1. Introduction
First time, the term listwaenites (known also as listvenites) was used by Rose [
1] to describe gold-bearing carbonate-silicate metasomatic rocks which outcrop in the Listvenya, Ural region. In general, rocks that have undergone alteration processes such carbonation, silicification, pyritization, and serpentinization of ultramafic rocks are referred to be listwaenites [
2,
3]. Listwaenites are associated with the majority of ophiolite massifs worldwide, and mainly occur along thrust fault and discontinuities that act as conduits for metasomatic fluids rich in CO2-, Ca and Si [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14].
In ultramafic rocks, mantle olivine and pyroxene are unstable near the surface and change to Listwaenites in the presence of CO2-rich fluids in the temperature range of 80 to 400 °C e.g., [
15,
16]. However, Falk and Kelemen [
17] estimated the temperature formation of Oman’s listwaenites at 80 to 130°C. The majority of earlier research indicates that many alteration stages along tectonic contact zones are typically linked to the transformation of ultramafic rocks from ophiolites to listwaenites. A second stage of metasomatic overprinting of serpentinites by carbonate and silica occurs after the first stage of metasomatic serpentinization of ultramafic rocks [
2,
17,
18,
19]. Solid carbonate and silica are the end products of mineral reactions with CO2 and water. The product of mineral reactions with CO
2 and water are solid carbonate and silica by the following reactions:
4Mg2SiO4 (Olivine) + CaMgSi2O6 (pyroxene) + 6H2O + CO2 = 3Mg3Si2O5(OH)4 (serpentine)+ CaCO3 (calcite)
This reaction often takes place in stages, e.g.,
4Mg2SiO4 + CaMgSi2O6 + 7H2O = 3Mg3Si2O5(OH)4 + Ca2+(aq) + 2OH-(aq)
in the subsurface, and then the reaction;
Ca2+(aq) + 2OH-(aq) + CO2 (aq or gas) = CaCO3 + H2O
depending on the pressure and temperature circumstances under which the reaction occurs, CO2 (g) represents CO2 in either gas or supercritical fluid form [
20,
21,
22].
The Semail ophiolite in Oman and the Neyriz ophiolite in Iran are remnants of the Tethyan oceanic lithosphere obducted tectonically on Oman and Iran toward the end of the Cretaceous, e [
23,
24,
25,
26]. Listwaenites from various regions of the Semail ophiolite in Oman have been the subject of numerous research (2,17,27-28]. Several studies proposed that the Listwaenite of Oman is a product of post-obductional extension e.g., [
2,
19], while Falk and Kelemen, [
17] proposed that listwaenite was formed during Late Cretaceous ophiolite obduction and ophiolite emplacement in the mantle wedge (95-75 Ma).
Neyriz, Kermanshah, Kurdistan, and Azerbaijani are the four regions where the Zagros ophiolite of Iran outcrops [
26,
29]. An important area for studying listwaenites and comprehending their geochemical properties in various geological contexts is the Islam Abad area which is primarily linked to the Neyriz ophiolites in Iran. In contrast to those in Oman, where considerable Listwaenites are mostly found in the Fenja region, the listwaenites in these ophiolites in Iran have not been as thoroughly explored e.g., [
25,
26].
Listwaenite rocks can provide insight into processes of carbon fluxes at the “leading edge of the mantle wedge” in subduction zones and enhanced mineral carbonation of peridotite. The prevalence of listwaenites in Oman and Iran underscores the geological complexity of these regions, offering valuable insights into their tectonic history. This paper aims to provide a comprehensive overview of the geochemistry and petrography of listwaenites in the two prominent regions of Fenja area in Oman and Islam Abad area in Iran. These areas are known for their extensive ophiolite complexes, which host listwaenites occurrences.
2. Methodology
100. samples were collected from the outcropping listwaenites and associated harzburgite and serpentinite in both Fanja area in Oman and Islam Abad in Iran. A representative set of 12 Listwaenite samples with different degrees of deformation were selected for analysis from Islam Abad area in Iran, 13 Listwaenite samples, 4 serpentinized harzburgite and 4 serpentinite samples were selected from the Fenja area in Oman. Cleaned rock chips from homogeneous sample parts were crushed in an agate mill after washing in distilled water and drying, excluding weathered rims and thicker veins. Major and trace element analysis were carried out using X-Ray fluorescence spectrometry (XRF) and ICP-OES at the ALS Albirq commercial Laboratories in Saudi Arabia. Sample powders were dissolved in a HCl-HNO3-HF mixture and then analyzed for major and trace elements with an Agilent 7500 inductively coupled plasma mass spectrometer (ICP-MS) then evaporated to dryness. The cycle of acid and drying was repeated 3 or 4 times. HClO4 was added during the evaporation stage to insure dissolution. Millipore-filtered distilled water was then added and spiked with 100 ppm Ag and Ta. Three aliquots of the spiked solution were analyzed using the ICP-OES. Multi-element USGS standard were used to calibrate the ICP-OES. Precision for major and minor elements content is estimated to be in the order of 2-5% for major elements and 5 % deviation from true values for trace elements. Analysis quality control and detection limits are given in Supplementary File S-1.
3. Field Description and Geological Setting
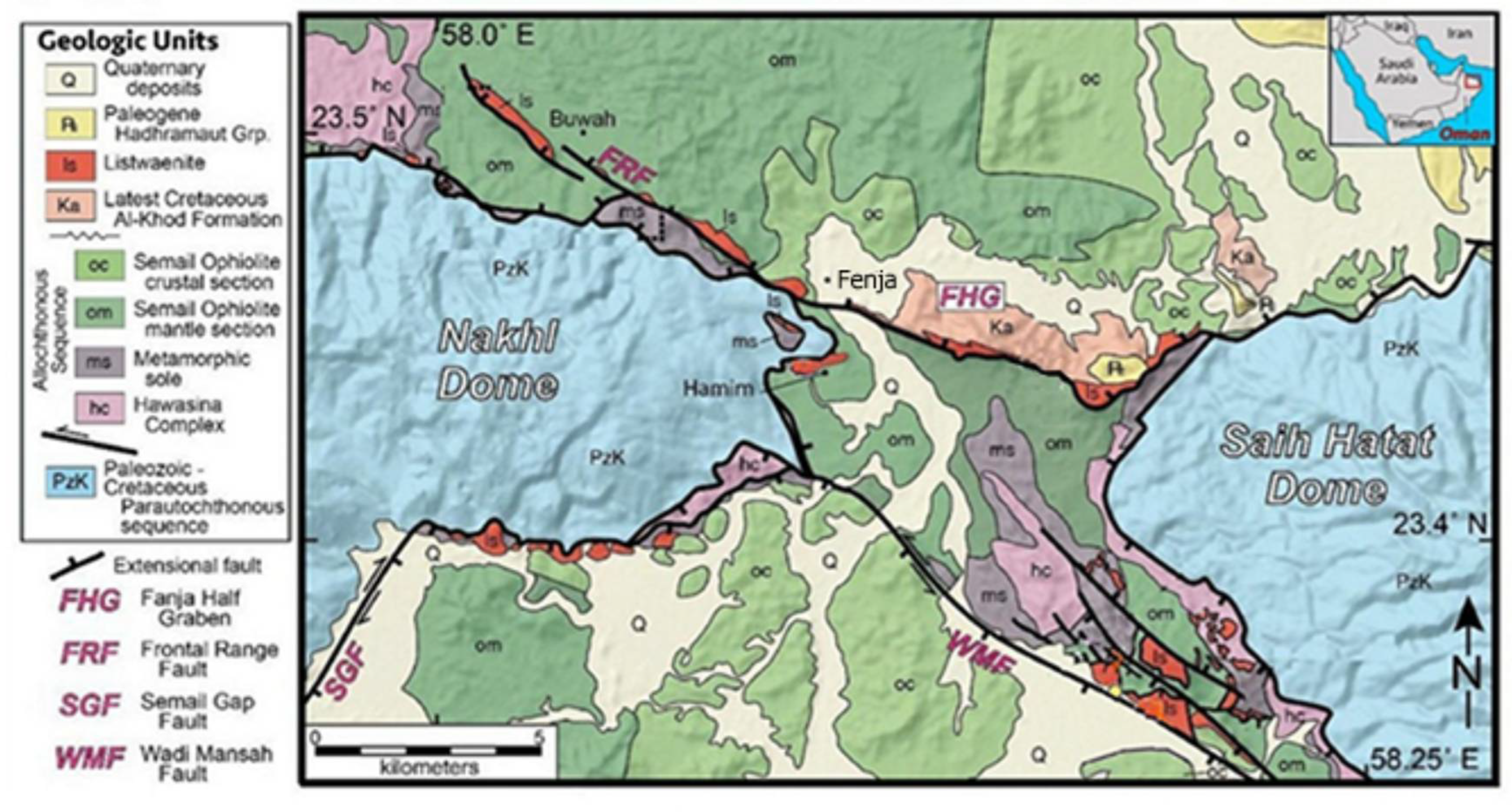
3.1. Fenja Area-Oman
Listwaenites in Fenja area are often found within the mantle harzburgites and serpentinites unites, mainly within the basal thrust of the ophiolite section above the underlying metamorphic sole. The majority of listwaenites are found along the frontal range fault and along the Wadi Mansah extensional faults (
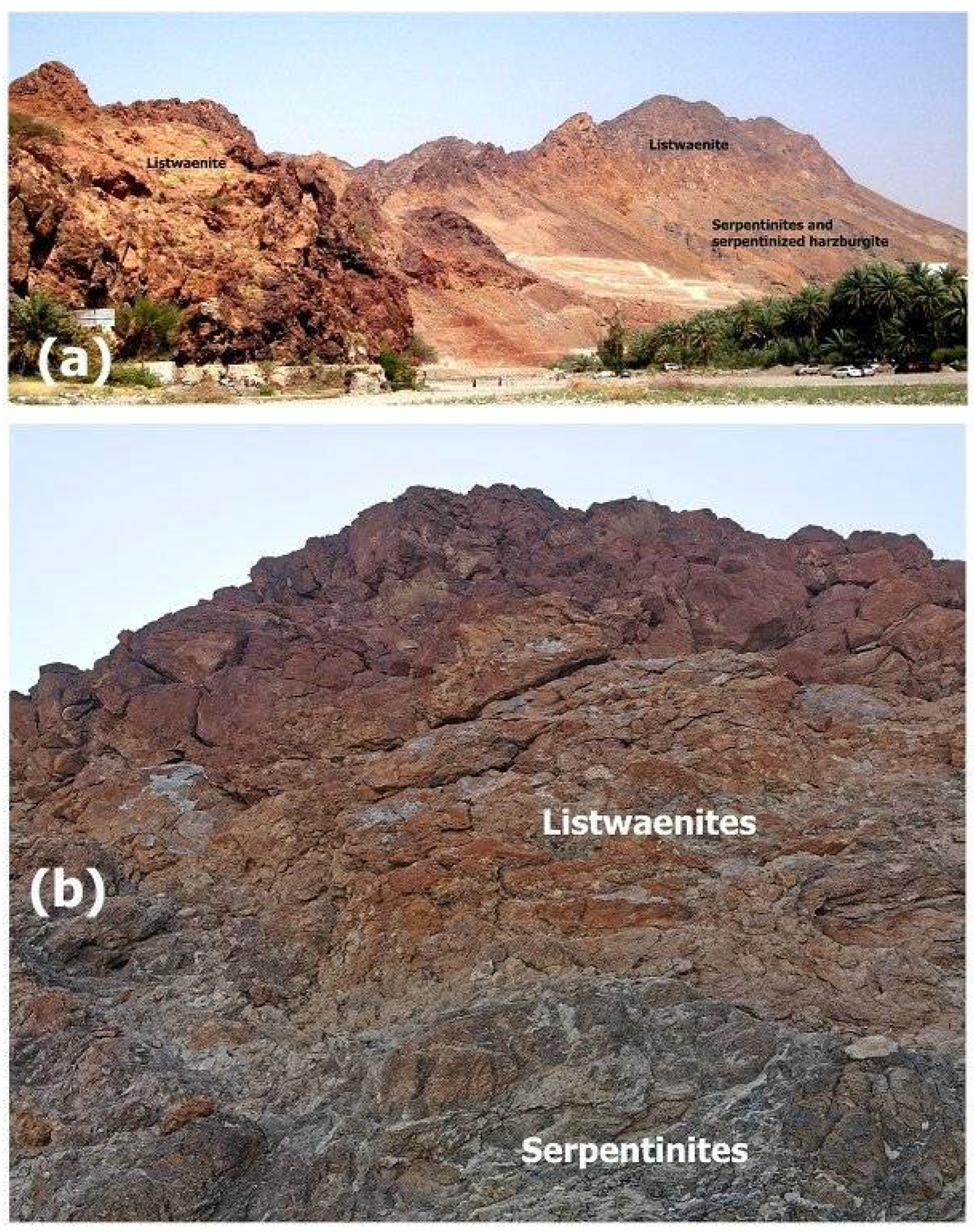
Figure 1). The listwaenites occur as a suite of WNW and NW- striking bodies that vary in thickness from 1 to 80 m and length from a few kilometers. The outcrops are more resistant to weathering than the harzburgite and serpentinite host rocks because of their yellow to dark reddish brown-, grey-, and orange-colored ridges (
Figure 2a). Listwaenite gradually transforms into serpentinite and serpentinized harzburgite in the field (
Figure 2b). Locally, the degree of alteration varies from slight stockwork and quartz-carbonate veining to substantially replaced carbonated and silicified rocks. Near the interface with the serpentinized harzburgites and serpentinites, the listwaenites are typically veined, brecciated, and severely broken. At the borders between the clast and matrix, individual clasts exhibit signs of repeated brecciation and re-cementation, such as the termination of clast-hosted veins. Upper mantle rocks in the area comprised of varying serpentinized harzburgite, serpentinite, pyroxenite dykes, subordinate dunite lenses. In the least serpentinized areas, common olivine remnants and extremely uncommon primary pyroxene are maintained. Serpentinization is often moderate to intense (80–90%). In most of the serpentinite samples under study, lizardite and chrysotile predominate as serpentine minerals.
