Submitted:
23 May 2024
Posted:
24 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. How DECT Imaging Works?
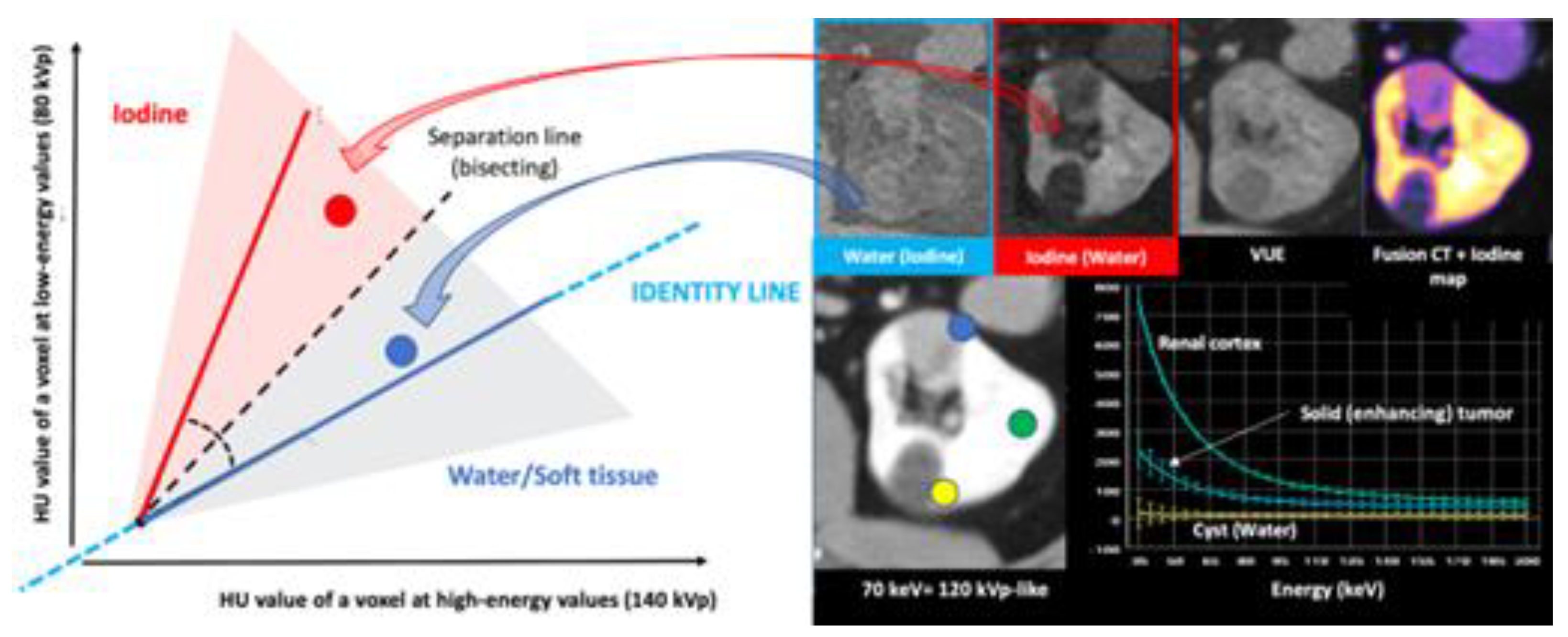
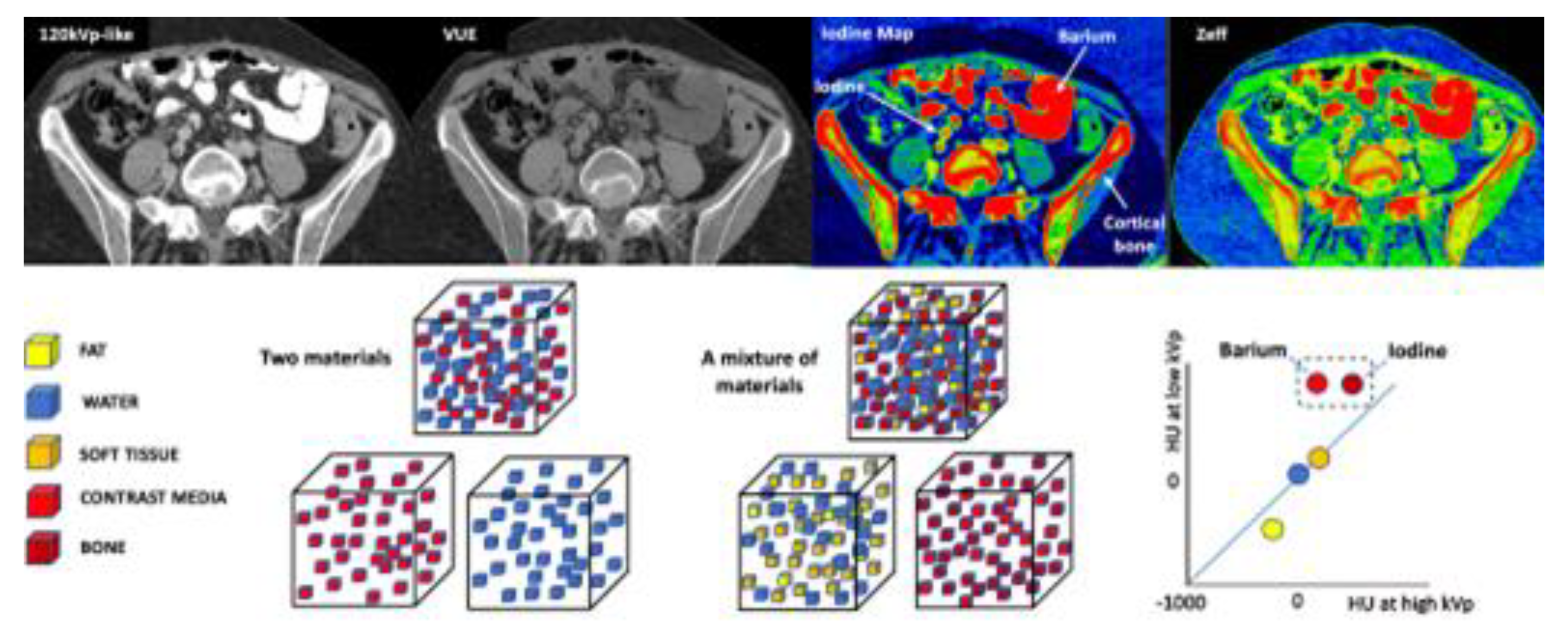
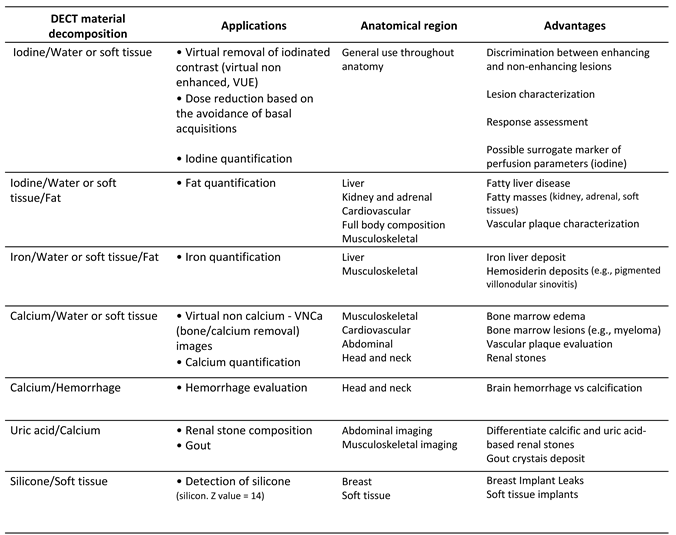
