Submitted:
03 June 2024
Posted:
05 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Chemicals and Reagents
2.3. Enzymatic Extract Preparation
2.4. Purification of the Enzymatic Extract
2.5. Determination of Proteolytic Activity of Alkaline Proteases:
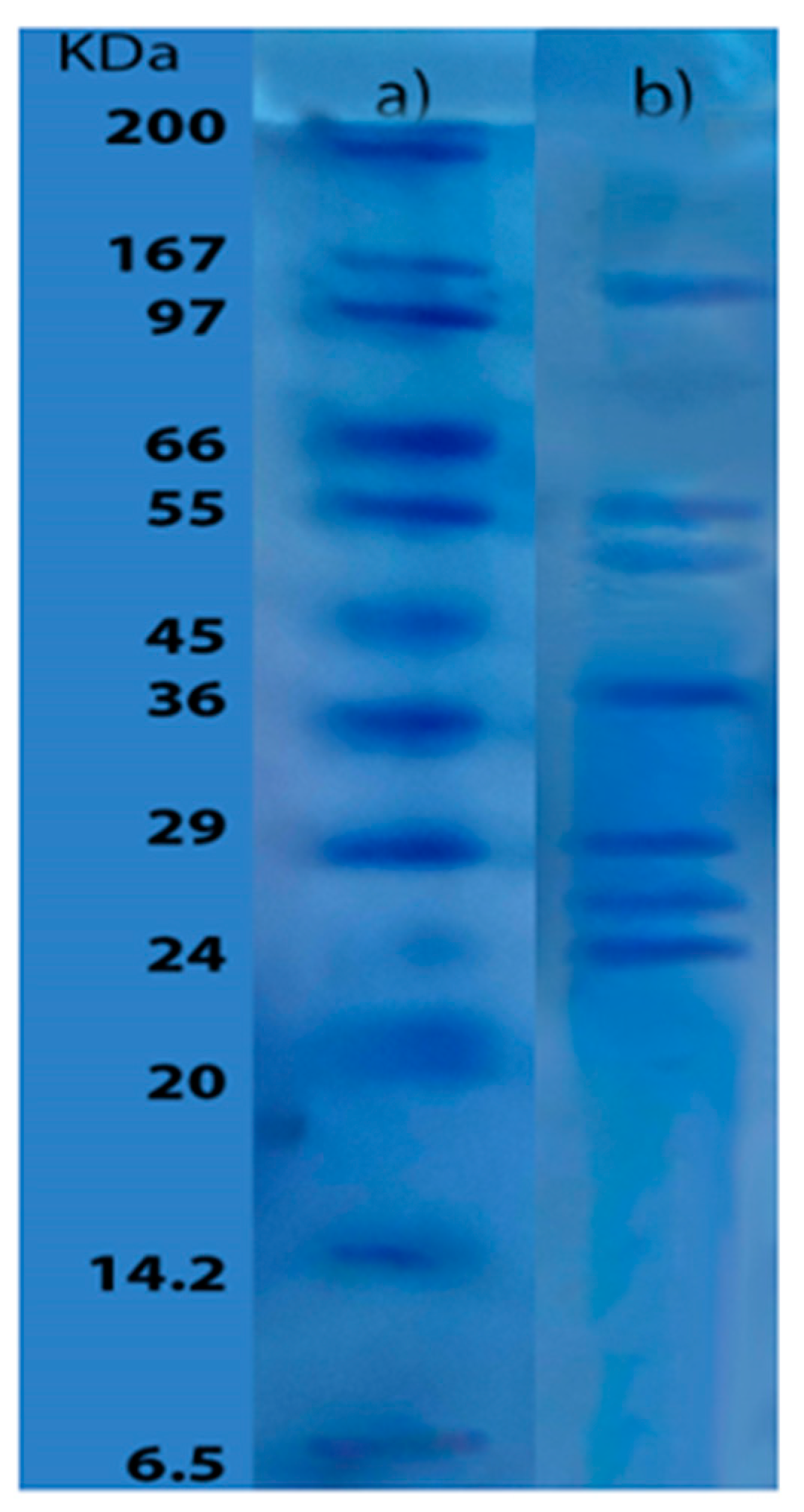
2.6. Molecular Sizes Determination
2.7. Gelatin Extraction from Yellowfin Tuna:
2.8. Amino Acid Analysis
2.9. Gel-Forming Properties
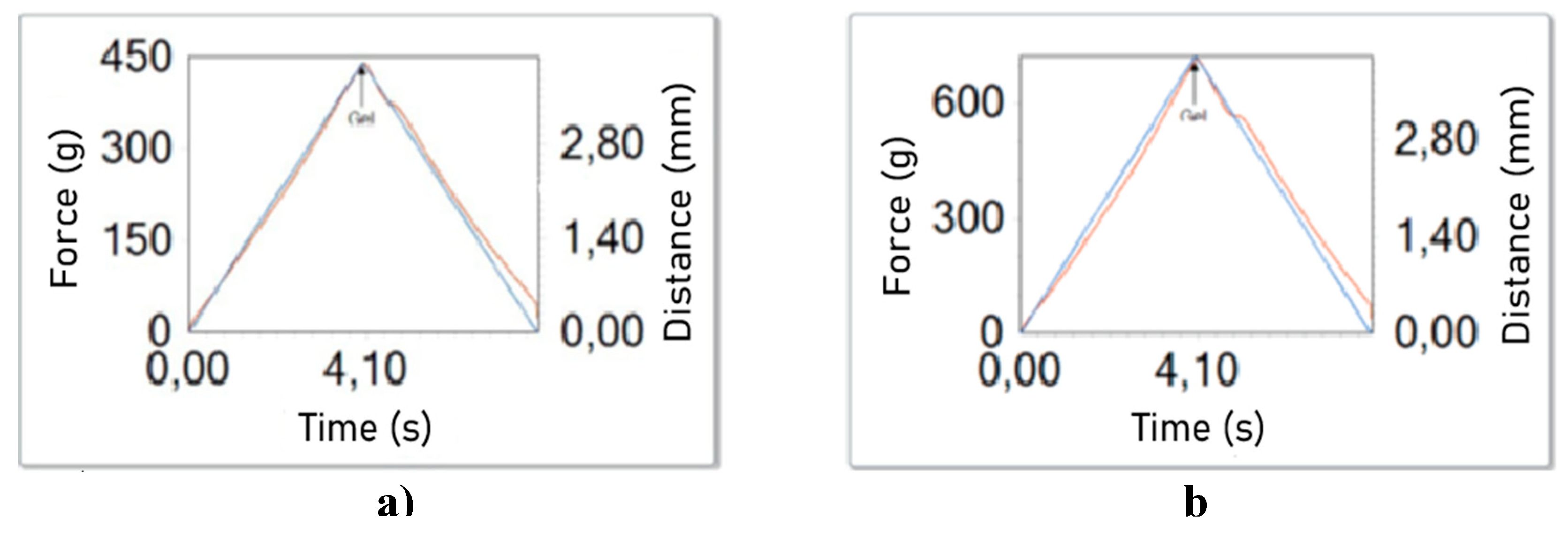