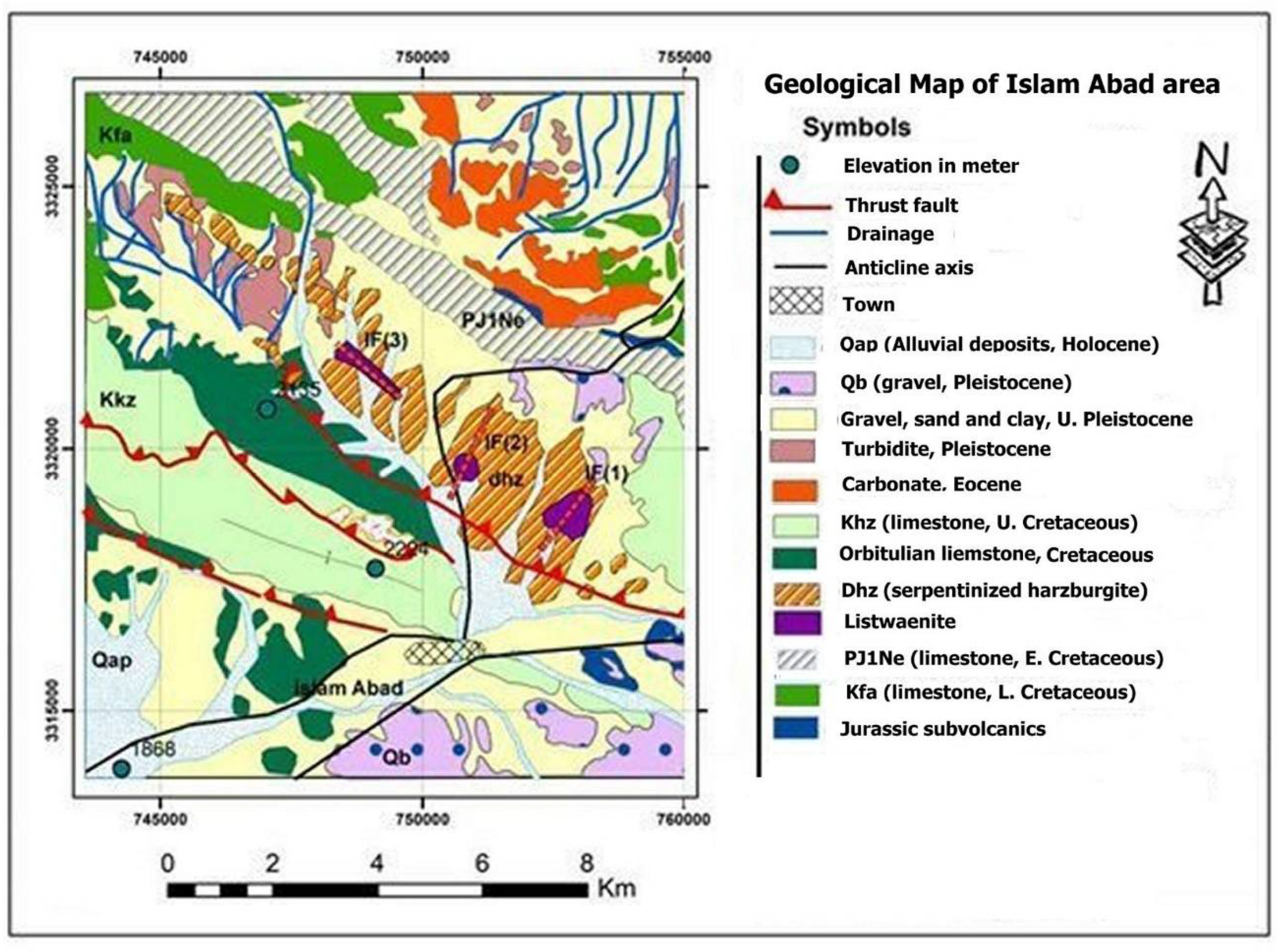
3.2. Islam Abad- Iran
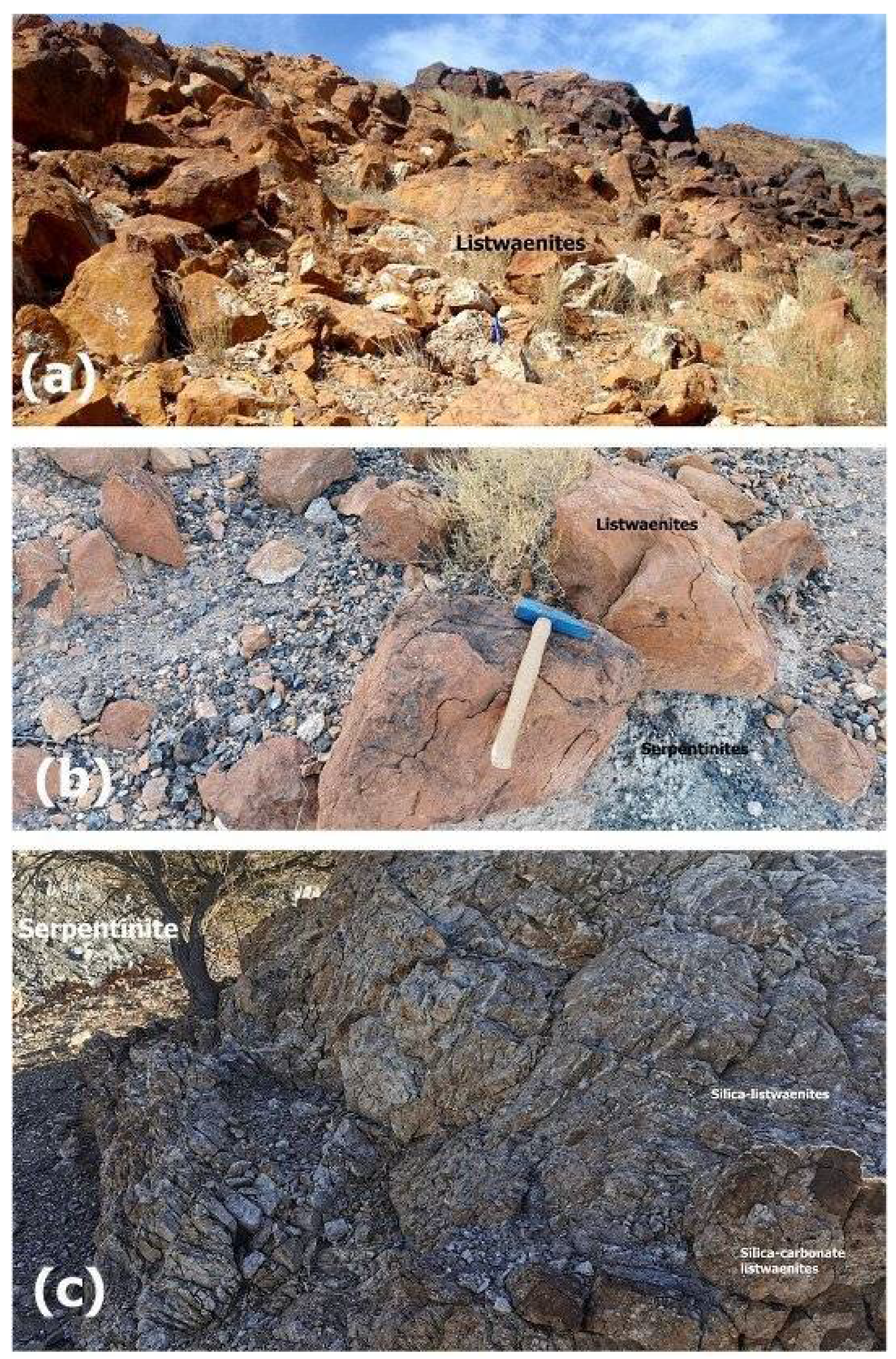
The Listwaenite is located near Islam Abad area, within the Neyriz ophiolite of Iran, close to Islam Abad city (
Figure 3). In this region, listwaenites are frequently found in conjunction with serpentinite and harzburgite that has undergone serpentinization. The Neyriz ophiolites in the region comprised of deep see sedimentary strata (radiolarian cherts) and the entire suite of ultramafic rocks (harzburgite, dunite, and pyroxenite) [
31,
32]. According to Monsef et al. [
33], the Neyriz ophiolite is geotectonically a component of the Iranian Zagros suture zone, which is the most deformed sector of the continuous collision between the Iranian and Arabian continents. Listwaenites are of variable-size, with erosion-resistant morphological peaks that primarily take the form of abrupt, sheet-like bodies that are 1-2 km broad and 5-20 m thick. The contact between carbonated serpentinite and the neighboring serpentinite occurs as a zone of increasing transition (
Figure 4a to c). A thin layer of harzburgite-rich alteration resembling gossan is present in the majority of instances. There are numerous secondary carbonate minerals (dolomite, magnesite, and calcite) and quartz veinlets in listwaenite rocks, which are fine-to- course- and macroscopically heterogeneous with frequent color transitions from dark red to orange, yellow greenish grey to pale grey on a centimeter to meter scale. Along the boundary between serpentines and listwaenites, stockwork structure is widespread, providing evidence for fluid passage and alteration activities (
Figure 4c). According to field investigations, Islam Abad listwaenites are connected to thrust fault system zones (
Figure 3). Three faults (IF1, IF2, IF3) have been found north of Islam Abad. Apart from sporadic little patches that are still evident inside the serpentinite bodies, ultramafic lithologies that outcrop near listwaenites have nearly entirely changed into serpentinite, as the transformation of the primary minerals into mineral assemblages characterized by opaque mineral phases and serpentine mineral groups is the result of the serpentinization of the ultramafic host rocks. Within serpentinite, many veins of calcite, dolomite, and serpentine intertwine to create a box-like structure.
4. Petrography
Listwaenites in Islam Abad and Fanja area show variable mineralogy with minerals commonly present include calcite, dolomite, magnesite, quartz, Cr-mica, serpentine, hematite, goethite, magnetite, and Cr-spinel. The results of optical microscopy led to distinguishing three types of listwaenites in both Fenja and Islam Abad areas, namely: silica-listwaenites, silica-carbonate-listwaenites and carbonate-listwaenites (
Figure 5 and
Figure 6), which are similar to listwaenites types occurring in other ophiolites worldwide [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11].
4.1. Carbonate Listwaenites
In the Fenja area in Oman, carbonate listwaenites are the most common types of listwaenites. Calcite, a small amount of quartz, goethite and hematite, serpentine, tiny chromite grains, and a few flakes of fuchsite make up the majority of this listwaenites. There are also a few veinlets of colloform magnesite and dolomite. Dolomite typically occurs in aggregate form and has textures ranging from unhedral to subhedral grains. Within the matrix, hematite is the alteration result of magnetite. Sometimes the oxide string distribution in listwaenites is similar to the distribution usually found in serpentine mesh (or hourglass) texture (
Figure 7a-b). The predominant minerals found in the transition zone between serpentinites and carbonate listwaenites include dolomite, lizardite, chrysotile, and a minor quantity of brown Cr-spinel. Several samples of this Listwaenite exhibit both macro- and micro- sized voids, with diameters ranging from a few microns to 60 μm. These cavities in the carbonate listwaenites give strong textural evidence for the alteration of less capable minerals that follows percolating fluids and early alteration-dissolution. The carbonate listwaenite from Islam Abad show coarse-grained calcite and several micro veins filled with iron hydroxides and relict Cr-spinel (
Figure 7c-d). The skeletal shape of brown Cr-spinel is unhedral to subhedral.
4.2. The Silica-Carbonate
In comparison to the other two listwaenites types, the silica-carbonate Listwaenite is less common. It is made up of dolomite, magnesite, calcite, traces of K-bearing minerals (fuchsite), quartz, and remnants of chrome spinel (
Figure 8a-b). The pseudomorphic mesh texture found in the protolith of harzburgite is still present. Samples from Fenja area consists mainly of fine to medium grained magnesite, Cr-spinel and several veins filled by quartz. The listwaenites exhibit a gradual carbonate replacement of the remaining serpentine. In comparison, the Listwaenites from Islam Abad consists mainly of coarse-grained dolomite, magnesite and quartz. Dolomite occurs as euhedral rhombic grains replacing serpentine minerals (
Figure 8c-d). Quartz grains usually replace dolomite rhombs.
4.3. Silica-Listvenites
This Listwaenite is found usually close to the transition zones between the Listwaenite bodies and the neighboring serpentinites. The Listwaenite from both Fenaj and Islam Abad shows similar petrographic features (
Figure 9 a-d). It is mainly formed of quartz occurring in two generations. The first generation is primarily made up of opal, chalcedony, cryptocrystalline quartz, serpentine, and remnants of magnetite and chrome spinels. The second generation of silica Listwaenite crosses over into the first generation and is nearly entirely devoid of oxide minerals. It features polycrystalline mosaic, columnar impingement, and comb textures. Quartz occurs as a groundmass that is crosscut polycrystalline and or amorphous quartz veins. The silica Listwaenite are usually brecciated and are porous. Most likely, the porosity indicates carbonate dissolution around the rims of the mesh.
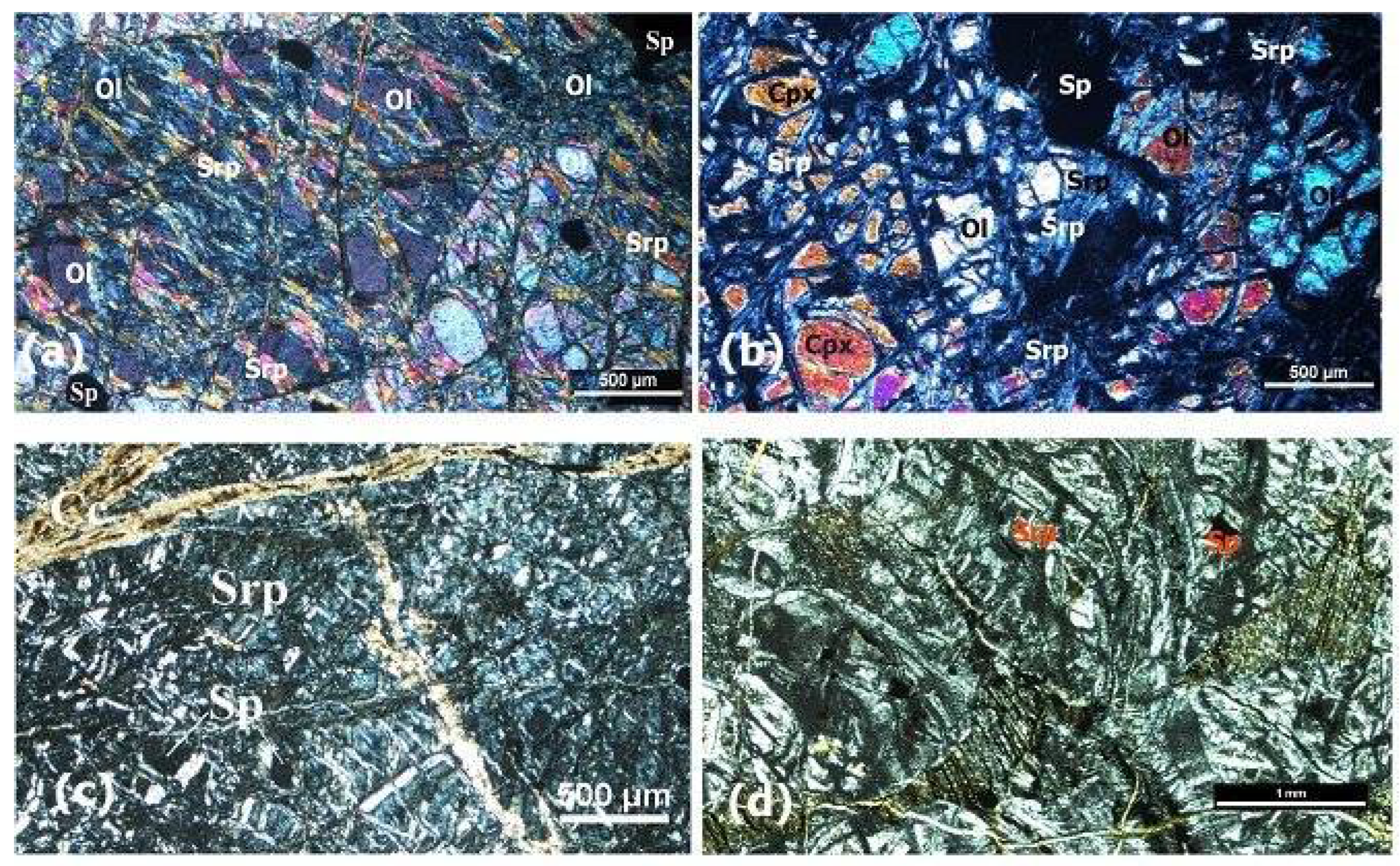
4.4. Serpentinized Harzburgites and Serpentinites
Serpentinized harzburgites are medium- to coarse-grained and are variably serpentinized (~30-40 vol%). Crystals of olivine that have been transformed into lizardite and chrysotile are globular to spherical when broken (
Figure 10-a-b). Serpentines exhibit mesh texture and mostly replace olivine and pyroxene in fractures and along cleavage lines (
Figure 10 c-d). Most serpentines are chrysotile and lizardite. There are isolated grains of chromite and Cr-spinel that are brecciating and, in certain places, have serpentine veins running through them. Mostly, hematite forms as tiny grains inside of fractures. In heavily serpentinized domains, magnetite typically develops along serpentine veins or on mesh rims. Kink band texture, a sign of tectonic stress, can be seen in certain serpentine minerals in these rocks. Little stages of chromite are seen, containing massive euhedral to anhedral crystals. A small amount of carbonate can be found in the serpentine matrix as spheroidal grains and veins filling Often, carbonate veins converge with or diverge into preceding serpentine veins.
