1. Introduction
Ro60 (also known as Sjögren's disease (SjD) autoantigen A2 (SSA2), RO60, or TROVE2) is an RNA-binding protein (RBP). Approximately 300 human RNA-binding proteins are known; however, only a few are characterized for their structure and function. Ro60 is essential for disease and development processes. Its distribution within cells varies between the nucleus, nucleolus, and cytoplasm. It is directly involved in maintaining cellular homeostasis, mediating RNA metabolism, stress responses and DNA replication. Although Ro60 is ubiquitously expressed, its expression can diminish with age and pathophysiological state [
1] . Ro60 is a core component of nucleocytoplasmic RNP complexes. Many RNA molecules such as YRNAs, Alu retroelement transcripts, LINE1 retroelement transcripts, and 5S rRNAs directly bind with Ro60. Ro60 interactions regulate stability, cellular distribution, and serve as quality control for these RNA molecules [
2,
3,
4] . Unlike sequence-specific RBPs, Ro60 scavenges small RNA molecules that fail to bind their cognate RBPs, thereby contributing to cellular homeostasis. Ro60 is evolutionarily conserved and found in all animal species, with orthologues also reported in some bacterial species [
5] . This review highlights how Ro60 mediates small RNA quality control, and how interactions between Ro60 and small RNA can lead to immunogenic exposure of Ro60 RNP complexes. Such exposure may drive inflammatory responses and autoantibody production.
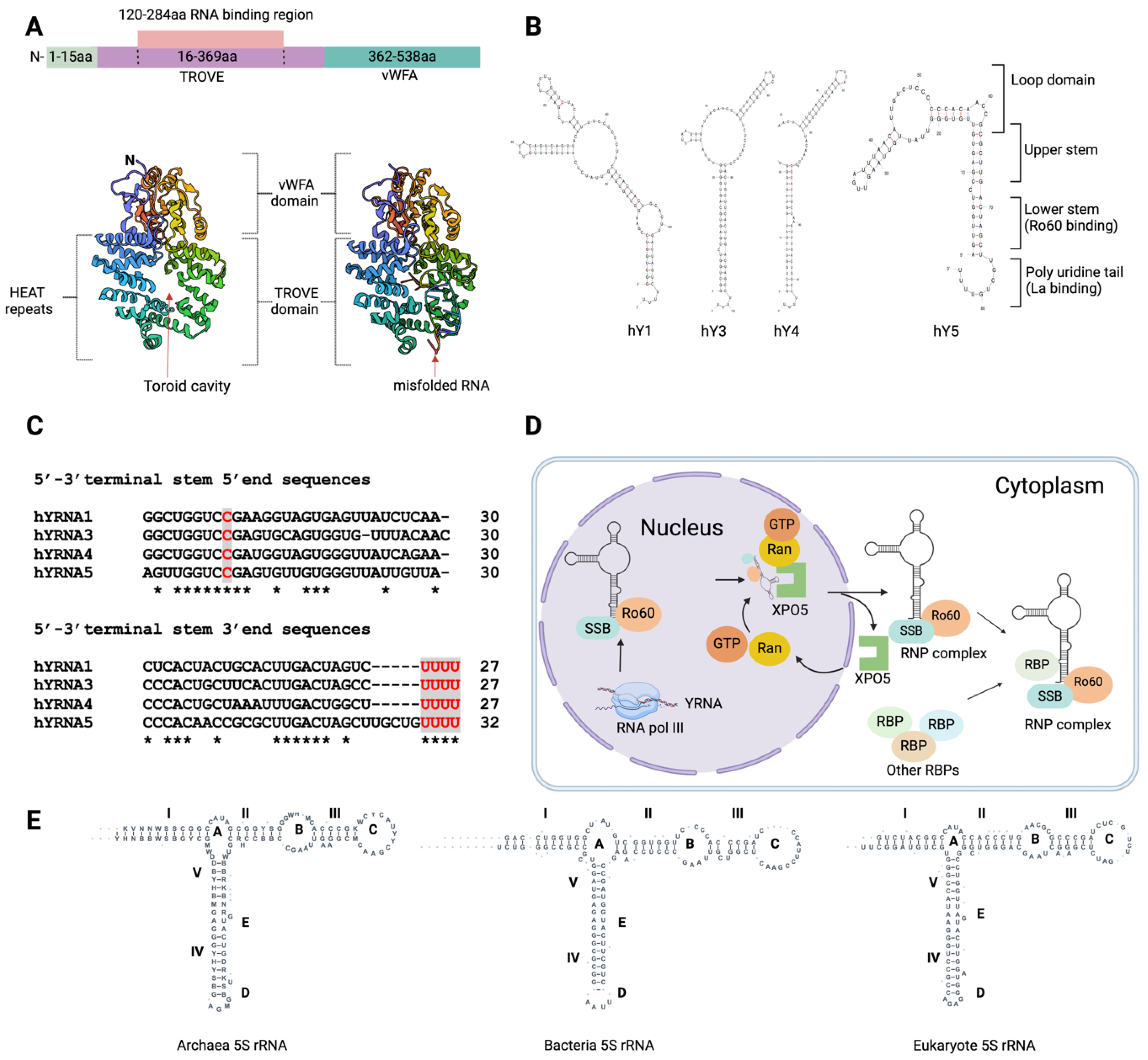
Figure 1.
Ro60 domain structure and small RNAs structural & functional surveillance for cellular distribution. (A) Ro60 is comprised of two major domains, vWFA and TROVE (1YVR &1YVP). The TROVE domain is composed of toroid HEAT repeats. (B) Schematic representation of human Ro60 and other autoantigenic RBPs bound to small RNAs (such as YRNA) and their nuclear-cytoplasmic transportation via XPO5/Ran GTPase [
18] . (C) UNAfold predicted secondary structure of human YRNAs (hY1, hY3, hY4 and hY5). (D) YRNAs terminal stem 5’ and 3’ end sequences. Ro60 binds to the 5'-3' stem-loop bulge, while La binds to the 3' terminal uridine oligos. (E) Secondary structure of 5S rRNAs from bacteria, archaea, and eukaryotes (structures adapted from the 5S rRNA database:
http://combio.pl/rrna/alignment/).
Figure 1.
Ro60 domain structure and small RNAs structural & functional surveillance for cellular distribution. (A) Ro60 is comprised of two major domains, vWFA and TROVE (1YVR &1YVP). The TROVE domain is composed of toroid HEAT repeats. (B) Schematic representation of human Ro60 and other autoantigenic RBPs bound to small RNAs (such as YRNA) and their nuclear-cytoplasmic transportation via XPO5/Ran GTPase [
18] . (C) UNAfold predicted secondary structure of human YRNAs (hY1, hY3, hY4 and hY5). (D) YRNAs terminal stem 5’ and 3’ end sequences. Ro60 binds to the 5'-3' stem-loop bulge, while La binds to the 3' terminal uridine oligos. (E) Secondary structure of 5S rRNAs from bacteria, archaea, and eukaryotes (structures adapted from the 5S rRNA database:
http://combio.pl/rrna/alignment/).
2. Structure of Ro60
Progress has been made in understanding the domain architecture of Ro60 by examining its counterparts in the African clawed frog (
Xenopus laevis) [
6,
7] and the extremophilic bacterium
Deinococcus radiodurans [
5] .
X. laevis Ro60, which shares 78% identity with human Ro60, comprises two distinct domains: the vWFA (von Willebrand factor A) domain and the TROVE (telomerase, Ro, vault) domain (
Figure 1A) [
7] . The vWFA domain is present in many proteins, including those that mediate cell adhesion, migration, homeostasis, signal transduction and immune defence mechanisms [
8] . In Ro60, the vWFA domain stabilises the central cavity of the TROVE domain [
7]. The vWFA domain contains a metal ion-dependent adhesion site (MIDAS; DxSxS…T…D), which binds divalent cations and mediates ligand binding and structural rearrangements. In integrins and other cell adhesion proteins, the vWFA domain functions as a ligand binding site [
9]. The vWFA domain also interacts with other domains such as NACHT-NTPase domain, telomerase-associated protein 1 (TEP1) N-terminal domain, and WD-40 repeats [
10]. The TROVE domain comprises seven α-helical repeats, arranged in a circular format with a central toroid hole of 10Å-15Å. This toroid hole can accommodate the binding of many small single-stranded RNA and misfolded RNA [
7]. The TROVE domain comprises ~300-500 residues and is present in TEP1 (also known as TROVE1) and Ro60. The central TROVE cavity is composed of positively charged amino acid residues that enable it to interact with negatively charged RNA molecules, particularly single-stranded RNAs (ssRNAs)[
10].
3. Ro60 controls the quality and cellular distribution of small RNAs
3.1. YRNAs
Ro60 is crucial for quality control, cellular distribution, and structural and functional surveillance of small RNAs including YRNAs and rRNAs. YRNAs are small (84-113 nucleotides (ntd) long) noncoding RNA molecules; in humans, there are four YRNAs: RNY1/hY1 (113 ntd; NR_004391.1), RNY3/hY3 (102 ntd; NR_004392.1), RNY4/hY4 (96 ntd; NR_004393.1), and RNY5/hY5 (85 ntd; NR_001571.2). YRNAs possess characteristic stem-loop structures with two main stems and single-stranded bulges. Ro60 and La/SSB (another SjD autoantigen and RBP) play essential roles in YRNA stabilisation, nuclear export, and cellular distribution.
