Submitted:
28 May 2024
Posted:
28 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Search Strategy
2.4. Study Selection
2.5. Quality Assessment
2.6. Data Extraction
3. Finalization of References and Study Characteristics
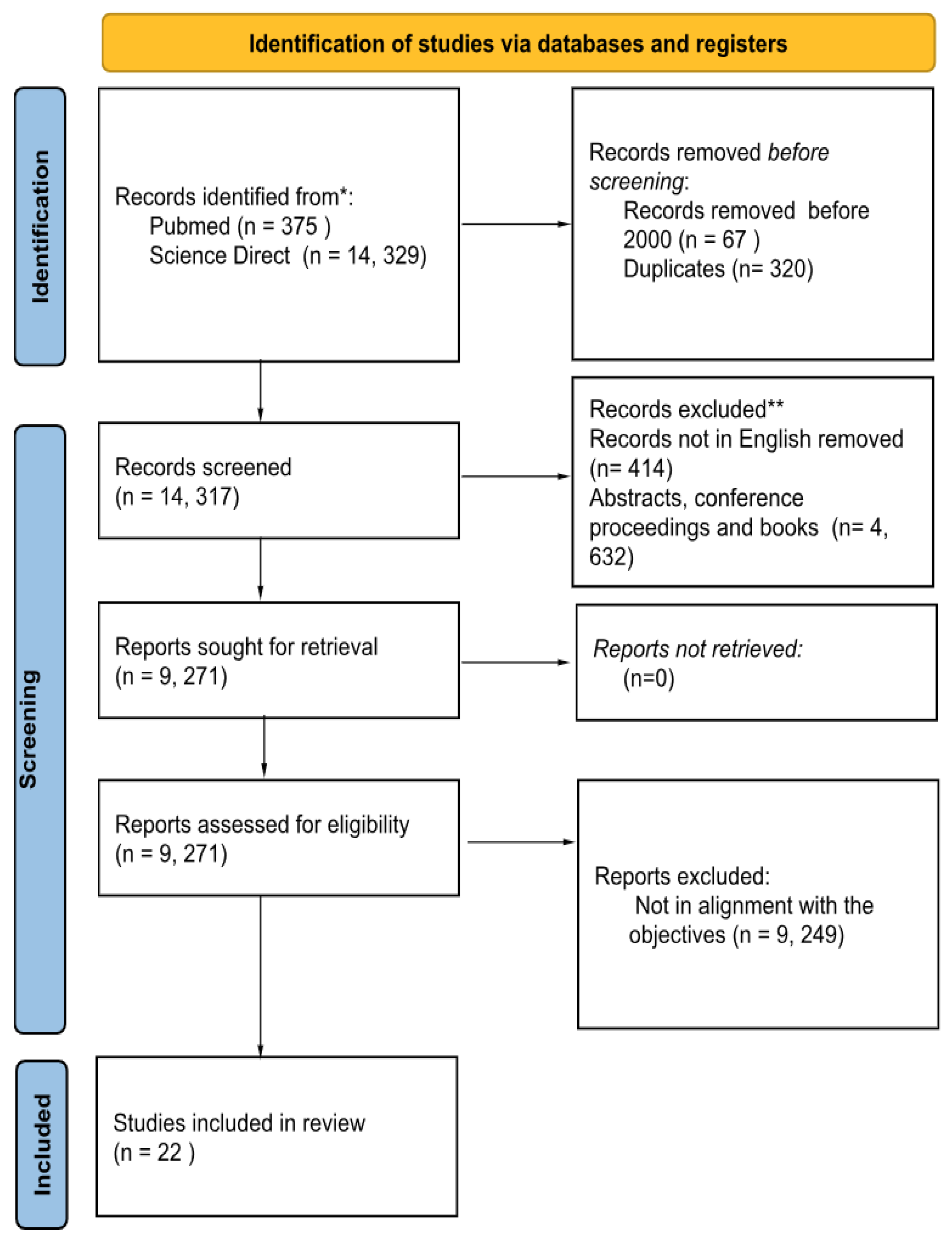
3.1. PRISMA Sheet and the Summary of Final Studies that Have Been Used for the Review
4. Results
4.1. Study Characteristic
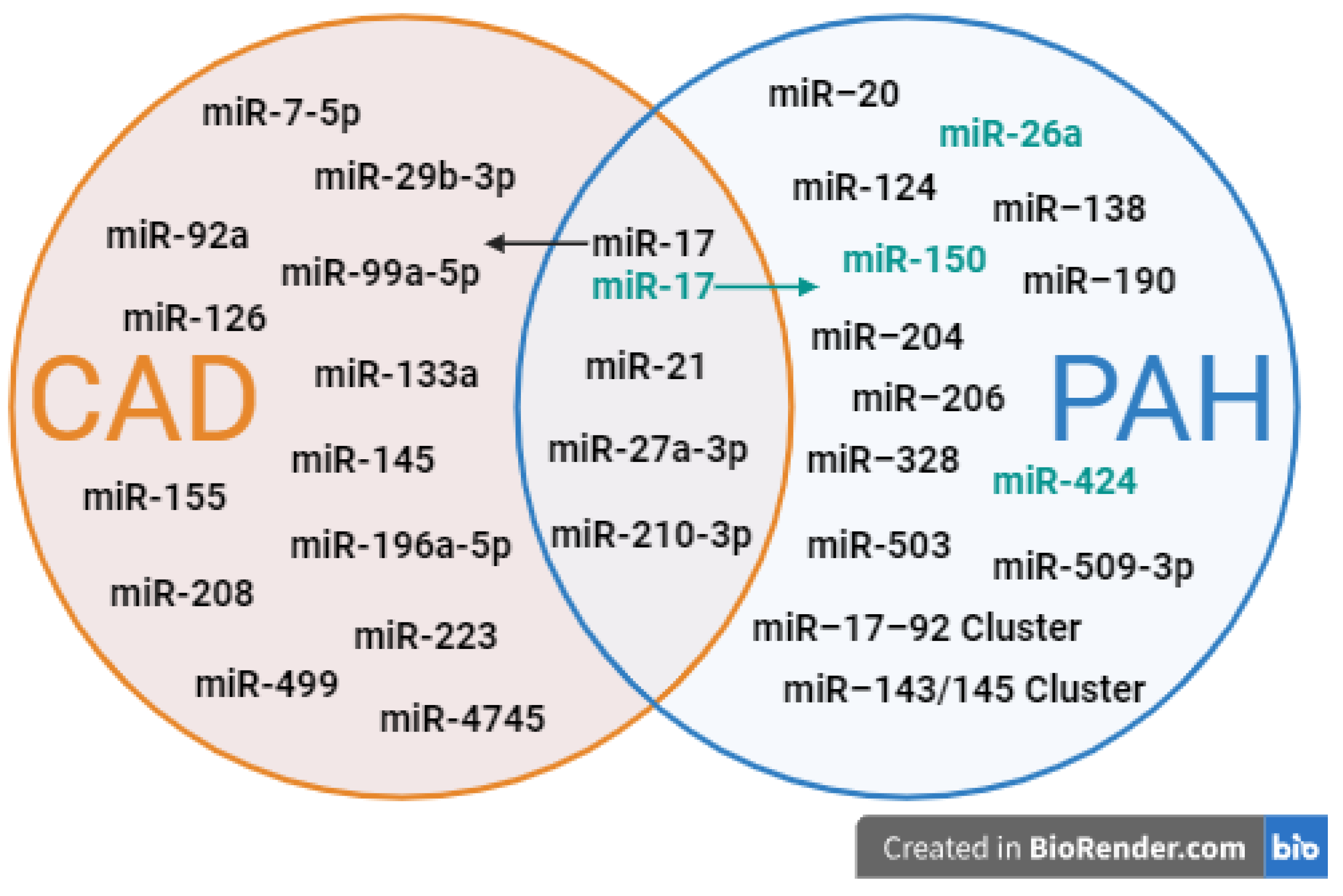
4.2. MiRNA Profiles in Cardiovascular Diseases
4.2.1. miRNA Profiles in CHD
4.2.2. miRNA Profiles in CAD
4.3. Role of miRNAs in Vascular Remodeling and Disease Pathogenesis
4.4. Diagnostic Potential of miRNAs in Cardiovascular Diseases
4.4.1. CAD
4.4.2. PAH
4.4.3. Other Cardiovascular Disease
5. Discussion
6. Limitations
7. Conclusions
References
- Wojciechowska, A.; Osiak, A.; Kozar-Kamińska, K. MicroRNA in Cardiovascular Biology and Disease. Adv Clin Exp Med 2017, 26, 868–874. [CrossRef]
- Duggal, B.; K. Gupta, M.; V. Naga Prasad, S. Potential Role of microRNAs in Cardiovascular Disease: Are They up to Their Hype? Current Cardiology Reviews 2016, 12, 304–310.
