Submitted:
11 June 2024
Posted:
12 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- Participants & Study Protocol
- Blood measures
- Targeted plasma metabolomics
- Stool sample collection and metagenomics processing
- Statistical analysis
3. Results
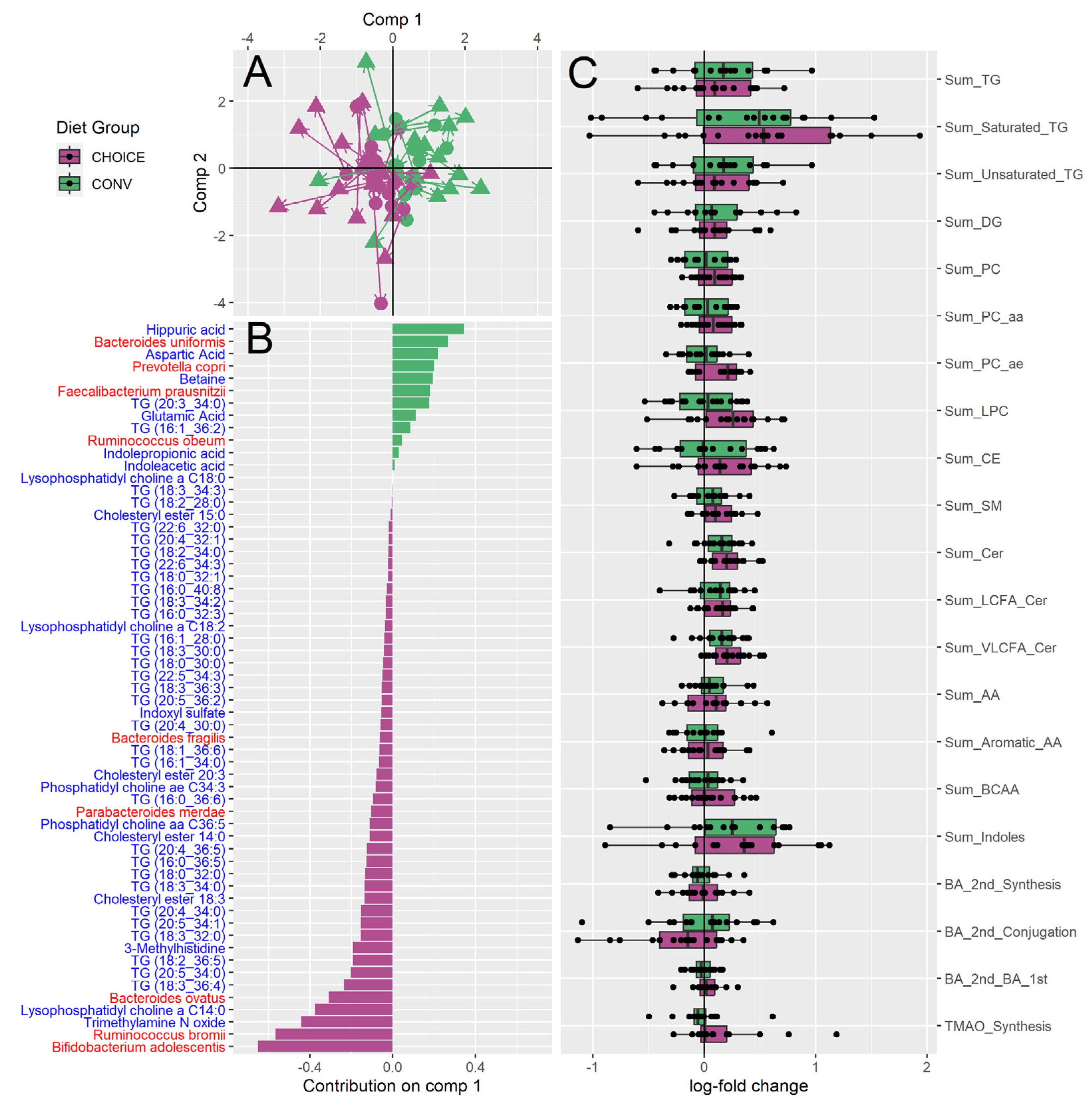
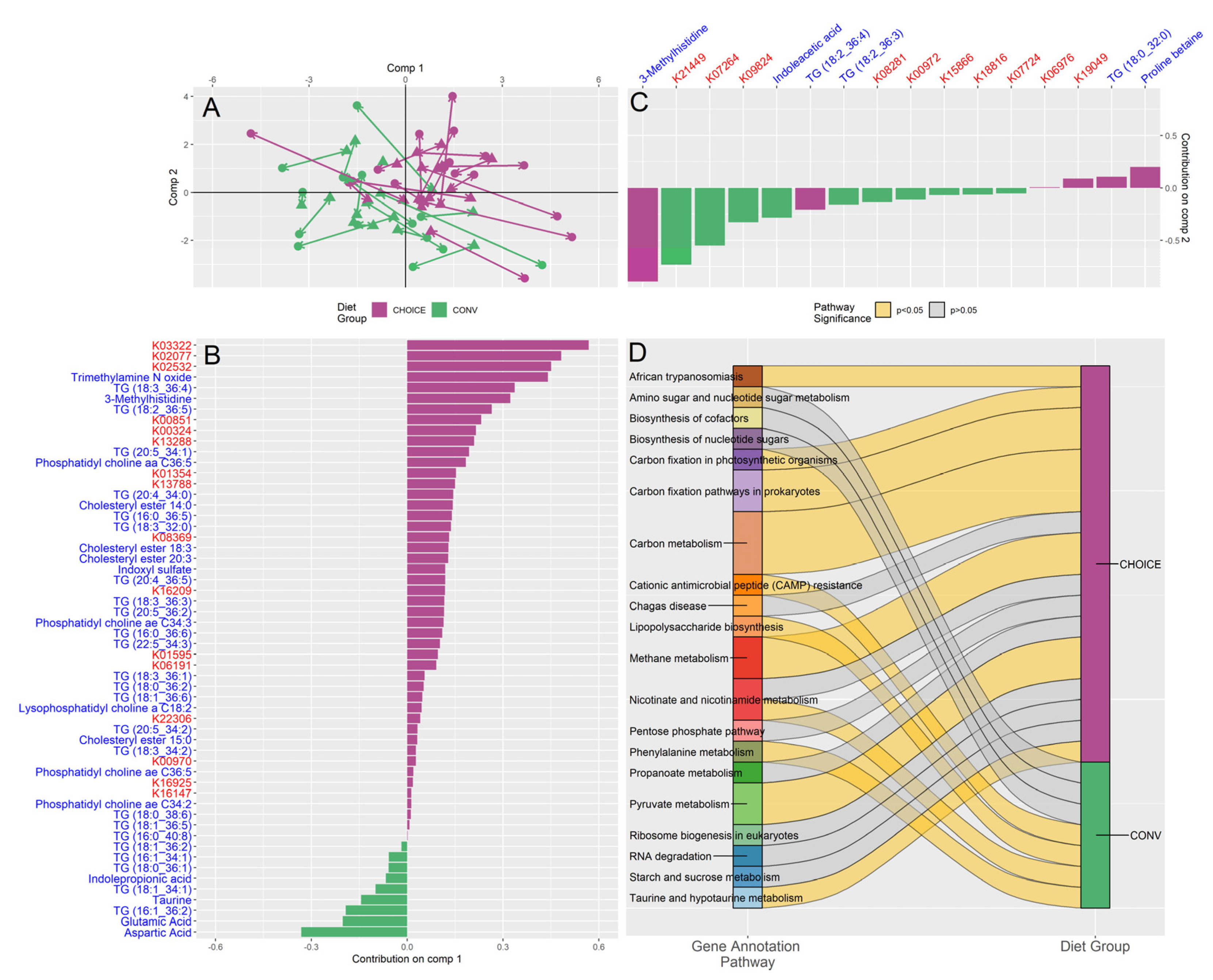
3.1. CHOICE Diet Enriches Carbohydrate Metabolizing Pathways in the Gut Microbiome, Altering Lipid Metabolism and Tryptophan Utilization Pathways
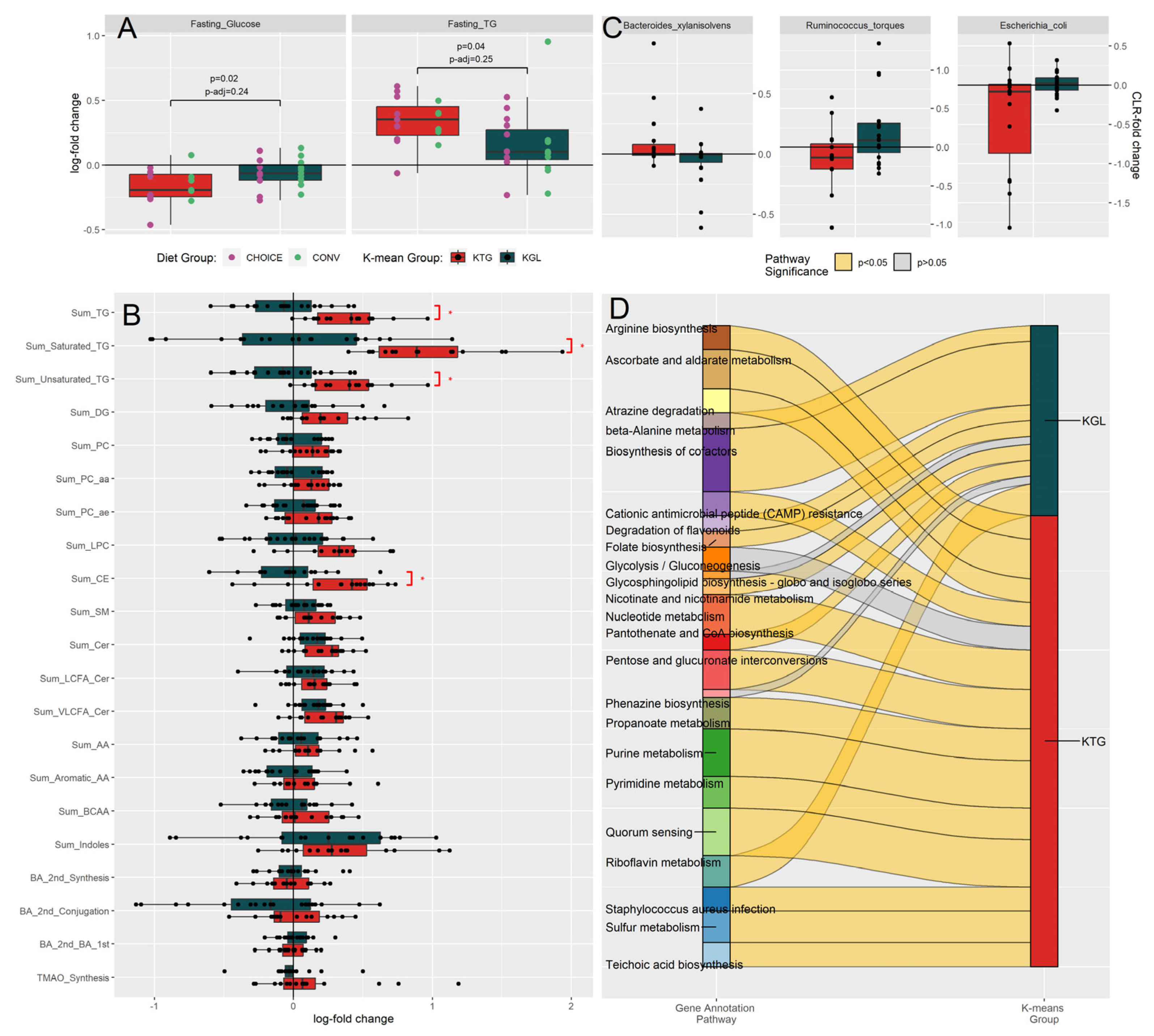
3.2. Bimodal Response to GDM Diet Intervention Is Characterized by a Relative Increase of Fasting TGs or Fasting Glucose in Participants Independent of Diet Treatment Group
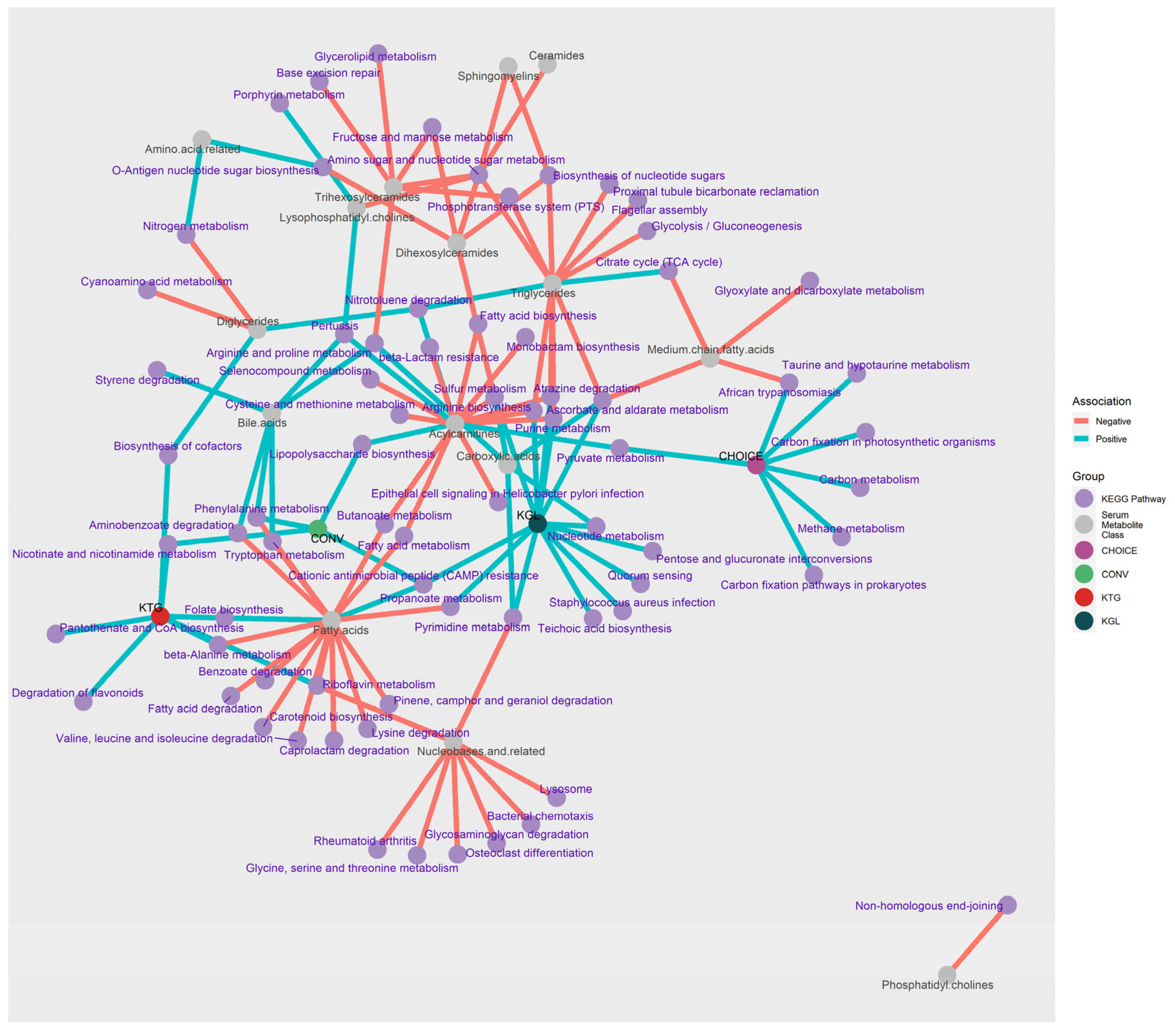
3.3. Microbiome Metabolic Pathways Are Negatively Associated with Host Plasma Lipid Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsu; Tain The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [CrossRef]
- Driscoll, A.; Gregory, E. Increases in Prepregnancy Obesity: United States, 2016–2019. NCHS Data Brief 2020, 392. [Google Scholar]
- Wang, M.C.; Freaney, P.M.; Perak, A.M.; Greenland, P.; Lloyd-Jones, D.M.; Grobman, W.A.; Khan, S.S. Trends in Prepregnancy Obesity and Association With Adverse Pregnancy Outcomes in the United States, 2013 to 2018. J Am Heart Assoc 2021, 10. [Google Scholar] [CrossRef]
- Sonagra, A.D. Normal Pregnancy- A State of Insulin Resistance. JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH 2014. [Google Scholar] [CrossRef]
- Gregory, E.C.; Ely, D.M. Trends and Characteristics in Gestational Diabetes: United States, 2016-2020. Natl Vital Stat Rep 2022, 71, 1–15. [Google Scholar]
- Black, M.H.; Sacks, D.A.; Xiang, A.H.; Lawrence, J.M. Clinical Outcomes of Pregnancies Complicated by Mild Gestational Diabetes Mellitus Differ by Combinations of Abnormal Oral Glucose Tolerance Test Values. Diabetes Care 2010, 33, 2524–2530. [Google Scholar] [CrossRef]
