Submitted:
26 May 2024
Posted:
27 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
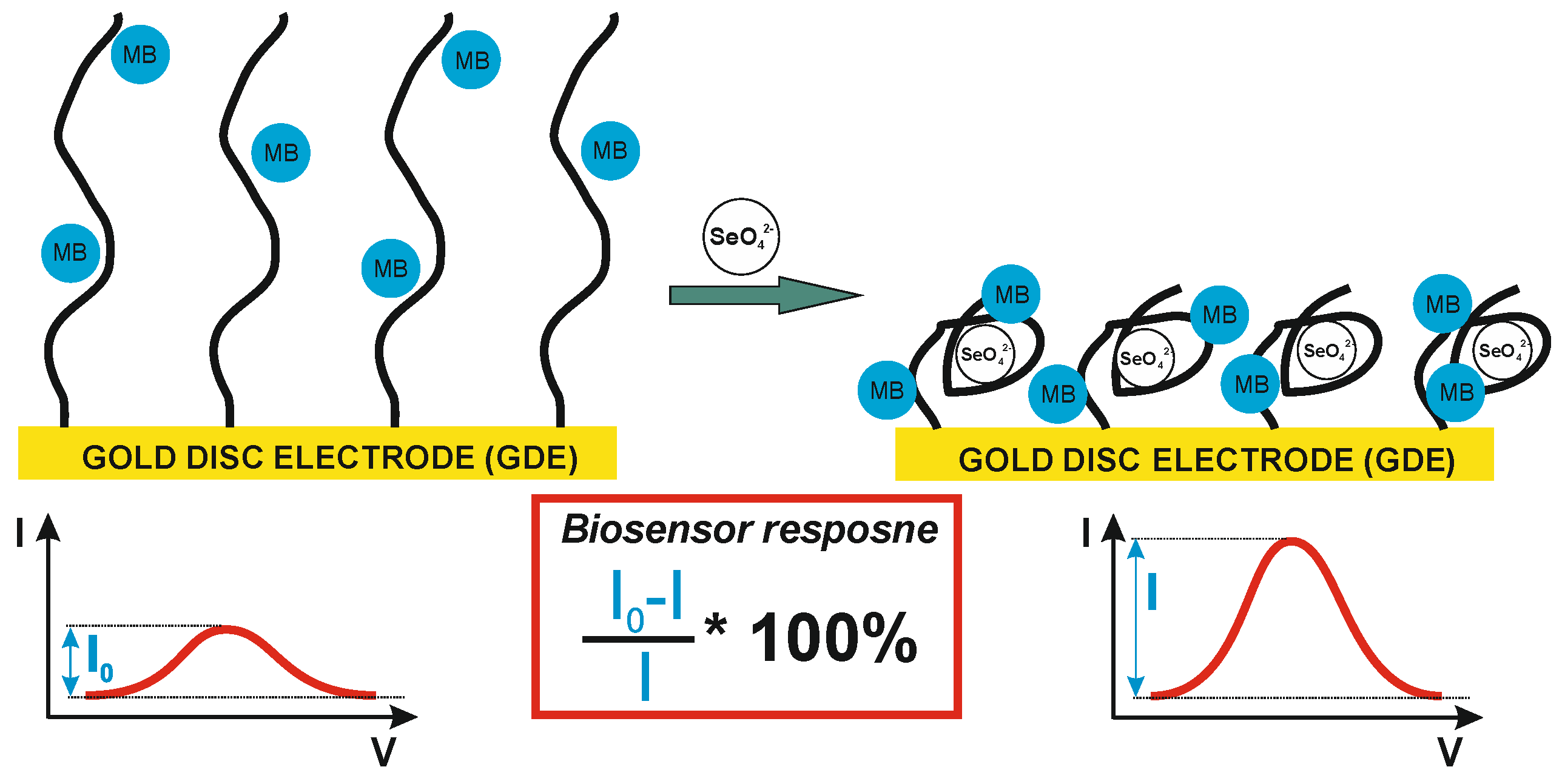
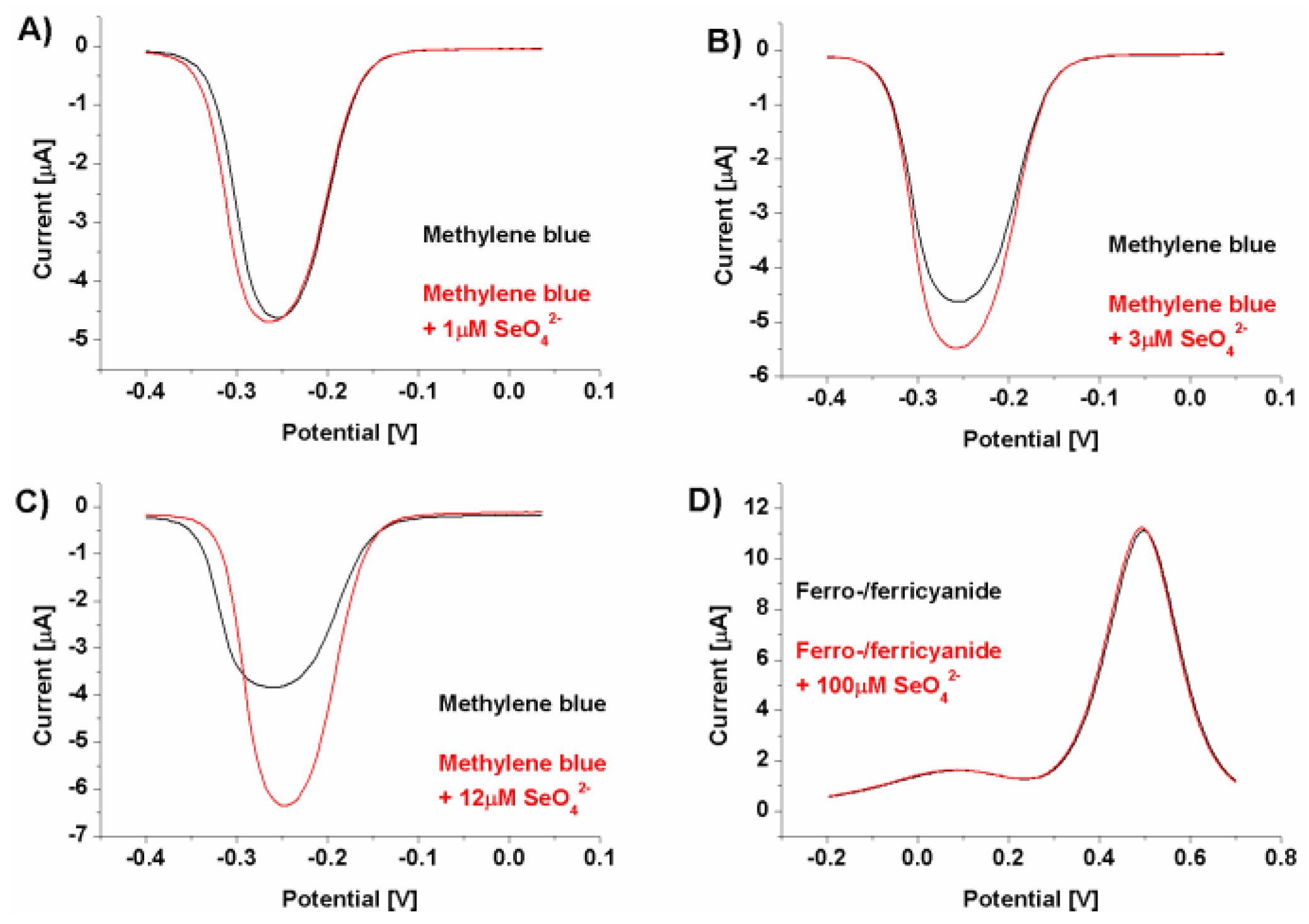
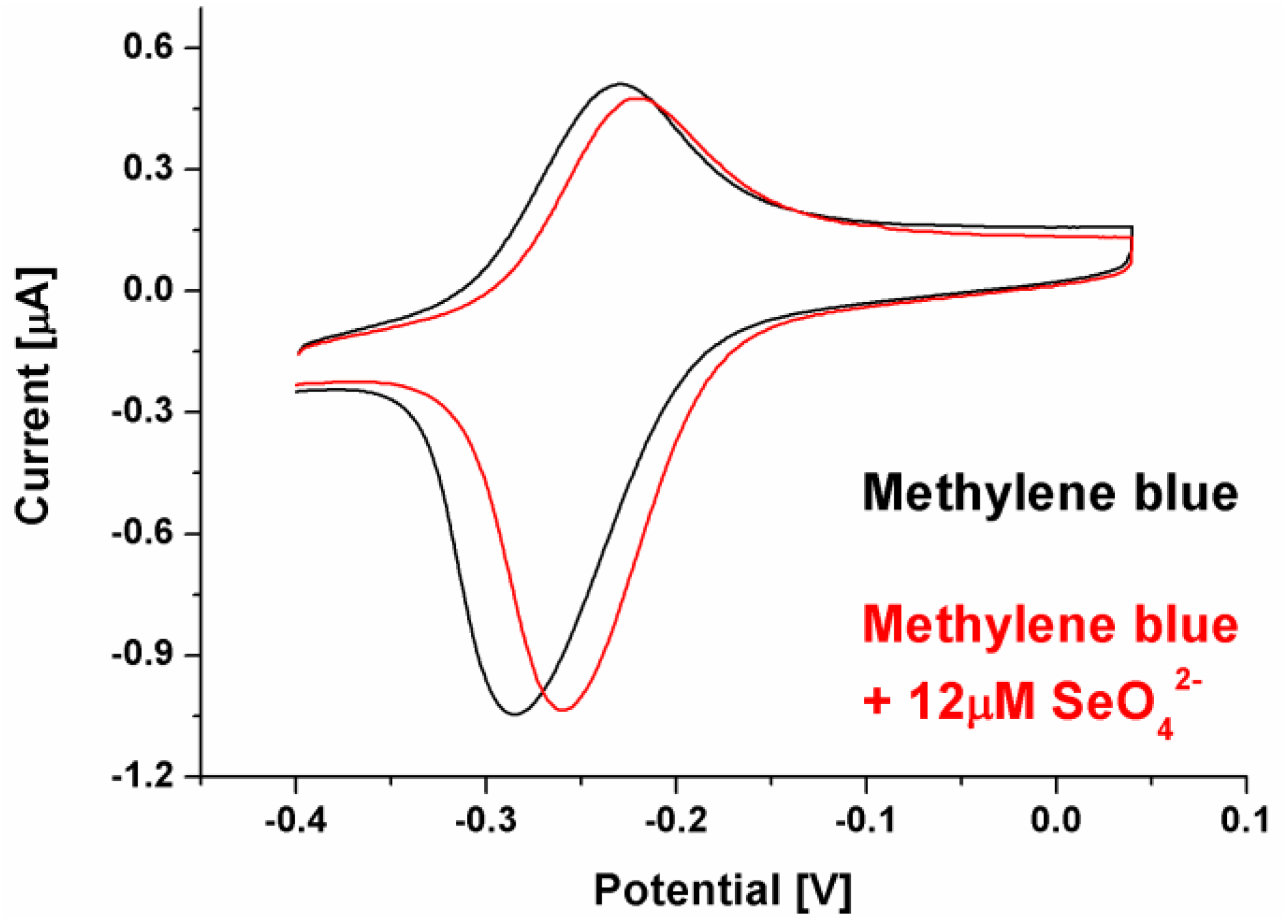
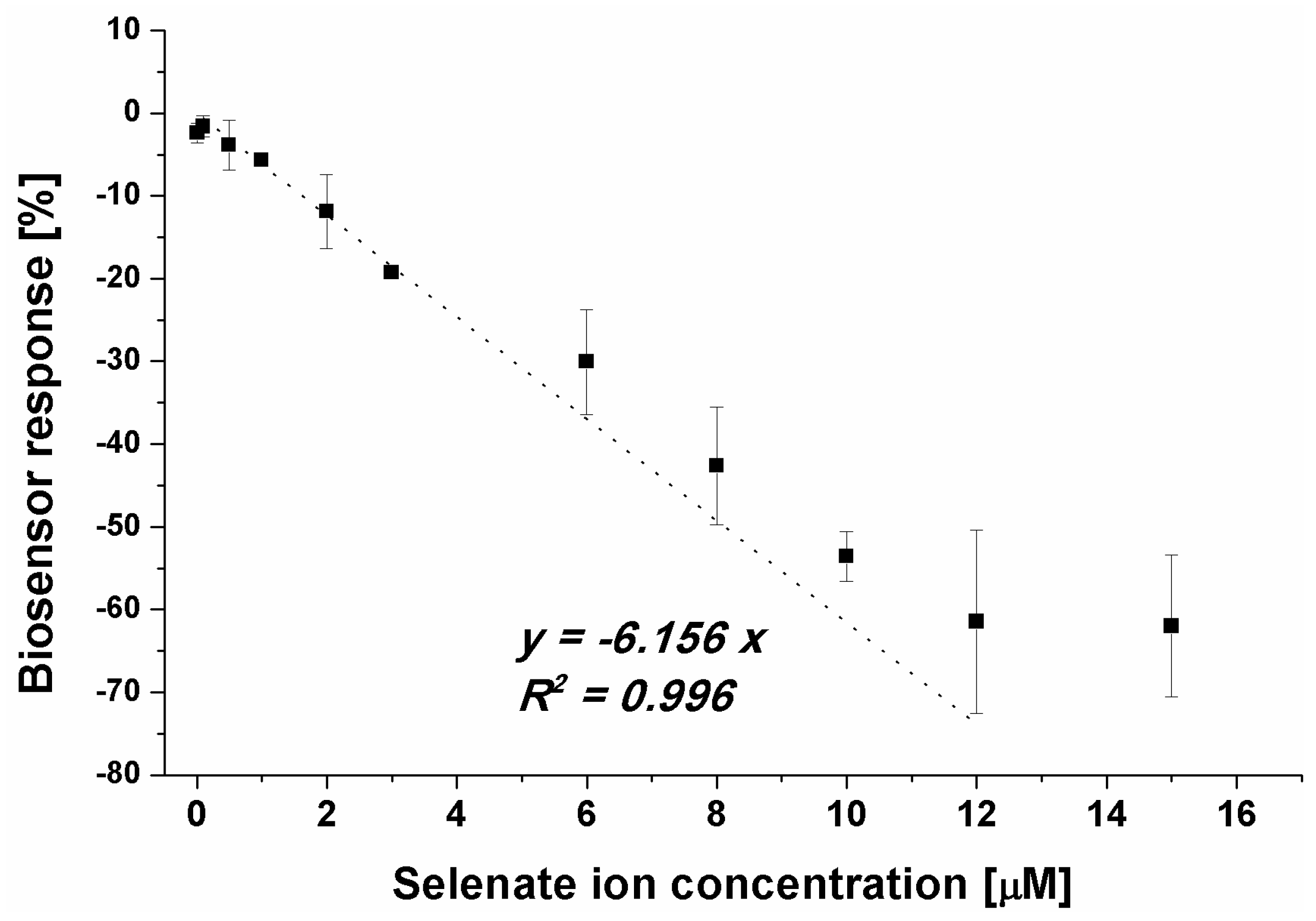
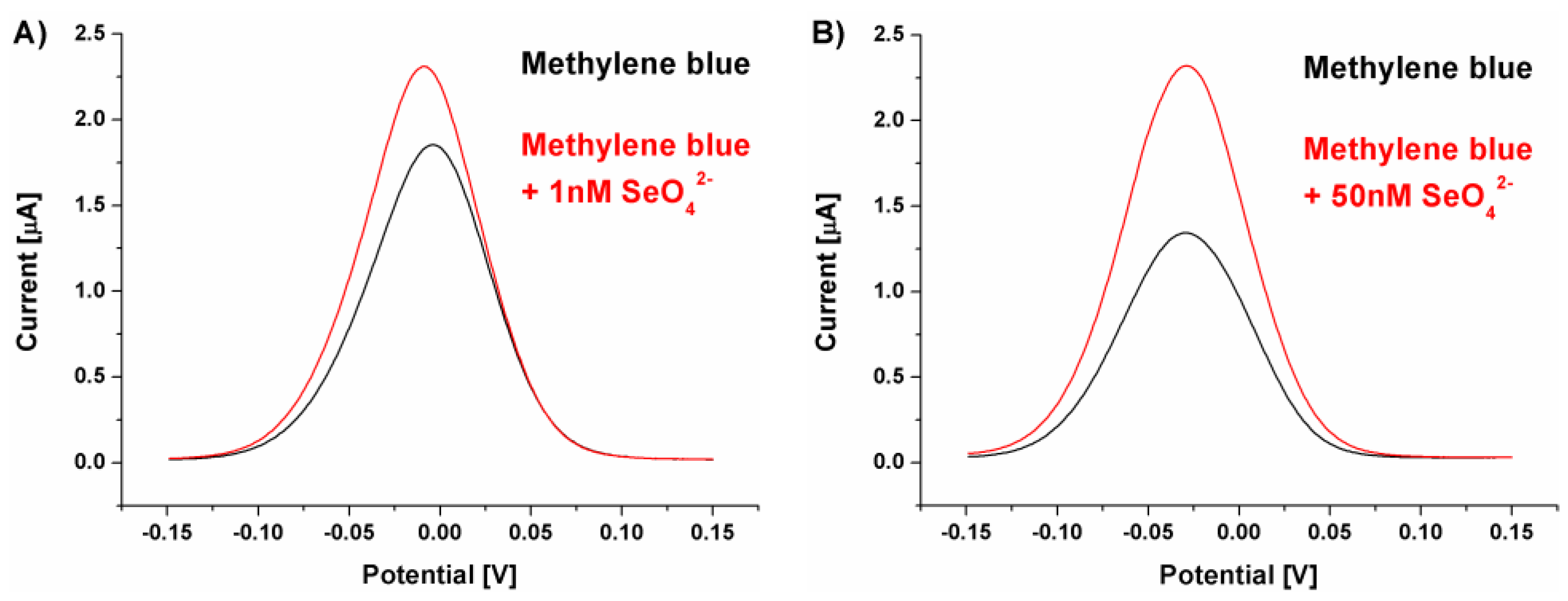
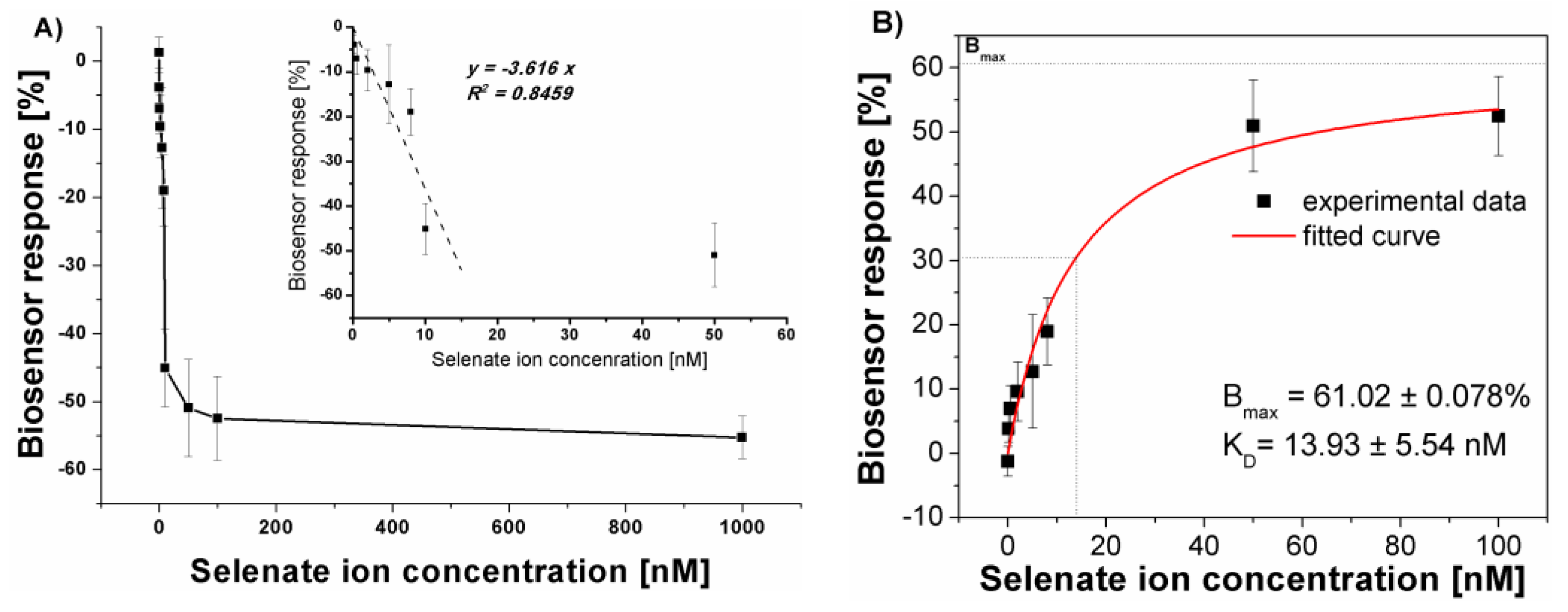
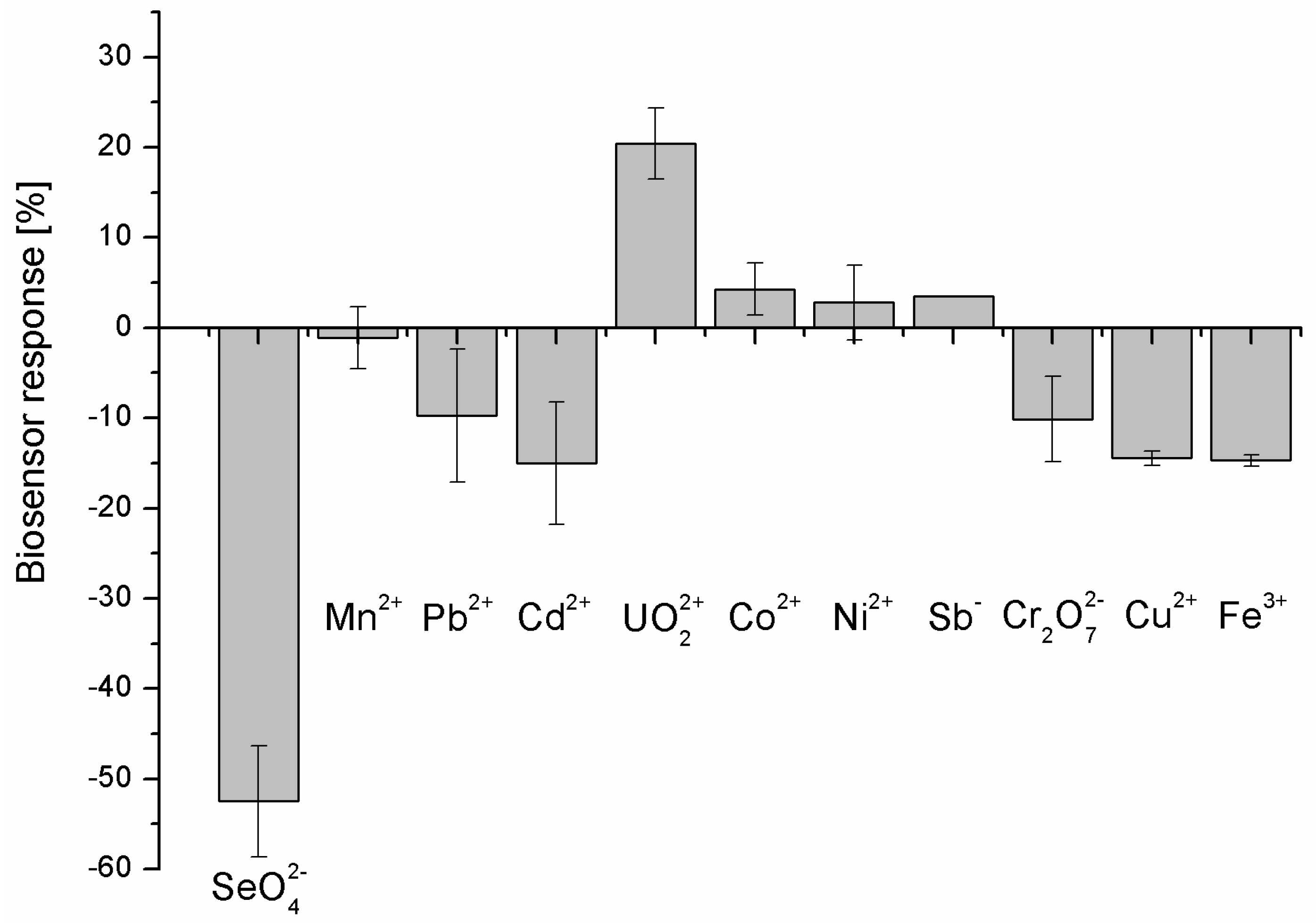
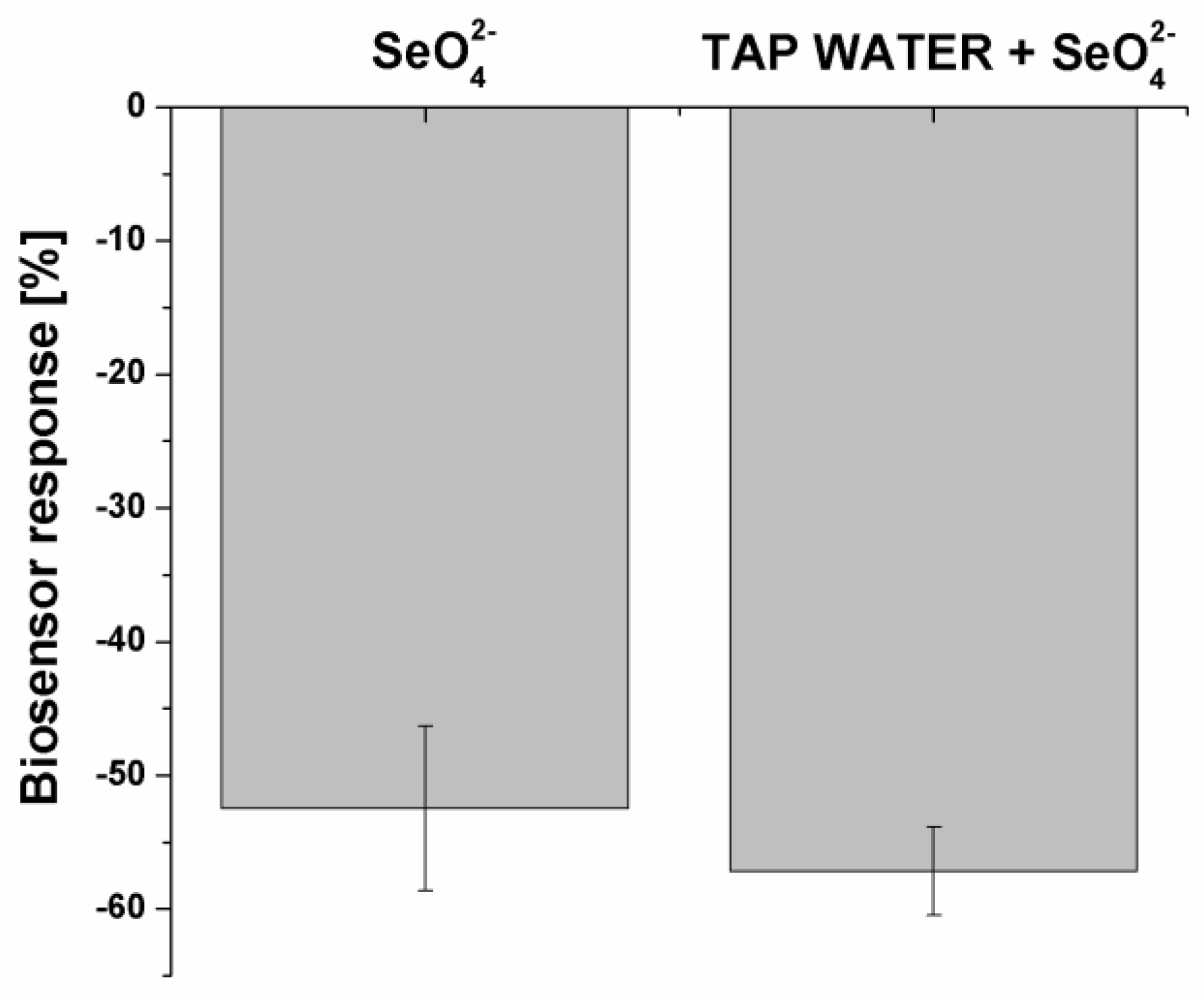
2. Results and Discussion
3. Materials and Methods
Reagents
Solutions
Gold Electrodes Cleaning and Modification
Electrochemical Measurements
4. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somogyi, Z.; Kádár, I.; Kiss, I.; Juríková, T.; Szekeres, L.; Balla, Š.; Nagy, P.; Bakonyi, G. Comparative toxicity of the selenate and selenite to the potworm Enchytraeus albidus (Annelida: Enchytraeidae) under laboratory conditions. Eur. J. Soil Biol. 2012, 50, 159–164. [Google Scholar] [CrossRef]
- Wake, B.D.; Bowie, A.R.; Butler, E.C.V.; Haddad, P.R. Modern preconcentration methods for the determination of selenium species in environmental water samples. TrAC Trends Anal. Chem. 2004, 23, 491–500. [Google Scholar] [CrossRef]
- Bleiman, N.; Mishael, Y.G. Selenium removal from drinking water by adsorption to chitosan–clay composites and oxides: Batch and columns tests. J. Hazard. Mater. 