5. Geochemistry
Table 1 presents the chemical analyses of serpentinized harzburgite, serpentinite, and listwaenites from Fenaj ophiolite in Oman.
Table 2. Shows the chemical analysis of listwaenites from Islam Abad area-Neyriz ophiolite in Iran. The averages of different types of listwaenites from Fenja and from Wadi Mansah in Oman [
17] and the average of listwaenites, harzburgites and serpentinite from Birjand ophiolite in eastern Iran [
26]) and Islam Abad area are also provided for comparison in supplementary file S-2.
Table 1 and
Table 2 and major oxide and trace elements variation diagrams (
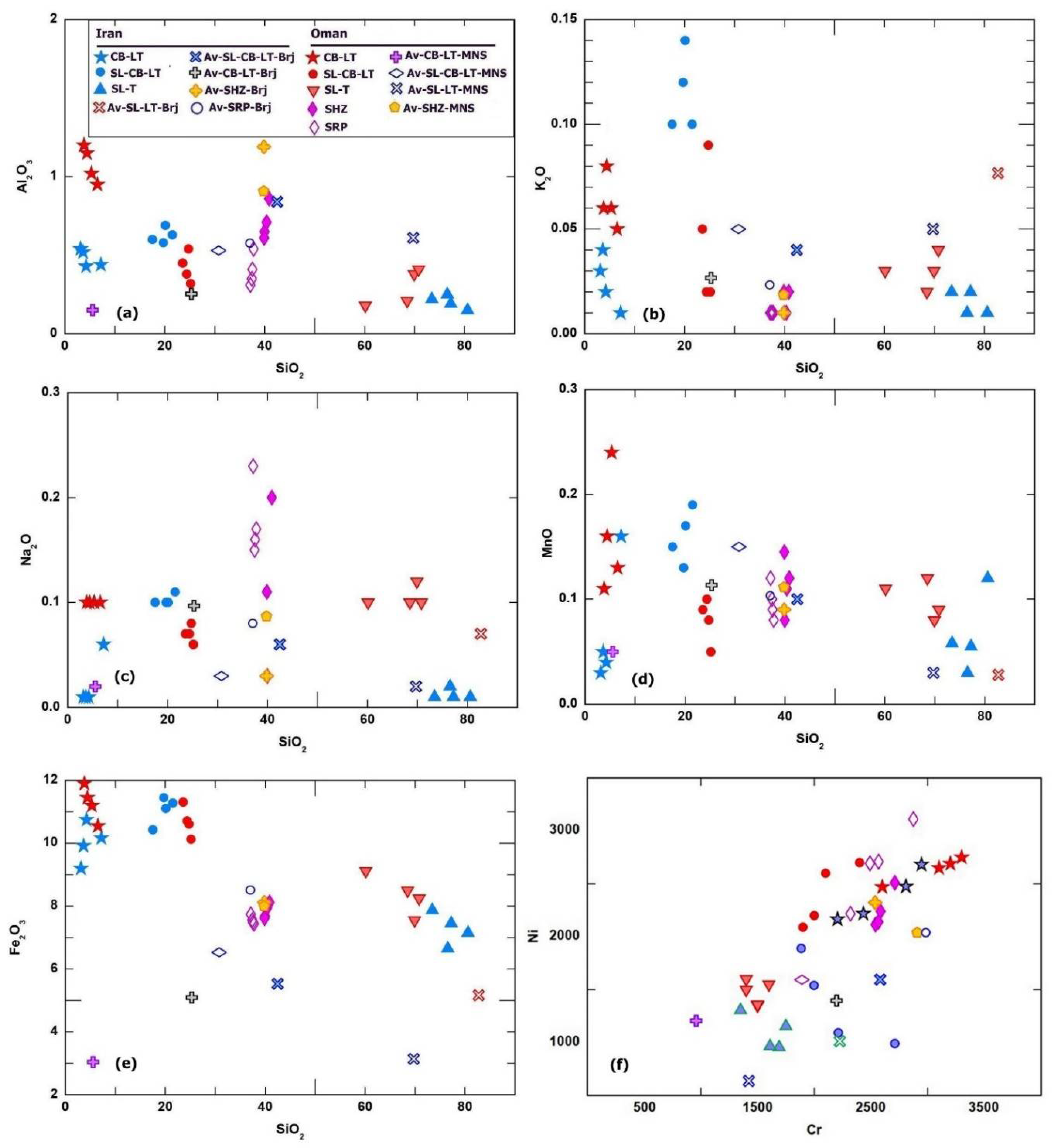
Figure 11a-f and supplementary file S-3) show that the different types of listwaenites from both Iran and Oman have variable major and trace element composition. All listwaenite types in both Oman and Iran have low Al
2O
3 (<1.2 wt.%), K
2O (<0.14 wt.%). Na
2O (<0.12 wt. %) and TiO
2 content (<0.04) (
Figure 11), as well as low high field strength elements incompatible lithophile trace elements and REE. K
2O is higher in the silica-carbonate from Iran compared to those in the silica-carbonate listwaenites from Oman and other two types of silica- and carbonate- listwaenites. The MnO, Na
2O, TiO
2 and Al
2O
3 contents in most samples overlap and compared to those contents in the associated serpentinites and harzburgites (supplementary file 3). Fe
2O
3 content is high (9.2-11.5 wt. %) in the carbonate and carbonate-silica listwaenites, while it is lower in the silica listwaenites and associated ultramafic rocks (6.6-9.1wt.%). The listwaenites from Oman show higher Fe
2O
3 contents than those from Iran. Silica-carbonate listwaenites plot as a distinct group between the field of silica- and carbonate-listwaenites. The three types of listwaenites are characterized by high Ni (1350-2750 ppm), Cr
2O
3 (0.2-0.45 wt. %) and Co (63-115 ppm). which overlaps the composition of host serpentinized harzburgites and serpentinites from the study area (
Table 1-2,
Figure 11f, and supplementary files S-3).
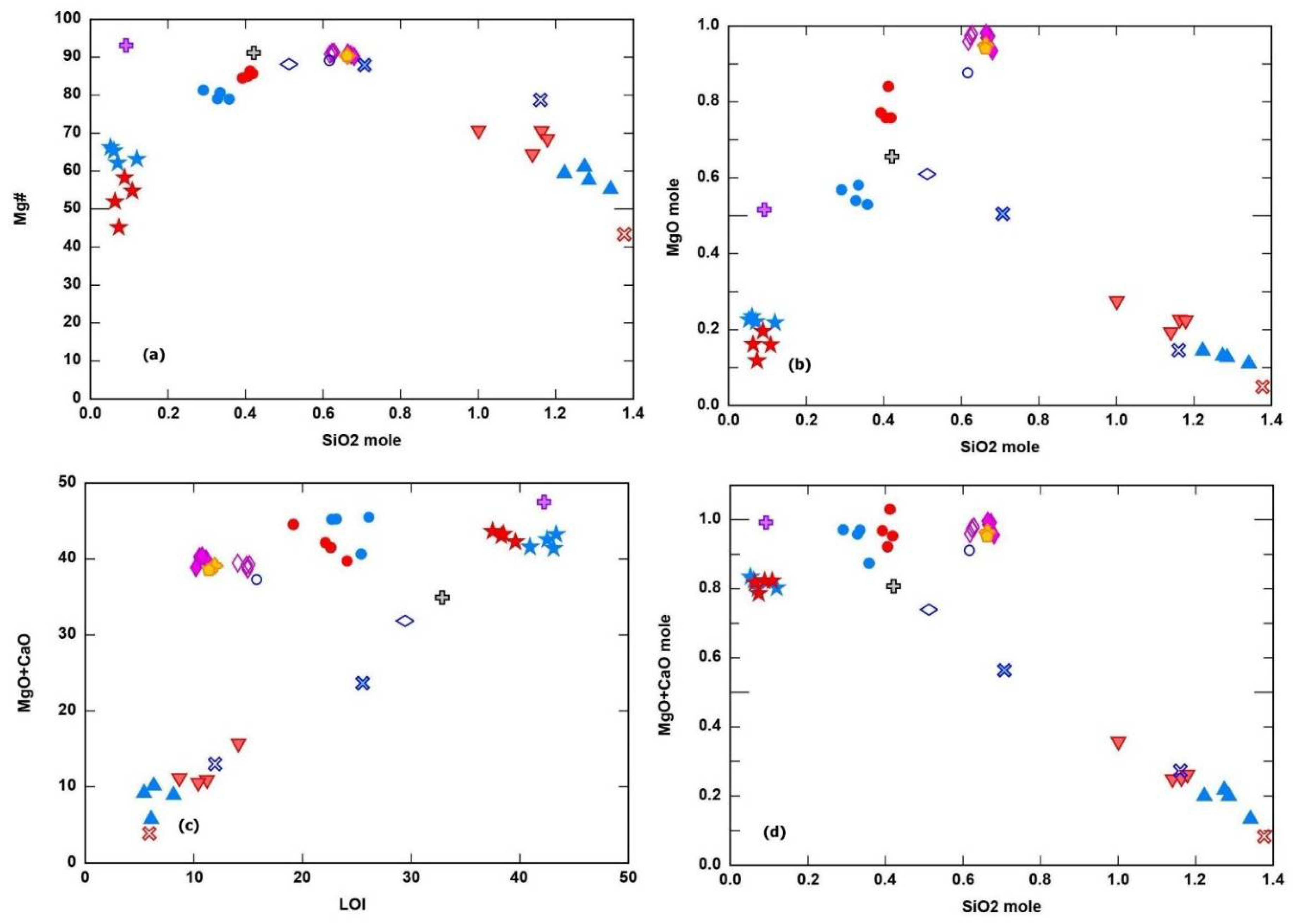
Carbonate- and silica- listwaenites in both Iran and Oman have low MgO (4.5 to 11.5 wt.%), while the carbonate-silica listwaenites from Iran have higher MgO (30.5 to 33.9 wt.%) than those from Oman (21.3 to 23.4 wt.%), but lower than MgO content in the associated serpentinites and harzburgite (35-40 wt.).The MgO + CaO wt.% in the carbonate and carbonate-silica listwaenites overlap the content in the associated serpentinites and harzburgites, while it is lower in the silica-listwaenites (
Figure 12a-d) The SiO
2 content show a wide range from 3 wt.% in the carbonate listwaenites to 80.6 wt.% in the silica listwaenites. The CaO content also shows a wide range from 1.3 wt.% in the silica listwaenites to 37.5 wt. % in the carbonate listwaenites. Higher LOI in carbonate and silica-carbonate listwaenites together with high MgO content in carbonate-silica listwaenites, is consistent with the presence of magnesite and dolomite as the most abundant carbonate mineral in the carbonate-silica listwaenites, and calcite in the carbonate listwaenites.
Figure 12c shows a clear positive relationship between LOI and the three types of studied listwaenites. Serpentinite compositions overlap and compared to serpentinized harzburgite protolith (
Table 1,
Figure 12a-d). Molar MgO, CaO and Mg# versus molar SiO
2 diagrams show similar variations of these oxides in the analyzed lithologies (
Figure 12b, d).
