Submitted:
27 May 2024
Posted:
28 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
| Year(s) | Researcher(s) | Clinical or experimental work |
|---|---|---|
| 1841 | Scherer | Discovery of hematoporphyrin by removing iron from dried blood |
| 1861-1871 | L.Pasteur, P. Bert | Discovery of phototoxicity |
| 1867 | J.L.W. Thudichum | Spektrum fluorescencji tej czerwonej substancji (hematoporfiryny) oraz fluorescencja |
| 1871 | F. Hoppe-Seyler | Giving a name to the red substance (hematoporphyrin) |
| 1874 | Schultz | Description of a patient with porphyria (errors in heme biosynthesis) |
| 1895-1903 | N.R.Finsen | Phototherapy (Nobel Prize 1903) |
| 1897-1904 | O. Raab, H. von Tappeiner | First reports about phototherapy |
| 1904 | H. von Tappeiner | Introduction of the term "photodynamic action" |
| 1903-1905 | - | The first "before and after" photos of patients (eosin exposure) |
| 1908-1913 | W. Hausmann, F. Meyer-Betz | Many PDT experiments with hematoporphyrin on paramecia, erythrocytes, mice, guinea pigs and humans |
| 1924 | - | A.Policard saw red porphyrin fluorescence in tumors, which was the first observation of tumors |
| 1925 | H. Fisher | The study of porphyrins, for which the Nobel Prize was awarded in 1929. |
| 1945 | S. Scwarz | Radiation hypersensitivity to porphyrins was noted |
| 1959 | D. Harman | A free radical theory of aging and disease has been proposed |
| 1960-1967 | R. Lipson, E. Baldes | HpD synthesis was performed |
| 1970 | H. Kautsky, G. Herzberg | Zauważono rolę aktywnego tlenu |
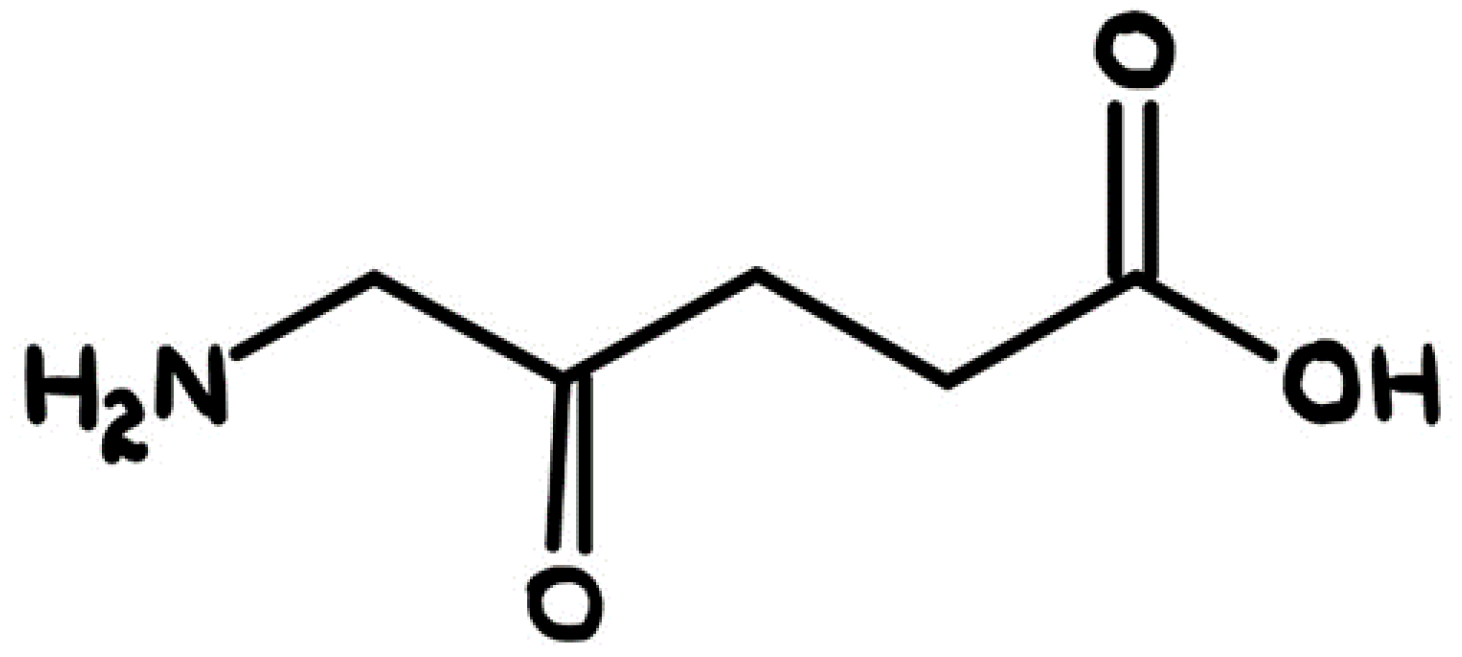
| 1975 | Z. Malik, M. Djaldetti | ALA was used to induce PpIX |
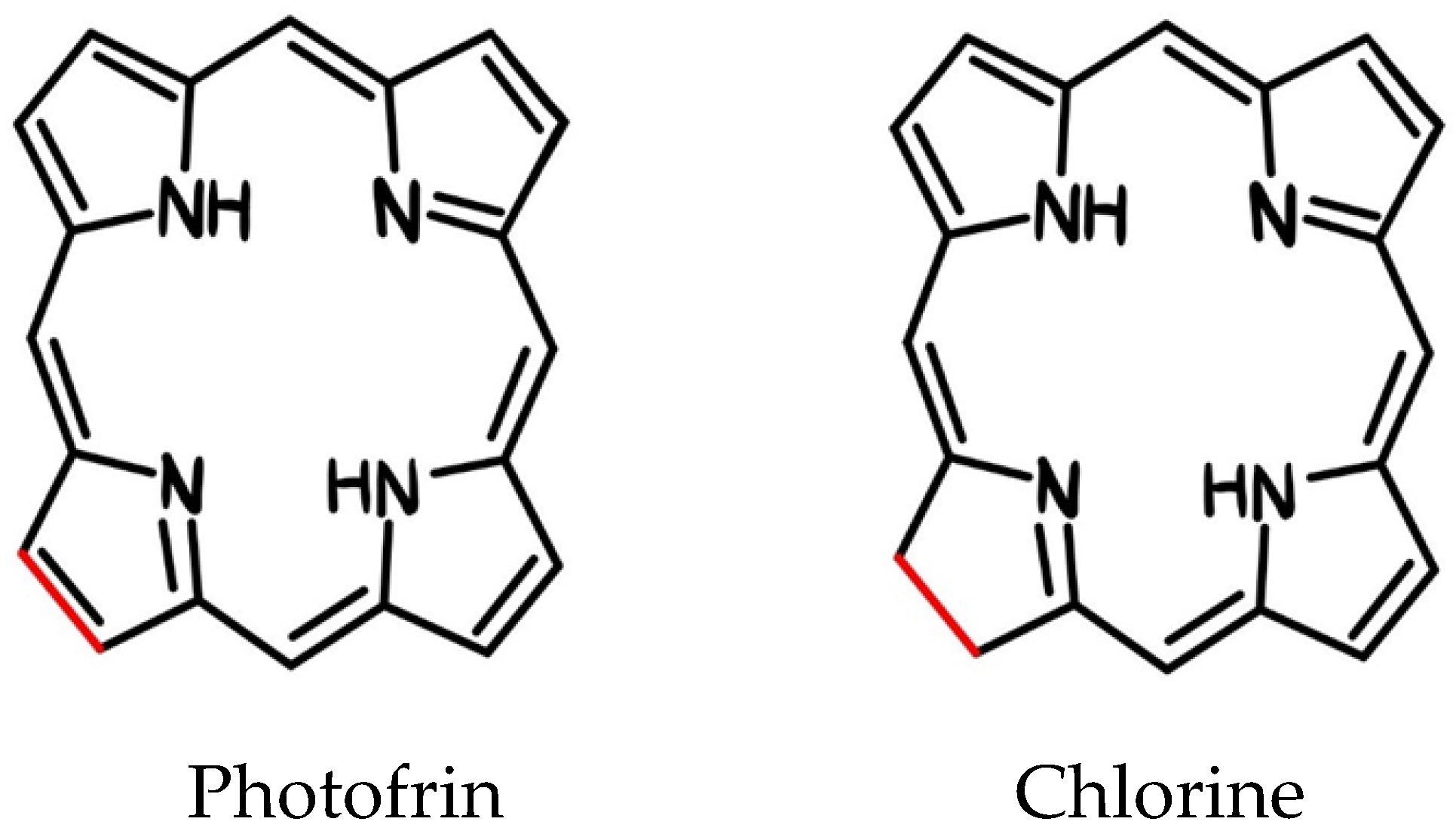
| 1983-1993 | T. J. Dougherty i inni | Photofrin was used |
| 1990 | J. Kennedy, R.Pottier | ALA has been used in the clinic |
| 1995 | cPDT with Photofrin, the first clinically approved photosensitizer for cancer treatment, was produced by Dougherty and his colleagues. Photofrin, which is a pure formulation of HpD, has received approval from the US Food and Drug Administration (FDA). | |
| 1993 | Photofrin becomes commercially available Representative approval of porphyrin sodium in Canada (bladder cancer) |
|
| 1994 | Representative approval of porphyrin sodium in Japan and the Netherlands (esophageal and lung cancers) | |
| 1995 | Representative approval of porphyrin sodium in the US and Canada (esophageal cancer) | |