Ro60 and La binding sites are conserved among all four YRNAs. YRNAs are stem bulge RNAs, with lower stem and upper stems (
Figure 1B). In the nucleus, YRNAs’ lower stem loops stably bind with RBPs, while upper stem loop regions mediate regulation of DNA replication [
11,
12] . La binds to YRNA’s 3' UCUUUU sequences, whilst Ro60 interacts with YRNA’s evolutionarily conserved lower stem (also known as the 5'-3' stem) region [
13]. The interaction between Ro60 and YRNAs relies on specific conserved nucleotide sequences, indicating a sequence-specific binding mechanism. The ninth nucleotide within the stem bulge at the 5'-3' terminus, within the conserved sequence "GGUC
CGA," is of critical importance (
Figure 1C) [
14,
15,
16] . The binding of Ro60 is essential for YRNA transport and quality control (
Figure 1D). YRNAs bind to the outer surface of the toroid ring, while single-stranded RNA nucleotide oligomers bind to the inside of the toroid hole [
7]. The Ro60 and La/SSB RNP complex exhibits considerable stability, with both Ro60 and La RBPs remaining in proximity due to protein interactions or the RBP-mediated stabilization of bound YRNAs. This interaction helps retain these two RBPs in the nucleus [
17]. Ro60-bound YRNA is exported to the cytoplasm in a process mediated by Ran GTPase [
18] (
Figure 1D).
Mature mRNA has 3' poly-A tail, while mature YRNAs have a poly-U tail. Polyadenylation marks YRNAs for exonuclease degradation. However, Ro60 protects YRNAs from polyadenylation [
19]. Importantly, YRNA binding to Ro60 facilitates binding of other small RNA molecules including 5S rRNA. 5S rRNA form complexes with Ro60 [
4,
20] , La/SSB [
20], L5, µL5, L11, µL18, and TFIIIA [
4]. Ro60 binding with 5S rRNA is prominent in the nucleoli, and such binding may be mediated by either protein-RNA or RNA-RNA interactions. The RNA-to-RNA interactions between the Ro60 RNP complex and 5S rRNA in the nucleus may be facilitated by physical interactions between hY4/hY5 and Ro60 [
4]. Ribosomal protein L5, which forms the L5-rRNA RNP complex, also binds to hY5, suggesting a possible association between 5S rRNA and YRNAs through L5-RNP/Ro60-RNP interactions. L5 plays a crucial role in the cellular trafficking of 5S rRNA and serves as a core component of the ribosomal machinery. YRNAs can physically interact with ribosome-unoccupied L5-5S rRNA RNP, thereby modulating its recruitment to Ro60. Human YRNAs vary in their internal loop region sequences and sizes (
Figure 1B). 5S rRNA interactions with Ro60 largely depend on the sequence and size of YRNAs' internal loop regions.
3.2.5. S rRNA
YRNA5, with its smaller internal loop region, is capable of binding with Ro60 and 5S rRNA, while larger internal loop regions create steric hindrance [
21]. The 5S rRNA is a fundamental component of the large ribosome subunit across all three domains of life: bacteria, archaea, and eukaryotes [
22]. All 5S rRNA molecules adopt a conserved secondary structure consisting of five double-stranded regions and five loops, including two hairpin loops, two internal loops, and a three-helix junction hinge region (
Figure 1E). In X. laevis, Ro60, in conjunction with the La/SSB protein, specifically binds to a variant of pre-5S rRNA that has nucleotide changes due to readthrough across the first termination signal, leading to 8-10 nucleotide extensions at the 3' end. The presence of Ro60 and La/SSB is essential for targeting these variant pre-5S rRNAs to discard pathways, which maintain a quality check on the structural and functional integrity of 5S rRNA moieties [
3]. The misfolded 5S rRNA precursor adopts an alternative helical structure, which allows it to bind to the central cavity of Ro60 via a single-stranded 3' extension [
7]. Similar to the misfolded defective pre-5S rRNA, wild type 5S rRNA can also adopt an alternative helical structure [
20]. Loss of Ro60 function in macrophages and lymphocytes can result in the misprocessing of 18S and 28S rRNAs, causing alterations in RNA length and promoting aberrant polyadenylation of these noncoding RNAs [
19]. Misprocessing of rRNAs can disrupt the protein biosynthesis machinery, as rRNAs play a crucial role in protein synthesis.
3.3. Alu and L1 retroelement transcripts
Up to half of the human genome is derived from retroelements, which share sequences, transcription and amplification characteristics with retroviruses; these include long interspersed nuclear elements (LINEs), short interspersed nuclear elements (SINEs), and endogenous retroviruses (ERVs). These elements undergo amplification through reverse transcription. Ro60 binds retroelement transcripts, and such binding reduces the ability of those transcripts to stimulate inflammation [
23]. Ro60 mounts qualitative and quantitative checks on retroelement transcription and amplification. Alu is the most common retroelement in primates [
24], and a small proportion of Alu elements are transcribed by RNA polymerase III. Alu transcripts have significant impacts on various cellular processes, including mRNA polyadenylation, RNA splicing, and adenosine deaminase acting on RNA (ADAR)-mediated A-to-I editing of RNA. Ro60 knockout cell lines had elevated Alu transcripts [
2]. In SLE patients, the level of Ro60 autoantibodies in the circulation correlated with the levels of circulating Alu RNA and expression of an IFN-stimulated gene signature [
2].
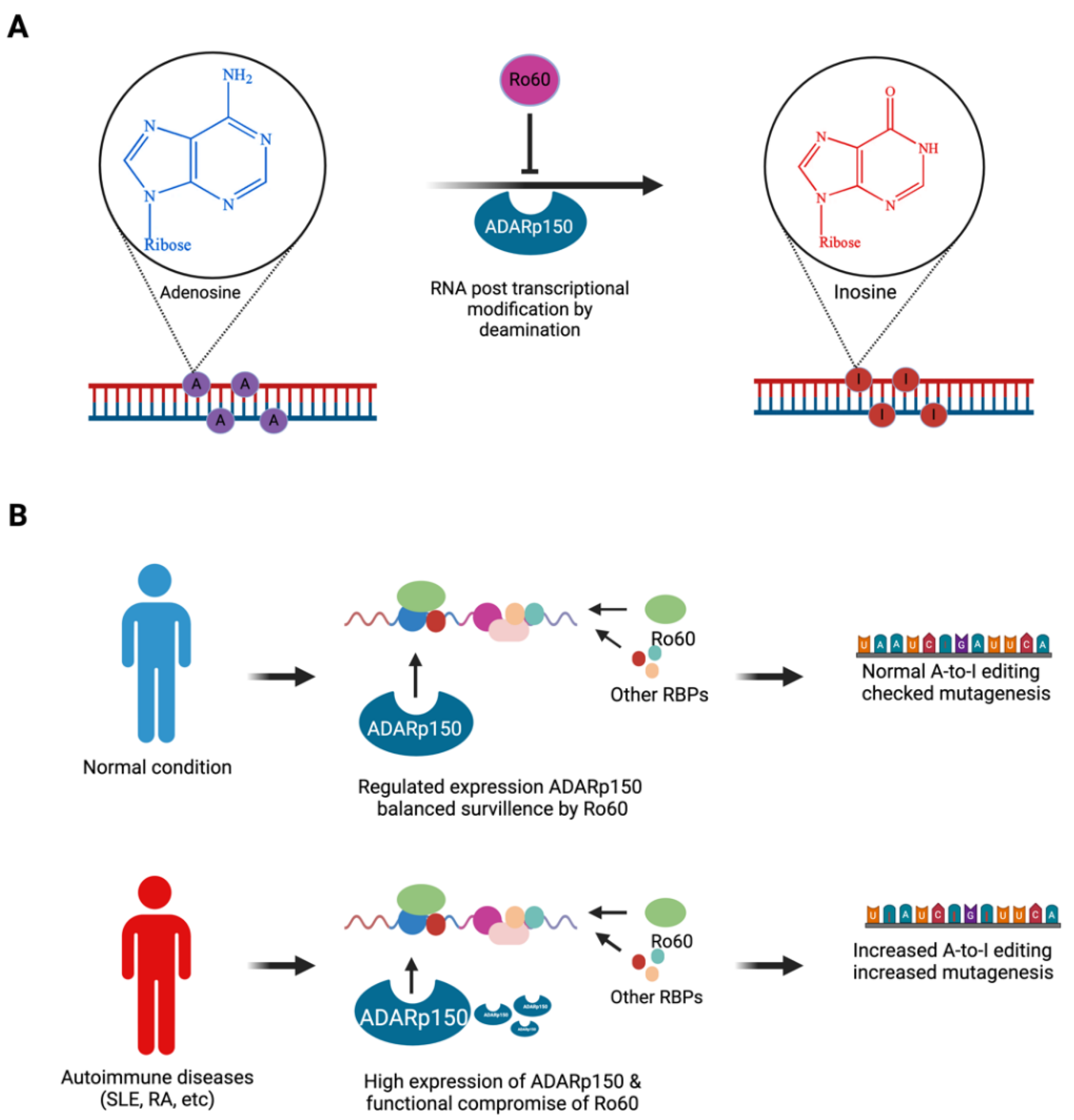
4. Ro60's role in RNA editing
RNA editing is a post-transcriptional modification process [
25], impacting protein-coding mRNA sequences, microRNA sequences, microRNA target sites, and nucleus-to-cytoplasm RNA export. Adenosine-to-inosine (A-to-I) modification is the most common RNA editing process, catalysed by ADARs [
26]; A-to-I modifications modulate developmental processes and disease progression, including autoimmune diseases [
27,
28] . ADAR enzymes (ADAR1, ADAR2, and ADAR3) are present in nearly in all tissues but particularly abundant in the central nervous system. Ro60 is one of several proteins that can modulate the efficiency and specificity of A-to-I RNA editing by ADARs [
29] (
Figure 2A). ADAR1-eCLIP analysis showed that Ro60 binds RNAs close to ADAR1 editing sites and modulates RNA editing positively and negatively. Dysregulation of RNA editing is observed in autoimmune diseases including rheumatoid arthritis (RA) and SLE. In SLE, loss of Ro60 function is associated with increased ADAR1p150, a specific isoform of ADAR1 [
30]; elevated ADAR1p150 expression drives RNA editing (
Figure 2B), potentially contributing to SLE pathogenesis [
30]. In RA, increased expression of ADAR1 and ADAR1p150 is associated with increased A-to-I modification of Alu retroelement transcripts [
31] (
Figure 2B). Ro60 RNP complexes also interact with other RNA editing enzymes, such as apolipoprotein-B mRNA editing enzyme catalytic polypeptide-like (APOBECs) [
32].
