
|
Clin Cardiol. 2022;45(7):717-722. |
| Real-world patients with coronary artery disease (CAD) and/or peripheral artery disease (PAD). | Dual pathway inhibition (DPI) using low-dose rivaroxaban and aspirin. | Effect of DPI on plasma inflammation and coagulation markers among patients with CAD and/or PAD. | IL-6, CRP, lipoprotein-associated phospholipase A2, copeptin, and GDF-15. | At the 24-week follow-up, there was a significant reduction in IL-6 and fibrinogen levels and a significant increase in GDF-15. | J Cardiovasc Pharmacol. 2023 Feb 1;81(2):129-133. |
| Cell, Mice or Combined models | Mono/Combination therapy | Condition Investigated | Inflammatory Markers Examined | Effect observed | Reference |
| KK-A(y) mouse model of type 2 diabetes mellitus. | Monotherapy (Rivaroxaban at doses of 5 or 10mg/kg) | Leukocyte-endothelial interaction and microthrombus formation in the context of type 2 diabetes mellitus. | Leukocyte-endothelial interaction and microthrombus formation in the context of type 2 diabetes mellitus. |
|
Thromb Res. 2014 Feb;133(2):276-80 |
| LPS-activated monocytes and THP-1 cells (a human monocytic cell line). | Monotherapy (either rivaroxaban or fondaparinux) | Tissue factor (TF) exposure on activated monocytes and macrophages involved in thrombosis through activation of factor X and cytokine release. | TF expression, prothrombinase activity, cytokine release in cell supernatants (with specific focus on IL-8 and TNFα). |
|
Exp Hematol Oncol. 2014 Dec 17;3(1):30. |
| Apolipoprotein E-deficient (ApoE-/-) mice | Monotherapy | Atherogenesis | PAR-1 and PAR-2 receptors, MMP-9, MMP-13, COX-2, TNF-a, and in vitro experiments that evaluated mRNA expression of IL-1b and TNF-a in mouse macrophages. |
|
Atherosclerosis. 2015;242(2):639-646. |
| Human umbilical vein endothelial cells (in vitro) An ischemic hind limb mouse model (in vivo) |
Monotherapy | The role of FXa in inducing cell senescence and its effect on tissue inflammation and regeneration. | Senescence-associated β-galactosidase, IGFBP-5, EGR-1, p53, and p16INK4a (via RT-qPCR array) and expression of cytokines ((IL-1b, IL-6, MCP-1 and ICAM-1) |
|
Sci Rep. 2016 Oct 18;6:35580. |
| Mouse model with polyurethane catheters placed unilaterally into the external jugular vein (EJV). | Monotherapy (either rivaroxaban or vehicle). | Dysfunction of indwelling central venous catheters (CVC) due to tissue ingrowth or clotting. | Plasma MCP-1 levels, EJV MMP-9 levels, cell proliferation (anti-Ki67), macrophage infiltration (anti-MAC387). |
|
Thromb Res. 2016 Aug;144:106-12 |
| Rat model of brain ischaemia/reperfusion injury using male Wistar rats. | Monotherapy (prestroke anticoagulation with rivaroxaban). | Influence of prestroke anticoagulation with rivaroxaban on stroke severity and associated effects on thrombo-inflammation. | Thrombin/antithrombin complex, intracerebral thrombus formation, CD68-immunoreactivity, expression of cytokines (IL-1b, TNF-alpha, INF-gamma), and adhesion molecules (specifically ICAM-1 and VCAM-1). |
|
Thromb Haemost. 2016 Apr;115(4):835-43. |
| Female SJL/J mice immunized with PLP139-151 to induce autoimmune experimental encephalomyelitis (EAE). | Monotherapy (either warfarin or rivaroxaban). | Effects of anticoagulants (warfarin and rivaroxaban) on autoimmune experimental encephalomyelitis (EAE) as a model for multiple sclerosis. | Neurological deficit scores, histopathological analyses of inflammatory lesions in the spinal cord. |
|
J Neuroinflammation. 2017 Jul 28;14(1):152. |
| Human umbilical vein endothelial cells that natively express protease-activated receptor-1 and -2 | Monotherapy | Function of rivaroxaban in the inactivated coagulation cascade and its role in altering gene transcripts, especially those of pro-inflammatory genes, upon FXa stimulation. |
|
|
J Pharmacol Sci. 2017;133(3):156-161. |