| 1996 | Representative approval of porphyrin sodium in France (esophageal cancer) | |
| 1997 | Representative approval of porphyrin sodium in Germany (lung cancer) | |
| 1998 | Representative approval of porphyrin sodium in the US and UK (lung cancer) | |
| 1999 | Photodynamic therapy (PDT) targeting antibody fragments (Neri) has been described. | |
| 2001 | Foscan approved in the EU for head/neck cancer in June, despite Scotia's collapse in January (Scotia/Biolitec) Visudyne as an effective first-line PDT for AMD (QLT). Foscan approved for the treatment of HNSCC in Europe. |
|
| 2003 | Photodynamic therapy (PDT) targeting the HER2 receptor using whole IgG antibodies. Telaporfin (NPe6) approved in Japan for photodynamic therapy of lung cancer |
|
| 2004 | Metwix (methylaminolevulinate, MAL) is approved by the US Food and Drug Administration (FDA) for use in photodynamic therapy (PDT) for the treatment of actinic keratosis (AK) and drug-induced carcinogenesis Polymer complex of chlorin e-6 with polyvinylpyrrolidone approved for photodynamic therapy in Russia |
|
| 2006 | randomized clinical trials of dPDT in northern Europe.. | |
| 2007 | HER2-targeted photodynamic therapy (PDT) using scFv. | |
| 2011 | ALA (10% topical gel) approved by EMA for the treatment of AK | |
| 2012 | Silicone phthalocyanin Pc4 has successfully completed Phase I clinical trials for topical application in photodynamic therapy (PDT). | |
| 2014 | Recommended photodynamic day therapy with MAL (16% topical cream) for the treatment of AK in Australia | |
| 2016 | Sellera published the first clinical study on veterinary photodynamic therapy (PDT) using antibacterial agents for the treatment of infected pododermatitis lesions in penguins EMA has extended the indications for the use of ALA (10% gel for topical use) to include the treatment of BCC and photodynamic day therapy |
|
| 2017 | FDA Approved Oral ALA for Fluorescent Guidance of Brain Tumor Resection (Non-PDT) | |
| 2018 | Padeliporfin is approved by the European Medicines Agency (EMA) for the treatment of prostate cancer. | |
| 2019 | Guided anticancer therapy FA@PDA using PDT | |
| 2020 | Anticancer therapy PTT + PDT | |
| 2021 | The use of chemotherapy in combination with PTT + PDT | |
| 2022 | PTA therapy | |
| 2023 | A group of scientists from the City University of Hong Kong (CityU) have made an important discovery by creating a new type of photo-oxidants that respond to near infrared. These substances are able to effectively eliminate cancer cells without requiring the presence of oxygen. | |