APOBECs (includes 3A, 3B, 3C, 3D, 3F, 3G, 3H, and AID) contribute to host defense by C to U conversions, which inhibit viral genomic integration [
33]. The association of APOBECs with Ro60 RNP suggests potential interplays between RNA editing and antiviral mechanisms. APOBEC3A, APOBEC3G, and AID expression were increased in salivary gland biopsies of SjD patients, compared to controls without SjD. APOBEC3A expression was elevated in the kidneys and blood cells of SLE patients [
30,
34] . Excessive RNA editing can be mutagenic. In SjD, higher expression of AID and APOBEC3G may contribute to an increased mutagenic load [
34]. In summary, the Ro60-RNP complex mediates modulation of A-to-I editing, and dysregulation of Ro60 functions associates with SjD, SLE, and RA pathogenesis.
5. Ro60 RNP complex and its extracellular presentation
The targeting of Ro60 RNPs by autoantibodies raises the question of whether and how Ro60 is exposed to the extracellular environment, thereby instigating an antibody response.
5.1. Components of the Ro60 RNP autoantigen complex
There is sparse evidence on the composition and structure of the Ro60 RNP-autoantibody complex. In autoimmune diseases such as SjD, SLE, RA, and systemic sclerosis (SSc), Ro60 autoantibodies often co-exist with autoantibodies targeting Ro52 (also called TRIM21) and La/SSB [
35]. The presence of these autoantibodies together might suggest the enrichment of Ro52 and La/SSB within autoantigenic RNP complexes. However, it is worth noting that Ro52 and Ro60 are distinct in their structure and function. Ro52 is an E3 ubiquitin ligase [
36], whilst Ro60 is an RNA-binding protein. It seems that there is no direct interaction between Ro52 and Ro60 autoantigens, and the historical artefacts and misunderstanding can be corrected by separating out these two autoantigens from the umbrella acronym ‘SSA.’ The ‘Ro’ in Ro60 and Ro52 is another historical artefact, referring to a patient identifier [
37]. One possibility is that calcium-dependent protein-protein bridging might play a role in bringing Ro52 into an autoimmune RNP complex [
38]. Ro60 RNP complexes are preloaded with a diverse range of small RNA moieties. Juste-Dolz et al [
38] reported that autoantibody-bound Ro60 undergoes a conformational change, such that antibody binding exposes a cryptic ‘Fc receptor’ within Ro60’s tertiary structure. Bound antibodies could then bridge additional Ro60 molecules to trigger immune complex (IC) growth. Antibodies bound to Ro60 may also bind via their Fc domain to Ro52’s PRY-SPRY domain [
36], possibly leading to artefactual detection of both autoantigens in Ro60 immunoprecipitants.
5.2. Ro60 RNP complex transportation and delivery to APCs
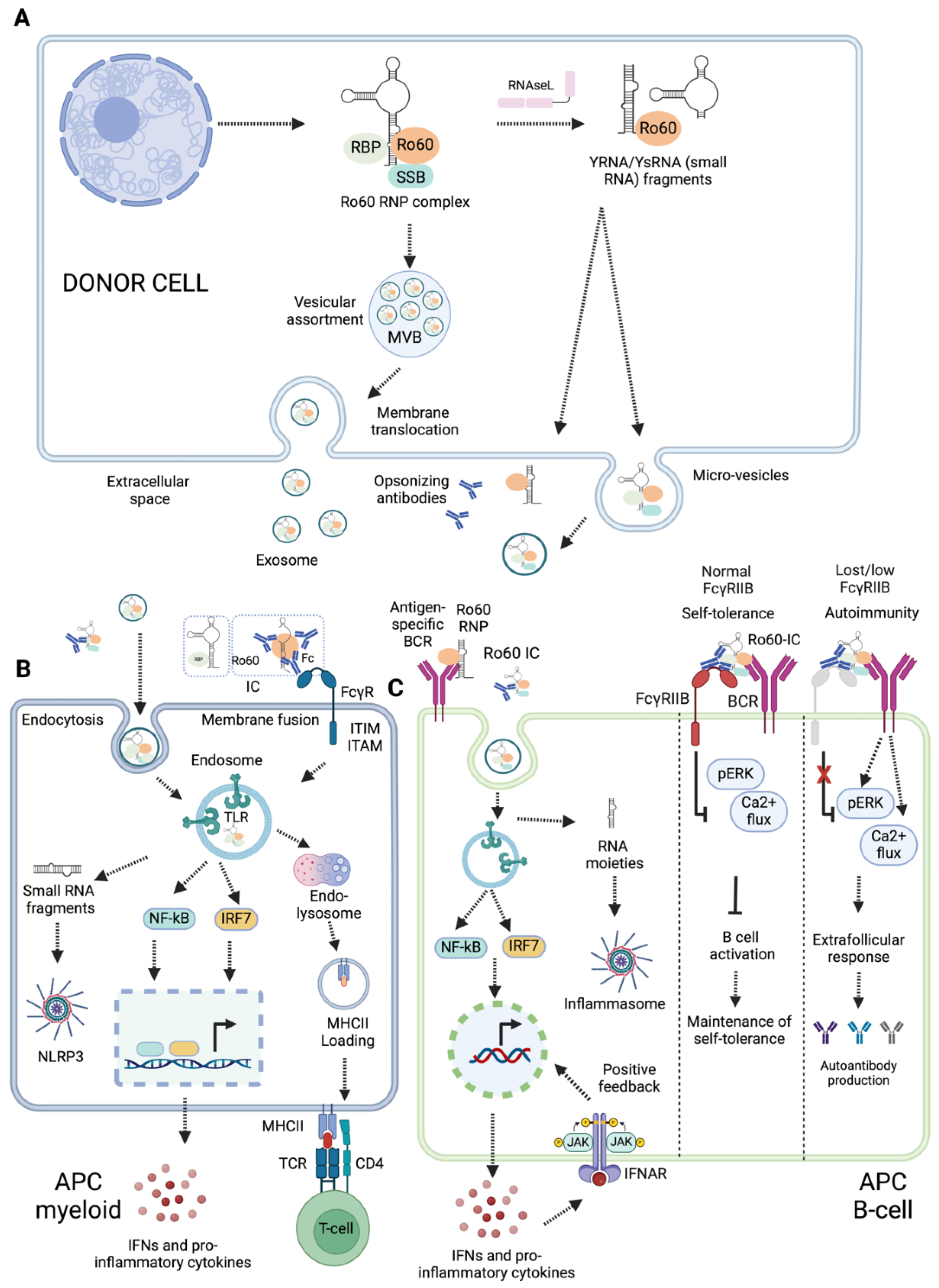
The Ro60-RNP complex is intracellular. The mechanisms for its delivery to antigen-presenting cells (APCs) are poorly understood. Based on available evidence, we propose three possible mechanisms through which the Ro60 RNP complex may be exposed in the extracellular space: small RNA-mediated extracellular translocation, extracellular vesicle-mediated delivery of Ro60 RNPs to APCs, or apoptotic and pyroptotic bleb-mediated exposure and delivery to APCs (
Figure 3A). Recent evidence suggests YRNAs are crucial for the XPO5/Ran GTPase-mediated transport of Ro60 from the nucleus to the cytoplasm and for its subcellular localization [
39] (
Figure 1D). In mouse fibroblasts, Ro60 RNPs translocated to the cell surface during early apoptosis in a manner dependent on their binding of mouse YRNA3 (mYR3) [
39]. A Ro60 mutant lacking mYR3 binding (H187S) was unable to translocate to the plasma membrane. Similarly, knockdown of mYR3 resulted in nuclear accumulation of Ro60 [
39]. However, a Ro60 mutant that retained mYR3 binding but lacked binding to 5S rRNA was still able to translocate to the plasma membrane [
39]. This suggests that both Ro60 and its bound small RNAs influence its localisation.