| In vitro study using human atherosclerotic plaques from carotid endarterectomy and vascular smooth muscle cells (VSMC) for experimentation. | Monotherapy | Progression and mechanisms of atherosclerotic plaques, specifically the role of coagulation factor Xa (FXa) in inducing endothelial cell senescence. | PARs, IGFBP-5, p53, and other inflammatory cytokines (IL-1b, IL-6, MCP-1, IGFBP-5). |
|
Sci Rep. 2017 Dec 7;7(1):17172 |
|
Monotherapy | Role of coagulation in acute lung injury (ALI) and the effect of rivaroxaban on it. |
|
|
Am J Transl Res. 2018;10(8):2335-2349. |
| Transluminal femoral artery injury in C57BL/6 mice induced by a straight wire. | Monotherapy | The role of pharmacological blockade of FXa in attenuating neointima formation following wire-mediated vascular injury. | IL-1β, IL-6, TNF-α, stromal cell-derived factor (SDF)-1, TGF-β1, granulocyte-macrophage colony stimulating factor (GM-CSF) |
|
Eur J Pharmacol. 2018;820:222-228. |
| Ten-week-old male CL57/B6 mice subjected to transverse aortic constriction (TAC) surgery | Monotherapy | Atrial fibrillation and inflammatory atrial fibrosis | mRNA levels of TNF-α, IL-6, IL-1β, MCP-1, cardiac PAR-2 expression |
|
J Cardiol. 2018;71(3):310-319. |
| Wild-type (WT) and PAR-2-/- mice subjected to left anterior descending artery (LAD) ligation. | Monotherapy | Cardiac injury and heart failure post-LAD ligation. | IL-6, IL-1β, and MPO(marker for neutrophil infliltration) |
|
Thromb Res. 2018;167:128-134. (***) |
| Male Albino rats | Monotherapy | Liver fibrosis induced by carbon tetrachloride | TNF-α, IL-1β and hydroxyproline | Rivaroxaban restored the inflammatory markers associated with liver fibrosis. | J Biochem Mol Toxicol. 2019;33(5):e22287. |
| Murine model of ischemic cardiomyopathy (ICM) using SR-BI KO/ApoeR61h/h mice (Hypo E mice). | Monotherapy | Effects of FXa inhibitors on atherosclerosis and cardiac remodelling post-MI in Hypo E mice. |
|
|
J Atheroscler Thromb. 2019;26(10):915-930. |
| MI (myocardial infarction) induced in wild-type mice through permanent ligation of the left anterior descending coronary artery. | Monotherapy (rivaroxaban added to regular chow diet). | Protective effects of rivaroxaban against cardiac remodeling after MI. | mRNA expression levels of TNF-α, TGF-β, PAR-1, IL-1 β, IL-6, MCP-1, MMP-2 MMP-9, PAR-2, A-type natriuretic peptides, B-type natriuretic peptides, and phosphorylation of extracellular signal-regulated kinase. |
|
Circ Rep. 2020 Mar 4;2(3):158-166. |
| Wistar rats weighing 200-250 g. | Combination therapy (Rivaroxaban with Sunitinib). | Nephrotoxicity induced by Sunitinib. | TNF-α/NFk-B signaling pathways, Malondialdehyde, Catalase, Glutathione, Glutathione reductase, Caspase-3, IL-17, MCP-1, IKBα. | Rivaroxaban treatment restored altered levels of inflammatory markers and exhibited nephroprotective effects against Sunitinib-induced nephrotoxicity by inhibiting oxidative stress-induced apoptosis and inflammation. | J Thromb Thrombolysis. 2020 Aug;50(2):361-370. |
| Male rats where inflammation was induced post-rivaroxaban therapy using LPS. | Monotherapy | LPS-induced acute vascular inflammatory response. |
|
|
PLoS One. 2020;15(12):e0240669. |
| In vitro study using the tissue factor-expressing prostate carcinoma cell line, 22Rv1. Whole blood was also stimulated with lipopolysaccharide (LPS) or phorbol-myristate-acetate (PMA). |
Monotherapy comparisons of rivaroxaban, dalteparin, and tinzaparin. | Cancer-associated thrombosis (CAT) and the influence of myeloperoxidase (MPO) on anticoagulant activity. | Tumor cell-induced procoagulant activity, platelet aggregation, and the impact of the cationic leukocyte-derived enzyme, MPO. Thrombin generation in plasma supernatants from LPS- or PMA-stimulated whole blood was also measured. |
|
J Thromb Haemost. 2020 Dec;18(12):3267-3279. |