- American Diabetes Association Management of Diabetes in Pregnancy. Diabetes Care 2017, 40, S114–S119. [CrossRef]
- Black, M.H.; Sacks, D.A.; Xiang, A.H.; Lawrence, J.M. Clinical Outcomes of Pregnancies Complicated by Mild Gestational Diabetes Mellitus Differ by Combinations of Abnormal Oral Glucose Tolerance Test Values. Diabetes Care 2010, 33, 2524–2530. [Google Scholar] [CrossRef]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front Endocrinol (Lausanne) 2020, 11. [Google Scholar] [CrossRef]
- Gorczyca, K.; Obuchowska, A.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Changes in the Gut Microbiome and Pathologies in Pregnancy. Int J Environ Res Public Health 2022, 19, 9961. [Google Scholar] [CrossRef]
- Kuang, Y.-S.; Lu, J.-H.; Li, S.-H.; Li, J.-H.; Yuan, M.-Y.; He, J.-R.; Chen, N.-N.; Xiao, W.-Q.; Shen, S.-Y.; Qiu, L.; et al. Connections between the Human Gut Microbiome and Gestational Diabetes Mellitus. Gigascience 2017, 6. [Google Scholar] [CrossRef]
- Lou, Y.C.; Olm, M.R.; Diamond, S.; Crits-Christoph, A.; Firek, B.A.; Baker, R.; Morowitz, M.J.; Banfield, J.F. Infant Gut Strain Persistence Is Associated with Maternal Origin, Phylogeny, and Traits Including Surface Adhesion and Iron Acquisition. Cell Rep Med 2021, 2, 100393. [Google Scholar] [CrossRef]
- Sugino, K.Y.; Hernandez, T.L.; Barbour, L.A.; Kofonow, J.M.; Frank, D.N.; Friedman, J.E. A Maternal Higher-Complex Carbohydrate Diet Increases Bifidobacteria and Alters Early Life Acquisition of the Infant Microbiome in Women with Gestational Diabetes Mellitus. Front Endocrinol (Lausanne) 2022, 13, 921464. [Google Scholar] [CrossRef]