2010, 183, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Ečimović, S.; Velki, M.; Vuković, R.; Štolfa Čamagajevac, I.; Petek, A.; Bošnjaković, R.; Grgić, M.; Engelmann, P.; Bodó, K.; Filipović-Marijić, V.; et al. Acute toxicity of selenate and selenite and their impacts on oxidative status, efflux pump activity, cellular and genetic parameters in earthworm Eisenia andrei. Chemosphere 2018, 212, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Palmer, I.S.; Olson, O.E. Relative Toxicities of Selenite and Selenate in the Drinking Water of Rats. J. Nutr. 1974, 104, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Wallschläger, D.; Roehl, R. Determination of inorganic selenium speciation in waters by ion chromatography-inductively coupled plasma-mass spectrometry using eluant elimination with a membrane suppressor. J. Anal. At. Spectrom. 2001, 16, 922–925. [Google Scholar] [CrossRef]
- Jackson, B.P.; Miller, W.P. Soluble Arsenic and Selenium Species in Fly Ash/Organic Waste-Amended Soils Using Ion Chromatography−Inductively Coupled Plasma Mass Spectrometry. Environ. Sci. Technol. 1999, 33, 270–275. [Google Scholar] [CrossRef]
- Yang, R.; Li, Q.; Zhou, W.; Yu, S.; Liu, J. Speciation Analysis of Selenium Nanoparticles and Inorganic Selenium Species by Dual-Cloud Point Extraction and ICP-MS Determination. Anal. Chem. 2022, 94, 16328–16336. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, A.H.; Tuzen, M.; Kazi, T.G. Ultrasonic assisted dispersive liquid-liquid microextraction method based on deep eutectic solvent for speciation, preconcentration and determination of selenium species (IV) and (VI) in water and food samples. Talanta 2017, 175, 352–358. [Google Scholar] [CrossRef]
