Submitted:
07 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
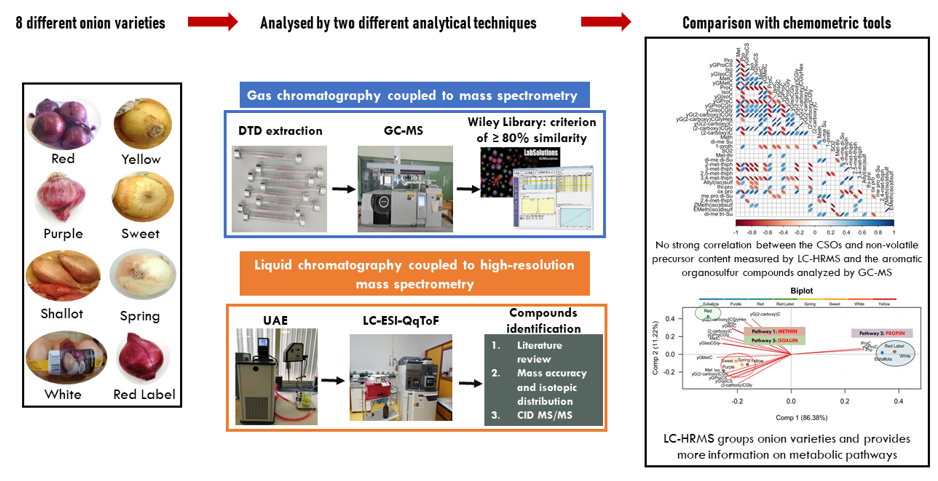
2. Materials and Methods
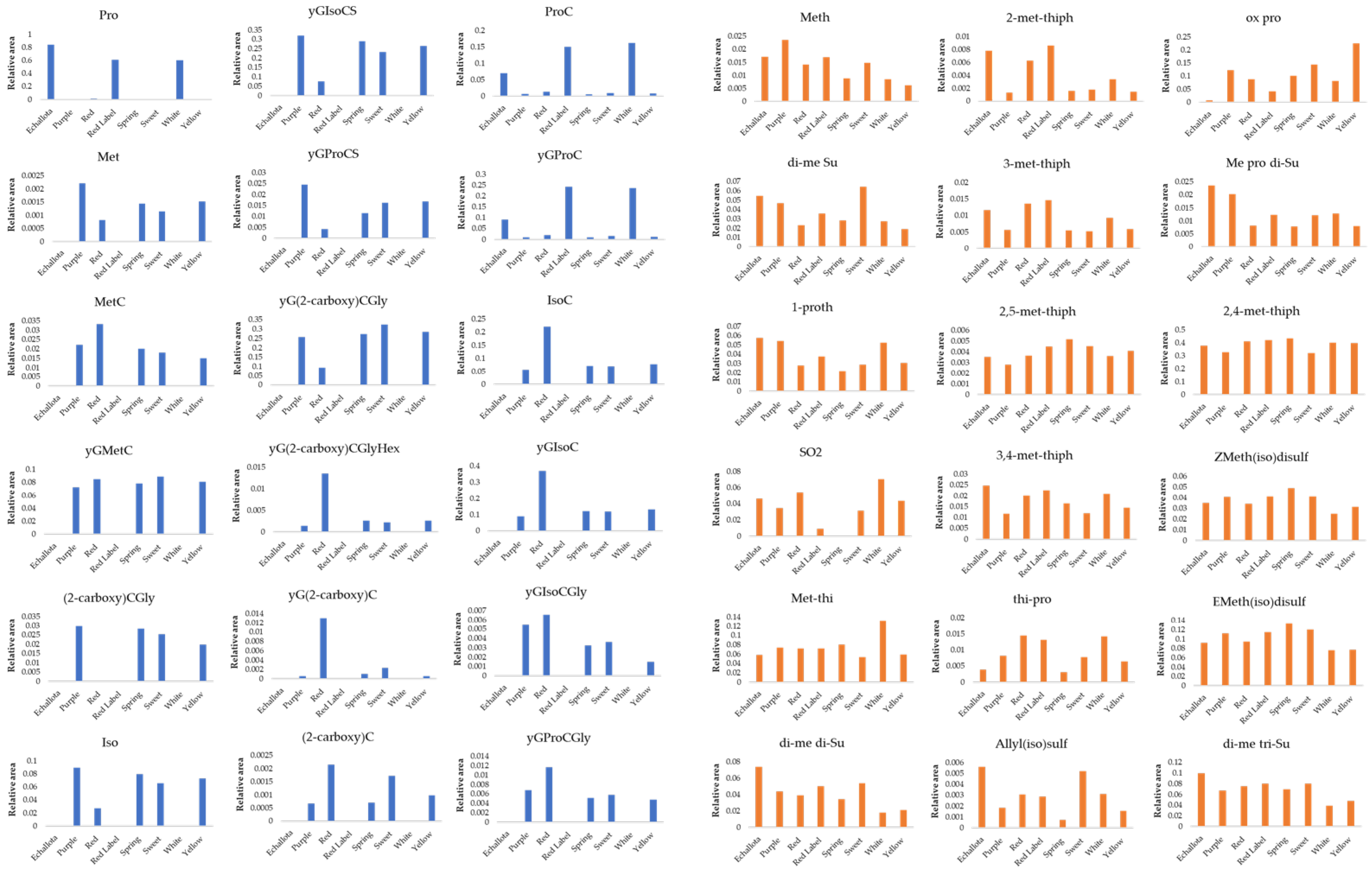
2.1. Onion Samples
2.2. Chemicals and Solvents
2.3. Study of Organosulfur Compounds Using GC-MS
2.4. Study of Organosulfur Compounds Using LC-HRMS
2.4.1. Extraction of Organosulfur Compounds
2.4.2. Analysis of Organosulfur Compounds by LC-HRMS
2.4.3. Identification of Organosulfur Compounds by LC-HRMS
- 1)
- 2)
- Then, from these previously reported compounds, the identification and characterization of compounds in the onion samples involved the evaluation of the mass error between the observed mass and the theoretical mass. To obtain accurate mass measurements mass spectrometers rely on calibration using ions of known m/z [31]. In this work, calibration of the instrument was performed externally before analysis with sodium formate solution. In addition, the calibration was validated by acquiring a post-calibration spectrum of the calibration solution itself (NaCOOH) and a known solution of leucine enkephalin. These calibration results showed a relative mass error of about 5 ppm. However, to ensure more accurate identification of the onion samples, candidate structures were considered with relative mass errors up to ± 10 ppm [32].
- 3)
- In addition, the isotopic pattern matching helped determine the chemical formula of the organosulfur compounds. Although the spectral patterns of isotopically generated ions are traditionally used as a secondary means of compound identification, in this work the careful examination of the theoretical patterns associated with a specific ion is also considered to be a powerful discriminator to uniquely identify chemical formulae [33].
- 4)
- Finally, tandem mass spectrometry analysis was employed to confirm the structure of the organosulfur compounds previously identified. Compounds for which reference MS/MS data could not be obtained were evaluated at the MS level only [34].