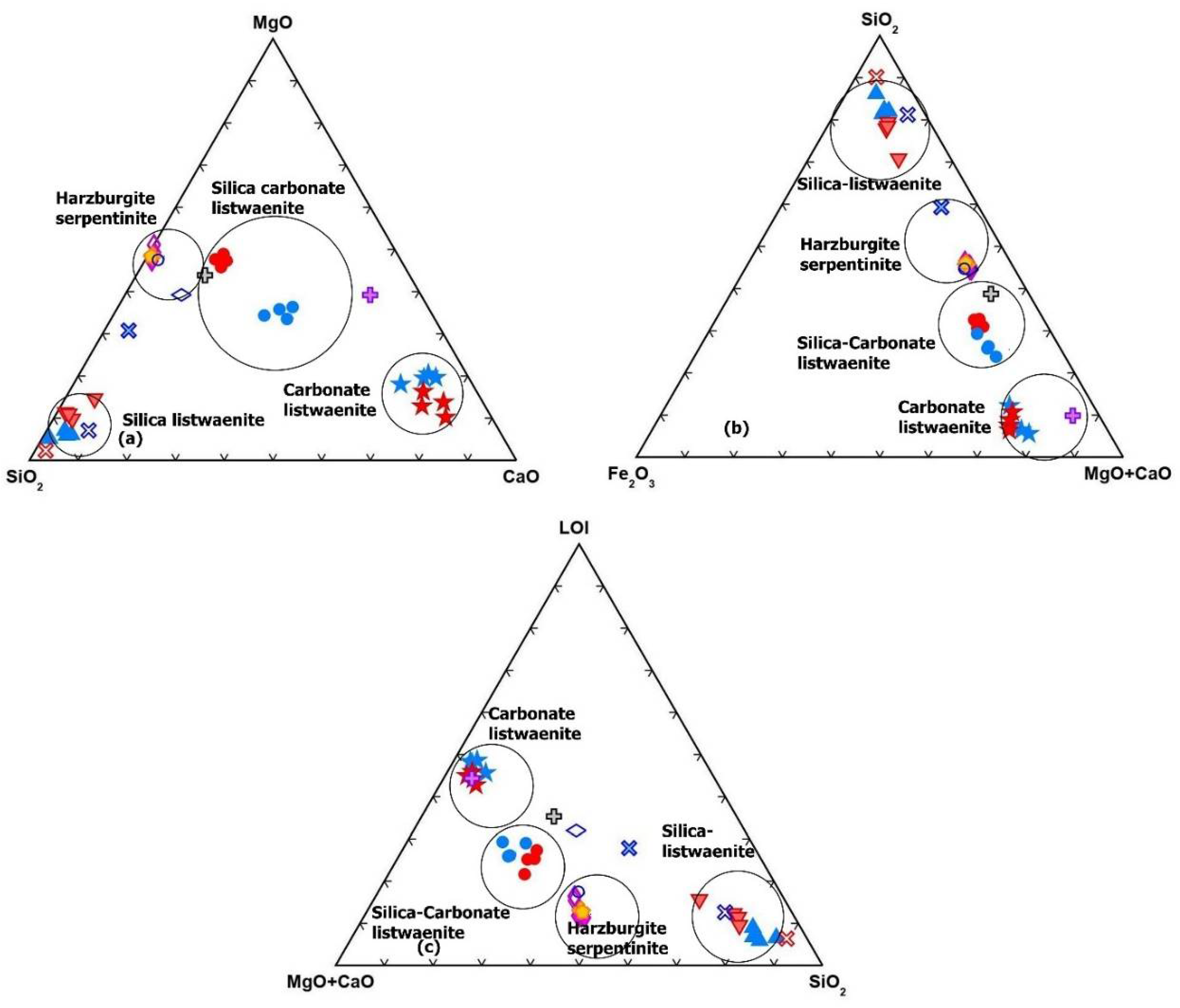
Triangular diagrams MgO–CaO–SiO
2 (
Figure 13a), SiO
2-Fe
2O3-CaO + Mg (
Figure 13b), MgO + CaO–LOI-SiO
2 (
Figure 13a-c) show that the Listwaenite samples can be classified into the three categories described above: silica-, carbonate-, and silica-carbonate listwaenites. Partially serpentinized harzburgites and serpentinites show comparable compositions and plot close to the center of MgO -SiO
2, MgO+CaO-SiO
2 and MgO + CaO-SiO
2-LOI tie lines (
Figure 13a-c).
Listwaenites have higher compositional variability, with the three Listwaenite fields mostly governed by changeable MgO + CaO-SiO2 and LOI, setting them apart from partially serpentinized harzburgites and serpentinites.
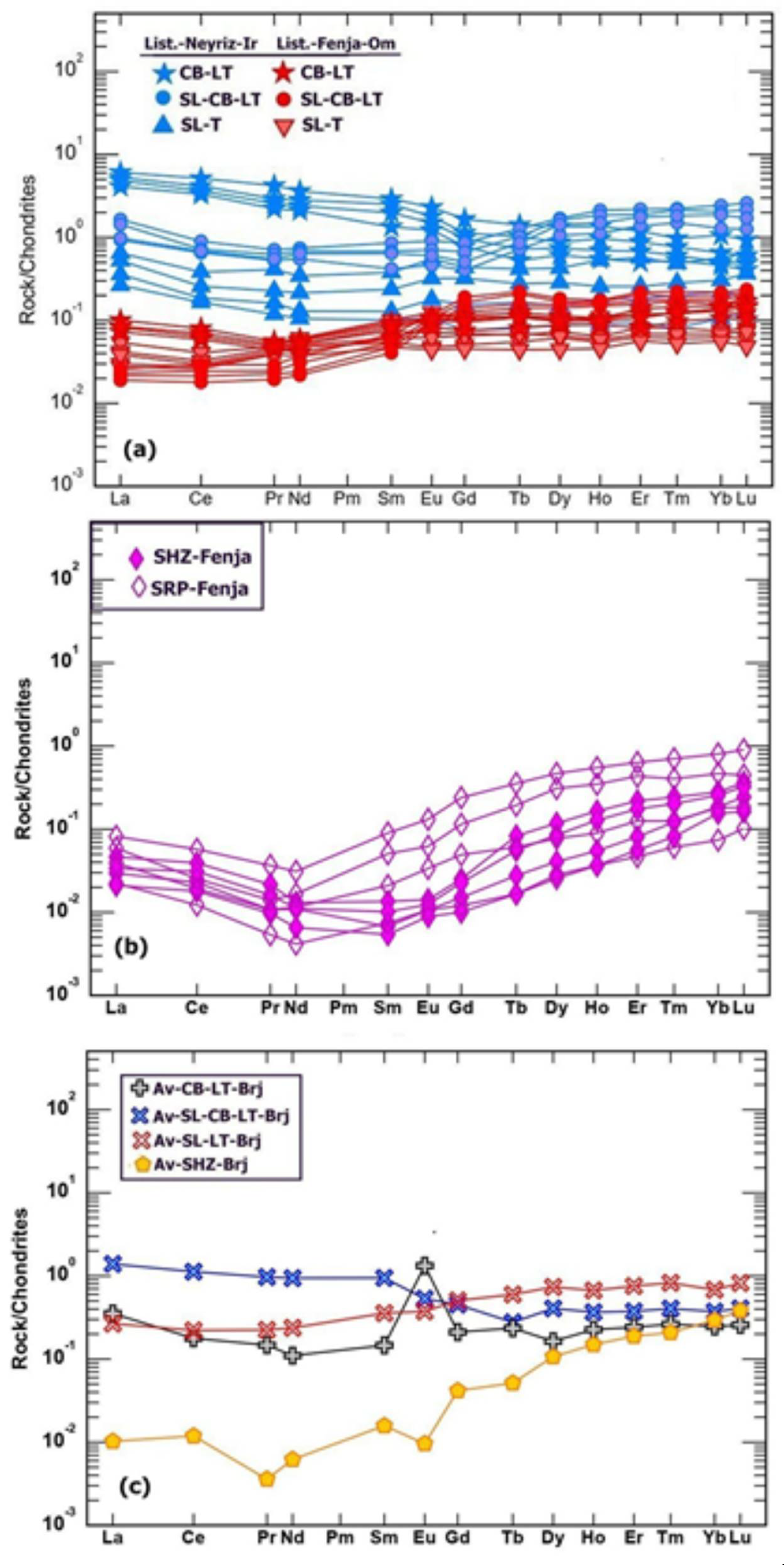
Figure 14a-b display the chondrite-normalized REE patterns for listwaenites, serpentinites, and harzburgites from Oman and Iran. All the examined lithologies have relatively similar rare earth element patterns, showing flat REE patterns that are comparable to those of related serpentinized harzburgite and serpentinite, which have a spoon pattern with lower LREE (Figure.). The carbonate listwaenites from Birgand ophiolite of Iran show a positive Eu anomaly (
Figure 14-c), whereas the serpentinite shows a negative anomaly, while the silica and silica-carbonate listwaenites show similar pattern to those from Oman and Islam Abad ophiolite. The REE contents of Iranian listwaenites are higher than those of Oman. All Listwaenite samples, however, have extremely low concentrations of rare earth elements; for example, the average REE total for listwaenites from Iran and Oman is 2.21 ppm and 1.82 ppm, respectively, while for serpentinites it is 0.26 and 0.27 ppm, and for serpentinized harzburgite from Iran and Oman it is 0.15 and 0.13 ppm respectively.
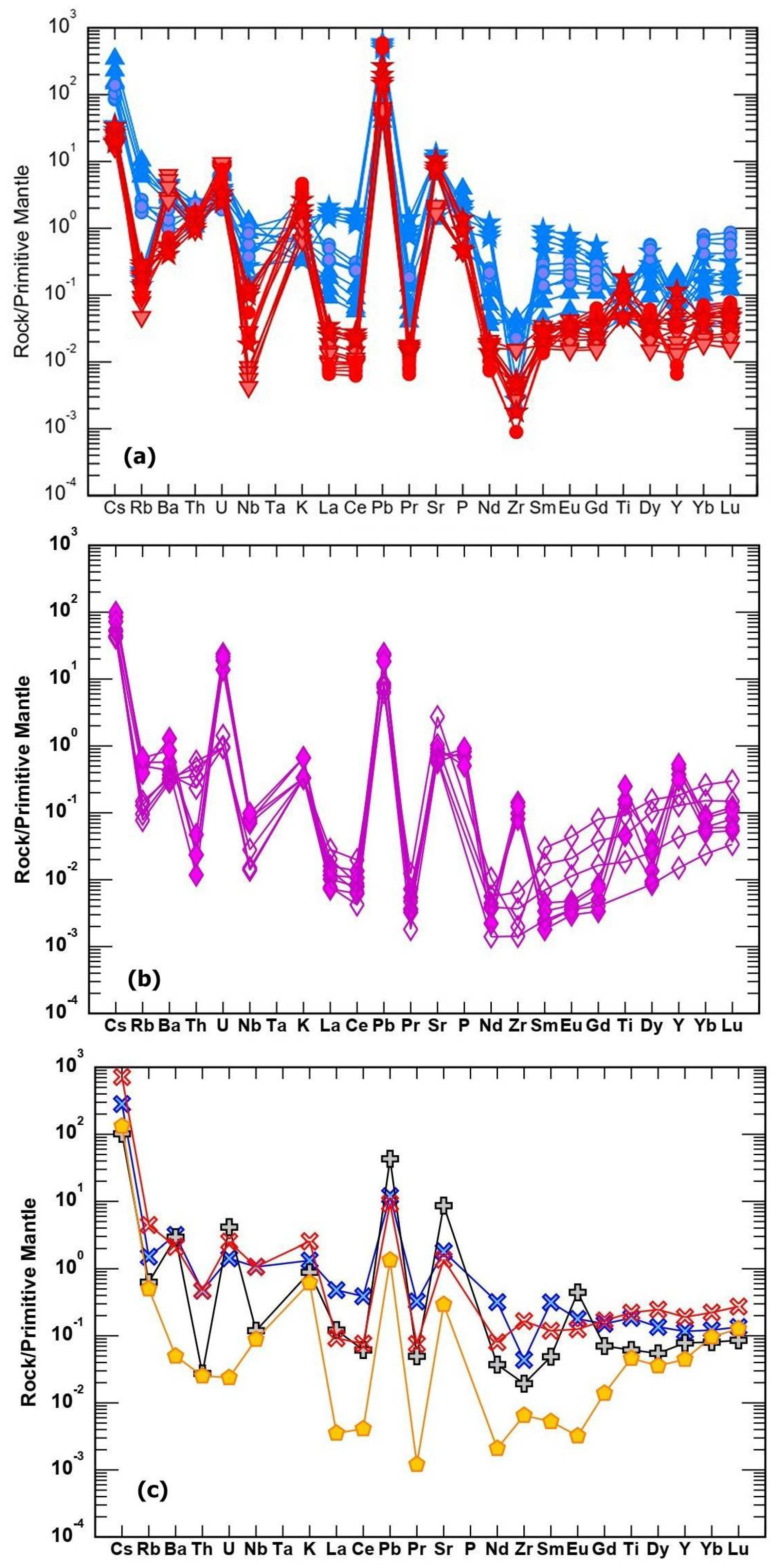
Figure 15a-c show-normalized diagrams of REE and trace elements in the studied lithologies. Compared to the harzburgite and serpentinite protolith, listwaenite lithologies from Islam Abad and Birjand ophiolites in Iran and Fanja in Oman show similar pattern but exhibit higher PM-normalized abundances (~5 to 900× PM) for Cs, U, K, Pb, and Sr (
Figure 15a, b). The trace element contents of Iranian listwaenites are somewhat higher than those of Oman. In partially serpentinized harzburgite and serpentinites, PM-normalized patterns clearly demonstrate enrichment (~5 to ~100× PM) in Cs, Ba, U, Pb, and Sr, whereas other trace element abundances are within or below PM values.
