1. Introduction
Dental bleaching is one of the most common procedures performed in aesthetic dentistry to reduce patients’ frustration with dental color color [
1,
2]. Among the dental bleaching techniques, the in-office approach that uses high-concentration hydrogen peroxide gels [
3,
4] is highly requested by patients since it provides faster results [
3,
5].
However, the main disadvantage of using more concentrated hydrogen peroxide gels is the increased rate of beaching-induced tooth sensitivity (TS) [
6,
7,
8,
9]. TS is generally related to the ability of hydrogen peroxide to penetrate through enamel and dentin after gel application [
10,
11] and to promote oxidative stress in pulp cells with the release of inflammatory mediators [
10,
12,
13,
14] as well as the intrinsic sensitivity to pain from each patient [
8]. Therefore, the higher the hydrogen peroxide concentration in dental pulp, the higher TS is observed [
3,
15], because the concentration of bleaching gel applied may influence the amount of hydrogen peroxide that reaches the pulp chamber [
15,
16]. For this reason, the addition of desensitizing or remineralizing agents in the in-office bleaching gels could be an effective alternative to reduce TS [
11,
17,
18,
19] by reducing the hydrogen peroxide diffusion in the pulp chamber.
Hydroxyapatite (HAp) is among the most investigated biomaterials today [
20,
21]. In Dentistry, this material is widely used due to its ability to obliterate dentin tubules [
22,
23,
24], and due to the remineralizing potential in enamel [
25,
26] which may be related to TS decrease [
27,
28,
29]. In addition, several nano-hydroxyapatite composites have been developed for various Dentistry uses [
20,
21,
30]. Composites and nanocomposites have promising mechanical and thermal properties [
31]. Therefore, the use of a nanocomposite containing hydroxyapatite whose benefits are already known, may be an effective alternative for TS control.
Regarding TS, it is known that hydrogen peroxide, in addition to causing pulp injuries, can lead to the direct activation of TRPA-1 receptors present in the C-fibers of the dental pulp, responsible for the detection of noxious stimulus [
32,
33]. Among the agents capable of inhibiting TRPA-1 receptors are capsaicinoides, such as capsaicin (CAP), which is a highly selective agonist of TRPA-1 receptors and can reduce the amount of neuropeptides released by nerve terminals [
34]. It also has a nociceptive effect in cases of pulp pain [
35,
36].
However, despite the results already known for the use of hydroxyapatite in TS, the CAP use for TS and pulpal pain control is poorly explored. As previously mentioned, HAp has been used for the formation of highly effective nanocomposites in the dental environment. For this reason, the synthesis of a nanocomposite capable of reducing hydrogen peroxide diffusion, concomitantly with the inhibition of receptors present in the dental pulp, could bring clinical benefit for the reduction of TS during dental bleaching procedures. When incorporated into the bleaching gel, this nanocomposite will remain in contact with the tooth structure for a longer time, enabling the simultaneous delivery of hydrogen peroxide and the desensitizing/remineralizing agents.
Therefore, the aim of this study was to obtain and characterize a HAp-CAP nanocomposite for physiochemical analysis. This nanocomposite is intended for use in an in-office dental bleaching gel. Additionally, we evaluated the effects of adding HAp-CAP to in-office dental bleaching, assessing its impact on reducing hydrogen peroxide diffusion into the pulp chamber and examining its influence on color alteration during the dental bleaching procedure.
2. Materials and Methods
2.1. Study Design
This study started with the obtaining of hydroxyapatite nanoparticles (Nano-HAp) for the subsequent synthesis of the Hydroxyapatite-Capsaicin Composite (HAp-CAP). The synthesis and characterization of Nano-HAp and HAp-CAP were carried out in the Industrial Pharmacy Laboratories at the State University of Ponta Grossa (UEPG). The pure CAP, Nano-Hap and HAp-CAP nanocomposite were characterized physiochemically to verify the properties before the synthesis of the hydroxyapatite-capsaicin nanocomposite. The in vitro permeation was performed using a suitably prepared human tooth.
2.2. Materials
CAP (C18H27NO3, 95% purity, 305.4 molecular weight) was purchased from Botanic Healthcare (Hyderabad, TG, India) and HAp previously synthesized with distilled water, ammonium hydroxide (NH4OH), phosphoric acid (H3PO4) and calcium nitrate PA (Ca(NO3)2.4H2O) were used as received. The analytical grade of other chemicals was used without any further purification. Water was purified in a Milli-Q Plus water purification system (Millipore, Bedford, MA, USA).
2.3. Synthesis of Hydroxyapatite Nanoparticles
HAps were synthesized through synthesis methods of precipitation. This method was carried out for obtaining HAp nanoparticles (Nano-HAp). This reaction was performed with the addition of 1.2 mol/L H
3PO
4 (P.A., 85% pure) in a suspension of 2.0 mol/L Ca(OH)
2 (P.A., 95% pure) with a Ca/P molar ratio of 1.67, in aqueous medium at 40 °C on magnetic stirring. The dropping speed of the H
3PO
4 solution was controlled at about 1 drop/s. After adding the acid solution, pH was adjusted to 10.0 using NH4OH (P.A., 28% pure). The precipitate was aged for 24 h, vacuum filtered, and the resulting precipitate was dried in an oven for 24 h at 100 °C. After drying, the HAp powders were ground in mortar and sieved through 200 mesh ASTM (75 μm) [
37].
2.4. Synthesis of HAp-CAP Nanocomposite
The HAp-CAP nanocomposite was prepared by the method of precipitation [
37]. The composite synthesis was performed in a 4:1 ratio using 3.2 g of the previously synthesized nano-HAp and 0.8 g of CAP in 99.5% ethyl alcohol (q.s.p) and zirconia beads at SpeedMixer
® (FlackTek SpeedMixer
® DAC 150.1 FV-K, Landrum, South Caroline) under agitation of 3000 rpm for 3 h to provide the grinding process. After this period, the zirconia beads were removed, and HAp-CAP was taken to the dry study at 35 °C for 48 h.
2.5. Physicochemical Characterization
2.5.1. Determination of the Particle Size, Polydispersity Index (PDI) and Zeta Potential
The particle size, PDI and zeta potential of the CAP, nano-HAp and HAp-CAP were determined by dynamic light scattering (Zs90 Nanoseries; Malvern, Malvern Instruments, UK). Before measurement, the samples were diluted using deionized water (1:1000). The refractive index of the dispersant was set to 1.33. Each sample was analyzed in triplicate, and the average value was used for analysis.
2.5.2. Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive X-ray Spectroscopy (EDS) Analysis
The pure CAP, nano-HAp, and HAp-CAP nanocomposite were diluted with deionized water and then dropped onto a plasma membrane (glow-discharge) carbon membrane grid and allowed to air dry. Then, the sample surfaces were metalized with 10 nm of a gold metallic alloy and analyzed in scanning electron microscope (MIRA3 LM, Tescan, Warrendale, PA, EUA) at an acceleration voltage of 10 kV. Images were acquired at a nominal magnification of 10kv. A qualitative distribution of the samples in the matrix was obtained by Energy dispersive X-ray spectroscopy (EDS) with a silicon drift detector - SDD (Oxford Instruments, High Wycombe, UK) at the SEM operating voltage of 10 kV. SEM and EDS examinations were performed in all groups by and a technician who was blinded to the experimental conditions under evaluation.
2.5.3. X-ray Powder Diffraction (XRPD)
The XRPD studies of the samples were carried out on an X-ray diffractometer (Ultima IV, Rigaku, The Wodland, TX, USA), using Bragg-Brentano geometry with Cu Kα radiation (I = 1.5418 Å) and a Peltier-cooled solid-state detector. All powder diffraction patterns were measured in continuous mode, using an 2θ angular range 50-80°, tube power 40 kV and 30 mA, step size 0.02° using a scan rate of 0.1° 2θ/min. All analyses were performed in triplicate.
2.5.4. Fourier-Transform Infrared Spectroscopy (FTIR)
The pure CAP, nano-Hap and HAp-CAP nanocomposite was assessed by FTIR using potassium bromide (KBr) pellets with 1 mg of the sample and 100 mg of spectroscopic grade KBr (2%, m/m) at the IR Prestige-21 equipment (SHIMADZU, Quito, Japan) in the range of 400-4000 cm-1 with a resolution of 4 cm-1 and 64 scans/min. The obtained spectrum was evaluated against the pure CAP, nano-HAp, and the HAp-CAP nanocomposite.