2.10. Gel Strength
2.11. Optimization of Gelatin Hydrolysis Conditions
2.12. Determionation of Protein Content
2.13. DPPH Radical Scavenging Activity
3. Results
3.1. Molecular Size Determination of enzymatic Extract
3.2. Amino acid Composition
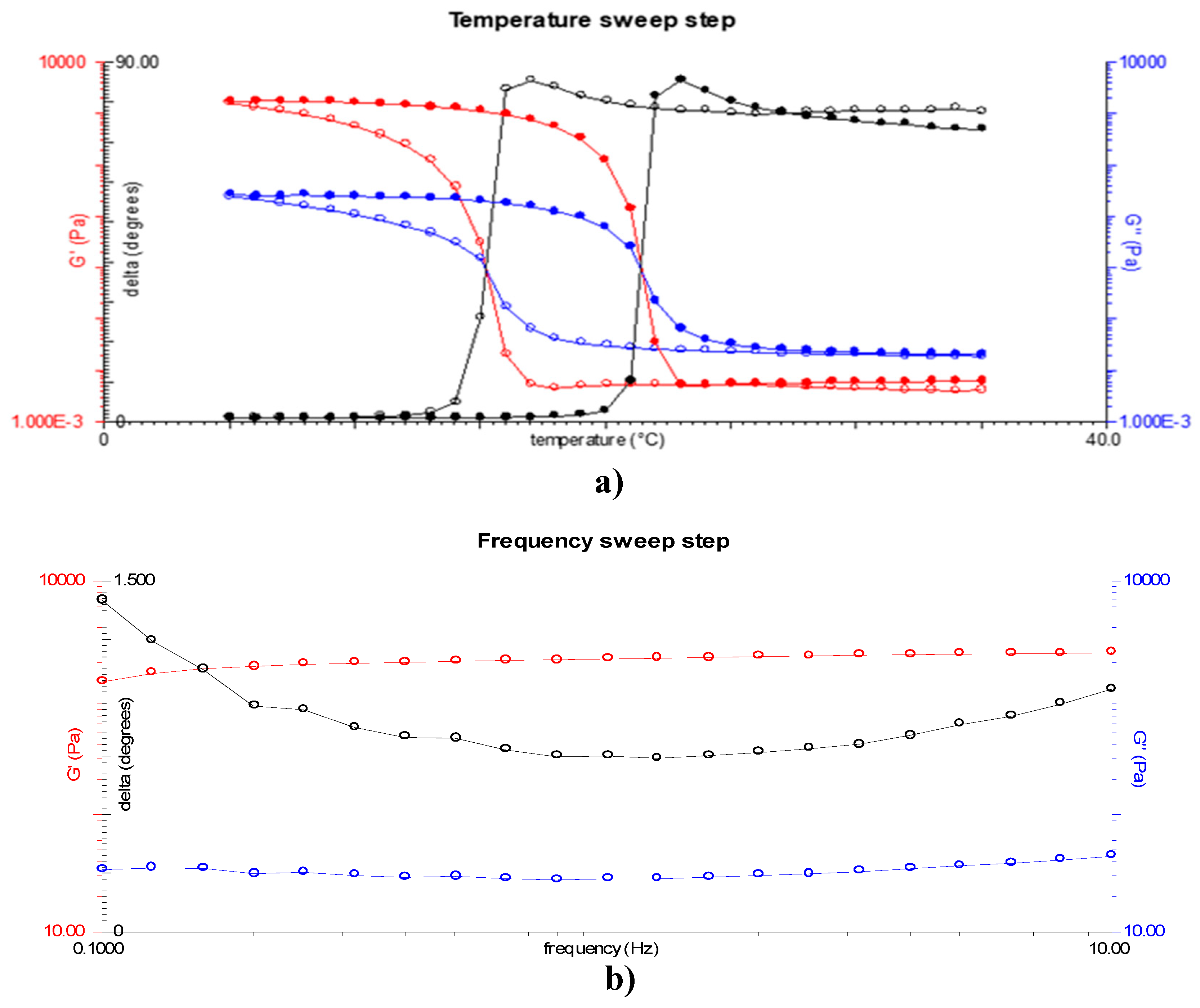
3.3. Viscoelastic Properties and Gel Strength
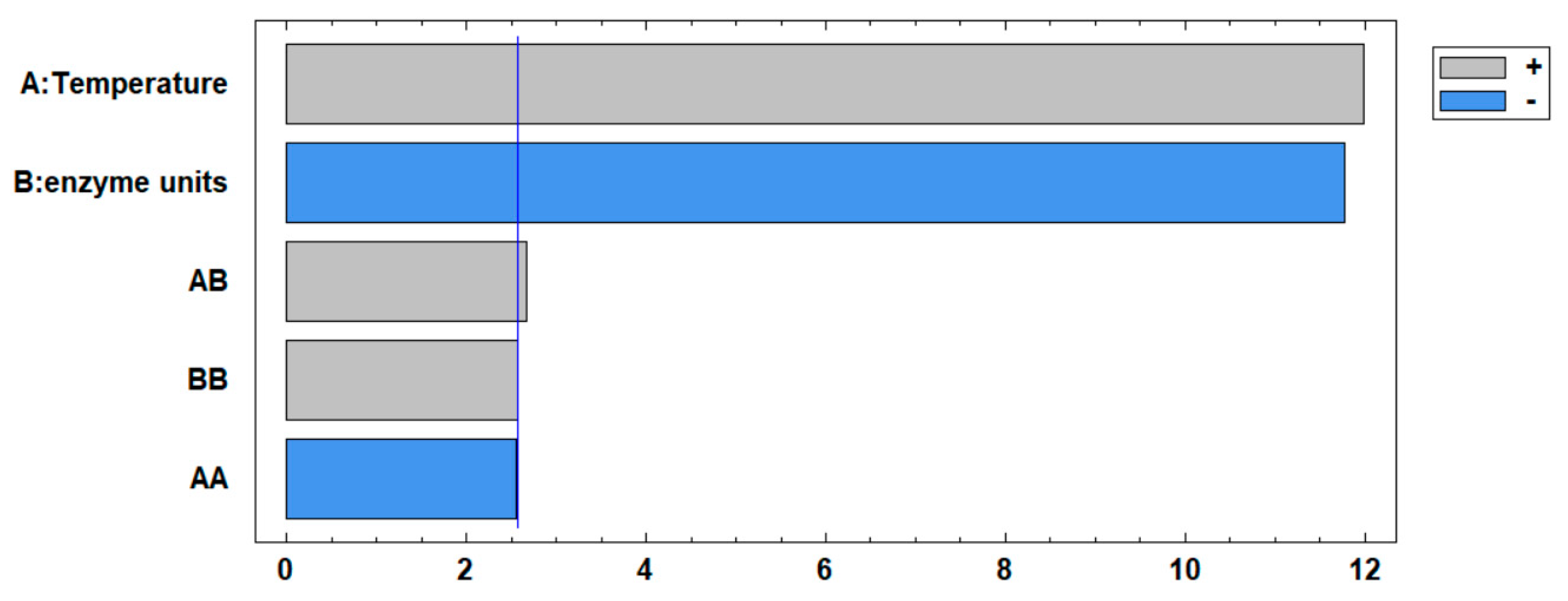
3.4. Optimization of Gelatin Hydrolysis Conditions
4. Discussion
4.1. Molecular Sizes Determination
4.2. Amino Acid Composition
4.3. Gel Strength
4.4. Viscoelastic Properties
4.5. Degree of Hydrolysis
4.6. Antioxidant Activity
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miyake, M.P.; Guillotreau, P.; Sun, C.-H.; Ishimura, G. Recent Developments in the Tuna Industry: Stocks, Fisheries, Management, Processing, Trade and Markets; Food and Agriculture Organization of the United Nations Rome, Italy, 2010; ISBN 9251066205.
