Submitted:
24 May 2024
Posted:
28 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals and Animal Care
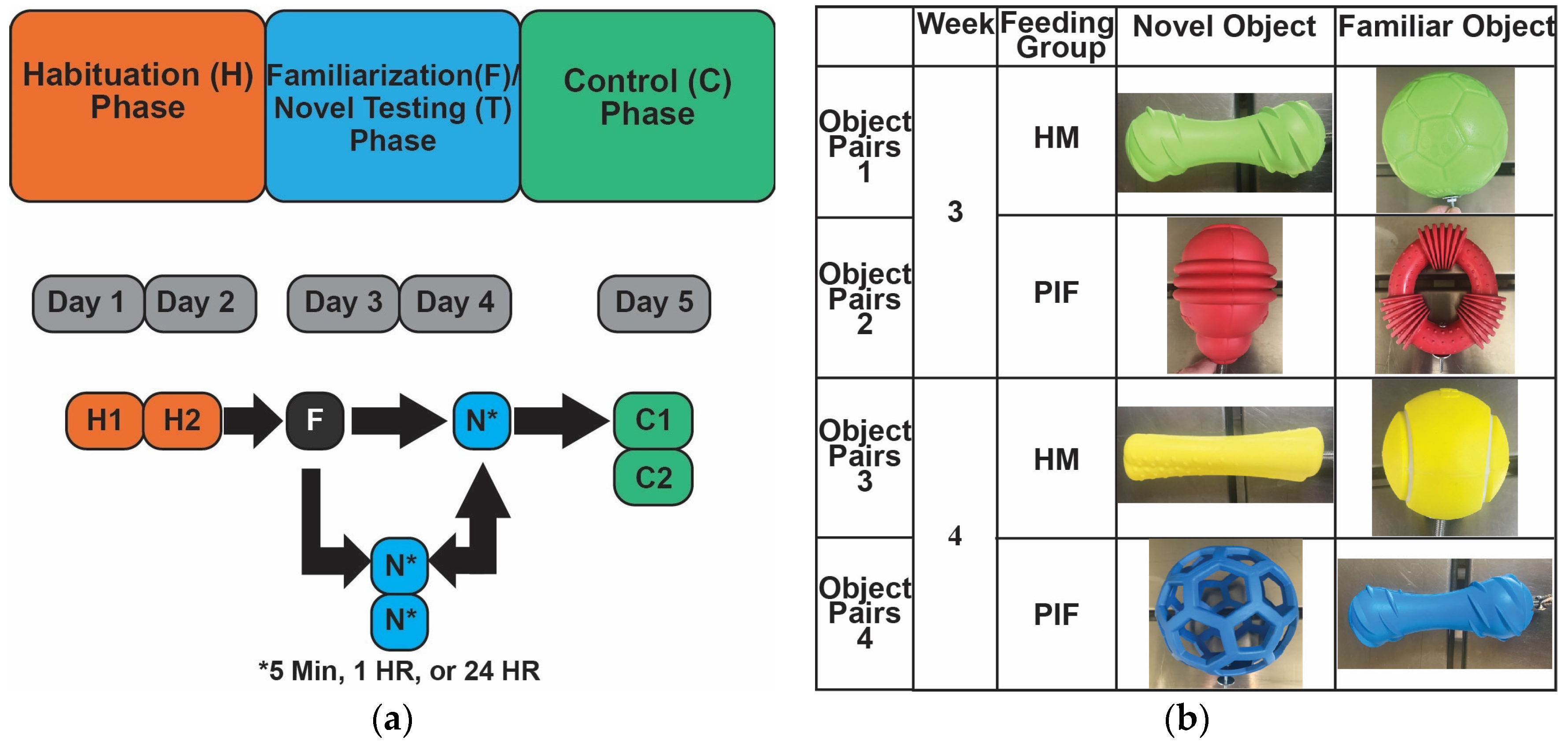
2.2. Functional Neurocognitive Assessment: Novel Object Recognition (NOR)
2.3. Euthanization and Sample Collection
