Submitted:
10 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
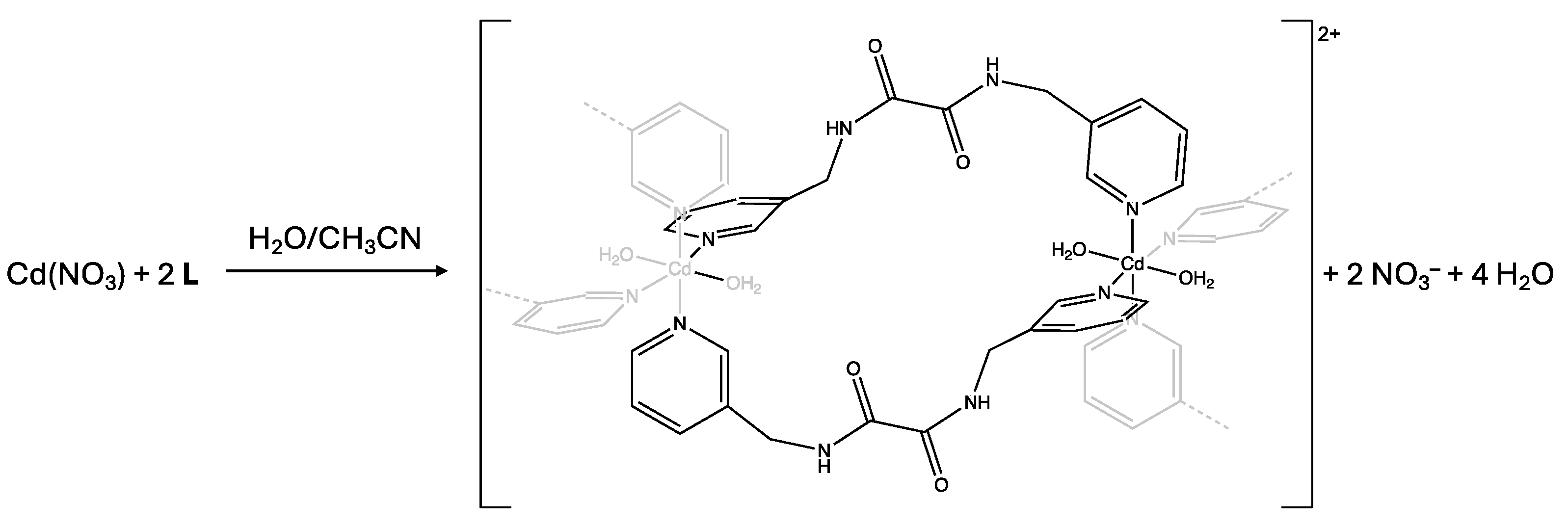
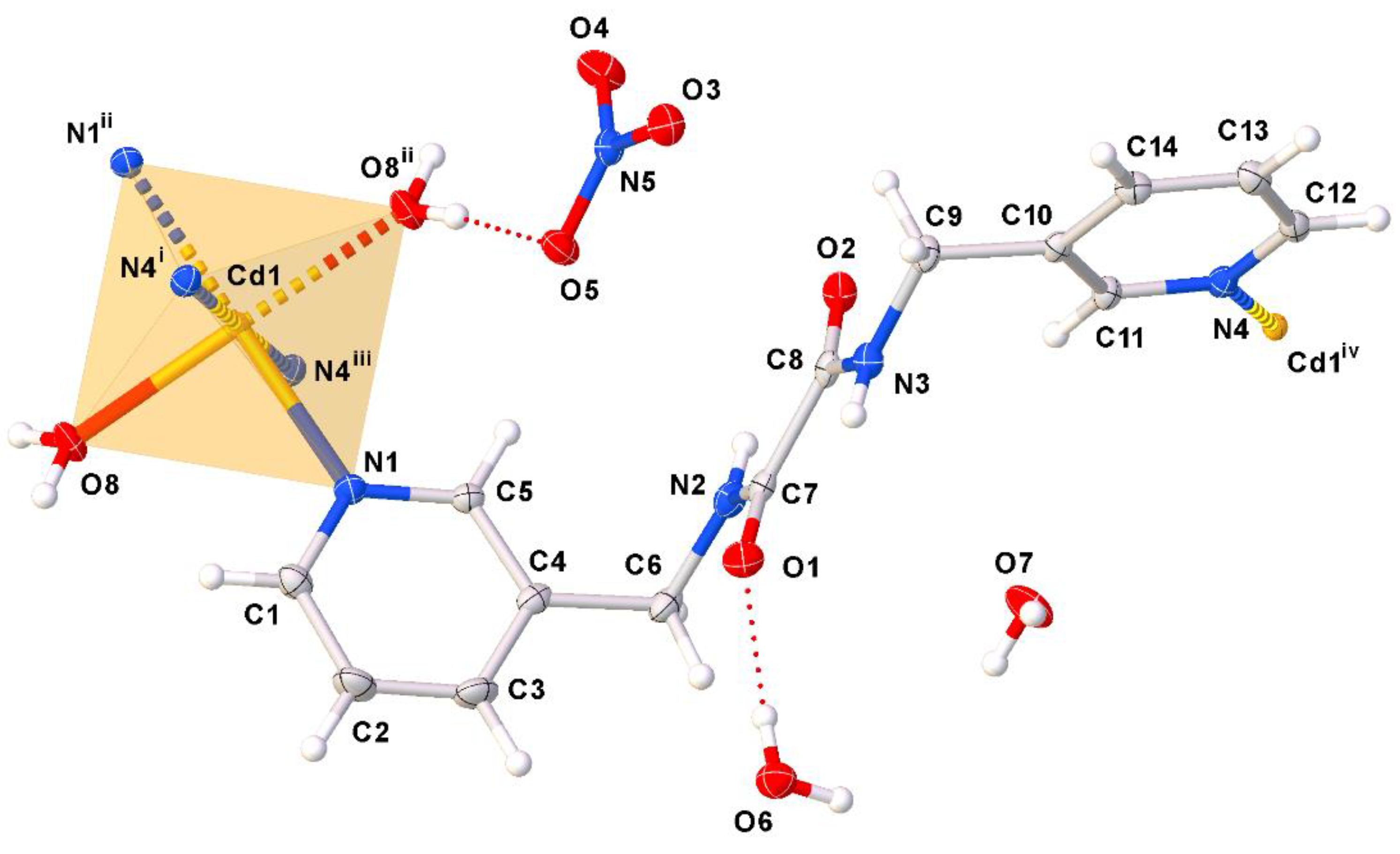
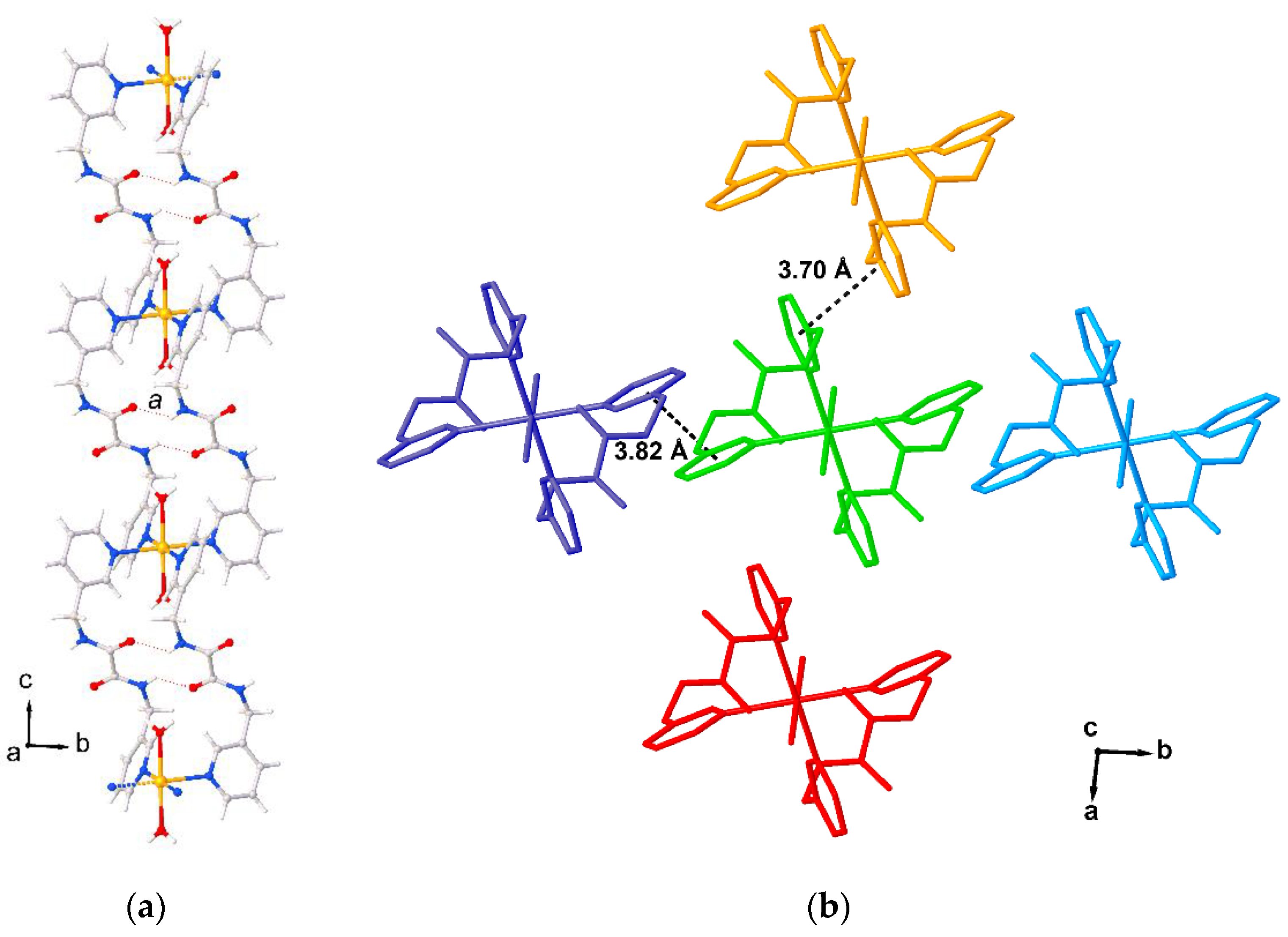
2. Results
3. Materials and Methods
3.1. General
3.2. Synthesis of Compound 1
4. Conclusions
Supplementary Materials
Funding
Conceptualisation
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, T.; Xiong, Y.; Feng, Y.; Guo, W.; Zhou, Y.; Zhao, J.; Jiang, T.; Shi, C.; Han, Y. Inhibition of LDH-A by Oxamate Enhances the Efficacy of Anti-PD-1 Treatment in an NSCLC Humanized Mouse Model. Front. Oncol. 2021, 11, 1033. [Google Scholar] [CrossRef]
- Miskimins, W.K.; Ahn, H.J.; Kim, J.Y.; Ryu, S.; Jung, Y.-S. Synergistic Anti-Cancer Effect of Phenformin and Oxamate. PLoS ONE 2014, 9, 85576. [Google Scholar] [CrossRef]
- Maiore, L.; Aragoni, M.C.; Carcangiu, G.; Cocco, O.; Isaia, F.; Lippolis, V.; Meloni, P.; Murru, A.; Slawin, A.M.Z.; Tuveri, E.; et al. Oxamate Salts as Novel Agents for the Restoration of Marble and Limestone Substrates: Case Study of Ammonium N-Phenyloxamate. New J. Chem. 2016, 40, 2768. [Google Scholar] [CrossRef]
- Pintus, A.; Aragoni, M.C.; Carcangiu, G.; Giacopetti, L.; Isaia, F.; Lippolis, V.; Maiore, L.; Meloni, P.; Arca, M. Density Functional Theory Modelling of Protective Agents for Carbonate Stones: A Case Study of Oxalate and Oxamate Inorganic Salts. New J. Chem. 2018, 42, 11593. [Google Scholar] [CrossRef]
- Maiore, L.; Aragoni, M.C.; Carcangiu, G.; Cocco, O.; Isaia, F.; Lippolis, V.; Meloni, P.; Murru, A.; Tuveri, E.; Arca, M. Synthesis, Characterization and DFT-Modeling of Novel Agents for the Protection and Restoration of Historical Calcareous Stone Substrates. J. Colloid Interface Sci. 2015, 448, 320. [Google Scholar] [CrossRef] [PubMed]