6. Discussion
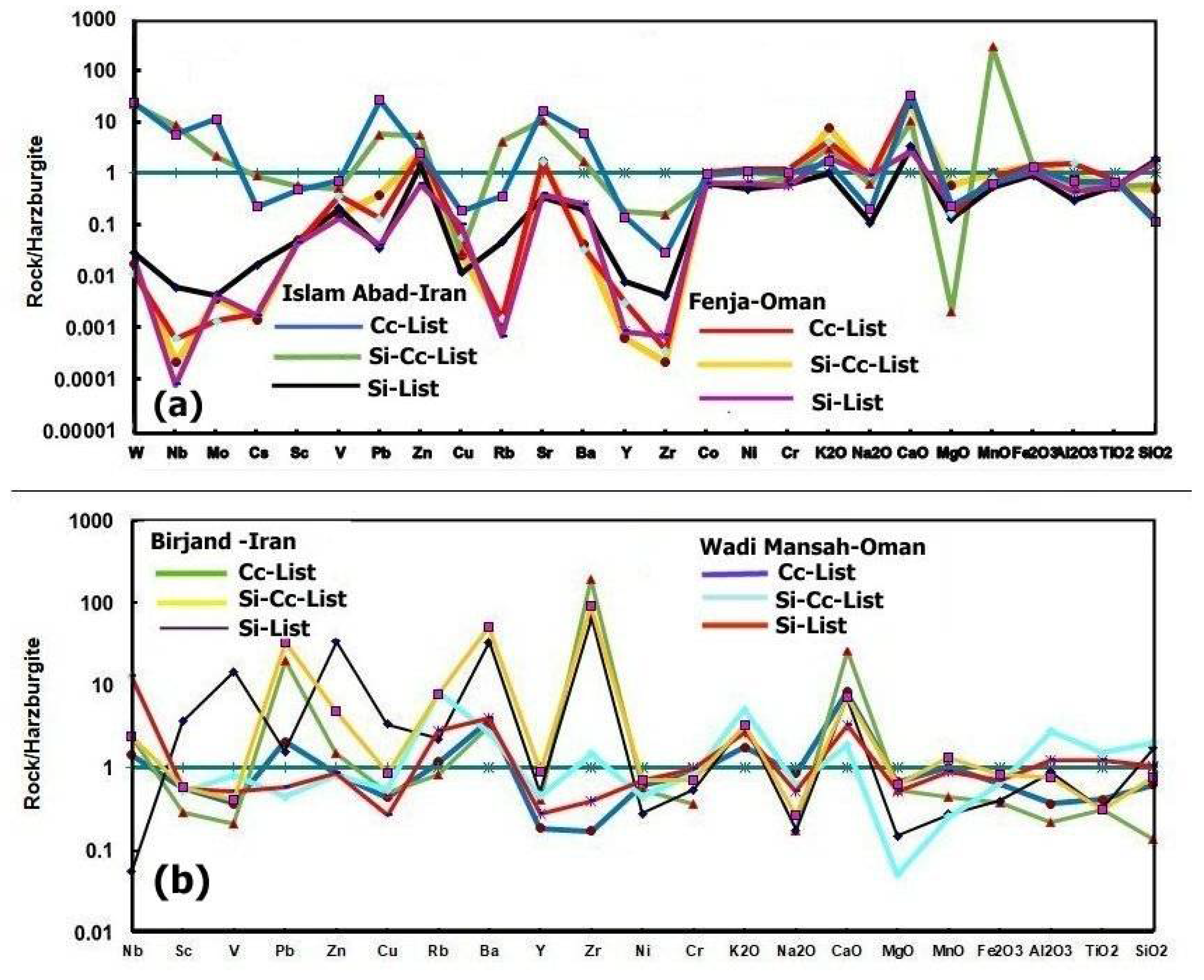
6.1. Element Mobility
The major, trace element, and REE harzburgite-normalized patterns in listwaenites generally correspond to the average harzburgite composition, with the exception of slight to moderate loss in SiO
2, TiO
2, Al
2O
3, MnO, MgO, Cu, V, and Nb, and high gains in CaO, K
2O, Ba, Zr, Sr, Pb, Zn, Mo, and W in the carbonate- and carbonate-silica listwaenites (
Figure 16). The levels of Cr, Ni, and Co are comparable to those found in harzburgites (
Figure 11f,
Table 1). By contrast, the silica listwaenites exhibit moderate to high losses in MgO, Na
2O, Zr, Y, Ba, Sr, Rb, Cu, Pb, Sc, Cs, Nb, Mo, and W, and low to moderate gains in SiO
2 and CaO. These differences could result from the hydrating fluids’ properties or could be inherited from the effects of serpentinization, carbonation, and silicification on the harzburgite protolith’s composition, which can cause these elements to be gained or lost before complete alteration. The harzburgite protolith may have interacted with fluids derived from sediment, as suggested by the observed enrichments in Ca and Sr [
39,
40,
41].
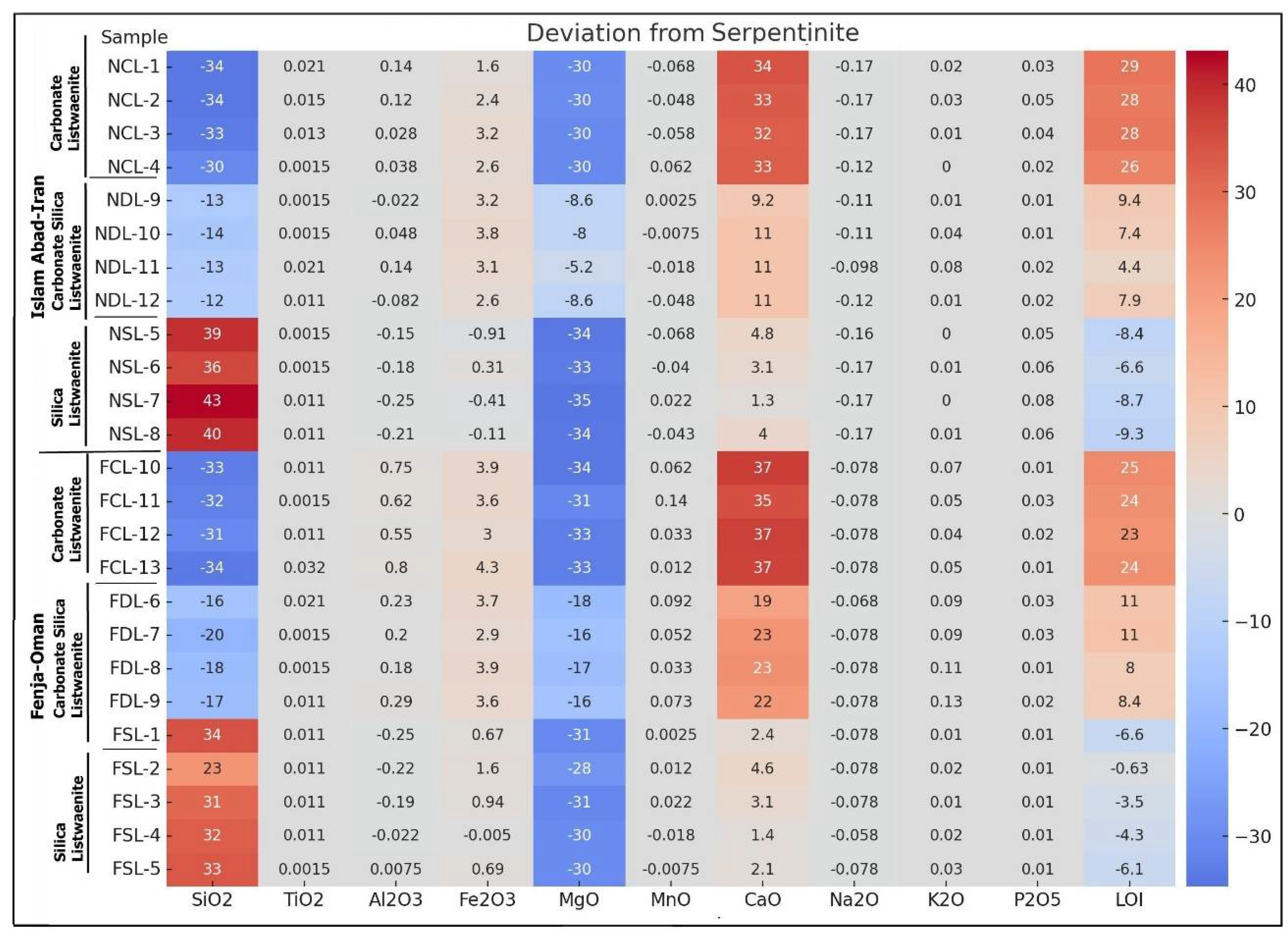
Figure 17a shows that the elements Ti, Al, Fe, Mn, Na, K and P are not deviated or show very slight deviation from the average serpentinite, while Si show high positive deviations in the silica listwaenites (+23 to +34 in Fenja and +36 to +43 in Islam Abad), but negative deviations (-12 to -34) in the carbonate and carbonate-silica- listwaenites. Ca and LOI show high positive deviation in the carbonate listwaenite and moderate positive deviation in the carbonate -silica-listwaenite and slight negative deviation in the silica listwaenites.
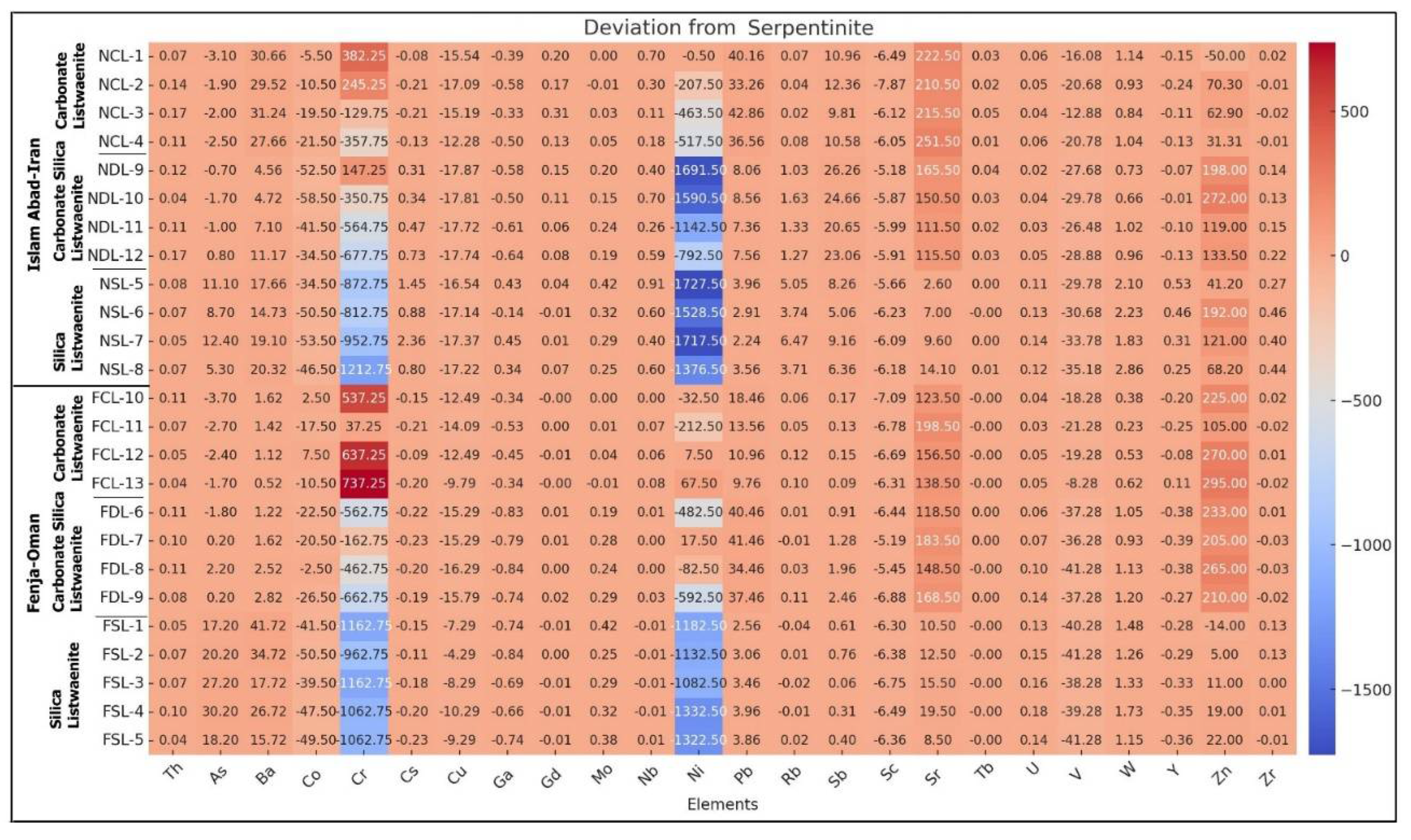
Figure 17b shows that Cr is more enriched in the Oman listwaenite, while Ni is more enriched in the Iranian listwaenite. Sr and Zn show moderate enrichment in the listwaenites, while other trace elements show only slight deviation from serpentinite.
The low Mg# and MgO depletion in silica listwaenites is most likely due to mobility of Mg as the Fe content of this type of listwaenites is comparable to that of silica-carbonate and carbonate listvenites (
Figure 12). This may indicate release of Mg from carbonate from the silica-carbonate listwaenites and replacement by Si from a later Si-rich fluid with lower pH. Mg mobility is not obvious in the carbonate-silica listwaenites as these elements show relatively similar concentrations to their harzburgite protolith, thus showing low mobility on the harzburgite-normalized patterns. However, Mg, Si and Ca mobility is obvious in the carbonate- and silica-listwaenites.
6.2. Origin of Listwaenites in Oman and Iran
Whole rock major and trace elements analysis, mineralogy and textural evidence indicate that the protolith of the investigated listwaenites were serpentinized mantle harzburgites and serpentinites, similar to those outcropping elsewhere in the ophiolite in Oman and Iran. In general, the harzburgites underwent an advanced alteration that resulted in their becoming serpentine in the first stage and listwaenites in the second.
The presence of quartz and carbonate-rich listwaenites is thought to be caused by an increase in CO2, CaO, and SiO
2 through hydrothermal fluids, which also alters the associated serpentinites and serpentinized harzburgite (Figue.13). These high values of CaO, MgO, SiO
2, and LOI in the listwaenites are hypothesized to have occurred during hydrothermal alteration. According to Spiridonov [
42] the alteration process comprises the replacement of silicates in ultramafic rocks with carbonate and silica where olivine and pyroxene in the ultramafic rocks donate the bivalent metal cations Fe, Mg. The relative concentration of clinopyroxene in the parent rock and the input of Ca in the hydrothermal fluid determine how the amount of Ca in the carbonate. Silica is liberated to form quartz as a result of the dissolution of the silicates and the fixing of Fe-Mg-Ca in carbonates [
4,
13,
43]. Figure. demonstrated that SiO2 and MgO have a negative association. This suggests that magnesium oxide has been removed from the environment with the introduction of hydrothermal fluids containing CO2 and the onset of listwaenitization processes. This is related to the high absorption of CO2 in harzburgite and serpentinites. These rocks have the capacity to produce surface carbonate, as demonstrated by their high absorption [
44].
According to Esfandiari et al. [
45], carbonate listwaenites are formed in the first stage of serpentinite alteration processes, followed by carbonate-silica listwaenites and silica listwaenites in the final stage. These conclusions are based on the temperature and pressure conditions required for the formation of carbonate and silica. The existence of three distinct Listwaenite types could be explained by variations in the protolith’s mineralogical and geochemical characteristics as well as by various stages of hydrothermal alteration that result in various stages of metasomatic replacement. Different types of listwaenites may arise because of fluctuations in pH, temperature, and CO2 fugacity. According to Ash and Arksey [
46] and Uçurum [
4], carbonate listwaenites form at high CO2 fugacity, pH range of 8–10, and moderate–high temperature. At high alkalinity conditions (pH > 11), primary silicate minerals lost material and created zones of high porosity, which led to the development of carbonate listwaenites [
2,
47]. Different pH and CO2 fugacity ranges may result in the formation of silica-carbonate listwaenites [e.g., 12]. For a stable quartz-dolomite assemblage with xCO2 values varied between 0.1 and 0.5 at 1 kbar, a maximum temperature ranges of 350 to 400 °C is recommended [
48]. After the mostly serpentine minerals dissolved during the first carbonate phase of alteration, silica listwaenites were created in the last stage by the precipitation of Si from the circulating hydrothermal fluids. Listwaenite gets a yellow-brown tint as a result of limonite development brought on by weathering and oxidation of Fe-oxides produced during serpentinization.