- Sugino, K.Y.; Hernandez, T.L.; Barbour, L.A.; Kofonow, J.M.; Frank, D.N.; Friedman, J.E. A Maternal Higher-Complex Carbohydrate Diet Increases Bifidobacteria and Alters Early Life Acquisition of the Infant Microbiome in Women with Gestational Diabetes Mellitus. Front Endocrinol (Lausanne) 2022, 13, 921464. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Farabi, S.S.; Fosdick, B.K.; Hirsch, N.; Dunn, E.Z.; Rolloff, K.; Corbett, J.P.; Haugen, E.; Marden, T.; Higgins, J.; et al. Randomization to a Provided Higher-Complex-Carbohydrate Versus Conventional Diet in Gestational Diabetes Mellitus Results in Similar Newborn Adiposity. Diabetes Care 2023, 46, 1931–1940. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin, No. 190: Gestational Diabetes Mellitus. Obstetrics and gynecology 2018, 131, e49–e64. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Farabi, S.S.; Fosdick, B.K.; Hirsch, N.; Dunn, E.Z.; Rolloff, K.; Corbett, J.P.; Haugen, E.; Marden, T.; Higgins, J.; et al. Randomization to a Provided Higher-Complex-Carbohydrate Versus Conventional Diet in Gestational Diabetes Mellitus Results in Similar Newborn Adiposity. Diabetes Care 2023, 46, 1931–1940. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin Sensitivity Indices Obtained from Oral Glucose Tolerance Testing: Comparison with the Euglycemic Insulin Clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Matsuda, M.; Balas, B.; DeFronzo, R.A. Muscle and Liver Insulin Resistance Indexes Derived from the Oral Glucose Tolerance Test. Diabetes Care 2007, 30, 89–94. [Google Scholar] [CrossRef]
- Bushnell, B. BBMap: A Fast, Accurate, Splice-Aware Aligner. undefined 2014. [Google Scholar]
- Truong, D.T.; Franzosa, E.A.; Tickle, T.L.; Scholz, M.; Weingart, G.; Pasolli, E.; Tett, A.; Huttenhower, C.; Segata, N. MetaPhlAn2 for Enhanced Metagenomic Taxonomic Profiling. Nature Methods 2015 12:10 2015, 12, 902–903. [Google Scholar] [CrossRef]