- FAO The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome. 2022; ISBN 978-92-5-136364-5.
- Yao, L.; Lu, J.; Qu, M.; Jiang, Y.; Li, F.; Guo, Y.; Wang, L.; Zhai, Y. Methodology and Application of PCR-RFLP for Species Identification in Tuna Sashimi. Food Sci. Nutr. 2020, 8, 3138–3146. [Google Scholar] [CrossRef]
- Herpandi, N.H.; Rosma, A.; Wan Nadiah, W.A. The Tuna Fishing Industry: A New Outlook on Fish Protein Hydrolysates. Compr. Rev. Food Sci. Food Saf. 2011, 10, 195–207. [Google Scholar] [CrossRef]
- Idowu, A.T.; Igiehon, O.O.; Idowu, S.; Olatunde, O.O.; Benjakul, S. Bioactivity Potentials and General Applications of Fish Protein Hydrolysates. Int. J. Pept. Res. Ther. 2020. [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and Realistic Opportunities in the Use of By-Products from Processing of Fish and Shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Ardiansyah, A.; Sahubawa, L. Restructuring Steak from Flakes of Yellowfin Tuna Meat Using Low Salt Microbial Transglutaminase (MTGase). In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing, 2020; Vol. 404, p. 12073.
- Ramakrishnan, S.R.; Jeong, C.-R.; Park, J.-W.; Cho, S.-S.; Kim, S.-J. A Review on the Processing of Functional Proteins or Peptides Derived from Fish By-Products and Their Industrial Applications. Heliyon 2023. [Google Scholar] [CrossRef]
- Nguyen, H.T.M.; Sylla, K.S.B.; Randriamahatody, Z.; Donnay-Moreno, C.; Moreau, J.; Tran, L.T.; Bergé, J.P. Enzymatic Hydrolysis of Yellowfin Tuna (Thunnus Albacares) by-Products Using Protamex Protease. Food Technol. Biotechnol. 2011, 49, 48–55. [Google Scholar]
- Guérard, F.; Dufosse, L.; De La Broise, D.; Binet, A. Enzymatic Hydrolysis of Proteins from Yellowfin Tuna (Thunnus Albacares) Wastes Using Alcalase. J. Mol. Catal. B Enzym. 2001, 11, 1051–1059. [Google Scholar] [CrossRef]
- Silva, E.V.C. da; Lourenço, L. de F.H.; Pena, R.S. Optimization and Characterization of Gelatin from Kumakuma ( Brachyplatystoma Filamentosum ) Skin. CyTA - J. Food 2017, 15. [CrossRef]
- Zulkaidah Siburian, W.; Raya Bandung, J.; Java, W.; Rochima, E.; Raya Bandung-Sumedang, J.; Yuli Andriani, I.; Praseptiangga, D.; Author, C.; Andriani, Y. Fish Gelatin (Definition, Manufacture, Analysis of Quality Characteristics, and Application): A Review. Int. J. Fish. Aquat. Stud. 2020, 8, 90–95. [Google Scholar]
- Ben Rebah, F.; Miled, N. Fish Processing Wastes for Microbial Enzyme Production: A Review. 3 Biotech 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Aguilar, J.G.; Sato, H.H. Microbial Proteases: Production and Application in Obtaining Protein Hydrolysates. Food Res. Int. 2018, 103. [Google Scholar] [CrossRef]
- Clerici, N.J.; Lermen, A.M.; Daroit, D.J. Agro-Industrial by-Products as Substrates for the Production of Bacterial Protease and Antioxidant Hydrolysates. Biocatal. Agric. Biotechnol. 2021, 37. [Google Scholar] [CrossRef]
- Singh, S.; Bajaj, B.K. Medium Optimization for Enhanced Production of Protease with Industrially Desirable Attributes from Bacillus Subtilis K-1. [CrossRef]
- Contesini, F.J.; de Melo, R.R.; Sato, H.H. An Overview of Bacillus Proteases: From Production to Application. Crit. Rev. Biotechnol. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Utilization of Seafood Processing By-Products for Production of Proteases by Paenibacillus Sp. TKU052 and Their Application in Biopeptides’ Preparation. Mar. Drugs 2020, 18. [CrossRef]
- Sathishkumar, R.; Ananthan, G.; Arun, J. Production, Purification and Characterization of Alkaline Protease by Ascidian Associated Bacillus Subtilis GA CAS8 Using Agricultural Wastes. Biocatal. Agric. Biotechnol. 2015, 4. [Google Scholar] [CrossRef]
- Contesini, F.J.; De Melo, R.R.; Sato, H.H. Critical Reviews in Biotechnology An Overview of Bacillus Proteases : From Production to Application An Overview of Bacillus Proteases : From Production to Application. Crit. Rev. Biotechnol. 2017, 0. [Google Scholar]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3. [Google Scholar] [CrossRef] [PubMed]