Islam Abad Listwaenite has a greater trace and REE element content than Fanja Listwaenite. According to Qiu & Zhu’s [
49], trace element abundance in Listwaenite rises as shear zone deformation advances. This suggests that increased deformation occurrences are connected to the rise in trace elements in the Islam Abad listwaenites. Carbonate in Listwaenite developed at 80–130
◦C [
17] or 50–250
◦C [
28] according to values of clumped isotopes and mineral paragenesis. While there is some overlap, these temperatures are often higher than the <65 C anticipated temperatures for Listwaenite formation [
50]. Most of the pressure during the creation of Listwaenite remains unknown. Falk and Kelemen [
17], however, employed typical subduction-zone geotherms and hypothesized that Listwaenite might have formed at pressures between 0.2 and 1.5 GPa based on the theory that the mineral developed in a subduction-zone scenario. At these pressures, the CO2 concentration necessary to stabilize the magnesite + quartz assemblage is only slightly higher than seawater CO2 concentrations. Equilibration of released water with carbonate in subducted sediments can yield fluids with sufficiently high CO2 concentrations to form magnesite-quartz Listwaenite upon reaction with harzburgites and the mass of fluid produced by compaction and partial dehydration reactions is sufficient to account for the observed mass of Listwaenite. These calculations are applicable to subduction zones worldwide. Globally, extensive carbonation of mantle wedge peridotite may be more common above subduction zones at the “leading edge of the mantle wedge” than has been previously recognized.
7. Conclusions
Islam Abad and Fanja listwaenites were formed due to hydrothermal alteration of serpentinized harzburgites and serpentinites along thrust faults and shear zones which acted as pathway for fluids. There are three distinct types of listwaenites: carbonate-, silica-carbonate-listwaenites, and silica-listwaenites. The higher trace elements contents in the Islam Abad listwaenites suggests that the degree of deformation in Islamabad area in Iran has been higher than the Fanja area of Oman. The ultramafic protolith is further supported by petrographic observation and by the enrichment mode of Cr, Ni, and Co in the listwaenites. The listwaenites alteration process occurred after the harzburgite suite serpentinized and the upward circulation of CO2, CaO, SiO2, and Fe2O3 rich hydrothermal fluids along fault zones. The alteration process is isocheimal in terms of the major and trace elements that are redistributed at different scales, with the exception of K, CO2, H2O, Mg, Ca, and Si.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplementary file S-1. Detection limit and quality control of chemical analysis. Supplementary file S-2. Average of listwaenites from Oman and Iran Supplementary file S-3a-e. Correlation matrix of major oxides and trace elements in the listwaenite from Fenja and Islam Abad area.
Author Contributions
S.Nasir.: field work, petrology, mineralogy, and writing the manuscript; K.Noori, field work, sampling, writing on geology of Islam Abad area; A Nasir: Data managements, interpretations ad plotting. All authors have read and agreed to the published version of the manuscript
Funding
This project is funded by Sultan Qaboos University grant No. IG/SCI/ETHS/24/02.
Acknowledgments
The authors would like to thank Dr. Arshad Ali for assisting in sample preparation. Thanks to M. Al Mahrooqi and M. Al Bousadi for assisting in the field work and rock sampling.
Conflicts of Interest
Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rose, G., 1837. Mineralogisch-Geognostische Reise Nach dem Ural, dem Altai und dem Kaspischen Meere, Volume 1: Reise nach dem Nordlichen Ural und dem Altai; Forgotten Books: Berlin, Germany.
- Nasir, S., Al Sayigh, A.R., Al Harthy, A., Al-Khirbash, S., Al-Jaaidi, O., Musllam, A., Al-Mishwat, A., Al-Bu’saidi, S., 2007. Mineralogical and geochemical characterization of listwaenite from the Semail Ophiolite, Oman. Chem. Erde Geochem. 67, (3), 213–228.
- Vural, A., Aydal, D., 2020. Soil geochemistry study of the listvenite area of Ayvacik (Çanakkale, Turkey), Caspian J. Environ. Sci. 18, 3, 205-215. [CrossRef]
- Uçurum, A., 2000. Listwaenites in Turkey: perspectives on formation and precious metal concentration with reference to occurrences in east central Anatoilia, Ofioliti, 25, 15-29.
- Vural, A., 2006. Investigation of gold enrichments in Bayramiç (Çanakkale, Turkey) and its near vicinity. PhD Dissertation, Ankara University, Ankara, Türkiye.
- Zoheir, B., & Lehmann, B., 2011. Listvenite–lode association at the Barramiya gold mine Eastern Desert, Egypt, Ore Geology Reviews, 39, 101-115.
- Kiliç, A.D., İnceöz, M., 2015. Mineralogical, geochemical and isotopic effect of silica in ultramaphic systems, eastern Anatolian Turkey. Geochemistry International, 53, 369-382.
- Vural, A., & Aydal D 2016. Using soil geochemistry for gold exploration: Ayvacık (Çanakkale-Northwest Turkey). 34th National and the 2nd International Geosciences Congress proceedings book. 22-24 Feb. 2016 Tehran, Iran.
- Belogub, E.V., Melekestseva, IY, Novoselov, KA, Zabotina, M V, Tret’yakov, GA, Zaykov, V.V., Yuminov, AM., 2017. Listvenite-related gold deposits of the South Urals (Russia): A review, Ore Geology Review, 85, 247-270.
- Yang, Y., Zhao, L., Zheng, R., Xu, Q., 2019. Evolution of the early Paleozoic Hongguleleng–Balkybey Ocean: Evidence from the Hebukesaier ophiolitic mélange in the northern West Junggar, NW China, Lithos, 324–325: 519-536.
- Ucurum, A., & Larson, L. T. (1999). Geology, Base-Precious Metal Concentration and Genesis of the Silica-Carbonate Alteration (Listwaenites) From Late Cretaceous Ophiolitic Mé- langes at Central East Turkey. Chemie der Erde, 59, 77-104.
- Boschi, C., Dini, A., Dallai, L., Ruggieri, G., Gianelli, G., 2009. Enhanced CO2-mineral sequestration by cyclic hydraulic fracturing and Si-rich fluid infiltration into serpentinites at Malentrata (Tuscany, Italy). Chem. Geol. 265, 209–226. [CrossRef]
- Halls, C., Zhao, R., 1995. Listvenite and related rocks: Perspectives on terminology and mineralogy with reference to an occurrence at Creggan Baun, Co. Mayo, Republic of Ireland. Mineralium Deposita 30, 303-313.
- 14. Hess HH (1933) The problem of serpentinization and the origin of certain chrysotile asbestos, talc and soapstone deposits. Econ Geol 28:634-657.
- Moody, J.B., 1976. Serpentinization: a review. Lithos 9, 125–138. [CrossRef]
- Takahashi, T., Morishita, T., Tamura, A., Harigane, Y., Kawamoto, T., 2020. Petrology of Oman listvenite (carbonated ultramafic rocks) and related serpentinite from the Hole BT1B of the Oman Drilling Project, Japan Geoscience Union JpGU-AGU Joint Meeting 2020. SCG56-14.
- Falk, E.S., Kelemen, P.B., 2015. Geochemistry and petrology of listvenite in the Semail ophiolite, Sultanate of Oman: Complete carbonation of peridotites during ophiolite emplacement. Geochimica et Cosmochimica Acta 160, 70-90. [CrossRef]
- Menzel, M.D., Urai, J.L., de Obeso, J.C., Kotowski, A., Manning, C.E., Kelemen, P.B., Kettermann, M., Jesus, A.P., Harigane, and the Oman Drilling Project Phase 1 Science Team, 2020. Brittle deformation of carbonated peridotite–insights from listvenites of the Samail Ophiolite (Oman Drilling Project Hole BT1B). Journal of Geophysical Research: Solid Earth 125, e2020JB020199. [CrossRef]
- Scharf, A., Bailey, C.M., Bolhar, R., Mattern, F., Ring, U., 2022. Post-obduction listwaenite genesis in the Oman Mountains inferred from strucutral analysis and U-Pb carbonate dating. Earth and Planetary Science Letters 595, 117756.
- Gerdemann SJ, O’Connor WK, Dahlin DC, Penner LR, Rush H (2007) Ex situ aqueous mineral carbonation. Environ Sci Technol 41:2587-2593.
- Jia, L. Anthony, E. J. (2002) Mineral carbonation and ZECA, Proceedings of the 6th international conference on greenhouse gas control technologies, Kyoto, Japan.
- O’Connor WK, Dahlin DC, Rush GE, Gerdemann SJ, Penner LR, Nilsen DN (2005) Aqueous mineral carbonation: Mineral availability, pretreatment, reaction parametrics, and process studies. Albany Research Center. US DOE: DOE/ARC-TR-04-002.
- Glennie, K. W., Boeuf, M. G. A., Clarke, M. W. H., Moody Stuart, M., Pilaar, W. F. H., & Reinhardt, B. M. (1974). Geology of the Oman Mountains. In Verhandelingen van het Koninklink Nederlands Geologisch Mijnbouwkundig Genootschap (Vol. 31, pp. 1–423). The Hague: Koninklink Nederlands Geologisch Mijnbouwkundig Genootschap.
- Searle, M., and Cox, J. (1999). Tectonic setting, origin, and obduction of the Oman ophiolite. Geological Society of America Bulletin, 111(1), 104–122. [CrossRef]
- Aftabi, A., and Zarinkoub, M.H., 2013. Petrogeochemistry of listvenite association in metaophiolites of Sahlabad region, eastern Iran: Implications for possible epigenetic Cu-Au ore exploration in metaophiolites, January 2013Lithos 156:186-203. [CrossRef]
- Boskabadi, A., Pitcairn, I.K., Leybourne, M.I., Teagle, D.A.H., Cooper M.J., Hadizadeh, H., Nasiri Bezenjani, R., Monazzami Bagherzadeh, R., 2020. Carbonation of ophiolitic ultramafic rocks: Listvenite formation in the Late Cretaceous ophiolites of eastern Iran, Lithos, 352–353, January 2020, 105307.
- Rajendran, S., Nasir, S., Kusky, T. M., Abduwasit, Gh., Gabr, S., El-Ghali, M.A.K.,2013. Detection of hydrothermal mineralized zones associated with listwaenites in Central Oman using ASTER data, Ore Geol. Rev. [CrossRef]
- Beinlich, A., Plumer, O., Boter, E., Muller, A., Kourim, F., Ziegler, M., Harigane, Y., Lafay, R., Kelemen, P.B., and the Oman Drilling Project Science Team, 2020. Ultramafic rock carbonation: Constraints from listvenite core BT1B, Oman Drilling Project. Journal of Geophysical Research: Solid Earth 125(6), e2019JB019060. [CrossRef]
- Niromand, M., Behyari, M., Rahimsouri, Y., 2021, Strain geometry and structural analysis of the Oshnavieh ophiolite: A new segment of the Neo-Tethys puzzle, Iranian Journal of Earth Sciences, Vol. 13, No. 4, 2021, 266-278. DOI: 10.30495/ijes.2021.685394.
- Villey, M., Le Métour, J., de Gramont, X., 1986. Geological map of Fanjah, sheet NF 40-3F, scale: 1: 100,000, with Explanatory notes: Directorate General of Minerals, Oman Ministry of Petroleum and Minerals.
- Babaei, H.A., Ghazi, A.M., Babaei, A., Latour, T.E., and Hassanipak, A.A., 2001, Geochemistry of arc volcanic rocks of the Zagros Crush Zone, Neyriz, Iran: Journal of Asian Earth Sciences, v. 19, p. 61–76. [CrossRef]
- Shafaii Moghadam, H. , Zaki Khedr M., , Chiaradia, M., St ern, R., Bakhshizad, F., Arai S., Chris J. Ottleyf, C., Akihiro Tamura, A., (2014). Supra-subduction zone magmatism of the Neyriz ophiolite, Iran: constraints from geochemistry and SrNd-Pb isotopes. International Geology Review, 56:11, 1395-1412, DOI: 10.1080/ 00206814.2014.942391.
- Monsef, I., Monsef R., João Mata, J. Zhiyong Zhang, Z., , Mortaza Pirouz,M., Mahnaz Rezaeian, M. Rasoul Esmaeili, R., Xiao, W. (2018) Evidence for an early-MORB to fore-arc evolution within the Zagros suture zone: Constraints from zircon U-Pb geochronology and geochemistry of the Neyriz ophiolite (South Iran). Gondwana Research 62 (2018) 287–305.
- Sabzehei, M., 1996, The geological map of Neyriz 1/100000 Series: Geological Survey of Iran.
- Oveisy, B., 2002. Geological map of Surian (1: 100,000), geological survey of Iran.
- Yusofi, T., Kargar, S., 1994. Geological map of Abadeh Tashk (1: 100,000), geological survey of Iran.
- McDonough, W.F., Sun, S.-S., 1995. The composition of the Earth. Chemical Geology 120, 223–253.
- Sun, S., and McDonough, W.F., 1989, Chemical and isotopic systematics of oceanic basalts: implications for mantle composition and processes, Geological Society London, Special Publication No. 42, pp. 313-345.
- Deschamps, F., Godard, M., Guillot, S., Hattori, K., 2013. Geochemistry of subduction zone serpentinites: a review. Lithos 178, 96–127.
- Kodolányi, J., Peke, T., Spandler,C., Kamber, B., Gméling, K. (2012). Geochemistry of ocean floor and fore-arc serpentinites: Constraints on the ultramafic input to subduction zones. Journal of Petrology, 53(2), 235-270.
- Peters, D., Bretscher, A., John, T., Scambelluri, M., Pettke, T., 2017. Fluid-mobile elements in serpentinites: Constraints on serpentinisation environments and element cycling in subduction zones. Chemical Geology 466, 654–666. [CrossRef]
- Spiridonov, E. M. (1991). Listvenites and zodites. International Geology Review, 33(4), 397–407. DOI: 10.1080/00206819109465698,.
- Sazanov, V. N., 1975- Listvenitization and mineralization (Listvenitizaciya iorudeneniye) lzdatelistvo Nauka (Science Publishers, Moscow).
- Power, I.M., Harrison, A.L., Dipple, G.M., Wilson, S.A., Kelemen, P.B., Hitch, M., Southam, G., 2013. Carbon mineralization: from natural analogues to engineered systems. Reviews in Mineralogy and Geochemistry 77(1), 305-360.
- Esfandiari, Z., Maccizadeh, M.A., Mehrnia, R., Taghipoor, B., 2013. Determining the origin of hydrothermal alteration of listvenite in the Northeast region Arsanjan, The 16th conference of Geological Society of Iran.
- Ash, C. H., & Arksey, R. L. (1991). The listwaenites-lode gold association in British Columbia. Geological Fieldwork 1989, paper 1990-1991.
- Neal, C., and G. Stanger (1983), Hydrogen generation from mantle source rocks in Oman, Earth Planet. Sci. Lett., 66, 315–320.
- Auclair, M., Gauthier, Trottier, J., Jebrak,, M., Chartrand, F. (1993); Mineralogy, geochemistry, and paragenesis of the Eastern Metals serpentinite-associated Ni-Cu-Zn deposit, Quebec Appalachians. Economic Geology, vol. 88, issue 1, pp. 123-138.