- Li, D.; Luo, R.; Liu, C.M.; Leung, C.M.; Ting, H.F.; Sadakane, K.; Yamashita, H.; Lam, T.W. MEGAHIT v1.0: A Fast and Scalable Metagenome Assembler Driven by Advanced Methodologies and Community Practices. Methods 2016. [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and Memory-Efficient Alignment of Short DNA Sequences to the Human Genome. Genome Biol 2009, 10, 1–10. [Google Scholar] [CrossRef]
- D, H.; GL, C.; PF, L.; ML, L.; FW, L.; LJ, H.; Hyatt, D.; Chen, G.L.; LoCascio, P.F.; Land, M.L.; et al. Prodigal: Prokaryotic Gene Recognition and Translation Initiation Site Identification. BMC Bioinformatics 2010, 11, 1–11. [Google Scholar] [CrossRef]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG Ortholog Assignment Based on Profile HMM and Adaptive Score Threshold. Bioinformatics 2020, 36, 2251–2252. [Google Scholar] [CrossRef]
- Pfaffel, O. ClustImpute: An R Package for k-Means Clustering with Build-in Missing Data Imputation. 2020. [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. MixOmics: An R Package for ‘omics Feature Selection and Multiple Data Integration. PLoS Comput Biol 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. The Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Ye, X.; Li, H.; Anjum, K.; Zhong, X.; Miao, S.; Zheng, G.; Liu, W.; Li, L. Dual Role of Indoles Derived From Intestinal Microbiota on Human Health. Front Immunol 2022, 13. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Rager, S.L.; Carrow, H.C.; Ananthanarayanan, A.; Callaghan, R.; Hart, L.R.; Li, T.; Ravisankar, P.; Brown, J.A.; Amir, M.; et al. Gut Bacteria-Derived Serotonin Promotes Immune Tolerance in Early Life. Sci Immunol 2024, 9, eadj4775. [Google Scholar] [CrossRef]
