Submitted:
05 June 2024
Posted:
06 June 2024
You are already at the latest version
Abstract
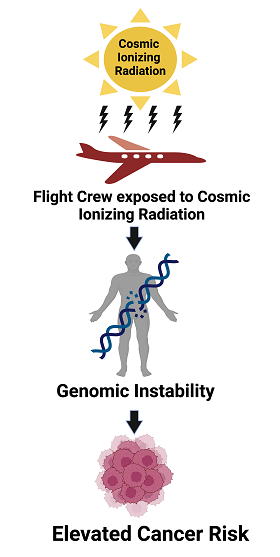
Keywords:
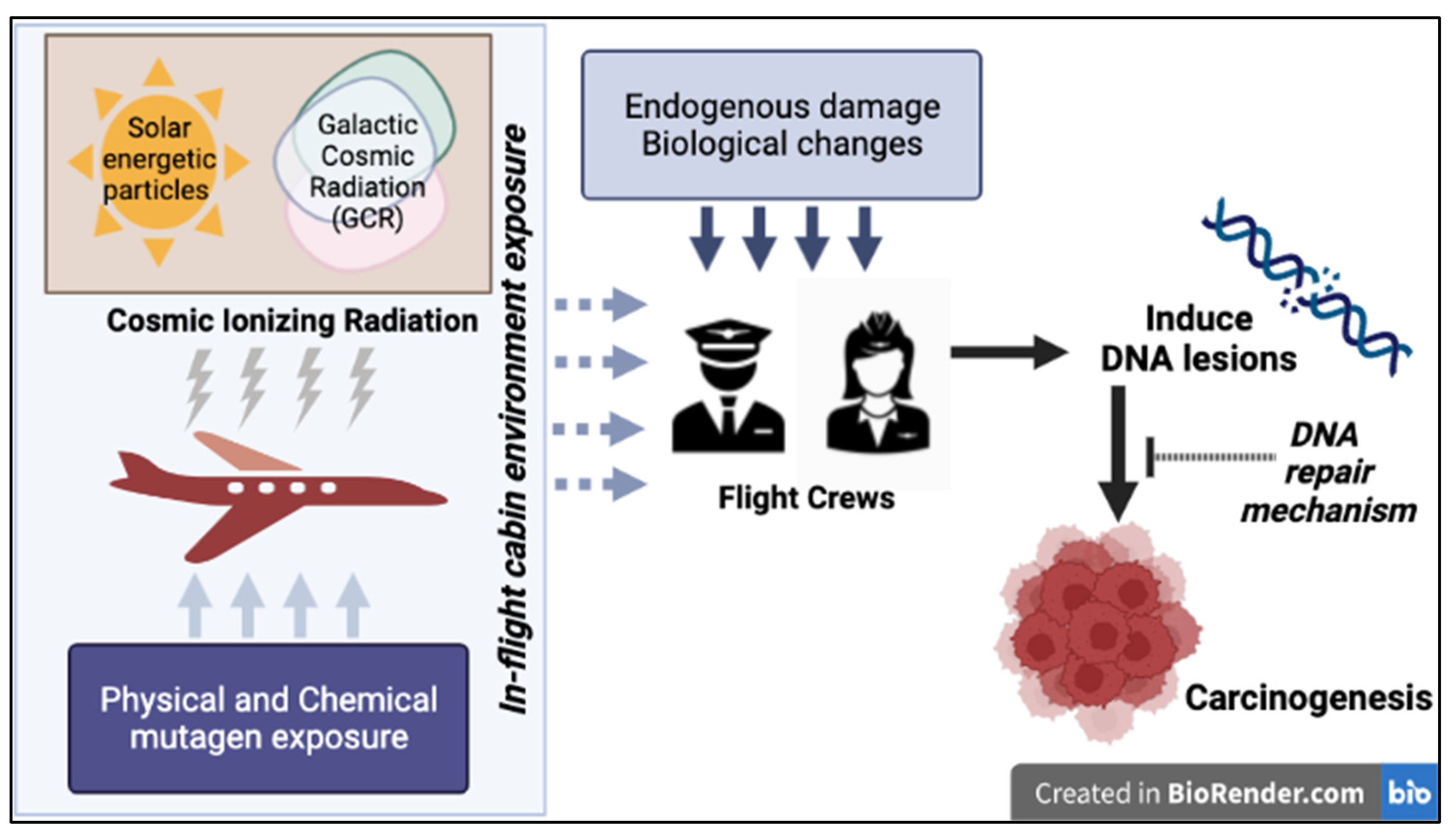
1. Introduction
2. Current Understanding of CIR Exposure during Flight Travel
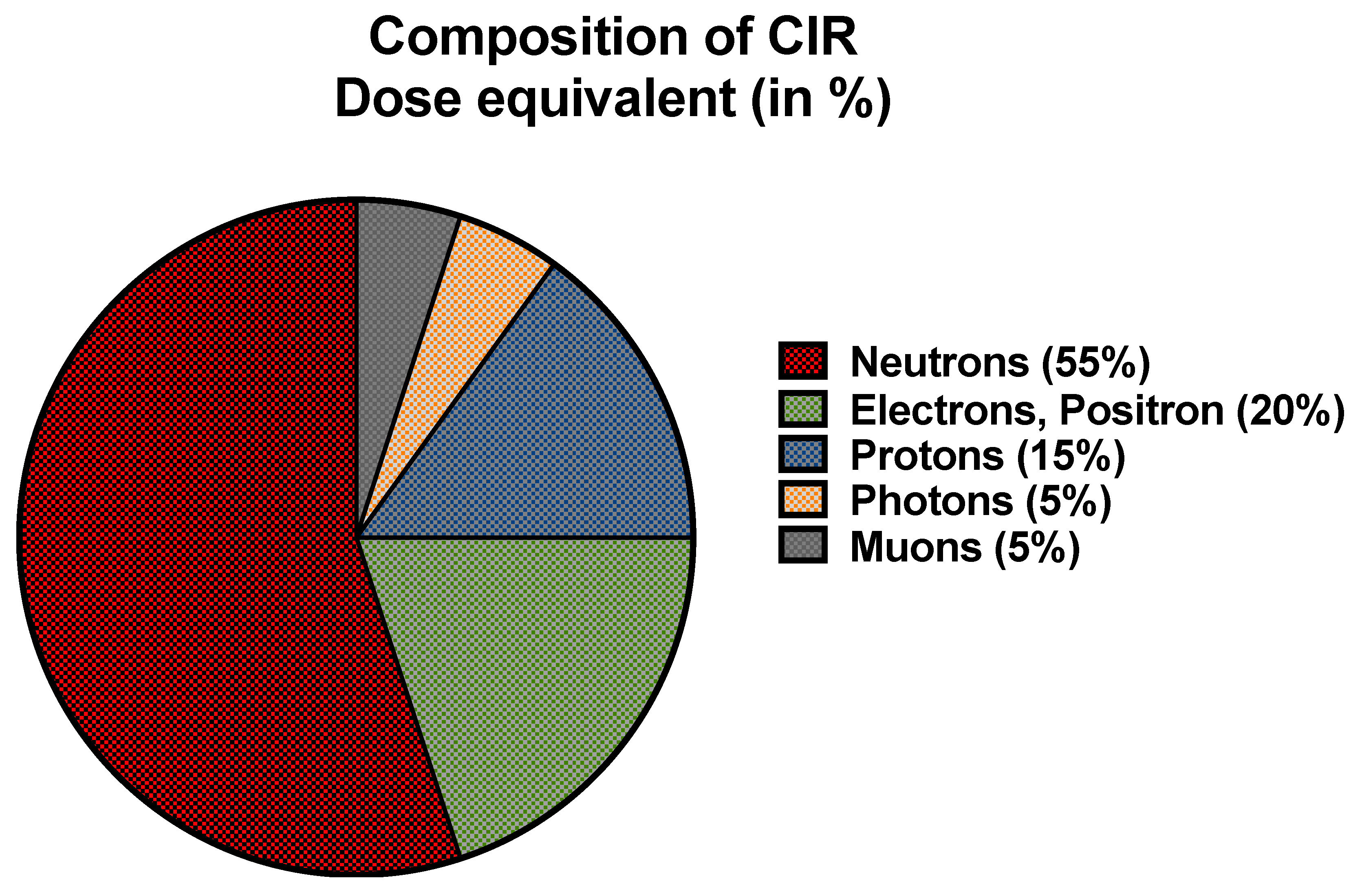
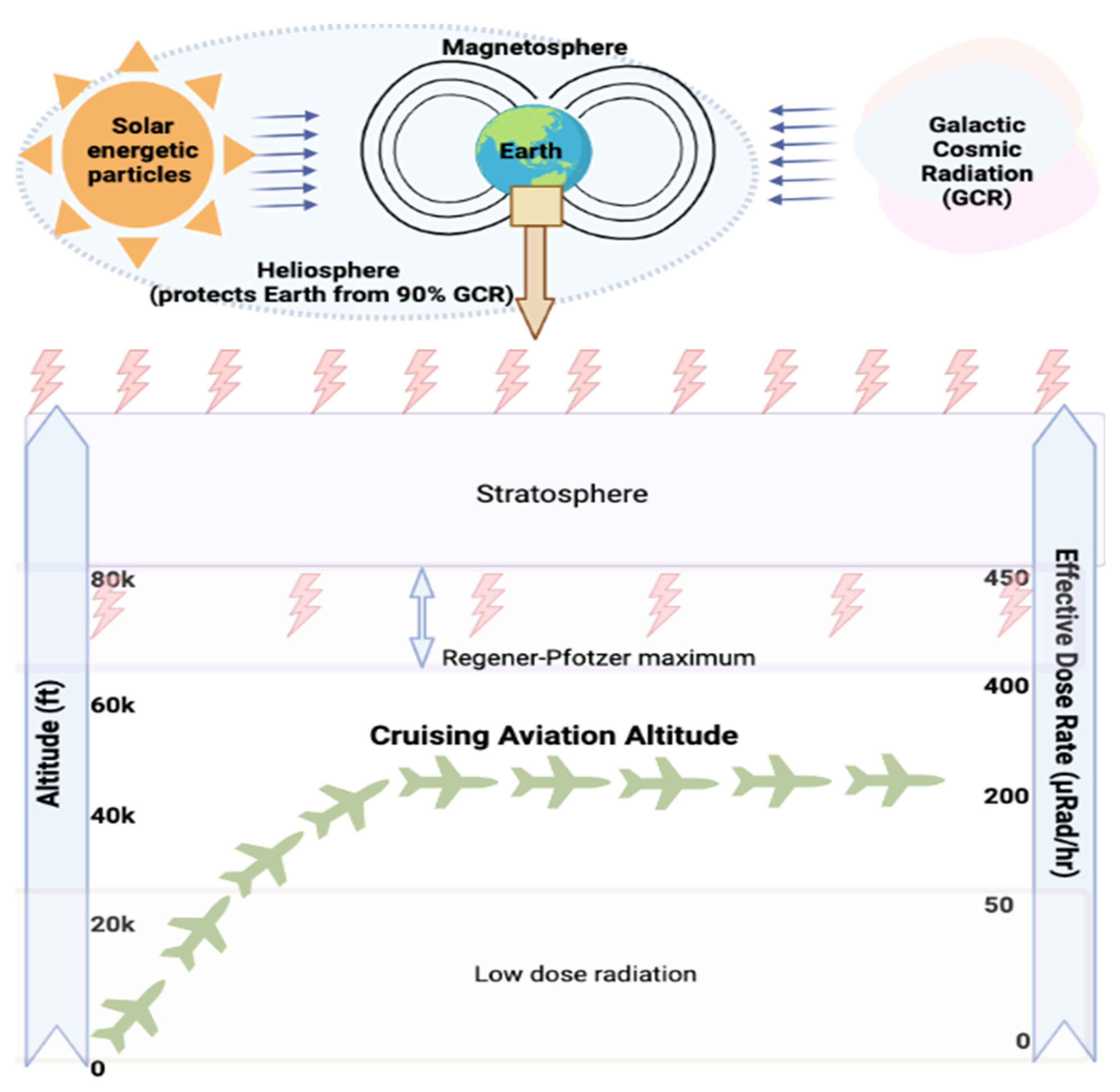
2.1. CIR Exposure during Flight
2.2. Methods for Measuring or Estimating CIR Exposure during Air Travel
3. Inter-Individual Variation in Response to Radiation Exposure
4. DNA Damage and Repair Mechanisms Associated with CIR Exposure in FC
4.1. Markers of DNA Damage and Genome Instability in FC that Are Consistent with the Expected Biological Effects of CIR Exposure
4.2. DNA Repair Mechanisms Involved in the Cellular Response to Damage Induced by CIR Components
5. Discussion and Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Council on Radiation Protection and Measurements Ionizing Radiation Exposure of the Population of the United States. Report No. 160. Council on Radiation Protection and Measurements (NCRP). Bethesda, MD:; 2009.
- Lin, E.C. Radiation Risk From Medical Imaging. Mayo Clin Proc 2010, 85, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- UNSCEAR Annex C. Biological Mechanisms Relevant for the Inference of Cancer Risks from Low-Dose and Low-Dose-RateradiationUNSCEAR 2020/2021 Report(Vienna: UNSCEAR); 2021.
- Aw, J. Cosmic Radiation and Commercial Air Travel. J Travel Med 2003, 10, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Goldhagen, P. Overview of Aircraft Radiation Exposure and Recent ER-2 Measurements. Health Phys 2000, 79, 526–544. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.; Fuller, N. History of the Solar Particle Event Radiation Doses On-Board Aeroplanes Using a Semi-Empirical Model and Concorde Measurements. Radiat Prot Dosimetry 2003, 104, 199–210. [Google Scholar] [CrossRef] [PubMed]
- McNeely, E.; Gale, S.; Tager, I.; Kincl, L.; Bradley, J.; Coull, B.; Hecker, S. The Self-Reported Health of U. S. Flight Attendants Compared to the General Population. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Scheibler, C.; Toprani, S.M.; Mordukhovich, I.; Schaefer, M.; Staffa, S.; Nagel, Z.D.; McNeely, E. Cancer Risks from Cosmic Radiation Exposure in Flight: A Review. Front public Heal 2022, 10. [Google Scholar] [CrossRef]