- Goldberg, S.; Martens, D.A.; Forster, H.S.; Herbel, M.J. Speciation of Selenium(IV) and Selenium(VI) using Coupled Ion Chromatography—Hydride Generation Atomic Absorption Spectrometry. Soil Sci. Soc. Am. J. 2006, 70, 41–47. [Google Scholar] [CrossRef]
- Huang, P.M.; Fujii, R. Selenium and Arsenic. 2018; pp. 793–831. [Google Scholar] [CrossRef]
- Finley, J.W.; Sigrid-Keck, A.; Robbins, R.J.; Hintze, K.J. Selenium Enrichment of Broccoli: Interactions between Selenium and Secondary Plant Compounds. J. Nutr. 2005, 135, 1236–1238. [Google Scholar] [CrossRef]
- Lavi, N.; Alfassi, Z.B. Determination of trace amounts of cadmium, cobalt, chromium, iron, molybdenum, nickel, selenium, titanium, vanadium and zinc in blood and milk by neutron activation analysis. Analyst 1990, 115, 817. [Google Scholar] [CrossRef] [PubMed]
- Utterback, P.L.; Parsons, C.M.; Yoon, I.; Butler, J. Effect of supplementing selenium yeast in diets of laying hens on egg selenium content. Poult. Sci. 2005, 84, 1900–1901. [Google Scholar] [CrossRef]
- Ando, M.; Takizawa, M.; Suwabe, S.; Yamato, S.; Shimada, K. Determination of Selenium in Human Serum by Liquid Chromatography/Electron Capture Atmospheric Pressure Chemical Ionization Mass Spectrometry after Acid Digestion and Derivatization Using 2,3-Diaminonaphthalene. Eur. J. Mass Spectrom. 2003, 9, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.; Manoochehri, M. A nanocomposite consisting of MIL-101(Cr) and functionalized magnetite nanoparticles for extraction and determination of selenium(IV) and selenium(VI). Microchim. Acta 2018, 185, 196. [Google Scholar] [CrossRef] [PubMed]