- Greyling, N.; Bordoloi, A.; Goosen, N.J. Optimising Enzymatic Conditions of Monkfish (Lophius Vomerinus) Heads Hydrolysis towards Potential Waste Biomass Valorisation. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Iosageanu, A.N.D.R.E.E.A.; Ilie, D.A.N.I.E.L.A.; Anton, E.D.; Craciunescu, O. The Effect of Fish Bone Bioactive Peptides on the Wound Healing Process: An in Vitro Study on Keratinocytes. Rom. Biotechnol 2021, 2692–2699. [Google Scholar] [CrossRef]
- He, Y.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Ten New Pentapeptides from Protein Hydrolysate of Miiuy Croaker (Miichthys Miiuy) Muscle: Preparation, Identification, and Antioxidant Activity Evaluation. LWT 2019, 105. [Google Scholar] [CrossRef]
- Mosquera, M.; Giménez, B.; Ramos, S.; López-Caballero, M.E.M.E.; Gómez-Guillén, M.D.C.M. del C.; Montero, P. Antioxidant, ACE-Inhibitory, and Antimicrobial Activities of Peptide Fractions Obtained From Dried Giant Squid Tunics. J. Aquat. Food Prod. Technol. 2016, 25. [CrossRef]
- Chen, Y.; Jin, H.; Yang, F.; Jin, S.; Liu, C.; Zhang, L.; Huang, J.; Wang, S.; Yan, Z.; Cai, X.; et al. Physicochemical, Antioxidant Properties of Giant Croaker (Nibea Japonica) Swim Bladders Collagen and Wound Healing Evaluation. Int. J. Biol. Macromol. 2019, 138. [Google Scholar] [CrossRef]
- Sepúlveda, C.T.; Zapata, J.E.; Martínez-Álvarez, O.; Alemán, A.; Montero, M.P.; Gómez-Guillén, M.C. The Preferential Use of a Soy-Rapeseed Lecithin Blend for the Liposomal Encapsulation of a Tilapia Viscera Hydrolysate. LWT 2021, 139. [Google Scholar] [CrossRef]
- González-Serrano, D.J.; Hadidi, M.; Varcheh, M.; Jelyani, A.Z.; Moreno, A.; Lorenzo, J.M. Bioactive Peptide Fractions from Collagen Hydrolysate of Common Carp Fish Byproduct: Antioxidant and Functional Properties. Antioxidants 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, H.; Krichen, F.; Kobbi, S.; Martinez-Alvarez, O.; Nedjar, N.; Bougatef, A.; Sila, A. Physicochemical and Biological Properties of Eel By-Products Protein Hydrolysates: Potential Application to Meat Product Preservation. Waste and Biomass Valorization 2020, 11. [Google Scholar] [CrossRef]
- Kumar, A.; Elavarasan, K.; Hanjabam, M.D.; Binsi, P.K.; Mohan, C.O.; Zynudheen, A.A.; Kumar K, A. Marine Collagen Peptide as a Fortificant for Biscuit: Effects on Biscuit Attributes. LWT 2019, 109. [Google Scholar] [CrossRef]
- Sisa, A.; Sotomayor, C.; Buitrón, L.; Gómez-Estaca, J.; Martínez-Alvarez, O.; Mosquera, M. Evaluation of By-Products from Agricultural, Livestock and Fishing Industries as Nutrient Source for the Production of Proteolytic Enzymes. Heliyon 2023, 9, e20735. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Mo, X.; Chen, X.; Han, D.; Zhao, C. Purification and Enzymatic Identification of an Acid Stable and Thermostable A-amylase from Rhizopus Microsporus. J. Inst. Brew. 2012, 118, 309–314. [Google Scholar] [CrossRef]
- El-Bendary, M.A.; Moharam, M.E.; Ali, T.H. Purification and Characterization of Milk Clotting Enzyme Produced by Bacillus Sphaericus. J. Appl. Sci. Res. 2007, 3, 695–699. [Google Scholar]
- Rieger, T.J.; de Oliveira, C.T.; Pereira, J.Q.; Brandelli, A.; Daroit, D.J. Proteolytic System of Bacillus Sp. CL18 Is Capable of Extensive Feather Degradation and Hydrolysis of Diverse Protein Substrates. Br. Poult. Sci. 2017, 58. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H.; von Jagow, G. Tricine-Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis for the Separation of Proteins in the Range from 1 to 100 KDa. Anal. Biochem. 1987. [CrossRef] [PubMed]
- Boran, G.; Regenstein, J.M. Fish Gelatin. Adv. Food Nutr. Res. 2010, 60, 119–143. [Google Scholar] [PubMed]
- Sifuentes-Penagos, G.; León-Vásquez, S.; Castillo, A. Hydrolysis of Proteins from Anchovy (Engraulis Ringens) Whole by Action of the ProtamexTM Enzyme. Sci. Agropecu. 2018, 9. [Google Scholar] [CrossRef]
- Salazar-Posada, C.; López-Padilla, A.; Cano-Salazar, J.A. Efecto Del PH y La Temperatura En La Hidrólisis Enzimática de Subproductos de La Industria Bovina. Rev. Lasallista Investig. 2013, 9. [Google Scholar]
- AOAC Association of Official Analytical Chemists 2005. Official Methods of Analysis 2005.
