Submitted:
03 June 2024
Posted:
04 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. State Of The Art
2. Materials and Methods
2.1. Apparatus
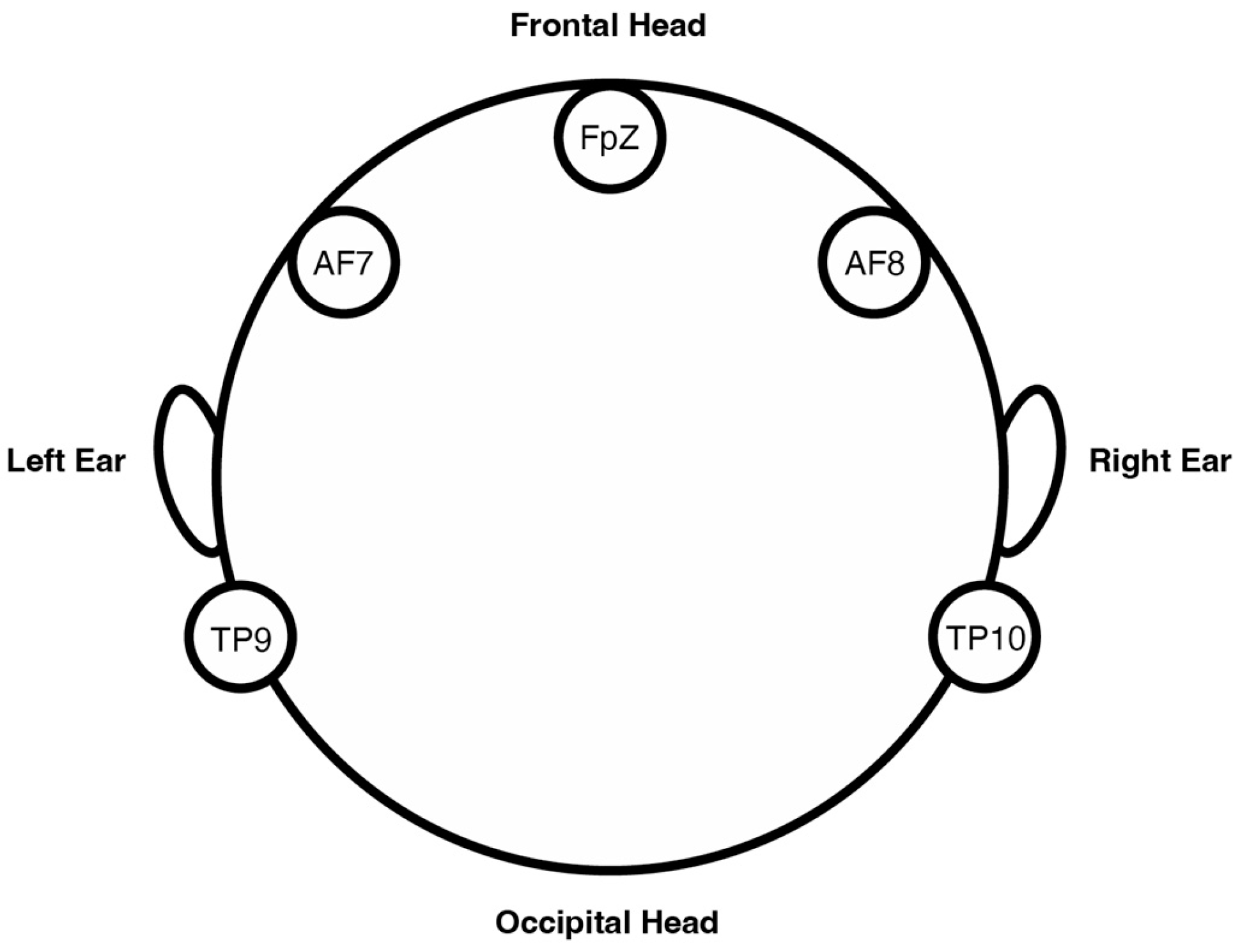
2.1.1. EEG Apparatus: Muse S (Gen 2)

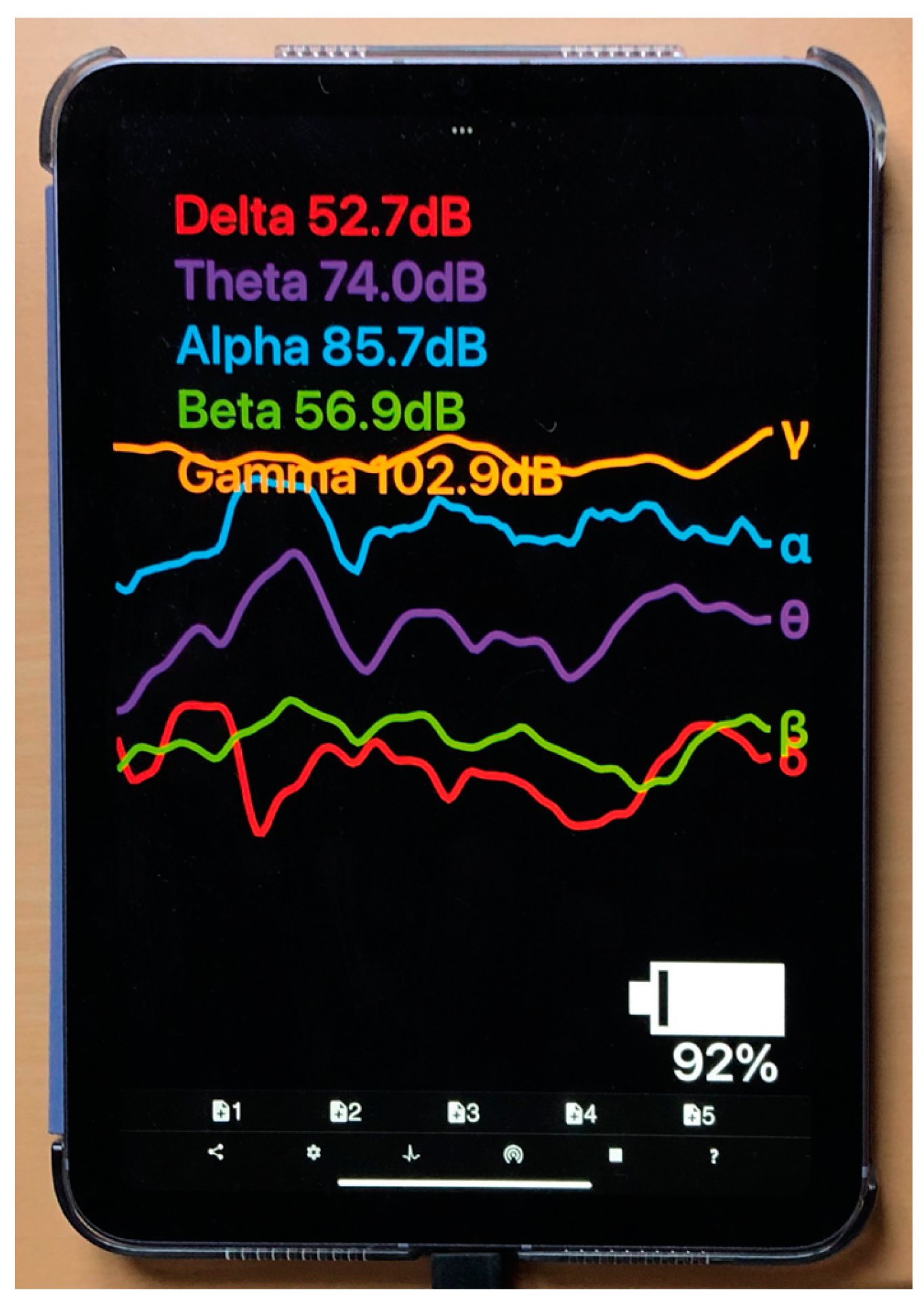
2.1.2. ECG Apparatus: Polar H10 Heart Rate Monitor
2.1.3. Questionnaire: Perceived Stress Scale PSS-10
2.1.4. Vibroacoustic Apparatus: Device & Audio

2.2. Method
2.2.1. Recruitment
2.2.2. Experimental Procedure
3. Results
3.1. Cognitive Stress (EEG)
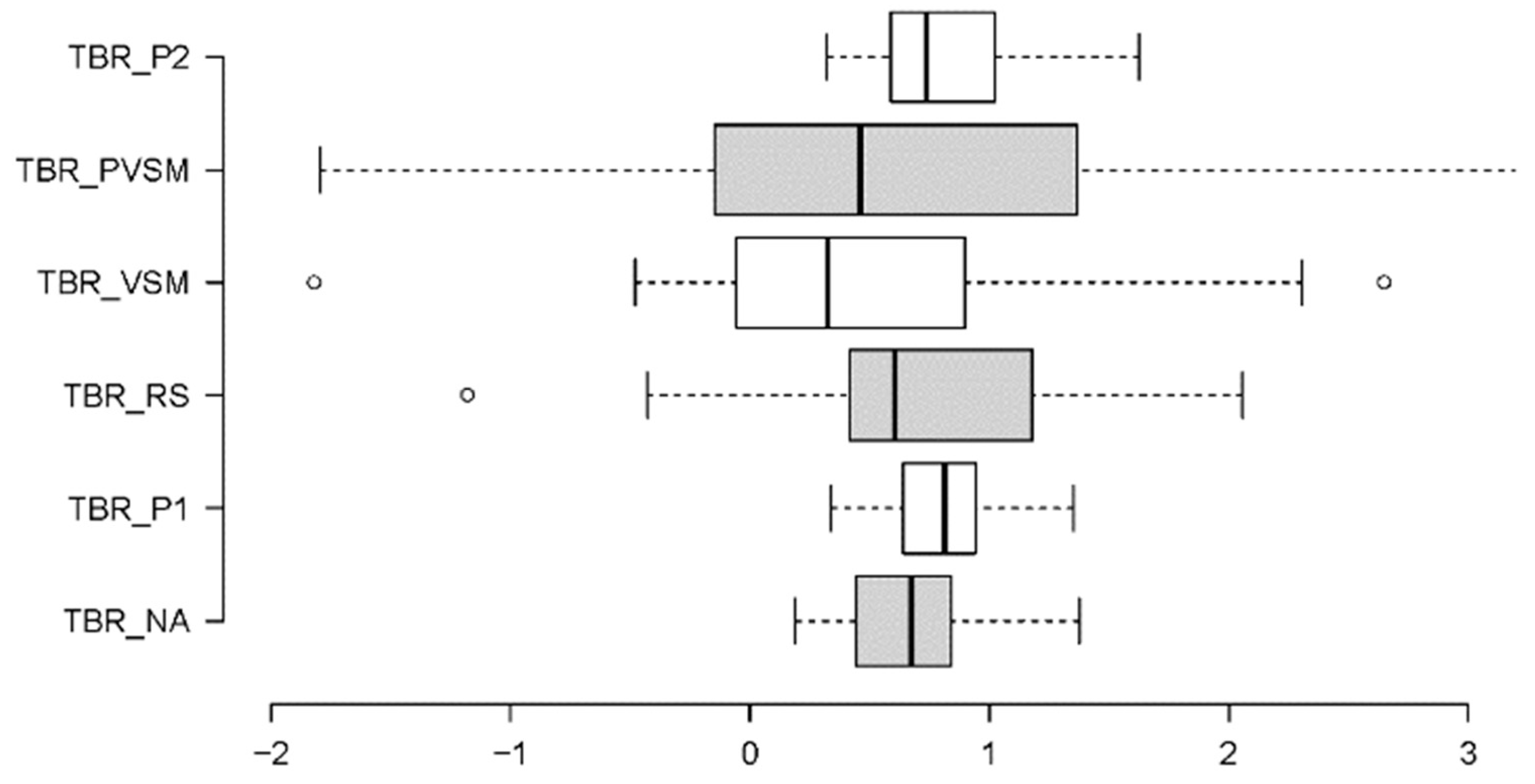
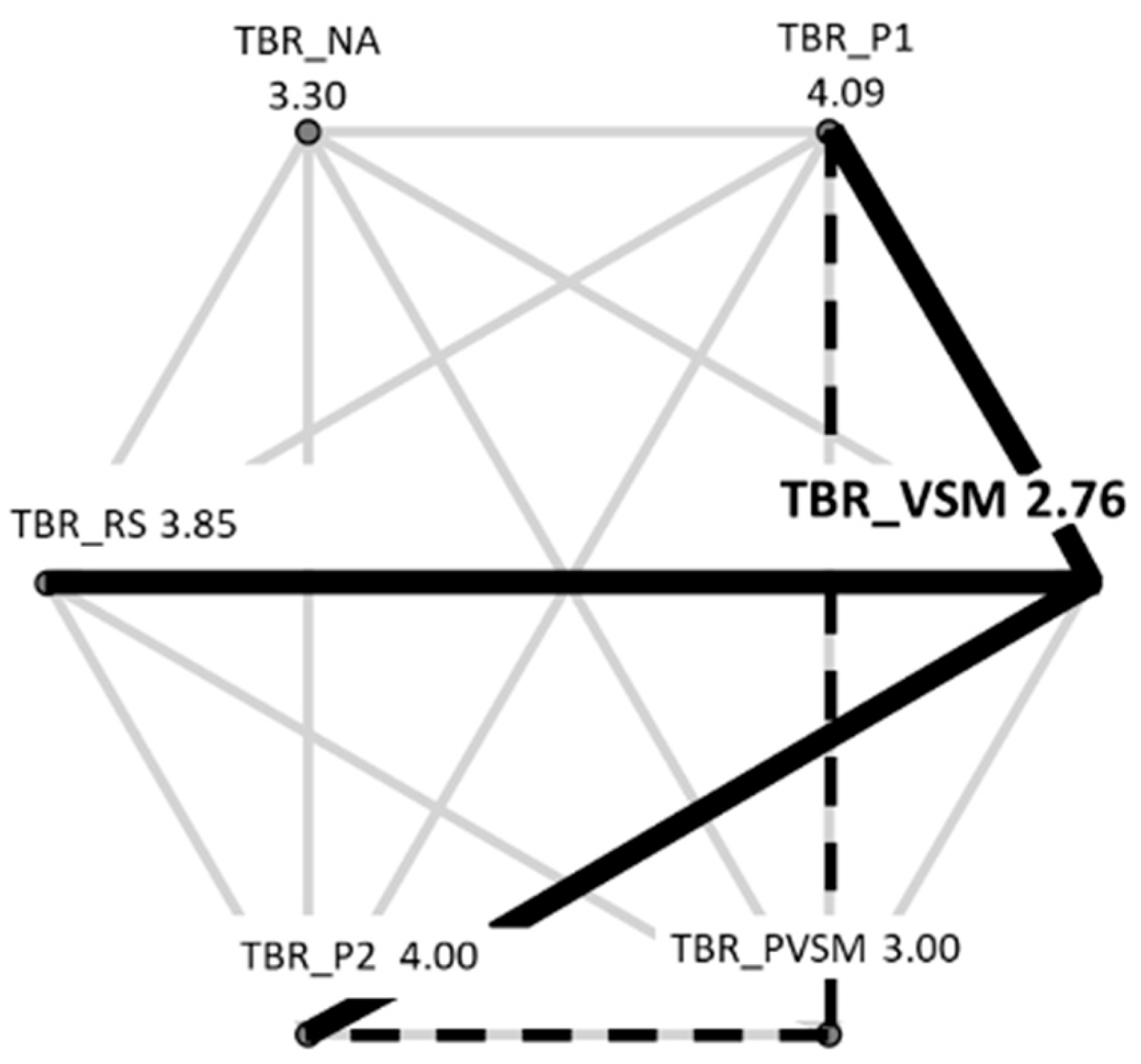
3.1.1. Theta/Beta Ratio (TBR) - Concentration and Focus
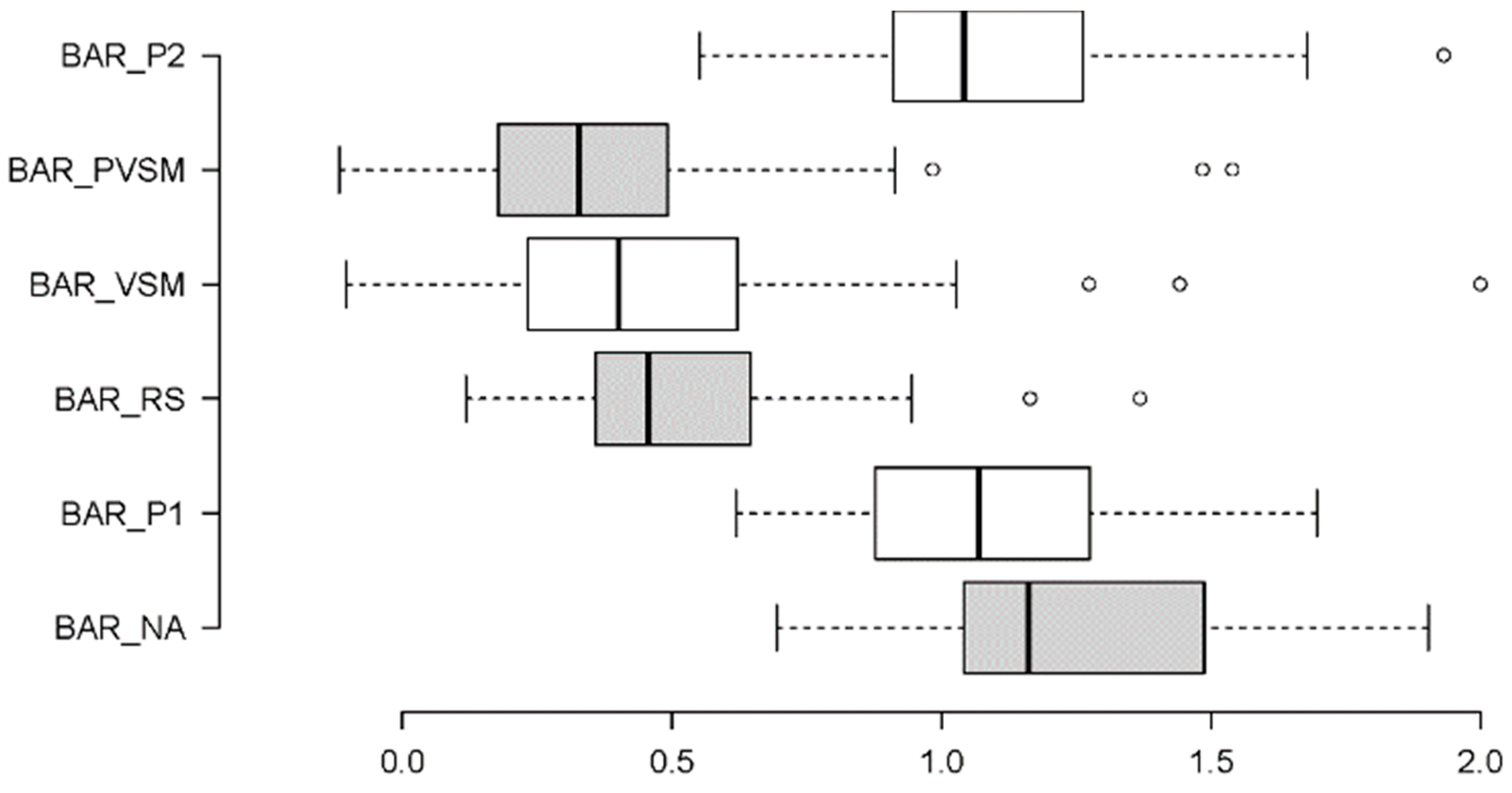
3.1.2. Beta/Alpha Ratio (BAR) - Arousal
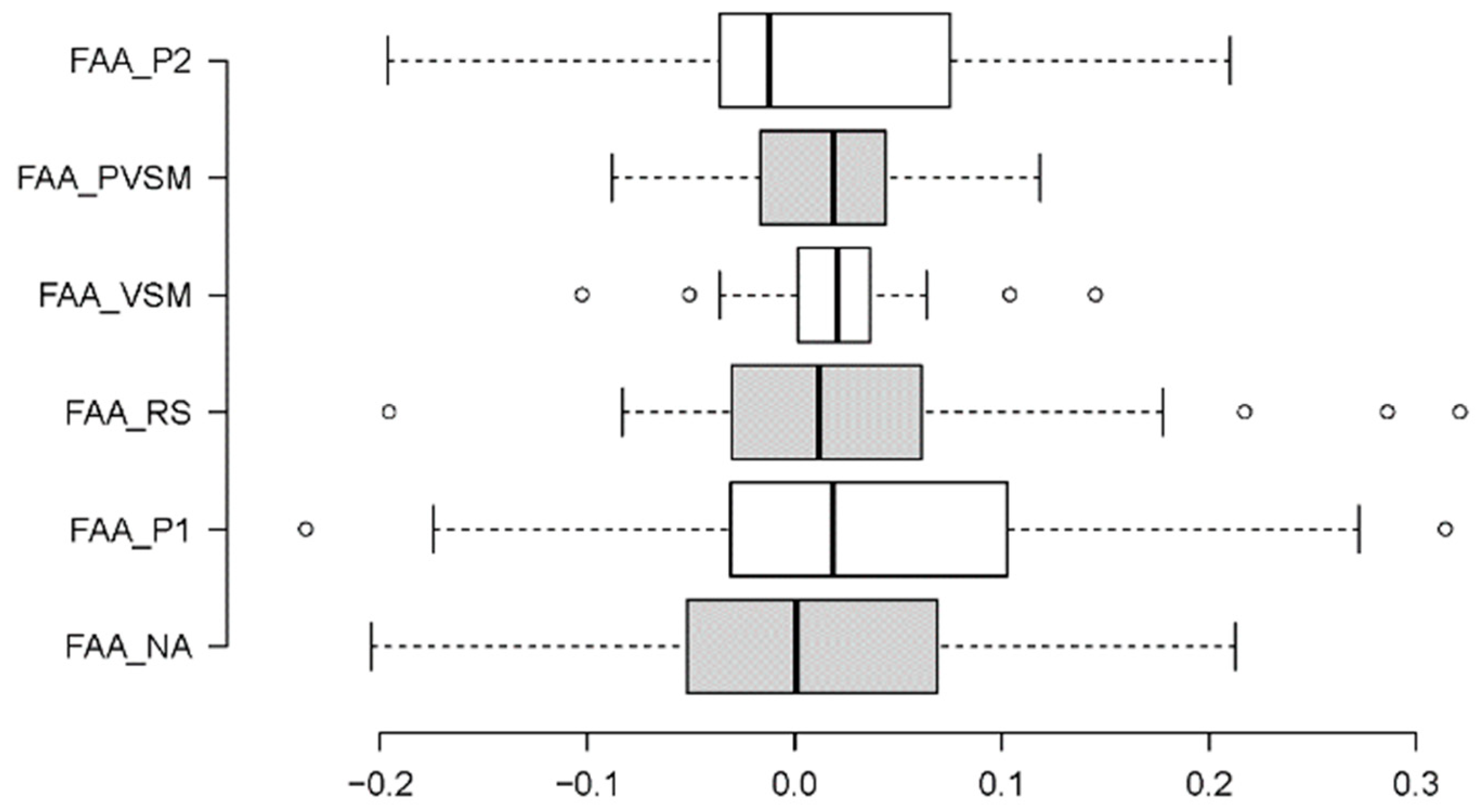
3.1.3. Frontal/Alpha Asymmetry (FAA) - Well-Being
3.1.4. Qualitative Data: First-Person Verbal Accounts
3.2. Physiological Stress (ECG)
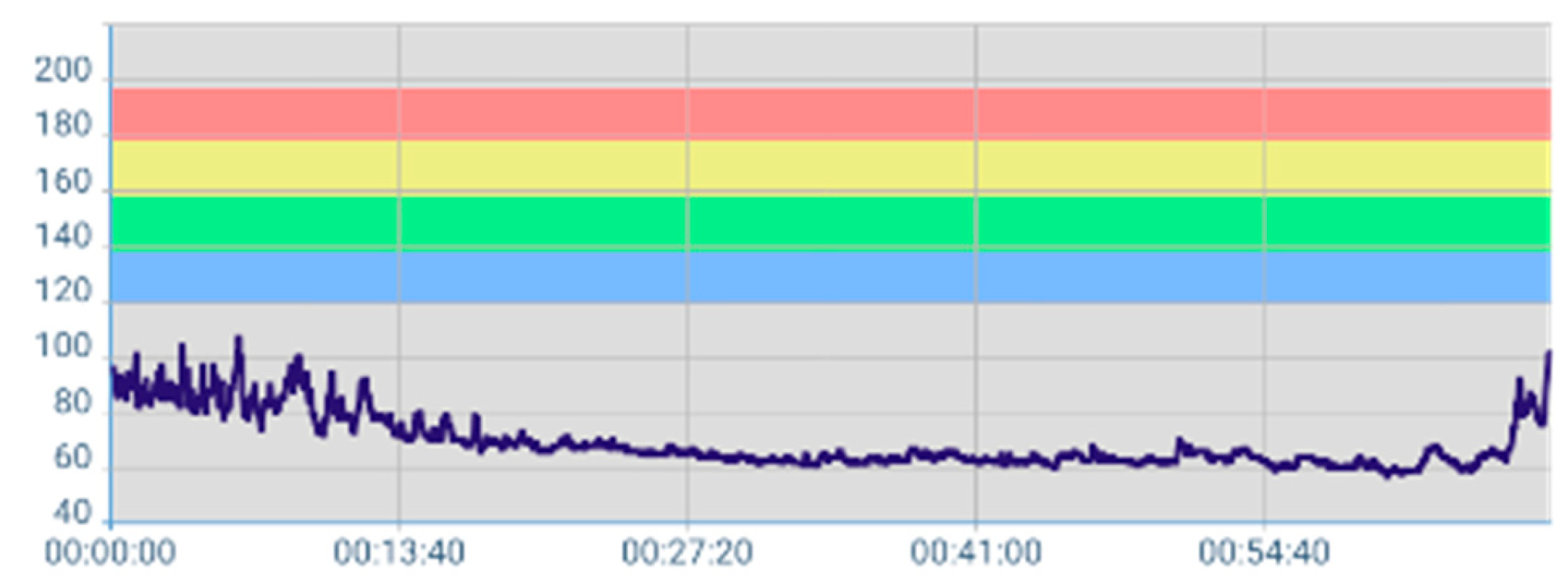
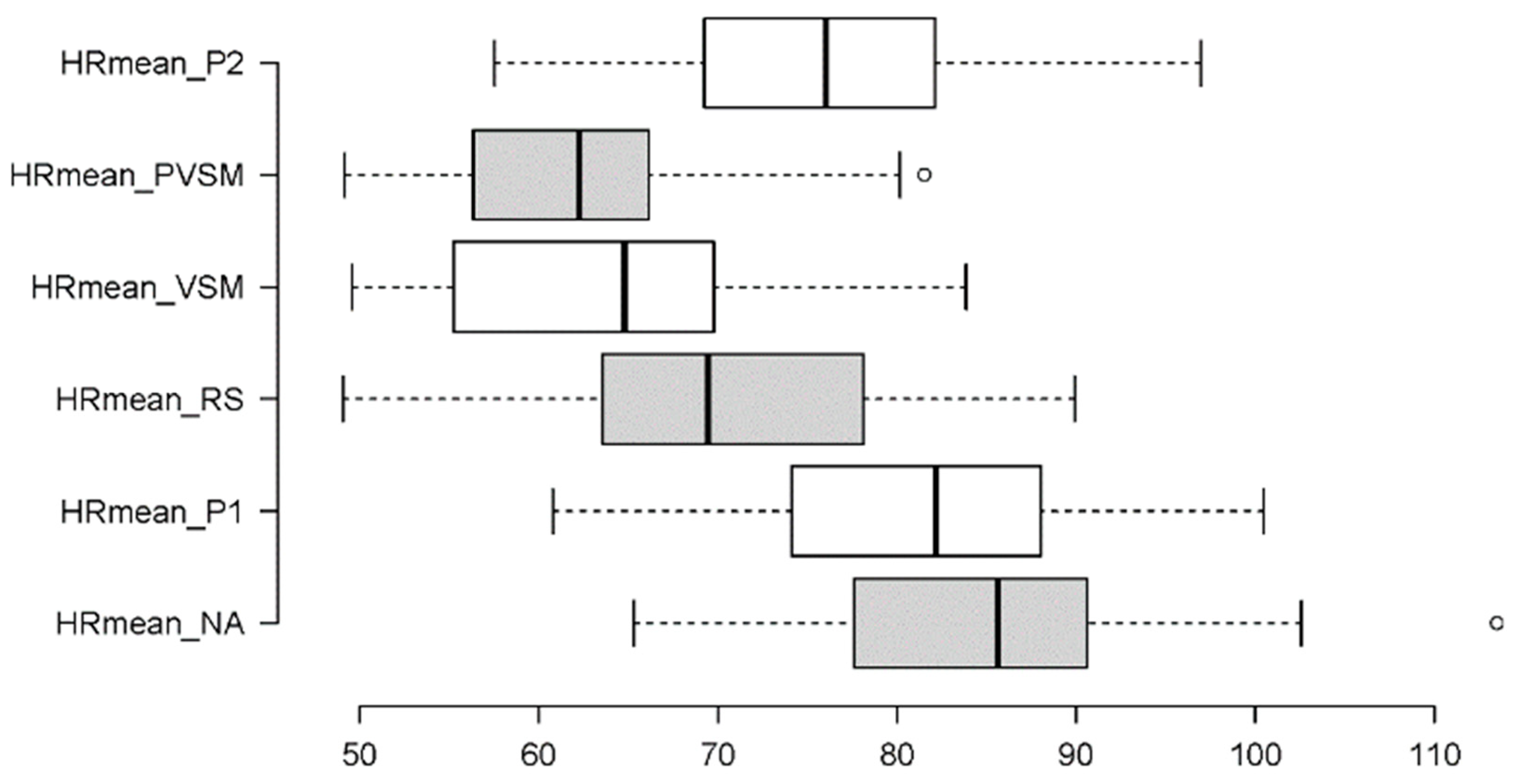
3.2.1. Mean HR
3.2.2. Mean HRV
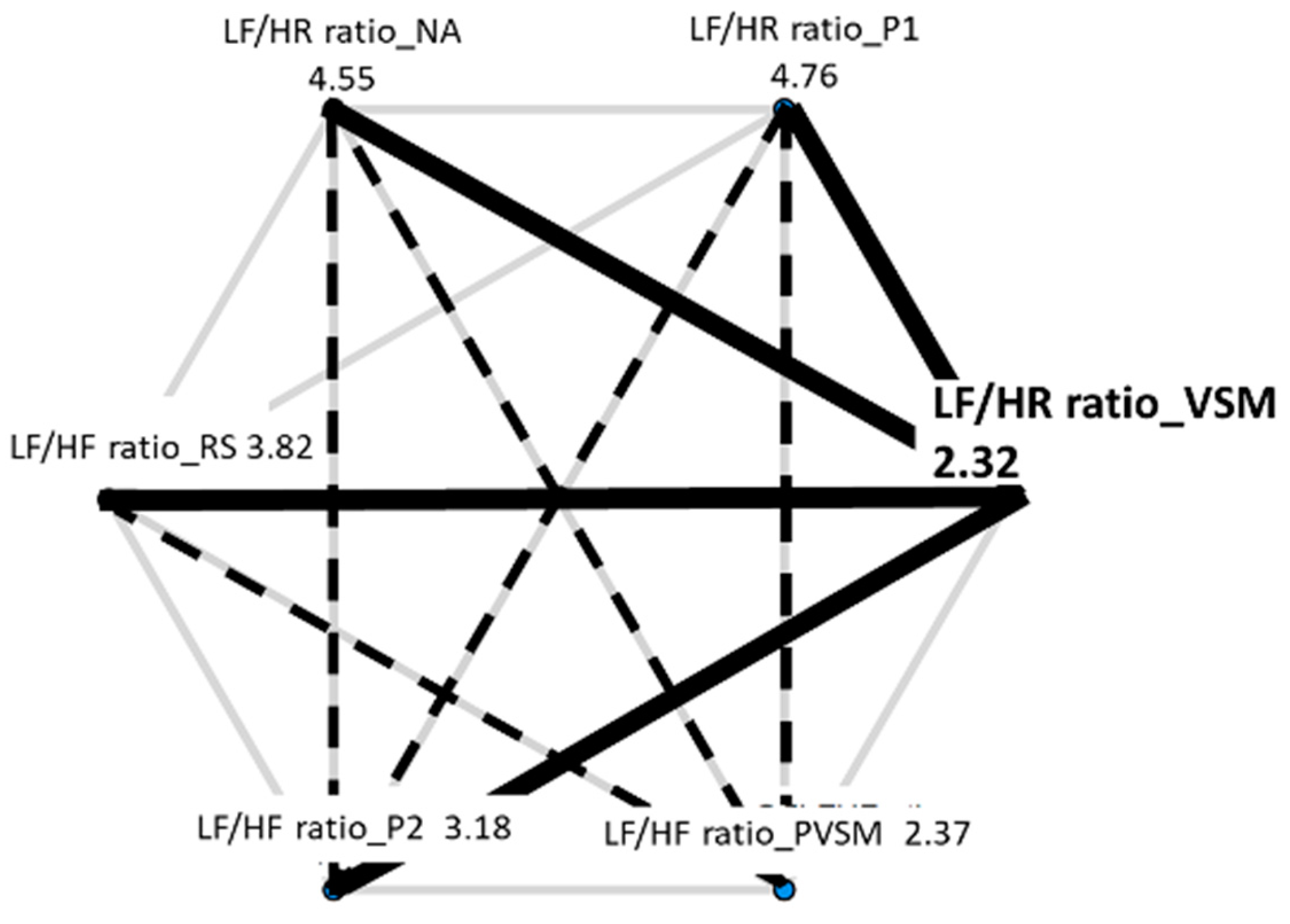
3.2.3. Sympathovagal Balance: LF/HF Ratio
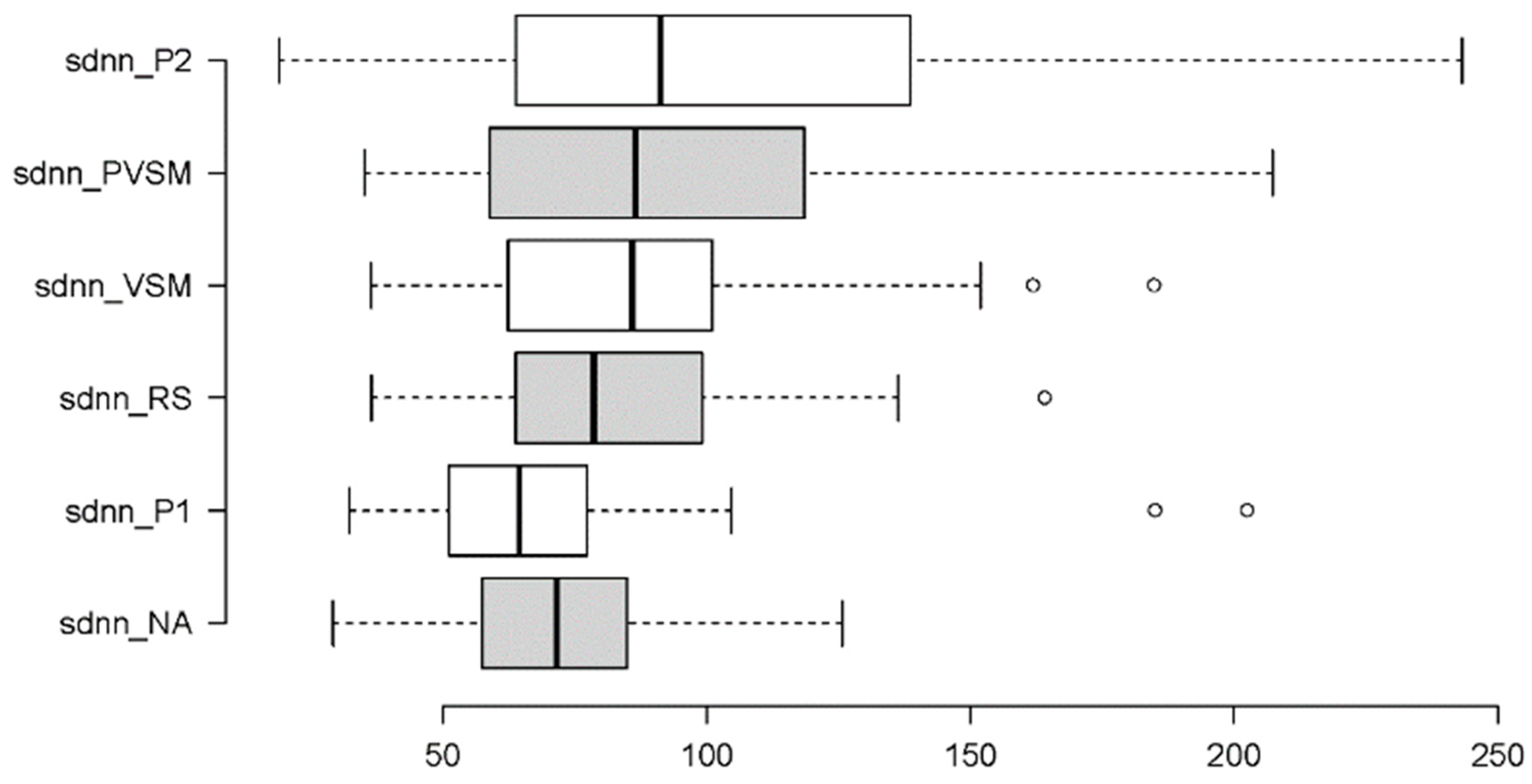
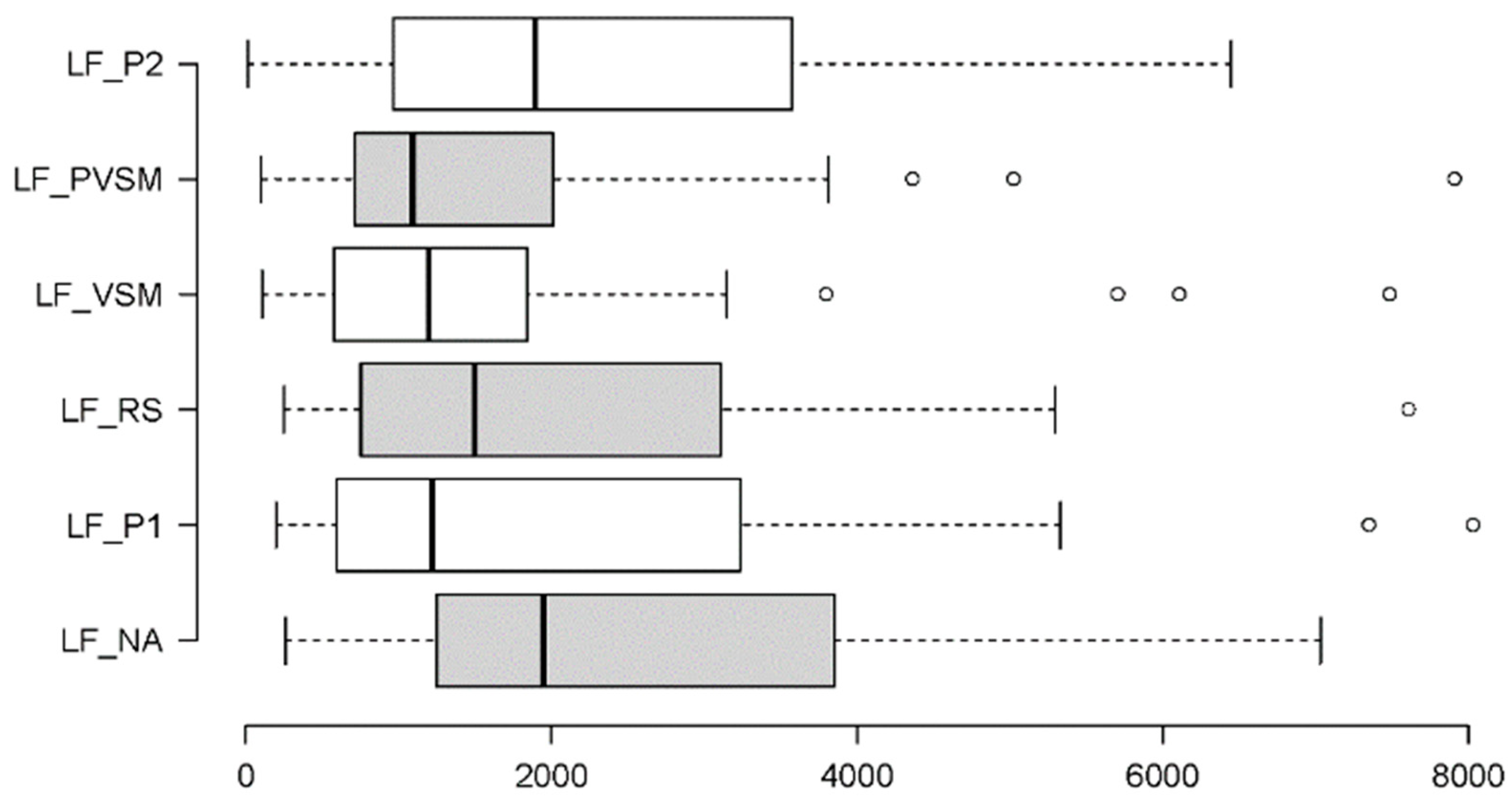
3.2.4. Sympathetic Activity: SDNN and LF
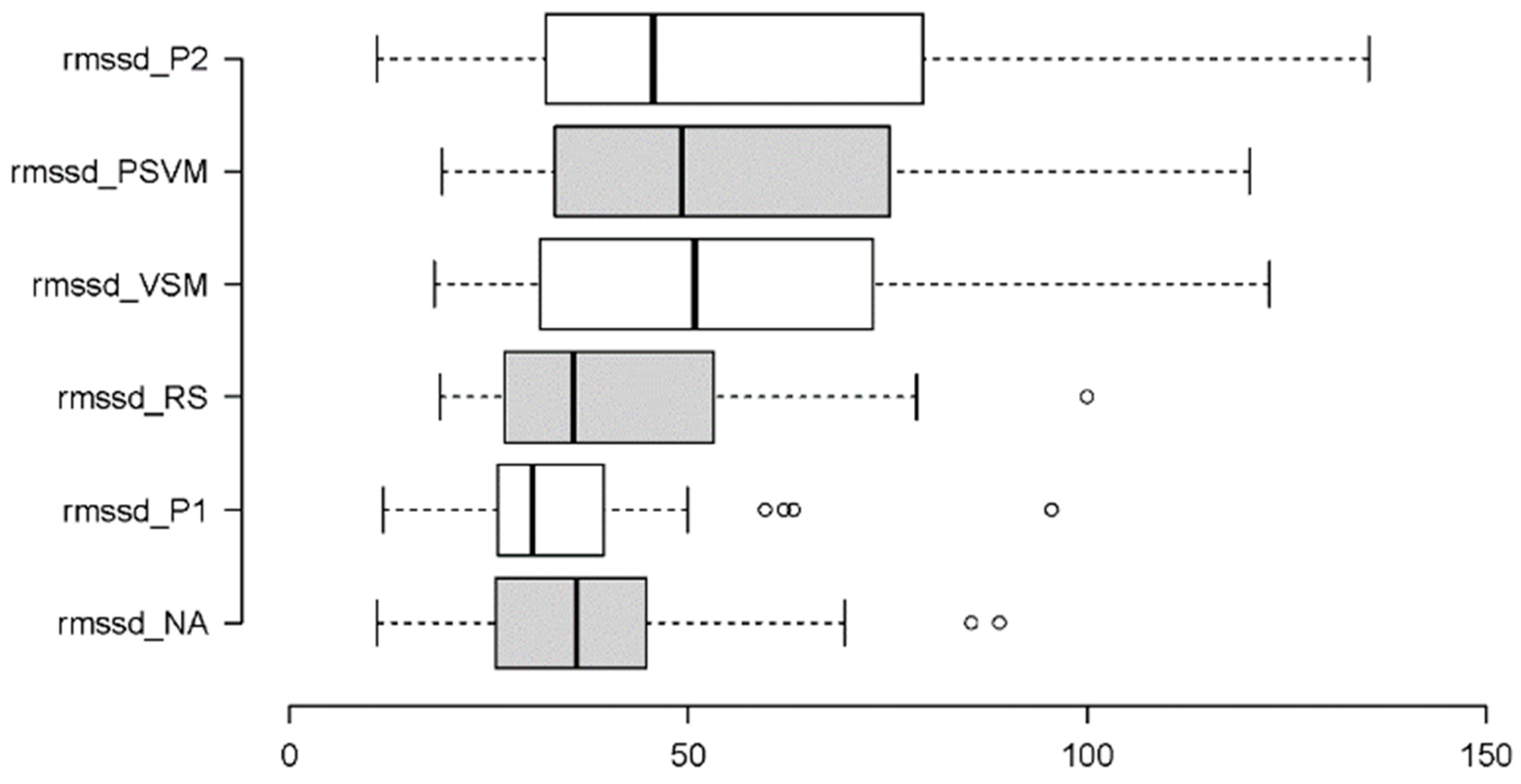
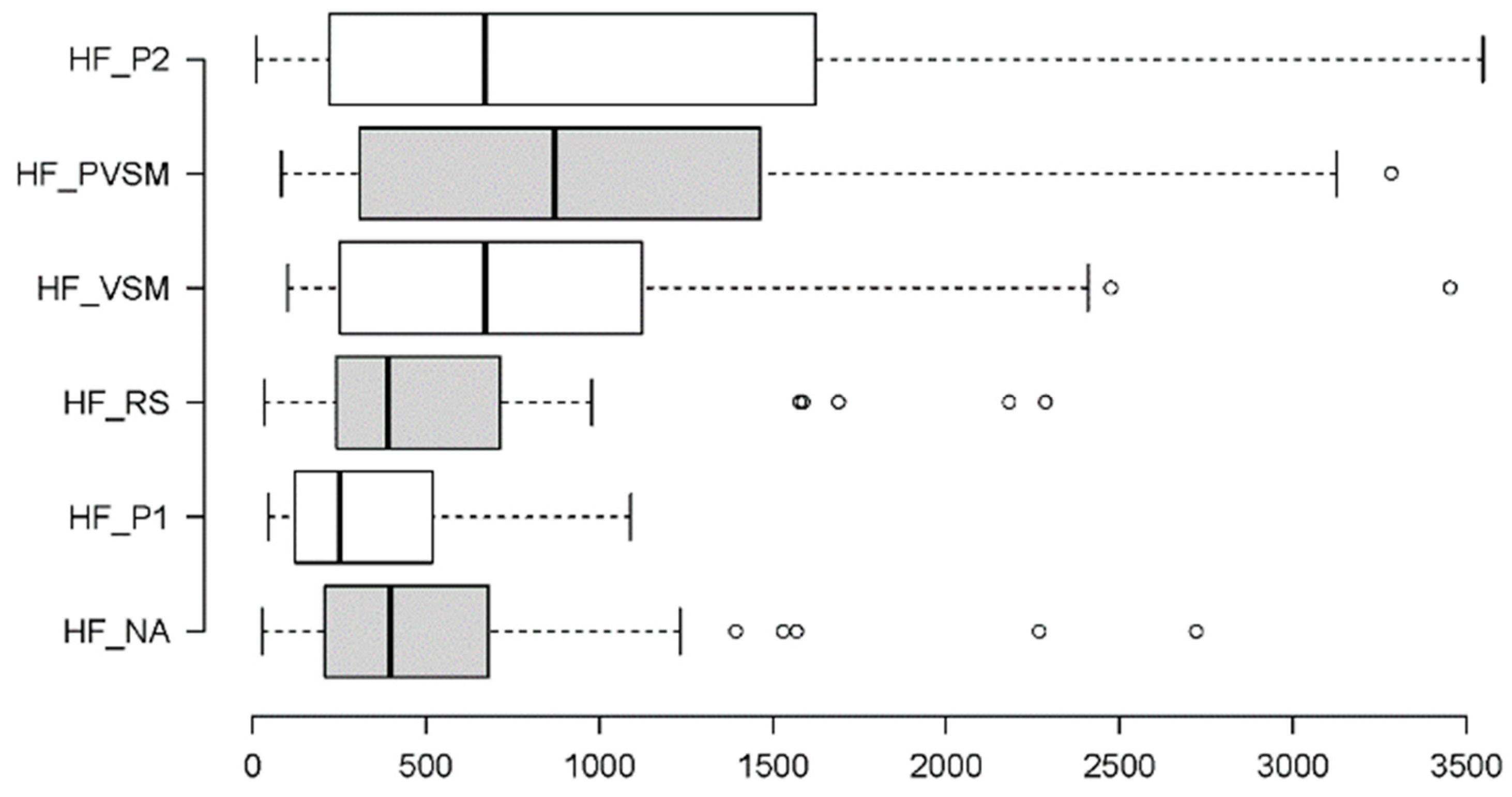
3.2.5. Parasympathetic Activity: RMSSD and HF
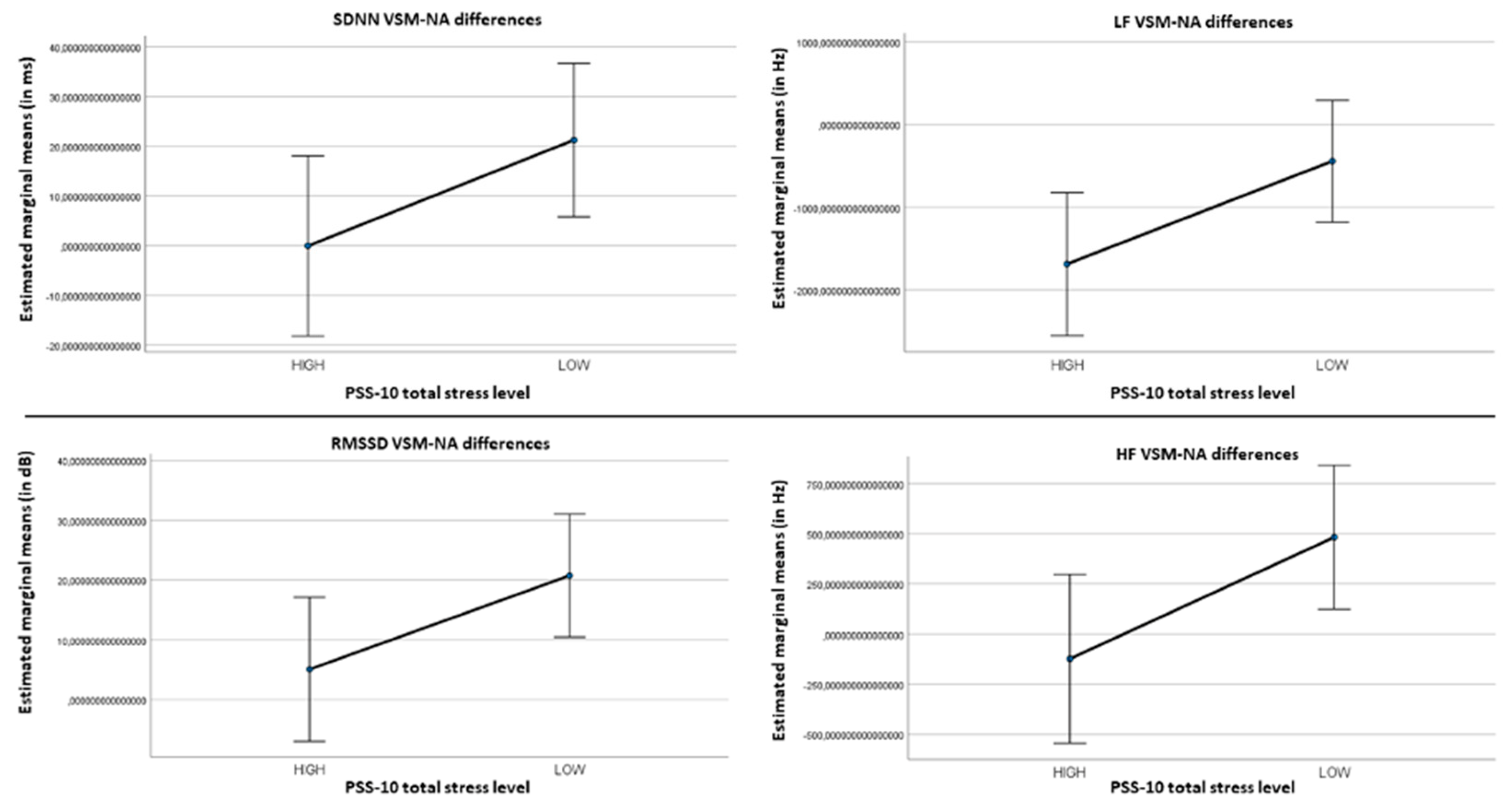
3.3. Psychological Stress
4. Discussion
4.1. Discussion of Cognitive-Stress Effects
4.2. Discussion of Physiological-Stress Effects
4.3. Discussion of Psychological-Stress Effects
4.4. Relevance and Implications
4.5. Limitations and Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nochaiwong, S.; Ruengorn, C.; Thavorn, K.; Hutton, B.; Awiphan, R.; Phosuya, C.; Ruanta, Y.; Wongpakaran, N.; Wongpakaran, T. Global Prevalence of Mental Health Issues Among the General Population During the Coronavirus Disease-2019 Pandemic: A Systematic Review and Meta-analysis. Sci Rep 2021, 11, 10173. [Google Scholar] [CrossRef] [PubMed]
- Gale, N. The Sociology of Traditional, Complementary and Alternative Medicine. Sociol Compass 2014, 8, 805–822. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Sasaki, J.E.; Wei, G.X.; Huang, T.; Yeung, A.S.; Neto, O.B.; Chen, K.W.; Hui, S.S. Effects of Mind-Body Exercises (Tai Chi/Yoga) on Heart Rate Variability Parameters and Perceived Stress: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. J Clin Med 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Danylova, T.V.; Shmarhun, V.M.; Vertel, A.V.; Matyushko, B.K.; Bondarenko, A.F.; Kychkyruk, T.; Petersen, J. Effects of the eastern mind-body practices on mental health during the covid-19 pandemic: When east meets west. Wiad Lek 2021, 74, 2850–2855. [Google Scholar] [CrossRef] [PubMed]