- Karaś, K.; Zioła-Frankowska, A.; Frankowski, M. New Method for Simultaneous Arsenic and Selenium Speciation Analysis in Seafood and Onion Samples. Molecules 2021, 26, 6223. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, J.; Yang, M.; Yang, G.; Wu, Y.; Fu, F. Speciation analysis of selenium in rice samples by using capillary electrophoresis-inductively coupled plasma mass spectrometry. Talanta 2011, 84, 983–988. [Google Scholar] [CrossRef]
- Rubinskaya, T.B.; Kovaleva, S.V.; Kulagin, E.M.; Gladyshev, V.P. Determination of Selenium(IV) by Stripping Voltammetry at a Mercury-Film Electrode. J. Anal. Chem. 2003, 58, 165–170. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Einaga, Y. Electrochemical Detection of Selenium (IV) and (VI) at Gold-Modified Diamond Electrodes. Electrocatalysis 2013, 4, 367–374. [Google Scholar] [CrossRef]
- Beni, V.; Collins, G.; Arrigan, D.W.M. Investigation into the voltammetric behaviour and detection of selenium(IV) at metal electrodes in diverse electrolyte media. Anal. Chim. Acta 2011, 699, 127–133. [Google Scholar] [CrossRef]
- Andrews, R.W.; Johnson, D.C. Voltammetric deposition and stripping of selenium(IV) at a rotating gold-disk electrode in 0.1M perchloric acid. Anal. Chem. 1975, 47, 294–299. [Google Scholar] [CrossRef]