- Nurilmala, M.; Adinugraha, S.C.; Jacoeb, A.M.; Susilawati, S.; Ochiai, Y. Evaluation of the Properties of Tuna Skin Gelatin as a Hard Capsule Material. Fish. Sci. 2020, 86. [Google Scholar] [CrossRef]
- Kaewdang, O.; Benjakul, S.; Prodpran, T.; Kaewmanee, T.; Kishimura, H. Characteristics of Gelatin Extracted from the Swim Bladder of Yellowfin Tuna (Thunnus Albacores) as Affected by Alkaline Pretreatments. J. Aquat. Food Prod. Technol. 2016, 25. [Google Scholar] [CrossRef]
- Alahmad, K.; Noman, A.; Xia, W.; Jiang, Q.; Xu, Y. Influence of the Enzymatic Hydrolysis Using Flavourzyme Enzyme on Functional, Secondary Structure, and Antioxidant Characteristics of Protein Hydrolysates Produced from Bighead Carp (Hypophthalmichthys Nobilis). Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Xu, Y.; AL-Bukhaiti, W.Q.; Abed, S.M.; Ali, A.H.; Ramadhan, A.H.; Xia, W. Influence of Enzymatic Hydrolysis Conditions on the Degree of Hydrolysis and Functional Properties of Protein Hydrolysate Obtained from Chinese Sturgeon (Acipenser Sinensis) by Using Papain Enzyme. Process Biochem. 2018, 67. [Google Scholar] [CrossRef]
- Wang, S.L.; Yeh, P.Y. Production of a Surfactant- and Solvent-Stable Alkaliphilic Protease by Bioconversion of Shrimp Shell Wastes Fermented by Bacillus Subtilis TKU007. Process Biochem. 2006, 41. [Google Scholar] [CrossRef]
- Patel, A.R.; Mokashe, N.U.; Chaudhari, D.S.; Jadhav, A.G.; Patil, U.K. Production Optimisation and Characterisation of Extracellular Protease Secreted by Newly Isolated Bacillus Subtilis AU-2 Strain Obtained from Tribolium Castaneum Gut. Biocatal. Agric. Biotechnol. 2019, 19. [Google Scholar] [CrossRef]
- Mahmoud, A.; Kotb, E.; Alqosaibi, A.I.; Al-Karmalawy, A.A.; Al-Dhuayan, I.S.; Alabkari, H. In Vitro and in Silico Characterization of Alkaline Serine Protease from Bacillus Subtilis D9 Recovered from Saudi Arabia. Heliyon 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Zia, M.A.; Iqbal, H.M.N. Purification and Kinetic Parameters Characterization of an Alkaline Protease Produced from Bacillus Subtilis through Submerged Fermentation Technique. World Appl. Sci. J. 2011, 12. [Google Scholar]
- Ramalingam, K.; Nandhi, P.; Murugan, R.; Venkatesan, R. Physical and chemical characterization of alkaline protease from bacillus subtilis vbc7 using agro waste as substrate. J. Microbiol. Biotechnol. Food Sci. 2022, 12. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, D.; Wang, Z.; Hou, J.; Tyagi, R.; Liang, Y.; Hu, Y. Purification and Characterization of a Novel, Highly Potent Fibrinolytic Enzyme from Bacillus Subtilis DC27 Screened from Douchi, a Traditional Chinese Fermented Soybean Food. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.S.; Chung, D.M.; Ryu, C.H.; Yoon, K.S.; Maeng, P.J.; Kim, S.H. Identification of Three Extracellular Proteases from Bacillus Subtilis KCTC 3014. J. Microbiol. Biotechnol. 2006, 16. [Google Scholar]
- Suh, H.J.; Lee, H.K. Characterization of a Keratinolytic Serine Protease from Bacillus Subtilis KS-1. J. Protein Chem. 2001, 20. [Google Scholar] [CrossRef] [PubMed]
- Binsi, P.K.; Shamasundar, B.A.; Dileep, A.O.; Badii, F.; Howell, N.K. Rheological and Functional Properties of Gelatin from the Skin of Bigeye Snapper (Priacanthus Hamrur) Fish: Influence of Gelatin on the Gel-Forming Ability of Fish Mince. Food Hydrocoll. 2009, 23. [Google Scholar] [CrossRef]
- S, J.; H, S.; S.M., R.; H, A.; F, K. Extraction and Evaluation of Gelatin from Yellow Fin Tuna (Thunnus Albacares) Skin and Prospect as an Alternative to Mammalian Gelatin. Iran. J. Fish. Sci. 2016, 18.