- Qi, Q.; Li, J.; Yu, B.; Moon, J.-Y.; Chai, J.C.; Merino, J.; Hu, J.; Ruiz-Canela, M.; Rebholz, C.; Wang, Z.; et al. Host and Gut Microbial Tryptophan Metabolism and Type 2 Diabetes: An Integrative Analysis of Host Genetics, Diet, Gut Microbiome and Circulating Metabolites in Cohort Studies. Gut 2022, 71, 1095–1105. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, C.; Gao, J. Extensive Summary of the Important Roles of Indole Propionic Acid, a Gut Microbial Metabolite in Host Health and Disease. Nutrients 2022, 15, 151. [Google Scholar] [CrossRef]
- de Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic Acid and Novel Lipid Metabolites Are Associated with a Lower Risk of Type 2 Diabetes in the Finnish Diabetes Prevention Study. Sci Rep 2017, 7, 46337. [Google Scholar] [CrossRef]
- Sehgal, R.; de Mello, V.D.; Männistö, V.; Lindström, J.; Tuomilehto, J.; Pihlajamäki, J.; Uusitupa, M. Indolepropionic Acid, a Gut Bacteria-Produced Tryptophan Metabolite and the Risk of Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 4695. [Google Scholar] [CrossRef]
- Pessa-Morikawa, T.; Husso, A.; Kärkkäinen, O.; Koistinen, V.; Hanhineva, K.; Iivanainen, A.; Niku, M. Maternal Microbiota-Derived Metabolic Profile in Fetal Murine Intestine, Brain and Placenta. BMC Microbiol 2022, 22, 46. [Google Scholar] [CrossRef]
- de Mello, V.D.; Lankinen, M.A.; Lindström, J.; Puupponen-Pimiä, R.; Laaksonen, D.E.; Pihlajamäki, J.; Lehtonen, M.; Uusitupa, M.; Tuomilehto, J.; Kolehmainen, M.; et al. Fasting Serum Hippuric Acid Is Elevated after Bilberry ( Vaccinium Myrtillus ) Consumption and Associates with Improvement of Fasting Glucose Levels and Insulin Secretion in Persons at High Risk of Developing Type 2 Diabetes. Mol Nutr Food Res 2017, 61, 1700019. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T. The Anti-Diabetic Potential of Betaine. Mechanisms of Action in Rodent Models of Type 2 Diabetes. Biomedicine & Pharmacotherapy 2022, 150, 112946. [Google Scholar] [CrossRef]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-Oxide (TMAO) in Human Health. EXCLI J 2021, 20, 301–319. [Google Scholar] [CrossRef]
- McArthur, K.L.; Zhang, M.; Hong, X.; Wang, G.; Buckley, J.P.; Wang, X.; Mueller, N.T. Trimethylamine N-Oxide and Its Precursors Are Associated with Gestational Diabetes Mellitus and Pre-Eclampsia in the Boston Birth Cohort. Curr Dev Nutr 2022, 6, nzac108. [Google Scholar] [CrossRef]
- Li, P.; Zhong, C.; Li, S.; Sun, T.; Huang, H.; Chen, X.; Zhu, Y.; Hu, X.; Peng, X.; Zhang, X.; et al. Plasma Concentration of Trimethylamine-N-Oxide and Risk of Gestational Diabetes Mellitus. Am J Clin Nutr 2018, 108, 603–610. [Google Scholar] [CrossRef]
- Svingen, G.F.T.; Schartum-Hansen, H.; Pedersen, E.R.; Ueland, P.M.; Tell, G.S.; Mellgren, G.; Njølstad, P.R.; Seifert, R.; Strand, E.; Karlsson, T.; et al. Prospective Associations of Systemic and Urinary Choline Metabolites with Incident Type 2 Diabetes. Clin Chem 2016, 62, 755–765. [Google Scholar] [CrossRef]
- Diaz, S.O.; Pinto, J.; Graça, G.; Duarte, I.F.; Barros, A.S.; Galhano, E.; Pita, C.; Almeida, M. do C.; Goodfellow, B.J.; Carreira, I.M.; et al. Metabolic Biomarkers of Prenatal Disorders: An Exploratory NMR Metabonomics Study of Second Trimester Maternal Urine and Blood Plasma. J Proteome Res 2011, 10, 3732–3742. [Google Scholar] [CrossRef]
- Barzilay, E.; Moon, A.; Plumptre, L.; Masih, S.P.; Sohn, K.-J.; Visentin, C.E.; Ly, A.; Malysheva, O.; Croxford, R.; Caudill, M.A.; et al. Fetal One-Carbon Nutrient Concentrations May Be Affected by Gestational Diabetes. Nutrition Research 2018, 55, 57–64. [Google Scholar] [CrossRef]