- Gangadhar, B.; Porandla, K. Yoga and Mental Health Services. Indian Journal of Psychiatry 2015, 57, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Singphow, C.; Purohit, S.; Tekur, P.; Bista, S.; Panigrahy, S.N.; Raghuram, N.; Nagendra, H.R. Effect of Yoga on Stress, Anxiety, Depression, and Spinal Mobility in Computer Users with Chronic Low Back Pain. Int J Yoga 2022, 15, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.; Stern, E.; Bushell, W.C.; Castle, R.D. Yoga and Pain: A Mind-Body Complex System. Front Pain Res (Lausanne) 2023, 4, 1075866. [Google Scholar] [CrossRef] [PubMed]
- Feneberg, A.C.; Nater, U.M. An Ecological Momentary Music Intervention for the Reduction of Acute Stress in Daily Life: A Mixed Methods Feasibility Study. Front Psychol 2022, 13, 927705. [Google Scholar] [CrossRef] [PubMed]
- Feneberg, A.C.; Stijovic, A.; Forbes, P.A.G.; Lamm, C.; Piperno, G.; Pronizius, E.; Silani, G.; Nater, U.M. Perceptions of Stress and Mood Associated With Listening to Music in Daily Life During the COVID-19 Lockdown. JAMA Netw Open 2023, 6, e2250382. [Google Scholar] [CrossRef] [PubMed]
- Valosek, L.; Wendt, S.; Link, J.; Abrams, A.; Hipps, J.; Grant, J.; Nidich, R.; Loiselle, M.; Nidich, S. Meditation Effective in Reducing Teacher Burnout and Improving Resilience: A Randomized Controlled Study. Frontiers in Education 2021, 6. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Frontiers in Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, R.J.S.; Band, G.P.H. Breath of Life: The Respiratory Vagal Stimulation Model of Contemplative Activity. Front Hum Neurosci 2018, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Kromenacker, B.W.; Sanova, A.A.; Marcus, F.I.; Allen, J.J.B.; Lane, R.D. Vagal Mediation of Low-Frequency Heart Rate Variability During Slow Yogic Breathing. Psychosom Med 2018, 80, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Chesky, K.S.; Michel, D.E. The Music Vibration Table (MVT ): Developing a Technology and Conceptual Model for Pain Relief. Music Therapy Perspectives 1991, 9, 32–38. [Google Scholar] [CrossRef]
- Naghdi, L.; Ahonen, H.; Macario, P.; Bartel, L. The Effect of Low-Frequency Sound Stimulation on Patients with Fibromyalgia: A Clinical Study. Pain Research & Management 2015, 20, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rüütel, E.; Vinkel, I.; Eelmäe, P. The Effect of Short-Term Vibroacoustic Treatment on Spasticity and Perceived Health Condition of Patients with Spinal Cord and Brain Injuries. Music and Medicine 2017, 9, 202–208. [Google Scholar] [CrossRef]