2.4. Statistical Analysis
3. Results and Discussion
3.1. DTD-GC-MS Analysis and Identification
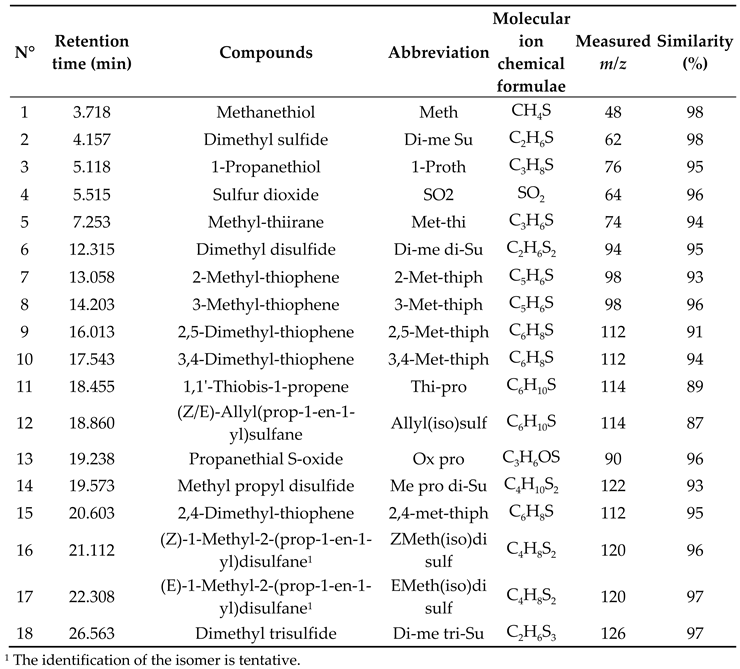
3.2. LC- ESI-QqToF Analysis and Identification
3.2. Quantification of Organosulfur Compounds
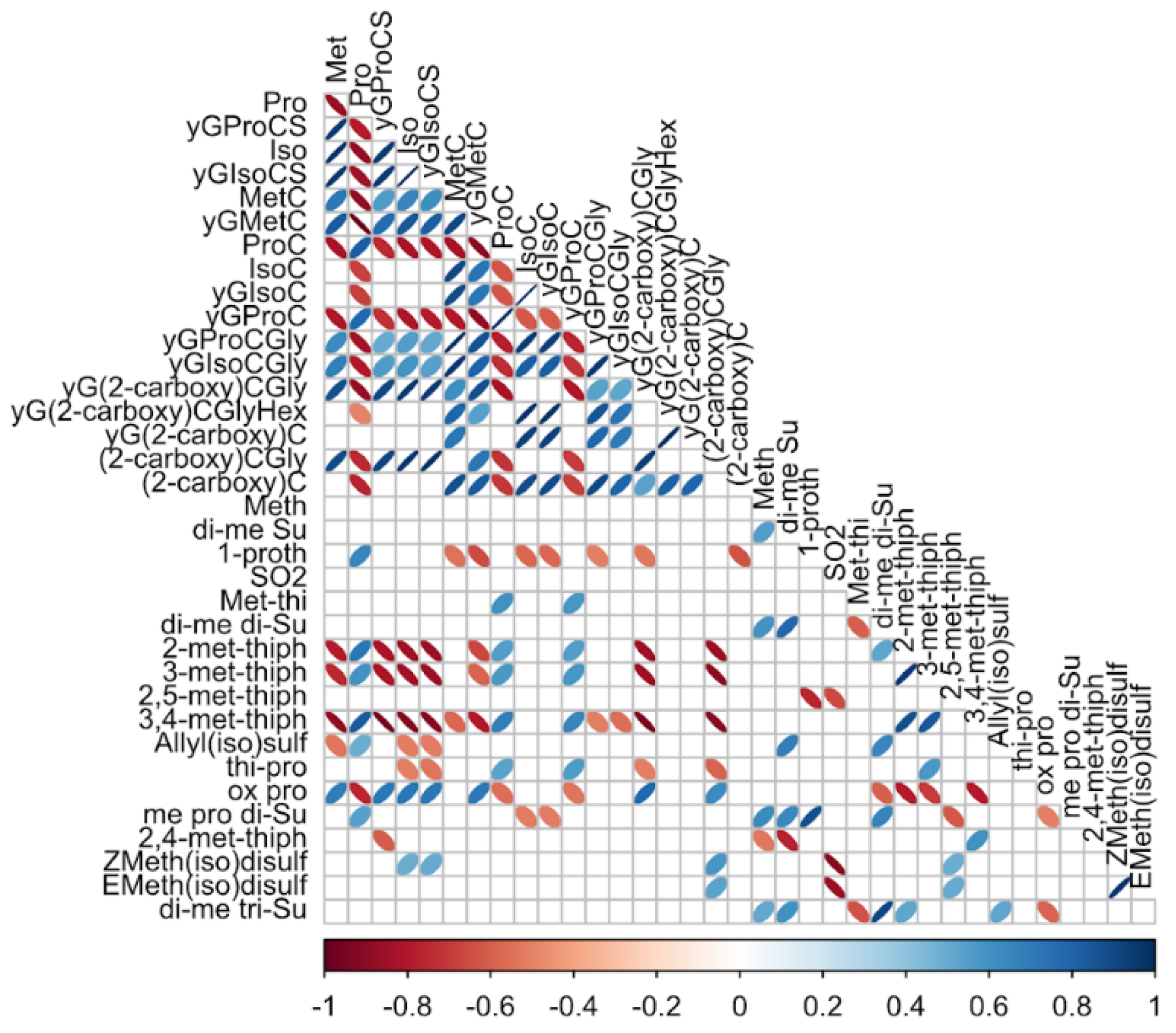
3.3. Correlations Between Organosulfur Compounds
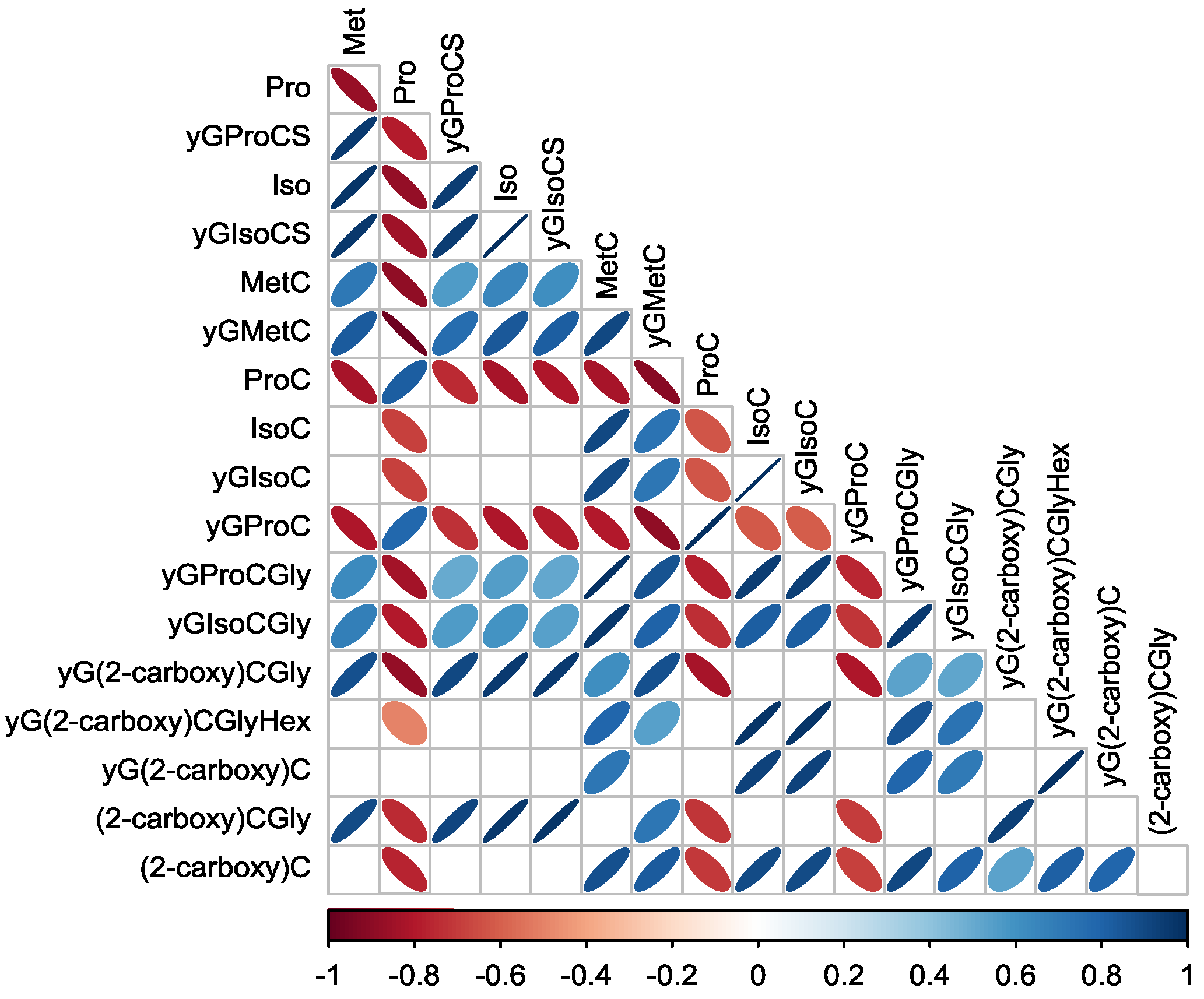
3.3.1. Correlations between Organosulfur Compounds Identified by LC- ESI-QqToF
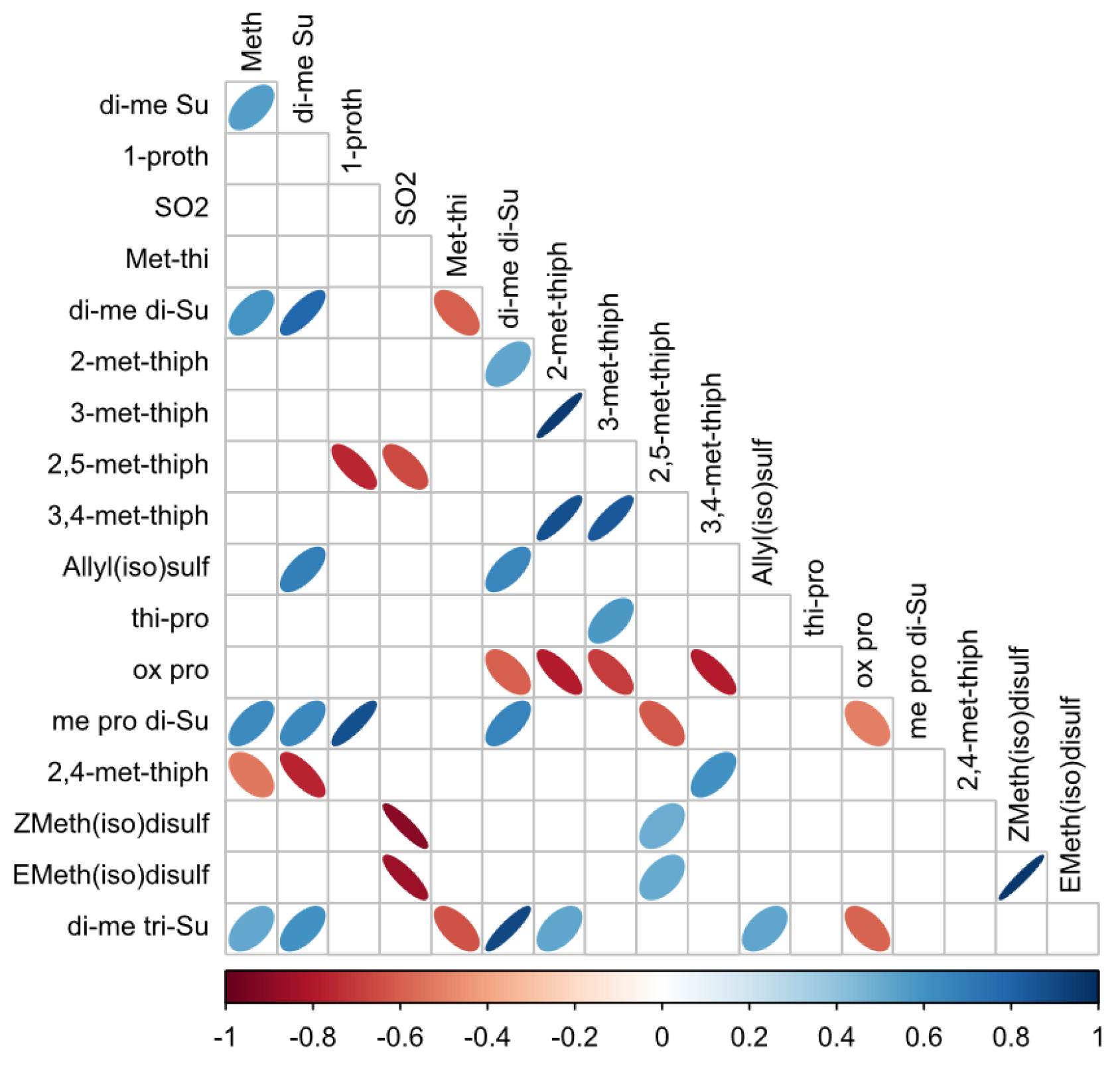
3.3.2. Correlations between Organosulfur Compounds Identified by DTD-GC-MS
3.3.3. Correlations between Organosulfur Compounds Identified with Both Techniques
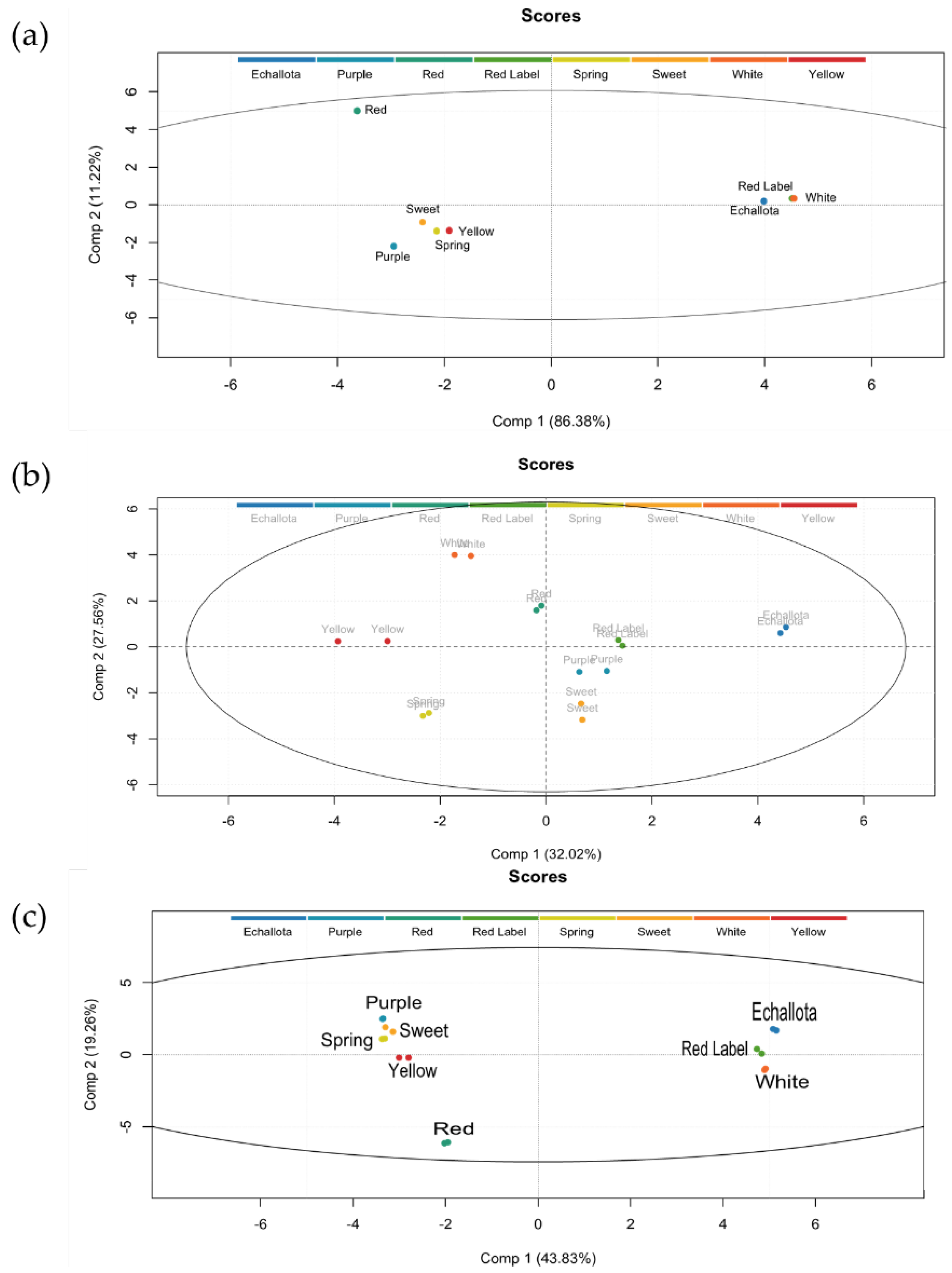
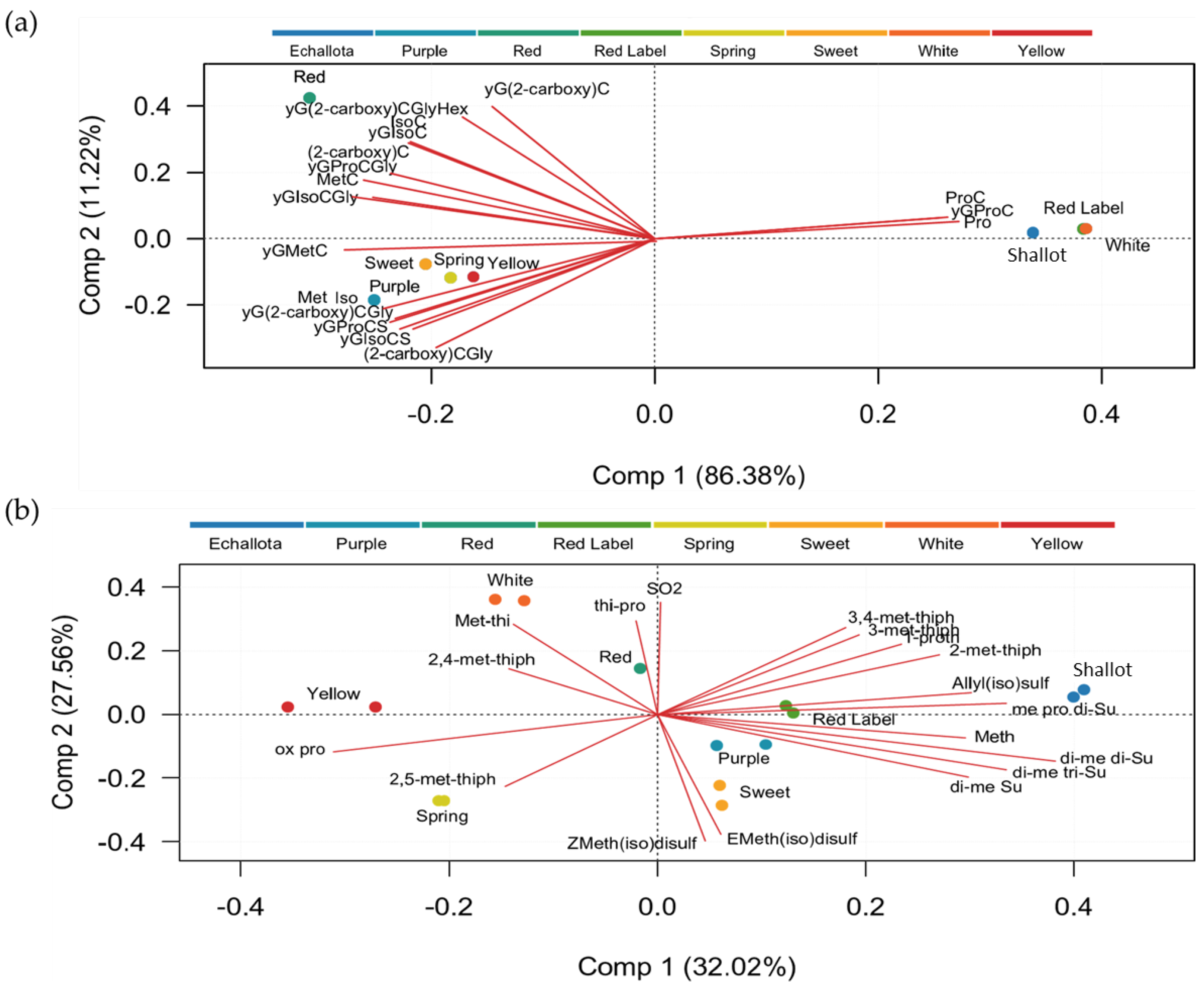