- Pintus, A.; Aragoni, M.C.; Carcangiu, G.; Caria, V.; Coles, S.J.; Dodd, E.; Giacopetti, L.; Gimeno, D.; Lippolis, V.; Meloni, P.; Murgia, S.; Navarro Ezquerra, A.; Podda, E.; Urru, C.; Arca, M. Ammonium N-(pyridin-2-ylmethyl)oxamate (AmPicOxam): A Novel Precursor of Calcium Oxalate Coating for Carbonate Stone Substrates. Molecules 2023, 28, 5768. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Elangovan, S.; Sang, R.; Spannenberg, A.; Jackstell, R.; Junge, K.; Li, Y.; Beller, M. Selective Catalytic Two-Step Process for Ethylene Glycol from Carbon Monoxide. Nat. Commun. 2016, 7, 1. [Google Scholar] [CrossRef]
- Zou, Y.Q.; Zhou, Q.Q.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Synthesis of Oxalamides by Acceptorless Dehydrogenative Coupling of Ethylene Glycol and Amines and the Reverse Hydrogenation Catalyzed by Ruthenium. Chem. Sci. 2020, 11, 7188. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, Y.; Zhang, L.; Guo, Y.; Ma, D. Oxalic Diamides and Tert-Butoxide: Two Types of Ligands Enabling Practical Access to Alkyl Aryl Ethers via Cu-Catalyzed Coupling Reaction. J. Am. Chem. Soc. 2019, 141, 3541. [Google Scholar] [CrossRef]
- Braun, M.; Frank, W.; Reiss, G.J.; Ganter, C. An N-Heterocyclic Carbene Ligand with an Oxalamide Backbone. Organometallics 2010, 29, 4418. [Google Scholar] [CrossRef]