- Campbell, E.; Burger, B.; Ala-Ruona, E. A Single-Case, Mixed Methods Study Exploring the Role of Music Listening in Vibroacoustic Treatment. Voices: A World Forum for Music Therapy 2019, 19, 27. [Google Scholar] [CrossRef]
- Hallihan, C.; Siegle, G.J. Effect of Vibroacoustic Stimulation on Athletes Recovering from Exercise. European Journal of Applied Physiology 2022, 122, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Rüütel, E.; Ratnik, M.; Tamm, E.; Zilensk, H. The Experience of Vibroacoustic Therapy in the Therapeutic Intervention of Adolescent Girls. Nordic Journal of Music Therapy 2004, 13, 33–46. [Google Scholar] [CrossRef]
- Braun Janzen, T.; Al Shirawi, M.I.; Rotzinger, S.; Kennedy, S.H.; Bartel, L. A Pilot Study Investigating the Effect of Music-Based Intervention on Depression and Anhedonia. Frontiers in Psychology 2019, 10. [Google Scholar] [CrossRef]
- Karkkainen, M.; Mitsui, J. The Effects of Sound Based Vibration Treatment on the Human Mind and Body The Physioacoustic Method. Journal of International Society of Life Information Science 2006, 24, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Kantor, J.; Campbell, E.A.; Kantorová, L.; Marečková, J.; Regec, V.; Karasová, K.; Sedláčková, D.; Klugar, M. Exploring Vibroacoustic Therapy in Adults Experiencing Pain: A Scoping Review. BMJ Open 2022, 12, e046591. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.; Hynynen, J.; Ala-Ruona, E. Vibroacoustic Treatment for Chronic Pain and Mood Disorders in a Specialised Healthcare Setting. Music and Medicine 2017, 9, 187–197. [Google Scholar] [CrossRef]
- Campbell, E.A.; Hynynen, J.; Burger, B.; Ala-Ruona, E. Exploring the Use of Vibroacoustic Treatment For Managing Chronic Pain and Comorbid Mood Disorders: A Mixed Methods Study. Nordic Journal of Music Therapy 2019, 28, 291–314. [Google Scholar] [CrossRef]
- Lehikoinen, P. The physioacoustic method. In Music Vibration and Health; Wigram, T., Dileo, C., Eds.; Jeffrey Books: Cherry Hill, NJ, 1997; pp. 206–216. [Google Scholar]
- Mosabbir, A.; Almeida, Q.J.; Ahonen, H. The Effects of Long-Term 40-Hz Physioacoustic Vibrations on Motor Impairments in Parkinson's Disease: A Double-Blinded Randomized Control Trial. Healthcare (Basel) 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G. The Effects of Vibroacoustic Music on Symptom Reduction. IEEE Engineering in Medicine and Biology Magazine, 1999; 97–100. [Google Scholar]
- Skille, O. VibroAcoustic Therapy. Music Therapy 1989, 8, 61–77. [Google Scholar] [CrossRef]
- Delmastro, F.; Martino, F.D.; Dolciotti, C. Physiological Impact of Vibro-Acoustic Therapy on Stress and Emotions through Wearable Sensors. In Proceedings of the 2018 IEEE International Conference on Pervasive Computing and Communications Workshops (PerCom Workshops), 19-23 March, 2018; pp. 621–626. [Google Scholar]