- Griffiths, R.F.; Powell, D.M.C. The Occupational Health and Safety of Flight Attendants. Aviat Space Environ Med 2012, 83, 514–521. [Google Scholar] [CrossRef]
- McNeely, E.; Mordukhovich, I.; Tideman, S.; Gale, S.; Coull, B. Estimating the Health Consequences of Flight Attendant Work: Comparing Flight Attendant Health to the General Population in a Cross-Sectional Study. BMC Public Health 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Bramlitt, E.T.; Shonka, J.J. Radiation Exposure of Aviation Crewmembers and Cancer. Health Phys 2015, 108, 76–86. [Google Scholar] [CrossRef]
- Toprani, S.M.; Scheibler, C.; Nagel, Z.D. Interplay Between Air Travel, Genome Integrity, and COVID-19 Risk Vis-a-Vis Flight Crew. Front Public Heal 2020, 8, 1–6. [Google Scholar] [CrossRef]
- McNeely, E.; Mordukhovich, I.; Staffa, S.; Tideman, S.; Coull, B. Legacy Health Effects among Never Smokers Exposed to Occupational Secondhand Smoke. 2019, 14. [CrossRef]
- Pinkerton, L.E.; Hein, M.J.; Anderson, J.L.; Little, M.P.; Sigurdson, A.J.; Schubauer-Berigan, M.K. Breast Cancer Incidence among Female Flight Attendants: Exposure–Response Analyses. Scand J Work Environ Heal 2016, 42, 538–546. [Google Scholar] [CrossRef] [PubMed]
- McNeely, E.; Mordukhovich, I.; Staffa, S.; Tideman, S.; Gale, S.; Coull, B. Cancer Prevalence among Flight Attendants Compared to the General Population. Environ Health 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Pukkala, E.; Helminen, M.; Haldorsen, T.; Hammar, N.; Kojo, K.; Linnersjö, A.; Rafnsson, V.; Tulinius, H.; Tveten, U.; Auvinen, A. Cancer Incidence among Nordic Airline Cabin Crew. Int J cancer 2012, 131, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, L.E.; Hein, M.J.; Anderson, J.L.; Christianson, A.; Little, M.P.; Sigurdson, A.J.; Schubauer-Berigan, M.K. Melanoma, Thyroid Cancer, and Gynecologic Cancers in a Cohort of Female Flight Attendants. 2018, 61, 572–581.