- Gómez-Estaca, J.; Montero, P.; Fernández-Martín, F.; Gómez-Guillén, M.C. Physico-Chemical and Film-Forming Properties of Bovine-Hide and Tuna-Skin Gelatin: A Comparative Study. J. Food Eng. 2009, 90. [Google Scholar] [CrossRef]
- Tümerkan, E.T.A.; Cansu, Ü.; Boran, G.; Mac Regenstein, J.; Özoğul, F. Physiochemical and Functional Properties of Gelatin Obtained from Tuna, Frog and Chicken Skins. Food Chem. 2019, 287, 273–279. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish Gelatin: Properties, Challenges, and Prospects as an Alternative to Mammalian Gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and Physical Properties of Gelatin Extracted from Different Marine Species: A Comparative Study. Food Hydrocoll. 2002, 16. [Google Scholar] [CrossRef]
- Nurilmala, M.; Hizbullah, H.H.; Karnia, E.; Kusumaningtyas, E.; Ochiai, Y. Characterization and Antioxidant Activity of Collagen, Gelatin, and the Derived Peptides from Yellowfin Tuna (Thunnus Albacares) Skin. Mar. Drugs 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Gu, Y.S.; Kim, S.B. Extracting Optimization and Physical Properties of Yellowfin Tuna (Thunnus Albacares) Skin Gelatin Compared to Mammalian Gelatins. Food Hydrocoll. 2005, 19. [Google Scholar] [CrossRef]
- Ningrum, A.; Widyastuti Perdani, A.; Supriyadi; Siti Halimatul Munawaroh, H.; Aisyah, S.; Susanto, E. Characterization of Tuna Skin Gelatin Edible Films with Various Plasticizers-essential Oils and Their Effect on Beef Appearance. J. Food Process. Preserv. 2021, 45, e15701.
- Ahmad, M.; Benjakul, S. Characteristics of Gelatin from the Skin of Unicorn Leatherjacket (Aluterus Monoceros) as Influenced by Acid Pretreatment and Extraction Time. Food Hydrocoll. 2011, 25. [Google Scholar] [CrossRef]
- Jellouli, K.; Balti, R.; Bougatef, A.; Hmidet, N.; Barkia, A.; Nasri, M. Chemical Composition and Characteristics of Skin Gelatin from Grey Triggerfish (Balistes Capriscus). LWT 2011, 44. [Google Scholar] [CrossRef]
- Gudmundsson, M.; Hafsteinsson, H. Gelatin from Cod Skins as Affected by Chemical Treatments. J. Food Sci. 1997, 62. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; al López-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Huang, M.; Cao, G.; Tao, F.; Shen, Q.; Zhou, G.; Yang, H. Repurposing Fish Waste into Gelatin as a Potential Alternative for Mammalian Sources: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 942–963. [Google Scholar] [CrossRef]
- Alfaro, A. da T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7.
- Montero, M.; Acosta, Ó.G.; Bonilla, A.I. Membrane Fractionation of Gelatins Extracted from Skin of Yellowfin Tuna (Thunnus Albacares): Effect on Molecular Sizes and Gelling Properties of Fractions. CYTA - J. Food 2022, 20. [CrossRef]
- Karayannakidis, P.D.; Chatziantoniou, S.E.; Zotos, A. Effects of Selected Process Parameters on Physical and Sensorial Properties of Yellowfin Tuna (Thunnus Albacares) Skin Gelatin. J. Food Process Eng. 2014, 37. [Google Scholar] [CrossRef]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An Overview of Gelatin Derived from Aquatic Animals: Properties and Modification. Trends Food Sci. Technol. 2017, 68. [Google Scholar] [CrossRef]
- Lv, L.-C.; Huang, Q.-Y.; Ding, W.; Xiao, X.-H.; Zhang, H.-Y.; Xiong, L.-X. Fish Gelatin: The Novel Potential Applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Jampilek, J.; Kos, J.; Kralova, K. Potential of Nanomaterial Applications in Dietary Supplements and Foods for Special Medical Purposes. Nanomaterials 2019, 9, 296. [Google Scholar] [CrossRef]
- Parvathy, U.; Binsi, P.K.; Visnuvinayagam, S.; Zynudheen, A.A.; Ninan, G.; Ravishankar, C.N. Functional Peptides from Yellowfin Tuna (Thunnus Albacares): Characterisation and Storage Stability Assessment. Indian J. Fish. 2020, 67, 69–79. [Google Scholar] [CrossRef]