- Joladarashi, D.; Thandavarayan, R.A.; Babu, S.S.; Krishnamurthy, P. Small Engine, Big Power: MicroRNAs as Regulators of Cardiac Diseases and Regeneration. International Journal of Molecular Sciences 2014, 15, 15891–15911. [CrossRef]
- Dlouhá, D.; Hubáček, J.A. Regulatory RNAs and Cardiovascular Disease – With a Special Focus on Circulating MicroRNAs. Physiol Res 2017, S21–S38. [CrossRef]
- Hkiri, S.; Mekni-Toujani, M.; Üstün, E.; Hosni, K.; Ghram, A.; Touil, S.; Samarat, A.; Sémeril, D. Synthesis of Novel 1,3,4-Oxadiazole-Derived α-Aminophosphonates/α-Aminophosphonic Acids and Evaluation of Their In Vitro Antiviral Activity against the Avian Coronavirus Infectious Bronchitis Virus. Pharmaceutics 2022, 15, 114. [CrossRef]
- Li, H.; Gao, F.; Wang, X.; Wu, J.; Lu, K.; Liu, M.; Li, R.; Ding, L.; Wang, R. Circulating microRNA-378 Levels Serve as a Novel Biomarker for Assessing the Severity of Coronary Stenosis in Patients with Coronary Artery Disease. Bioscience Reports 2019, 39, BSR20182016. [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [CrossRef]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) Version 2018 for Information Professionals and Researchers. Education for Information 2018, 34, 285–291. [CrossRef]
- Shi, L.; Liao, J.; Liu, B.; Zeng, F.; Zhang, L. Mechanisms and Therapeutic Potential of microRNAs in Hypertension. Drug Discovery Today 2015, 20, 1188–1204. [CrossRef]
- Santulli, G. MicroRNAs and Endothelial (Dys) Function: MICRORNAS AND ENDOTHELIAL (Dys) FUNCTION. J. Cell. Physiol. 2016, 231, 1638–1644. [CrossRef]
- Welten, S.M.J.; Goossens, E.A.C.; Quax, P.H.A.; Nossent, A.Y. The Multifactorial Nature of microRNAs in Vascular Remodelling. Cardiovasc Res 2016, 110, 6–22. [CrossRef]
- Fang, Y.; Yeh, C. Role of microRNAs in Vascular Remodeling. CMM 2015, 15, 684–696. [CrossRef]
- Henning, R.J. Cardiovascular Exosomes and MicroRNAs in Cardiovascular Physiology and Pathophysiology. J. of Cardiovasc. Trans. Res. 2021, 14, 195–212. [CrossRef]
- Ali, S.S.; Kala, C.; Abid, M.; Ahmad, N.; Sharma, U.S.; Khan, N.A. Pathological microRNAs in Acute Cardiovascular Diseases and microRNA Therapeutics. Journal of Acute Disease 2016, 5, 9–15. [CrossRef]
- Jones Buie, J.N.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Fan, H. The Role of miRNAs in Cardiovascular Disease Risk Factors. Atherosclerosis 2016, 254, 271–281. [CrossRef]
- Sayed, A.S.M.; Xia, K.; Salma, U.; Yang, T.; Peng, J. Diagnosis, Prognosis and Therapeutic Role of Circulating miRNAs in Cardiovascular Diseases. Heart, Lung and Circulation 2014, 23, 503–510. [CrossRef]
- Udali, S.; Guarini, P.; Moruzzi, S.; Choi, S.-W.; Friso, S. Cardiovascular Epigenetics: From DNA Methylation to microRNAs. Molecular Aspects of Medicine 2013, 34, 883–901. [CrossRef]
- Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Oikonomou, E.; Tsigkou, V.; Paschou, S.A.; Vlasis, K.; Marinos, G.; Vavuranakis, M.; Stefanadis, C.; et al. MicroRNAs in Cardiovascular Disease. Hellenic Journal of Cardiology 2020, 61, 165–173. [CrossRef]
- Johnson, J.L. Elucidating the Contributory Role of microRNA to Cardiovascular Diseases (a Review). Vascular Pharmacology 2019, 114, 31–48. [CrossRef]
- Bienertova-Vasku, J.; Novak, J.; Vasku, A. MicroRNAs in Pulmonary Arterial Hypertension: Pathogenesis, Diagnosis and Treatment. Journal of the American Society of Hypertension 2015, 9, 221–234. [CrossRef]
- Patterson, A.J.; Song, M.A.; Choe, D.; Xiao, D.; Foster, G.; Zhang, L. Early Detection of Coronary Artery Disease by Micro-RNA Analysis in Asymptomatic Patients Stratified by Coronary CT Angiography. Diagnostics 2020, 10, 875. [CrossRef]
- Kanuri, S.H.; Ipe, J.; Kassab, K.; Gao, H.; Liu, Y.; Skaar, T.C.; Kreutz, R.P. Next Generation MicroRNA Sequencing to Identify Coronary Artery Disease Patients at Risk of Recurrent Myocardial Infarction. Atherosclerosis 2018, 278, 232–239. [CrossRef]