- Schüz, J. Airline Crew Cohorts: Is There More to Learn Regarding Their Cancer Risk? Occup Environ Med 2014, 71, 307. [Google Scholar] [CrossRef] [PubMed]
- Nuclear Regulatory Commission (NRC) Biological Effects of Radiation. U.S. Nuclear Regulatory Commission Backgrounder (2017); 2017.
- ICRP International Commission on Radiological Protection (ICRP). Publication 103: Recommendations of the International Commission on Radiological Protection. Radiat Prot Dosim 2008, 129, 500–507. [Google Scholar]
- Sridharan, D.M.; Asaithamby, A.; Blattnig, S.R.; Costes, S. V.; Doetsch, P.W.; Dynan, W.S.; Hahnfeldt, P.; Hlatky, L.; Kidane, Y.; Kronenberg, A.; et al. Evaluating Biomarkers to Model Cancer Risk Post Cosmic Ray Exposure. Life Sci Sp Res 2016, 9, 19–47. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T.; Barnes, D.E. Repair of Endogenous DNA Damage. Cold Spring Harb Symp Quant Biol 2000, 65, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 7th Edition; Wolters Kluwer Health Adis (ESP), 2012; ISBN 10: 1608311937.
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological Consequences of Radiation-Induced DNA Damage: Relevance to Radiotherapy. Clin Oncol (R Coll Radiol) 2013, 25, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Nagel, Z.D.; Chaim, I.A.; Samson, L.D. Inter-Individual Variation in DNA Repair Capacity: A Need for Multi-Pathway Functional Assays to Promote Translational DNA Repair Research. DNA Repair (Amst) 2014, 19, 199–213. [Google Scholar] [CrossRef]
- Nelson, B.C.; Dizdaroglu, M. Implications of DNA Damage and DNA Repair on Human Diseases. Mutagenesis 2020, 35, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.S.; Jorgensen, T.J.; Kennedy, A.R.; Boice, J.D.; Shapiro, A.; Hu, T.C.C.; Moyer, B.R.; Grace, M.B.; Kelloff, G.J.; Fenech, M.; et al. Mitigating the Risk of Radiation-Induced Cancers: Limitations and Paradigms in Drug Development. J Radiol Prot 2014, 34. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.T. Radiation Protection Aspects of the Cosmic Radiation Exposure of Aircraft Crew. 2004, 109, 349–355.
- Silva, R.; Folgosa, F.; Soares, P.; Pereira, A.S.; Garcia, R.; Gestal-Otero, J.J.; Tavares, P.; Gomes Da Silva, M.D.R. Occupational Cosmic Radiation Exposure in Portuguese Airline Pilots: Study of a Possible Correlation with Oxidative Biological Markers. Radiat Environ Biophys 2013, 52, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Mishev, A. Short- and Medium-Term Induced Ionization in the Earth Atmosphere by Galactic and Solar Cosmic Rays. Int J Atmos Sci 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Usoskin, I.G.; Desorgher, L.; Velinov, P.; Storini, M.; Flückiger, E.O.; Bütikofer, R.; Kovaltsov, G.A. Ionization of the Earth’s Atmosphere by Solar and Galactic Cosmic Rays. Acta Geophys 2009 571 2008, 57, 88–101. [Google Scholar] [CrossRef]
- Phillips, T.; Johnson, S.; Koske-Phillips, A.; White, M.; Yarborough, A.; Lamb, A.; Herbst, A.; Molina, F.; Gilpin, J.; Grah, O.; et al. Space Weather Ballooning. Sp Weather 2016, 14, 697–703. [Google Scholar] [CrossRef]
- Hands, A.D.P.; Ryden, K.A.; Mertens, C.J. The Disappearance of the Pfotzer-Regener Maximum in Dose Equivalent Measurements in the Stratosphere. Sp Weather 2016, 14, 776–785. [Google Scholar] [CrossRef]
- Desmaris, G. Cosmic Radiation in Aviation: Radiological Protection of Air France Aircraft Crew. Ann ICRP 2016, 45, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Blettner, M.; Grosche, B.; Zeeb, H. Occupational Cancer Risk in Pilots and Flight Attendants: Current Epidemiological Knowledge. Radiat Environ Biophys 1998, 37, 75–80. [Google Scholar] [CrossRef]
- Friedberg, W., Faulkner, DN., Snyder, L., Darden, EB Jr., and O’Brien, K. Galactic Cosmic Radiation Exposure and Associated Health Risks for Air Carrier Crewmembers. Aviat Sp Env Med 1989, 60, 1104–1108.
- Dreger, S.; Wollschläger, D.; Schafft, T.; Hammer, G.P.; Blettner, M.; Zeeb, H. Cohort Study of Occupational Cosmic Radiation Dose and Cancer Mortality in German Aircrew, 1960-2014. 2020, 77, 285–291.
- Federal Aviation Administration (FAA) In-Flight Radiation Exposure. US Department of Transportation, FAA Advisory Circular 120–61B.; 2014.
- Bottollier-Depois, J.F.; Chau, Q.; Bouisset, P.; Kerlau, G.; Plawinski, L.; Lebaron-Jacobs, L. Assessing Exposure to Cosmic Radiation on Board Aircraft. Adv Sp Res 2003, 32, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, P.J. Estimated Individual Annual Cosmic Radiation Doses for Flight Crews. Aviat Space Environ Med 1998, 69, 621–625. [Google Scholar] [PubMed]
- Grajewski, B.; Yong, L.C.; Bertke, S.J.; Bhatti, P.; Little, M.P.; Ramsey, M.J.; Tucker, J.D.; Ward, E.M.; Whelan, E.A.; Sigurdson, A.J.; et al. Chromosome Translocations and Cosmic Radiation Dose in Male U.S. Commercial Airline Pilots. Aerosp Med Hum Perform 2018, 89, 616–625. [Google Scholar] [CrossRef] [PubMed]
- EURADOS Comparison of Codes Assessing Radiation Exposure of Aircraft Crew Due to Galactic Cosmic Radiation, EURADOS Report 2012-03.; Braunschweig, 2012.