2.4. Quantification of Microglial Morphology
2.5. Systemic Inflammatory/Anti-inflammatory Markers
2.6. Statistical Analysis
2.6.1. NOR Cognitive Testing
2.6.2. Piglet Body Weights, Brain Morphology, and Systemic Cytokines
3. Results
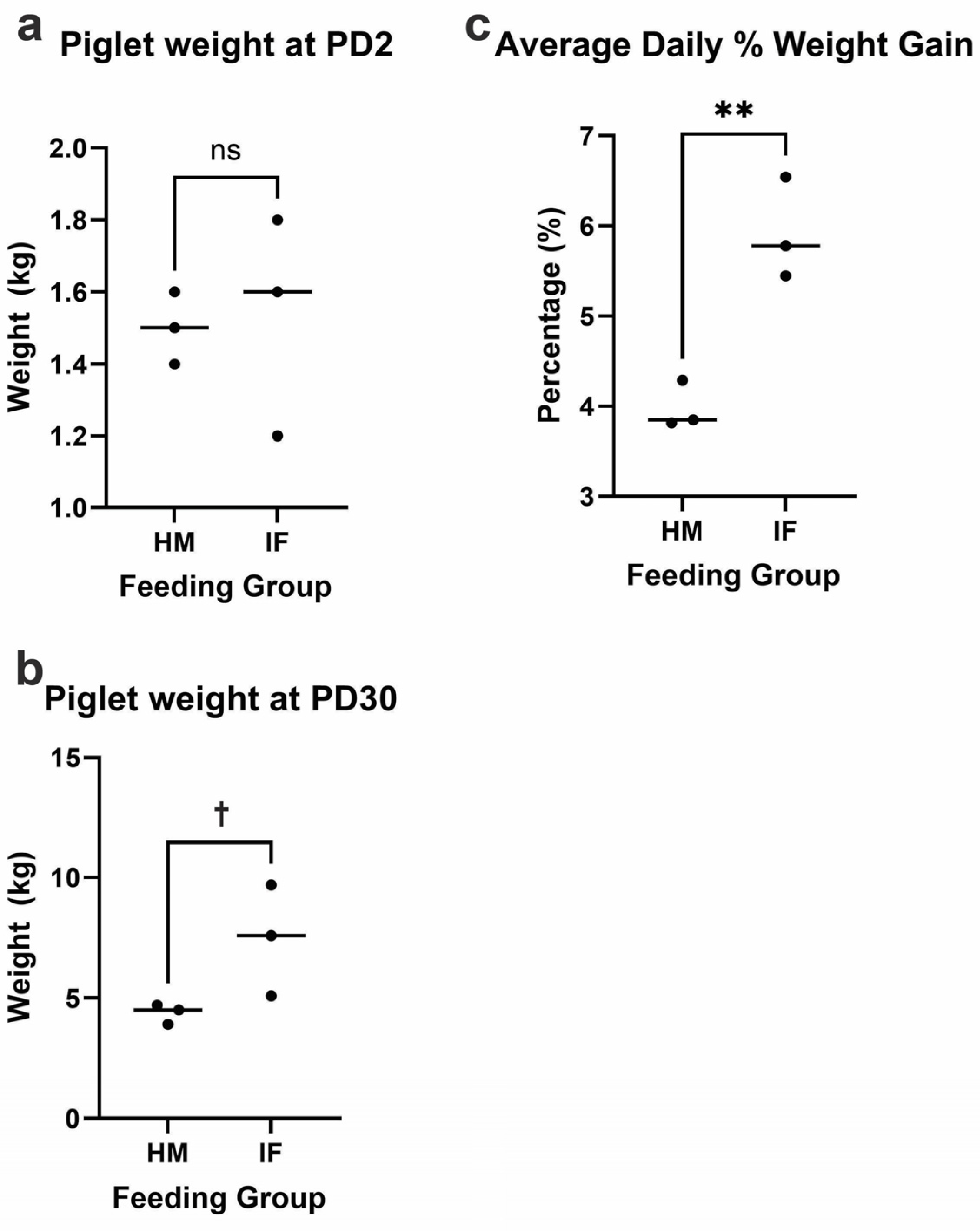
3.1. Piglet Weights