3.4. Principal Component Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallor, C.; Thomas, B. Resource allocation and the origin of flavor precursors in onion bulbs. J HORTIC SCI BIOTECH 2008, 83, 191–198. [Google Scholar] [CrossRef]
- Block, E. The Organosulfur Chemistry of the Genus Allium - Implications for the Organic Chemistry of Sulfur. Angew. Chemie Int. Ed. English 1992, 31, 1135–1178. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2015; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Marefati, N.; Ghorani, V.; Shakeri, F.; Boskabady, M.; Kianian, F.; Rezaee, R.; Boskabady, M.H. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm Biol. 2021, 59, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Dorant, E.; Van den Brandt, P.A.; Goldbohm, E.A.; Sturmans, F. Consumption of onions and a reduced risk of stomach carcinoma. Gastroenterology 1996, 110, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ülger, T.G.; Çakiroglu, F.P. The effects of onion (Allium cepa L.) dried by different heat treatments on plasma lipid profile and fasting blood glucose level in diabetic rats. Avicenna J Phytomed, 2020, 10, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, E.M.; Sabry, A.; Khalil, W.K.B. Neuroprotective effects of onion and garlic root extracts against Alzheimer’s disease in rats: antimicrobial, histopathological, and molecular studies. Biotechnologia 2022, 103, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, N.V.; Belinskaia, D.A.; Ukolov, A.I.; Jenkins, R.O.; Avdonin, P.V. Chapter 41 - Organosulfur Compounds as Nutraceuticals. In Nutraceuticals: Efficacy, Safety and Toxicity. 2016, Ramesh, C.G., Editorial Elsevier Inc., 555–568, ISBN:9780128210383.