- Toprani, S.M.S.M.; Das, B. Radio-Adaptive Response of Base Excision Repair Genes and Proteins in Human Peripheral Blood Mononuclear Cells Exposed to Gamma Radiation. Mutagenesis 2015, 30, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Zabuga, O.; Socol, Y. Health Impacts of Low-Dose Ionizing Radiation: Current Scientific Debates and Regulatory Issues. Dose Response 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Toprani, S.M.; Das, B. Role of Base Excision Repair Genes and Proteins in Gamma-Irradiated Resting Human Peripheral Blood Mononuclear Cells. Mutagenesis 2015, 30, 247–261. [Google Scholar] [CrossRef]
- Calabrese, E.J. The Additive to Background Assumption in Cancer Risk Assessment: A Reappraisal. Environ Res 2018, 166, 175–204. [Google Scholar] [CrossRef]
- Toprani, S.M.; Das, B. Radio-Adaptive Response, Individual Radio-Sensitivity and Correlation of Base Excision Repair Gene Polymorphism (HOGG1, APE1, XRCC1, and LIGASE1) in Human Peripheral Blood Mononuclear Cells Exposed to Gamma Radiation. Environ Mol Mutagen 2020, 61, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Komov, O.; Krasavin, E.; Nasonov, E.; Mel’nikov, L.; Shmakov, N.; Cunh, M.; Testa, E.; Beuve, M. Relationship between Radioadaptive Response and Individual Radiosensitivity to Low Doses of Gamma Radiation: An Extended Study of Chromosome Damage in Blood Lymphocytes of Three Donors. Int J Radiat Biol 2018, 94, 54–61. [Google Scholar] [CrossRef]
- Nenoi, M.; Wang, B.; Vares, G. In Vivo Radioadaptive Response: A Review of Studies Relevant to Radiation-Induced Cancer Risk. Hum Exp Toxicol 2015, 34, 272. [Google Scholar] [CrossRef]
- Bolzán, A.D.; Bianchi, M.S.; Giménez, E.M.; Flaqué, M.C.D.; Ciancio, V.R. Analysis of Spontaneous and Bleomycin-Induced Chromosome Damage in Peripheral Lymphocytes of Long-Haul Aircrew Members from Argentina. Mutat Res 2008, 639, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC) Radiation Emergencies Factsheet – Radiation and Pregnancy: A Fact Sheet for the Public. Department of Health and Human Services, Updated 15 Nov 2011; 2011.
- Williams, P.; Fletcher, S. Health Effects of Prenatal Radiation Exposure. Am Fam Physician 2011, 82, 488–493. [Google Scholar]
- United Nations. Scientific Committee on the Effects of Atomic Radiation.; United Nations. General Assembly. Genetic and Somatic Effects of Ionizing Radiation : United Nations Scientific Committee on the Effects of Atomic Radiation : 1986 Report to the General Assembly, with Annexes. 1986, 366.
- Valentin, J.; Cox, R.; Streffer, C. Biological Effects after Prenatal Irradiation (Embryo and Fetus). 2003, 33, 1–206. [CrossRef]
- Saada, M.; Sanchez-Jimenez, E.; Roguin, A. Risk of Ionizing Radiation in Pregnancy: Just a Myth or a Real Concern? Europace 2023, 25, 270. [Google Scholar] [CrossRef] [PubMed]
- Grajewski, B.; Whelan, E.A.; Lawson, C.C.; Hein, M.J.; Waters, M.A.; Anderson, J.L.; Macdonald, L.A.; Mertens, C.J.; Tseng, C.Y.; Cassinelli, R.T.; et al. Miscarriage among Flight Attendants. Epidemiology 2015, 26, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Cone, J.E.; Vaughan, L.M.; Huete, A.; Samuels, S.J. Reproductive Health Outcomes among Female Flight Attendants: An Exploratory Study. J Occup Environ Med 1998, 40, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lauria, L.; Ballard, T.J.; Caldora, M.; Mazzanti, C.; Verdecchia, A. Reproductive Disorders and Pregnancy Outcomes among Female Flight Attendants. Aviat Sp Env Med 2006, 77, 533–539. [Google Scholar]
- Irgens, Å.; Irgens, L.M.; Reitan, J.B.; Haldorsen, T.; Tveten, U. Pregnancy Outcome among Offspring of Airline Pilots and Cabin Attendants. Scand J Work Environ Health 2003, 29, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, W.; Chan, A.; Li, C.; He, X.; Cui, L.; Lv, Y.; Liu, J.; Guo, X. An Epidemiological Study of Reproductive Health in Female Civil Aviation Employees. Aviat Space Environ Med 2013, 84, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, A.R.; Barnes, J.; Ejiogu, N.; Foster, K.; Brant, L.J.; Zonderman, A.B.; Evans, M.K. Age, Sex, and Race Influence Single-Strand Break Repair Capacity in a Human Population. Free Radic Biol Med 2008, 45, 1631–1641. [Google Scholar] [CrossRef]
- Garm, C.; Moreno-Villanueva, M.; Bürkle, A.; Petersen, I.; Bohr, V.A.; Christensen, K.; Stevnsner, T. Age and Gender Effects on DNA Strand Break Repair in Peripheral Blood Mononuclear Cells. 2013, 12, 58–66. [CrossRef]
- Scott, D.; Barber, J.B.P.; Levine, E.L.; Burrill, W.; Roberts, S.A. Radiation-Induced Micronucleus Induction in Lymphocytes Identifies a High Frequency of Radiosensitive Cases among Breast Cancer Patients: A Test for Predisposition? Br J Cancer 1998, 77, 614–620. [Google Scholar] [CrossRef]
- Distel, L.V.R.; Neubauer, S.; Keller, U.; Sprung, C.N.; Sauer, R.; Grabenbauer, G.G. Individual Differences in Chromosomal Aberrations after in Vitro Irradiation of Cells from Healthy Individuals, Cancer and Cancer Susceptibility Syndrome Patients. Radiother Oncol 2006, 81, 257–263. [Google Scholar] [CrossRef]
- Köberle, B.; Koch, B.; Fischer, B.M.; Hartwig, A. Single Nucleotide Polymorphisms in DNA Repair Genes and Putative Cancer Risk. Arch Toxicol 2016 9010 2016, 90, 2369–2388. [Google Scholar] [CrossRef]