- Kantor, J.; Vilímek, Z.; Vítězník, M.; Smrčka, P.; Campbell, E.A.; Bucharová, M.; Grohmannová, J.; Špinarová, G.; Janíčková, K.; Du, J.; et al. Effect of Low Frequency Sound Vibration on Acute Stress Response in University Students - Pilot Randomized Controlled Trial. Front Psychol 2022, 13, 980756. [Google Scholar] [CrossRef] [PubMed]
- Kantor, J.; Vilímek, Z.; Du, J.; Campbell, E. Effects of Vibroacoustic Therapy on Physiological Responses Related to Stress and its Measurements in Adults a Scoping Review Protocol. Zdravotnícke listy 2022, 10. [Google Scholar]
- Vilímek, Z.; Kantor, J.; Krejčí, J.; Janečka, Z.; Jedličková, Z.; Nekardová, A.; Botek, M.; Bucharová, M.; Campbell, E.A. The Effect of Low Frequency Sound on Heart Rate Variability and Subjective Perception: A Randomized Crossover Study. Healthcare (Basel) 2022, 10, 1024. [Google Scholar] [CrossRef]
- Brodsky, W. Post-Exposure Effects of Music-Generated Vibration and Whole-Body Acoustic Stimulation among Symphony Orchestra Musicians. Psychology of Music 2000, 28, 98–115. [Google Scholar] [CrossRef]
- Rüütel, E. The Psychophysiological Effects of Music and Vibroacoustic Stimulation. Nordic Journal of Music Therapy 2002, 11, 16–26. [Google Scholar] [CrossRef]
- Koike, Y.; Hoshitani, M.; Tabata, Y.; Seki, K.; Nishimura, R.; Kano, Y. Effects of Vibroacoustic Therapy on Elderly Nursing Home Residents with Depression. Journal of Physical Therapy Science 2012, 24, 291–294. [Google Scholar] [CrossRef]
- Cavallo, F.; Rovini, E.; Dolciotti, C.; Radi, L.; Ragione, R.D.; Bongioanni, P.; Fiorini, L. Physiological response to Vibro-Acoustic stimulation in healthy subjects: a preliminary study. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, Canada, 20-24 July 2020; 2020; pp. 5921–5924. [Google Scholar]
- Campbell, E.A.; Hynynen, J.; Burger, B.; Vainionpää, A.; Ala-Ruona, E. Vibroacoustic treatment to improve functioning and ability to work: a multidisciplinary approach to chronic pain rehabilitation. Disability and Rehabilitation 2021, 43, 2055–2070. [Google Scholar] [CrossRef] [PubMed]
- VibroAcoustics Bass Module. Available online: https://vibroacoustics.dk/product (accessed on 1st May 2024).
- Fernandez, M. Acoustics and Universal Movement. In Music Vibration and Health; Wigram, T., Dileo, C., Eds.; Jeffrey Books: Cherry Hill, NJ, 1997; pp. 11–26. [Google Scholar]
- Ala-Ruona, E.; Punkanen, M.; Campbell, E. Vibroacoustic Therapy: Conception, Development, and Future Directions. Musiikkiterapia 2015, 30, 48–71. [Google Scholar]