| 2024 | The latest preclinical studies have confirmed the effectiveness of 5-ALA in the treatment of gliomas in brain organoids, leading to the death of cancer cells while not negatively affecting healthy cells. Applying PDT to 3D tumor models |
|
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Analysis Process
2.4. Analysis Categories
- History of the development of photodynamic therapy
- Application of photodynamic therapy in various fields of medicine
- Scientific discoveries influencing the development of this method
- Technological innovations and their impact on the effectiveness of therapy photodynamic
2.5. Systematization of Data
3. Results
3.1. Mechanism of Action of PDT
3.2. Development of PDT Technology
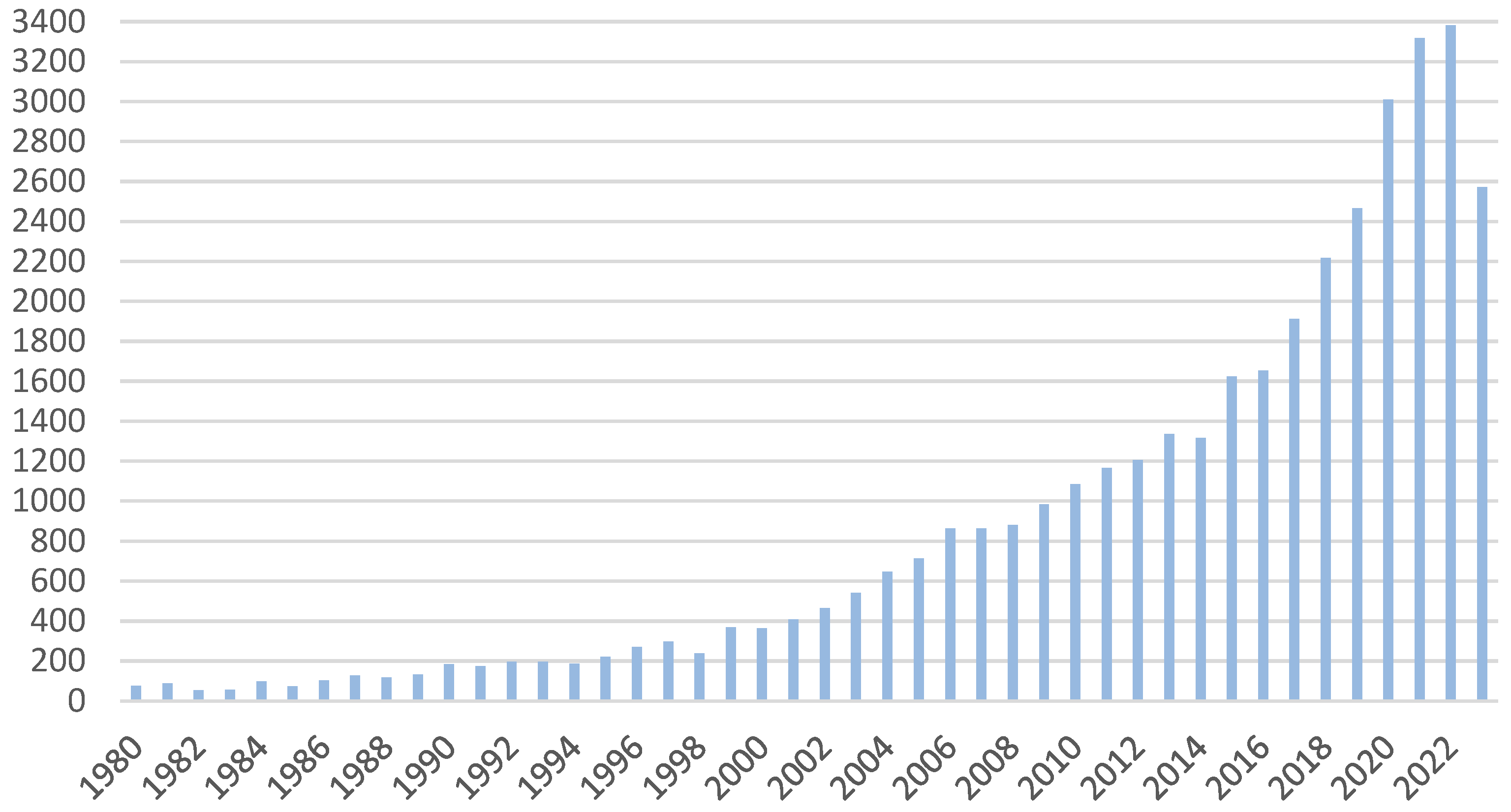
3.2.1. Photodynamic Therapy in the Years 1990–1995
3.2.2. Photodynamic Therapy in the Years 1996–2000
3.2.3. Photodynamic Therapy in 2001–2005
3.2.4. Photodynamic Therapy in 2006–2010
3.2.5. Photodynamic Therapy in 2011–2015
3.2.6. Photodynamic Therapy in 2016–2020
3.2.7. Photodynamic Therapy in 2021–2023
3.3. Natural Photosensitizers
- Selective accumulation in tumor tissue: The photosensitizer should be able to selectively accumulate in the area of tumor tissue, minimizing the effect on healthy tissues.
- No phototoxic effects in healthy tissues: The photosensitizer should not cause undesirable phototoxic effects in healthy tissues, which means that it cannot damage healthy cells when exposed to light.
- Appropriate absorption bands: The absorption bands of a photosensitizer should not coincide with the absorption bands of the body's natural pigments, such as melanin or hemoglobin, or with the absorption bands of water in the area close to infrared.
- Efficient generation of singlet oxygen and oxidative reactions: The photosensitizer should be able to efficiently generate singlet oxygen and other oxidative reactions that are crucial in the destruction of cancer cells.
- Minimal side effects: The photosensitizer should not cause significant side effects that may be harmful to the patient.
- Low toxicity and easy elimination: The photosensitizer should be low toxic and easily removed from the body after completion of therapy to minimize side effects and burden on the patient's body.