3.2. Cognitive Function – NOR Testing
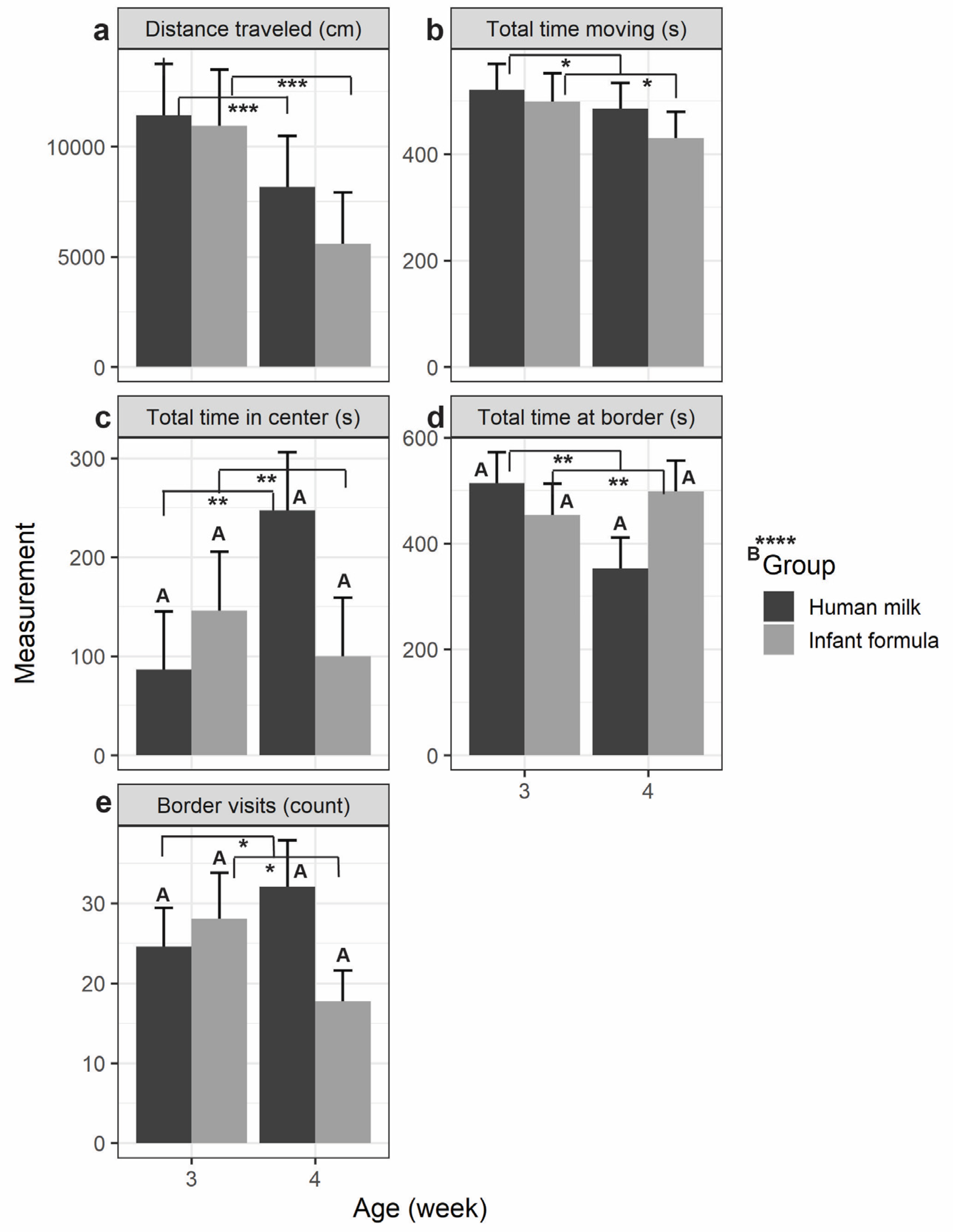
3.2.1. NOR Habituation Phase
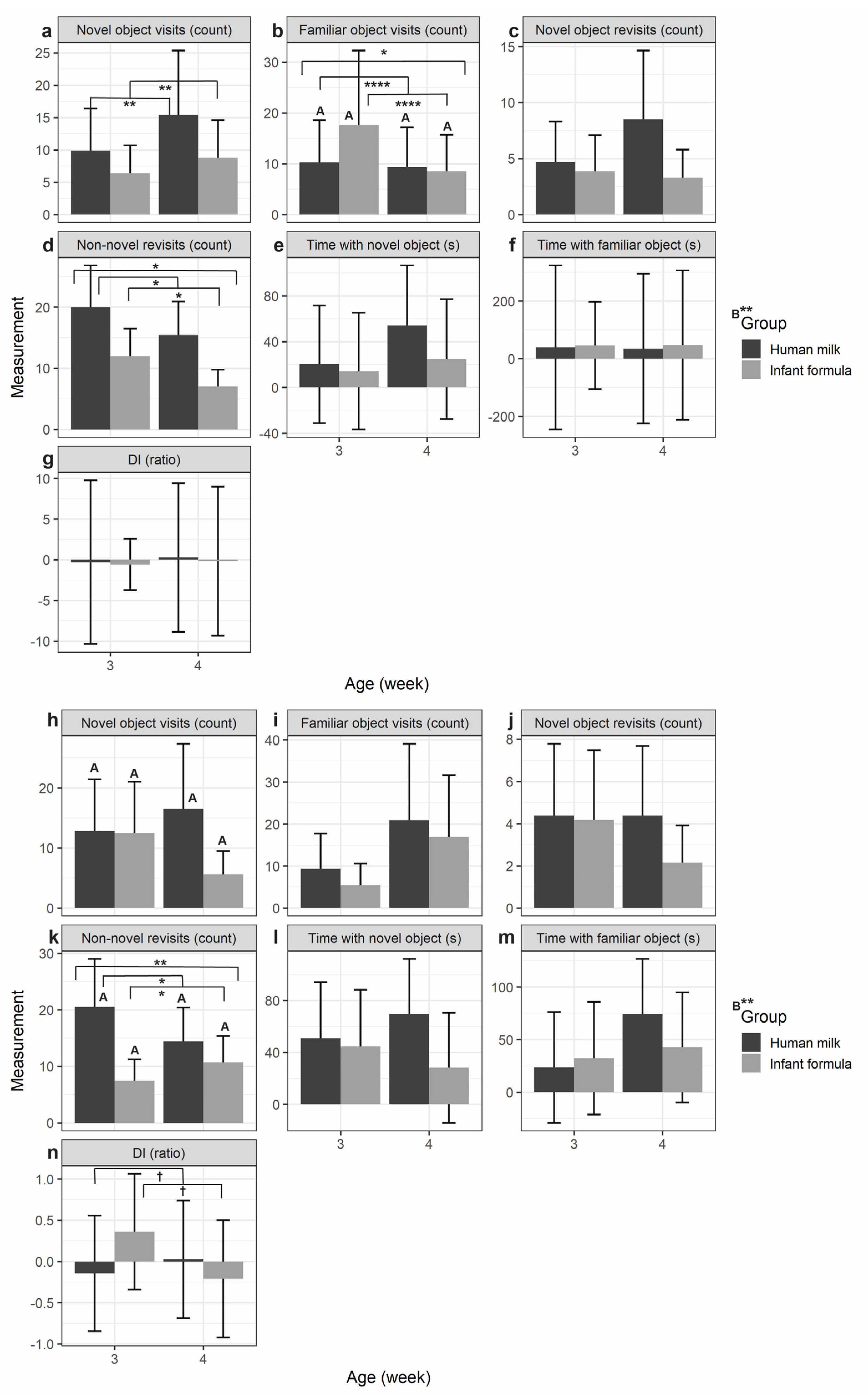
3.2.2. NOR Novel & Control Testing Phases
3.3. Piglet Brain Weights
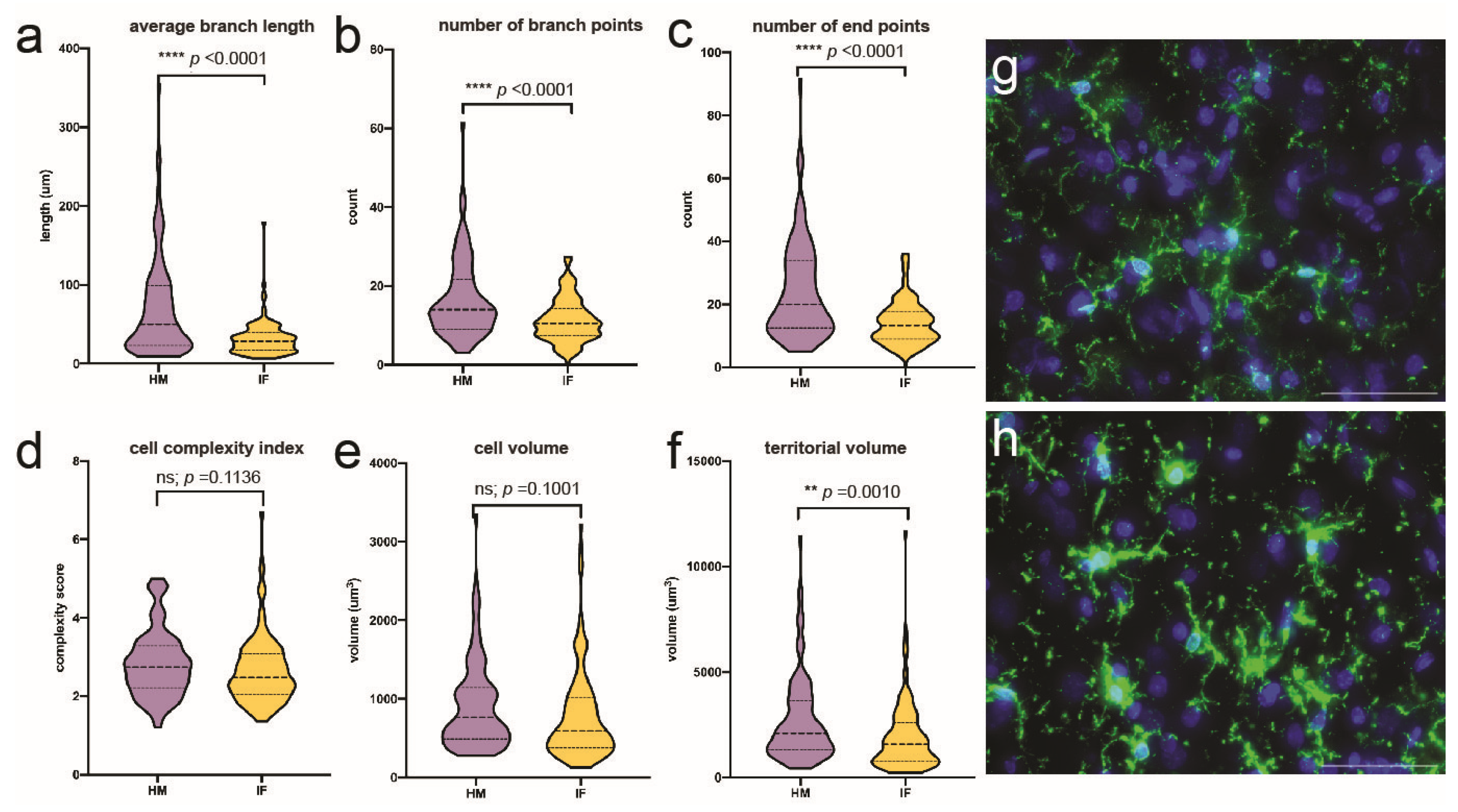
3.4. Piglet Microglial Morphology
3.5. Systemic inflammatory/Anti-inflammatory Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Animal Care and Use Committee Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Preterm birth. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.html. (2020, October 30).