- Attar, E.T. Review of electroencephalography signals approaches for mental stress assessment. Neurosciences (Riyadh) 2022, 27, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Akinrodoye, M.A.; Lui, F. Neuroanatomy, Somatic Nervous System. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556027/ (accessed on 2nd May 2024).
- Waxenbaum, J.A.; Reddy, V.; Varacallo, M. Anatomy, Autonomic Nervous System. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539845/ (accessed on 2nd May 2024).
- Balzarotti, S.; Biassoni, F.; Colombo, B.; Ciceri, M.R. Cardiac vagal control as a marker of emotion regulation in healthy adults: A review. Biological Psychology 2017, 130, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Tracey, K.J. The Vagus Nerve and the Inflammatory Reflex-Linking Immunity and Metabolism. Nat Rev Endocrinol 2012, 8, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Wilfong, A.A.; Schultz, R.J. Vagus Nerve Stimulation for Treatment of Epilepsy in Rett Syndrome. Dev Med Child Neurol 2006, 48, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, M.; Rigby, A.; Marson, A.G. Vagus Nerve Stimulation for Focal Seizures. Cochrane Database Syst Rev 2022, 7, Cd002896. [Google Scholar] [CrossRef]
- Elger, G.; Hoppe, C.; Falkai, P.; Rush, A.J.; Elger, C.E. Vagus Nerve Stimulation is Associated with Mood Improvements in Epilepsy Patients. Epilepsy Res 2000, 42, 203–210. [Google Scholar] [CrossRef]
- Rush, A.J.; George, M.S.; Sackeim, H.A.; Marangell, L.B.; Husain, M.M.; Giller, C.; Nahas, Z.; Haines, S.; Simpson, R.K., Jr.; Goodman, R. Vagus Nerve Stimulation (VNS) for Treatment-Resistant Depressions: A Multicenter Study. Biol Psychiatry 2000, 47, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Sobocki, J.; Fourtanier, G.; Estany, J.; Otal, P. Does Vagal Nerve Stimulation Affect Body Composition and Metabolism? Experimental Study of a New Potential Technique in Bariatric Surgery. Surgery 2006, 139, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Yuan, H.; Najib, U.; Ailani, J.; Morais, A.L.; Mathew, P.G.; Liebler, E.; Tassorelli, C.; Diener, H.C. Non-Invasive Vagus Nerve Stimulation for Primary Headache: A Clinical Update. Cephalalgia 2020, 40, 1370–1384. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardóttir, G.A.; Nielsen, P.M.; Rønager, J.; Wang, A.G. A Pilot Study on High Amplitude Low Frequency-Music Impulse Stimulation as an Add-On Treatment for Depression. Brain Behav 2019, 9, e01399. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Tong, W.; Collins, D.J.; Ibbotson, M.R.; Prawer, S.; Stamp, M. Mechanisms and Applications of Neuromodulation Using Surface Acoustic Waves-A Mini-Review. Front Neurosci 2021, 15, 629056. [Google Scholar] [CrossRef] [PubMed]
- MindMonitor Technical Manual. Available online: https://mind-monitor.com/Technical_Manual.php (accessed on 5th April 2023).