- Qiu, T., and Zhu, Y., 2018, Listwaenite in the Sartohay ophiolitic mélange (Xinjiang, China): A genetic model based on petrology, U-Pb chronology and trace element geochemistry, Lithos 302–303 (2018) 427–446.
- Wilde, A., Simpson, L., Hanna, S., 2002. Preliminary study of Cenozoic hydrothermal alteration and platinum deposition in the Oman Ophiolite, in Jessell, M. J., ed., General Contributions. Journal of the Virtual Explorer 6, 7-13.
Figure 1.
Geological map showing the location of major outcrops of listwaenites in Fenja area modified after [
19,
30].
Figure 1.
Geological map showing the location of major outcrops of listwaenites in Fenja area modified after [
19,
30].
Figure 2.
Field photo showing an example of listwaenites ridge in Fenja area (a) and contact between listwaenites and serpentinites (b).
Figure 2.
Field photo showing an example of listwaenites ridge in Fenja area (a) and contact between listwaenites and serpentinites (b).
Figure 3.
Geological map of Neyriz ophiolite in Islam Abad area Modified after [
34,
35,
36].
Figure 3.
Geological map of Neyriz ophiolite in Islam Abad area Modified after [
34,
35,
36].
Figure 4.
Field photos showing orange brown calcite listwaenites (a), contact between listwaenites and serpentinite (b) and silica- and (c) silica-carbonate listwaenites from Islam Abad area.
Figure 4.
Field photos showing orange brown calcite listwaenites (a), contact between listwaenites and serpentinite (b) and silica- and (c) silica-carbonate listwaenites from Islam Abad area.
Figure 5.
Photos of rock samples from Fenja area. (a) Carbonate Listwaenite, (b) Silica-carbonate Listwaenite with white veins and clasts of silica, (c ) Silica rich listwaenites.
Figure 5.
Photos of rock samples from Fenja area. (a) Carbonate Listwaenite, (b) Silica-carbonate Listwaenite with white veins and clasts of silica, (c ) Silica rich listwaenites.
Figure 6.
Photos of rock samples from Islam Abad area. (a) Carbonate Listwaenite, (b) Silica-carbonate Listwaenite with white veins and clasts of silica, (c) Silica rich listwaenites.
Figure 6.
Photos of rock samples from Islam Abad area. (a) Carbonate Listwaenite, (b) Silica-carbonate Listwaenite with white veins and clasts of silica, (c) Silica rich listwaenites.
Figure 7.
Microscopic images of carbonate listwaenites from Fenja area showing fin grained calcite matrix with micro veins filled with iron hydroxides and wide veins filled with calcite (Cc) replacing serpentinite mesh (a-xpl and b-ppl). Macroscopic images of carbonate listwaenites from Islam Abad area (a-xpl and b-ppl) showing relict of Cr-spinel (Sp) and several micro-veins filled with secondary iron hydroxides and small patches of serpentinite relicts.
Figure 7.
Microscopic images of carbonate listwaenites from Fenja area showing fin grained calcite matrix with micro veins filled with iron hydroxides and wide veins filled with calcite (Cc) replacing serpentinite mesh (a-xpl and b-ppl). Macroscopic images of carbonate listwaenites from Islam Abad area (a-xpl and b-ppl) showing relict of Cr-spinel (Sp) and several micro-veins filled with secondary iron hydroxides and small patches of serpentinite relicts.
Figure 8.
Microscopic images of silica-carbonate listwaenites from Fenja area showing fin grained matrix of magnesite (Mgs) and late stage quartz (Qz) filling veins and relict spinel (Sp) (a-xpl and b-ppl). Microscopic images of silica-carbonate listwaenites from Islam Abad area showing coarse-grained rhombic dolomite (Do), magnesite (Mgs) and later stage quartz replacing carbonate minerals.
Figure 8.
Microscopic images of silica-carbonate listwaenites from Fenja area showing fin grained matrix of magnesite (Mgs) and late stage quartz (Qz) filling veins and relict spinel (Sp) (a-xpl and b-ppl). Microscopic images of silica-carbonate listwaenites from Islam Abad area showing coarse-grained rhombic dolomite (Do), magnesite (Mgs) and later stage quartz replacing carbonate minerals.
Figure 9.
Microscopic images of silica-listwaenites from Fenja area . (a-xpl and b-ppl) showing early stage fin grained quartz matrix and later stage of coarse grained quartz (Qz) filling veins. filled with iron hydroxides and wide veins filled with calcite replacing serpentinite mesh. Microscopic images of silica- listwaenites from Islam Abad area (c-xpl and d-ppl) showing fine grained early stage quartz replacing relict serpentine (Srp) and Cr-spinel (Sp) and later stage coarse-grained quartz.
Figure 9.
Microscopic images of silica-listwaenites from Fenja area . (a-xpl and b-ppl) showing early stage fin grained quartz matrix and later stage of coarse grained quartz (Qz) filling veins. filled with iron hydroxides and wide veins filled with calcite replacing serpentinite mesh. Microscopic images of silica- listwaenites from Islam Abad area (c-xpl and d-ppl) showing fine grained early stage quartz replacing relict serpentine (Srp) and Cr-spinel (Sp) and later stage coarse-grained quartz.
Figure 10.
Microscopic images of serpentinized harzburgite from Fenja area and (a) and Islam Abad (b) showing medium-grained olivine (Ol) altered to serpentine (Srp) and scattered spinel grains (Sp). 10c-d macroscopic images of serpentinites from Fenja (c) and Islam Abad area (d) showing alteration of olivine to serpentine (Srp) and calcite (Cc) filling veins cutting serpentine minerals.
Figure 10.
Microscopic images of serpentinized harzburgite from Fenja area and (a) and Islam Abad (b) showing medium-grained olivine (Ol) altered to serpentine (Srp) and scattered spinel grains (Sp). 10c-d macroscopic images of serpentinites from Fenja (c) and Islam Abad area (d) showing alteration of olivine to serpentine (Srp) and calcite (Cc) filling veins cutting serpentine minerals.
Figure 11.
a-f. Binary variation diagrams of SiO2 vs. selected major oxides (a-e) and Cr vs. Ni (f) in listwaenites, serpentinites and harzburgite from Fenja and Islam Abad.
Figure 11.
a-f. Binary variation diagrams of SiO2 vs. selected major oxides (a-e) and Cr vs. Ni (f) in listwaenites, serpentinites and harzburgite from Fenja and Islam Abad.
Figure 12.
a-d. Binary variation diagrams (a) SiO
2 mole vs. Mg#, (b) SiO
2 mole vs MgO mole, (c) LOI vs. MgO + CaO and (d) SiO
2 mole vs MgO + CaO mole. Symbols as in
Figure 11.
Figure 12.
a-d. Binary variation diagrams (a) SiO
2 mole vs. Mg#, (b) SiO
2 mole vs MgO mole, (c) LOI vs. MgO + CaO and (d) SiO
2 mole vs MgO + CaO mole. Symbols as in
Figure 11.
Figure 13.
Triangular diagrams. (a) SiO
2-CaO-MgO, (b) Fe
2O
3-MgO+CaO-SiO
2 and (c) MGO+CaO-SiO
2-LOI. Symbols as in
Figure 11.
Figure 13.
Triangular diagrams. (a) SiO
2-CaO-MgO, (b) Fe
2O
3-MgO+CaO-SiO
2 and (c) MGO+CaO-SiO
2-LOI. Symbols as in
Figure 11.
Figure 14.
(a) REE normalization diagram after McDonough and Sun [
37] of listwaenite lithologies from Fenja and Islam Abad (Neyriz ophiolite) (b) REE pattern of serpentinite and harzburgite from Fenja area, (c) REE pattern of listwaenite and harzburgite from Birjand area (Brj), Iran [
26].
Figure 14.
(a) REE normalization diagram after McDonough and Sun [
37] of listwaenite lithologies from Fenja and Islam Abad (Neyriz ophiolite) (b) REE pattern of serpentinite and harzburgite from Fenja area, (c) REE pattern of listwaenite and harzburgite from Birjand area (Brj), Iran [
26].
Figure 15.
(a) PM-normalized diagrams race elements after Sun and McDonough [
38] in listwaenite lithologies in Fenja and Islam Abad area. (b) and in serpentinite and harzburgite from Fenja area (b) (c) listwaenites in Birjand area in Iran (c) [
26]. Symbol as in
Figure 14.
Figure 15.
(a) PM-normalized diagrams race elements after Sun and McDonough [
38] in listwaenite lithologies in Fenja and Islam Abad area. (b) and in serpentinite and harzburgite from Fenja area (b) (c) listwaenites in Birjand area in Iran (c) [
26]. Symbol as in
Figure 14.
Figure 16.
a-b. (a) Major and trace elements contents in Listwaenite lithologies from Fenja and Islam Abad area normalized by harzburgite from Fenja area, compared to Listwaenite lithologies from Birjand ophiolite in Iran [
26] and Wadi Mansah in Samail ophiolite [
17] (Figure. 16b). Cc-List: carbonate listwaenite, Si-Cc-List: Carbonate-Silica listwaenite, Si-List.: Silica listwaenite.
Figure 16.
a-b. (a) Major and trace elements contents in Listwaenite lithologies from Fenja and Islam Abad area normalized by harzburgite from Fenja area, compared to Listwaenite lithologies from Birjand ophiolite in Iran [
26] and Wadi Mansah in Samail ophiolite [
17] (Figure. 16b). Cc-List: carbonate listwaenite, Si-Cc-List: Carbonate-Silica listwaenite, Si-List.: Silica listwaenite.
Figure 17.
a. Positive and negative deviation of major elements in listwaenites relative to serpentinite.
Figure 17.
a. Positive and negative deviation of major elements in listwaenites relative to serpentinite.
Figure 17.
b. Positive and negative deviation of trace elements in listwaenites relative to serpentinite.
Figure 17.
b. Positive and negative deviation of trace elements in listwaenites relative to serpentinite.
Table 1.
Major and trace element analysis of listwaenites from Fenja area-Oman.
Table 1.
Major and trace element analysis of listwaenites from Fenja area-Oman.
| Sample |
FCL-10 |
FCL-11 |
FCL-12 |
FCL-13 |
FDL-6 |
FDL-7 |
FDL-8 |
FDL-9 |
FSL-1 |
FSL-2 |
FSL-3 |
FSL-4 |
FSL-5 |
| Type |
Carbonate-Listwaenite |
Silica carbonate listwaenites |
Silica listwaenites |
| SiO2 wt.% |
4.4 |
5.3 |
6.5 |
3.8 |
21.5 |
17.5 |
19.7 |
20.1 |
71.8 |
60.13 |
68.5 |
69.9 |
70.8 |
| TiO2 |
0.02 |
0.01 |
0.02 |
0.04 |
0.03 |
0.01 |
0.01 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.01 |
| Al2O3 |
1.15 |
1.02 |
0.95 |
1.2 |
0.63 |
0.6 |
0.58 |
0.69 |
0.15 |
0.18 |
0.21 |
0.38 |
0.41 |