- Jotani, M.M.; Zukerman-Schpector, J.; Madureira, L.S.; Poplaukhin, P.; Arman, H.D.; Miller, T.; Tiekink, E.R.T. Structural, Hirshfeld surface and theoretical analysis of two conformational polymorphs of N,N′-bis(pyridin-3-ylmethyl)oxalamide. Z. Krist. Cryst. Mater. 2016, 231, 415. [Google Scholar] [CrossRef]
- DeHaven, B.A.; Chen, A.L.; Shimizu, E.A.; Salpage, S.R.; Smith, M.D.; Shimizu, L.S. Interplay between Hydrogen and Halogen Bonding in Cocrystals of Dipyridinylmethyl Oxalamides and Tetrafluorodiiodobenzenes. Cryst. Growth Des. 2019, 19, 5776. [Google Scholar] [CrossRef]
- Zeng. , Q.; Li, M.; Wu, D.; Lei, S.; Liu, C.; Piao, L.; Yang, Y.; An, S.; Wang, C. Organic−Inorganic Hybrid Aligned by the Ligand−Ligand Hydrogen Bonds by Using Pyridyl-Substituted Oxalamides as the Building Blocks. Cryst. Growth Des. 2008, 8, 869. [Google Scholar] [CrossRef]
- Hu, J. -H.; Hsu, H. -H.; Chen, Y. -W.; Chen, W. -H.; Liu, S. -M. Zinc(II) coordination polymers with mixed ligands: Synthesis, structures and evaluation on metal sensing. J. Mol. Struct. 2023, 1289, 135896. [Google Scholar] [CrossRef]
- Lee, W. -T.; Liao, T. -T.; Chen, J. -D. Nickel(II) Coordination Polymers Supported by Bis-pyridyl-bis-amide and Angular Dicarboxylate Ligands: Role of Ligand Flexibility in Iodine Adsorption. Int. J. Mol. Sci. 2022, 23, 3603. [Google Scholar] [CrossRef] [PubMed]
- Schauer, C.L.; Matwey, E.; Fowler, F.W.; Lauher, J. Controlled Spacing of Metal Atoms via Ligand Hydrogen Bonds. J. Am. Chem. Soc. 1997, 119, 10245. [Google Scholar] [CrossRef]
- Wheaton, C.A.; Puddephatt, R.J. Complexes of gold(I) with a chiral diphosphine and bis(pyridine) ligands: Isomeric macrocycles and a polymer. Polyhedron 2016, 120, 88. [Google Scholar] [CrossRef]