- Garcia-Moreno, F.M.; Bermudez-Edo, M.; Garrido, J.L.; Rodríguez-Fórtiz, M.J. Reducing Response Time in Motor Imagery Using A Headband and Deep Learning. Sensors 2020, 20, 6730. [Google Scholar] [CrossRef] [PubMed]
- Schaffarczyk, M.; Rogers, B.; Reer, R.; Gronwald, T. Validity of the Polar H10 Sensor for Heart Rate Variability Analysis during Resting State and Incremental Exercise in Recreational Men and Women. Sensors (Basel) 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Gilgen-Ammann, R.; Schweizer, T.; Wyss, T. RR interval signal quality of a heart rate monitor and an ECG Holter at rest and during exercise. Eur J Appl Physiol 2019, 119, 1525–1532. [Google Scholar] [CrossRef]
- Speer, K.E.; Semple, S.; Naumovski, N.; McKune, A.J. Measuring Heart Rate Variability Using Commercially Available Devices in Healthy Children: A Validity and Reliability Study. Eur J Investig Health Psychol Educ 2020, 10, 390–404. [Google Scholar] [CrossRef]
- Heart Rate Monitor. Available online: https://play.google.com/store/apps/details?id=com.bmi.hr_monitor&hl=en&gl=US (accessed on 20th May 2024).
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr Cardiol Rev 2021, 17, e160721189770. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. Journal of Health and Social Behavior 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res (Korean Soc Nurs Sci) 2012, 6, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Fox, R.S.; Mills, S.D.; Roesch, S.C.; Sadler, G.R.; Klonoff, E.A.; Malcarne, V.L. Reliability and validity of the Perceived Stress Scale-10 in Hispanic Americans with English or Spanish language preference. J Health Psychol 2019, 24, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.M.; Brähler, E.; Dreier, M.; Reinecke, L.; Müller, K.W.; Schmutzer, G.; Wölfling, K.; Beutel, M.E. The German version of the Perceived Stress Scale – psychometric characteristics in a representative German community sample. BMC Psychiatry 2016, 16, 159. [Google Scholar] [CrossRef] [PubMed]
- Nordin, M.; Nordin, S. Psychometric evaluation and normative data of the Swedish version of the 10-item perceived stress scale. Scand J Psychol 2013, 54, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, A.; Dalgaard, V.L.; Nielsen, K.J.; Andersen, J.H.; Zachariae, R.; Olsen, L.R.; Jørgensen, A.; Christiansen, D.H. Cross-cultural adaptation and validation of the Danish consensus version of the 10-item Perceived Stress Scale. Scand J Work Environ Health 2015, 41, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. Journal of Environmental Psychology 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Ratcliffe, E.; Gatersleben, B.; Sowden, P.T. Bird Sounds and their Contributions to Perceived Attention Restoration and Stress Recovery. Journal of Environmental Psychology 2013, 36, 221–228. [Google Scholar] [CrossRef]
- Buxton, R.T.; Pearson, A.L.; Allou, C.; Fristrup, K.; Wittemyer, G. A Synthesis of Health Benefits of Natural Sounds and their Distribution in National Parks. Proceedings of the National Academy of Sciences 2021, 118, e2013097118. [Google Scholar] [CrossRef]
- Ratcliffe, E. Sound and Soundscape in Restorative Natural Environments: A Narrative Literature Review. Frontiers in Psychology 2021, 12. [Google Scholar] [CrossRef]
- Gould van Praag, C.D.; Garfinkel, S.N.; Sparasci, O.; Mees, A.; Philippides, A.O.; Ware, M.; Ottaviani, C.; Critchley, H.D. Mind-Wandering and Alterations to Default Mode Network Connectivity when Listening to Naturalistic Versus Artificial Sounds. Scientific Reports 2017, 7, 45273. [Google Scholar] [CrossRef]
- Nozaradan, S. Exploring how musical rhythm entrains brain activity with electroencephalogram frequency-tagging. Philos Trans R Soc Lond B Biol Sci 2014, 369, 20130393. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M.H.; McIntosh, G.C.; Hoemberg, V. Neurobiological Foundations of Neurologic Music Therapy: Rhythmic Entrainment and the Motor System. Front Psychol 2014, 5, 1185. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Hoefle, S.; Monteiro, M.C.; Moll, J.; Keller, P.E. Neural Correlates of Listening to Varying Synchrony Between Beats in Samba Percussion and Relations to Feeling the Groove. Front Neurosci 2022, 16, 779964. [Google Scholar] [CrossRef]
- Stupacher, J.; Matthews, T.E.; Pando-Naude, V.; Foster Vander Elst, O.; Vuust, P. The Sweet Spot Between Predictability and Surprise: Musical Groove in Brain, Body, and Social Interactions. Front Psychol 2022, 13, 906190. [Google Scholar] [CrossRef]
- Bartel, L.R.; Chen, R.; Alain, C.; Ross, B. Vibroacoustic Stimulation and Brain Oscillation: From Basic Research to Clinical Application. Music and Medicine 2017, 9, 153. [Google Scholar] [CrossRef]
- Fries, P. Neuronal Gamma-Band Synchronization as a Fundamental Process in Cortical Computation. Annu Rev Neurosci 2009, 32, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Jirakittayakorn, N.; Wongsawat, Y. Brain Responses to 40-Hz Binaural Beat and Effects on Emotion and Memory. International Journal of Psychophysiology 2017, 120, 96–107. [Google Scholar] [CrossRef]
- Fooks, C.; Niebuhr, O. Assessing Vibroacoustic Sound Massage Through The Biosignal of Human Speech: Evidence of Improved Wellbeing. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Seoul, South Korea, 14-19 April 2024; 2024; pp. 11401–11405. [Google Scholar]
- Wang, H.-M.; Huang, S.-C. SDNN/RMSSD as a Surrogate for LF/HF: A Revised Investigation. Modelling and Simulation in Engineering 2012, 2012. [Google Scholar] [CrossRef]
- JP, S.F.G. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 2017, Sep 28. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, R.; Adams, J.; Baker, K.; Daufin, E.K.; Ellington, C.; Fitts, E.; Himsel, J.; Holladay, L.; Okeowo, D. How College Students Spend Their Time Communicating. International Journal of Listening 2008, 22, 13–28. [Google Scholar] [CrossRef]
- Sandler, H.; Tamm, S.; Fendel, U.; Rose, M.; Klapp, B.F.; Bösel, R. Positive Emotional Experience: Induced by Vibroacoustic Stimulation Using a Body Monochord in Patients with Psychosomatic Disorders: Is Associated with an Increase in EEG-Theta and a Decrease in EEG-Alpha Power. Brain Topogr 2016, 29, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, N.; Yazdani, S.; Vesin, J.M.; Schmitt, L.; Millet, G.P. RMSSD Is More Sensitive to Artifacts Than Frequency-Domain Parameters: Implication in Athletes' Monitoring. J Sports Sci Med 2022, 21, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Massaro, S.; Pecchia, L. Heart Rate Variability (HRV) Analysis: A Methodology for Organizational Neuroscience. Organizational Research Methods 2019, 22, 354–393. [Google Scholar] [CrossRef]
- Fooks, C.; Niebuhr, O. Assessing Effects of Vibroacoustic Stimulation Compared to a Guided Mindfulness Meditation Using The Biosignal of Human Speech. 2025; manuscript in preparation; to be submitted. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.; Walach, H.; Offenbächer, M.; Lynch, S.; Kohls, N. Measuring Mindfulness: A Rasch Analysis of the Freiburg Mindfulness Inventory. Religions 2011, 2, 693–706. [Google Scholar] [CrossRef]
- Kaske, E.A.; Chen, C.S.; Meyer, C.; Yang, F.; Ebitz, B.; Grissom, N.; Kapoor, A.; Darrow, D.P.; Herman, A.B. Prolonged Physiological Stress Is Associated With a Lower Rate of Exploratory Learning That Is Compounded by Depression. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2023, 8, 703–711. [Google Scholar] [CrossRef]
- Whiston, A.; Igou, E.R.; Fortune, D.G.; Semkovska, M. Longitudinal interactions between residual symptoms and physiological stress in the remitted symptom network structure of depression. Acta Psychologica 2023, 241, 104078. [Google Scholar] [CrossRef] [PubMed]
- Pate, B.S.; Smiley, C.E.; Harrington, E.N.; Bielicki, B.H.; Davis, J.M.; Reagan, L.P.; Grillo, C.A.; Wood, S.K. Voluntary wheel running as a promising strategy to promote autonomic resilience to social stress in females: Vagal tone lies at the heart of the matter. Autonomic Neuroscience 2024, 253, 103175. [Google Scholar] [CrossRef] [PubMed]
- Satyjeet, F.; Naz, S.; Kumar, V.; Aung, N.H.; Bansari, K.; Irfan, S.; Rizwan, A. Psychological Stress as a Risk Factor for Cardiovascular Disease: A Case-Control Study. Cureus 2020, 12, e10757. [Google Scholar] [CrossRef]
- Kumar, R.; Rizvi, M.R.; Saraswat, S. Obesity and Stress: A Contingent Paralysis. Int J Prev Med 2022, 13, 95. [Google Scholar] [CrossRef]
- Dakanalis, A.; Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Vasios, G.K.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. The Association of Emotional Eating with Overweight/Obesity, Depression, Anxiety/Stress, and Dietary Patterns: A Review of the Current Clinical Evidence. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P. Interoceptive signals from the heart and coronary circulation in health and disease. Auton Neurosci 2024, 253, 103180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Van Someren, E.J.W.; Xu, Z.; Ren, Z.; Tang, L.; Li, C.; Lei, X. Identifying the insomnia-related psychological issues associated with hyperarousal: A network perspective. International Journal of Psychophysiology 2024, 195, 112276. [Google Scholar] [CrossRef] [PubMed]
- Touch the Sound. Available online: https://vibroacoustics.dk/ (accessed on 1st May).