- Fakude, C.T.; Arotiba, O.A.; Moutloali, R.; Mabuba, N. Nitrogen-doped Graphene Electrochemical Sensor for Selenium (IV) in Water. Int. J. Electrochem. Sci. 2019, 14, 9391–9403. [Google Scholar] [CrossRef]
- Idris, A.O.; Mabuba, N.; Nkosi, D.; Maxakato, N.; Arotiba, O.A. Electrochemical detection of selenium using glassy carbon electrode modified with reduced graphene oxide. Int. J. Environ. Anal. Chem. 2017, 97, 534–547. [Google Scholar] [CrossRef]
- Jain, R.; Thakur, A.; Kumar, P.; Pooja, D. Au/ZnO nanocomposites decorated ITO electrodes for voltammetric sensing of selenium in water. Electrochim. Acta 2018, 290, 291–302. [Google Scholar] [CrossRef]
- Ali, A.G.; Altahan, M.F.; Beltagi, A.M.; Hathoot, A.A.; Abdel-Azzem, M. Voltammetric and impedimetric determinations of seleniumiv by an innovative gold-free poly(1-aminoanthraquinone)/multiwall carbon nanotube-modified carbon paste electrode. RSC Adv. 2022, 12, 4988–5000. [Google Scholar] [CrossRef]
- John, N.; Abraham, K.E. Detection of Selenium and Nickel Metal Ion in Water Using Mn3O4-Cn-Modified Electrode. Int. J. Electrochem. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Guidelines for drinking-water quality: Fourth edition incorporating the first and second addenda [Internet]; World Health Organization: Geneva, 2022; ISBN 9789240045064.
- Motlagh, M.K.; Noroozifar, M.; Kraatz, H.-B. Highly sensitive and selective detection of selenate in water samples using an enzymatic gold nanodendrite biosensor. Can. J. Chem. 2023, 101, 440–448. [Google Scholar] [CrossRef]