- Heimers, A. Chromosome Aberration Analysis in Concorde Pilots. Mutat Res 2000, 467, 169–176. [Google Scholar] [CrossRef]
- Romano, E.; Ferrucci, L.; Nicolai, F.; Derme, V.; De Stefano, G.F. Increase of Chromosomal Aberrations Induced by Ionising Radiation in Peripheral Blood Lymphocytes of Civil Aviation Pilots and Crew Members. Mutat Res 1997, 377, 89–93. [Google Scholar] [CrossRef]
- Nicholas, J.S.; Butler, G.C.; Davis, S.; Bryant, E.; Hoel, D.G.; Mohr, L.C. Stable Chromosome Aberrations and Ionizing Radiation in Airline Pilots. Aviat Space Environ Med 2003, 74, 953–956. [Google Scholar]
- Cavallo, D.; Marinaccio, A.; Perniconi, B.; Tomao, P.; Pecoriello, V.; Moccaldi, R.; Iavicoli, S. Chromosomal Aberrations in Long-Haul Air Crew Members. Mutat Res 2002, 513, 11–15. [Google Scholar] [CrossRef]
- Yong, L.C.; Sigurdson, A.J.; Ward, E.M.; Waters, M.A.; Whelan, E.A.; Petersen, M.R.; Bhatti, P.; Ramsey, M.J.; Ron, E.; Tucker, J.D. Increased Frequency of Chromosome Translocations in Airline Pilots with Long-Term Flying Experience. Occup Environ Med 2009, 66. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Y.; Liu, J.; Zhang, H.; Guo, Y.; Zhang, Y.; Duan, S.; Peng, X.; Sun, T.; Jia, B.; et al. Influence of Cosmic Radiation on Lymphocyte Micronucleus, Serum Lipid Peroxide and Antioxidation Capacity in Aircrew Members. Chinese Sci Bull 2002 478 2002, 47, 647–653. [Google Scholar] [CrossRef]
- Zwingmann, I.; Welle, I.; van Herwijen, M.; Engelen, J.; Schilderman, P.; Smid, T.; Kleinjans, J. Oxidative DNA Damage and Cytogenetic Effects in Flight Engineers Exposed to Cosmic Radiation - PubMed. Env Mol Mutagen 1998, 32, 121–129. [Google Scholar] [CrossRef]
- Nickoloff, J.A.; Sharma, N.; Taylor, L. Clustered DNA Double-Strand Breaks: Biological Effects and Relevance to Cancer Radiotherapy. Genes (Basel) 2020, 11. [Google Scholar] [CrossRef]
- Nuszkiewicz, J.; Woźniak, A.; Szewczyk-Golec, K. Ionizing Radiation as a Source of Oxidative Stress—The Protective Role of Melatonin and Vitamin D. Int J Mol Sci 2020, 21, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Georgakilas, A.G. Formation of Clustered DNA Damage after High-LET Irradiation: A Review. J Radiat Res 2008, 49, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jella, K.K.; Jaafar, L.; Li, S.; Park, S.; Story, M.D.; Wang, H.; Wang, Y.; Dynan, W.S. Exposure to Galactic Cosmic Radiation Compromises DNA Repair and Increases the Potential for Oncogenic Chromosomal Rearrangement in Bronchial Epithelial Cells. Sci Rep 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Asaithamby, A.; Chen, D.J. Mechanism of Cluster DNA Damage Repair in Response to High-Atomic Number and Energy Particles Radiation. Mutat Res 2011, 711, 87. [Google Scholar] [CrossRef]
- Campa, A.; Alloni, D.; Antonelli, F.; Ballarini, F.; Belli, M.; Dini, V.; Esposito, G.; Facoetti, A.; Friedland, W.; Furusawa, Y.; et al. DNA Fragmentation Induced in Human Fibroblasts by 56Fe Ions: Experimental Data and Monte Carlo Simulations. Radiat Res 2009, 171, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Nagamatsu, A.; Nenoi, M.; Fujimori, A.; Kakinuma, S.; Katsube, T.; Wang, B.; Tsuruoka, C.; Shirai, T.; Nakamura, A.J.; et al. Space Radiation Biology for “Living in Space. ” Biomed Res Int 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Juerb, D.; Zwar, M.; Giesen, U.; Nolte, R.; Kriesen, S.; Baiocco, G.; Puchalska, M.; van Goethem, M.J.; Manda, K.; Hildebrandt, G. Comparative Study of the Effects of Different Radiation Qualities on Normal Human Breast Cells. Radiat Oncol 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Dai, X. The Relationship between DNA Single-Stranded Damage Response and Double-Stranded Damage Response. Cell Cycle 2018, 17, 73–79. [Google Scholar] [CrossRef]
- Ward, J.F. Some Biochemical Consequences of the Spatial Distribution of Ionizing Radiation-Produced Free Radicals. Radiat Res 1981, 86, 185–195. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, C.; Sun, F.; Wei, W.; Hu, B.; Wang, J. Both Complexity and Location of DNA Damage Contribute to Cellular Senescence Induced by Ionizing Radiation. PLoS One 2016, 11. [Google Scholar] [CrossRef]
- Prasanna, P.G.S.; Escalada, N.D.; Blakely, W.F. Induction of Premature Chromosome Condensation by a Phosphatase Inhibitor and a Protein Kinase in Unstimulated Human Peripheral Blood Lymphocytes: A Simple and Rapid Technique to Study Chromosome Aberrations Using Specific Whole-Chromosome DNA Hybridization Probes for Biological Dosimetry. Mutat Res 2000, 466, 131–141. [Google Scholar] [CrossRef]
- Pujol-Canadell, M.; Perrier, J.R.; Cunha, L.; Shuryak, I.; Harken, A.; Garty, G.; Brenner, D.J. Cytogenetically-Based Biodosimetry after High Doses of Radiation. PLoS One 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Leatherbarrow, E.L.; Harper, J. V.; Cucinotta, F.A.; O’Neill, P. Induction and Quantification of Gamma-H2AX Foci Following Low and High LET-Irradiation. Int J Radiat Biol 2006, 82, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rose Li, Y.; Halliwill, K.D.; Adams, C.J.; Iyer, V.; Riva, L.; Mamunur, R.; Jen, K.Y.; del Rosario, R.; Fredlund, E.; Hirst, G.; et al. Mutational Signatures in Tumours Induced by High and Low Energy Radiation in Trp53 Deficient Mice. Nat Commun 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Nasonova, E.; Czerski, K.; Kutsalo, P.; Pereira, W.; Krasavin, E. Production and Distribution of Chromosome Aberrations in Human Lymphocytes by Particle Beams with Different LET. Radiat Environ Biophys 2019, 58, 99. [Google Scholar] [CrossRef]