All cells synthesize and secrete extracellular vesicles (EVs) for short and long-distance cell-to-cell communication; these can deliver biomolecules, including nucleic acids, lipids, and proteins [
40]. EVs may serve as sources of autoantigens, contributing to a breach of self-tolerance. For example, EVs (exosomes) derived from epithelial cells of SjD patients were enriched in autoantigens such as La/SSB, Ro/SSA, and Sm RNPs [
41] (
Figure 3A). APCs can capture EVs by phagocytosis or receptor mediated endocytosis (
Figure 3B), process the antigens, and present them to T lymphocytes, thereby initiating an immune response [
41]. EVs carry Ro60-binding small RNAs and small RNA-derived fragments [
42,
43] . Their YRNA content can vary across source cells, as shown for PBMCs vs. neutrophils [
42]. EVs derived from murine cardiosphere cells were found to be enriched in YRNA4 fragments [
44], while EVs derived from cell lines were rich in YRNA5 fragments [
45]. Enrichment of specific autoantigens and small RNA molecules in EVs may contribute to breaching self-tolerance in autoimmune diseases.
Cell death may also expose Ro60 RNP complexes to the extracellular space. Apoptosis can occur in response to a wide range of stimuli, such as DNA damage, growth factor deficiency, and viral infection, and is considered non-immunogenic. However, immunogenic apoptosis triggers are less well understood. During apoptosis, subcellular redistribution of specific autoantigens, including Ro60, Ro52, and La/SSB antigens, has been observed [
46]. Autoantigenic targets associated with SLE are packaged into two types of blebs, originating from the fragmented nucleus and endoplasmic reticulum, in response to UV damage [
47]. Extracellular exposure of cryptic nuclear neoantigen epitopes, such as Ro60 and La/SSB, during apoptosis can lead to immune activation [
46,
48] . The exposed neoantigen epitopes from nuclear antigens are referred to as apotopes [
49]. The mechanism by which these epitopes become exposed in the extracellular environment can be investigated in vitro using stimulants that promote apoptosis, such as tumour necrosis factor-alpha (TNFα). Induction of apoptosis can also result in the cleavage of nuclear antigens [
50], potentially exposing neoepitopes. Pyroptotic and necroptotic cell death are pro-inflammatory lytic mechanisms that may release cytosolic contents.
6. Innate immune sensing of Ro60 RNP complexes and inflammation
APCs can engulf Ro60 RNP complexes with bound small RNA moieties. These small RNA molecules can engage with pattern recognition receptors (PRRs), serving as ‘danger’ signals to drive inflammation and adaptive immune responses
(Figure 3B). APCs can internalize Ro60-IC via Fc and complement receptors. Following uptake, RNAs derived from the Ro60 RNP complex might activate endosomal toll-like receptors (TLRs) and cytoplasmic RNA sensors (
Figure 3B). The outcomes of innate immune activation might depend on types of small RNA contained within Ro60 RNP complex; some small RNAs may promote pro-inflammatory responses [
51], potentially modulating immune responses. In this section, we emphasise the roles of Ro60 RNP complexes containing small RNAs in activating endosomal TLRs and cytoplasmic RNA sensors.
6.1. Endosomal TLR-mediated responses
Endosomal TLRs (TLR3, TLR7, and TLR8) recognize diverse viral RNA moieties and instigate host protective responses. TLR3 recognizes dsRNA, while both TLR7 and TLR8 sense ssRNAs. Fcγ receptor mediated IC endocytosis may expose Ro60 RNP-autoantibody complexes to endosomal TLRs (
Figure 3B). ICs containing Ro60 RNPs from apoptotic fibroblasts stimulated macrophages via TLR7, inducing the macrophages to secrete TNFα [
39]. Neonatal lupus, characterized by heart conduction abnormalities and foetal congenital heart block (CHB), is caused by transfer of maternal autoantibodies across the placenta [
52]. Irrespective of underlying rheumatic conditions, over 85% of women who give birth to babies with CHB have anti-Ro/anti-La/SSB antibodies, although only 2% of babies born from mothers with anti-Ro/anti-La/SSB antibodies develop CHB (42, 52, 53). This suggests that additional factors beyond autoantibodies are necessary for the development of neonatal lupus [
54]. Clancy et al. demonstrated that fibrosis induction in CHB may begin with TLR signalling. Ro60, bound RNAs (pre-5S rRNA and hY3), and anti-Ro60 IgG from CHB stimulated macrophages to secrete TNF-α in a manner dependent on FcγR and TLR7 [
54].
SLE-associated nephritis is commonly marked by immunoglobulin deposition and leukocyte recruitment at glomeruli. Administration of anti-TLR7 mAbs diminishes autoantibody production in lupus-prone mice, suggesting that RNA-stimulated TLR7 signaling promotes leukocyte recruitment and autoantibody production in SLE [
55]. ICs containing Ro60 and associated small RNAs (Alu transcripts, YRNAs) might engage with autoreactive B cells in a manner dependent on antigen-specific BCR, complement receptors, FcγRIIB and TLR7 [
56] (
Figure 3C). TLR recognition was dependent on RNA sequence complementarity or uridine content, and Fc receptor engagement was required to bring the ICs into the endosomal compartment for TLR recognition.
6.2. Activation of Inflammasomes
Inflammasomes are a group of innate intracellular immune receptors, which are activated in response to multiple damage-associated and pathogen-associated stimuli, including viral nucleic acids [
57]. Inflammasome activation leads to caspase-1-mediated processing and release of inflammatory cytokines IL-1β and IL-18, and to pro-inflammatory pyroptotic cell death. Potential inflammasome roles in autoimmune disease pathogenesis have been reviewed extensively [
58,
59,
60,
61] . In RA, dysregulated fibroblast-macrophage crosstalk drives release of pro-inflammatory cytokines including TNFα, IL-1β and IL-18. It is hypothesised that dysregulated pyroptotic cell death may contribute to driving this autoimmune disease [
60]. SjD patients’ PBMCs had increased expression of the NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome components ASC and caspase-1, as well as the NLRP3-induced cytokines IL-1β and IL-18, compared to healthy controls [
62] (
Figure 3B). Furthermore, immunofluorescent staining of salivary-gland (SG)-infiltrating macrophages from SjD patients showed evidence of increased pyroptotic activity by ASC speck formation, compared to healthy controls. Increased gene expression of inflammasome components was then correlated with anti-Ro/SSA positivity and higher SG focal scores, suggestive of increased disease severity [
63]. We speculate that pathogens which enter the body orally may activate innate immune pathways such as pyroptosis leading to epithelial cell death [
64]. For SjD pathogenesis, this may drive antigenic exposure, contributing to excessive autoimmune inflammation and SG dysfunction.
It remains unclear whether Ro60 RNPs can directly contribute to NLRP3 inflammasome activation and pyroptotic cell death. The NLRP3 inflammasome can be activated by cytosolic viral RNA [
65], and it has been proposed that endogenous RNAs and retroelements may also be able to activate NLRP3 [
66,
67] . As Ro60 is known to bind endogenous retroelement transcripts [
23], it is possible that Ro60 may stabilise or promote retroelement-specific NLRP3 activity in a manner similar to that reported for DEAD/H-box RNA binding proteins [
68,
69] . Furthermore, NLRP3 is a key target of TLR7 signaling, and Ro60-associated RNAs may augment NLRP3 inflammasome expression indirectly through TLR7 [
70]. Potential Ro60-NLRP3 interactions are worth further investigation.
6.3. Ro60 antigen presentation to T cells
APCs can process and present peptides from internalized Ro60 to CD4+T cells through the major histocompatibility complex (MHC)II pathway. APCs and other cells can process and present endogenous Ro60 to CD8+ T cells, through MHCI. In normal conditions, self-tolerance mechanisms should prevent immune responses to autologous Ro60. Ro60 antigenic epitopes for T cells have been studied in various autoimmune conditions. In SjD, a dominant Ro60 epitope is the peptide sequence ELYKEKALSVETEKLLKYLEAV, between amino acids 211-232 within the RNA-binding TROVE domain [
71]. In SLE, Ro60 epitopes were identified in amino acids 169-190, also in the TROVE domain [
71,
72,
73] . MHCII expression is predominantly a feature of APCs, and its expression on non-APCs can be defined as a pathogenic feature. Immunization of mice with adjuvant-emulsified Ro60_316-335 led to infiltration of T and B cells into lacrimal and salivary glands, as well as induction of ectopic MHCII expression, consistent with a SjD-like process [
74,
75] . Characterising Ro60 epitopes may help to understand the immune response and develop targeted therapeutic approaches for autoimmune disease where Ro60 is an autoantigen.