- Zhu, G.; Gou, J.; Klee, H.; Huang, S. Next-Gen Approaches to Flavor-Related Metabolism. Annu Rev Plant Biol. 2019, 29, 187–212. [Google Scholar] [CrossRef] [PubMed]
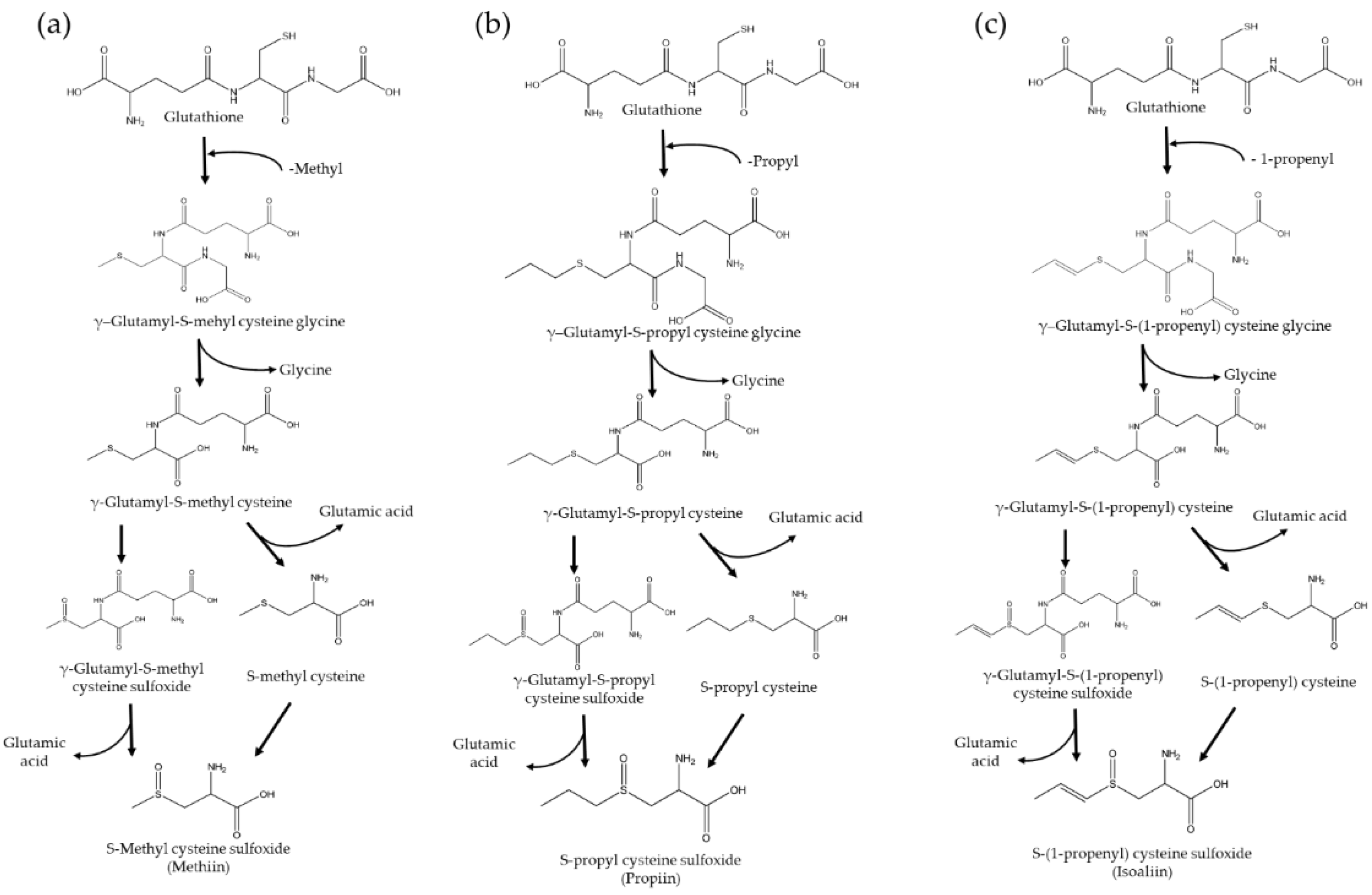
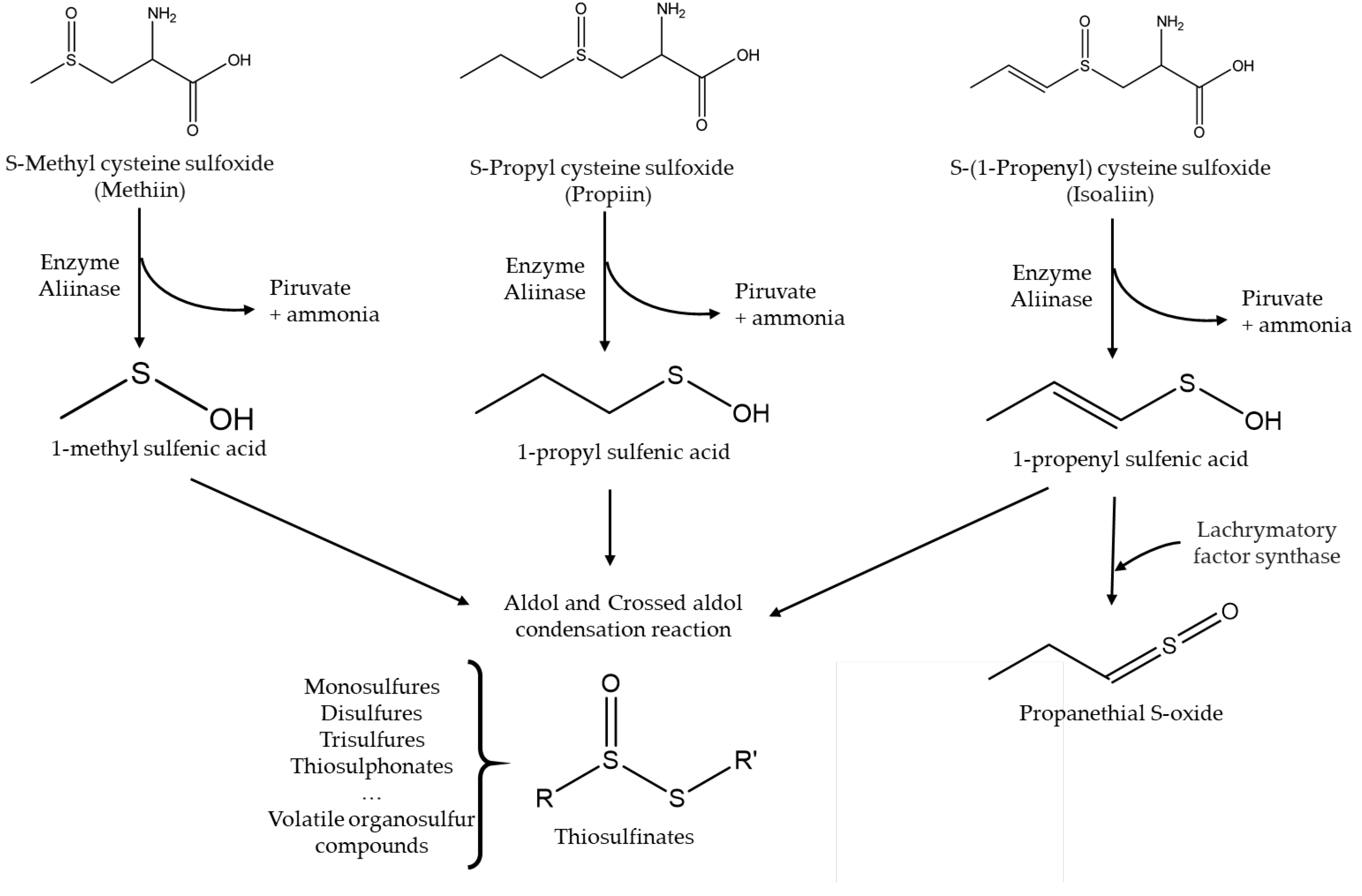
- Jones, M.G.; Hughes, J.; Tregova, A.; Milne, J.; Tomsett, A.B.; Collin, H.A. Biosynthesis of the flavour precursors of onion and garlic. J Exp Bot. 2004, 55, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.R.; Moates, G.K.; Ng, A.; Rhodes, M.J.C.; Smith, A.C.; Waldron, K.W. Quantitative analysis of flavour precursors and pyruvate levels in different tissues and cultivars of onion (Allium cepa). Food Chem. 1999, 64, 257–261. [Google Scholar] [CrossRef]
- Silvaroli, J.A.; Pleshinger, M.J.; Banerjee, S.; Kiser, P.D.; Golczak, M. Enzyme That Makes You Cry-Crystal Structure of Lachrymatory Factor Synthase from Allium cepa. ACS Chem Bio. 2017, 12, 2296–2304. [Google Scholar] [CrossRef]
- Hill, C.R.; Haoci Liu, A.; McCahon, L.; Zhong, L.; Shafaei, A.; Balmer, L.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. S-methyl cysteine sulfoxide and its potential role in human health: a scoping review. Crit Rev Food Sci Nutr. 2023, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kubec, R.; Svobodová, M.; Velı́šek, J. Gas chromatographic determination of S-alk(en)ylcysteine sulfoxides. J Chromatogr A 1999, 862, 85–94. [Google Scholar] [CrossRef]
- Machová, M.; Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Release of Volatile Compounds from Sliced Onion Analysed by Gas Chromatography Coupled to Mass Spectrometry and Its Antimicrobial Activity. J. Food Nutr. Res. 2019, 58, 393–400, ISSN:1336-8672. [Google Scholar]
- Kim, N.Y.; Park, M.H.; Jang, E.Y.; Lee, J.H. Volatile Distribution in Garlic (Allium Sativum L.) by Solid Phase Microextraction (SPME) with Different Processing Conditions. Food Sci. Biotechnol. 2011, 20, 775–782. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barbero, G.F. Application of Direct Thermal Desorption–Gas Chromatography–Mass Spectrometry for Determination of Volatile and Semi-Volatile Organosulfur Compounds in Onions: A Novel Analytical Approach. Pharmaceuticals 2023, 16, 715. [Google Scholar] [CrossRef] [PubMed]
- Mitrová, K.; Hrbek, V.; Svoboda, P.; Hajšlová, J.; Ovesná, J. Antioxidant activity, s-alk(en)yl-l-cysteine sulfoxide and polyphenol content in onion (allium cepa L.) cultivars are associated with their genetic background. Czech J. Food Sci. 2016, 34, 127–132. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Shin, D.; Yoo, M. Change in Organosulfur Compounds in Onion (Allium Cepa L.) during Heat Treatment. Food Sci. Biotechnol. 2016, 25, 115–119. [Google Scholar] [CrossRef]
- Böttcher, C.; Krähmer, A.; Stürtz, M.; Widder, S.; Schulz, H. Comprehensive Metabolite Profiling of Onion Bulbs (Allium Cepa) Using Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry. Metabolomics 2017, 13, 1–15. [Google Scholar] [CrossRef]