- Chen, W. -J.; Lee, C. -Y.; Huang, Y. -H.; Chen, J.D. Cd(II) and Co(II) coordination polymers constructed from N,N’-Bis(3-pyridylmethyl)oxalamide and 1,4-naphthalenedicarboxylic acid. Polyhedron 2022, 223, 115991. [Google Scholar] [CrossRef]
- Liao, T. -T.; Lin, S. -Y.; Chen, J. -D. Co(II) coordination polymers supported by a benzenetetracarboxylate and bis-pyridyl-bis-amides with different flexibilities. CrystEngComm 2023, 25, 1723. [Google Scholar] [CrossRef]
- Qin, Z.; Jennings, M.C.; Puddephatt, R.J. Self-Assembly in Palladium(II) and Platinum(II) Chemistry: The Biomimetic Approach. Inorg. Chem. 2003, 42, 1956. [Google Scholar] [CrossRef]
- Tan, Y.S.; Yeo, C.I.; Kwong, H.C.; Tiekink, E.R.T. Unusual {⋯HNC2O⋯HCnO}, n = 1 or 2, synthons predominate in the molecular packing of one-dimensional coordination polymers, {Cd[S2P(OR)2]2(3LH2)}n, for R = Me and Et, but are precluded when R = i-Pr; 3LH2 = N,N′-bis(3-pyridylmethyl)oxalamide. CrystEngComm 2022, 24, 2992. [Google Scholar] [CrossRef]
- Podda, E.; Dodd, E.; Arca, M.; Aragoni, M.C.; Lippolis, V.; Coles, S.J.; Pintus, A. N,N’-Dipropyloxamide. Molbank, 2024, 2024, M1753. [Google Scholar] [CrossRef]
- Podda, E.; Dodd, E.; Arca, M.; Aragoni, M.C.; Lippolis, V.; Coles, S.J.; Pintus, A. N,N’-Dibutyloxamide. Molbank, 2023, 2023, M1677. [Google Scholar] [CrossRef]
- CSD. ConQuest Software, Version 2024.1.0; The Cambridge Crystallographic Data Centre: Cambridge, UK, 2024. [Google Scholar]