- Gill, S.V., May-Benson, T.A., Teasdale, A., & Munsell, E. G. Birth and developmental correlates of birth weight in a sample of children with potential sensory processing disorder. BMC Pediatr 13, 29 (2013). [CrossRef]
- Peterson, B. S. et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000 Oct 18;284(15):1939-47. [CrossRef]
- Romero, R., Dey, S. K., & Fisher, S. J. Preterm labor: one syndrome, many causes. Science 345(6198), 760-5 (2014). [CrossRef]
- Chen, Z. et al. Impact of early term and late preterm birth on infants’ neurodevelopment: evidence from a cohort study in Wuhan, China. BMC Pediatr 22, 251 (2022). [CrossRef]
- Sansavini, A. et al. The effect of gestational age on developmental outcomes: a longitudinal study in the first 2 years of life. Child: Care Health Dev. 37(1), 26-36 (2011). [CrossRef]
- Rakic, P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 145(1), 61-83 (1972). [CrossRef]
- Rakic, P. A. small step for the cell—a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18(9), 383-388 (1995). [CrossRef]
- Sidman, R. L., & Rakic, P. "Development of the human central nervous system,” in Histology and Histopathology of the Nervous System. (W. Haymaker and R. D. Adams, Springfield, IL: C.C. Thomas, 3-145, 1982).
- Kostović, I., & Rakic, P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J. Neurosci. 4(1), 25-42 (1984). [CrossRef]
- Kostović, I., & Rakic, P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J. Comp. Neurol. 297(3), 441-470 (1990). [CrossRef]
- Hüppi, P. S. et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. An. Neurol.: Official J. Am. Neurol. Assoc. Child Neurol. Soc. 43(2), 224-235 (1998). [CrossRef]
- Kidokoro, H. et al. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 134(2), e444-e453 (2014). [CrossRef]
- Fanaro, S. Feeding intolerance in the preterm infant. Early Hum. Dev. 89, S13-S20 (2013). [CrossRef]
- Gibertoni, D. et al. Positive effect of human milk feeding during NICU hospitalization on 24 month neurodevelopment of very low birth weight infants: an Italian cohort study. PLoS One 10(1), e0116552 (2015). [CrossRef]
- Belfort, M. B. et al. Breast milk feeding, brain development, and neurocognitive outcomes: a 7-year longitudinal study in infants born at less than 30 weeks' gestation. J. Pediatr. 177, 133-139 (2016). [CrossRef]
- Andres, A. et al. Developmental status of 1-year-old infants fed breast milk, cow’s milk formula, or soy formula. Pediatrics, 129(6), 1134-1140 (2012). [CrossRef]
- Oddy, W. H. et al. Breast feeding and cognitive development in childhood: a prospective birth cohort study. Pediatr. Perinat. Epidemiol. 17(1), 81-90 (2003). [CrossRef]
- Ou, X. et al. Voxel-based morphometry and fMRI revealed differences in brain gray matter in breastfed and milk formula–fed children. Am. J. Neuroradiol. 37(4), 713-719 (2016). [CrossRef]
- Luby, J. L., Belden, A. C., Whalen, D., Harms, M. P., & Barch, D. M. Breastfeeding and childhood IQ: The mediating role of gray matter volume. J. Am. Acad. Child Adolesc. Psychiatry 55(5), 367-375 (2016). [CrossRef]
- Straub, N., Grunert, P., Northstone, K., & Emmett, P. Economic impact of breast-feeding-associated improvements of childhood cognitive development, based on data from the ALSPAC. British J. Nutr. 122(s1), S16-S21 (2019). [CrossRef]
- Der, G., Batty, G. D., & Deary, I. J. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ 333(7575), 945 (2006). [CrossRef]
- Boutwell, B. B., Young, J. T., & Meldrum, R. C. On the positive relationship between breastfeeding & intelligence. Dev. Psychol. 54(8), 1426 (2018). [CrossRef]
- Kramer, M. S. et al. Breastfeeding and child cognitive development: New evidence from a large randomized trial. Arch. General Psychiatry 65, 578-584 (2008). [CrossRef]
- Lind, N. M. et al. The use of pigs in neuroscience: modeling brain disorders. Neurosci. Biobehav. Rev. 31(5), 728-751 (2007). [CrossRef]
- Gonzalez, L. M., Boeser, A. J., & Blikslager, A. T. Porcine models of digestive disease: the future of large animal translational research. Trans. Res. 166(1), 12-27 (2015). [CrossRef]
- Cohen-Wolkowiez, M. et al. Early and late onset sepsis in late preterm infants. Pediatr. Infect. Dis. J. 28(12), 1052 (2009). [CrossRef]