- Zhu, L.; Chen, T.; Ye, W.; Wang, J.-Y.; Zhou, J.-P.; Li, Z.-Y.; Li, C.-C. Circulating miR-182-5p and miR-5187-5p as Biomarkers for the Diagnosis of Unprotected Left Main Coronary Artery Disease. J. Thorac. Dis. 2019, 11, 1799–1808. [CrossRef]
- McCaffrey, T.A.; Toma, I.; Yang, Z.; Katz, R.; Reiner, J.; Mazhari, R.; Shah, P.; Falk, Z.; Wargowsky, R.; Goldman, J.; et al. RNAseq Profiling of Blood from Patients with Coronary Artery Disease: Signature of a T Cell Imbalance. Journal of Molecular and Cellular Cardiology Plus 2023, 4, 100033. [CrossRef]
- Yao, Y. Combination of PBMC miR-19b-5p, miR-221, miR-25-5p and Hypertension Correlates with Increased Risk of Heart Failure in Coronary Heart Disease Patients. Anatol J Cardiol 2018. [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Dávalos, A.; Gil-Zamorano, J.; Gonzálvez, M.; García-Almagro, F.J.; Ruiz Ros, J.A.; Tomás-Barberán, F.A.; Espín, J.C.; et al. One-Year Supplementation with a Grape Extract Containing Resveratrol Modulates Inflammatory-Related microRNAs and Cytokines Expression in Peripheral Blood Mononuclear Cells of Type 2 Diabetes and Hypertensive Patients with Coronary Artery Disease. Pharmacological Research 2013, 72, 69–82. [CrossRef]
- Li, Y.; Ren, W.; Wang, X.; Yu, X.; Cui, L.; Li, X.; Zhang, X.; Shi, B. MicroRNA-150 Relieves Vascular Remodeling and Fibrosis in Hypoxia-Induced Pulmonary Hypertension. Biomedicine & Pharmacotherapy 2019, 109, 1740–1749. [CrossRef]
- Witarto, B.S.; Visuddho, V.; Aldian, F.M.; Atmaja, M.S.S.; Ariyanto, M.V.; Witarto, A.P.; Wungu, C.D.K.; Susilo, H.; Alsagaff, M.Y.; Rohman, M.S. Blood-Based Circulating microRNAs as Diagnostic Biomarkers for Subclinical Carotid Atherosclerosis: A Systematic Review and Meta-Analysis with Bioinformatics Analysis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2023, 17, 102860. [CrossRef]
- Tang, P. Clinical Diagnostic Value of Circulating Serum miR-509-3p in Pulmonary Arterial Hypertension with Congenital Heart Disease. Hellenic Journal of Cardiology 2020, 61, 26–30. [CrossRef]
- Agiannitopoulos, K.; Samara, P.; Papadopoulou, M.; Efthymiadou, A.; Papadopoulou, E.; Tsaousis, G.N.; Mertzanos, G.; Babalis, D.; Lamnissou, K. miRNA Polymorphisms and Risk of Premature Coronary Artery Disease. Hellenic Journal of Cardiology 2021, 62, 278–284. [CrossRef]
- Small, E.M.; Olson, E.N. Pervasive Roles of microRNAs in Cardiovascular Biology. Nature 2011, 469, 336–342. [CrossRef]
- Cheng, Y.; Ji, R.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNAs Are Aberrantly Expressed in Hypertrophic Heart: Do They Play a Role in Cardiac Hypertrophy? The American Journal of Pathology 2007, 170, 1831–1840. [CrossRef]
- Lakkisto, P.; Dalgaard, L.T.; Belmonte, T.; Pinto-Sietsma, S.-J.; Devaux, Y.; de Gonzalo-Calvo, D. Development of Circulating microRNA-Based Biomarkers for Medical Decision-Making: A Friendly Reminder of What Should NOT Be Done. Critical Reviews in Clinical Laboratory Sciences 2023, 60, 141–152. [CrossRef]
- de Gonzalo-Calvo, D.; Pérez-Boza, J.; Curado, J.; Devaux, Y. Challenges of microRNA-based Biomarkers in Clinical Application for Cardiovascular Diseases. Clin Transl Med 2022, 12, e585. [CrossRef]
- Chen, K.-Y.; Liu, Z.; Lu, J.-H.; Yang, S.-Y.; Hu, X.-Y.; Liang, G.-Y. The Function of Circular RNAs in Myocardial Ischemia–Reperfusion Injury: Underlying Mechanisms and Therapeutic Advancement. Cardiovasc Drugs Ther 2024. [CrossRef]
- Zhang, X.; Schulze, P.C. MicroRNAs in Heart Failure: Non-Coding Regulators of Metabolic Function. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2016, 1862, 2276–2287. [CrossRef]
- Sugatani, T.; Hruska, K.A. MicroRNA-223 Is a Key Factor in Osteoclast Differentiation. J of Cellular Biochemistry 2007, 101, 996–999. [CrossRef]