2.5.5. Thermogravimetric Analysis (TGA)
The TGA analysis was conducted in a TA thermal analysis analyzer (Labsys Evo STA, Setaram Instrumentation, Caluire, France), was used to obtain information of the material’s thermal degradation. Samples of 10 ± 0.1 mg were heated from 20 to 500 °C under nitrogen atmosphere at a flow rate of 30 mL/min-1. All the experiments were conducted at 10 °C/min-1 and the thermogravimetric and derivative (TG/DTG) curves were recorded. The hybrid nanocomposites compositions were also calculated from the residues obtained at the end of the TGA. The equipment was previously calibrated with copper sulfate pentahydrate.
2.5.6. Determination of Differential Scanning Calorimetry (DSC)
The DSC analysis of the samples was performed using a thermal analysis equipment (Labsys Evo STA, Setaram Instrumentation, Caluire, France) out at a maximum temperature of 500 °C. The sample was packed in a well-sealed aluminum pan and then heated from - 100 °C to 120 °C at a rate of 10 °C/min-1. The equipment was previously calibrated with indium.
2.6. Addition of HAp-CAP in an In-Office Bleaching Gel: Hydrogen Peroxide Diffusion Inside the Pulp Chamber and Bleaching Efficacy Evaluation
2.6.1. Ethical Committees and teeth Selection
After the previous physiochemical analysis, the 5% HAp-CAP nanocomposite was added to an in-office bleaching gel. An in vitro study of hydrogen peroxide diffusion and color alteration was performed to justify the HAp-CAP nanocomposite use in Dentistry. The methodology used for this purpose is clearly consolidated [
11,
16,
18,
38,
39]. The study was submitted and approved by the Research Ethics Committee of the State University of Ponta Grossa (# 5.435.477) and has used eighteen premolars without morphological alterations or enamel cracks obtained from the Tooth Bank of the State University of Ponta Grossa.
2.6.2. Bleaching Gels Preparation
Two in-office bleaching gels were prepared containing 3% sodium alginate (Biotec, Biotec - Comércio de Produtos para laboratório Eireli, São José dos Pinhais, Brazil), 0.05% E.D.T.A (Synth, Synth Diadema, Brazil), 0.4% citric acid (Biotec, Biotec - Comércio de Produtos para laboratório Eireli, São José dos Pinhais, Brazil), 25% propyleneglycol (Quimidrol, Quimidrol - Produtos Químicos para a Indústria, Pinhais, Brazil) and purified water as vehicle. In one of these gels, 35% pure hydrogen peroxide (HP 35%) was also added. In the other one, 5% HAp-CAP nanocomposite (HP + HAp-CAP) was also added besides the 35% HP. In order to confirm the initial concentrations inside the prepared gels and compare them, those were titrated with a standardized potassium permanganate solution before the bleaching procedure, and both gels showed the same final concentration of 35% hydrogen peroxide. All measurements were assessed in triplicate (data not shown).
After being prepared, both gels were filled in identical white syringes, and coded to be used for bleaching the teeth selected.
2.6.3. Sample Size Calculation
This stage of the study is based on the quantification of HP inside the pulp cavity. According to previous literature [
17], an average of 0.399 ± 0.119 μg/mL of HP was found in the pulp chamber post-in-office dental bleaching with HP 35% gels. Thus, using a bilateral test with an alpha of 0.05 and a power of 80%, six teeth were needed in each group to detect a difference of 0.200 μg/mL.
2.6.4. Sample Preparation and Dental Bleaching Procedure
The teeth were randomly assigned to three groups (n = 6) according to the experimental bleaching gel used: hydrogen peroxide 35% (positive control) or hydrogen peroxide Hap-Cap 35% (HP + Hap-CAP). An additional group not exposed to bleaching treatment was evaluated (control). The roots of all teeth were removed approximately 3 mm from the cement-enamel junction, using a low-speed diamond disk (Isomet 1000, Buehler Ltd., Lake Bluff, USA). Following, the pulp tissue was rinsed with deionized water from a Millipore Milli-Q (MS2000) system (Gehaka, São Paulo, SP, Brazil), and the access to the pulp cavity was expanded using a # 1014 spherical drill (KG Sorensen, Barueri, SP, Brazil) [
11,
38].
A standard analytical curve was drawn from a 5,000 μg/mL stock solution prepared from a concentrated solution (34% - 36% HP, LABSYNTH, Diadema, SP, Brazil). This solution was diluted in an acetate buffer solution (pH = 4.5) and titrated using a potassium permanganate solution to determine the analytical grade and the actual concentration of the solution. Based on this initial concentration, serial volumetric dilutions of 0.000-0.404 μg/mL were performed to draw the analytical curve. The known concentrations of hydrogen peroxide were obtained using a Cary UV-Vis 50 spectrophotometer (Varian, Palo Alto, CA, USA) [
16,
38].
To dental bleaching procedure, the teeth had their occlusal surface fixed on a wax plate, allowing access to the pulp cavity, and had their vestibular area isolated by photopolymerizable resin barrier (Top Dam, FGM, Joinville, SC, Brazil). So, a 25 μL aliquot of the acetate buffer (pH = 4.5) was inserted into the pulp cavity of each tooth to absorb and preserve any hydrogen peroxide that may penetrate the pulp cavity during bleaching treatment. The bleaching gels were applying for an only session of 50 min.
After the procedure, the acetate buffer solution in the pulp cavity of each sample was aspirated using a micropipette and transferred to a glass tube. This procedure was repeated with the acetate buffer to clean the pulp cavity, and this was solution transferred to the same glass tube. So, 100 μL of 0.5 mg / mL (Leucocrystal Violet, Sigma Chemical Co., St Louis, MO, USA) and 50 μL of 1 mg / mL of horseradish peroxidase enzyme (Peroxidase Type VI-A, Sigma Chemical Co., St. Louis, MO, USA) were added to the glass tube, along with deionized water (2.725 μL). This sequence was repeated separately for each tooth. The resulting solution was measured using a Cary 50 UV-Vis spectrophotometer (Varian, Palo Alto, CA, USA) [
16,
38]. According to Beer's Law, absorbance directly corresponds to the concentration; therefore, the concentration of hydrogen peroxide (μg/mL) was determined by comparison with the calibration curve already obtained.
During the bleaching procedure, the pH of each bleaching gel was measured immediately and 50 minutes post-application. For this, a 6 mm circular pHmeter and a flat surface pH electrode (Extech pH100: ExStik pH Meter; Extech Instruments, Nashua, NH, USA) were positioned in each tooth bleached and held in position until stabilization. Three measurements were carried out on each tooth [
38,
40].
The bleaching efficacy evaluation was measured before the bleaching procedure and one week post bleaching protocol. It was performed for all groups, using a digital spectrophotometer (VITA Easyshade Advance 4.0, VITA Zahnfabrik, Bad Säckingen, Germany). To this, the color parameters were measured (L*, a*, and b*), and the color change before (baseline) and after treatment (one week) was given by the difference between the colors measured with the spectrophotometer using the CIELab formula [
41]:
Perceptual changes will be accepted when the differences in the initial and after-bleaching colors present ∆E
ab > 2.7 [
42].
The obtained data were analyzed using the Kolmogorov–Smirnov test to assess whether they were normally distributed and using the Barlett test to verify the assumption of equality of variances (not shown data). The data of the amount of the hydrogen peroxide concentration (µg/mL) detected inside the pulp cavity were subjected to t-test to compare the values obtained in different groups. The bleaching efficacy were submitted to t-test to compare different bleaching gels. Statistical significance was set at α = 0.05. The results have been converted into graphs.
3. Results and Discussion
The HAp-CAP nanocomposite was suitably obtained by the precipitation method. After drying, all the obtained formulations were in powder with a white color. It was known that composites and nanocomposites exhibit promising mechanical and thermally advantageous properties [
31]. The finds of the characterization tests highlight the superior properties of the HAp-CAP nanocomposite in comparison to the individual materials.
3.1. Physicochemical Characterization
3.1.1. Determination of the Particle Size, Zeta Potential and Polydispersion Index (PDI)
Regarding the average diameter, suitable nanometric-scaled size and low PDI which indicated a narrow distribution [
43,
44] were recorded in
Table 1. PDI values close to 0 are considered monodisperse and greater than 0.5 indicate heterogeneous dispersion.
Considering the zeta potential (ζ), the colloidal structures showed negative values higher than − 35 mV. According to the considered system, ζ can be positive (cationic) or negative (anionic). Its absolute value gives valuable information on the stability of a colloid. For |ζ| typically larger than 30 mV, the high electric charge that is formed on the surface of the incipient droplets causes strong repellant forces between them, preventing further particle aggregation and consequently providing the system with a suitable stability [
45,
46].