- Rhyu, D.Y.; Park, S.H. Characterization of alkyl thiosulfinate in Allium hookeri root using HPLC-ESI-MS. J Korean Soc Appl Biol Chem. 2013, 56, 457–459. [Google Scholar] [CrossRef]
- Tsuge, K.; Kataoka, M.; Seto, Y. Determination of S-methyl-, S-propyl-, and S-propenyl-L-cysteine sulfoxides by gas chromatography-mass spectrometry after tert-butyldimethylsilylation. J Agric Food Chem. 2002, 50, 4445–4451. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Wang, Z.; Zhang, X.; Ni, Y. Modified method for rapid quantitation of S-alk(en)yl-L-cysteine sulfoxide in yellow onions (Allium cepa L.). J Agric Food Chem. 2007, 55, 5429–5435. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.A.; Locatelli, D.A.; González, R.E.; Cavagnaro, P.F.; Camargo, A.B. Analytical Methods for Bioactive Sulfur Compounds in Allium: An Integrated Review and Future Directions. J. Food Compos. Anal. 2017, 61, 4–19. [Google Scholar] [CrossRef]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Carrera, C.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Flavonol composition and antioxidant activity of onions (Allium cepa L.) based on the development of new analytical ultrasound-assisted extraction methods. Antioxidants 2021, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Weng, R.; Sheng, X.; Wang, X.; Zhang, W.; Qian, Y.; Qiu, J. Profiling of organosulfur compounds and amino acids in garlic from different regions of China. Food Chem. 2020, 305, 125499. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Ma, H.; Zhang, X.; Zhang, Z.; Wang, Y. Metabolomics and transcriptomics analyses provides insights into S-alk(en)yl cysteine sulfoxides (CSOs) accumulation in onion (Allium cepa). Sci Hortic. 2023, 310, 111727. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Pereira-Caro, G.; Ludwig, I.A.; Motilva, M.J.; Moreno-Rojas, J.M. Bioavailability of Organosulfur Compounds after the Ingestion of Black Garlic by Healthy Humans. Antioxidants 2023, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rojas, J.M.; Moreno-Ortega, A.; Ordóñez, J.L.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Pereira-Caro, G. Development and Validation of UHPLC-HRMS Methodology for the Determination of Flavonoids, Amino Acids and Organosulfur Compounds in Black Onion, a Novel Derived Product from Fresh Shallot Onions (Allium Cepa Var. Aggregatum). LWT 2018, 97, 376–383. [Google Scholar] [CrossRef]