| Ang II-infused ApoE-/- mice and calcium chloride-induced AAA models, as well as human aortic endothelial cells. | Monotherapy | AAA | IL-6, IL-8, IL-1β, MCP-1, MMP-2 as well as adhesive molecules were examined in relation to FXa stimulation and rivaroxaban treatment. |
|
Vascul Pharmacol. 2021;136:106818. |
| Young adult male Wistar Albino type rats with surgically induced Achilles tendon injury followed by primary repair. | Monotherapy (rivaroxaban vs. nadroparin calcium vs. no medication) | Effects of antithrombotic-adjusted prophylactic doses on Achilles tendon healing | Inflammatory cells, capillary vessels, fibroblasts, degrees of inflammation, neovascularization, fibroblastic activity, and collagen fibre sequencing for histopathological evaluation. |
|
J Hand Microsurg. 2021;15(2):133-140. |
| The investigation consisted of two studies: Study 1: PAR2 deficient (PAR2-/-) and wild-type mice infused with angiotensin II (Ang II) or a vehicle. Study 2: Spontaneously hypertensive rats (SHRs) treated after 8h of right atrial rapid pacing. |
Monotherapy (either rivaroxaban, warfarin, or vehicle). | Role of PAR2 signalling in AF arrhythmogenesis and the potential ameliorating effect of rivaroxaban on atrial inflammation and AF prevention. | mRNA expression of collagen1 and collagen3, gene expression of inflammatory (TNF-α, MCP-1, TGF-β, Col1a1, Col31, F2R and F2l1) and fibrosis-related biomarkers in the atrium. |
|
Circ J. 2021;85(8):1383-1391. |
| Pilot, single-centre, randomized, double-blind, placebo-controlled, crossover study with subjects having sickle cell anaemia. | Monotherapy (either rivaroxaban or placebo). | Sickle cell disease (SCD) and its association with coagulation activation. | Thrombin-antithrombin complex, D-dimer, inflammatory (hs-CRP, IL-6, IL-2 and IL-8) and endothelial activation markers, measures of microvascular blood flow. |
|
Transfusion. 2021 Jun;61(6):1694-1698. (***) |
| Male Ldlr-/- mice fed a western-type diet to induce atherosclerosis. | Combination of aspirin (given in water) and rivaroxaban (given in the diet) compared to each agent alone. | Atherosclerosis in Ldlr-/- mice. | Expression of 55 proteins in the aorta and plasma (specific proteins not listed in provided information). |
|
Atherosclerosis. 2022 Mar;345:7-14 |
| LPS stimulation of PBMCs or citrate-anticoagulated whole blood. | Monotherapy with PACMA-31 or DMSO vehicle. | Regulation of TF in monocytes by Protein disulphide isomerase (PDI) and the effect of PACMA-31, a specific PDI inhibitor. | TF expression (antigen, procoagulant activity, mRNA), release of IL-6 and TNFα, and LPS-induced signaling pathways. |
|
Thromb Res. 2022;220:48-59. (***) |
| In vivo: Various strains of mice (wild-type and NLRP3 knockout) fed with standard chow or a high-fat diet. In vitro: examination with mice aortic endothelial cells (MAECs) and smooth muscle cells (MOVASs). |
Monotherapy | Therapeutic role of rivaroxaban in attenuating vascular lesions and dysfunction in type 2 diabetes mellitus mice | NLRP3 inflammasome activation, vascular tension, intima-media thickness, collagen deposition, PAR-1, PAR-2, mitogen-activated protein kinase (MAPK), NF-κB. |
|
J Cell Physiol. 2022 Aug;237(8):3369-3380. |
| ICH induced by collagenase injection into the striatum of wild type (C57BL/6J) anticoagulated mice (warfarin or rivaroxaban) and Mmp10 -/- mice. | Monotherapy using either prothrombin complex concentrate (PCC) or CM-352 (MMP-fibrinolysis inhibitor). | Intracranial hemorrhage (ICH) associated with oral anticoagulants (specifically rivaroxaban). | PAI-1, IL-6, neutrophil infiltration, thrombin-activatable fibrinolysis inhibitor (TAFI) activation. |
|
Thromb Haemost. 2022 Aug;122(8):1314-1325 |
| Human Model or Population | Mono/Combination therapy | Condition Investigated | Inflammatory Markers Examined | Effect observed | Reference |