3.1.2. Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive X-ray Spectroscopy (EDS) Analysis
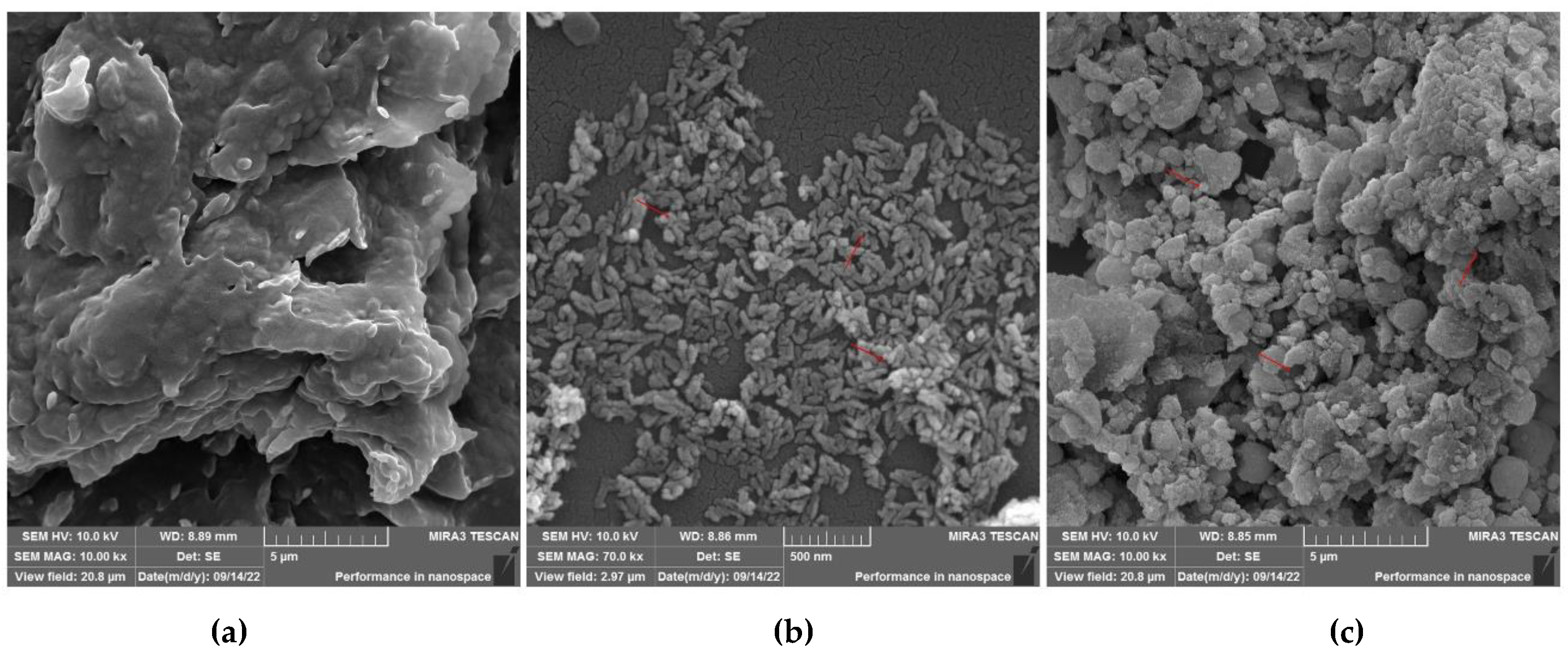
SEM analysis was performed in order to investigate the shape and surface of materials. Photomicrographs performed for pure CAP, nano-HAp and HAp-CAP nanocomposite after drying are depicted in
Figure 1 (a, b and c, respectively). CAP raw material (
Figure 1a) is organized as monoclinic crystals (rectangular plates), as expected [
45]. While the analysis of samples obtained through the precipitation method presented agglomerates of particles of nanometric size (
Figure 1b).
The HAp-CAP nanocomposite (
Figure 1c) mainly presented elongated particles agglomerates and smooth surface. No pores were observed. These images confirmed the HAp-CAP nanocomposite formation through the aforementioned method, as well as the nano-sized dimensions of these formulations.
3.1.3. X-ray Powder Diffraction (XRPD)
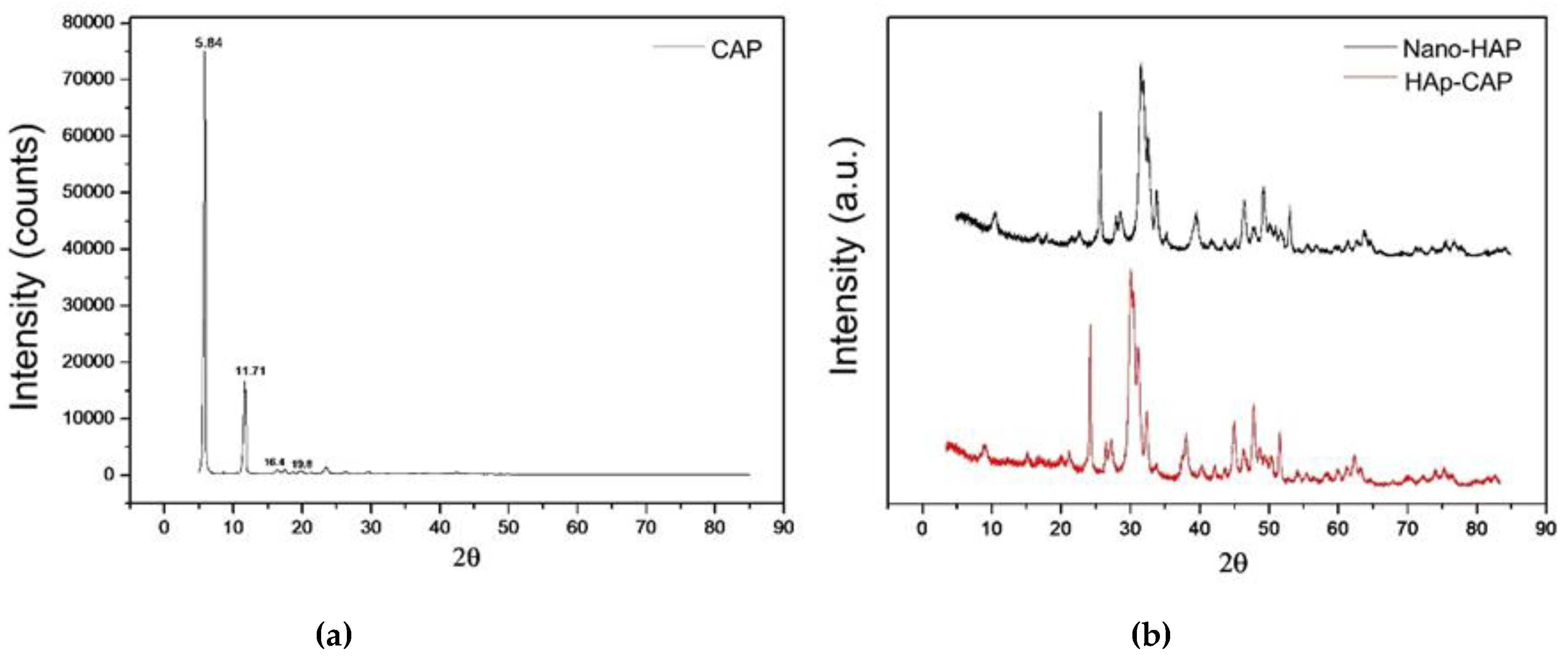
Figure 2 summarizes the XRPD patterns of CAP, nano-Hap and HAp-CAP. Pure drug (CAP) showed intense diffraction peaks at Bragg angles 2θ = 5.84° (24,000), 11.71° (4500), 16.4° (1240), and 19.8° (1870) which confirmed the crystalline structure of capsaicinoids characterized by sharp and well-defined peaks [
45]. The XRPD pattern of nano-HAp presented a single crystalline phase corresponding to HAp, with characteristic peaks at 10.84°, 21.79°, 28.89°, 31.73°, 32.86°, and 39.74° [
37].
The HAp-CAP nanocomposite obtained showed crystalline diffraction patterns similar to nano-HAp. These results indicate that the procedure provided a remarkable decrease of the crystalline diffraction peaks of capsaicinoids leading to drug amorphization. The preparation of nanocomposite containing capsaicinoids as amorphous solids, when compared to the original crystalline structure of the drug, may facilitate the dissolution process which is desirable mainly due capsaicinoids have low solubility in aqueous media [
45].