- Huo, X.; Li, J.; Cao, Y.-F.; Li, S.-N.; Shao, P.; Leng, J.; Li, W.; Liu, J.; Yang, K.; Ma, R.C.W.; et al. Trimethylamine N-Oxide Metabolites in Early Pregnancy and Risk of Gestational Diabetes: A Nested Case-Control Study. J Clin Endocrinol Metab 2019, 104, 5529–5539. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of Gut Microbiota in Adult Patients with Type 2 Diabetes and Healthy Individuals. Microb Pathog 2017, 111, 362–369. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Li, X.-F.; Wang, R.-L. Bifidobacterium Adolescentis Supplementation Ameliorates Visceral Fat Accumulation and Insulin Sensitivity in an Experimental Model of the Metabolic Syndrome. British Journal of Nutrition 2012, 107, 1429–1434. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.; Bergeron, N.; Krauss, R.M.; Jansson, J.K.; Tiedje, J.M. Metagenomic Insights into the Degradation of Resistant Starch by Human Gut Microbiota. Appl Environ Microbiol 2018, 84. [Google Scholar] [CrossRef]
- Wei, J.; Qing, Y.; Zhou, H.; Liu, J.; Qi, C.; Gao, J. 16S RRNA Gene Amplicon Sequencing of Gut Microbiota in Gestational Diabetes Mellitus and Their Correlation with Disease Risk Factors. J Endocrinol Invest 2021, 45, 279–289. [Google Scholar] [CrossRef]
- Sun, Z.; Pan, X.; Li, X.; Jiang, L.; Hu, P.; Wang, Y.; Ye, Y.; Wu, P.; Zhao, B.; Xu, J.; et al. The Gut Microbiome Dynamically Associates with Host Glucose Metabolism throughout Pregnancy: Longitudinal Findings from a Matched Case-Control Study of Gestational Diabetes Mellitus. Advanced Science 2023, 10. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Leite, A.Z.; Rodrigues, N. de C.; Gonzaga, M.I.; Paiolo, J.C.C.; de Souza, C.A.; Stefanutto, N.A.V.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Matheucci Junior, E.; et al. Detection of Increased Plasma Interleukin-6 Levels and Prevalence of Prevotella Copri and Bacteroides Vulgatus in the Feces of Type 2 Diabetes Patients. Front Immunol 2017, 8. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Liu, P.-Y.; Huang, M.-C.; Chang, C.-I.; Chen, H.-Y.; Chou, Y.-H.; Tsai, C.-N.; Lin, C.-H. Abundance of Prevotella Copri in Gut Microbiota Is Inversely Related to a Healthy Diet in Patients with Type 2 Diabetes. J Food Drug Anal 2023, 31. [Google Scholar] [CrossRef]
- De Bandt, J.-P.; Coumoul, X.; Barouki, R. Branched-Chain Amino Acids and Insulin Resistance, from Protein Supply to Diet-Induced Obesity. Nutrients 2022, 15, 68. [Google Scholar] [CrossRef]
- Liang, H.; Hussey, S.E.; Sanchez-Avila, A.; Tantiwong, P.; Musi, N. Effect of Lipopolysaccharide on Inflammation and Insulin Action in Human Muscle. PLoS One 2013, 8, e63983. [Google Scholar] [CrossRef]
- Pedro, M.N.; Magro, D.O.; da Silva, E.U.P.P.; Guadagnini, D.; Santos, A.; de Jesus Pedro, R.; Saad, M.J.A. Plasma Levels of Lipopolysaccharide Correlate with Insulin Resistance in HIV Patients. Diabetol Metab Syndr 2018, 10, 5. [Google Scholar] [CrossRef]
- White, P.J.; Newgard, C.B. Branched-Chain Amino Acids in Disease. Science (1979) 2019, 363, 582–583. [Google Scholar] [CrossRef]
- Wang, J.; Si, Y.; Wu, C.; Sun, L.; Ma, Y.; Ge, A.; Li, B. Lipopolysaccharide Promotes Lipid Accumulation in Human Adventitial Fibroblasts via TLR4-NF-ΚB Pathway. Lipids Health Dis 2012, 11, 139. [Google Scholar] [CrossRef]
- Devlin, A.M.; Singh, R.; Wade, R.E.; Innis, S.M.; Bottiglieri, T.; Lentz, S.R. Hypermethylation of Fads2 and Altered Hepatic Fatty Acid and Phospholipid Metabolism in Mice with Hyperhomocysteinemia. Journal of Biological Chemistry 2007, 282, 37082–37090. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A. V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