| Patients who had elective orthopaedic surgical procedures, specifically total hip or knee arthroplasty. | Monotherapy (Dabigatran vs. low-molecular weight heparin) | Thromboembolic complications post-total hip or knee arthroplasty |
|
No significant difference in the incidence of local or systemic inflammatory complications between dabigatran and low-molecular weight heparin | Pol Orthop Traumatol. 2012 (***) |
|
Monotherapy | Impact of dabigatran on pro-inflammatory cytokines, growth factors, and chemokines; exploring its potential anti-inflammatory effects. | Angiogenin, CXCL1/GRO alpha, IL-1 beta/IL-1F2, human IL-6, IL-8/CXCL8, CCL2/MCP-1, TGF- beta 1, TNF-alpha, VEGF. |
In summary, the study suggests that dabigatran could have anti-inflammatory effects by reducing the expression of pro-inflammatory cytokines, growth factors, and chemokines
|
Life Sci. 2020 Dec 1;262:118474. |
| Cell, Mice or Combined models | Mono/Combination therapy | Condition Investigated | Inflammatory Markers Examined | Effect observed | Reference |
| Female C57BL/6 mice | Mono therapy |
|
|
|
Arthritis Rheum. 2011 May;63(5):1416-25. |
| Transgenic atherosclerosis-prone mice with diminished coagulant or hypercoagulable phenotype (FII(-/WT):ApoE(-/-) and TM(Pro/Pro):ApoE(-/-)) | Monotherapy (Dabigatran etexilate or recombinant activated protein C (APC)) |
|
|
|
PLoS One. 2013;8(2):e55784. |
|
Monotherapy (Dabigatran) |
|
|
|
Front Aging Neurosci. 2013 May 9;5:19. |
|
Monotherapy |
|
|
|
J Pharmacol Exp Ther. 2014 Nov;351(2):288-97. |
|
Monotherapy with the thrombin inhibitor dabigatran at a dosage of 1.2 g/kg/day. |
|
|
Conclusion: The study concludes that dabigatran, a thrombin inhibitor, effectively reduces vascular oxidative stress and inflammation.
|
Arch Med Sci. 2014 Feb 24;10(1):154-60. |
|
Monotherapy |
|
The mean lesion area in the aortic sinus and the innominate artery.
|
The study concluded that inhibition of thrombin with dabigatran etexilate retards the initiation and progression of atherosclerotic lesions in ApoE-/- mice. The treatment also improved endothelial function and reduced the accumulation of macrophages within the vascular wall.
|
Drug Des Devel Ther. 2015 Sep 10;9:5203-11. |
|
Monotherapy using the oral thrombin inhibitor dabigatran (10 mg/g) |
|
|
The study found that while dabigatran was successful in achieving systemic thrombin inhibitory activity similar to human trials, it did not significantly mitigate allergic lung inflammation triggered by HDM in a murine asthma model. Given the limited effects of dabigatran on the evaluated markers of inflammation and asthma pathology, the study suggests that dabigatran might not be a suitable candidate for clinical evaluation in asthma treatment.
|
Am J Physiol Lung Cell Mol Physiol. 2015 Oct 15;309(8):L768-75. (***) |
|
Monotherapy (Dabigatran etexilate (DE) or Warfarin) |
|
|
|
J Neuroimmunol. 2016 Aug 15;297:159-68. |
|
Monotherapy with dabigatran etexilate (DE) at a dosage of 15 mg/kg. |
|
|
Conclusion: The study concluded that prophylactic anticoagulation with dabigatran etexilate improves outcomes following ischemic stroke in rats by reducing thrombin-induced inflammation and thrombus formation.
|
Curr Neurovasc Res. 2016;13(3):199-206. |
| Vascular leak-dependent mice model for pulmonary fibrosis | Monotherapy |
|
|
In summary, dabigatran exhibited significant anti-inflammatory effects in a vascular leak-dependent mouse model for fibrotic lung disease.
|
JCI Insight. 2017 May 4;2(9):e86608. |
|
Monotherapy |
|
|
In summary, dabigatran treatment showed anti-inflammatory effects in a high-fat diet-induced obesity model in mice. It limited weight gain, reduced systemic inflammation, and decreased macrophage counts in adipose tissue, thereby potentially suppressing the progression of obesity-associated diseases.