- Brunse, A., Abbaspour, A., & Sangild, P. T. Brain barrier disruption and region-specific neuronal degeneration during necrotizing enterocolitis in preterm pigs. Dev. Neurosci. 40(3), 198-208 (2018). [CrossRef]
- Shennan, A. T., Dunn, M. S., Ohlsson, A., Lennox, K., & Hoskins, E. M. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 82(4), 527-532 (1988). [CrossRef]
- Yang, C., Hawkins, K. E., Doré, S., & Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol-Cell Physiol. 316(2), C135-C153 (2019). [CrossRef]
- Wang, L. W., Tu, Y. F., Huang, C. C., & Ho, C. J. JNK signaling is the shared pathway linking neuroinflammation, blood–brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J. Neuroinflamm. 9(1), 1-17 (2012). [CrossRef]
- Dammann, O., Durum, S., & Leviton, A. Do white cells matter in white matter damage? Trends Neurosci. 24(6), 320-324 (2001). [CrossRef]
- Okazaki, K. et al. Elevation of cytokine concentrations in asphyxiated neonates. Neonatology 89(3), 183-189 (2006). [CrossRef]
- Vadodaria, K. C. et al. Altered neuronal support and inflammatory response in bipolar disorder patient-derived astrocytes. Stem Cell Rep. 16(4), 825-835 (2021). [CrossRef]
- Shi, L., Fatemi, S. H., Sidwell, R. W., & Patterson, P. H. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 23(1), 297-302 (2003). [CrossRef]
- Wang, L. Y., Tu, Y. F., Lin, Y. C., & Huang, C. C. CXCL5 signaling is a shared pathway of neuroinflammation and blood–brain barrier injury contributing to white matter injury in the immature brain. J. Neuroinflamm. 13(1), 1-15 (2016). [CrossRef]
- Kunugi, H., Urushibara, T., Murray, R. M., Nanko, S., & Hirose, T. Prenatal underdevelopment and schizophrenia: a case report of monozygotic twins. Psychiatry and Clin. Neurosci. 57(3), 271-274 (2003). [CrossRef]
- Miron, V. E. Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination. J Leukoc Biol 101, 1103-1108 (2017). [CrossRef]
- Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691-705 (2012). [CrossRef]
- Hughes, A. N. & Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat Neurosci 23, 1055-1066 (2020). [CrossRef]
- Li, Q. et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 101, 207-223 e210 (2019). [CrossRef]
- Church, J. S., Milich, L. M., Lerch, J. K., Popovich, P. G. & McTigue, D. M. E6020, a synthetic TLR4 agonist, accelerates myelin debris clearance, Schwann cell infiltration, and remyelination in the rat spinal cord. Glia 65, 883-899 (2017). [CrossRef]
- Xu, T. et al. The roles of microglia and astrocytes in myelin phagocytosis in the central nervous system. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 271678X221137762 (2022). [CrossRef]
- Bar, E. & Barak, B. Microglia roles in synaptic plasticity and myelination in homeostatic conditions and neurodevelopmental disorders. Glia 67, 2125-2141 (2019). [CrossRef]
- Antunes, M., & Biala, G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cog. Processing 12(2), 93-110 (2012). [CrossRef]
- Fleming, S. A., & Dilger, R. N. (2017). Young pigs exhibit differential exploratory behavior during novelty preference tasks in response to age, sex, and delay. Behav. Brain Res. 321(50-60). [CrossRef]
- Kent, A. L., Wright, I. M., Abdel-Latif, M. E., New South, W. & Australian Capital Territory Neonatal Intensive Care Units Audit, G. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics 129, 124-131 (2012). [CrossRef]
- Peacock, J. L., Marston, L., Marlow, N., Calvert, S. A. & Greenough, A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res 71, 305-310 (2012). [CrossRef]
- VanRyzin, J. W., Marquardt, A. E., Pickett, L. A. & McCarthy, M. M. Microglia and sexual differentiation of the developing brain: A focus on extrinsic factors. Glia (2019). [CrossRef]
- VanRyzin, J. W., Pickett, L. A. & McCarthy, M. M. Microglia: Driving critical periods and sexual differentiation of the brain. Dev Neurobiol 78, 580-592 (2018). [CrossRef]
- Le Dividich, J., & Noblet, J. Colostrum intake and thermoregulation in the neonatal pig in relation to environmental temperature. Neonatology 40(3-4), 167-174 (1981). [CrossRef]
- Mount, L. E. Environmental temperature preferred by the young pig. Nature 199(4899), 1212-1213 (1963). [CrossRef]
- Brown, J. E. Nutrition during lactation: Infant nutrition. In Nutrition through the life cycle (7th edn), 157-158 (Cengage Learning, 2020).