- Romson, J.; Emmer, Å. Mass calibration options for accurate electrospray ionization mass spectrometry. Int. J. Mass Spectrom. 2021, 467, 116619. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of phenolic compounds from medicinal plants (Hops and Juniper Berries) and their antioxidant activity. Foods, 2020, 9, 1–25. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Baruca Arbeiter, A.; Peeters, K.; Miklavčič Višnjevec, A.; Jenko Pražnikar, Z. HPLC-DAD-ESI-QTOF-MS Determination of Bioactive Compounds and Antioxidant Activity Comparison of the Hydroalcoholic and Water Extracts from Two Helichrysum italicum Species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Kerebba, N.; Rautenbach, F.; Kumar Singh, S.; Dua, K.; Oguntibeju, O.O. UPLC-ESI-QTOF-MS phenolic compounds identification and quantification from ethanolic extract of Myrtus communis ‘Variegatha’: In vitro antioxidant and antidiabetic potentials. Arab. J. Chem. 2023, 16, 104447. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical. 2022, Computing, Vienna, Austria. https://www.R-project.org/.
- RStudio Team. RStudio: Integrated Development Environment for R. 2022, RStudio, PBC, Boston, MA. http://www.rstudio.com/.
- Wei, T.; Simko, V. R package 'corrplot': Visualization of a Correlation Matrix. (Version 0.92). 2021. Available from https://github.com/taiyun/corrplot.
- Kucheryavskiy, S. mdatools – R package for chemometrics. Chemom. Intell. Lab. Syst. 2020, 198, 103937. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Ludwig, I.A.; Motilva, M.J.; Moreno-Rojas, J.M.; Pereira-Caro, G. Urinary excretion of organosulfur compounds after acute ingestion of black onion. Food Funct. 2023, 14, 5023–5031. [Google Scholar] [CrossRef]
- Randle, W.M.; Lancaster, J.E.; Shaw, M.L.; Sutton, K.H.; Hay, R.L.; Bussard, M.L. Quantifying Onion Flavor Compounds Responding to Sulfur Fertility-Sulfur Increases Levels of Alk(En)Yl Cysteine Sulfoxides and Biosynthetic Intermediates. J. Am. Soc. Hortic. Sci. 1995, 120, 1075–1081. [Google Scholar] [CrossRef]
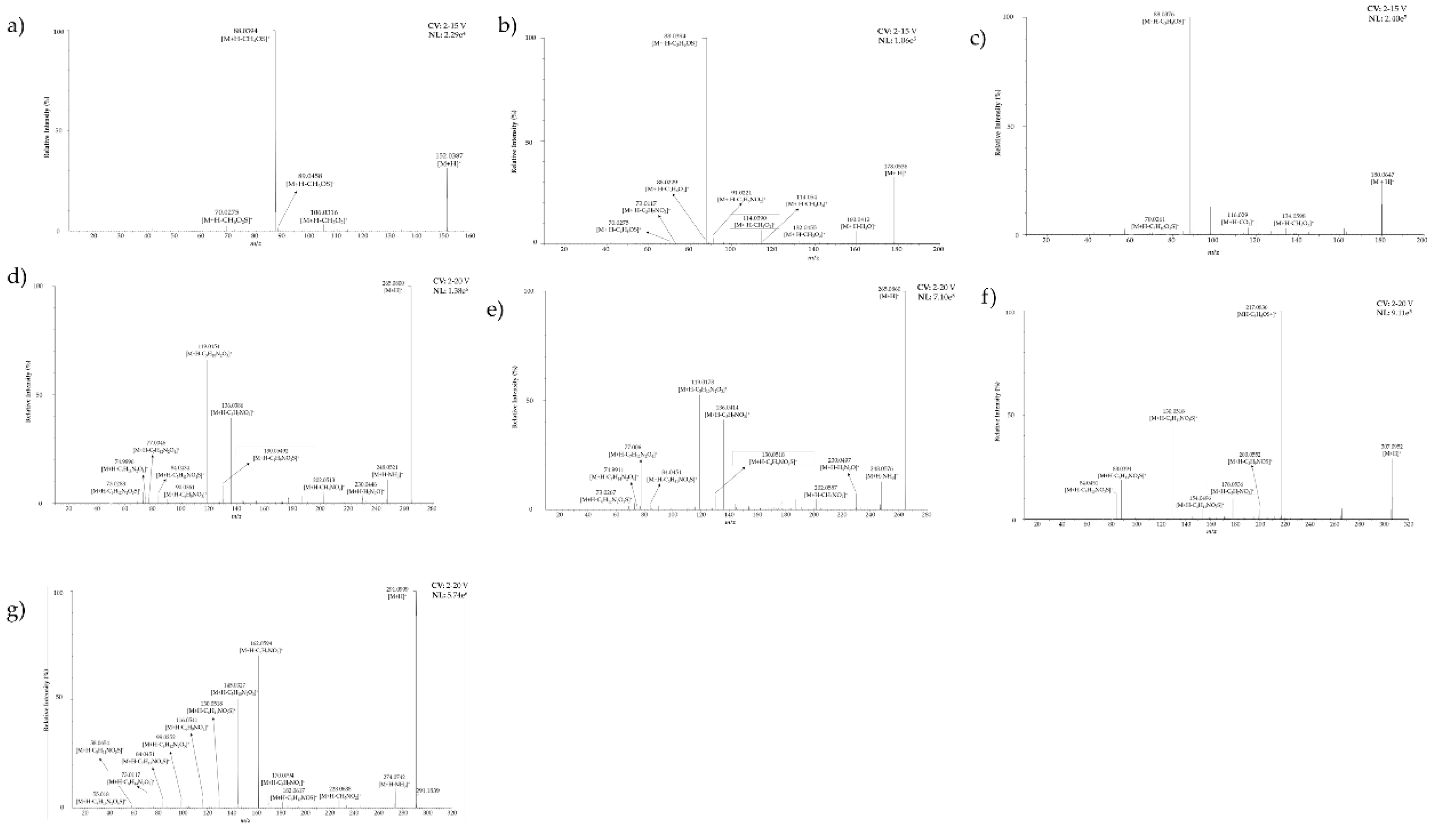
| N° | Retention time (min) | Compounds | Abbreviation | Ion formulae | Measured m/z | Calculated m/z | Absolute error δ (ppm) | Product ions formula (Relative intensity [%], elemental composition) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.908 | Propiin | Pro | C6H14NO3S | 180.0705 | 180.0694 | 6.1088 | 70.0261 (1, C3H4NO+), 88.0376 (100, C3H6NO2+), 116.0290 (3, C5H8OS+), 134.0598 (3, C5H12NOS+), 180.0647 (23, C6H14NO3S+) |
| 2 | 3.145 | Methiin | Met | C4H10NO3S | 152.0385 | 152.0381 | 2.6309 | 70.0275 (2, C3H4NO+), 88.0392 (100, C3H6NO2+), 89.0458 (3, C3H7NO2+.), 106.0316 (8, C3H8NOS+), 152.0369 (47, C4H10NO3S+) |
| 3 | 3.331 | S-Methyl cysteine | MetC | C4H10NO2S | 136.0438 | 136.0432 | 4.4104 | No MSMS data available |
| 4 | 3.331 | y-glutamyl-S-methyl-L-cysteine | yGMetC | C9H17N2O5S | 265.0866 | 265.0858 | 3.0179 | 73.0276 (3, C3H5O2+), 74.9911 (1, C2H3OS+), 77.0062 (1, C2H5OS +), 84.0451 (2, C2H6NO+), 90.0379 (2, C3H8NS+), 119.0178 (53, C4H7O2S+), 130.0518 (7, C5H8NO3S+), 136.0414 (41, C4H10NO2S+), 202.0557 (5, C8H12NO3S+), 230.0497 (7, C9H12NO4S+), 248.0576 (13, C9H14NO5S+), 265.0860 (100, C9H17N2O5S+) |