- Motlagh, M.K.; Noroozifar, M.; Sodhi, R.N.S.; Kraatz, H. Development of a Bacterial Enzyme-Based Biosensor for the Detection and Quantification of Selenate. Chem. – A Eur. J. 2022, 28. [Google Scholar] [CrossRef]
- Kolliopoulos, A.V.; Metters, J.P.; Banks, C.E. Electroanalytical sensing of selenium(iv) utilising screen printed graphite macro electrodes. Anal. Methods 2013, 5, 851. [Google Scholar] [CrossRef]
- Ruscito, A.; DeRosa, M.C. Small-Molecule Binding Aptamers: Selection Strategies, Characterization, and Applications. Front. Chem. 2016, 4. [Google Scholar] [CrossRef]
- Carothers, J.M.; Goler, J.A.; Kapoor, Y.; Lara, L.; Keasling, J.D. Selecting RNA aptamers for synthetic biology: investigating magnesium dependence and predicting binding affinity. Nucleic Acids Res. 2010, 38, 2736–2747. [Google Scholar] [CrossRef]
- Ziółkowski, R.; Górski, Ł. Electrochemical metal sensors with DNA receptor layers. Curr. Anal. Chem. 2014, 10, 600–608. [Google Scholar] [CrossRef]
- Jarczewska, M.; Szymczyk, A.; Zajda, J.; Olszewski, M.; Ziółkowski, R.; Malinowska, E. Recent Achievements in Electrochemical and Optical Nucleic Acids Based Detection of Metal Ions. Molecules 2022, 27, 7481. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, C.; Ma, T.; Liu, X.; Chen, Z.; Li, S.; Deng, Y. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J. Nanobiotechnology 2021, 19, 166. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Mirihana Arachchilage, G.; Basu, S. Metal Cations in G-Quadruplex Folding and Stability. Front. Chem. 2016, 4. [Google Scholar] [CrossRef]