| Photosensitizer | Nanoparticle | Results | In vivo/ In vitro |
|---|---|---|---|
| Photofrin | F3 – Polymer targeted particles | • High rate of uptake of nanoparticles by cells • Significant improvement in survival rate (MDA-MB-435 cell line—breast cancer, 9L rat gliomas) |
In vitro [78] |
| Nanoporous zinc oxide | • Increased ROS generation • Increased cytotoxic effect (Cell line A549 - lung cancer) |
In vitro [79] In vivo |
|
| Liposomy | • Higher phototoxic effect of liposomal photofrin compared to the free drug (Athymic nude rats, Cr:NIH-rna strain with U97 cells) |
In vivo [80] | |
| Photoporphyrin IX | Gold particles | • Increased cytotoxic effect of conjugates (HeLa cell line - cervical cancer) • Increased apoptosis (HeLa cell line - cervical cancer) • Increased single oxygen generation (male Newborn Medical Research Institute [NMRI] mice) |
In vitro [81] In vitro [82] In vivo [83] |
| Polyethyleneimine nanoparticles | • Ability to generate single oxygen upon exposure to light with a wavelength of 635 nm | In vitro [84] | |
| Carbon particles | • Increased single oxygen generation • Additional bioluminescence effect • Increased phototoxic effect (MMC-7721 cell line - hepatocellular carcinoma) |
In vitro [85] | |
| Nanoparticles with a silver core and a silica coating | • Increased single oxygen generation (U251MG cell line - astrocyma glioblastoma, HepG2 cell line - hepatocellular carcinoma) |
In vitro [86] | |
| Polymerosomes | • Increased cytotoxic effect • Selective cytotoxic effect on melanoma cells (Cell line A375 - malignant melanoma) |
In vitro [87] | |
| Micelle poli(etylenoglikolu) - polikaprolaktonu (PEG-PCL) | • Synergistic activity with erlotinib (MDA-MB-231 cell line - breast cancer) |
In vitro [88] |
| Photosensitizer | Nanoparticle | Results | In vivo/ In vitro |
|---|---|---|---|
| Chlorine e6 | Lipidots | • Reduced dark toxicity • Retained phototoxicity (CAL-33 cell line - squamous cell carcinoma of the tongue) |
In vitro [89] |
| Superparamagnetic iron oxide partition nanoclusters (SPION) | • High solubility in water • Single oxygen generation preserved • Significant delay in tumor growth (4T1 cell line - breast tumor mice, female nude mice carrying 4T1) |
In vitro [90] In vivo |
|
| Methoxy-poly(ethylene glycol)-poly(D,L-lactide) (mPEG-PLA-Ce6) | • Increased single oxygen generation • Increased cellular internalization (A549 cell line - lung cancer, monolayers and 3D spheres) |
In vitro [91] | |
| Verteporfirin | Poly(D,L-lactide-co-glycolide) | • Size dependent toxicity • Increased phototoxic effect for smaller nanopartitions • Efficiently controlled tumor growth by small nanopartitions loaded with verteporfin (EMT-6 cell line - mammary tumor mice, SKH1 female nude mice) |
In vitro [92] In vivo |
| 2-[1-heksyloksyetylowy]-2-devinyl pyrofeoforbid-a (HPPH) | Functionalized polyacrylamide (AFPAA) | • Efficient en-capsulation, post-loading or HPPH conjugation • Highest phototoxicity and single oxygen production for the post-loaded form • No dark toxicity observed • Tumor location in a murine colorectal cancer model (PC-3 cell line - prostate cancer, MDA-MB-435S cell line - melanoma, Mice carrying human glioblastoma U87MG) |
In vitro [93] In vivo |
| Compound | Nazwa | Absorption [nm] | Application |
|---|---|---|---|
| Porfimer sodium salt | Photofrin | 632 | Canada (1993) - bladder cancer USA (1995) - esophageal cancer USA (1998) - lung cancer USA (2003) - Barrett's esophagus Japan - cervical cancer Europe, Canada, Japan, USA, Great Britain - endobronchial cancer |
| 5-aminolevulinic acid (ALA) | Levulan | 632 | USA (1999) - actinic keratosis |
| Aminolewulinian metylu (MAL) | Metvixia | - | USA (2004) - actinic keratosis |
| Heksaminolewulinian (HAL) | Cysviev | - | USA (2010) - diagnosis of bladder cancer |
| A derivative of benzoporphyrin Monoacid ring A (BPD-MA) |
Visudine | 689 | USA (age-related macular degeneration 1999) - |
| Meta-tetra(hydroxyphenyl)chlorin (m-THPC) | Foscan | 652 | Europe - neck and head cancer |
| ethyl tin ethiopurpurine | Purlytin | 664 |
Clinical trials - breast adenocarcinoma, basal cell carcinoma, Kaposi's sarcoma, age-related macular degeneration |
| N-aspartylochloryna e6 (NPe6) | Laserphyrin, Litx | 664 | Japan (2003) - lung cancer |
| 2-(1-heksyloksyetylo)-2-dewinyl pirofoforbid (HPPH) | Photochlor | 665 | Clinical trials - esophageal cancer, basal cell carcinoma, lung cancer, Barrett's esophagus |
| Bakteriopheoforbide palladu (WST09) | Tookad | 763 | Clinical trials - prostate cancer |
| WST11 | Stakel | - | Clinical trials - prostate cancer |
| Motexafina lutet (Lu-Tex) | Lutrin, Optrin, Antrin | 732 | Clinical trials - prostate cancer, age-related macular degeneration, breast cancer, cervical cancer, arterial disease |
| Tetrasulfonic aluminum phthalocate (APkS4) | Photosens | 676 | Russia (2001) - stomach, skin, lips, oral cavity, tongue, breast cancer |
| Silicon phthalocyanine (Pc4) | - | 675 | Clinical tests - practical keratosis, Bowen's disease, skin cancer, mycosis |
4. Discussion
4.1. Advances in Imaging Techniques and Diagnostics Supporting PDT
- Imaging using fluorescence techniques:
- Optical imaging:
- Advanced microscopy imaging:
- Molecular diagnostics:
- Imaging using hybrid technologies:
4.2. The Most Important Centers Specializing in the Treatment of Skin Problems with Photodynamic Therapy in the World
| Country | Research Centre |
|---|---|
| Austria | Wiener Privatklinik Rudolfinerhaus Privatklinik Döbling Private Clinic Akh Vienna General Hospital Graz Ragnitz Private Clinic Private Clinic Konfraternetat Leech Pivate Clinic |
| China | Fuda Cancer Hospital |
| Czech Republik | Motol University Hospital University Hospital Brno |
| France | American Hospital of Paris Oncological Institut Gustave Roussy |
| Germany | Dermatologikum Berlin Solingen City Hospital University Hospital Rechts der Isar Nuremberg Hospital Helios Medical Group University Hospital of Köln Charité University Hospital Frankfurt University Hospital Essen University Hospital Meoclinic |
| India | Gleneagles Global Hospitals BLK Super Speciality Hospital Apollo Hospitals Fortis Hospital Artemis Hospitals Manipal Hospitals Group Medanta Hospital Wockhardt Hospitals |
| Izrael | Sourasky Medical Center (Ichilov) Assuta Medical Center Sheba Medical Center Rambam Hospital Herzliya Medical Center Hadassah Medical Center Yitzhak Rabin Medical Center Schneider Children’s Medical Center Shaare Zedek Medical Center Ramat Aviv Medical Center Assaf Harofeh Medical Center Meir Medical Center |
| Italy | Salvator Mundi International Hospital |
| Korea | JK Plastic Surgery Clinic Asan Medical Center Severance Hospital Gachon University Gil Medical Center Cheil General Hospital & Women’s Healthcare Center Kyung Hee University Hospital (KUIMS) Seoul National University Hospital Soon Chun Hyang University Hospital Inha University Hospital Chung-Ang University Hospital |
| Litwa | Abromiskes Rehabilitation Center Medical Diagnostic and Treatment Centre |
| Poland | Krakow University Hospital |
| Singapur | Raffles hospital |
| Spain | Hospital Quirónsalud Barcelona Clínica Universidad de Navarra |
| Tajland | Bumrungrad International Hospital |
| Turkey | Medicana Hospitals Group Medistate Hospital Medipol Mega University Hospital Memorial Hospital Medical Park Hospitals Group Koc University Hospital Acibadem Maslak Hospital Liv Hospital Anadolu Medical Center Hisar Intercontinental Hospital Gaziosmanpasa Private Clinic Memorial Şişli Hospital Medical Park Gebze Clinic Memorial Bahçelievler Hospital Memorial Hospital Ataşehir Medical Park Göztepe Hospital Acibadem Taksim Clinic Medical Park Fatih Hospital |
4.3. Side Effects and Complications of PDT
4.4. The Future of PDT
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- 1. Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380-387. [CrossRef]
- 2. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250-281. [CrossRef]
- 3. Podbielska H, Sieroń A, Stręk W, eds. Diagnostyka i terapia fotodynamiczna. Wrocław: Wydawnictwo Medyczne Urban & Partner; 2004.