- Pan, Y.; Liang, H.; Liu, H.; Li, D.; Chen, X.; Li, L.; Zhang, C.-Y.; Zen, K. Platelet-Secreted MicroRNA-223 Promotes Endothelial Cell Apoptosis Induced by Advanced Glycation End Products via Targeting the Insulin-like Growth Factor 1 Receptor. The Journal of Immunology 2014, 192, 437–446. [CrossRef]
- Jin, W.; Wang, L.; Cheng, S.; Lv, H. Prognostic Value of microRNA-378 in Esophageal Cancer and Its Regulatory Effect on Tumor Progression. Exp Ther Med 2021, 22, 704. [CrossRef]
- Wang, J.; Li, Y.; Ma, Q.; Huang, J. miR-378 in Combination with Ultrasonic Irradiation and SonoVue Microbubbles Transfection Inhibits Hepatoma Cell Growth. Mol Med Report 2020. [CrossRef]
- Schauer, S.N.; Sontakke, S.D.; Watson, E.D.; Esteves, C.L.; Donadeu, F.X. Involvement of miRNAs in Equine Follicle Development. REPRODUCTION 2013, 146, 273–282. [CrossRef]
- Sun, C.; Liu, W.; Lu, Z.; Li, Y.; Liu, S.; Tang, Z.; Yan, Y.; Li, Z.; Feng, H.; Zhang, D.; et al. Hepatic miR-378 Modulates Serum Cholesterol Levels by Regulating Hepatic Bile Acid Synthesis. Theranostics 2021, 11, 4363–4380. [CrossRef]
- Li, J.; Chen, M.; Wang, J.; Lu, L.; Li, X.; Le, Y. MicroRNA Profiling in Chinese Children with Henoch-Schonlein Purpura and Association between Selected microRNAs and Inflammatory Biomarkers. Acta Paediatrica 2021, 110, 2221–2229. [CrossRef]
- Zhang, Y.; Zhao, Y.; Liu, L.; Su, H.; Dong, D.; Wang, J.; Zhang, Y.; Chen, Q.; Li, C. MicroRNA-19b Promotes Nasopharyngeal Carcinoma More Sensitive to Cisplatin by Suppressing KRAS. Technol Cancer Res Treat 2018, 17, 153303381879365. [CrossRef]
- Ma, Y.; Ma, M.; Sun, J.; Li, W.; Li, Y.; Guo, X.; Zhang, H. CHIR-99021 Regulates Mitochondrial Remodelling via β-Catenin Signalling and miRNAs during Endodermal Differentiation. Journal of Cell Science 2019, jcs.229948. [CrossRef]
- Morris, V.A.; Zhang, A.; Yang, T.; Stirewalt, D.L.; Ramamurthy, R.; Meshinchi, S.; Oehler, V.G. MicroRNA-150 Expression Induces Myeloid Differentiation of Human Acute Leukemia Cells and Normal Hematopoietic Progenitors. PLoS ONE 2013, 8, e75815. [CrossRef]
- Ling, Z.; Fan, G.; Yao, D.; Zhao, J.; Zhou, Y.; Feng, J.; Zhou, G.; Chen, Y. MicroRNA-150 Functions as a Tumor Suppressor and Sensitizes Osteosarcoma to Doxorubicin-induced Apoptosis by Targeting RUNX2. Exp Ther Med 2019. [CrossRef]
- Zhang, T. microRNA-150 Inhibits Human CD133-Positive Liver Cancer Stem Cells through Negative Regulation of the Transcription Factor c-Myb. Int J Oncol 2011. [CrossRef]
- Bai, D.; Sun, H.; Wang, X.; Lou, H.; Zhang, J.; Wang, X.; Jiang, L. MiR-150 Inhibits Cell Growth In Vitro and In Vivo by Restraining the RAB11A/WNT/β-Catenin Pathway in Thyroid Cancer. Med Sci Monit 2017, 23, 4885–4894. [CrossRef]
- Wang, F.; Ren, X.; Zhang, X. Role of microRNA-150 in Solid Tumors. Oncology Letters 2015, 10, 11–16. [CrossRef]
- Wang, F.; Wu, D.; Xu, Z.; Chen, J.; Zhang, J.; Li, X.; Chen, S.; He, F.; Xu, J.; Su, L.; et al. miR-182-5p Affects Human Bladder Cancer Cell Proliferation, Migration and Invasion through Regulating Cofilin 1. Cancer Cell Int 2019, 19, 42. [CrossRef]
- Zhang, Z.; Wang, C.; Liu, T.; Tang, Z.; Yan, R.; Zhang, C.; Cheng, C.; Wang, J.; Wang, H.; Huang, H.; et al. miRNA-182-5p Promotes Human Bladder Cancer Proliferation and Migration through the FOXF2/SHH Axis. neo 2022, 69, 321–330. [CrossRef]
- Lee, H.J.; Lee, E.J.; Seo, M. Galpha12 Protects Vascular Endothelial Cells from Serum Withdrawal-Induced Apoptosis through Regulation of miR-155. Yonsei Med J 2016, 57, 247. [CrossRef]
- Muhammad, F.; Trivett, A.; Wang, D.; Lee, D.J. Tissue-specific Production of MicroRNA-155 Inhibits Melanocortin 5 Receptor-dependent Suppressor Macrophages to Promote Experimental Autoimmune Uveitis. Eur J Immunol 2019, 49, 2074–2082. [CrossRef]