3.1.4. Fourier-Transform Infrared Spectroscopy (FTIR)
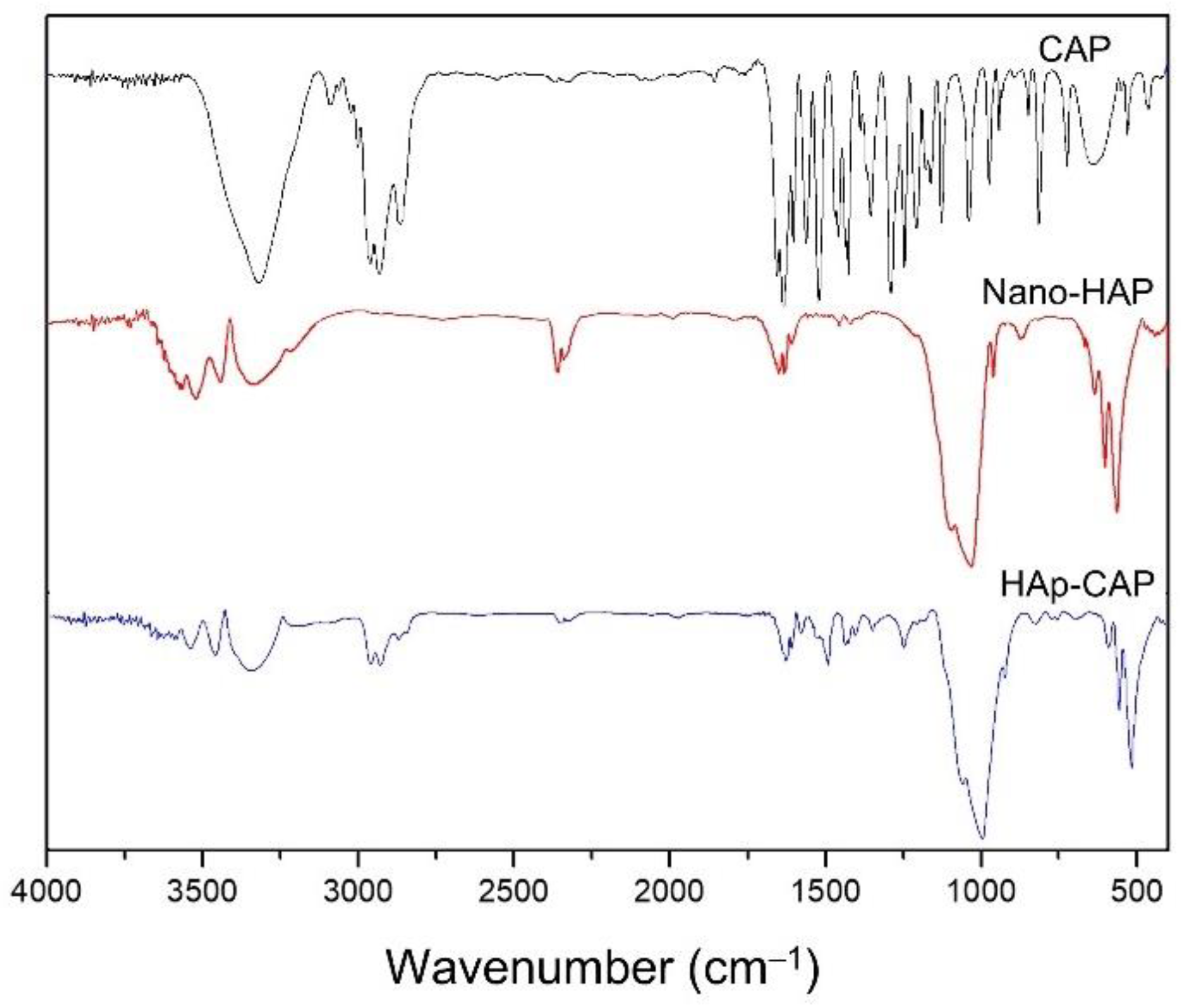
FTIR studies were carried out to explore the type of interaction between CAP and HAp. FTIR spectra of composite CAP are represented in
Figure 3. Capsaicin ((6E)-N-[(4-Hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide) is an alkaloid of the group of capsaicinoids, and its molecule contains three major fragments—aromatic head, amide linkage, and a hydrophobic tail. CAP is depicted to peak at 3310 cm
-1 due to broadened N-H and O-H stretch (
Figure 3). The peaks showed at 1627 cm
-1 represent C═O stretch and at 1516 cm
-1 for N-H bends. The peak shown at 1284 cm
-1 represents C-N bonds stretching, and the peak depicted at 807 cm
-1 for out of plane C-H bend, in agreement with the previously reported [
45].
FTIR spectra of the nano-HAp and HAp-CAP showed similar patterns (Figure. 3). Broad bands at 3432 and 1642 cm
−1 were attributed to adsorbed water, while the sharp peak at 3571 cm
−1 was assigned to the stretching vibration of the lattice OH
− ions and the medium sharp peak at 633 cm
−1 was assigned to the O—H deformation mode. Some typical bands for PO
43− appeared at 470, 568, 602, 964, 1041, and 1093 cm
−1. These assignments confirm that these nanoparticulate products were successfully synthesized and were consistent to HAp [
37]. Nanocomposite showed an overlap of the raw materials signals, and no novel signal was evidenced for these formulations. In that sense, no chemical reactions occurred during the nanocomposite process.
3.1.5. Thermogravimetric Analysis
Figure 4 summarizes the steps of thermal degradation verified for pure CAP (
Figure 4a) and Nano-HAp and HAp-CAP (
Figure 4b).
Figure 4a show the thermal stability curve (TGA) or the rate of weight loss (DTG) for the CAP. The CAP is a kind of amide, and the thermal stability of which is relatively poor. Three degradation steps can be seen from
Figure 4a. The capsaicin firstly shows initial weight loss at 150 °C and shows the highest degradation degree at 230 °C. This may be due to the rupture and degradation of the amide group in the CAP. The second step starts at 250 °C and degrades intensively at 310 °C. This can be ascribed to the decomposition of the alkene and methyl group in this material. The third step starts at 390 °C and shows highest degradation at 450 °C. This may be deduced to the hydrolysis of the remaining organic compounds in the residue. On the other side, when the TGA profile of the HAp-CAP composite, it was possible to observed that thermal stability of the CAP is greatly improved after reaction of HAp and CAP. Its degradation occurs in only one step. This step starts at about 300 °C. From the
Figure 4, we also can see that the intensive degree was decreased after reaction on the surface of CAP.
3.1.6. Determination of Differential Scanning Calorimetry (DSC)
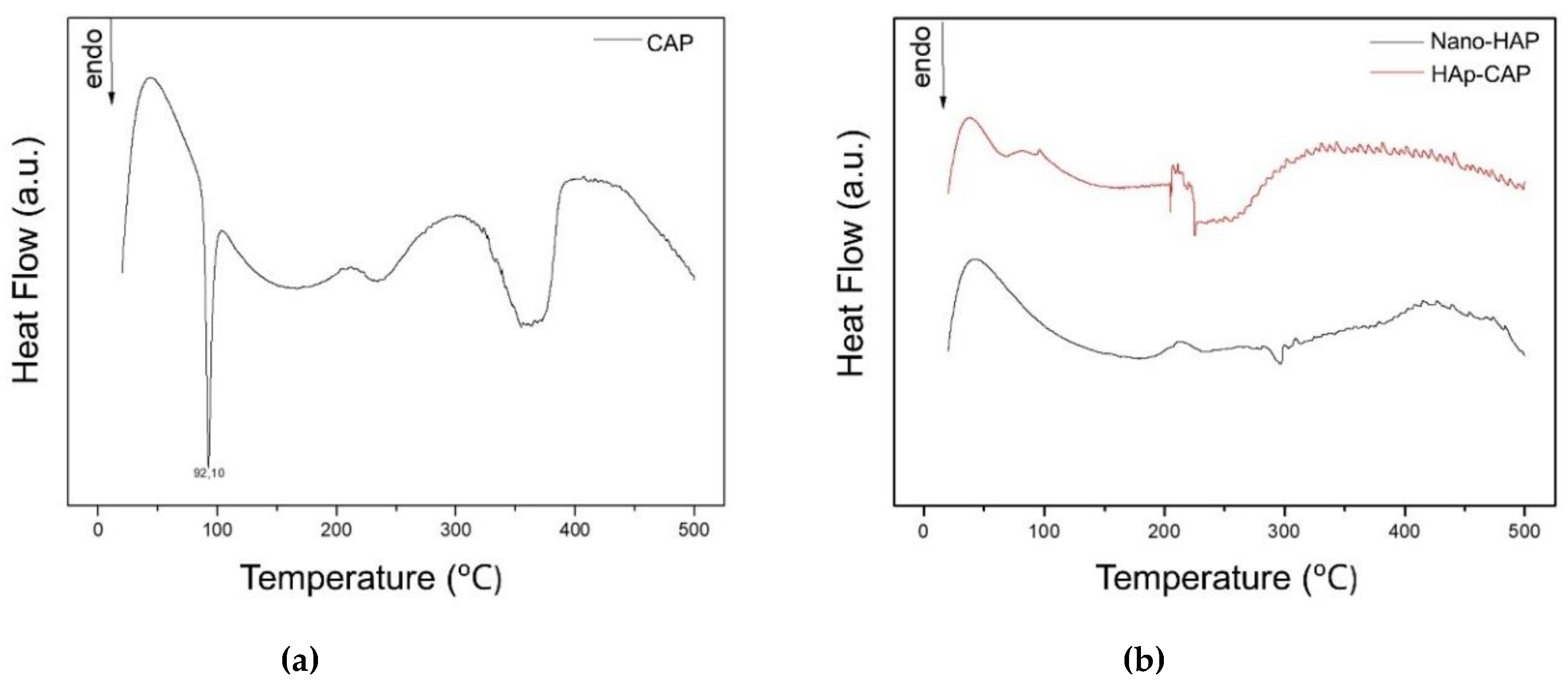
DSC is a technique for the thermal analysis of a broad diversity of materials. Changes in material properties with changes in the temperature can give valuable information about the drug-excipient interactions and new entities formation. The DSC curves of all experimental groups are displayed in
Figure 5.