- 4. Osmałek T, Gośliński T. Rozwój badań dotyczących fotodynamicznej terapii onkologicznej. Farm Pol. 2009;65(8):549–552.
- 5. Herschel W. Experiments on the Refrangibility of the Invisible Rays of the Sun. Philos Trans R Soc Lond. 1800;90:284-292.
- 6. Cauvin JF. Des bienfaits de l’insolation. Rozprawa Ph.D. Thesis, University of Paris, France. 1815.
- 7. Finsen NR. Phototherapy. London: Edward Arnold; 1901.
- 8. Gøtzsche PC. Niels Finsen's treatment for lupus vulgaris. J R Soc Med. 2011;104(1):41–42. [CrossRef]
- 9. Raab O. Uber die Wirkung fluoreszierender Stoffe auf Infusorien. Z Biol. 1900;39:524–546.
- 10. Allison RR, Downie GH, Cuenca R, Hu XH, Jh Childs C, Sibata CH. Photosensitizers in clinical PDT. Photodiagnosis Photodyn Ther. 2004;1(1):27–42. [CrossRef]
- 11. Acroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74(5):656–669. [CrossRef]
- 12. MacCormack MA. Photodynamic Therapy. Adv Dermatol. 2006;22:219–258. [CrossRef]
- 13. Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380-387. [CrossRef]
- 14. Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38(8):2628-2635.
- 15. Ge R, Ahn JC, Shin JI, Bahk CW, He P, Chung PS. An in vitro and in vivo study of combination therapy with Photogem®-mediated photodynamic therapy and cisplatin on mouse cancer cells (CT-26). Photomed Laser Surg. 2010;29(3):155-160. [CrossRef]
- 16. Allison RR, Moghissi K. Photodynamic therapy (PDT): PDT mechanisms. Clin Endosc. 2013;46:24. [CrossRef]
- 17. Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B Biol. 2009;96:1-8. [CrossRef]
- 18. Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part two—cellular signaling, cell metabolism and modes of cell death. Photodiagn Photodyn Ther. 2005;2:1-23. [CrossRef]
- 19. A. Nowak-Stepniowska, P. Pergoł, A. Padzik-Graczyk [Photodynamic method of cancer diagnosis and therapy--mechanisms and applications] Postepy Biochem., 59 (2013), pp. 53-63.
- 20. Z. Luksiene Photodynamic therapy: mechanism of action and ways to improve the efficiency of treatment Med. (Kaunas), 39 (2003), pp. 1137-1150.
- 21. A. Juzeniene, J. Moan The history of PDT in Norway Photodiagn. Photodyn. Ther., 4 (2007), pp. 3-11. [CrossRef]
- 22. Fonseca SM, Pina J, Arnaut LG, Seixas de Melo J, Burrows HD, Chattopadhyay N, Alcácer L, Charas A, Morgado J, Monkman AP, Asawapirom U, Scherf U, Edge R, Navaratnam S. Triplet-state and singlet oxygen formation in fluorene-based alternating copolymers. J Phys Chem B. 2006;110:8278-8283. [CrossRef]
- 23. D. Kessel, N.L. Oleinick Photodynamic therapy and cell death pathways Methods Mol. Biol. (2010), pp. 35-46. [CrossRef]
- 24. E. Buytaert, M. Dewaele, P. Agostinis Molecular effectors of multiple cell death pathways initiated by photodynamic therapy Biochim. Biophys. Acta Rev. Cancer., 1776 (2007), pp. 86-107. [CrossRef]
- 25. N. Mehraban, H.S. Freeman Developments in PDT sensitizers for increased selectivity and singlet oxygen production Mater. (Basel Switz.), 8 (2015), pp. 4421-4456. [CrossRef]
- 26. Dugan M, Crawford E, Nseyo U. Photodynamic therapy (PDT) after transurethal resection (tur) for superficial papillary bladder carcinoma (SBC): a randomized trial. Proc ASCO. 1991;10:173.
- 27. Nseyo U, Dehaven J, Dougherty T. Photodynamic therapy (PDT) in the management of patients with resistant superficial bladder cancer: a long term experience. J Clin Laser Med Surg. 1998;16:61–68. [CrossRef]
- 28. Lightdale CJ, Heier SK, Marcon NE, McCoughan JS, Jr, Gerdes H, Overholt BF, et al. Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd:YAG laser for palliation of esophageal cancer: a multicenter randomized trial. Gastrointest Endosc. 1995;42:507–512. [CrossRef]
- 29. Biel M. Photodynamic therapy and the treating of head and neck cancers. J Clin Laser Radiat Surg. 1996;14:239–244. [CrossRef]
- 30. Overholt BF, Panjehpour M. Photodynamic therapy for Barrett’s esophagus. Gastrointest Endosc Clin N Am. 1997;7:207–220. [CrossRef]
- 31. Van den Bergh, H. (2001). Photodynamic therapy of age-related macular degeneration: History and principles. Seminars in Ophthalmology, 16(4), 181–200. [CrossRef]
- 32. Sterry W, Stockfleth E. Malignant epithelial tumors. In: Braun-Falco’s Dermatology. 3rd ed. Berlin, Heidelberg: Springer-Verlag; 2009. pp. 1357–1376.
- 33. Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. Epidermal and appendageal tumors. In: Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York: McGrawHill; 2012. pp. 1007–1094.