|
J Clin Invest. 2017 Aug 1;127(8):3152-3166. |
|
Mono therapy (dabigatran administered either during the onset of fibrotic phase (late treatment) or inflammatory phase (early treatment)). |
|
|
|
Clin Exp Rheumatol. 2017 Sep-Oct;35 Suppl 106(4):35-39. (***) |
|
Monotherapy (Dabigatran) |
|
|
|
Acta Ophthalmol. 2018 Aug;96(5):452-458. |
|
MonotherapyGroup 1: Control (No treatment)Group 2: Dabigatran etexilate (10 mg/kg orally for 7 days)Group 3: Bemiparin sodium (250 IU/kg subcutaneously for 7 days) |
|
|
|
World Neurosurg. 2019 Jun;126:e731-e735. (***) |
|
Monotherapy |
|
|
|
Atherosclerosis. 2019 Aug;287:81-88. |
|
Combination therapy (Dabigatran with gentamicin) |
|
|
|
PLoS One. 2019 Apr 19;14(4):e0215333. |
|
Monotherapy (Dabigatran) |
|
|
|
J Thromb Haemost. 2019 Mar;17(3):538-550. |
|
Monotherapy (Dabigatran) |
|
|
|
Biochem Biophys Rep. 2020 Nov 19;24:100862. |
|
Monotherapy (Dabigatran) |
|
|
|
Front Mol Neurosci. 2020 Jun 30;13:114. |
|
- Monotherapy (Dabigatran etexilate) |
|
|
|
Vascul Pharmacol. 2020 |
|
Monotherapy |
|
|
|
Eur J Pharmacol. 2021 Feb 15;893:173838. |
|
Monotherapy |
|
|
|
Liver Transpl. 2021 Feb;27(3):363-384. |
|
Mono therapy (warfarin, dabigatran, and heparin are each used independently). |
|
|
|
Theranostics. 2021 Feb 20;11(9):4251-4261 |
|
Monotherapy Control (Western-type diet only) Dabigatran etexilate (duration: 6 or 18 weeks) Warfarin (duration: 6 or 18 weeks) |
|
|
|
J Thromb Haemost. 2021 May;19(5):1348-1363. |
|
Monotherapy (Dabigatran etexilate or Warfarin) |
|
|
|
J Thromb Haemost. 2021 May;19(5):1348-1363. |
| Retinoic acid (RA)-differentiated human neuroblastoma cell line SH-SY5Y | Monotherapy (250 nM Dabigatran with/without 10-100 nM Thrombin) |
|
|
|
Cereb Circ Cogn Behav. 2021 May 6;2:100014 |
|
Monotherapy |
|
|
In summary, elevated thrombin activity appears to contribute to tissue damage and dysfunction in Crohn’s disease. The study highlights the potential therapeutic benefits of inhibiting thrombin and protease-activated receptor-1 as a strategy for treating inflammatory bowel disease.
|
J Crohns Colitis. 2021 May 4;15(5):787-799. |
| Pig coronary artery stenting model | Combination therapy (dabigatran, aspirin, and clopidogrel) |
|
|
In summary, the short-term peri-interventional triple therapy with dabigatran, aspirin, and clopidogrel enhanced endothelium-dependent vasodilation and reduced thrombin generation and tissue burden, showing anti-inflammatory effects in a porcine model of coronary artery stenting.