| 5 | 3.348 | Isoaliin | Iso | C6H12NO3S | 178.0542 | 178.0538 | 2.2465 | 70.0275 (1, C3H4NO+), 73.0117 (1, C3H5S+), 88.0229 (3, C3H6NS+), 88.0394 (100, C3H6NO2+), 91.0221 (4, C3H7O+), 105.0016 (3, C3H5O2S+), 114.039 (8, C5H8NO2+), 118.0678 (6, C5H12NS+), 123.0119 (4, C3H7O3S+), 132.0455 (2, C5H10NOS+), 146.0599 (7, C6H12NOS+), 160.0412 (5, C6H10NO2S+), 178.0536 (30, C6H12NO3S+) |
| 6 | 3.348 | γ-Glutamyl-S-(1-propenyl) cysteine sulfoxide | yGIsoCS | C11H19N2O6S | 307.0981 | 307.0964 | 5.5357 | 84.0451 (12, C4H6NO+), 88.0394 (19, C3H6NO2+), 130.0518 (46, C5H8NO3+), 154.0496 (5, C7H8NO3+), 178.0536 (10, C6H12NO3S+), 200.0552 (5, C8H10NO5+), 217.0836 (100, C8H13N2O5+), 307.0952 (29, C11H19N2O6S+) |
| 7 | 3.449 | S-(2-carboxypropil) cysteine-glycine | (2-carboxy)CGly | C9H17N2O5S | 265.0866 | 265.0858 | 3.0179 | 73.0283 (5, C3H5O2+), 74.9896 (1, C2H3OS+), 77.0046 (3, C2H5OS+), 84.0434 (4, C2H6NO+), 90.0361 (2, C3H8NS+), 119.0154 (66, C4H7O2S+), 130.0492 (8, C5H8NO3S+), 136.0386 (39, C4H10NO2S+), 202.0513 (5, C8H12NO3S+), 230.0446 (4, C9H12NO4S+), 248.0521 (11, C9H14NO5S+), 265.0800 (100, C9H17N2O5S+) |
| 8 | 3.398 | γ-Glutamyl-S-propyl cysteine sulfoxide | yGProCS | C11H21N2O6S | 309.1130 | 309.1120 | 3.2351 | No MSMS data available |
| 9 | 3.449 | γ–Glutamyl-S-(2-carboxy propyl) cysteine glycine | yG(2-carboxy)CGly | C14H24N3O8S | 394.1303 | 394.1284 | 4.8208 | No MSMS data available |
| 10 | 3.534 | γ–Glutamyl-S-(2-carboxypropyl) cysteine glycine hexoside | yG(2-carboxy)CGlyHex | C20H34N3O13S | 556.1827 | 556.1812 | 2.6970 | No MSMS data available |
| 11 | 3.567 | γ–Glutamyl-S-(2-carboxypropyl) cysteine | yG(2-carboxy)C | C12H21N2O7S | 337.1080 | 337.1069 | 1.7799 | No MSMS data available |
| 12 | 3.584 | S-(2-carboxypropyl) cysteine | (2-carboxy)C | C7H14NO4S | 208.0648 | 208.0643 | 2.4031 | No MSMS data available |
| 13 | 4.075 | S-Propylcysteine | ProC | C6H14NO2S | 164.0752 | 164.0745 | 4.2664 | No MSMS data available |
| 14 | 4.091 | γ-Glutamyl-S-propyl cysteine | yGProC | C11H21N2O5S | 293.1176 | 293.1171 | 1.7058 | No MSMS data available |
| 15 | 4.818 | S-(1-Propenyl) cysteine | IsoC | C6H12NO2S | 162.0590 | 162.0589 | 0.6171 | No MSMS data available |
| 16 | 4.835 | γ-Glutamyl-S-(1-propenyl) cysteine | yGIsoC | C11H19N2O5S | 291.1022 | 291.1015 | 2.4047 | 55.018 (1, C3H3O+), 58.0654 (1, C3H8N+), 73.0117 (8, C3H5S+), 84.0451 (3, C4H6NO+), 99.0252 (3, C5H7S+), 116.0541 (4, C5H10NS+), 130.0518 (4, C5H8NO3+), 145.0327 (50, C6H9O2S+), 162.0594 (70, C6H12NO2S+), 170.0794 (8, C8H12NOS+), 182.0617 (3, C9H12NOS+), 228.0688 (4, C10H14NO3S+), 274.0742 (9, C11H16NO5S+), 291.0999 (100, C9H17N2O5S+) |
| 17 | 7.777 | γ–Glutamyl-S-(S-1-propenyl)cysteine glycine | yGIsoCGly | C13H22N3O6S2 | 380.0971 | 380.0950 | 5.5249 | No MSMS data available |
| 18 | 8.825 | γ–Glutamyl-S-(S-propyl)cysteine-glycine | YGProCGly | C13H24N3O6S2 | 382.1113 | 382.1107 | 1.5702 | No MSMS data available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