The CAP (
Figure 5a) showed a sharp endothermic event at 92, 10°, attributed to its melting point, representing CAP crystalline nature, as well as previously reported [
47]. The typical melting event of CAP was not observed in the DSC curves when Hap-CAP nanocomposite synthesized (
Figure 5b). This thermal behavior suggests that an amorphization of the drug occurred. This result was reinforced by the XRPD patterns, in which no crystalline peak of FA was observed for drug-loaded microparticles.
3.2. Hydrogen Peroxide Diffusion Inside the Pulp Chamber and Bleaching Efficacy
The relationship between TS and the hydrogen peroxide concentration in the pulp chamber is well established. This is because a higher diffusion of hydrogen peroxide into the pulp chamber corresponds to an increased level of TS [
3,
15]. Hence, the primary aim of this research was to create an innovative material with suitable physicochemical properties to be used in a bleaching gel for in-office use. While there is a lack of studies assessing the simultaneous use of the two materials employed in the composite synthesis (HAp and CAP), it is established that their individual application has demonstrated positive outcomes in addressing adverse effects on the tooth surface [
11,
48] and influencing pulp receptors [
35,
36].
After successfully obtaining the HAp-CAP nanocomposite, we decided to add it to an in-office bleaching gel in an attempt to reduce the diffusion of hydrogen peroxide into the pulp chamber, as well as the inhibition of receptors present in the dental pulp. The bleaching products used for in-office dental bleaching trigger alterations to the enamel surface after the bleaching procedure [
11,
49], avoiding the peroxides that easily cross enamel and dentin, which allow free radicals to reach the pulp where they produce an inflammatory reaction with the release of different inflammatory mediators [
13,
14], known as the TS. Thus, the higher the hydrogen peroxide concentration, the higher its diffusion into the pulp chamber [
15,
16] as well as a greater development of TS [
3,
15]. In addition, the acidity found in bleaching gels also seems to be related to the amount of hydrogen peroxide that reaches the pulp [
38].
Consequently, the optimal solution for reducing TS involves combining agents capable of both desensitizing the dental pulp (CAP) and remineralizing the dental surface (HAp) during dental bleaching. Unfortunately, due to the impossibility of assessing TS in vitro, it was evaluated the evaluation of the amount of hydrogen peroxide in the pulp chamber. The expectation was that incorporating a HAp-CAP nanocomposite into an in-office bleaching gel would reduce its penetration into the pulp chamber by depositing material on the tooth surface.
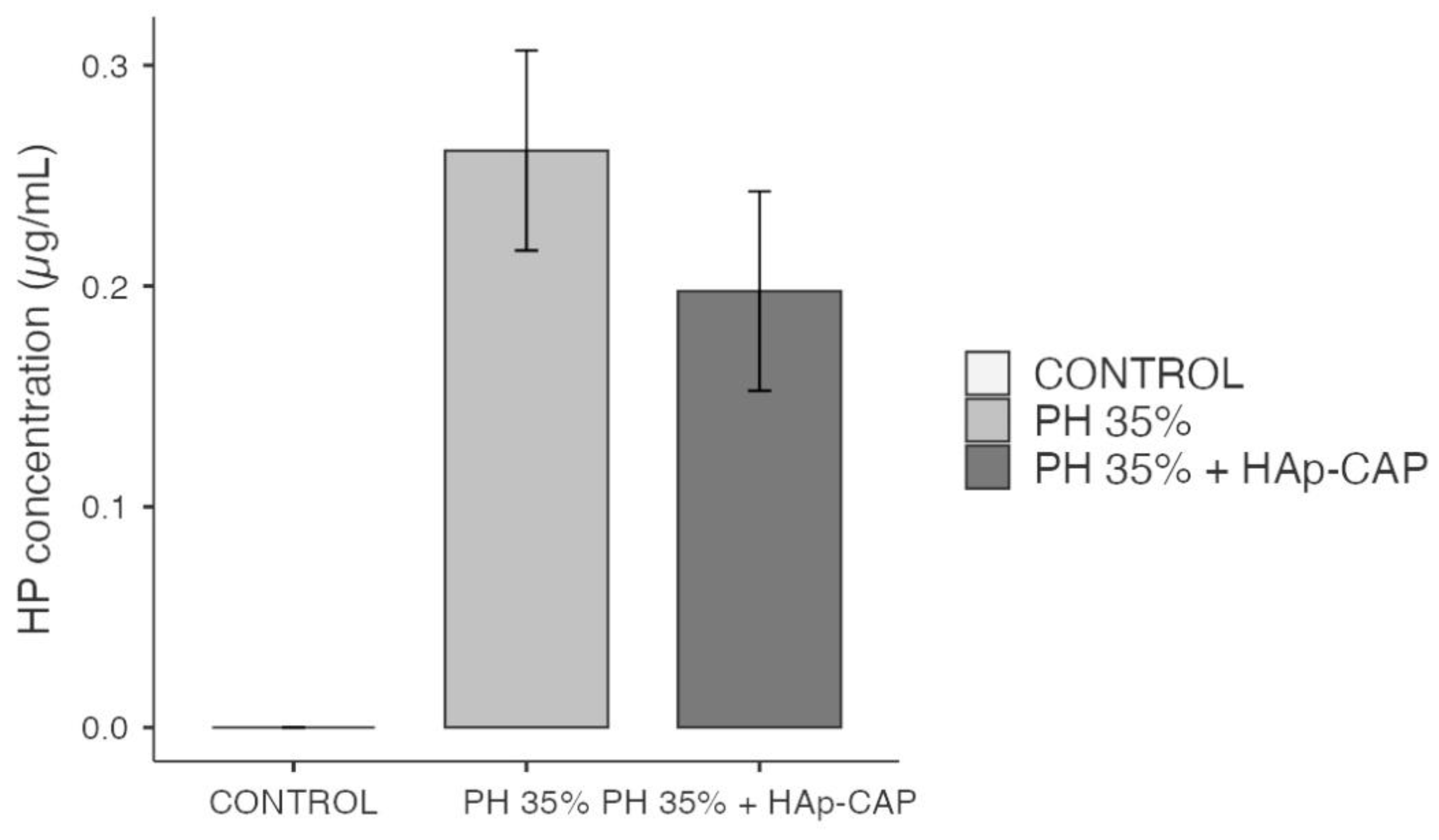
Figure 6 shows the amount of hydrogen peroxide that diffused into the pulp chamber. Both bleaching gels evaluated showed a significant and greater amount of hydrogen peroxide inside the pulp chamber when compared to control (p < 0.001). However, besides a slight decrease in the hydrogen peroxide values within the pulp chamber was noted when an HAp-CAP nanocomposite was added to the bleaching gel, there was no significant difference when compared to in-office bleaching gel without the addition of HAp-CAP (p > 0.05).
Several reasons can explain the present results. The primary reason behind this occurrence could be attributed to the simultaneous application of hydrogen peroxide. This suggests that although the composite material remained in contact with the tooth surface, the hydrogen peroxide reaction might have dominated or hindered the composite's effect. Another important fact to mention is that the gel containing HAp-CAP was only evaluated against hydrogen peroxide diffusion and color alteration. This leads us to believe that it should also be evaluated in terms of its effects on the tooth surface. Earlier studies reveal that nanoparticles, especially HAp, has an ability to adhere to the enamel surface by a protective layer for enamel [
50], so the investigation of the HAp-CAP effects on the dental surface has been encouraged.