- Han, Y.; Byun, S.H.; Park, J.H.; Kim, S.B. Bioactive Properties of Enzymatic Hydrolysates from Abdominal Skin Gelatin of Yellowfin Tuna (Thunnus Albacares). Int. J. Food Sci. Technol. 2015, 50. [Google Scholar] [CrossRef]
- Viji, P.; Phannendra, T.S.; Jesmi, D.; Madhusudana Rao, B.; Dhiju Das, P.H.; George, N. Functional and Antioxidant Properties of Gelatin Hydrolysates Prepared from Skin and Scale of Sole Fish. J. Aquat. Food Prod. Technol. 2019, 28. [Google Scholar] [CrossRef]
- Wardani, D.W.; Ningrum, A. Manikharda; Vanidia, N.; Munawaroh, H.S.H.; Susanto, E.; Show, P.L. In Silico and in Vitro Assessment of Yellowfin Tuna Skin (Thunnus Albacares) Hydrolysate Antioxidation Effect. Food Hydrocoll. Heal. 2023, 3. [CrossRef]
- Cai, B.; Wan, P.; Chen, H.; Huang, J.; Ye, Z.; Chen, D.; Pan, J. Purification and Identification of Novel Myeloperoxidase Inhibitory Antioxidant Peptides from Tuna (Thunnas Albacares) Protein Hydrolysates. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Nguyen, B.C.; Nguyen, H.M.X.; Nguyen, K.H.N.; Kha, T.C. Functional Properties of Yellowfin Tuna (Thunnus Albacares) Skin Collagen Hydrolysate Fraction Obtained By Ultrafiltration Purification. Curr. Res. Nutr. Food Sci. 2021, 9. [Google Scholar] [CrossRef]
- Thilanja, G.P.D.D.S.; Dissanayake, K.S.M.; Kariyawasam, M.G.T.R.; Abeyrathne, E.D.N.S. Extraction of Crude Collagen from Yellowfin Tuna ( Thunnus Albacares ) Skin and Determination of the Functional Properties of Its Hydrolysates. J. Technol. Value Addit. 2020, 2. [Google Scholar]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant Properties of a Radical-Scavenging Peptide Purified from Enzymatically Prepared Fish Skin Gelatin Hydrolysate. J. Agric. Food Chem. 2005, 53. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.I.; Ho, H.Y.; Chu, Y.J.; Chow, C.J. Characteristic and Antioxidant Activity of Retorted Gelatin Hydrolysates from Cobia (Rachycentron Canadum) Skin. Food Chem. 2008, 110. [Google Scholar] [CrossRef]
- Nagano, H.; To, K.A. Purification of Collagenase and Specificity of Its Related Enzyme from Bacillus Subtilis FS-2. Biosci. Biotechnol. Biochem. 2000, 64. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakul, S.; Ghomi, M.R. The Effects of Pretreatments on Antioxidative Activities of Protein Hydrolysate from the Muscle of Brownstripe Red Snapper (Lutjanus Vitta). LWT 2011, 44. [Google Scholar] [CrossRef]
- Qiu, Y.T.; Wang, Y.M.; Yang, X.R.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Gelatin and Antioxidant Peptides from Gelatin Hydrolysate of Skipjack Tuna (Katsuwonus Pelamis) Scales: Preparation, Identification and Activity Evaluation. Mar. Drugs 2019, 17. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Yachai, M.; Visessanguan, W.; Shahidi, F.; Hayes, K.D. Amino Acid Composition and Antioxidative Peptides from Protein Hydrolysates of Yellow Stripe Trevally (Selaroides Leptolepis). J. Food Sci. 2009, 74. [Google Scholar] [CrossRef] [PubMed]
| Amino acids | g/100g dry sample |
|---|---|
| Asp+Asn | 5.26 |
| Thr | 3.29 |
| Ser | 3.77 |
| Glu+Gln | 9.81 |
| Pro | 12.17 |
| Gly | 22.93 |
| Ala | 10.49 |
| Cys | 0.22 |
| Val | 1.83 |
| Met | 2.06 |
| Ile | 0.84 |
| Leu | 2.53 |
| Tyr | 0.50 |
| Phe | 14 |
| His | 5 |
| Lys | 26 |
| Arg | 51 |
| OH-Pro | 73 |
| OH-Lys | 6 |
| Total | 1000 |
| Temperature (°C) | Enzymatic Units (IU) | Hydrolysis degree (% DH) |
Antioxidant activity (%) |
|---|---|---|---|
| 70 | 6.5 | 16.66 | 28.65 ± 0.16 |
| 70 | 4 | 15.40 | 31.71 ± 0.41 |
| 60 | 4 | 16.06 | 28.01 ± 0.49 |
| 70 | 1.5 | 12.77 | 35.96 ± 0.22 |
| 60 | 6.5 | 18.30 | 26.60 ± 0.55 |
| 60 | 4 | 16.24 | 28.15 ± 0.45 |
| 60 | 1.5 | 13.46 | 33.26 ± 0.32 |
| 50 | 4 | 17.16 | 22.32 ± 0.54 |
| 60 | 4 | 16.10 | 27.65 ± 0.88 |
| 50 | 6.5 | 19.81 | 17.64 ± 0.38 |
| 50 | 1.5 | 16.35 | 29.77 ± 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