- Gaussian 16 (rev. C.01), Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.V.; Bloino, J.; Janesko, B.G.; Gomperts, R.; Mennucci, B.; Hratchian, H.P.; Ortiz, J.V.; Izmaylov, A.F.; Sonnenberg, J.L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V.G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J.A., Jr.; Peralta, J.E.; Ogliaro, F.; Bearpark, M.J.; Heyd, J.J.; Brothers, E.N.; Kudin, K.N.; Staroverov, V.N.; Keith, T.A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.P.; Burant, J.C.; Iyengar, S.S.; Tomasi, J.; Cossi, M.; Millam, J.M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J.W.; Martin, R.L.; Morokuma, K.; Farkas, O.; Foresman, J.B.; Fox, D.J. Gaussian, Inc., Wallingford CT, 2016.
- Adamo, C.; Barone, V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models, J. Chem. Phys. 1998, 108, 664. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn, Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Podda, E.; Chaudhari, S.; Bhasin, A.K.K.; Bhasin, K.K.; Coles, S.J.; Orton, J.B.; Isaia, F.; Lippolis, V.; Pintus, A.; Slawin, A.M.Z.; Woollins, J. D.; Arca, M. An experimental and theoretical insight into I2/Br2 oxidation of bis(pyridin-2-yl)diselane and ditellane. Chem. – Asian. J. 2023, 18, e202300836. [Google Scholar] [CrossRef] [PubMed]
- Arca, M. GaussMem 2024. https://massimiliano-arca.itch.io/gaussmem.
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural localized molecular orbitals. J. Chem. Phys. 1985, 83, 1736. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899. [Google Scholar] [CrossRef]
- GaussView, Version 6, Dennington, Roy; Keith, Todd, A.; Millam, John, M. Semichem Inc., Shawnee Mission, KS, 2016.
- APEX3, SAINT, Bruker AXS Inc.: Madison (WI), USA, 2015.
- SADABS, Bruker AXS Inc., Madison (WI), USA, 2016.