6.4. B cell activation by Ro60 RNP
Self-reactive B cells can engage with Ro60 RNP complex and its derivates (
Figure 3C). These interactions can include those between Ro60-specific BCR and Ro60 RNP complexes, antigen-specific BCR and Ro60 immune complexes (ICs), FcγRIIB and Ro60 ICs, and co-engagement of FcγRIIB and antigen-specific BCR (
Figure 3C). BCR and IC engagement can promote endosomal TLR signalling, potentially inducing an IFNα positive feedback loop and driving extrafollicular B cell responses, germinal centre reactions, and autoantibody production [
76] (
Figure 3C). B cells express the inhibitory receptor FcγRIIB, which may provide negative feedback to as a self-tolerance mechanism. FcγRIIB co-engagement with BCR-IC negatively regulates BCR-TLR7 endosomal response pathways [
77]. Additional interactions between Ro60-RNP complexes and B cells can occur via complement receptors, which can reduce B cell activation thresholds whilst FcγRIIB engagement dampens B cell activation and autoimmunity [
78,
79] . SLE patients are reported to have reduced expression of FcγRIIB [
79]. Dysregulation of BCR and FcγRIIB signalling promotes an autoinflammatory loop in an Interleukin-1 Receptor-Associated Kinase 4 dependent manner [
80]. B cell-intrinsic TLR7 signalling promotes anti-RNA antibody synthesis and lupus pathogenesis in lupus-prone mice [
81]. It will be important to uncover whether and how Ro60 RNP complexes promote TLR7 signalling to drive pathogenesis in other autoimmune diseases.
7. Ro60 autoantibodies in diagnosis and monitoring of autoimmune disease
Ro60 is an extractable nuclear antigen, and serum Ro60 autoantibodies are common diagnostic markers for various autoimmune disease including SjD, SLE, SSc, and neuropsychiatric SLE (49, 82, 83). Anti-Ro60 autoantibodies may co-exist with autoantibodies targeting other RNPs, such as La/SSB, and the E3 ubiquitin ligase Ro52 (TRIM21). Not all diagnostic testing distinguishes autoantibodies to Ro60 from those targeting Ro52; these may be grouped under the term ‘SSA’ or ‘SSA/Ro’, with ‘Ro’ referring to a particular patient serum sample [
37]. Ro60 autoantibodies can be detected years before the clinical onset of autoimmune disease [
84,
85] . Blood is the most common biosample used in diagnosis of autoimmune disease; however, depending on autoimmune condition, tears, saliva, and CSF may also be used [
86,
87] . Anti-Ro/SSA antibodies are not standalone markers; therefore, detection of anti-SSA antibodies is accompanied with other diagnostic modalities [
88,
89] . However, sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of these diagnostic approaches may vary, and combinations of methods are recommended in clinical settings [
89]. Using sera specimens of 184 autoimmune disease patients and 50 controls, Qin et al. evaluated the analytical and clinical performance of line immunoassay, multiplex bead-based flow fluorescent immunoassay, and magnetic bar code immunoassay in detection of antinuclear antibodies, including those targeting Ro60 and Ro52; they found that the three assays showed good agreement [
90]. Diagnostic accuracy is an important parameter in autoantibody detection, requiring consistent refinement of validation assays.
The classification of autoimmune patients into different categories can be achieved through approaches like counter immune electrophoresis followed by line immunoblotting to stratify patients into anti-Ro60
low and anti-Ro60
high groups. Ro60
low patients’ anti-Ro60 antibodies have fewer somatic mutations with restricted heavy variable gene use [
89]. The diagnostic and prognostic significance of anti-Ro60 autoantibodies is not uniformly established, and comparative measures of sensitivity and specificity are lacking. Anti-Ro60 in combination with anti-RLP0, anti-RLP1, and anti-RLP2 autoantibodies in CSF may serve as diagnostic markers for neuropsychiatric SLE [
88]. In SjD, combined reactivity of anti-Ro52, anti-Ro60, and anti-La is associated with increased lymphoma risk [
91]. Overall, the combinations of autoantibodies and their levels provide valuable information for disease classification, estimation of disease severity, and prediction of associated complications. It is also imperative to understand whether anti-Ro60 antibody levels in autoimmune patients vary with time and disease severity. Derksen
et al monitored anti-Ro60 antibodies over a period of 80 months spanning two pregnancies in an SLE patient, and found that anti-Ro60 antibody levels fluctuated over time in a manner unrelated to disease severity index, anti-dsDNA antibody level, or immunosuppressive therapies [
92]. Contrarily, Lindop
et al reported that in the case of SjD, anti-Ro60 antibody levels remained stable from months to years [
84].
Understanding autoantibody dynamics may be useful for monitoring and devising new therapies for autoimmune diseases. RA patients with a positive anti-SSA diagnosis are more likely to develop anti-drug antibodies, limiting the efficacy of the TNF-α antagonists Infliximab [
93] and Adalimumab [
94]. Whilst an ‘anti-SSA positive’ diagnosis could mean Ro52
+/Ro60
-, Ro52
-/Ro60
+, or Ro52
+/Ro60
+, differential diagnosis of Ro60 and Ro52 antibodies has diagnostic and prognostic utility [
82,
95] . Ro52
-/Ro60
+ patients were more likely to have SLE, whilst Ro52
+/Ro60
+ patients were more likely to have SjD; Ro52
+/Ro60
- patients were more likely to have other inflammatory diseases [
82]. Furthermore, in SjD, differential and combinatorial positive diagnosis of anti-Ro60, anti-Ro52, and anti-La/SSB antibodies associates with different clinical features, suggesting separate assessment of anti-Ro52 and anti-Ro60 antibodies for efficient patient stratification to allow development of tailored treatment plans [
95]. To overcome all the potential confusions, it may be advisable to discontinue the use of the SSA acronym in favour of specifically designating Ro60 and Ro52.
8. Commensals, Ro60 cross-reactivity, and autoimmunity
Commensal microorganisms—our microbiome—reside in and on various part of the body, including the gastrointestinal tract, skin, oral cavity, and other mucosal surfaces. The diversity and composition of the microbiome varies among different individuals and across different sites within the body of same individual. The human gastrointestinal tract can contain 10
13-10
14 microorganisms, equalling or exceeding the number of human cells in the body, and up to ~100 times more genetic information than the human genome [
96]. Dysbiosis, i.e. changes in microbiome composition or overgrowth of certain microbes, has been associated with various pathophysiological conditions, including autoimmune diseases [
97].
Ro60 is evolutionarily conserved, and its orthologues are present in commensal bacteria. Greiling et al. identified Ro60 orthologues in subsets of microbes that colonise the human oral mucosa, skin, and gut [
98]. Antibodies from Ro60-seropositive SLE patient sera immunoprecipitated bacterial Ro60 RNPs from the normal microbiome constituent species,
Propionobacterium propionicum (
Pp, oral and skin microbiomes) and
Bacteroides thetaiotaomicron (
Bt, gut microbiome). The immunoprecipitated RNPs included bacterial YRNAs. Peptides from
Pp and
Bt Ro60 also stimulated Ro60-reactive T cell clones from SLE patients. Germ-free mice developed T and B cell responses cross-reactive to human Ro60 following colonisation with Bt [
98]. In another study, Szymula et al. reported that peptides derived from the TROVE and vWFA domains of bacterial Ro60 orthologues could stimulate T cell hybridomas specific for human Ro60 epitopes [
99]. The bacterium
Capnocytophaga ochracea, which is part of the normal oral microbiome, was identified as a potential source of cross-reactive Ro60 peptides [
99]. These reports suggest that Ro60-autoreactive cells may respond to normal constituents of the human microbial flora; importantly, patients with Ro60 autoimmunity did not have readily identifiable changes in microbiome makeup compared to healthy volunteers [
98]. Antigenic cross-reactivity of orthologues and dysbiosis should be considered as potential causal mechanisms that may disrupt self-tolerance and, if left unchecked, lead to autoimmunity.
9. Conclusions and future perspectives
Ro60 is a highly conserved protein which binds and controls the cellular distribution of small RNAs, in particular YRNAs and rRNAs. Importantly, Ro60 provides cellular surveillance by binding retroelements such as Alu transcripts, which may otherwise drive inflammation, excessive RNA editing, mutagenesis, and possible malignancies. When bound to YRNAs, Ro60 RNPs may also contribute to innate immune processes such as NLRP3 inflammasome activation. However, our understanding of these possible interactions is sparse and warrants further investigation. Ro60 as an autoantigen is well-established in multiple autoimmune diseases including SjD, SLE, RA and SSc, whereby anti-Ro60 autoantibodies are commonly detected. However, the mechanisms by which Ro60 is targeted by the immune response remain unclear. In this review we outline various potential mechanisms including secretion of Ro60 RNP complexes within EVs, exposure during immunogenic forms of cell death, TLR-mediated stimulation, and possible cross-reactivity from commensal microorganisms. Further studies should explore whether such processes truly drive anti-Ro60 autoantibody production. In vitro studies could investigate possible contributions of Ro60 RNPs to forms of pro-inflammatory lytic cell death, and how EVs containing Ro60 RNPs are taken up and enable a breach in self-tolerance. Together, these studies will contribute to our understanding of how and why Ro60 becomes an autoantigen.
Author Contributions
Writing—original draft preparation, RSM and ELJ; writing—review and editing, RSM, ELJ, and LBD; figure preparation, RSM; funding acquisition, LBD and RSM. All authors have read and agreed to the published version of the manuscript.