| Fe2O3 |
11.45 |
11.2 |
10.55 |
11.9 |
11.28 |
10.43 |
11.45 |
11.11 |
8.23 |
9.12 |
8.5 |
7.55 |
8.25 |
| MgO |
4.76 |
7.9 |
6.45 |
6.5 |
21.35 |
22.9 |
21.76 |
23.4 |
8.45 |
11.1 |
7.8 |
9.14 |
9.05 |
| MnO |
0.16 |
0.24 |
0.13 |
0.11 |
0.19 |
0.15 |
0.13 |
0.17 |
0.1 |
0.11 |
0.12 |
0.08 |
0.09 |
| CaO |
37.5 |
35.14 |
37.2 |
36.8 |
19.3 |
22.6 |
23.45 |
21.85 |
2.45 |
4.6 |
3.1 |
1.4 |
2.1 |
| Na2O |
0.1 |
0.1 |
0.1 |
0.1 |
0.11 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.12 |
0.1 |
| K2O |
0.08 |
0.06 |
0.05 |
0.06 |
0.1 |
0.1 |
0.12 |
0.14 |
0.02 |
0.03 |
0.02 |
0.03 |
0.04 |
| P2O5 |
0.01 |
0.03 |
0.02 |
0.01 |
0.03 |
0.03 |
0.01 |
0.02 |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
| LOI |
39.6 |
38.3 |
37.5 |
38.5 |
25.4 |
26.1 |
22.7 |
23.1 |
8.1 |
14.1 |
11.2 |
10.4 |
8.65 |
| Total |
99.23 |
99.3 |
99.47 |
99.02 |
99.92 |
100.52 |
100.01 |
100.7 |
99.43 |
99.5 |
99.58 |
99.03 |
99.51 |
| Mg# |
45.17 |
58.3 |
54.79 |
51.98 |
78.95 |
81.31 |
79.02 |
80.67 |
67.05 |
70.69 |
64.52 |
70.58 |
68.5 |
| Sc ppm |
4.01 |
4.32 |
4.41 |
4.79 |
4.66 |
5.91 |
5.65 |
4.22 |
4.8 |
4.72 |
4.35 |
4.61 |
4.74 |
| As |
3.1 |
4.1 |
4.4 |
5.1 |
5 |
7 |
9 |
7 |
24 |
27 |
34 |
37 |
25 |
| Ba |
3.9 |
3.7 |
3.4 |
2.8 |
3.5 |
3.9 |
4.8 |
5.1 |
44 |
37 |
20 |
29 |
18 |
| Co |
115 |
95 |
120 |
102 |
90 |
92 |
110 |
86 |
71 |
62 |
73 |
65 |
63 |
| Cu |
5.8 |
4.2 |
5.8 |
8.5 |
3 |
3 |
2 |
2.5 |
11 |
14 |
10 |
8 |
9 |
| Zn |
275 |
155 |
320 |
345 |
283 |
255 |
315 |
260 |
36 |
55 |
61 |
69 |
72 |
| Ni |
2650 |
2470 |
2690 |
2750 |
2200 |
2700 |
2600 |
2090 |
1500 |
1550 |
1600 |
1350 |
1360 |
| Ga |
0.75 |
0.56 |
0.64 |
0.75 |
0.26 |
0.3 |
0.25 |
0.35 |
0.35 |
0.25 |
0.4 |
0.43 |
0.35 |
| Cr |
3100 |
2600 |
3200 |
3300 |
2000 |
2400 |
2100 |
1900 |
1400 |
1600 |
1400 |
1500 |
1500 |
| V |
36 |
33 |
35 |
46 |
17 |
18 |
13 |
17 |
14 |
13 |
16 |
15 |
13 |
| Rb |
0.13 |
0.12 |
0.19 |
0.17 |
0.08 |
0.059 |
0.1 |
0.18 |
0.03 |
0.08 |
0.05 |
0.06 |
0.09 |
| Sr |
150 |
225 |
183 |
165 |
145 |
210 |
175 |
195 |
37 |
39 |
42 |
46 |
35 |
| Zr |
0.06 |
0.02 |
0.05 |
0.02 |
0.05 |
0.01 |
0.01 |
0.02 |
0.17 |
0.17 |
0.04 |
0.05 |
0.03 |
| Sb |
0.41 |
0.37 |
0.39 |
0.33 |
1.15 |
1.52 |
2.2 |
2.7 |
0.85 |
1 |
0.3 |
0.55 |
0.64 |
| Cs |
0.21 |
0.15 |
0.27 |
0.16 |
0.14 |
0.13 |
0.16 |
0.17 |
0.21 |
0.25 |
0.18 |
0.16 |
0.13 |
| Nb |
0.013 |
0.08 |
0.07 |
0.09 |
0.019 |
0.016 |
0.015 |
0.039 |
0.006 |
0.005 |
0.004 |
0.003 |
0.02 |
| Y |
0.22 |
0.17 |
0.34 |
0.53 |
0.04 |
0.03 |
0.04 |
0.15 |
0.14 |
0.13 |
0.09 |
0.07 |
0.06 |
| La |
0.0201 |
0.0238 |
0.0172 |
0.0195 |
0.0055 |
0.0051 |
0.0045 |
0.0061 |
0.0071 |
0.0128 |
0.0099 |
0.0091 |
0.0065 |
| Ce |
0.043 |
0.049 |
0.036 |
0.039 |
0.015 |
0.013 |
0.011 |
0.018 |
0.017 |
0.025 |
0.02 |
0.018 |
0.016 |
| Pr |
0.0048 |
0.0052 |
0.0041 |
0.0045 |
0.0023 |
0.0021 |
0.0018 |
0.0027 |
0.0037 |
0.0045 |
0.0043 |
0.004 |
0.0036 |
| Nd |
0.026 |
0.027 |
0.019 |
0.024 |
0.014 |
0.011 |
0.01 |
0.017 |
0.022 |
0.028 |
0.024 |
0.023 |
0.017 |
| Sm |
0.013 |
0.014 |
0.009 |
0.012 |
0.008 |
0.007 |
0.006 |
0.01 |
0.008 |
0.015 |
0.011 |
0.009 |
0.007 |
| Eu |
0.0059 |
0.0061 |
0.0047 |
0.0057 |
0.006 |
0.0055 |
0.0051 |
0.0065 |
0.0027 |
0.007 |
0.004 |
0.0035 |
0.0025 |
| Gd |
0.02 |
0.023 |
0.015 |
0.019 |
0.033 |
0.028 |
0.025 |
0.038 |
0.01 |
0.022 |
0.014 |
0.013 |
0.009 |
| Tb |
0.0041 |
0.0045 |
0.0029 |
0.0039 |
0.0075 |
0.0057 |
0.0051 |
0.008 |
0.002 |
0.004 |
0.0024 |
0.0025 |
0.0016 |
| Dy |
0.026 |
0.028 |
0.022 |
0.024 |
0.041 |
0.038 |
0.035 |
0.045 |
0.015 |
0.025 |
0.018 |
0.016 |
0.011 |
| Ho |
0.0056 |
0.0058 |
0.0048 |
0.0054 |
0.0091 |
0.0085 |
0.0081 |
0.0096 |
0.003 |
0.006 |
0.0035 |
0.0032 |
0.0025 |
| Er |
0.019 |
0.022 |
0.013 |
0.017 |
0.033 |
0.028 |
0.025 |
0.035 |
0.01 |
0.019 |
0.013 |
0.011 |
0.009 |
| Tm |
0.0029 |
0.0036 |
0.0024 |
0.0031 |
0.0048 |
0.0044 |
0.0042 |
0.0055 |
0.0015 |
0.003 |
0.002 |
0.0017 |
0.0013 |
| Yb |
0.023 |
0.024 |
0.017 |
0.022 |
0.031 |
0.028 |
0.025 |
0.035 |
0.01 |
0.021 |
0.012 |
0.011 |
0.009 |
| Lu |
0.0037 |
0.0042 |
0.0025 |
0.0035 |
0.0054 |
0.0051 |
0.0046 |
0.0058 |
0.0015 |
0.0029 |
0.002 |
0.0018 |
0.0012 |
| Mo |
0.13 |
0.14 |
0.17 |
0.12 |
0.32 |
0.41 |
0.37 |
0.42 |
0.55 |
0.38 |
0.42 |
0.45 |
0.51 |
| W |
1.1 |
0.95 |
1.25 |
1.34 |
1.77 |
1.65 |
1.85 |
1.92 |
2.2 |
1.98 |
2.05 |
2.45 |
1.87 |
| Pb |
19 |
14.1 |
11.5 |
10.3 |
41 |
42 |
35 |
38 |
3.1 |
3.6 |
4 |
4.5 |
4.4 |
| Th |
0.14 |
0.11 |
0.09 |
0.08 |
0.15 |
0.13 |
0.14 |
0.12 |
0.09 |
0.11 |
0.1 |
0.13 |
0.08 |
| U |
0.06 |
0.05 |
0.07 |
0.07 |
0.08 |
0.09 |
0.12 |
0.16 |
0.15 |
0.17 |
0.18 |
0.2 |
0.16 |
Table 2.
Major and trace element analysis of listwaenites from Islam Abad-Neyriz ophiolite-Iran.
Table 2.
Major and trace element analysis of listwaenites from Islam Abad-Neyriz ophiolite-Iran.
| Sample |
NCL-1 |
NCL-2 |
NCL-3 |
NCL-4 |
NDL-9 |
NDL-10 |
NDL-11 |
NDL-12 |
NSL-5 |
NSL-6 |
NSL-7 |
NSL-8 |
| Type |
Carbonate listwaenites |
Silica carbonate listwaenites |
Silica listwaenites |
| SiO2 wt.% |
3.12 |
3.63 |
4.22 |
7.19 |
24.37 |
23.56 |
24.74 |
25.15 |
76.54 |
73.44 |
80.59 |
77.23 |
| TiO2 |
0.03 |
0.024 |
0.021 |
0.01 |
0.01 |
0.01 |
0.03 |
0.02 |
0.01 |
0.01 |
0.02 |
0.02 |
| Al2O3 |
0.54 |
0.52 |
0.43 |
0.44 |
0.38 |
0.45 |
0.54 |
0.32 |
0.25 |
0.22 |
0.15 |
0.19 |
| Fe2O3 |
9.2 |
9.92 |
10.75 |
10.17 |
10.71 |
11.31 |
10.61 |
10.13 |
6.65 |
7.87 |
7.15 |
7.45 |
| MgO |
9.11 |
9.45 |
8.9 |
8.8 |
30.55 |
31.09 |
33.88 |
30.55 |
5.27 |
5.81 |
4.45 |
5.11 |
| MnO |
0.03 |
0.05 |
0.04 |
0.16 |
0.1 |
0.09 |
0.08 |
0.05 |
0.03 |
0.058 |
0.12 |
0.055 |
| CaO |
34.14 |
33.11 |
32.5 |
32.78 |
9.18 |
11.05 |
10.66 |
10.95 |
4.85 |
3.09 |
1.31 |
4.07 |
| Na2O |
0.01 |
0.01 |
0.01 |
0.06 |
0.07 |
0.07 |
0.08 |
0.06 |
0.02 |
0.01 |
0.01 |
0.01 |
| K2O |
0.03 |
0.04 |
0.02 |
0.01 |
0.02 |
0.05 |
0.09 |
0.02 |
0.01 |
0.02 |
0.01 |
0.02 |
| P2O5 |
0.03 |
0.05 |
0.04 |
0.02 |
0.01 |
0.01 |
0.02 |
0.02 |
0.05 |
0.06 |
0.08 |
0.06 |
| LOI |
43.36 |
42.52 |
43.12 |
40.95 |
24.1 |
22.1 |
19.15 |
22.6 |
6.3 |
8.12 |
6.06 |
5.4 |
| Total |
99.6 |
99.32 |
100.05 |
100.59 |
99.5 |
99.79 |
99.88 |
99.87 |
99.98 |
98.71 |
99.96 |
99.61 |
| Mg# |
66.25 |
65.37 |
62.13 |
63.17 |
84.97 |
84.49 |
86.35 |
85.67 |
61.1 |
59.4 |
55.23 |
57.62 |
| Sc ppm |
4.61 |
3.23 |
4.98 |
5.05 |
5.92 |
5.23 |
5.11 |
5.19 |
5.44 |
4.87 |
5.01 |
4.92 |
| As |
3.7 |
4.9 |
4.8 |
4.3 |
6.1 |
5.1 |
5.8 |
7.6 |
17.9 |
15.5 |
19.2 |
12.1 |
| Ba |
32.94 |
31.8 |
33.52 |
29.94 |
6.84 |
7 |
9.38 |
13.45 |
19.94 |
17.01 |
21.38 |
22.6 |
| Co |
107 |
102 |
93 |
91 |
60 |
54 |
71 |
78 |
78 |
62 |
59 |
66 |
| Cu |
2.75 |
1.2 |
3.1 |
6.01 |
0.42 |
0.48 |
0.57 |
0.55 |
1.75 |
1.15 |
0.92 |
1.07 |
| Zn |
|
120.3 |
112.9 |
81.31 |
248 |
322 |
169 |
183.5 |
91.2 |
242 |
171 |
118.2 |
| Ni |
2682 |
2475 |
2219 |
2165 |
991 |
1092 |
1540 |
1890 |
955 |
1154 |
965 |
1306 |
| Ga |
0.7 |
0.51 |
0.76 |
0.59 |
0.51 |
0.59 |
0.48 |
0.45 |
1.52 |
0.95 |
1.54 |
1.43 |
| Cr |
2945 |
2808 |
2433 |
2205 |
2710 |
2212 |
1998 |
1885 |
1690 |
1750 |
1610 |
1350 |
| V |
38.2 |
33.6 |
41.4 |
33.5 |
26.6 |
24.5 |
27.8 |
25.4 |
24.5 |
23.6 |
20.5 |
19.1 |
| Rb |
0.14 |
0.11 |
0.09 |
0.15 |
1.1 |
1.7 |
1.4 |
1.34 |
5.12 |
3.81 |
6.54 |
3.78 |
| Sr |
249 |
237 |
242 |
278 |
192 |
177 |
138 |
142 |
29.1 |
33.5 |
36.1 |
40.6 |
| Zr |
0.06 |
0.03 |
0.02 |
0.03 |
0.178 |
0.164 |
0.191 |
0.254 |
0.31 |
0.5 |
0.44 |
0.48 |
| Sb |
11.2 |
12.6 |
10.05 |
10.82 |
26.5 |
24.9 |
20.89 |
23.3 |
8.5 |
5.3 |
9.4 |
6.6 |
| Cs |
0.285 |
0.149 |
0.153 |
0.235 |
0.667 |
0.703 |
0.834 |
1.097 |
1.812 |
1.244 |
2.719 |
1.162 |
| Nb |
0.71 |
0.31 |
0.119 |
0.194 |
0.41 |
0.71 |
0.27 |
0.6 |
0.92 |
0.61 |
0.41 |
0.61 |
| Y |
0.27 |
0.18 |
0.31 |
0.29 |
0.35 |
0.41 |
0.32 |
0.29 |
0.95 |
0.88 |
0.73 |
0.67 |
| La |
1.253 |
1.144 |
1.441 |
0.976 |
0.39 |
0.34 |
0.22 |
0.233 |
0.124 |
0.063 |
0.085 |
0.153 |
| Ce |
2.552 |
2.241 |
3.154 |
2.031 |
0.551 |
0.442 |
0.401 |
0.42 |
0.158 |
0.101 |
0.114 |
0.235 |
| Pr |
0.271 |
0.239 |
0.392 |
0.206 |
0.066 |
0.058 |
0.049 |
0.051 |
0.021 |
0.011 |
0.015 |
0.038 |
| Nd |
1.293 |
1.096 |
1.651 |
0.961 |
0.339 |
0.312 |
0.251 |
0.292 |
0.098 |
0.048 |
0.059 |
0.157 |
| Sm |
0.371 |
0.294 |
0.431 |
0.198 |
0.127 |
0.101 |
0.062 |
0.095 |
0.035 |
0.015 |
0.019 |
0.056 |
| Eu |
0.101 |
0.084 |
0.13 |
0.069 |
0.051 |
0.04 |
0.026 |
0.034 |
0.018 |
0.006 |
0.01 |
0.031 |
| Gd |
0.225 |
0.195 |
0.331 |
0.155 |
0.171 |
0.133 |
0.08 |
0.101 |
0.064 |
0.015 |
0.031 |
0.087 |
| Tb |
0.037 |
0.026 |
0.051 |
0.019 |
0.046 |
0.037 |
0.026 |
0.031 |
0.01 |
0.003 |
0.006 |
0.015 |
| Dy |
0.215 |
0.182 |
0.331 |
0.151 |
0.418 |
0.398 |
0.261 |
0.351 |
0.07 |
0.021 |
0.037 |
0.105 |
| Ho |
0.053 |
0.038 |
0.074 |
0.03 |
0.116 |
0.101 |
0.061 |
0.082 |
0.014 |
0.005 |
0.01 |
0.029 |
| Er |
0.141 |
0.109 |
0.191 |
0.081 |
0.35 |
0.301 |
0.221 |
0.281 |
0.041 |
0.015 |
0.029 |
0.091 |
| Tm |
0.019 |
0.015 |
0.024 |
0.013 |
0.055 |
0.053 |
0.037 |
0.045 |
0.007 |
0.002 |
0.005 |
0.012 |
| Yb |
0.105 |
0.081 |
0.171 |
0.074 |
0.39 |
0.327 |
0.207 |
0.301 |
0.049 |
0.017 |
0.035 |
0.091 |
| Lu |
0.015 |
0.012 |
0.023 |
0.01 |
0.064 |
0.053 |
0.031 |
0.042 |
0.009 |
0.003 |
0.005 |
0.016 |
| Mo |
0.13 |
0.12 |
0.16 |
0.18 |
0.33 |
0.28 |
0.37 |
0.32 |
0.55 |
0.45 |
0.42 |
0.38 |
| W |
1.86 |
1.65 |
1.56 |
1.76 |
1.45 |
1.38 |
1.74 |
1.68 |
2.82 |
2.95 |
2.55 |
3.58 |
| Pb |
40.7 |
33.8 |
43.4 |
37.1 |
8.6 |
9.1 |
7.9 |
8.1 |
4.5 |
3.45 |
2.78 |
4.1 |
| Th |
0.1 |
0.17 |
0.21 |
0.14 |
0.16 |
0.08 |
0.15 |
0.2 |
0.12 |
0.1 |
0.09 |
0.11 |
| U |
0.085 |
0.07 |
0.065 |
0.079 |
0.04 |
0.06 |
0.05 |
0.07 |
0.13 |
0.15 |
0.16 |
0.14 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).