- Wang, Y.; Luan, B.-Q.; Yang, Z.; Zhang, X.; Ritzo, B.; Gates, K.; Gu, L.-Q. Single Molecule Investigation of Ag+ Interactions with Single Cytosine-, Methylcytosine- and Hydroxymethylcytosine-Cytosine Mismatches in a Nanopore. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Hossain, Z.; Huq, F. Studies on the interaction between Cd2+ ions and DNA. J. Inorg. Biochem. 2002, 90. [Google Scholar] [CrossRef]
- Rossberg, A.; Abe, T.; Okuwaki, K.; Barkleit, A.; Fukuzawa, K.; Nakano, T.; Mochizuki, Y.; Tsushima, S. Destabilization of DNA through interstrand crosslinking by UO22+. Chem. Commun. 2019, 55. [Google Scholar] [CrossRef]
- Torigoe, H.; Miyakawa, Y.; Ono, A.; Kozasa, T. Positive cooperativity of the specific binding between Hg2+ ion and T:T mismatched base pairs in duplex DNA. Thermochim. Acta 2012, 532. [Google Scholar] [CrossRef]
- Kohlberger, M.; Gadermaier, G. SELEX: Critical factors and optimization strategies for successful aptamer selection. Biotechnol. Appl. Biochem. 2022, 69, 1771–1792. [Google Scholar] [CrossRef]
- Edsall, J.T. The size and shape of protein molecules. In Fortschritte der Chemischen Forschung Volume 1; Springer Berlin Heidelberg: Berlin, Heidelberg; pp. 119–174. [CrossRef]
- Rahm, M.; Hoffmann, R.; Ashcroft, N.W. Atomic and Ionic Radii of Elements 1 –96. Chem. – A Eur. J. 2016, 22, 14625–14632. [Google Scholar] [CrossRef]
- https://basepairbio.com/multiplex-selex-venn-multiplex-selex/.