The second explanation was that the pH did not remain stable during the application period and was slightly acidic at the end of the bleaching procedure, which may have contributed to a greater diffusion of hydrogen peroxide in the pulp chamber. The pH behavior of the two in-office bleaching gels evaluated was evaluated only qualitatively but demonstrates an acidic pH initial, varying from 5.1 to 5.3 in the hydrogen peroxide 35%, and 6.3 to 5.3 to HP + HAp-CAP bleaching gels, which demonstrates a small drop in pH after 50-minutes application. As previously observed, less acidic or alkaline materials have lower amounts of hydrogen peroxide in the pulp chamber [
17,
18,
38]. Therefore, the addition of HAp-CAP nanocomposite to alkaline bleaching agents should be tested in the future, as well as its use in less concentrated bleaching agents for at-home use.
Additionally, it's noteworthy that the most effective efforts in reducing TS have been documented when desensitizing agents are administered prior to dental bleaching [
51,
52]. This observation might prompt further exploration using the HAp-CAP nanocomposite in topical desensitizing approaches.
Nevertheless, the hydrogen peroxide levels observed in this research align with previous studies [
17,
18], particularly when concentrations closer to 35% were employed, as higher concentrations of hydrogen peroxide are typically linked to increased rates of its diffusion.
We should also mention that the concentration of HAp-CAP introduced into the bleaching gel was 5%. Previous studies have shown that concentrations up to 5% of remineralizing or desensitizers were normally employed to provide satisfactory results in reducing TS [
53,
54,
55,
56,
57], without compromising treatment safety. However, this is a preliminary study and does not dispense with the need to evaluate different concentrations of the HAp-CAP in the bleaching gels.
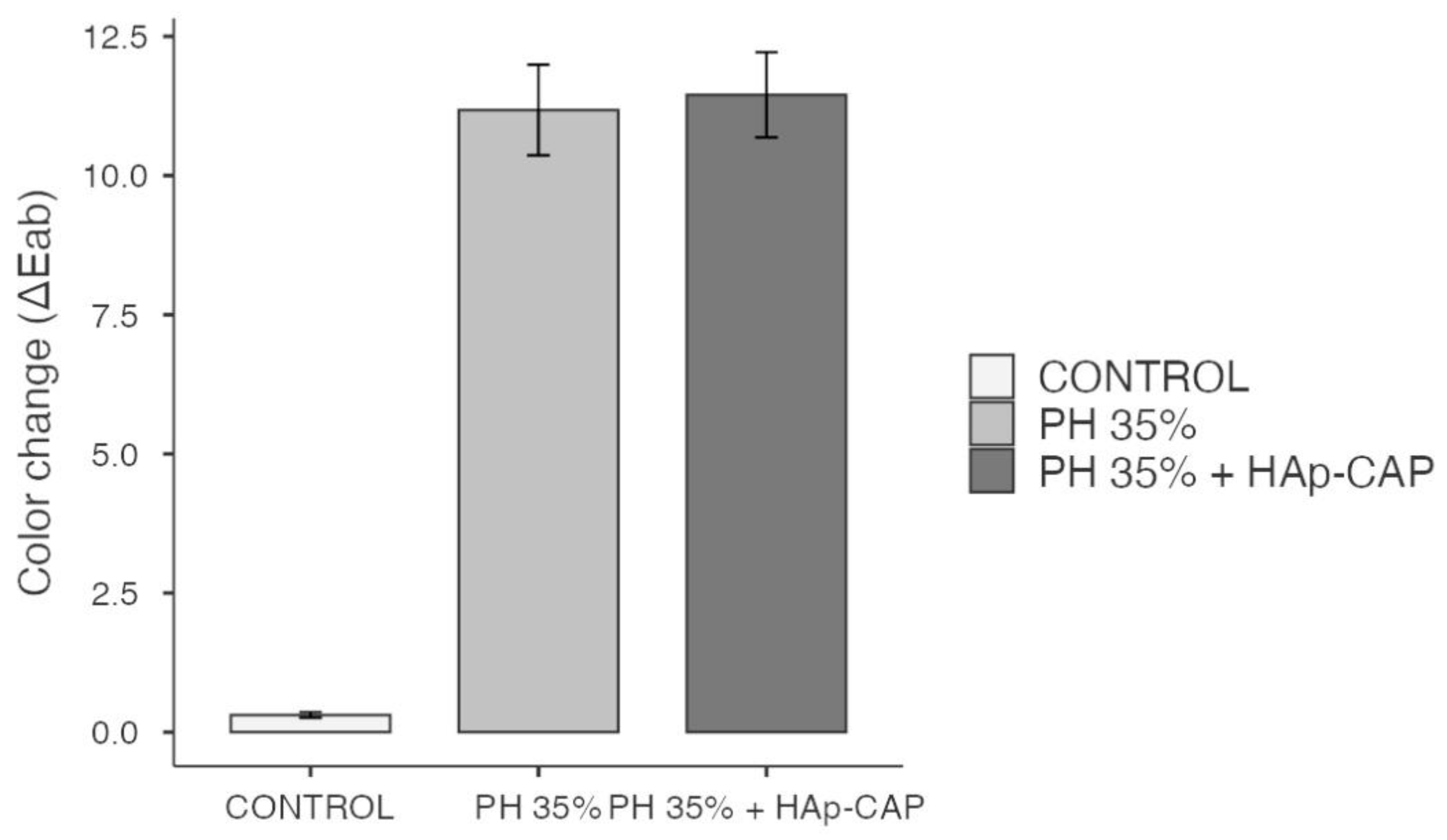
Taking into consideration that a potential interference in the bleaching efficacy can be observed when remineralizing products are used in dental bleaching [
58] it’s wort to mention that the use of HAp-CAP was not able to interfere in the bleaching efficacy observed in the present study. Regarding the bleaching efficacy, both bleaching gels used showed higher bleaching efficacy when compared to the control (p < 0.05). However, it's important to mention that, the addition of HAp-CAP to the bleaching gel did not affect their bleaching effect, since both in-office bleaching gels showed similar bleaching efficacy (
Figure 7). Observe that, the values of ∆E
ab obtained are in according with previous findings [
11,
18], where a high hydrogen peroxide concentration was used. From a dental point of view, this is a determining factor for the use recommendation of HAp-CAP in bleaching gels.
Additionally, while the methodology employed to measure the hydrogen peroxide concentration in the pulp chamber during dental bleaching yields crucial data, there have not been studies thus far correlating the in vitro pulp hydrogen peroxide levels with the occurrence of TS in patients. Therefore, it's important to mention some additional limitations regarding the present study: antioxidants capable of inhibiting TRPA-1 receptors and reducing the release of neuropeptides from nerve terminals cannot be assessed in the laboratory. Consequently, it's crucial that future clinical studies evaluate the use of the HAp-CAP nanocomposite when added to in-office bleaching gel as well as when applied prior to in-office bleaching gel.
It's also worth noting that in the current study, the primary factor influencing diffusion might have been hydroxyapatite rather than capsaicin, underscoring the need for clinical studies to assess the effects of HAp on pulp fibers and sensitivity reduction. Finally, it's important to mention that to date, the most successful attempts to reduce tooth sensitivity have been observed when desensitizing agents were applied before bleaching, a fact that may encourage further studies and the pursuit of more satisfactory outcomes regarding hydrogen peroxide reduction in the pulp chamber.
4. Conclusions
The HAp-CAP nanocomposite physicochemical analyses confirmed that it showed mean diameter, polydispersity index, and zeta potential within the standards for nanoscale. Morphological and surface analysis showed that HAp-CAP nanocomposite had a spherical shape with a smooth surface. Structural analysis confirmed that there was no chemical reaction between CAP and HAp. All these physicochemical characterization showed that the synthesis process of the HAp-CAP composite was successfully carried out. The addition of HAp-CAP nanocomposite in an in-office bleaching gel showed a slight reduction of the amount of hydrogen peroxide inside the pulp chamber and did not interfere in the color effectiveness after in-office dental bleaching.
Author Contributions
“Conceptualization, K.L.S., M.R., J.M.N, A.D.L. and P.V.F.; methodology, K.L.S, D.H., M.W.F., M.R., J.M.N; formal analysis, K.L.S., J.M.N. M.W.F; investigation, K.L.S., D.H., M.W.F, J.M.N.; data curation, K.L.S. and J.M.N; writing—original draft preparation, K.L.S, M.W.F., J.M.N. and A.D.L.; writing—review and editing, K.L.S. A.D.L. and P.V.F.; supervision, A.D.L. and P.V.F.; project administration, K.L.S.; funding acquisition, A.D.L and P.V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by National Council for Scientific and Technological Development (CNPq) (313704/2019-8, 402043/2022-7, and 168149/2022-2) and to Araucaria Foundation as well as the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES, Finance Code 001).