|
Front Cardiovasc Med. 2021 Jul 2;8:690476. |
|
Monotherapy |
|
|
|
Front Pharmacol. 2022 Feb 28;13:834472(***) |
| Rat model of complete Freund’s adjuvant (CFA)-induced arthritis. | Monotherapy |
|
|
|
Int J Mol Sci. 2022 Sep 7;23(18):10297. |
| Mouse model (T7K24R trypsinogen mutant mouse and T7D23A mouse) | Monotherapy |
|
|
|
JCI Insight. 2022 Nov 8;7(21):e161145. |
| Pig stenting coronary artery model | Combination therapy: Dabigatran with dual antiplatelet therapy (DAPT) (clopidogrel 75 mg + aspirin 100 mg) |
|
|
|
J Pers Med. 2023 Jan 31;13(2):280 |
| Model or Population | NOACs used | Condition Investigated | Inflammatory Markers Examined | Effect observed | Reference |
|---|---|---|---|---|---|
| The study utilized the Berkeley (BERK) mouse model of Sickle Cell Disease (SCD) which has specific genetic modifications involving human and murine globins. For certain experiments, wild-type (WT) mice and PAR-1 and PAR-2 deficient mice were used. Additionally, bone marrow (BM) transplantation was conducted where irradiated PAR-1 and PAR-2 mice received bone marrow cells from either the BERK or WT control mice. |
Dabigatran and Rivaroxaban | Sickle Cell Disease (SCD) | IL-6, sVCAM-1 was assessed in relation to the activation of TF in sickle mice, MPO which is an enzyme primarily found in neutrophils and is released during the activation of neutrophils. |
|
Blood. 2014;123(11):1747-1756. |
| Human umbilical vascular endothelial cells (HUVECs) | Dabigatran and Rivaroxaban | Transcriptional changes in HUVECs when exposed to thrombin, focusing on the expression levels of preselected pro-inflammatory genes. | ELAM-1, VCAM-1, ICAM-1, MCP-1, IL-8, CXCL1, CXCL2 and TF |
|
Thromb Res. 2016 Jun;142:44-51 |
| 44 patients with acute ischemic stroke patients who were newly prescribed anti-thrombotic agents. | Dabigatran (n =12) and Apixaban (n =14) with antiplatelet agents (n-18) used as control. | Acute ischemic stroke | Hs-CRP, IL-6, pentraxin – 3 |
|
Clin Transl Med. 2018 Jan 12;7(1):2. |
| Polymorphonuclear leukocytes (PMNL) isolated from heparinized venous blood taken from non-smoking, healthy adults. | Dabigatran and Rivaroxaban | Inflammatory response by NOACs | reactive oxygen species (rOS), elastase release, cytosolic calcium flux, neutrophil extracellular trap (NET) formation, cell viability | No significant pro-inflammatory effects of dabigatran or rivaroxaban at concentrations of up to 10 µM was observed on the indicated PMNL markers. | Activation. Pharmaceuticals (Basel). 2018 May 14;11(2):46. |
| 187 patients with non-valvular atrial fibrillation (NVAF) | Dabigatran (n = 96) and Rivaroxaban (n= 91) | NVAF | Hs-CRP, pentraxin-3, IL-1β, IL-6, IL-18, TNF-α, MCP-1,GDF-15, and soluble thrombomodulin. |
|
Heart Vessels. 2019 Jun;34(6):1002-1013 |
| Male Wistar rats weighing between 250 to 350 g | Apixaban, Dabigatran and Rivaroxaban | Ischemia-reperfusion injury in the kidneys | IL-1β and TNF-α. |
|
Turk Gogus Kalp Damar Cerrahisi Derg. 2022 Apr 27;30(2):184-191. |
| Human umbilical vascular endothelial cells (HUVECs) | Dabigatran and Rivaroxaban | Inflammatory activation in endothelial cells caused by 25-hydroxycholesterol (25-OHC), which is associated with atherosclerosis. | Anti-inflammatory cytokines: TGF-β IL-37 IL-35 (specifically its subunits EBI3 and p35) Pro-inflammatory cytokines: IL-18 IL-23 |
25-OHC Effects:
|
Clin Exp Pharmacol Physiol. 2022 Aug;49(8):805-812. |
|
Animal model: 60 C57B/6J mice divided into groups: control (CON) group, atrial fibrillation (AF) group, AF + edoxaban group, and AF + rivaroxaban group. Human Model: Erythrocytes of patients with atrial fibrillation |
Edoxaban and Rivaroxaban | Atrial fibrillation | TNF-α, IL-1β, IL-6, and IL-10. | Both edoxaban and rivaroxaban reduced inflammation in atrial fibrillation, where the effect of edoxaban was superior to that of rivaroxaban. | Front Pharmacol. 2022;13:904317. |
| A syngeneic mouse model comprising male BALB/c mice that were inoculated with colon cancer Colon26 cells. | Dabigatran, Edoxaban and Rivaroxaban | Effect of NOACs on tumour progression in colorectal cancer. | Appraisal of tissue-factor, plasminogen activator inhibitor – 1, IL-6 and MMP-2 in the plasma of Colon26-inoculated mice. | All NOACs displayed anti-inflammatory effect, edoxaban displayed a superior ability not just to suppress tumor growth but also to mitigate elevated levels of inflammatory markers. | TH Open. 2023;7(1):e1-e13. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