- 34. Morton CA, Szeimies RM, Sidoroff A, Braathen LR. European guidelines for topical photodynamic therapy part 1: Treatment delivery and current indications - actinic keratoses, Bowen’s disease, basal cell carcinoma. J Eur Acad Dermatol Venereol. 2013;27:536–544. [CrossRef]
- 35. Hamblin MR. Photodynamic Therapy for Cancer: What's Past is Prologue. Photochem Photobiol. 2020 May;96(3):506-516. Epub 2020 Jan 7. [CrossRef] [PubMed] [PubMed Central]
- 36. Jurklies B, Anastassiou G, Ortmans S, Schüler A, Schilling H, Schmidt-Erfurth U, Bornfeld N. Photodynamic therapy using verteporfin in circumscribed choroidal haemangioma. Br J Ophthalmol. 2003 Jan;87(1):84-9. [CrossRef] [PubMed] [PubMed Central]
- 37. Berr F. Photodynamic therapy for cholangiocarcinoma. Semin Liver Dis. 2004 May;24(2):177-87. [CrossRef] [PubMed]
- 38. Moore CM, Pendse D, Emberton M. Photodynamic therapy for prostate cancer--a review of current status and future promise. Nat Clin Pract Urol. 2009 Jan;6(1):18-30. [CrossRef] [PubMed]
- 39. Usuda J, Kato H, Okunaka T, Furukawa K, Tsutsui H, Yamada K, Suga Y, Honda H, Nagatsuka Y, Ohira T, Tsuboi M, Hirano T. Photodynamic therapy (PDT) for lung cancers. J Thorac Oncol. 2006 Jun;1(5):489-93. [PubMed]
- 40. Smijs TGM, Bouwstra JA, Schuitmaker HJ, Talebi M, Pavel S. A novel ex vivo skin model to study the susceptibility of the dermatomycete Trichophyton rubrum to photodynamic treatment in different growth phases. J Antimicrob Chemother. 2007;59:433-440. [CrossRef]
- 41. Berking C, Herzinger T, Flaig MJ, Brenner M, Borelli C, Degitz K. The efficacy of photodynamic therapy in actinic cheilitis of the lower lip: a prospective study of 15 patients. Dermatol Surg. 2007 Jul;33(7):825-30. [CrossRef] [PubMed]
- 42. Morton C, Horn M, Leman J, Tack B, Bedane C, Tjioe M, Ibbotson S, Khemis A, Wolf P. Comparison of topical methyl aminolevulinate photodynamic therapy with cryotherapy or Fluorouracil for treatment of squamous cell carcinoma in situ: Results of a multicenter randomized trial. Arch Dermatol. 2006 Jun;142(6):729-35. [CrossRef] [PubMed]
- 43. Zane C, Capezzera R, Sala R, Venturini M, Calzavara-Pinton P. Clinical and echographic analysis of photodynamic therapy using methylaminolevulinate as sensitizer in the treatment of photodamaged facial skin. Lasers Surg Med. 2007 Mar;39(3):203-9. [CrossRef] [PubMed]
- 44. Wildeman MA, Nyst HJ, Karakullukcu B, Tan BI. Photodynamic therapy in the therapy for recurrent/persistent nasopharyngeal cancer. Head Neck Oncol. 2009 Dec 17;1:40. [CrossRef] [PubMed] [PubMed Central]
- 45. Allison RR, Sheng C, Cuenca R, Bagnato VS, Austerlitz C, Sibata CH. Photodynamic therapy for anal cancer. Photodiagnosis Photodyn Ther. 2010 Jun;7(2):115-9. Epub 2010 May 7. [CrossRef] [PubMed]
- 46. Jerjes W, Upile T, Hamdoon Z, Mosse CA, Akram S, Hopper C. Photodynamic therapy outcome for oral dysplasia. Lasers Surg Med. 2011 Mar;43(3):192-9. [CrossRef] [PubMed]
- 47. Yu YE, Kuohung V, Gilchrest BA, Penrose C, Shim H. Photodynamic therapy for treatment of hand warts. Dermatol Surg. 2012 May;38(5):818-20. [CrossRef] [PubMed]
- 48. Bombeccari GP, Guzzi G, Gualini F, Gualini S, Santoro F, Spadari F. Photodynamic therapy to treat periimplantitis. Implant Dent. 2013 Dec;22(6):631-8. [CrossRef] [PubMed]
- 49. Sotiriou E, Apalla Z, Vrani F, Lallas A, Chovarda E, Ioannides D. Photodynamic therapy vs. imiquimod 5% cream as skin cancer preventive strategies in patients with field changes: a randomized intraindividual comparison study. J Eur Acad Dermatol Venereol. 2015 Feb;29(2):325-329. Epub 2014 Apr 23. [CrossRef] [PubMed]
- 50. Shafirstein G, Rigual NR, Arshad H, Cooper MT, Bellnier DA, Wilding G, Tan W, Merzianu M, Henderson BW. Photodynamic therapy with 3-(1'-hexyloxyethyl) pyropheophorbide-a for early-stage cancer of the larynx: Phase Ib study. Head Neck. 2016 Apr;38 Suppl 1(Suppl 1):E377-83. Epub 2015 Jun 29. [CrossRef] [PubMed] [PubMed Central]
- 51. Zhao Y, Tu P, Zhou G, Zhou Z, Lin X, Yang H, Lu Z, Gao T, Tu Y, Xie H, Zheng Q, Gu Y, Tao J, Zhu X. Hemoporfin Photodynamic Therapy for Port-Wine Stain: A Randomized Controlled Trial. PLoS One. 2016 May 26;11(5):e0156219. [CrossRef] [PubMed] [PubMed Central]
- 52. Maździarz A, Osuch B, Kowalska M, Nalewczyńska A, Śpiewankiewicz B. Photodynamic therapy in the treatment of vulvar lichen sclerosus. Photodiagnosis Photodyn Ther. 2017 Sep;19:135-139. Epub 2017 May 17. [CrossRef] [PubMed]
- 53. Fan L, Yin R, Lan T, Hamblin MR. Photodynamic therapy for rosacea in Chinese patients. Photodiagnosis Photodyn Ther. 2018 Dec;24:82-87. Epub 2018 Aug 15. [CrossRef] [PubMed] [PubMed Central]
- 54. Fisher C, Ali Z, Detsky J, Sahgal A, David E, Kunz M, Akens M, Chow E, Whyne C, Burch S, Wilson BC, Yee A. Photodynamic Therapy for the Treatment of Vertebral Metastases: A Phase I Clinical Trial. Clin Cancer Res. 2019 Oct 1;25(19):5766-5776. Epub 2019 Jul 2. [CrossRef] [PubMed]
- 55. Niazi FH, Koppolu P, Tanvir SB, Samran A, Alqerban A. Clinical efficacy of photodynamic therapy in the treatment of necrotizing ulcerative periodontitis among HIV seropositive patients: A randomized controlled clinical trial. Photodiagnosis Photodyn Ther. 2020 Mar;29:101608. Epub 2019 Nov 23. [CrossRef] [PubMed]