- Shen, S.-M.; Jiang, H.; Zhao, J.-N.; Shi, Y. Down-Regulation of miR-155 Inhibits Inflammatory Response in Human Pulmonary Microvascular Endothelial Cells Infected with Influenza A Virus by Targeting Sphingosine-1-Phosphate Receptor 1. Chinese Medical Journal 2020, 133, 2429–2436. [CrossRef]
- Casciaro, M.; Di Salvo, E.; Brizzi, T.; Rodolico, C.; Gangemi, S. Involvement of miR-126 in Autoimmune Disorders. Clin Mol Allergy 2018, 16, 11. [CrossRef]
| Category of study designs | Methodological quality criteria | Responses | |||
| Yes | No | Can’t tell | Comments | ||
|
Screening questions (for all types) |
S1. Are there clear research questions? | ✔ | |||
| S2. Does the collected data allow me to address the research questions? | ✔ | ||||
| Further appraisal may not be feasible or appropriate when the answer is ‘No’ or ‘Can’t tell’ to one or both screening questions. | |||||
| 1. Qualitative | 1.1. Is the qualitative approach appropriate to answer the research question? | ✔ | Shi et al., 2015 [9]; Santulli et al., 2016 [10]; Welten et al., 2016 [11]; Fang and Yeh, 2015 [12]; Henning, 2021 [13]; Ali et al., 2016 [14]; Buie et al., 2016 [15]; Sayed et al., 2014 [16]; Udali et al., 2013 [17]; Siasos et al., 2020 [18]; Johnson, 2019 [19]; Bienertova-Vasku et al., 2015 [20] |
||
| 1.2. Are the qualitative data collection methods adequate to address the research question? | ✔ | ||||
| 1.3. Are the findings adequately derived from the data? | ✔ | ||||
| 1.4. Is the interpretation of results sufficiently substantiated by data? | ✔ | ||||
| 1.5. Is there coherence between qualitative data sources, collection, analysis and interpretation? | ✔ | ||||
| 2. Quantitative randomized controlled trials | 2.1. Is randomization appropriately performed? | ✔ | Patterson et al., 2020 [21]; Kanuri et al., 2018 [22]; Zhu et al., 2019 [23]; McCaffrey et al., 2023[24] |
||
| 2.2. Are the groups comparable at baseline? | ✔ | ||||
| 2.3. Are there complete outcome data? | ✔ | ||||
| 2.4. Are outcome assessors blinded to the intervention provided? | ✔ | ||||
| 2.5 Did the participants adhere to the assigned intervention? | ✔ | ||||
| 3. Quantitative non-randomized | 3.1. Are the participants representative of the target population? | ✔ | Yao et al., 2018 [25]; Tomé-Carneiro et al., 2013 [26]; Li et al., 2019 [27]; Witarto et al., 2023 [28]; Tang, 2020 [29]; Agiannitopoulos et al., 2021 [30] |
||
| 3.2. Are measurements appropriate regarding both the outcome and intervention (or exposure)? | ✔ | ||||
| 3.3. Are there complete outcome data? | ✔ | ||||
| 3.4. Are the confounders accounted for in the design and analysis? | ✔ | ||||
| 3.5. During the study period, is the intervention administered (or exposure occurred) as intended? | ✔ | ||||
| Nature of study | Total number of patients | Intervention | Conclusion | Reference |
|---|---|---|---|---|
| Observational Study | 98 | To compare miRNA profiles in PBMCs of CHD patients with and without HF and evaluate the significance of DEMs for HF risk in CHD patients. | CHD patients with and without HF could be differentiated according to PBMC miRNA profiles, and the combination of PBMC miR19b-5p, miR-221, miR-25-5p, and hypertension correlates with an increased HF risk in CHD patients. | Yao et al., 2018 [25] |
| Review Article | - | Summarize the current knowledge regarding the mechanisms of miRNAs in cardiovascular remodelling, focusing specifically on hypertension. |
The multiplexed detection of circulating miRNAs should be combined to compensate for the traits according to the study goal and specific miRNA features. |
Shi et al., 2015 [9] |
| Observational Study | 35 | Examine molecular changes in PBMCs after one-year daily intake of 8 mg RES-enriched grape extract (GE-RES) in hypertensive male T2DM patients. | Long-term grape extract with RES reduces pro-inflammatory cytokine expression and inflammation-related miRs in immune cells of T2DM hypertensive patients, supporting an immunomodulatory treatment effect. | Tomé-Carneiro et al., 2013 [26] |