- Nair, S.; Engelbrecht, M.; Miles, X.; Ndimba, R.; Fisher, R.; du Plessis, P.; Bolcaen, J.; Nieto-Camero, J.; de Kock, E.; Vandevoorde, C. The Impact of Dose Rate on DNA Double-Strand Break Formation and Repair in Human Lymphocytes Exposed to Fast Neutron Irradiation. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Grayson, J.; Lyons, T. Brain Cancer, Flying, and Socioeconomic Status: A Nested Case-Control Study of USAF Aircrew - PubMed. Aviat Sp Env Med 1996, 67, 1152–1154. [Google Scholar]
- Mortazavi, S. The Safety Issues of Onboard Wi-Fi: Possible Interactions of Oxidative Stress-Causing High Altitude, Cosmic Radiation, and Wi-Fi Radiation. React Oxyg Species 2017, 4, 441–444. [Google Scholar] [CrossRef]
- Akdag, M.Z.; Dasdag, S.; Canturk, F.; Karabulut, D.; Caner, Y.; Adalier, N. Does Prolonged Radiofrequency Radiation Emitted from Wi-Fi Devices Induce DNA Damage in Various Tissues of Rats? J Chem Neuroanat 2016, 75, 116–122. [Google Scholar] [CrossRef]
- Bodewein, L.; Schmiedchen, K.; Dechent, D.; Stunder, D.; Graefrath, D.; Winter, L.; Kraus, T.; Driessen, S. Systematic Review on the Biological Effects of Electric, Magnetic and Electromagnetic Fields in the Intermediate Frequency Range (300 Hz to 1 MHz). Environ Res 2019, 171, 247–259. [Google Scholar] [CrossRef]
- Cavallo, D.; Tomao, P.; Marinaccio, A.; Perniconi, B.; Setini, A.; Palmi, S.; Iavicoli, S. Evaluation of DNA Damage in Flight Personnel by Comet Assay. 2002, 516, 148–152. [CrossRef]
- Nelson, G. Fundamental Space Radiobiology. Gravit Sp Biol Bull 2003, 16, 29–36. [Google Scholar]
- Seth, I.; Schwartz, J.L.; Stewart, R.D.; Emery, R.; Joiner, M.C.; Tucker, J.D. Neutron Exposures in Human Cells: Bystander Effect and Relative Biological Effectiveness. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Iliakis, G.; Wang, H.; Perrault, A.R.; Boecker, W.; Rosidi, B.; Windhofer, F.; Wu, W.; Guan, J.; Terzoudi, G.; Panteliasc, G. Mechanisms of DNA Double Strand Break Repair and Chromosome Aberration Formation. Cytogenet Genome Res 2004, 104, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bauchinger, M. Quantification of Low-Level Radiation Exposure by Conventional Chromosome Aberration Analysis. Mutat Res Genet Toxicol 1995, 339, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Cologne, J.; Sugiyama, H.; Hamasaki, K.; Tatsukawa, Y.; French, B.; Sakata, R.; Misumi, M. Chromosome Aberrations among Atomic-Bomb Survivors Exposed in Utero: Updated Analysis Accounting for Revised Radiation Doses and Smoking. Radiat Environ Biophys 2022, 61, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Ainsbury, E.A.; Livingston, G.K.; Abbott, M.G.; Moquet, J.E.; Hone, P.A.; Jenkins, M.S.; Christensen, D.M.; Lloyd, D.C.; Rothkamm, K. Interlaboratory Variation in Scoring Dicentric Chromosomes in a Case of Partial-Body x-Ray Exposure: Implications for Biodosimetry Networking and Cytogenetic “Triage Mode” Scoring. Radiat Res 2009, 172, 746–752. [Google Scholar] [CrossRef] [PubMed]
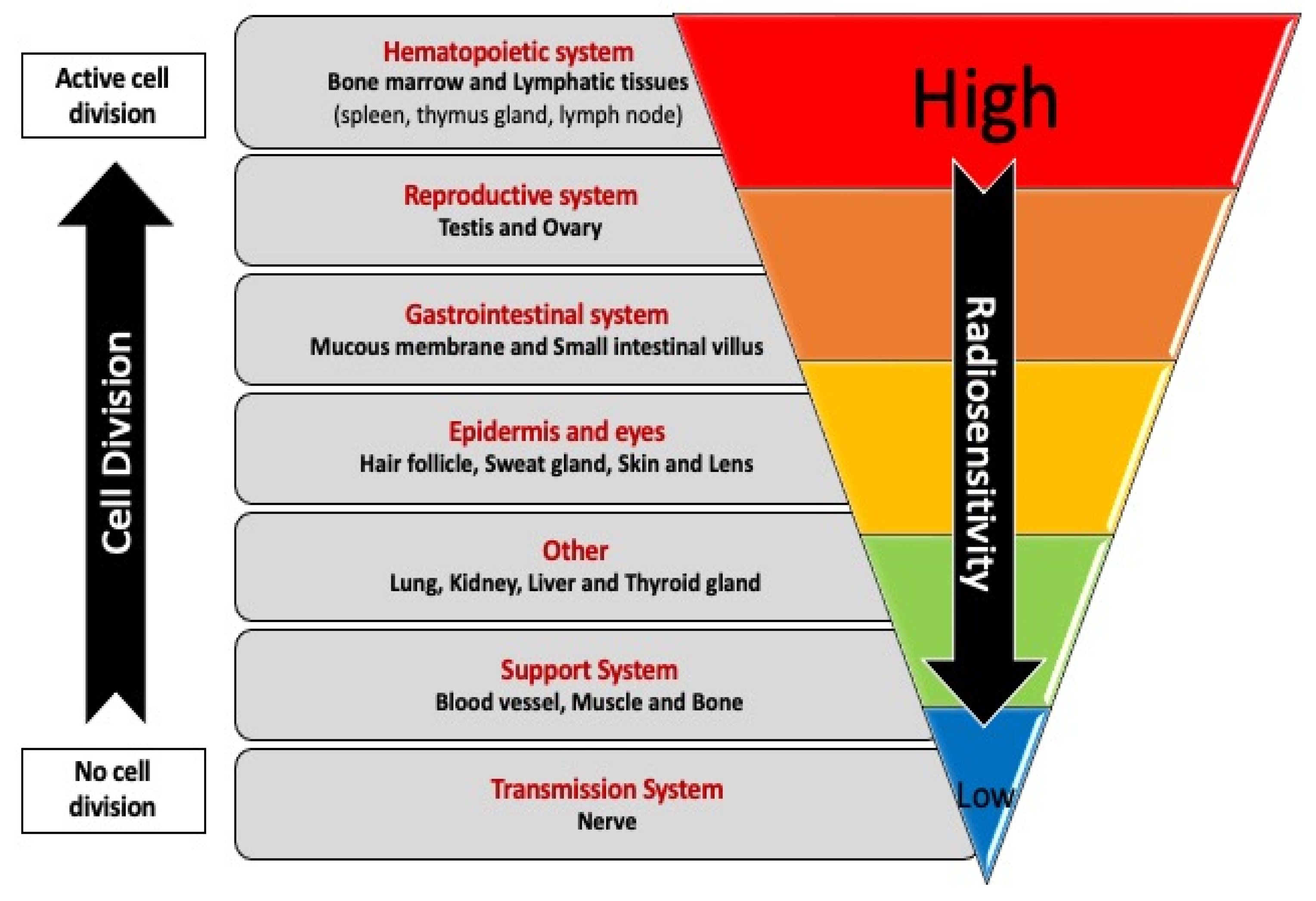
- CA, K.; Heintz, P.; Sandoval, D.; Chambers, G.; Adolphi, N.; Paffett, K. Radiation Effects on Tissues and Organs; John Wiley & Sons, Ltd., 2014.
- Ministry of the Environment Government of Japan (JCN1000012110001) Radiosensitivity of Organs and Tissues [MOE]. In Booklet to Provide Basic Information Regarding Health Effects of Radiation; 2013.
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat Rev Mol Cell Biol 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.M.; Powell, D.R.; Li, Z.; Bell, J.S.K.; Barwick, B.G.; Feng, H.; McCrary, M.R.; Dwivedi, B.; Kowalski, J.; Dynan, W.S.; et al. Galactic Cosmic Radiation Induces Persistent Epigenome Alterations Relevant to Human Lung Cancer. Sci Rep 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Wilson, D.M. DNA Damage and Associated DNA Repair Defects in Disease and Premature Aging; Am J Hum Genet, 2019; Vol. 105, pp. 237–257.
- Limoli, C.L.; Ponnaiya, B.; Corcoran, J.J.; Giedzinski, E.; Kaplan, M.I.; Hartmann, A.; Morgan, W.F. Genomic Instability Induced by High and Low LET Ionizing Radiation. Adv Space Res 2000, 25, 2107–2117. [Google Scholar] [CrossRef]
- Y, Y.; Dai, W. Genomic Instability and Cancer. J Carcinog Mutagen 2015, 5, 1000165. [Google Scholar]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ Mol Mutagen 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer) IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco Smoke and Involuntary Smoking. Lyon (FR): International Agency for Research on Cancer; 2004. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 83.); 2004.
- IARC (International Agency for Research on Cancer) IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Painting, Firefighting, and Shiftwork. Lyon (FR): International Agency for Research on Cancer; 2010. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 98.); 2010.