- Sheldrick, G.M. SHELXT - Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3. [Google Scholar]
- Dolomanov, O. v.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Re-finement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339. [Google Scholar] [CrossRef]
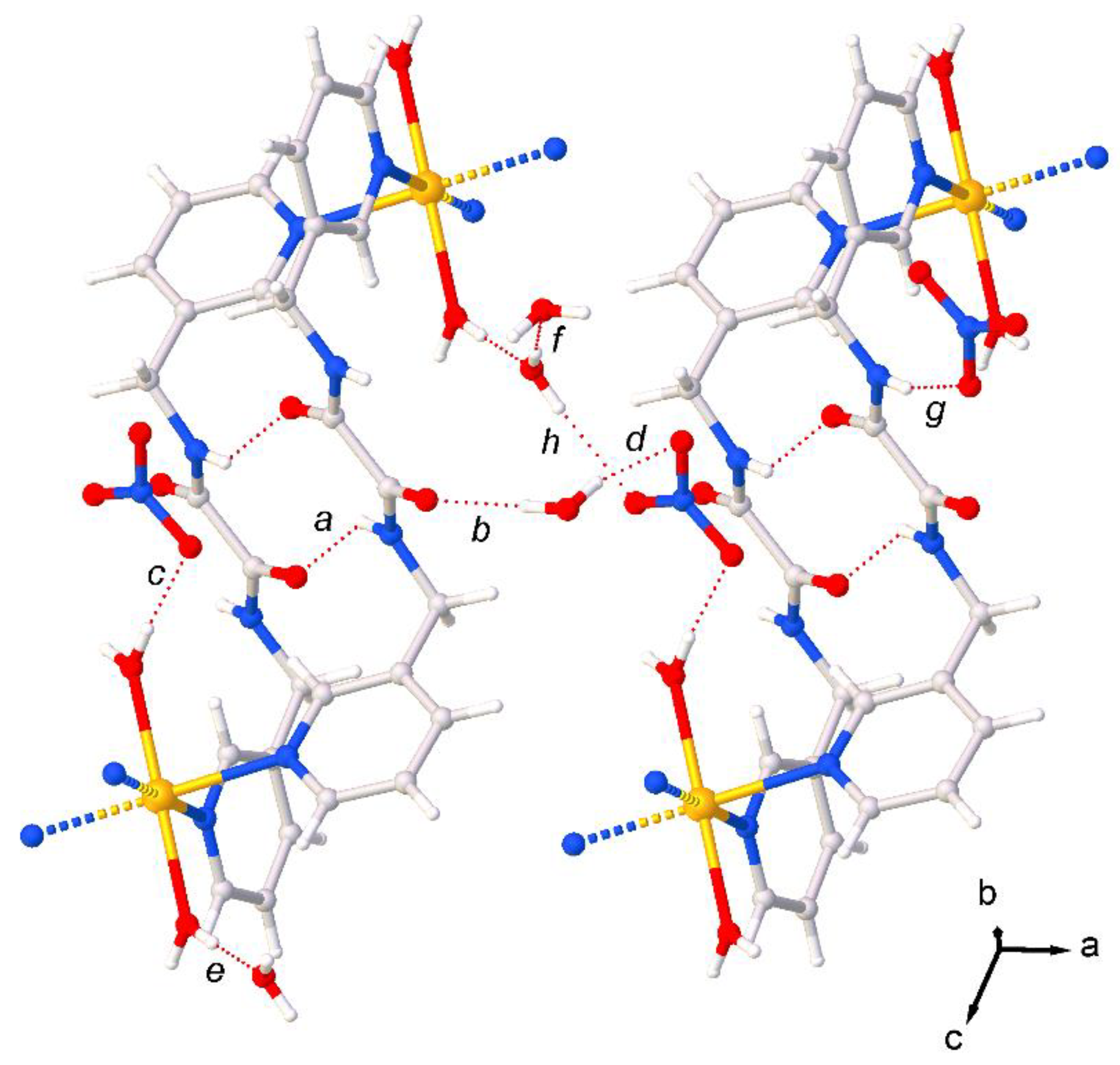
| Interaction A–B···C | dA–B (Å) | dB∙∙∙C (Å) | dA∙∙∙C (Å) | αA–B···C (°) | |
| a | N2–H2···O2iii | 0.83(2) | 2.159(2) | 2.851(2) | 140.7(2) |
| b | O6–H6C···O1 | 0.78(2) | 2.039(2) | 2.815(2) | 172.0(2) |
| c | O8ii–H8Bii···O5 | 0.80(2) | 1.98(2) | 2.775(2) | 177.8(2) |
| d | O6–H6D···O3v | 0.81(2) | 2.08(2) | 2.876(2) | 166.2(2) |
| e | O8–H8A···O7i | 0.84(2) | 1.84(2) | 2.674(2) | 175.7(2) |
| f | O7–H7A···O6vi | 0.81(2) | 1.959(2) | 2.765(2) | 177.3(2) |
| g | N3v–H3v···O3vi | 0.82(2) | 2.140(2) | 2.937(2) | 163.6(2) |
| h | O7–H7B···O4v | 0.80(2) | 2.079(2) | 2.872(2) | 172.7(2) |
| Symmetry codes: i = +x, +y, 1+z; ii = 1−x, 1−y, 2−z; iii = 1−x, 1−y, 1−z; v = 1+x, +y, +z; vi = 2−x, 2−y, 1−z. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