| Pilot Study | 32 | assess miRNA expression profiles in patients with or without CAD as selected by coronary CT angiography (CTA) | miRNA expression patterns in whole blood as selected on the basis of coronary CTA and risk scores vary significantly depending on the subject phenotype. Thus, profiling miRNA may improve early detection of CAD. | Patterson et al., 2020 [21] |
| Cohort study | 437 | Next generation miRNA sequencing to identify CAD patients at risk of recurrent myocardial infarction | MiRNA next generation sequencing demonstrates altered fingerprint profile of whole blood miRNA expression among subjects with subsequent recurrent thrombotic events on standard medical therapy ('non-responders'), as compared to subjects with no recurrent cardiovascular events. | Kanuri et al., 2018 [22] |
| Cohort study | 65 | High-throughput sequencing (NGS) was initially employed to compare circulating miRNA expression profiles in uLMCAD patients to that in patients without CAD to identify candidate miRNA biomarkers. | circulating miR-182-5p and miR-5187-5p were suitable diagnostic biomarkers for uLMCAD, both potentially providing diagnostic information for discriminating uLMCAD patients from non-CAD population prior to invasive diagnostic coronary angiography (CAG). | Zhu et al., 2019 [23] |
| Review Article | - | the complex interactions linking miRs, expression of genes, and molecular pathways leading to endothelial dysfunction. | The potential therapeutic use of miRs is currently being explored through several approaches, including inhibition and over-expression, in many cardiovascular disorders. | Santulli et al., 2016 [10] |
| Review Article | - | discuss the multifactorial role of miRNAs and miRNA clusters that were reported to play a role in multiple forms of vascular remodelling and are clearly linked to cardiovascular disease. | Understanding the contribution of these miRNAs to the entire spectrum of vascular remodelling processes is important, especially as these miRNAs may have great potential as therapeutic targets for treatment of various cardiovascular diseases. |
Welten et al., 2016 [11] |
| Review Paper | - | the function and mechanisms of miRs, which are highly expressed in various cells types, especially endothelial and smooth muscle cells, which are closely involved in the process of vascular remodeling. |
miRs play crucial roles in vascular remodeling | Fang and Yeh, 2015 [12] |
| Review Paper | - | the role of exosomes and miRNAs in normal myocardium, myocardial injury and infarction, atherosclerosis, and the importance of circulating miRNAs as biomarkers of cardiac diseas | Increased understanding of exosome content and function will facilitate their development as novel therapeutic strategies for the treatment of patients with atherosclerotic cardiovascular diseases. | Henning, 2021 [13] |
| Investigational study | 24 | investigate the beneficial effect of miR-150 on PH in hypoxia-induced rats, PASMCs and PAECs. | miR-150 protects against hypoxia-induced pulmonary vascular remodeling, fibrosis, abnormal proliferation of PASMCs and PAECs, which suggests miR-150 as a promising therapeutic target for PH. | Li et al., 2019 [27] |
| Review Paper | - | Determining the number of miRNAs involved in pathophysiology of various cardiovascular diseases, and diagnostic and therapeutic potentials of miRNAs in these diseases. | extensive researches are required to explore the therapeutic and diagnostic values of miRNAs as successful as classical approaches. | Ali et al., 2016 [14] |
| Review Paper | - | examines the role of miRNAs as either biomarkers or potential contributors to the pathophysiology of these aforementioned risk factors. | Patients with CAD have differentially expressed miRNAs due to a variety of signaling pathways activated during disease processes. | Buie et al., 2016 [15] |