- 56. Zhang H, Shi L, Zhang Y, Wang P, Zhang G, Cao Y, Zhou Z, Wang X. Modified photodynamic therapy to minimize pain in the treatment of condylomata acuminata: A prospective, randomized, self-controlled study. Photodiagnosis Photodyn Ther. 2020 Dec;32:101915. Epub 2020 Jul 4. [CrossRef] [PubMed]
- 57. Hassan SNE, Hussein TM, Eldeeb ME. Photodynamic therapy using methylene blue and intense pulsed light versus intense pulsed light alone in treatment of verruca: A randomized controlled study. Photodiagnosis Photodyn Ther. 2021 Dec;36:102541. Epub 2021 Sep 20. [CrossRef] [PubMed]
- 58. Buzzá HH, Stringasci MD, de Arruda SS, Crestana RHS, de Castro CA, Bagnato VS, Inada NM. HPV-induced condylomata acuminata treated by Photodynamic Therapy in comparison with trichloroacetic acid: A randomized clinical trial. Photodiagnosis Photodyn Ther. 2021 Sep;35:102465. Epub 2021 Jul 29. [CrossRef] [PubMed]
- 59. Vellappally S, Mahmoud MH, Alaqeel SM, Alotaibi RN, Almansour H, Alageel O, Hashem M, Fouad H, Saadaldin S, Sukumaran A. Efficacy of antimicrobial photodynamic therapy versus antiviral therapy in the treatment of herpetic gingivostomatitis among children: Aa randomized controlled clinical trial. Photodiagnosis Photodyn Ther. 2022 Sep;39:102895. Epub 2022 Apr 30. [CrossRef] [PubMed]
- 60. Swapna LA, Alawad AO, Abdullah AlAmri L, Sayed Abdul N, Qamar Z, Vempalli S, Niazi FH. Efficacy of 5-aminolevulinic acid-mediated photodynamic therapy in patients with nicotine stomatitis. Photodiagnosis Photodyn Ther. 2023 Mar;41:103152. Epub 2022 Dec 2. [CrossRef] [PubMed]
- 61. Bezerra DT, La Selva A, Cecatto RB, Deana AM, Prates RA, Bussadori SK, Mesquita-Ferrari RA, Motta LJ, Fernandes KPS, Martimbianco ALC, Frochot C, Pereira BJ, Rossi F, Mimica MJ, Horliana ACRT. Antimicrobial Photodynamic Therapy in the Nasal Decolonization of Maintenance Hemodialysis Patients: A Pilot Randomized Trial. Am J Kidney Dis. 2023 May;81(5):528-536.e1. Epub 2022 Nov 14. [CrossRef] [PubMed]
- 62. Shao J, Hu M, Wang W, Pan Z, Zhao D, Liu J, Lv M, Zhang Y, Li Z. Indocyanine green based photodynamic therapy for keloids: Fundamental investigation and clinical improvement. Photodiagnosis Photodyn Ther. 2024 Feb;45:103903. Epub 2023 Nov 19. [CrossRef] [PubMed]
- 63. Fernandez-Montero A, Zuaznabar J, Pina-Sanchez M, Maestro S, Martin-Navarro L, Muñoz-Rodríguez N, Olagüe C, Pastrana M, Martínez-Fernández M, Camps G, Rodriguez JA, Marchese FP, Zazpe J, Pozuelo M, Del Pozo JL, Quiroga J, Pineda-Lucena A, Reina G, Kolenda J, Moreno-Galarraga L, Gonzalez-Aseguinolaza G, Rua M, Smerdou C, Carmona-Torre F, Argemi J. Photodynamic nasal SARS-CoV-2 decolonization shortens infectivity and influences specific T-Cell responses. Front Cell Infect Microbiol. 2023 Jan 25;13:1110467. [CrossRef] [PubMed] [PubMed Central]
- 64. Li X, Lee S, Yoon J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem Soc. 2018;47:1174−1188. [CrossRef]
- 65. Dyrała A, Kaleta-Richter M, Cylupa K, Porwoł P, Kawczyk-Krupka A. Zastosowanie hiperycyny w terapii fotodynamicznej – przegląd badań preklinicznych. Acta Bio-Optica et Informatica Medica. 2015;209-215.
- 66. Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. [CrossRef]
- 67. Lu Y, Sun W, Du J, Fan J, Peng X. Immuno-photodynamic Therapy (IPDT): Organic Photosensitizers and Their Application in Cancer Ablation. ACS Au. 2023;3:682–699. [CrossRef]
- 68. Evensen JF, Moan J, Winkelman JW. Toxic and phototoxic effects of tetraphenylpophinesulphonate and haematoporphyrin derivative in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;51:477-491. [CrossRef]
- 69. Berg K, Bommer JC, Moan J. Evaluation of sulfonated aluminum phthalocyanines for use in photochemotherapy. A study on the relative efficiencies of photoinactivation. Photochem Photobiol. 1989;49:587-594. [CrossRef]
- 70. Ma L, Moan J, Berg K. Evaluation of a new photosensitizer, meso-tetra-hydroksyphenyl-chlorin, for use in photodynamic therapy: comparison of its photobiological properties with those of two other photosensitizers. Int J Cancer. 1994;57:883-888. [CrossRef]
- 71. Cunderlikova B, Gangeskar L, Moan J. Acid-base properties of chlorin e6: relation to cellular uptake. J Photochem Photobiol B. 1999;53:81-90. [CrossRef]
- 72. Friberg EG, Cunderlikova B, Pettersen EO, Moan J. PH effects on the cellular uptake of four photosensitizing drugs evaluated for use in photodynamic therapy of cancer. Cancer Lett. 2003;195:73-80. [CrossRef]
- 73. Cunderlikova B, Sikurova L, Moan J. PH, serum proteins and ionic strength influence the uptake of merocyanine 540 by WiDr cells and its interaction with membrane structures. Bioelectrochemistry. 2003;59:1-10. [CrossRef]
- 74. Uzdensky AB, Bragin DE, Kolosov MS, Kubin A, Loew HG, Moan J. Photodynamic effect of hypericin and a water-soluble derivative on isolated crayfish neuron and surrounding glial cells. J Photochem Photobiol B. 2003;72:27-33. [CrossRef]
- 75. Peng Q, Brown SB, Moan J, Nesland JM, Wainwright M, Griffiths J, Dixon B, Cruse-Sawyer J, Vernon D. Biodistribution of a methylene blue derivative in tumor and normal tissue of rats. J Photochem Photobiol B. 1993;20:63-71. [CrossRef]
- 76. Graczykowa A. Fotodynamiczna metoda rozpoznawania i leczenia nowotworów. 1999.