Funding
R.S.M. is an industrial postdoc fellow receiving a career development fellowship from the Oxford-Bristol Myers Squibb-Celgene Research Fellowship Programme, and has received additional funding from EULAR (No. Q323RSV101). ELJ is supported by a KTRR Studentship from the Kennedy Trust for Rheumatology Research (KENN 19 20 21), and by a Henni Mester Fellowship (University College, University of Oxford). The funders had no part in the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Radziszewska A, Webb K, Peckham H, Ioannou Y. Ro60 Expression Decreases with Age in Peripheral Blood Mononuclear Cells of Children and Adolescents and Correlates with Tlr7 Stimulation in Pdcs of Prepubertal Children. Ann Rheum Dis. 2017;76:389.
- Hung, T.; Pratt, G.A.; Sundararaman, B.; Townsend, M.J.; Chaivorapol, C.; Bhangale, T.; Graham, R.R.; Ortmann, W.; Criswell, L.A.; Yeo, G.W.; et al. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science 2015, 350, 455–459, . [CrossRef]
- A O'Brien, C.; Wolin, S.L. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors.. Genes Dev. 1994, 8, 2891–2903, . [CrossRef]
- Campos-Almaraz, M.; Fraire-Velazquez, S.; Moreno, J.; Herrera-Esparza, R. The 5S rRNA is associated with Ro60 ribonucleoprotein and is co-precipitated with hYRNAs by anti-Ro antibodies.. Autoimmunity 1999, 31, 95–101, . [CrossRef]
- Ramesh, A.; Savva, C.G.; Holzenburg, A.; Sacchettini, J.C. Crystal Structure of Rsr, an Ortholog of the Antigenic Ro Protein, Links Conformational Flexibility to RNA Binding Activity. 2007, 282, 14960–14967, . [CrossRef]
- Fuchs, G.; Stein, A.J.; Fu, C.; Reinisch, K.M.; Wolin, S.L. Structural and biochemical basis for misfolded RNA recognition by the Ro autoantigen. Nat. Struct. Mol. Biol. 2006, 13, 1002–1009, . [CrossRef]
- Stein, A.J.; Fuchs, G.; Fu, C.; Wolin, S.L.; Reinisch, K.M. Structural Insights into RNA Quality Control: The Ro Autoantigen Binds Misfolded RNAs via Its Central Cavity. Cell 2005, 121, 529–539, . [CrossRef]
- Colombatti, A.; Bonaldo, P.; Doliana, R. Type A Modules: Interacting Domains Found in Several Non-Fibrillar Collagens and in Other Extracellular Matrix Proteins. Matrix 1993, 13, 297–306, . [CrossRef]
- Whittaker, C.A.; Hynes, R.O. Distribution and Evolution of von Willebrand/Integrin A Domains: Widely Dispersed Domains with Roles in Cell Adhesion and Elsewhere. Mol. Biol. Cell 2002, 13, 3369–3387, . [CrossRef]
- Bateman, A.; Kickhoefer, V. The TROVE module: A common element in telomerase, Ro and Vault ribonucleoproteins. BMC Bioinform. 2003, 4, 49–49, . [CrossRef]
- Wang, I.; Kowalski, M.P.; Langley, A.R.; Rodriguez, R.; Balasubramanian, S.; Hsu, S.-T.D.; Krude, T. Nucleotide Contributions to the Structural Integrity and DNA Replication Initiation Activity of Noncoding Y RNA. Biochemistry 2014, 53, 5848–5863, . [CrossRef]
- Kowalski, M.P.; Krude, T. Functional roles of non-coding Y RNAs. Int. J. Biochem. Cell Biol. 2015, 66, 20–29, . [CrossRef]
- Mathews, M.B.; Francoeur, A.M. La Antigen Recognizes and Binds to the 3′-Oligouridylate Tail of a Small RNA. Mol. Cell. Biol. 1984, 4, . [CrossRef]
- Green, C.D.; Long, K.S.; Shi, H.; Wolin, S.L. Binding of the 60-kDa Ro autoantigen to Y RNAs: Evidence for recognition in the major groove of a conserved helix. RNA 1998, 4, 750–765, . [CrossRef]
- Wolin, S.L.; A Steitz, J. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs.. Proc. Natl. Acad. Sci. 1984, 81, 1996–2000, . [CrossRef]
- Pruijn, G.J.; Siobbe, R.L.; Venrooij, W.J. Analysis of protein-RNA interactions within Ro ribonucleoprotein complexes. Nucleic Acids Res. 1991, 19, 5173–5180, . [CrossRef]
- Langley, A.R.; Chambers, H.; Christov, C.P.; Krude, T. Ribonucleoprotein Particles Containing Non-Coding Y RNAs, Ro60, La and Nucleolin Are Not Required for Y RNA Function in DNA Replication. PLOS ONE 2010, 5, e13673, . [CrossRef]
- Rutjes, S.A.; Lund, E.; VAN DER Heijden, A.; Grimm, C.; VAN Venrooij, W.J.; Pruijn, G.J. Identification of a novel cis-acting RNA element involved in nuclear export of hY RNAs. RNA 2001, 7, 741–752, . [CrossRef]
- Spurlock CF, 3rd, Tossberg JT, Guo Y, Sriram S, Crooke PS, 3rd, Aune TM. Defective structural RNA processing in relapsing-remitting multiple sclerosis. Genome Biol. 2015;16(1):58.
- Shi, H.; A O'Brien, C.; Van Horn, D.J.; Wolin, S.L. A misfolded form of 5S rRNA is complexed with the Ro and La autoantigens.. 1996, 2, 769–84.
- Hogg JR, Collins K. Human Y5 RNA specializes a Ro ribonucleoprotein for 5S ribosomal RNA quality control. Genes Dev. 2007;21(23):3067-72.
- Szymanski, M.; Zielezinski, A.; Barciszewski, J.; Erdmann, V.A.; Karlowski, W.M. 5SRNAdb: an information resource for 5S ribosomal RNAs. Nucleic Acids Res. 2016, 44, D180–D183, . [CrossRef]
- Hung, T.; Pratt, G.A.; Sundararaman, B.; Townsend, M.J.; Chaivorapol, C.; Bhangale, T.; Graham, R.R.; Ortmann, W.; Criswell, L.A.; Yeo, G.W.; et al. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science 2015, 350, 455–459, . [CrossRef]
- Deininger, P.L. Alu elements: know the SINEs. Genome Biol. 2011, 12, 236, . [CrossRef]
- Zinshteyn B, Nishikura K. Adenosine-to-inosine RNA editing. Wiley Interdiscip Rev Syst Biol Med. 2009;1(2):202-9.
- A Savva, Y.; E Rieder, L.; A Reenan, R. The ADAR protein family. Genome Biol. 2012, 13, 252–252, . [CrossRef]
- Vlachogiannis, N.I.; Tual-Chalot, S.; Zormpas, E.; Bonini, F.; Ntouros, P.A.; Pappa, M.; Bournia, V.-K.; Tektonidou, M.G.; Souliotis, V.L.; Mavragani, C.P.; et al. Adenosine-to-inosine RNA editing contributes to type I interferon responses in systemic sclerosis. J. Autoimmun. 2021, 125, 102755, . [CrossRef]
- Li, Q.; Gloudemans, M.J.; Geisinger, J.M.; Fan, B.; Aguet, F.; Sun, T.; Ramaswami, G.; Li, Y.I.; Ma, J.-B.; Pritchard, J.K.; et al. RNA editing underlies genetic risk of common inflammatory diseases. Nature 2022, 608, 569–577, . [CrossRef]
- Tariq, A.; Garncarz, W.; Handl, C.; Balik, A.; Pusch, O.; Jantsch, M.F. RNA-interacting proteins act as site-specific repressors of ADAR2-mediated RNA editing and fluctuate upon neuronal stimulation. Nucleic Acids Res. 2013, 41, 2581–2593, . [CrossRef]
- Quinones-Valdez, G.; Tran, S.S.; Jun, H.-I.; Bahn, J.H.; Yang, E.-W.; Zhan, L.; Brümmer, A.; Wei, X.; Van Nostrand, E.; Pratt, G.A.; et al. Regulation of RNA editing by RNA-binding proteins in human cells. Commun. Biol. 2019, 2, 19, . [CrossRef]
- Vlachogiannis, N.; Gatsiou, A.; Silvestris, D.A.; Stamatelopoulos, K.; Tektonidou, M.G.; Gallo, A.; Sfikakis, P.P.; Stellos, K. Increased adenosine-to-inosine RNA editing in rheumatoid arthritis. J. Autoimmun. 2020, 106, 102329, . [CrossRef]
- Vasudevan, A.A.J.; Smits, S.H.; Höppner, A.; Häussinger, D.; Koenig, B.W.; Münk, C. Structural features of antiviral DNA cytidine deaminases. Biol. Chem. 2013, 394, 1357–1370, . [CrossRef]
- Gallois-Montbrun, S.; Kramer, B.; Swanson, C.M.; Byers, H.; Lynham, S.; Ward, M.; Malim, M.H. Antiviral Protein APOBEC3G Localizes to Ribonucleoprotein Complexes Found in P Bodies and Stress Granules. J. Virol. 2007, 81, 2165–2178, . [CrossRef]
- Mavragani, C.P.; Kirou, K.A.; Nezos, A.; Seshan, S.; Wild, T.; Wahl, S.M.; Moutsopoulos, H.M.; Crow, M.K. Expression of APOBEC family members as regulators of endogenous retroelements and malignant transformation in systemic autoimmunity. Clin. Immunol. 2021, 223, 108649, . [CrossRef]
- Fayyaz A, Kurien BT, Scofield RH. Autoantibodies in Sjogren's Syndrome. Rheum Dis Clin North Am. 2016;42(3):419-34.