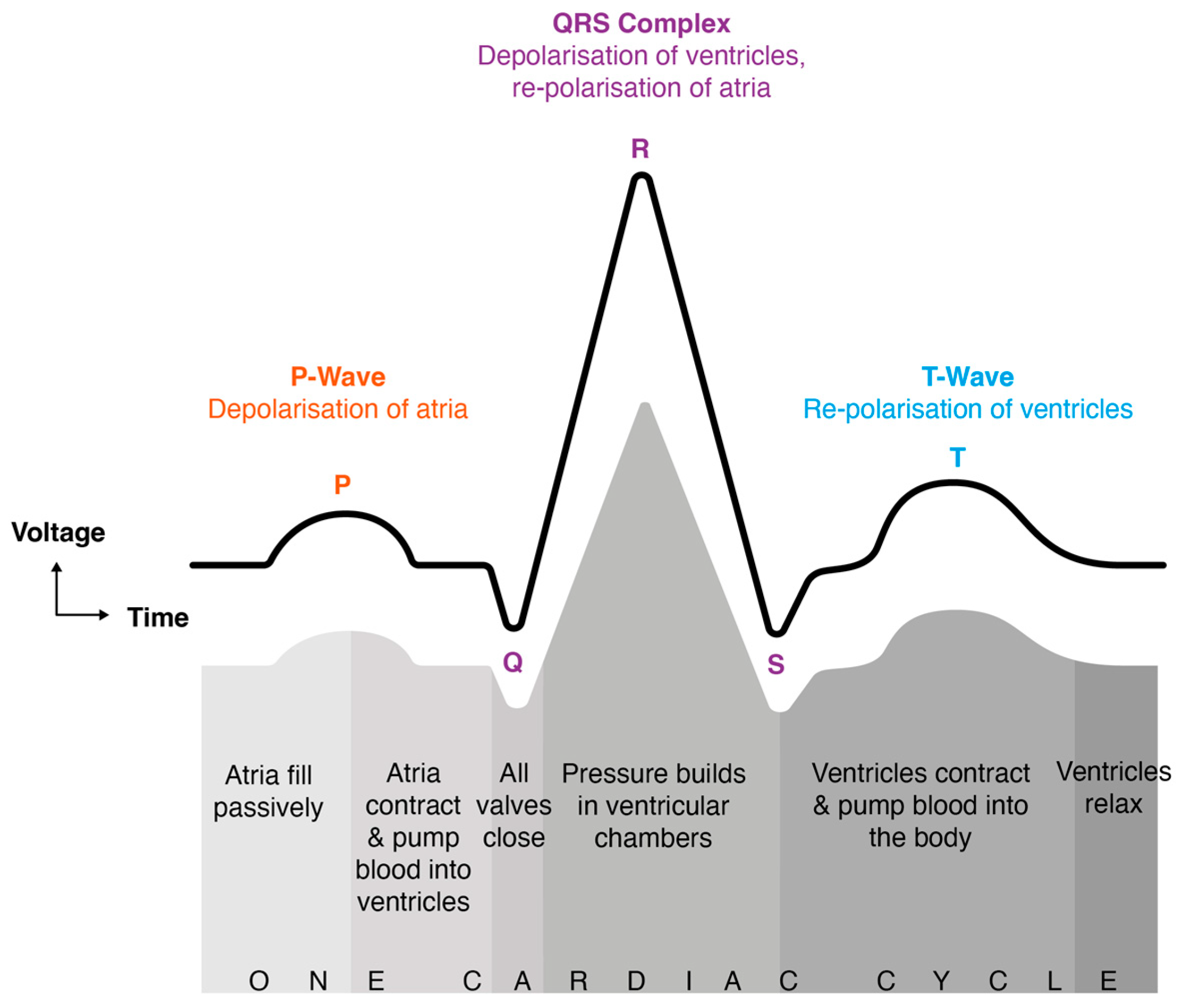
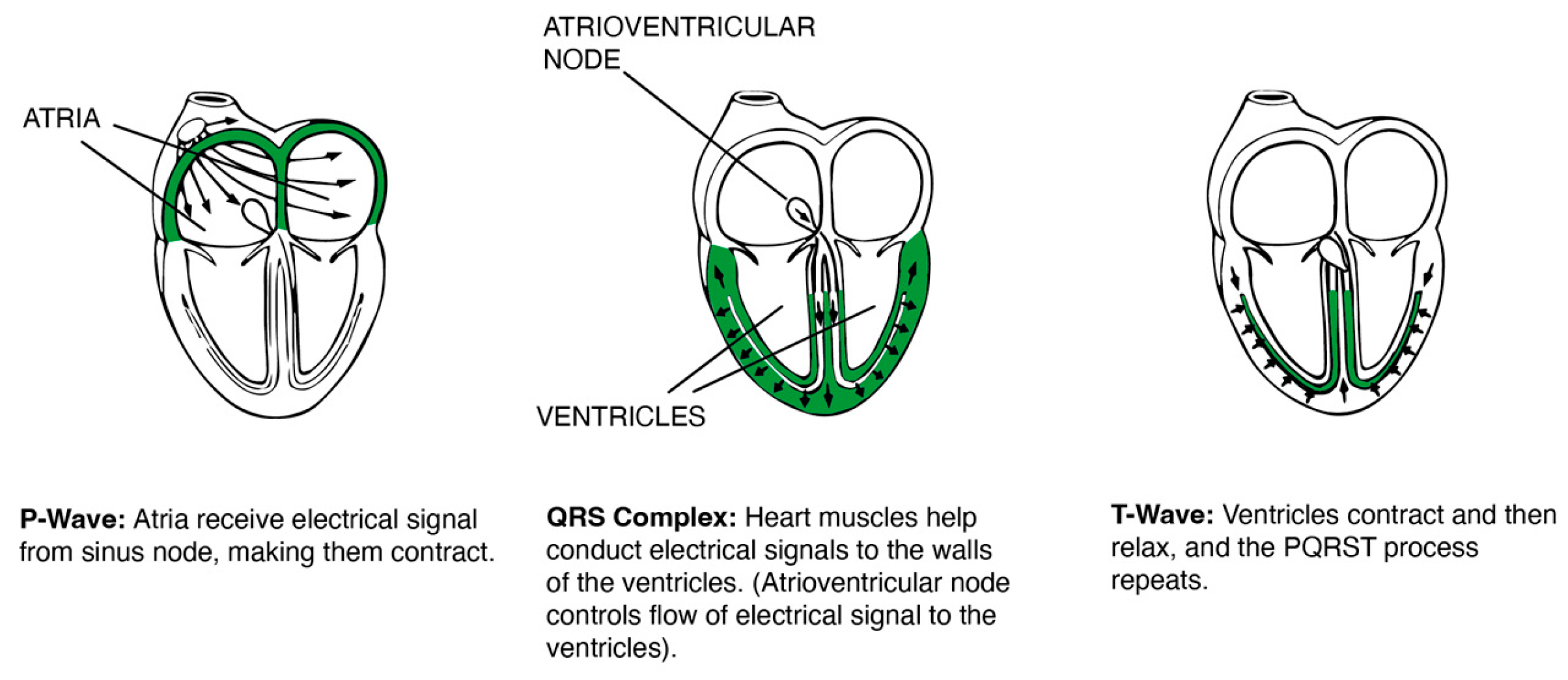
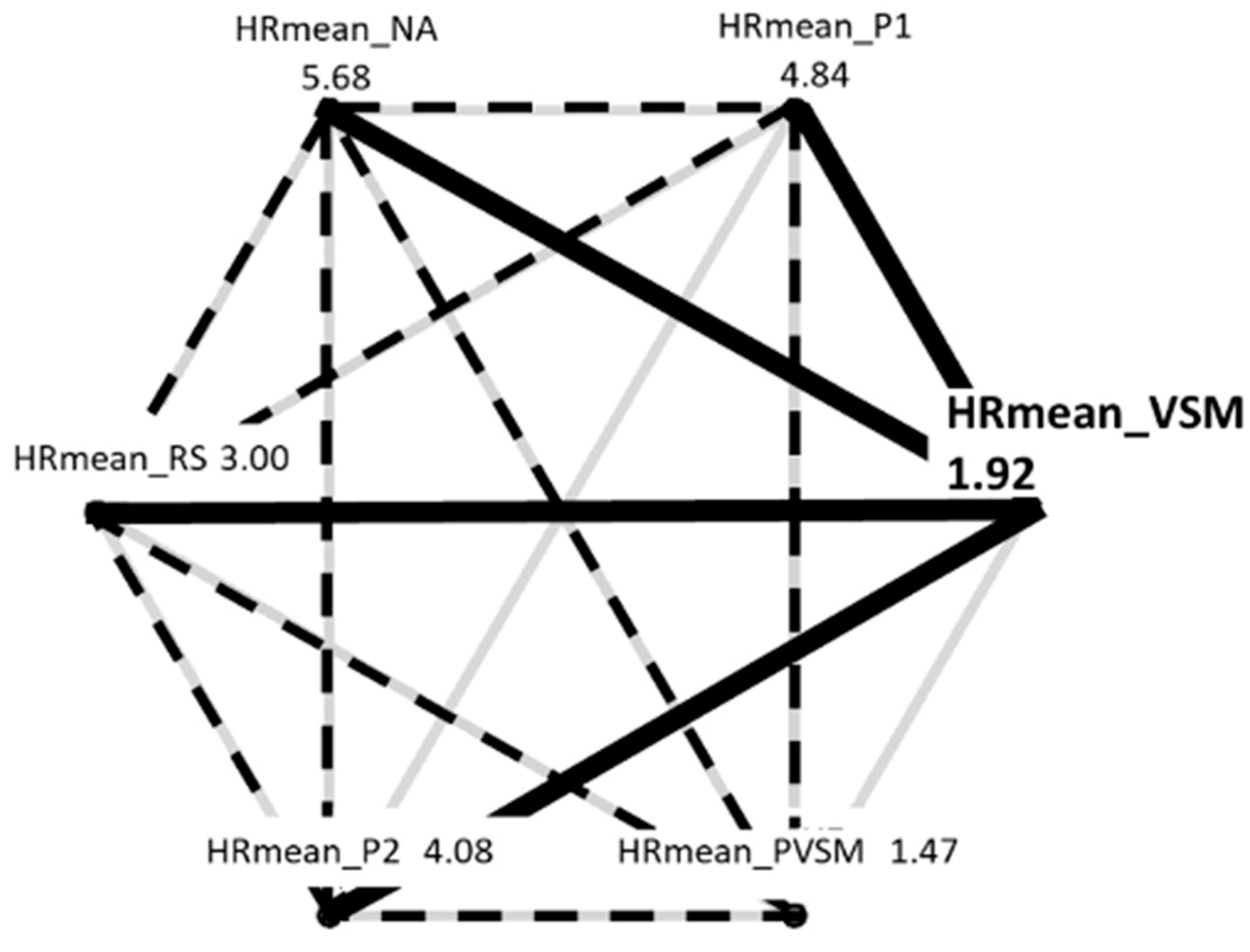
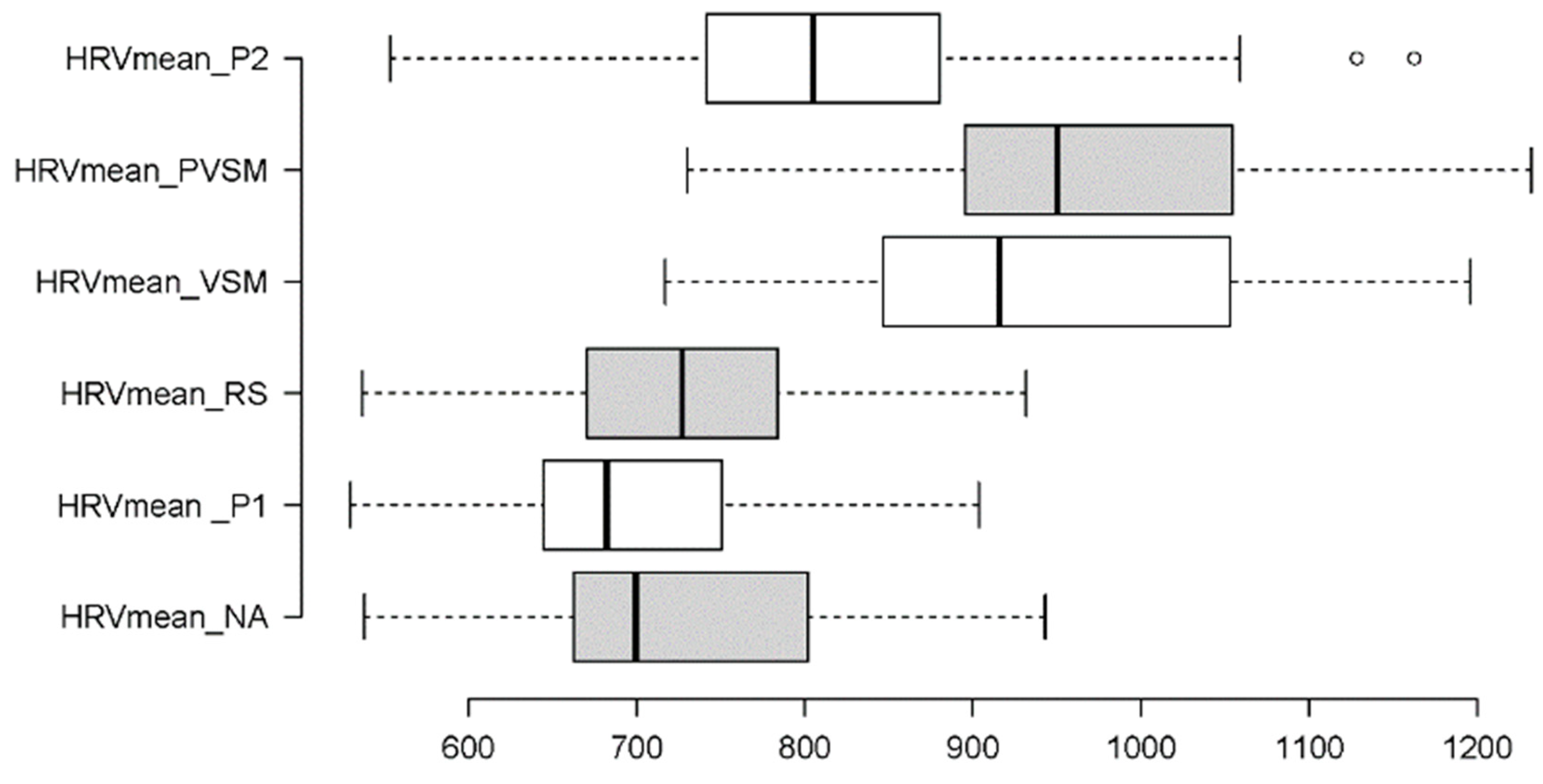
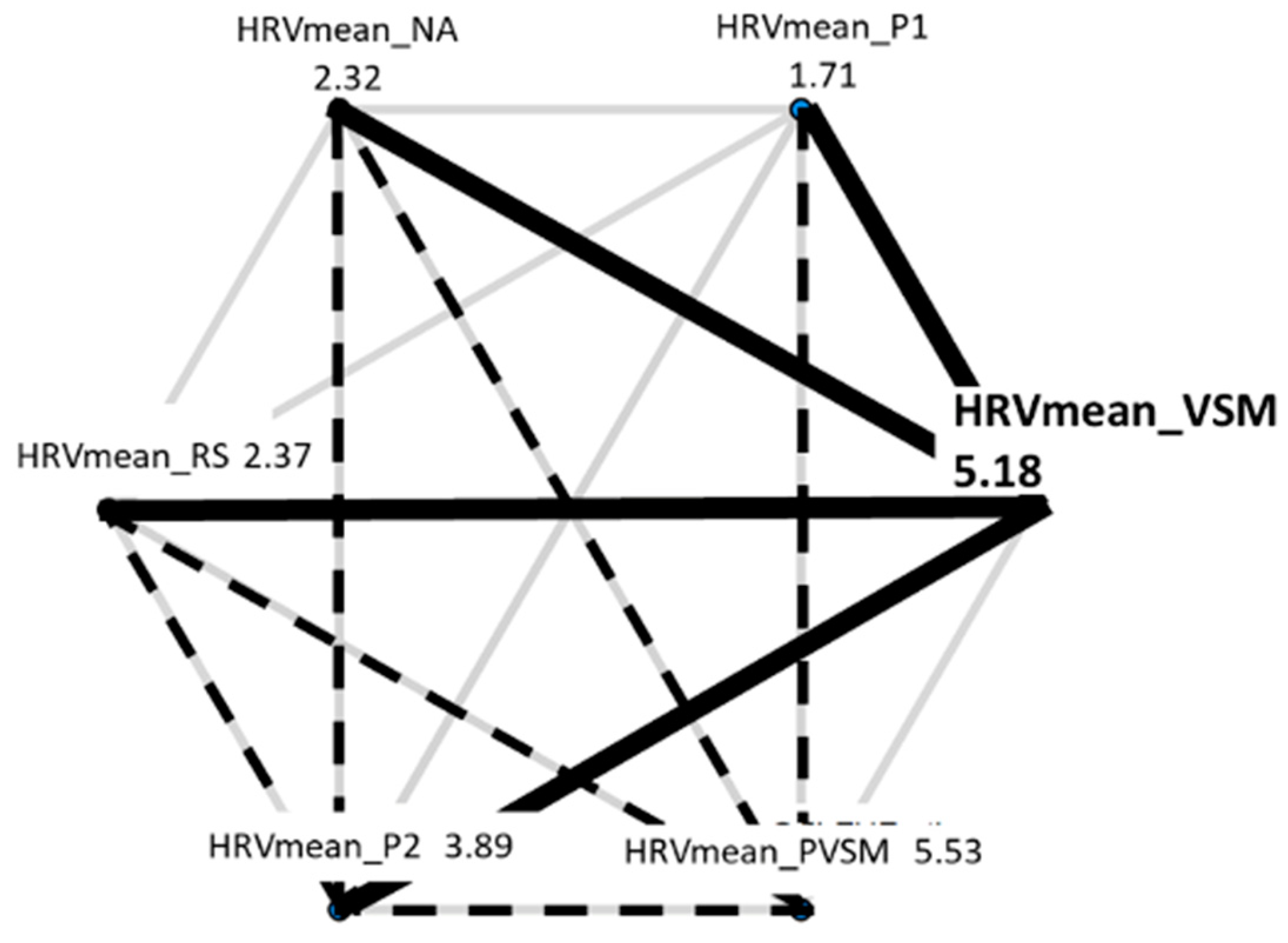
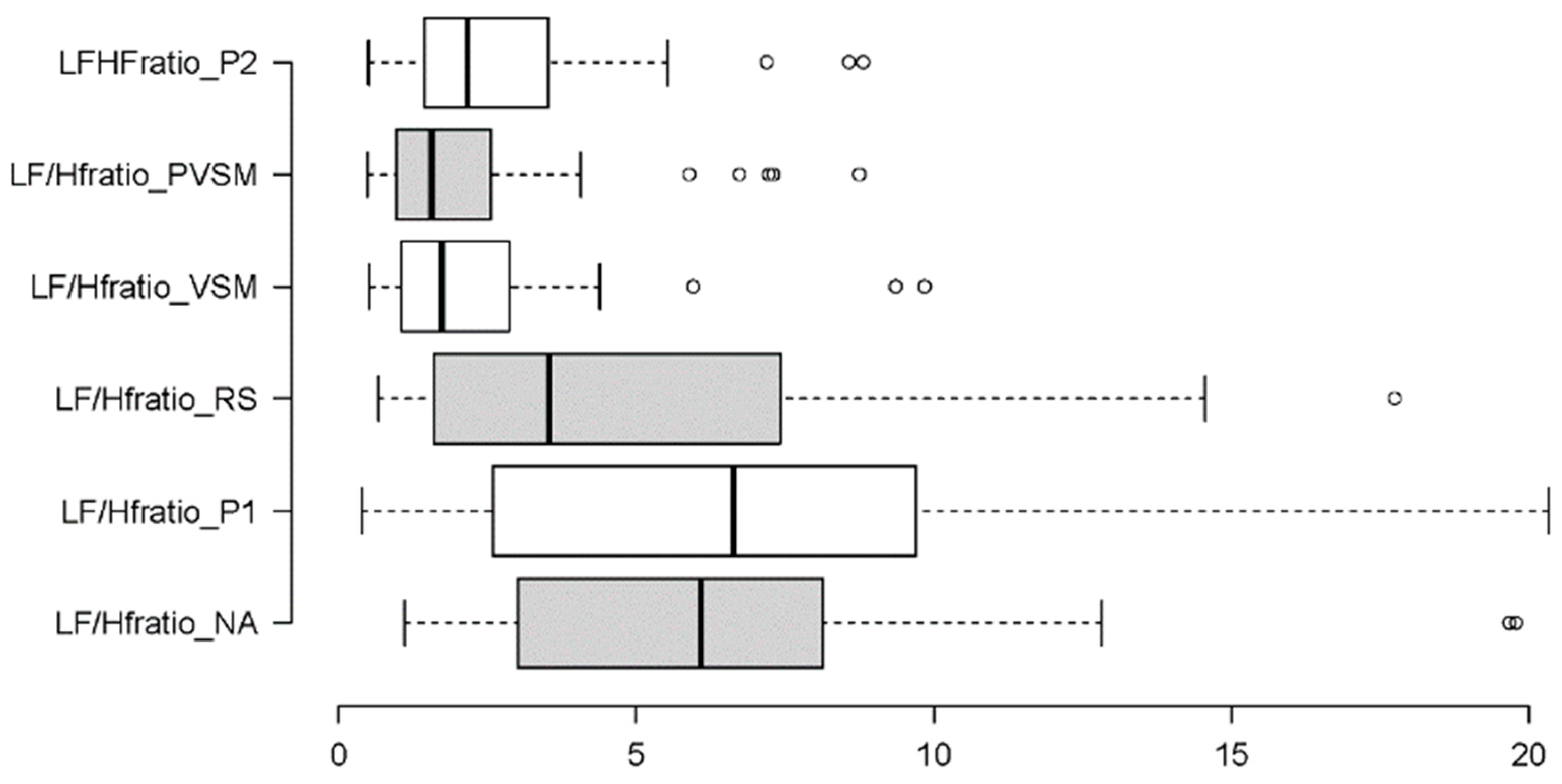
| Stage | Duration (minutes) | Eyes | Posture | Activity | Purpose |
|---|---|---|---|---|---|
| Stage 1 Normal Activity (NA) |
5 | Open | Sat, chair. | Study introduction. | Neutralise elevated heart rate. |
| Stage 2 Prosody 1 (P1) |
5 | Open | Sat, VSM module. | Reading speech passage. | Speech prosody analysis. (Not discussed). |
| Stage 3 Rest-State (RS) |
2 | Closed | Supine position, VSM module. |
No activity required. |
Control for basal heart rate, physiological stress and cognitive stress. |
| Stage 4 VSM Stimulation (VSM) |
45 | Closed | Supine position, VSM module. |
Vibroacoustic Sound Massage and vibrotactile soundscape. |
Independent variable. |
| Stage 5 Post-VSM (PVSM) |
5 | Closed | Supine position, VSM module. |
No activity required. | Durational measure for VSM effects. |
|
Stage 6 Prosody 2 (P2) |
5 | Open | Sat, VSM module. | Reading speech passage. | Speech prosody analysis. (Not discussed). |
| Theta/Beta Ratio (TBR) - concentration and focus | Beta/Alpha Ratio (BAR) - arousal and relaxation | Frontal/Alpha Asymmetry (FAA) - well-being |
|---|---|---|
| “I craved rhythm, interesting how rhythm doesn’t come through aurally, but physically through vibrations, body craves it, then gets it” | “Not awake but not sleeping, in-between” | “Cathartic” |
| “Could really match my breath work” | “Hyperactive in my head, rested in my body” | “Really relaxing” |
| “Not thinking about anything, zone into how much my bodies feeling” | “Disconnected from my body” | “Extremely empowering” |
| “Zoning into sounds themselves” | ”Felt like flying translated into your body” | “Very pleasant” |
| “Sometimes my thoughts were somewhere else, going in and out of zones” | “After the vibrations it feels numb in a good way, you can’t feel the borders of your body” | “Felt familiar, reminded me of home” |
| “Mind wondering but still aware of a lot of things” | ” It felt liberating” | “I feel super calm and open” |
| “Pulled down again by thought” | ”Complete serenity” | “Clear mind-body connection” |
| “Distracted me from (ADHD) medication, thinking ‘what is happening with my body?’” | “Body felt super relaxed and calm” | “100% enjoyable” |
| “There were phases when I was really relaxed, couldn’t think about anything, then all of a sudden really aware” | “Feeling of wanting to transcend, feeling my whole - a unity between my mind and body” | “My tension and stress feels released” |
| “Once in a while came back to focusing on what’s going on, the music, vibrations” | “Takes away the racing mind” | “Sounds were very calming, especially the birds” |
| “Totally immersive” | “Feel like you’re floating” | “Nice waves sounds, all ambient relaxing sounds” |
| “Felt super creative” | “No idea what kind of state I was in” | “Felt like a weighted blanket - safe and comfortable” |
| “Saw shapes, forms and colours that followed the vibrations in music” | “Vibrations going through the body” | “The vibration resonated with my body deeply” |