- 77. Kübler AC. Medical Laser Application. 2005;20(1):37-45. [CrossRef]
- 78. Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Ross BD. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res. 2006;12(22):6677–6686. [CrossRef]
- 79. Fakhar-E-Alam M, Ali SMU, Ibupoto ZH, Kimleang K, Atif M, Kashif M, Willander M. Sensitivity of A-549 human lung cancer cells to nanoporous zinc oxide conjugated with Photofrin. Lasers Med Sci. 2012;27(3):607–614. [CrossRef]
- 80. Jiang F, Lilge L, Grenier J, Li Y, Wilson MD, Chopp M. Photodynamic therapy of U87 human glioma in nude rat using liposome-delivered photofrin. Lasers Surg Med. 1998;22(2):74–80. [CrossRef]
- 81. Savarimuthu WP, Gananathan P, Rao AP, Manickam E, Singaravelu G. Protoporphyrin IX-gold nanoparticle conjugates for targeted photodynamic therapy–An in-vitro study. J Nanosci Nanotechnol. 2014;15(8):5577–5584. [CrossRef]
- 82. Juárez AAS, Alvarado EM, Gallegos ER. Cell death induced by photodynamic therapy with the conjugate of gold nanoparticles-PpIX in HeLa cell line. In AIP conference proceedings. 2019;2099:040008. [CrossRef]
- 83. Ashjari M, Dehfuly S, Fatehi D, Shabani R, Koruji M. Efficient functionalization of gold nanoparticles using cysteine conjugated protoporphyrin IX for singlet oxygen production in vitro. RSC Adv. 2015;5(127):104621–104628. [CrossRef]
- 84. Ning LG, Liu P, Wang B, Li CM, Kang ET, Lu ZS, Xu LQ. Hydrothermal derived protoporphyrin IX nanoparticles for inactivation and imaging of bacteria strains. J Colloid Interface Sci. 2019;549:72–79. [CrossRef]
- 85. Yang K, Wang C, Liu C, Ding S, Tian F, Li F. Bioluminescence-initiated photodynamic therapy bridged on high-luminescent carbon dots-conjugated protoporphyrin IX. J Mater Sci. 2019;54(4):3383–3391. [CrossRef]
- 86. Lismont M, Dreesen L, Heinrichs B, Páez CA. Protoporphyrin IX-functionalized AgSiO2 Core-Shell nanoparticles: Plasmonic enhancement of fluorescence and singlet oxygen production. Photochem Photobiol. 2016;92(2):247–256. [CrossRef]
- 87. Wang M, Geilich BM, Keidar M, Webster TJ. Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome-mediated photodynamic therapy and cold atmospheric plasma. Int J Nanomedicine. 2017;12:4117–4127. [CrossRef]
- 88. Yan L, Miller J, Yuan M, Liu JF, Busch TM, Tsourkas A, Cheng Z. Improved photodynamic therapy efficacy of Protoporphyrin IX-loaded polymeric micelles using Erlotinib pretreatment. Biomacromolecules. 2017;18(6):1836–1844. [CrossRef]
- 89. Hinger D, Navarro F, Käch A, Thomann JS, Mittler F, Couffin AC, Maake C. Photoinduced effects of m-tetrahydroxyphenylchlorin loaded lipid nanoemulsions on multicellular tumor spheroids. J Nanobiotechnology. 2016;14(1):68. [CrossRef]
- 90. Amirshaghaghi A, Yan L, Miller J, Daniel Y, Stein JM, Busch TM, Tsourkas A. Chlorin e6-coated superparamagnetic Iron oxide nanoparticle (SPION) nanoclusters as a Theranostic agent for dual-mode imaging and photodynamic therapy. Sci Rep. 2019;9(1):2613. [CrossRef]
- 91. Kumari P, Rompicharla SVK, Bhatt H, Ghosh B, Biswas S. Development of chlorin e6-conjugated poly(ethylene glycol)-poly(d,l-lactide) nanoparticles for photodynamic therapy. Nanomedicine. 2019;14(7):819–834. [CrossRef]
- 92. Konan-Kouakou YN, Boch R, Gurny R, Allémann E. In vitro and in vivo activities of verteporfin-loaded nanoparticles. J Control Release. 2005;103(1):83–91. [CrossRef]
- 93. Wang S, Fan W, Kim G, Hah HJ, Lee YEK, Kopelman R, Pandey RK. Novel methods to incorporate photosensitizers into nanocarriers for cancer treatment by photodynamic therapy. Lasers Surg Med. 2011;43(7):686–695. [CrossRef]
- 94. Li L, Chen Y, Chen W, Tan Y, Chen H, Yin J. Photodynamic therapy based on organic small molecular fluorescent dyes. Chinese Chem Lett. 2019;30(10):1689-1703. [CrossRef]
- 95. Pogue BW, Gibbs-Strauss S, Valdés PA, Samkoe K, Roberts DW, Paulsen KD. Review of Neurosurgical Fluorescence Imaging Methodologies. IEEE J Sel Top Quantum Electron. 2010;25(1):1-16. [CrossRef]
- 96. Jing R, Wang Q, Chen L, Li G, Li R, Zhang L, Zhang H, Zuo B, Seow Y, Qiao X, Wang B, Xu J, Chen J, Song T. Functional imaging and targeted drug delivery in mice and patient tumors with a cell nucleolus-localizing and tumor-targeting peptide. Biomaterials. 2022;289:1-12. [CrossRef]
- 97. Fukushi J, Makagiansar IT, Stallcup WB. NG2 Proteoglycan Promotes Endothelial Cell Motility and Angiogenesis via Engagement of Galectin-3 and α3β1 Integrin. Mol Biol Cell. 2004;15(8):3580-3590. [CrossRef]
- 98. Bando Y, Grimm C, Cornejo VH, Yuste R. Genetic voltage indicators. BMC Biol. 2019;17:71. [CrossRef]
- 99. Morton CA, McKenna KE, Rhodes LE. British Association of Dermatologists' guidelines for the management of squamous cell carcinoma in situ (Bowen's disease) 2014. Br J Dermatol. 2014;170(2):245-260. [CrossRef]
- 100. Biel MA. Photodynamic therapy of head and neck cancers. Methods Mol Biol. 2010;635:281-293. [CrossRef]
- 101. Pervaiz S, Olivo M. Art and science of photodynamic therapy. Clin Exp Pharmacol Physiol. 2006;33(5-6):551-556. [CrossRef]
- 102. Allison RR, Bagnato VS, Cuenca R, Downie GH, Sibata CH. The future of photodynamic therapy in oncology. Future Oncol. 2006;2:53-71. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