- Jones EL, Laidlaw SM, Dustin LB. TRIM21/Ro52 - Roles in Innate Immunity and Autoimmune Disease. Frontiers in immunology. 2021;12:738473.
- Clark, G.; Reichlin, M.; Tomasi, T.B. Characterization of a Soluble Cytoplasmic Antigen Reactive with Sera from Patients with Systemic Lupus Erythmatosus. J. Immunol. 1969, 102, 117–122, . [CrossRef]
- Juste-Dolz, A.; Nascimento, N.M.D.; Monzó, I.; Grau-García, E.; Román-Ivorra, J.A.; Lopez-Paz, J.L.; Escorihuela, J.; Puchades, R.; Morais, S.; Gimenez-Romero, D.; et al. New structural insights into the role of TROVE2 complexes in the on-set and pathogenesis of systemic lupus erythematosus determined by a combination of QCM-D and DPI. Anal. Bioanal. Chem. 2019, 411, 4709–4720, . [CrossRef]
- Reed, J.H.; Sim, S.; Wolin, S.L.; Clancy, R.M.; Buyon, J.P. Ro60 Requires Y3 RNA for Cell Surface Exposure and Inflammation Associated with Cardiac Manifestations of Neonatal Lupus. J. Immunol. 2013, 191, 110–116, . [CrossRef]
- Gao, Y.; Qin, Y.; Wan, C.; Sun, Y.; Meng, J.; Huang, J.; Hu, Y.; Jin, H.; Yang, K. Small Extracellular Vesicles: A Novel Avenue for Cancer Management. Front. Oncol. 2021, 11, . [CrossRef]
- Kapsogeorgou, E.K.; Abu-Helu, R.F.; Moutsopoulos, H.M.; Manoussakis, M.N. Salivary gland epithelial cell exosomes: A source of autoantigenic ribonucleoproteins. Arthritis Rheum. 2005, 52, 1517–1521, . [CrossRef]
- Driedonks, T.A.P.; Mol, S.; De Bruin, S.; Peters, A.L.; Zhang, X.; Lindenbergh, M.F.S.; Beuger, B.M.; Van Stalborch, A.-M.D.; Spaan, T.; De Jong, E.C.; et al. Y-RNA subtype ratios in plasma extracellular vesicles are cell type-specific and are candidate biomarkers for inflammatory diseases. J. Extracell. Vesicles 2020, 9, doi:10.1080/20013078.2020.1764213.
- Driedonks, T.A.P.; Nolte-'t Hoen, E.N.M. Circulating Y-RNAs in Extracellular Vesicles and Ribonucleoprotein Complexes; Implications for the Immune System. Front. Immunol. 2019, 9, 3164–3178, . [CrossRef]
- Cambier, L.; De Couto, G.; Ibrahim, A.; Echavez, A.K.; Valle, J.; Liu, W.; Kreke, M.; Smith, R.R.; Marbán, L.; Marbán, E. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 2017, 9, 337–352, doi:10.15252/emmm.201606924.
- Chakrabortty, S.K.; Prakash, A.; Nechooshtan, G.; Hearn, S.; Gingeras, T.R. Extracellular vesicle-mediated transfer of processed and functional RNY5 RNA. RNA 2015, 21, 1966–1979, . [CrossRef]
- Ohlsson M, Jonsson R, Brokstad KA. Subcellular redistribution and surface exposure of the Ro52, Ro60 and La48 autoantigens during apoptosis in human ductal epithelial cells: a possible mechanism in the pathogenesis of Sjogren's syndrome. Scand J Immunol. 2002;56(5):456-69.
- Rosen, A.; Casciola-Rosen, L.; Ahearn, J. Novel packages of viral and self-antigens are generated during apoptosis.. J. Exp. Med. 1995, 181, 1557–1561, . [CrossRef]
- Pan, Z.; Davis, K.; Maier, S.; Bachmann, M.P.; Kim-Howard, X.R.; Keech, C.; Gordon, T.P.; McCluskey, J.; Farris, A.D. Neo-epitopes are required for immunogenicity of the La/SS-B nuclear antigen in the context of late apoptotic cells. Clin. Exp. Immunol. 2006, 143, 237–248, . [CrossRef]
- Reed, J.H.; Jackson, M.W.; Gordon, T.P. A B cell apotope of Ro 60 in systemic lupus erythematosus. Arthritis Rheum. 2008, 58, 1125–1129, . [CrossRef]
- McArthur C, Wang Y, Veno P, Zhang J, Fiorella R. Intracellular trafficking and surface expression of SS-A (Ro), SS-B (La), poly(ADP-ribose) polymerase and alpha-fodrin autoantigens during apoptosis in human salivary gland cells induced by tumour necrosis factor-alpha. Arch Oral Biol. 2002;47(6):443-8.
- Hizir, Z.; Bottini, S.; Grandjean, V.; Trabucchi, M.; Repetto, E. RNY (YRNA)-derived small RNAs regulate cell death and inflammation in monocytes/macrophages. Cell Death Dis. 2017, 8, e2530–e2530, . [CrossRef]
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274.
- Buyon JP, Saxena A, Izmirly PM, Cuneo B, Wainwright B. Neonatal lupus: Clinical spectrum, biomarkers, pathogenesis, and approach to treatment. Systemic Lupus Erythematosus (Second Edition). Academic Press. 2021:507-19.
- Clancy, R.M.; Alvarez, D.; Komissarova, E.; Barrat, F.J.; Swartz, J.; Buyon, J.P. Ro60-Associated Single-Stranded RNA Links Inflammation with Fetal Cardiac Fibrosis via Ligation of TLRs: A Novel Pathway to Autoimmune-Associated Heart Block. J. Immunol. 2010, 184, 2148–2155, . [CrossRef]
- Murakami, Y.; Fukui, R.; Tanaka, R.; Motoi, Y.; Kanno, A.; Sato, R.; Yamaguchi, K.; Amano, H.; Furukawa, Y.; Suzuki, H.; et al. Anti-TLR7 Antibody Protects Against Lupus Nephritis in NZBWF1 Mice by Targeting B Cells and Patrolling Monocytes. Front. Immunol. 2021, 12, . [CrossRef]
- Green, N.M.; Moody, K.-S.; Debatis, M.; Marshak-Rothstein, A. Activation of Autoreactive B Cells by Endogenous TLR7 and TLR3 RNA Ligands. J. Biol. Chem. 2012, 287, 39789–39799, . [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687, doi:10.1038/nm.3893.
- Shin, J.I.; Lee, K.H.; Joo, Y.H.; Lee, J.M.; Jeon, J.; Jung, H.J.; Shin, M.; Cho, S.; Kim, T.H.; Park, S.; et al. Inflammasomes and autoimmune and rheumatic diseases: A comprehensive review. J. Autoimmun. 2019, 103, 102299, . [CrossRef]
- Chen, B.; Wang, Y.; Chen, G. New Potentiality of Bioactive Substances: Regulating the NLRP3 Inflammasome in Autoimmune Diseases. Nutrients 2023, 15, 4584, . [CrossRef]
- Demarco, B.; Danielli, S.; Fischer, F.A.; Bezbradica, J.S. How Pyroptosis Contributes to Inflammation and Fibroblast-Macrophage Cross-Talk in Rheumatoid Arthritis. Cells 2022, 11, 1307, . [CrossRef]
- Shaw, P.J.; McDermott, M.F.; Kanneganti, T.-D. Inflammasomes and autoimmunity. Trends Mol. Med. 2011, 17, 57–64, . [CrossRef]
- Kim SK, Choe JY, Lee GH. Enhanced expression of NLRP3 inflammasome-related inflammation in peripheral blood mononuclear cells in Sjogren's syndrome. Clin Chim Acta. 2017;474:147-54.
- Vakrakou AG, Boiu S, Ziakas PD, Xingi E, Boleti H, Manoussakis MN. Systemic activation of NLRP3 inflammasome in patients with severe primary Sjogren's syndrome fueled by inflammagenic DNA accumulations. J Autoimmun. 2018;91:23-33.
- Verstappen, G.M.; Pringle, S.; Bootsma, H.; Kroese, F.G.M. Epithelial–immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis. Nat. Rev. Rheumatol. 2021, 17, 333–348, . [CrossRef]
- Zhao, C.; Zhao, W. NLRP3 Inflammasome-A Key Player in Antiviral Responses. Front. Immunol. 2020, 11, 211, . [CrossRef]
- Mu X, Ahmad S, Hur S. Endogenous Retroelements and the Host Innate Immune Sensors. Adv Immunol. 2016;132:47-69.