- Sankar, K.; Kuzmanović, U.; Schaus, S.E.; Galagan, J.E.; Grinstaff, M.W. Strategy, Design, and Fabrication of Electrochemical Biosensors: A Tutorial. ACS Sensors 2024. [Google Scholar] [CrossRef]
- Szymczyk, A.; Soliwodzka, K.; Moskal, M.; Różanowski, K.; Ziółkowski, R. Further insight into the possible influence of electrode blocking agents on the stem-loop based electrochemical DNA sensor parameters. Sensors Actuators B Chem. 2022, 354, 131086. [Google Scholar] [CrossRef]
- Wong, K.L.; Liu, J. Factors and methods to modulate DNA hybridization kinetics. Biotechnol. J. 2021, 16. [Google Scholar] [CrossRef]
- Krishnamurthy, R. Role of pK a of Nucleobases in the Origins of Chemical Evolution. Acc. Chem. Res. 2012, 45, 2035–2044. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Bradu, C.; Boussouga, Y.-A.; Aliaskari, M.; Schäfer, A.I.; Das, S.; Wilson, L.D.; Ike, M.; Inoue, D.; et al. Methods for selenium removal from contaminated waters: a review. Environ. Chem. Lett. 2022, 20, 2019–2041. [Google Scholar] [CrossRef]
- Szymczyk, A.; Popiołek, M.; Krzemiński, J.; Olszewski, M.; Ziółkowski, R.; Malinowska, E. Identification of medium- and mechanism-related pitfalls towards improved performance and applicability of electrochemical mercury(II) aptasensors. Microchim. Acta 2024, 191, 189. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Pinto, I.S.S.; Soares, E.V.; Soares, H.M.V.M. (Un)suitability of the use of pH buffers in biological, biochemical and environmental studies and their interaction with metal ions—A review. RSC Adv. 2015, 5, 30989–31003. [Google Scholar] [CrossRef]
- Powell, K.J.; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Sjöberg, S.; Wanner, H. Chemical speciation of environmentally significant heavy metals with inorganic ligands. Part 1: The Hg2+ Cl−, OH−, CO32−, SO42−, and PO43− aqueous systems (IUPAC Technical Report). Pure Appl. Chem. 2005, 77. [Google Scholar] [CrossRef]
- Zhang, X.; Servos, M.R.; Liu, J. Surface Science of DNA Adsorption onto Citrate-Capped Gold Nanoparticles. Langmuir 2012, 28, 3896–3902. [Google Scholar] [CrossRef]
- Ziółkowski, R.; Górski, Ł.; Oszwałdowski, S.; Malinowska, E. Electrochemical uranyl biosensor with DNA oligonucleotides as receptor layer. Anal. Bioanal. Chem. 2012, 402, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Jarczewska, M.; Kierzkowska, E.; Ziółkowski, R.; Górski, T.; Malinowska, E. Electrochemical oligonucleotide-based biosensor for the determination of lead ion. Bioelectrochemistry 2015, 101, 35–41. [Google Scholar] [CrossRef]
- Ziółkowski, R.; Uścińska, A.; Mazurkiewicz-Pawlicka, M.; Małolepszy, A.; Malinowska, E. Directly-thiolated graphene based electrochemical sensor for Hg(II) ion. Electrochim. Acta 2019, 305, 329–337. [Google Scholar] [CrossRef]
- Cristiano, E.; Hu, Y.-J.; Sigfried, M.; Kaplan, D.; Nitsche, H. A Comparison of Point of Zero Charge Measurement Methodology. Clays Clay Miner. 2011, 59, 107–115. [Google Scholar] [CrossRef]
- Thevendran, R.; Navien, T.N.; Meng, X.; Wen, K.; Lin, Q.; Sarah, S.; Tang, T.-H.; Citartan, M. Mathematical approaches in estimating aptamer-target binding affinity. Anal. Biochem. 2020, 600. [Google Scholar] [CrossRef]
- Kim, S.S.; Min, J.H.; Lee, J.K.; Baik, M.H.; Choi, J.-W.; Shin, H.S. Effects of pH and anions on the sorption of selenium ions onto magnetite. J. Environ. Radioact. 2012, 104, 1–6. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