Acknowledgments
The authors express gratitude to the Multi-user Laboratory Complex at the State University of Ponta Grossa for lab facilities.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Maran B.M.; Vochikovski L.; Hortkoff D.R.A.; Stanislawczuk R.; Loguercio A.D.; Reis A. Bleaching sensitivity with a desensitizing in-office bleaching gel: a randomized double-blind clinical trial. Quintessence Int 2020, 51:788-797. [CrossRef]
- Kielbassa A.M.; Maier M.; Gieren A.K.; Eliav E. Tooth sensitivity during and after vital tooth bleaching: A systematic review on an unsolved problem. Quintessence Int 2015, 46:881-897. [CrossRef]
- Maran B.M.; Matos T.P.; de Castro A.D.S.; Vochikovski L.; Amadori A.L.; Loguercio A.D.; Reis A.; Berger S.B. In-office bleaching with low/medium vs. high concentrate hydrogen peroxide: A systematic review and meta-analysis. J Dent 2020, 103:103499. [CrossRef]
- da Costa J.B.; McPharlin R.; Paravina R.D.; Ferracane J.L. Comparison of At-home and In-office Tooth Whitening Using a Novel Shade Guide. Oper Dent 2010, 35:381-388. [CrossRef]
- Irusa K.; Alrahaem I.A.; Ngoc C.N.; Donovan T. Tooth whitening procedures: A narrative review. Dent Rev 2022, 2:100055. [CrossRef]
- de Sá JL.; Silva J.S.; Herkrath F.J.; Favoreto M.W.; Reis A.; Silva L.M.; Loguercio A.D.; Martins L.M. In-office bleaching with complete cervical third protection protocol: A split-mouth, double-blind, randomized clinical trial. Am J Dent 2021, 34:281-285.
- Meireles S.S.; Santos M.E.; Lustosa I.M.C.; Leite E.L.L. Effects of a reduced in-office bleaching protocol with 37.5% hydrogen peroxide on effectiveness and tooth sensitivity: A double-blind randomized clinical trial. J Esthet Restor Dent 2021, 33:824-831.
- Rezende M., Loguercio A.D.; Kossatz S.; Reis A. Predictive factors on the efficacy and risk/intensity of tooth sensitivity of dental bleaching: A multi regression and logistic analysis. J Dent 2016, 45:1-6. [CrossRef]
- de Geus J.L.; Wambier L.M.; Kossatz S.; Loguercio A.D.; Reis A. At-home vs In-office Bleaching: A Systematic Review and Meta-analysis. Oper Dent 2016, 41:341-356. [CrossRef]
- Torres C.R.; Zanatta R.F.; Godoy M.M.; Borges A.B. Influence of Bleaching Gel Peroxide Concentration on Color and Penetration through the Tooth Structure. J Contemp Dent Pract 2021, 22:479-483.
- Acuña E.A.O.; Parreiras S.O.; Favoreto M.W.; Cruz G.P.; Gomes A.; Borges C.P.F.; Loguercio A.D.; Reis A. In-office bleaching with a commercial 40% hydrogen peroxide gel modified to have different pHs: Color change, surface morphology, and penetration of hydrogen peroxide into the pulp chamber. J Esthet Rest Dent 2022, 34:322-327. [CrossRef]
- Soares D.G.; Basso F.G.; Hebling J.; de Souza Costa C.A. Concentrations of and application protocols for hydrogen peroxide bleaching gels: Effects on pulp cell viability and whitening efficacy. J Dent 2014, 42:185-198. [CrossRef]
- Cintra L.T.; Benetti F.; da Silva Facundo A.C.; Ferreira L.L.; Gomes-Filho J.E.; Ervolino E.; Rahal V.; Briso A.L. The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod 2013, 39:1576-1580. [CrossRef]
- Caviedes-Bucheli J.; Munoz H.R.; Azuero-Holguin M.M.; Ulate E. Neuropeptides in dental pulp: the silent protagonists. J Endod 2008, 34:773-788.
- Cardenas A.F.M.; Maran B.M.; Araújo L.C.R.; de Siqueira F.S.F.; Wambier L.M.; Gonzaga C.C.; Loguercio A.D.; Reis A. Are combined bleaching techniques better than their sole application? A systematic review and meta-analysis. Clin Oral Investig 2019, 23:3673-3689. [CrossRef]
- Carneiro T.S.; Favoreto M.W.; Bernardi L.G.; Bandeca M.C.; Borges C.; Reis A.; Loguercio A.D. Application Tip and Concentration of a Self-mixing Bleach: Hydrogen Peroxide Inside the Pulp Chamber, Color Change, and Amount of Bleaching Gel Used. Oper Dent 2023, 48:146-154. [CrossRef]
- Mena-Serrano A.P.; Parreiras S.O.; do Nascimento E.M.; Borges C.P.; Berger S.B.; Loguercio A.D.; Reis A. Effects of the concentration and composition of in-office bleaching gels on hydrogen peroxide penetration into the pulp chamber. Oper Dent 2015, 40:E76-82. [CrossRef]
- Balladares L.; Alegria-Acevedo L.F.; Montenegro-Arana A.; Arana-Gordillo L.A.; Pulido C.; Salazar-Gracez M.T.; Reis A.; Loguercio A.D. Effects of pH and Application Technique of In-office Bleaching Gels on Hydrogen Peroxide Penetration into the Pulp Chamber. Oper Dent 2019, 44:659-667. [CrossRef]
- Parreiras S.O.; Favoreto M.W.; Lenz R.E.; Serra M.E.; Borges C.P.F.; Loguercio A.D.; Reis A. Effect of Prior Application of Desensitizing Agent on the Teeth Submitted to In-Office Bleaching. Braz Dent J 2020, 31:236-243. [CrossRef]
- Mazumder S.; Nayak A.K.; Ara T.J.; Hasnain M.S. 7 - Hydroxyapatite composites for dentistry. In Applications of Nanocomposite Materials in Dentistry, 1st ed.; Asiri A.M., Inamuddin, Mohammad A. Publisher: Woodhead Publishing, Sawston, 2019; Volume 1, pp. 123-143.
- Bordea I.R.; Candrea S.; Alexescu G.T.; Bran S.; Băciuț M.; Băciuț G.; Lucaciu O.; Dinu C.M.; Todea D.A. Nano-hydroxyapatite use in dentistry: a systematic review. Drug Metab Rev 2020, 52:319-332. [CrossRef]
- Shetty S.; Kohad R.; Yeltiwar R. Hydroxyapatite as an In-Office Agent for Tooth Hypersensitivity: A Clinical and Scanning Electron Microscopic Study. J Periodontol 2010, 81:1781-1789. htpps://10.1902/jop.2010.100172.
- Huamán-Mujica K.; Castañeda-Vía J.; Bermúdez García V.; Dominguez J.; Landauro C.; Quispe-Marcatoma J.; Tay Chu Jon L. Dentinal tubules obliteration using a toothpaste with nano-hydroxyapatite obtained from chicken eggshell. J Stoma 2022, 75:147-154. [CrossRef]
- Kulal R.; Jayanti I.; Sambashivaiah S.; Bilchodmath S. An In-vitro Comparison of Nano Hydroxyapatite, Novamin and Proargin Desensitizing Toothpastes - A SEM Study. J Clin Diagn Res 2016, 10:ZC51-ZC54. [CrossRef]
- Moharam L.M.; Khadr S.; Abdou A.; Nagi S.M. Effect of Arginine and nano-hydroxyapatite application on the hypersensitivity and color change of bleached enamel: A randomized controlled clinical trial. J Clin Exp Dent 2022, 14:e499-e505. [CrossRef]
- Clift F. Artificial methods for the remineralization of hydroxyapatite in enamel. Mater Today Chem 2021, 21:100498. [CrossRef]
- Wang L.; Magalhães A.C.; Francisconi-dos-Rios L.F.; Calabria M.P.; Araújo D.F.G.; Buzalaf M.A.R.; Lauris J.R.P.; Pereira J.C. Treatment of Dentin Hypersensitivity Using Nano-Hydroxyapatite Pastes: A Randomized Three-Month Clinical Trial. Oper Dent 2016, 41:E93-E101. [CrossRef]
- Gopinath N.M.; John J.; Nagappan N.; Prabhu S.; Kumar E.S. Evaluation of Dentifrice Containing Nano-hydroxyapatite for Dentinal Hypersensitivity: A Randomized Controlled Trial. J Int Oral Health 2015, 7:118-122.