- Tarallo, V.; Hirano, Y.; Gelfand, B.D.; Dridi, S.; Kerur, N.; Kim, Y.; Gil Cho, W.; Kaneko, H.; Fowler, B.J.; Bogdanovich, S.; et al. DICER1 Loss and Alu RNA Induce Age-Related Macular Degeneration via the NLRP3 Inflammasome and MyD88. Cell 2012, 149, 847–859, . [CrossRef]
- Li, J.; Hu, L.; Liu, Y.; Huang, L.; Mu, Y.; Cai, X.; Weng, C. DDX19A Senses Viral RNA and Mediates NLRP3-Dependent Inflammasome Activation. 2015, 195, 5732–5749, . [CrossRef]
- Mitoma, H.; Hanabuchi, S.; Kim, T.; Bao, M.; Zhang, Z.; Sugimoto, N.; Liu, Y.-J. The DHX33 RNA Helicase Senses Cytosolic RNA and Activates the NLRP3 Inflammasome. Immunity 2013, 39, 123–135, . [CrossRef]
- Lee, J.; Mohammad, N.; Lu, Y.; Kang, K.; Han, K.; Brantly, M. Alu RNA induces NLRP3 expression through TLR7 activation in α-1-antitrypsin–deficient macrophages. J. Clin. Investig. 2022, 7, . [CrossRef]
- Routsias, J.G.; Tzioufas, A.G.; Sakarellos-Daitsiotis, M.; Sakarellos, C.; Moutsopoulos, H.M. Epitope mapping of the Ro/SSA60KD autoantigen reveals disease-specific antibody-binding profiles. Eur. J. Clin. Investig. 1996, 26, 514–521, . [CrossRef]
- McClain, M.T.; Heinlen, L.D.; Dennis, G.J.; Roebuck, J.; Harley, J.B.; A James, J. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat. Med. 2005, 11, 85–89, . [CrossRef]
- Wolin, S.L.; Reinisch, K.M. The Ro 60 kDa autoantigen comes into focus: Interpreting epitope mapping experiments on the basis of structure. Autoimmun. Rev. 2006, 5, 367–372, . [CrossRef]
- Yin J, Zheng J, Deng F, Zhao W, Chen Y, Huang Q, et al. Gene Expression Profiling of Lacrimal Glands Identifies the Ectopic Expression of MHC II on Glandular Cells as a Presymptomatic Feature in a Mouse Model of Primary Sjogren's Syndrome. Front Immunol. 2018;9:2362.
- Speight PM, Cruchley A, Williams DM. Epithelial HLA-DR expression in labial salivary glands in Sjogren's syndrome and non-specific sialadenitis. J Oral Pathol Med. 1989;18(3):178-83.
- Fillatreau, S.; Manfroi, B.; Dörner, T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021, 17, 98–108, . [CrossRef]
- Avalos AM, Uccellini MB, Lenert P, Viglianti GA, Marshak-Rothstein A. FcgammaRIIB regulation of BCR/TLR-dependent autoreactive B-cell responses. Eur J Immunol. 2010;40(10):2692-8.
- Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13(2):277-85.
- Barlev AN, Malkiel S, Kurata-Sato I, Dorjee AL, Suurmond J, Diamond B. FcgammaRIIB regulates autoantibody responses by limiting marginal zone B cell activation. J Clin Invest. 2022;132(17).
- Corzo, C.A.; Varfolomeev, E.; Setiadi, A.F.; Francis, R.; Klabunde, S.; Senger, K.; Sujatha-Bhaskar, S.; Drobnick, J.; Do, S.; Suto, E.; et al. The kinase IRAK4 promotes endosomal TLR and immune complex signaling in B cells and plasmacytoid dendritic cells. Sci. Signal. 2020, 13, . [CrossRef]
- Cosgrove, H.A.; Gingras, S.; Kim, M.; Bastacky, S.; Tilstra, J.S.; Shlomchik, M.J. B cell–intrinsic TLR7 expression drives severe lupus in TLR9-deficient mice. J. Clin. Investig. 2023, 8, . [CrossRef]
- Robbins A, Hentzien M, Toquet S, Didier K, Servettaz A, Pham BN, et al. Diagnostic Utility of Separate Anti-Ro60 and Anti-Ro52/TRIM21 Antibody Detection in Autoimmune Diseases. Front Immunol. 2019;10:444.
- Menendez A, Gomez J, Caminal-Montero L, Diaz-Lopez JB, Cabezas-Rodriguez I, Mozo L. Common and specific associations of anti-SSA/Ro60 and anti-Ro52/TRIM21 antibodies in systemic lupus erythematosus. ScientificWorldJournal. 2013;2013:832789.
- Lindop R, Arentz G, Bastian I, Whyte AF, Thurgood LA, Chataway TK, et al. Long-term Ro60 humoral autoimmunity in primary Sjogren's syndrome is maintained by rapid clonal turnover. Clin Immunol. 2013;148(1):27-34.
- Heinlen, L.D.; McClain, M.T.; Ritterhouse, L.L.; Bruner, B.F.; Edgerton, C.C.; Keith, M.P.; James, J.A.; Harley, J.B. 60 kD Ro and nRNP A Frequently Initiate Human Lupus Autoimmunity. PLOS ONE 2010, 5, e9599, . [CrossRef]
- Tetsuka, S.; Suzuki, T.; Ogawa, T.; Hashimoto, R.; Kato, H. Anti-Ro/SSA Antibodies May Be Responsible for Cerebellar Degeneration in Sjogren’s Syndrome. J. Clin. Med. Res. 2021, 13, 113–120, . [CrossRef]
- Ching, K.; Burbelo, P.; Gonzalez-Begne, M.; Roberts, M.; Coca, A.; Sanz, I.; Iadarola, M. Salivary anti-Ro60 and anti-Ro52 Antibody Profiles to Diagnose Sjögren’s Syndrome. J. Dent. Res. 2011, 90, 445–449, . [CrossRef]
- Hu, C.; Huang, W.; Chen, H.; Song, G.; Li, P.; Shan, Q.; Zhang, X.; Zhang, F.; Zhu, H.; Wu, L.; et al. Autoantibody Profiling on Human Proteome Microarray for Biomarker Discovery in Cerebrospinal Fluid and Sera of Neuropsychiatric Lupus. PLOS ONE 2015, 10, e0126643, . [CrossRef]
- Lee, A.Y.S.; Beroukas, D.; Brown, L.; Lucchesi, C.; Kaur, A.; Gyedu, L.; Hughes, N.; Ng, Y.H.; Saran, O.; Gordon, T.P.; et al. Identification of a unique anti-Ro60 subset with restricted serological and molecular profiles. Clin. Exp. Immunol. 2021, 203, 13–21, . [CrossRef]
- Qin, Y.; Fan, C.; Wang, Y.; Feng, M.; Liang, Z.; Zhao, X.; Gao, C.; Luo, J. Analytical and clinical performance of different platforms simultaneously detecting 15 antinuclear antibodies. J. Clin. Lab. Anal. 2022, 36, e24554, . [CrossRef]
- Zampeli, E.; Mavrommati, M.; Moutsopoulos, H.M.; Skopouli, F.N. Anti-Ro52 and/or anti-Ro60 immune reactivity: autoantibody and disease associations. 2020, 38, S134–S141.
- Derksen RH, Meilof JF. Anti-Ro/SS-A and anti-La/SS-B autoantibody levels in relation to systemic lupus erythematosus disease activity and congenital heart block. A longitudinal study comprising two consecutive pregnancies in a patient with systemic lupus erythematosus. Arthritis Rheum. 1992;35(8):953-9.
- Hagiwara, S.; Tsuboi, H.; Honda, F.; Takahashi, H.; Kurata, I.; Ohyama, A.; Yagishita, M.; Abe, S.; Kurashima, Y.; Kaneko, S.; et al. Association of anti-Ro/SSA antibody with response to biologics in patients with rheumatoid arthritis. Mod. Rheumatol. 2016, 26, 857–862, . [CrossRef]
- Chen, P.-K.; Lan, J.-L.; Chen, Y.-M.; Chen, H.-H.; Chang, S.-H.; Chung, C.-M.; Rutt, N.H.; Tan, T.-M.; Mamat, R.N.R.; Anuar, N.D.; et al. Anti-TROVE2 Antibody Determined by Immune-Related Array May Serve as a Predictive Marker for Adalimumab Immunogenicity and Effectiveness in RA. J. Immunol. Res. 2021, 2021, 1–13, . [CrossRef]
- Deroo L, Achten H, De Boeck K, Genbrugge E, Bauters W, Roels D, et al. The value of separate detection of anti-Ro52, anti-Ro60 and anti-SSB/La reactivities in relation to diagnosis and phenotypes in primary Sjogren's syndrome. Clin Exp Rheumatol. 2022;40(12):2310-1317.
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836, . [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258, . [CrossRef]
- Greiling, T.M.; Dehner, C.; Chen, X.; Hughes, K.; Iñiguez, A.J.; Boccitto, M.; Ruiz, D.Z.; Renfroe, S.C.; Vieira, S.M.; Ruff, W.E.; et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci. Transl. Med. 2018, 10, . [CrossRef]
- Szymula A, Rosenthal J, Szczerba BM, Bagavant H, Fu SM, Deshmukh US. T cell epitope mimicry between Sjogren's syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin Immunol. 2014;152(1-2):1-9.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).