| “I saw colours that made sense with the sounds” | “The stronger the vibration the more relaxed I was, I couldn’t think” | “Feel physically something in my body taken out of me - I feel very light” |
| “Don’t know where my head went, felt I was tripping” | “The more vibration, the less aware I was” | “A yummy space - feels like a good place - like you’re being mothered somehow” |
| “Felt like a psychedelic trip” | “Lost track of time” | “Felt like being in my mother’s womb - very safe” |
| “I felt medicated or drugged in some way” | “Seeing the sound waves going through my body in dancing waves” | “Delightful - reminded me of being a child” |
| “Blurred lines between otherness and myself” | ||
| “Felt I was letting go of thoughts, allowing the experience and sound to come over me lead to deep relaxation” | ||
| “Stimulated - really relaxing but intense at the same time” |
| Main effect of |
Test statistics |
p | Effect size | Sig. multiple comp. tests |
Test statistics |
p | Effect size |
|---|---|---|---|---|---|---|---|
| SDNN | F[5,160] = 7.267 | < 0.001 | ηp² = 0.164 | VSM vs. NA | t[37] = 2.062 | 0.046 | d = 36.78 |
| VSM vs. P1 | t[37] = 2.075 | 0.046 | d = 42.04 | ||||
| VSM vs. P2 | t[37] = -2.266 | 0.030 | d = 51.84 | ||||
| NA vs. PVSM | t[37] = 3.746 | 0.001 | d = 8.26 | ||||
| NA vs. P2 | t[37] = 3.463 | 0.001 | d = 7.81 | ||||
| P1 vs. RS | t[37] = 1.987 | 0.050 | d = 7.50 | ||||
| P1 vs. PVSM | t[37] = 4.459 | < 0.001 | d = 7.09 | ||||
| P1 vs. P2 | t[37] = 3.855 | < 0.001 | d = 7.18 | ||||
| RS vs. P2 | t[37] = 2.777 | 0.009 | d = 4.61 | ||||
| LF | χ²[5] = 21.038 | < 0.001 | W = 0.11 | VSM vs. NA | W = 4.108 | < 0.001 | r = 0.67 |
| VSM vs. P1 | W = 2.269 | 0.023 | r = 0.39 | ||||
| VSM vs. RS | W = 2.453 | 0.014 | r = 0.40 | ||||
| VSM vs. P2 | W = -3.004 | 0.003 | r = 0.49 | ||||
| PVSM vs. NA | W = 3.066 | 0.002 | r = 0.50 | ||||
| PVSM vs. P2 | W = -1.962 | 0.050 | r = 0.32 | ||||
| Main effect of | Test statistics |
p | Effect size | Sig. multiple comp. tests |
Test statistics |
p | Effect size |
|---|---|---|---|---|---|---|---|
| RMSSD | χ²[5] = 44.180 | < 0.001 | W = 0.24 | VSM vs. NA | W = 3.250 | < 0.001 | r = 0.53 |
| VSM vs. P1 | W = 3.740 | 0.023 | r = 0.61 | ||||
| VSM vs. RS | W = 2.453 | 0.014 | r = 0.40 | ||||
| NA vs. PVSM | W = 4.108 | < 0.001 | r = 0.67 | ||||
| NA vs. P2 | W = 4.599 | < 0.001 | r = 0.67 | ||||
| P1 vs. PVSM | W = 4.599 | < 0.001 | r = 0.75 | ||||
| P1 vs. P2 | W = 4.108 | < 0.001 | r = 0.75 | ||||
| RS vs. PVSM | W = -3.311 | < 0.001 | r = 0.54 | ||||
| RS vs. P2 | W = -3.311 | < 0.001 | r = 0.54 | ||||
| HF | χ²[5] = 37.263 | < 0.001 | W = 0.20 | VSM vs. P1 | W = 3.740 | < 0.001 | r = 0.61 |
| VSM vs. RS | W = 2.207 | 0.027 | r = 0.36 | ||||
| NA vs. PVSM | W = -2.942 | 0.003 | r = 0.48 | ||||
| NA vs. P2 | W = -2.820 | 0.005 | r = 0.46 | ||||
| P1 vs. PVSM | W = -4.660 | < 0.001 | r = 0.76 | ||||
| P1 vs. P2 | W = -4.782 | < 0.001 | r = 0.78 | ||||
| RS vs. PVSM | W = -3.250 | 0.002 | r = 0.53 | ||||
| RS vs. P2 | W = -3.127 | 0.001 | r = 0.51 | ||||
| Measure | TBR | BAR | FAA | HR mean | HRV mean | LF/HF ratio | SDNN | LF | RMSSD | HF |
|---|---|---|---|---|---|---|---|---|---|---|
| Rho [36] | 0.02 | 0.13 | -0.19 | 0.07 | -0.04 | -0.07 | -0.34* | -0.32* | -0.17 | -0.20 |
| p | 0.89 | 0.44 | 0.24 | 0.65 | 0.79 | 0.66 | 0.03 | 0.05 | 0.31 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