- da Silva, J. DNA Damage Induced by Occupational and Environmental Exposure to Miscellaneous Chemicals. Mutat Res Rev Mutat Res 2016, 770, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Brzozowska, B.; Sollazzo, A.; Lundholm, L.; Lisowska, H.; Haghdoost, S.; Wojcik, A. Simultaneous Induction of Dispersed and Clustered DNA Lesions Compromises DNA Damage Response in Human Peripheral Blood Lymphocytes. PLoS One 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Soren, D.C.; Toprani, S.M.; Jain, V.; Saini, D.; Das, B. Quantitation of Genome Damage and Transcriptional Profile of DNA Damage Response Genes in Human Peripheral Blood Mononuclear Cells Exposed in Vitro to Low Doses of Neutron Radiation. Int J Radiat Res 2019, 17, 1–14. [Google Scholar]
- Roobol, S.J.; van den Bent, I.; van Cappellen, W.A.; Abraham, T.E.; Paul, M.W.; Kanaar, R.; Houtsmuller, A.B.; van Gent, D.C.; Essers, J. Comparison of High- and Low-LET Radiation-Induced DNA Double-Strand Break Processing in Living Cells. Int J Mol Sci 2020, 21, 1–19. [Google Scholar] [CrossRef]
- Slatter, M.A.; Gennery, A.R. Update on DNA-Double Strand Break Repair Defects in Combined Primary Immunodeficiency. Curr Allergy Asthma Rep 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Lewis, S.; Wlodarski, M.W. DNA Repair Syndromes and Cancer: Insights Into Genetics and Phenotype Patterns. Front Pediatr 2020, 8. [Google Scholar] [CrossRef]
- Davis, A.J.; Chen, B.P.C.; Chen, D.J. DNA-PK: A Dynamic Enzyme in a Versatile DSB Repair Pathway. DNA Repair (Amst) 2014, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Winters, T.A.; Jung, M.; Purkayastha, S.; Cavalli, L.R.; Chasovkikh, S.; Haddad, B.R.; Dritschilo, A. Radiation-Generated Short DNA Fragments May Perturb Non-Homologous End-Joining and Induce Genomic Instability. J Radiat Res 2011, 52, 309–319. [Google Scholar] [CrossRef]
- Yuan, Y.; Britton, S.; Delteil, C.; Coates, J.; Jackson, S.P.; Barboule, N.; Frit, P.; Calsou, P. Single-Stranded DNA Oligomers Stimulate Error-Prone Alternative Repair of DNA Double-Strand Breaks through Hijacking Ku Protein. Nucleic Acids Res 2015, 43, 10264–10276. [Google Scholar] [CrossRef]
- Schmid, T.E.; Dollinger, G.; Beisker, W.; Hable, V.; Greubel, C.; Auer, S.; Mittag, A.; Tarnok, A.; Friedl, A.A.; Molls, M.; et al. Differences in the Kinetics of Gamma-H2AX Fluorescence Decay after Exposure to Low and High LET Radiation. Int J Radiat Biol 2010, 86, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.P.; Hirakawa, H.; Nakajima, N.I.; Moore, S.; Nie, J.; Sharma, N.; Sugiura, M.; Hoki, Y.; Araki, R.; Abe, M.; et al. Low- and High-LET Ionizing Radiation Induces Delayed Homologous Recombination That Persists for Two Weeks before Resolving. Radiat Res 2017, 188, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Grim, S.; Smith, L.E.; Kim, P.M.; Nickoloff, J.A.; Goloubeva, O.G.; Morgan, W.F. Ionizing Radiation Induces Delayed Hyperrecombination in Mammalian Cells. Mol Cell Biol 2004, 24, 5060–5068. [Google Scholar] [CrossRef] [PubMed]
- Grosse, N.; Fontana, A.O.; Hug, E.B.; Lomax, A.; Coray, A.; Augsburger, M.; Paganetti, H.; Sartori, A.A.; Pruschy, M. Deficiency in Homologous Recombination Renders Mammalian Cells More Sensitive to Proton versus Photon Irradiation. Int J Radiat Oncol Biol Phys 2014, 88, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.H.; Shim, E.Y.; Lee, S.E. Microhomology-Mediated End Joining: Good, Bad and Ugly. Mutat Res - Fundam Mol Mech Mutagen 2018.
- Wood, R.D.; Doublié, S. DNA Polymerase θ (POLQ), Double-Strand Break Repair, and Cancer. DNA Repair (Amst) 2016, 44, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Smith, C.M.; Simpson, D.A.; Gupta, G.P. Targeting Non-Homologous and Alternative End Joining Repair to Enhance Cancer Radiosensitivity. Semin Radiat Oncol 2022, 32, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Krokan, H.E.; Bjørås, M. Base Excision Repair. Cold Spring Harb Perspect Biol 2013, 5, 1–22. [Google Scholar] [CrossRef]
- Yang, N.; Galick, H.; Wallace, S.S. Attempted Base Excision Repair of Ionizing Radiation Damage in Human Lymphoblastoid Cells Produces Lethal and Mutagenic Double Strand Breaks. DNA Repair (Amst) 2004, 3, 1323–1334. [Google Scholar] [CrossRef]
- Fung, H.; Demple, B. Distinct Roles of Ape1 Protein in the Repair of DNA Damage Induced by Ionizing Radiation or Bleomycin. J Biol Chem 2011, 286, 4968–4977. [Google Scholar] [CrossRef]
- Eccles, L.J.; O’Neill, P.; Lomax, M.E. Delayed Repair of Radiation Induced Clustered DNA Damage: Friend or Foe? Mutat Res 2011, 711, 134–141. [Google Scholar] [CrossRef]
- Rajaraman, P.; Bhatti, P.; Doody, M.M.; Simon, S.L.; Weinstock, R.M.; Linet, M.S.; Rosenstein, M.; Stovall, M.; Alexander, B.H.; Preston, D.L.; et al. Nucleotide Excision Repair Polymorphisms May Modify Ionizing Radiation-Related Breast Cancer Risk in US Radiologic Technologists. Int J cancer 2008, 123, 2713–2716. [Google Scholar] [CrossRef] [PubMed]
- Terzidis, M.A.; Ferreri, C.; Chatgilialoglu, C. Radiation-Induced Formation of Purine Lesions in Single and Double Stranded DNA: Revised Quantification. Front Chem 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Raja, S.; Van Houten, B. The Involvement of Nucleotide Excision Repair Proteins in the Removal of Oxidative DNA Damage. Nucleic Acids Res 2020, 48, 11227–11243. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P. How Does Hormesis Impact Biology, Toxicology, and Medicine? NPJ aging Mech Dis 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.J.; Alhamed, A.; Sveiven, S.; Almutairy, A.; Klimas, N.G.; Abreu, M.; Sullivan, K.; Grant, S.G. Preliminary Evidence for a Hormetic Effect on DNA Nucleotide Excision Repair in Veterans with Gulf War Illness. Mil Med 2020, 185, E47–E52. [Google Scholar] [CrossRef] [PubMed]
- Kottemann, M.C.; Smogorzewska, A. Fanconi Anaemia and the Repair of Watson and Crick DNA Crosslinks. Nature 2013, 493, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Sanlorenzo, M.; Wehner, M.R.; Linos, E.; Kornak, J.; Kainz, W.; Posch, C.; Vujic, I.; Johnston, K.; Gho, D.; Monico, G.; et al. The Risk of Melanoma in Airline Pilots and Cabin Crew: A Meta-Analysis. JAMA Dermatology 2015, 151, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Institute, P. Women Pilot Statistics: Female Representation in Aviation - Pilot Institute. Available online: https://pilotinstitute.com/women-aviation-statistics/#:~:text=This is a massive 151,compared to 6.03%25 in 2021. (accessed on 3 January 2023).
- National Institute for Occupational Safety and Health (NIOSH) Hierarchy of Controls. NIOSH Workplace Safety and Health Topics; 2015.