| Review Paper | - | examine more recent data regarding circulating miRNAs and their potential roles in diagnosis, prognosis and therapeutic strategies for cardiovascular diseases. | circulating miRNAs are resistant to endogenous ribonuclease activity and can be present in human plasma or serum in a remarkably stable form. | Sayed et al., 2014 [16] |
| Review Paper | - | the role of epigenetics, from DNA methylation to miRNAs, in major cardiovascular diseases such as ischemic heart disease, hypertension, heart failure and stroke. | DNA methylation as well as other epigenetic mechanisms such as post-translational histone modifications and non-coding RNA-mediated mechanisms including miRNAs, are fascinating, novel approaches to be explored for a better understanding of cardiovascular disease pathogenesis and for the possible definition of useful, unique biomarkers for disease. |
Udali et al., 2013 [17] |
| Cohort study | 177 | employed Illumina RNAseq and network co-expression analysis to identify systematic changes underlying CAD. | The pattern of changes is consistent with stress-related changes in the maturation of T and Treg cells, possibly due to changes in the immune synapse. | McCaffrey et al., 2023 [24] |
| Bioinformatics study | 2542 | investigate the diagnostic performance of circulating miRNAs in detecting subclinical carotid atherosclerosis. | Circulating miRNAs had excellent accuracy in detecting subclinical carotid atherosclerosis, suggesting their utilisation as novel diagnostic tools. | Witarto et al., 2023 [28] |
| Diagnostic study | 350 | evaluate the diagnostic value of circulating serum miR-509-3p in PAH (pulmonary arterial hypertension) with congenital heart disease. | The expression of miR-509-3p decreased in the serum of patients with PAH along with congenital heart disease. | Tang, 2020 [29] |
| Review Article | - | present the most recent data concerning the role of miRNAs as potential novel biomarkers for cardiovascular disease | the discovery of stable circulating miRNAs launches a new generation of most promising biomarkers for CVD. |
Siasos et al., 2020 [18] |
| Review Article | - | knowledge on tissue and circulating miRNA expression changes identified to be involved in four key cardiovascular diseases; atherosclerosis, abdominal aortic aneurysms, restenosis, and pulmonary arterial hypertension | utilising a custom selection of miRNA inhibitors and/or mimics may provide an appealing therapeutic approach to manage single and co-existing cardiovascular diseases and their clinical complications. | Johnson, 2019 [19] |
| case-control study | 400 | investigate whether four well-studied miRNA polymorphisms in non-Caucasian populations, namely miR146a G>C (rs2910164), miR149 C>T (rs2292832), miR196a2 C>T (rs11614913) and miR499 A>G (rs3746444), contribute to the risk for the development of premature CAD in the Greek population. | at least two of the studied polymorphisms, miR196a2 C>T (rs11614913) and miR499 A>G (rs3746444), as well as the miR146C-miR149C-miR196T-miR499G allele combination, could represent useful biomarkers of CAD and/or MI susceptibility in the Greek population. | Agiannitopoulos et al., 2021 [30] |
| Review Article | - | the role of miRNAs in the development of PAH as well as on their potential use as biomarkers and therapeutic tools in both experimental PAH models and in humans. | the current rapid development of our understanding of the actual biological roles of miRNAs in PAH development as well as partial successes in the field of RNAi therapy clearly justify an optimistic approach to future developments. | Bienertova-Vasku et al., 2015 [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