- Urschel, K. L., Shoveller, A. K., Uwiera, R. R., Pencharz, P. B., & Ball, R. O. Citrulline is an effective arginine precursor in enterally fed neonatal piglets. J. Nutr. 136(7), 1806-1813 (2006). [CrossRef]
- Wykes, L. J., Ball, R. O., & Pencharz, P. B. Development and validation of a total parenteral nutrition model in the neonatal piglet. J. Nutr. 123(7), 1248- 1259 (1993). [CrossRef]
- Burrin, D. G. et al. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am. J. Clin. Nutr. 71(6), 1603-1610 (2000). [CrossRef]
- Bos, C. et al. Intestinal lysine metabolism is driven by the enteral availability of dietary lysine in piglets fed a bolus meal. Am. J. Physiol.-Endocrinol. Metabol. 285(6), E1246-E1257 (2003). [CrossRef]
- National Research Council. Nutrient requirements of swine (National Academies Press, 2012).
- Kajimoto, M. et al. Inhaled nitric oxide reduces injury and microglia activation in porcine hippocampus after deep hypothermic circulatory arrest. J. Thoracic Cardiovasc. Surg. 161(6), e485-e498 (2020). [CrossRef]
- Lim, R. R., Hainsworth, D. P., Mohan, R. R., & Chaurasia, S. S. Characterization of a functionally active primary microglial cell culture from the pig retina. Exp. Eye Res. 185, 107670 (2019). [CrossRef]
- Fumagalli, S., Perego, C., Ortolano, F., & De Simoni, M. G. CX3CR1 deficiency induces an early protective inflammatory environment in ischemic mice. Glia 61(6), 827-842 (2013). [CrossRef]
- Rice, R. A. et al. Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia 65(6), 931-944 (2017). [CrossRef]
- Howard, C. V., & Reed, M. G. Unbiased stereology: Three-dimensional measurement in microscopy (Advanced Methods) 2nd edn. (QTP Publications, 2010).
- York, E. M., LeDue, J. M., Bernier, L. P., & MacVicar, B. A. 3DMorph automatic analysis of microglial morphology in three dimensions from ex vivo and in vivo imaging. Eneuro, 5(6) (2018). [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. https://www.R-project.org/ (2022).
- Bates, D., Maechler, M., Bolker, B., & Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat Softw, 67(1), 1-48. (2015). [CrossRef]
- Kuznetsova, A., Brockhoff, P.B., Christensen, R.H.B. (2017). “lmerTest Package: Tests in Linear Mixed Effects Models.” J. Stat Softw, 82(13), 1-26. [CrossRef]
- Shapiro, S. S., & Wilk, M. B. An analysis of variance test for normality (complete samples). Biometrika 52(3-4), 591-611 (1965). [CrossRef]
- Niblock, M.M. et al. Comparative anatomical assessment of the piglet as a model for the developing human medullary serotonergic system. Brain Res Rev. 50(1), 169-183 (2005). [CrossRef]
- Knickmeyer R. C., Gouttard S. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 28(47),12176–12182 (2008). [CrossRef]
- Haigh, A., Chou, J. Y., & O'Driscoll, K. (2020). Variations in the behavior of Pigs during an open field and novel object test. Front. Vet. Sci. 607(7; 2020). [CrossRef]
- Brewer, J. S., Bellinger, S. A., Joshi, P., & Kleven, G. A. Enriched open field facilitates exercise and social interaction in 2 strains of guinea pigs (Cavia porcellus). J. Am. Assoc. Lab. Anim. Sci. 53(4), 344–355 (2014).