- Radon, K.; Goldberg, M.; Becklake, M. Healthy Worker Effect in Cohort Studies on Chronic Bronchitis. Scand J Work Environ Health 2002, 28, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Prasongtanakij, S.; Wood, D.K.; Weingeist, D.M.; Fessler, J.; Navasummrit, P.; Ruchirawat, M.; Engelward, B.P. Cometchip: A High-Throughput 96-Well Platform for Measuring DNA Damage in Microarrayed Human Cells. J Vis Exp 2014. [Google Scholar] [CrossRef]
- Garty, G.; Chen, Y.; Salerno, A.; Turner, H.; Zhang, J.; Lyulko, O.; Bertucci, A.; Xu, Y.; Wang, H.; Simaan, N.; et al. The RABIT: A Rapid Automated Biodosimetry Tool for Radiological Triage. Health Phys 2010, 98, 209–217. [Google Scholar] [CrossRef]
- Nagel, Z.D.; Margulies, C.M.; Chaim, I.A.; McRee, S.K.; Mazzucato, P.; Ahmad, A.; Abo, R.P.; Butty, V.L.; Forget, A.L.; Samson, L.D. Multiplexed DNA Repair Assays for Multiple Lesions and Multiple Doses via Transcription Inhibition and Transcriptional Mutagenesis. Proc Natl Acad Sci U S A 2014. [Google Scholar] [CrossRef] [PubMed]
- Nagel, Z.D.; Beharry, A.A.; Mazzucato, P.; Kitange, G.J.; Sarkaria, J.N.; Kool, E.T.; Samson, L.D. Fluorescent Reporter Assays Provide Direct, Accurate, Quantitative Measurements of MGMT Status in Human Cells. PLoS One 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Nagel, Z.D.; Engelward, B.P.; Brenner, D.J.; Begley, T.J.; Sobol, R.W.; Bielas, J.H.; Stambrook, P.J.; Wei, Q.; Hu, J.J.; Terry, M.B.; et al. Towards Precision Prevention: Technologies for Identifying Healthy Individuals with High Risk of Disease. Mutat Res - Fundam Mol Mech Mutagen 2017, 800–802, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Cheong, A.; Nagel, Z.D. Human Variation in DNA Repair, Immune Function, and Cancer Risk. Front Immunol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Hornhardt, S.; Rößler, U.; Sauter, W.; Rosenberger, A.; Illig, T.; Bickeböller, H.; Wichmann, H.E.; Gomolka, M. Genetic Factors in Individual Radiation Sensitivity. DNA Repair (Amst) 2014, 16, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Kehm, R.; Santella, R.M.; Brenner, D.J.; Terry, M.B. DNA Repair Phenotype and Cancer Risk: A Systematic Review and Meta-Analysis of 55 Case–Control Studies. Sci Reports 2022 121 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Luxton, J.J.; McKenna, M.J.; Lewis, A.; Taylor, L.E.; George, K.A.; Dixit, S.M.; Moniz, M.; Benegas, W.; Mackay, M.J.; Mozsary, C.; et al. Telomere Length Dynamics and DNA Damage Responses Associated with Long-Duration Spaceflight. Cell Rep 2020, 33. [Google Scholar] [CrossRef]
- Marnell, C.S.; Bick, A.; Natarajan, P. Clonal Hematopoiesis of Indeterminate Potential (CHIP): Linking Somatic Mutations, Hematopoiesis, Chronic Inflammation and Cardiovascular Disease. J Mol Cell Cardiol 2021, 161, 98–105. [Google Scholar] [CrossRef]
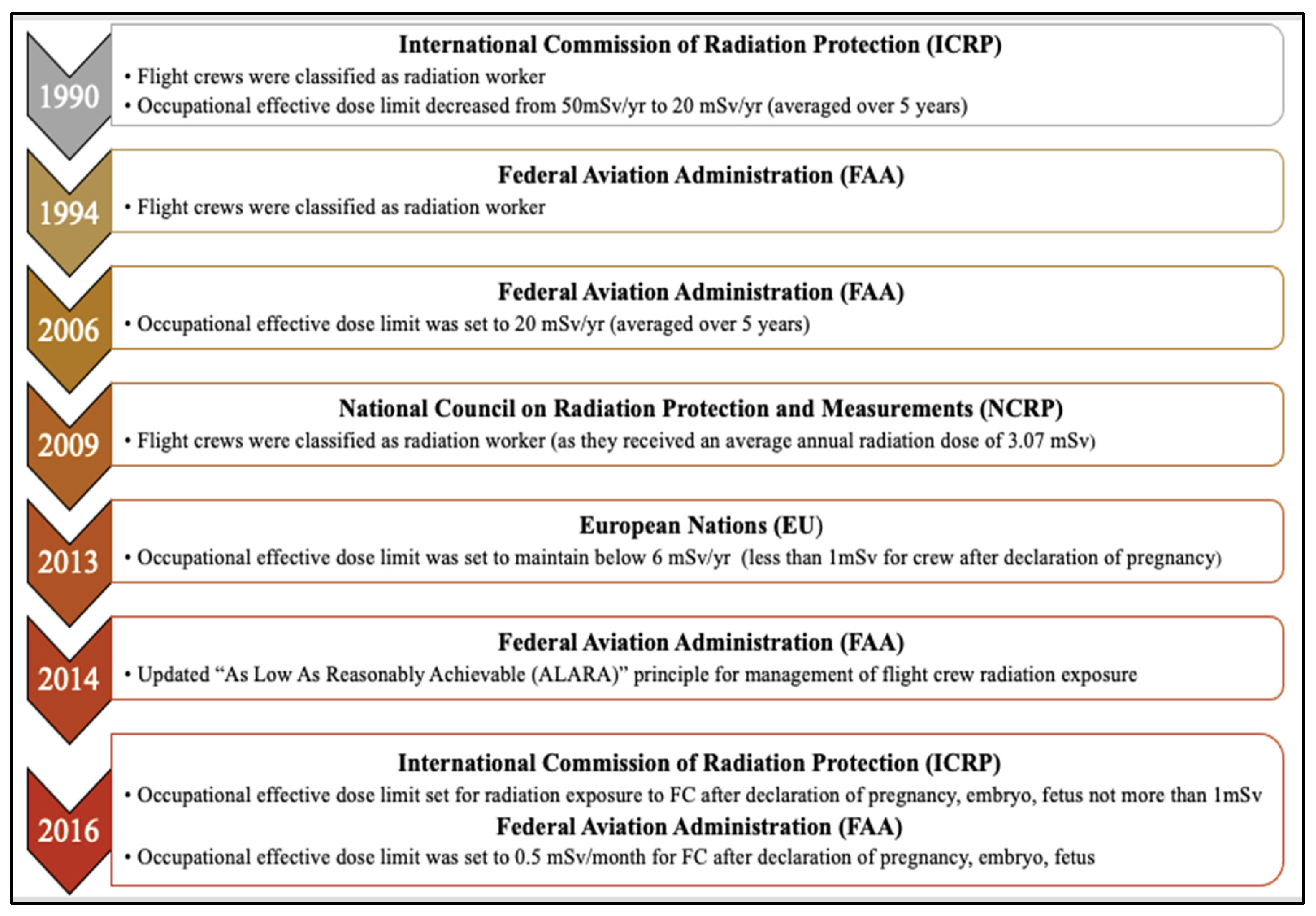
| Exposure Limits | International Commission of Radiation Protection (ICRP) [20] |
European Nations (EU) [43] |
U.S, National Council on Radiation Protection and Measurements (NCRP) [1] | U.S, Nuclear Regulatory Commission, (NRC) [19] |
U.S, Federal Aviation Administration (FAA) [39] |
|---|---|---|---|---|---|
| General Public | 1 mSv/y | 1 mSv/y | 1 mSv/y | 1 mSv/y | 1 mSv/y |
|
Pregnant Women and Unborn Fetus |
1 mSv after declaration of pregnancy | 1 mSv after declaration of pregnancy | 5 mSv and no more than 0.5 mSv in any month |
5 mSv and no more than 0.5 mSv in any month |
1 mSv and no more than 0.5 mSv in any month |
| Occupational Exposure | 20 mSv/y* | 20 mSv/y* employer required monitoring and administrative controls to maintain <6 mSv/y |
50 mSv/y | 50 mSv/y | 20 mSv/y* recommendation for FC to self-monitor without requirements for the employer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