- Vano M.; Derchi G.; Barone A.; Pinna R.; Usai P.; Covani U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: a double-blind randomized controlled trial. Clin Oral Investig 2018, 22:313-320. [CrossRef]
- Izzetti R.; Gennai S.; Nisi M.; Gulia F.; Miceli M.; Giuca M.R. Clinical Applications of Nano-Hydroxyapatite in Dentistry. Appl Sci 2022, 12:10762. [CrossRef]
- Reduwan Billah S.M. Composites and Nanocomposites. In Functional Polymers, 1st ed.; Jafar Mazumder M.A.; Sheardown H.; Al-Ahmed A. C. Springer International Publishing, 2019; Volume 1, pp. 1-67.
- Markowitz K. Pretty painful: Why does tooth bleaching hurt? Med Hypotheses 2010, 74:835-840. [CrossRef]
- Hossain M.Z.; Bakri M.M.; Yahya F.; Ando H.; Unno S.; Kitagawa J. The Role of Transient Receptor Potential (TRP) Channels in the Transduction of Dental Pain Int J Mol Sci 2019, 20:526. [CrossRef]
- Catalfamo L.M.; Marrone G.A.O.; Basilicata M.; Vivarini I.A.O.; Paolino V.; Della-Morte D.; De Ponte F.S.; Di Daniele F.; Quattrone D.; De Rinaldis D.; Bollero P.; Di Daniele N.A.O.; Noce A.A.O. The Utility of Capsicum annuum L. in Internal Medicine and In Dentistry: A Comprehensive Review. Int J Environ Rest Public Health 2022, 19:11187. [CrossRef]
- Raoof M.; Ashrafganjoui E.; Kooshki R.; Abbasnejad M.; Haghani J.; Amanpour S.; Zarei M.R. Effect of chronic stress on capsaicin-induced dental nociception in a model of pulpitis in rats. Archi Oral Biol 2018, 2018, 85:154-159. [CrossRef]
- Hargreaves K.M.; Jackson D.L.; Bowles W.R. Adrenergic Regulation of Capsaicin-sensitive Neurons in Dental Pulp. J Endod 2003, 29:397-399. [CrossRef]
- Pupo Y.M.; Leite L.M.B.; Senegaglia A.C.; Antunes L.; Nadal J.M.; de Lara E.L.; Saito R.E.; Antunes S.R.M.; Lacerda W.F.; Farago P.V. Effect of Hydroxyapatite Microspheres, Amoxicillin-Hydroxyapatite and Collagen-Hydroxyapatite Composites on Human Dental Pulp-Derived Mesenchymal Stem Cells. Mater 2021, 14:7515. [CrossRef]
- da Silva K.L.; Favoreto M.W.; Centenaro G.G.; Bernardi L.G.; Borges C.P.F.; Reis A.; Loguercio A.D. Can all highly concentrated in-office bleaching gels be used as a single-application? Clin Oral Investig 2023, 27:3663-3671. [CrossRef]
- Favoreto M.W.; Parreiras S.O.; Wendlinger M.; Carneiro T.S.; Lenhani M.I.; Borges C.P.F.; Loguercio A.D. Evaluation of hydrogen peroxide permeability, color change, and physical–chemical properties on the in-office dental bleaching with different mixing tip. J Esthet Restor Dent 2023. [CrossRef]
- Bernardi L.G.; Favoreto M.W.; Carneiro T.S.; Mena-Serrano A.; Borges C.P.F.; Reis A.; Loguercio A.D. Use of an applicator brush with high concentration bleaching gels. Clini Oral Investig 2022, 26:6387-6395. [CrossRef]
- De L'Eclairage CI. Recommendations on uniform color spaces, color-difference equations, psychometric color terms. 1978.
- Paravina R.D.; Ghinea R.; Herrera L.J.; Bona A.D.; Igiel C.; Linninger M.; Sakai M.; Takahashi H.; Tashkandi E.; Perez M.M. Color Difference Thresholds in Dentistry. J Esthet Restor Dent 2015, 27:S1-S9. [CrossRef]
- Shekunov B. Nanoparticle technology for drug delivery: from nanoparticles to cutting-edge delivery strategies - part I. Drugs 2005, 8:399-401.
- Avadi M.R.; Sadeghi A.M.; Mohammadpour N.; Abedin S.; Atyabi F.; Dinarvand R.; Rafiee-Tehrani M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomed 2010, 6:58-63. [CrossRef]
- Almeida M.A.; Nadal J.M.; Grassiolli S.; Paludo K.S.; Zawadzki S.F.; Cruz L.; Paula J.P.; Farago P.V. Enhanced gastric tolerability and improved anti-obesity effect of capsaicinoids-loaded PCL microparticles. Mater Sci Eng C Mater Biol Appl 2014, 40:345-356. [CrossRef]
- Azouz L.; Dahmoune F.; Rezgui F.; G'Sell C. Full factorial design optimization of anti-inflammatory drug release by PCL-PEG-PCL microspheres. Mater Sci Eng C Mater Biol Appl 2016, 58:412-419. [CrossRef]
- Gomes M.L.S.; da Silva Nascimento N.; Borsato D.M.; Pretes A.P.; Nadal J.M.; Novatski A.; Gomes R.Z.; Fernandes D.; Farago P.V.; Zanin S.M.W. Long-lasting anti-platelet activity of cilostazol from poly(epsilon-caprolactone)-poly(ethylene glycol) blend nanocapsules. Mater Sci Eng C Mater Biol Appl 2019, 94:694-702. [CrossRef]
- Xu B.; Li Q.; Wang Y. Effects of pH values of hydrogen peroxide bleaching agents on enamel surface properties. Oper Dent 2011, 36:554-562. htpps://doi.org/10.2341/11-045-1.
- Azrak B.; Callaway A.; Kurth P.; Willershausen B. Influence of bleaching agents on surface roughness of sound or eroded dental enamel specimens. J Esthet Restor Dent 2010, 22:391-399. [CrossRef]
- Andrade A.C.; Tenuta L.M.; Borges A.B.; Torres C.R. Effect of a hydrogen peroxide bleaching agent with calcium and phosphorus-containing salts on enamel surface hardness and roughness. Am J Dent 2021, 34:215-221. https://10.1111/j.1708-8240.2010.00372.x.
- Favoreto M.W.; Carneiro T.S.; Forville H.; Burey A.; Simas F.D.; Loguercio A.D.; Reis A. Use of calcium-containing bioactive desensitizers in dental bleaching: A systematic review and meta-analysis. J Am Dent Assoc 2023, 154:245-259 e212. [CrossRef]
- Rezende M.; Coppla F.M.; Chemin K.; Chibinski A.C.; Loguercio A.D.; Reis A. Tooth Sensitivity After Dental Bleaching With a Desensitizer-containing and a Desensitizer-free Bleaching Gel: A Systematic Review and Meta-analysis. Oper Dent 2019, 44:E58-E74. [CrossRef]
- Vilela A.P.; Rezende M.; Terra R.M.O.; da Silva K.L.; Sutil E.; Calixto A.L.; Reis A.; Loguercio A.D.; Farago P.V. Effect of topical application of nanoencapsulated eugenol on dental sensitivity reduction after in-office dental bleaching: a randomized, triple-blind clinical trial. J Esthet Restor Dent 2021, 33:660-667. [CrossRef]
- Burey A., Sutil E.; Nunez M.A; Mendez-Bauer M.L.; Rezende M.; Reis A.; Gomes O.M.M.; Farago P.V.; Loguercio A.D. Assessm;nt of the effect of experimental bleaching agent with nano-bioactive material on postoperative sensitivity: A randomized, triple blind clinical trial. J Esthet Restor Dent 2021, 33:764-774. [CrossRef]
- Pintado-Palomino K.; Peitl Filho O.; Zanotto E.D.; Tirapelli C. A clinical, randomized, controlled study on the use of desensitizing agents during tooth bleaching. J Dent 2015, 43:1099-1105. [CrossRef]
- Nanjundasetty J.K.; Ashrafulla M. Efficacy of desensitizing agents on postoperative sensitivity following an in-office vital tooth bleaching: A randomized controlled clinical trial. J Conserv Dent 2016, 19:207-211. [CrossRef]
- de Castro Oliveira L.; Marchetti V.M.; de Souza E.S.R.F.; Delbem A.C.B.; Souza M.T.; Ganss B.; Theodoro L.H.; Fagundes T.C. In vitro dentin permeability and tubule occlusion of experimental in-office desensitizing materials. Clin Oral Investig 2023, 27:1265-1276. [CrossRef]
- Palé M.; Mayoral J.R.; Llopis J.; Vallès M.; Basilio J.; Roig M. Evaluation of the effectiveness of an in-office bleaching system and the effect of potassium nitrate as a desensitizing agent. Odontology 2014, 102:203-210. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).