- Mormède, P., Dantzer, R., Bluthe, R. M., Caritez, J. C. Differences in adaptive abilities of three breeds of Chinese pigs. Behavioural and neuroendocrine studies. Genet Sel Evol 16(1), 85-102 (1984). [CrossRef]
- Conrad, M. S., Dilger, R. N., & Johnson, R. W. (2012). Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: a longitudinal MRI study. Dev. Neurosci. 34(4), 291–298 (2012). [CrossRef]
- Belfort, M. B. et al. Breast milk feeding, brain development, and neurocognitive outcomes: a 7-year longitudinal study in infants born at less than 30 weeks' gestation. J. Pediatr. 177, 133-139 (2016). [CrossRef]
- Huang, J. et al. Early feeding of larger volumes of formula milk is associated with greater body weight or overweight in later infancy. Nutr. J. 17(1), 1-9 (2018). [CrossRef]
- Hopkins, D., Steer, C. D., Northstone, K., & Emmett, P. M. Effects on childhood body habitus of feeding large volumes of cow or formula milk compared with breastfeeding in the latter part of infancy. Am. J. of Clin. Nutr. 102(5), 1096-1103 (2015). [CrossRef]
- Deoni, S. C. et al. Breastfeeding and early white matter development: A cross-sectional study. Neuroimage 82, 77-86 (2013). [CrossRef]
- Kafouri, S. et al. Breastfeeding and brain structure in adolescence. International journal of epidemiology, 42(1), 150-159 (2013. [CrossRef]
- Isaacs, E. B. et al. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr. Res. 67(4), 357-362 (2010). [CrossRef]
- Barger, N. et al. Microglia: An Intrinsic Component of the Proliferative Zones in the Fetal Rhesus Monkey (Macaca mulatta) Cerebral Cortex. Cereb Cortex 29, 2782-2796 (2019). [CrossRef]
- Cunningham, C. L., Martinez-Cerdeno, V. & Noctor, S. C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33, 4216-4233 (2013). [CrossRef]
- Kracht, L. et al. Human fetal microglia acquire homeostatic immune-sensing properties early in development. Science 369, 530-537 (2020). [CrossRef]
- Hanisch, U. K. & Kettenmann, H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10, 1387-1394 (2007). [CrossRef]
- Dinarello, C. A. Biologic basis for interleukin-1 in disease. Blood 87(6), 2095–2147 (1996).
- Besedovsky, H. O., & del Rey, A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr. Rev. 17(1), 64-102 (1996). [CrossRef]
- Haas, H. S., & Schauenstein, K. Neuroimmunomodulation via limbic structures—the neuroanatomy of psychoimmunology. Prog. Neurobio. 51(2), 195-222 (1997). [CrossRef]
- Rothwell, N. J., & Hopkins, S. J. Cytokines and the nervous system II: actions and mechanisms of action. Trends Neurosci. 18(3), 130-136 (1995). [CrossRef]
- Schneider, H. et al. A neuromodulatory role of interleukin-1β in the hippocampus. Proc. Natl. Acad. Sci. 95(13), 7778-7783 (1998). [CrossRef]
- Yirmiya, R., & Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 25(2), 181-213 (2011). [CrossRef]
- Milner, B. Hemispheric specialization: Scope and limits. In The Neurosciences: the third study program (Schmitt, F. O., Worden, F. G.) 75-89 (MIT Press, Cambridge, MA, 1974).
- Purves, D. et al. (eds.). Long-Term Synaptic Potentiation. In Neuroscience (2nd edn), Chapter 35 (Sinauer Associates, Sunderland, MA, 2001).
- Lenz, K. M., Nugent, B. M., Haliyur, R., & McCarthy, M. M. Microglia are essential to masculinization of brain and behavior. J. Neurosci. 33(7), 2761-2772 (2013). [CrossRef]
- McDonough, A. et al. Ischemic preconditioning induces cortical microglial proliferation and a transcriptomic program of robust cell cycle activation. Glia 68(1), 76-94 (2020). [CrossRef]
- Satoh, J. I. et al. TMEM119 marks a subset of microglia in the human brain. Neuropathol. 36(1), 39-49 (2016). [CrossRef]
- Hamner, M. A. et al. Microglial depletion abolishes ischemic preconditioning in white matter. Glia 70(4), 661-674 (2022). [CrossRef]
| Variable | Nutritional Group | Age | Nutrition-by-age |
|---|---|---|---|
|
Habituation Variables Distance Traveled Total Time Moving Total Center Time Total Border Time Border Visits |
|||
| 0.176 | 8.77 x 10-4*** | 0.347 | |
| 0.169 | 0.037* | 0.485 | |
| 0.224 | 0.003** | 9.75 x 10-6**** | |
| 0.231 | 0.002** | 1.00 x 10-5**** | |
| 0.369 | 0.015* | 1.94 x 10-5**** | |
|
Novel Variables Novel Object Visits Familiar Object Visits Novel Object Revisits Non-novel Object Revisits Time with Novel Object Time with Familiar Object DI |
|||
| 0.356 | 0.001** | 0.603 | |
| 0.036* | 1.87 x 10-7**** | 0.002** | |
| 0.102 | 0.283 | 0.853 | |
| 0.033* | 0.023* | 0.123 | |
| 0.442 | 0.373 | 0.760 | |
| 0.582 | 0.918 | 0.893 | |
| 0.371 | 0.224 | 0.839 | |
|
Control Variables Novel Object Visits Familiar Object Visits Novel Object Revisits Non-novel Object Revisits Time with Novel Object Time with Familiar Object DI |
|||
| 0.847 | 0.321 | 1.28 x 10-4*** | |
| 0.649 | 0.107 | 0.579 | |
| 0.847 | 0.919 | 0.121 | |
| 0.001** | 0.010* | 0.005** | |
| 0.428 | 0.935 | 0.312 | |
| 0.599 | 0.424 | 0.169 | |
| 0.666 | 0.072† | 0.193 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