2.1. How is DECT Able to Characterize and Quantify Materials?
2.2. More Image Types are Available with DECT Than with Single-Energy CT
2.2.1. Material-Selective Images
2.2.1.1. Material-Labeling
2.2.1.2. Material-Subtraction
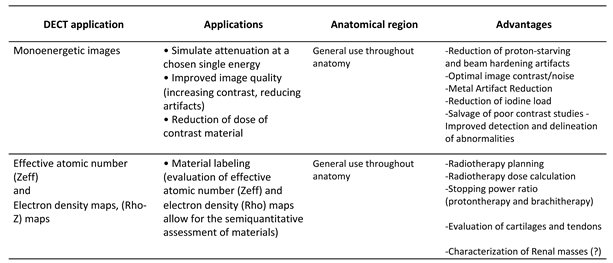
2.2.2. Energy-Selective Images
2.2.3. Polichromatic-like Images
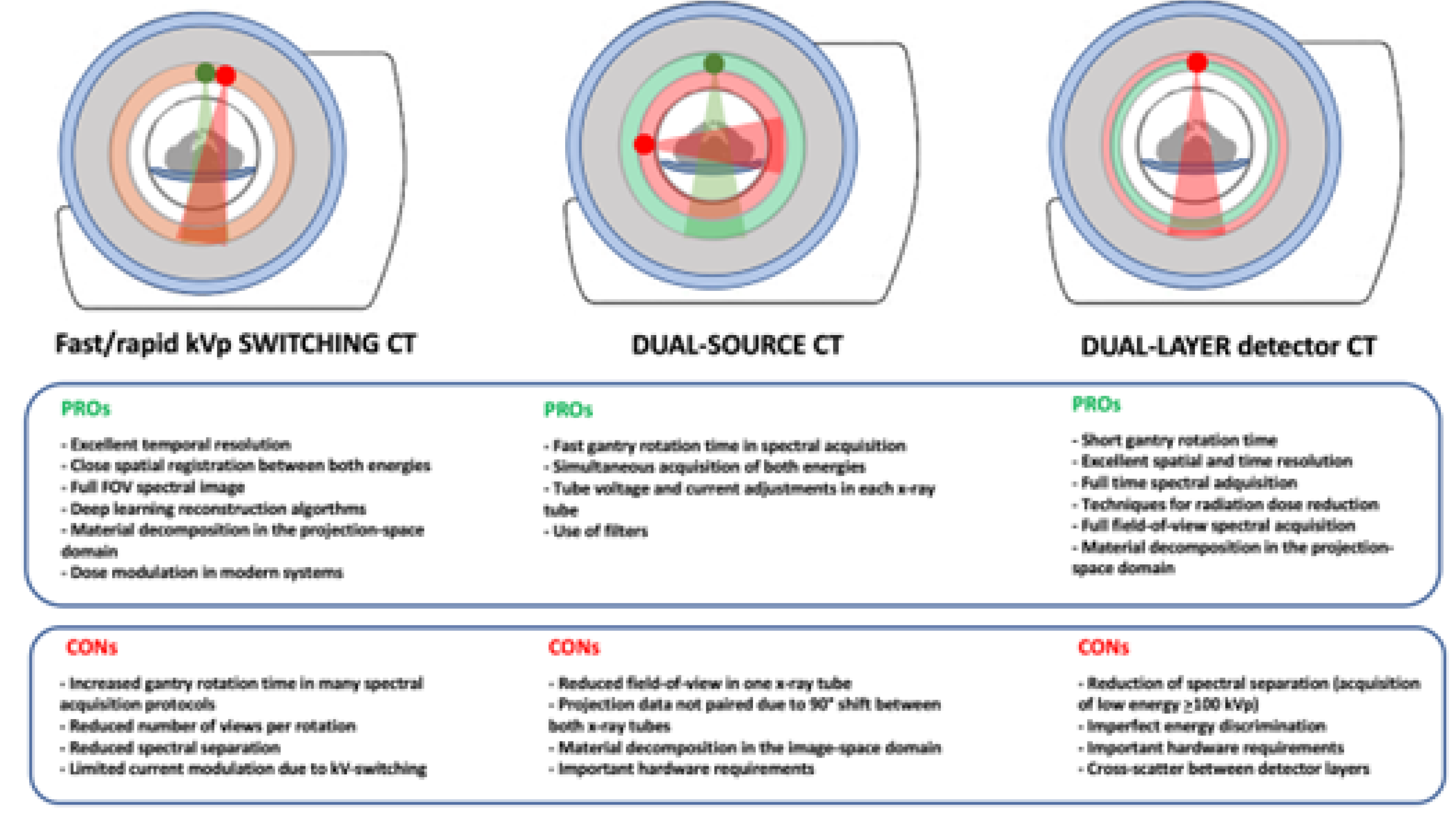
2.3. Technical Solutions for Acquiring DECT Imaging
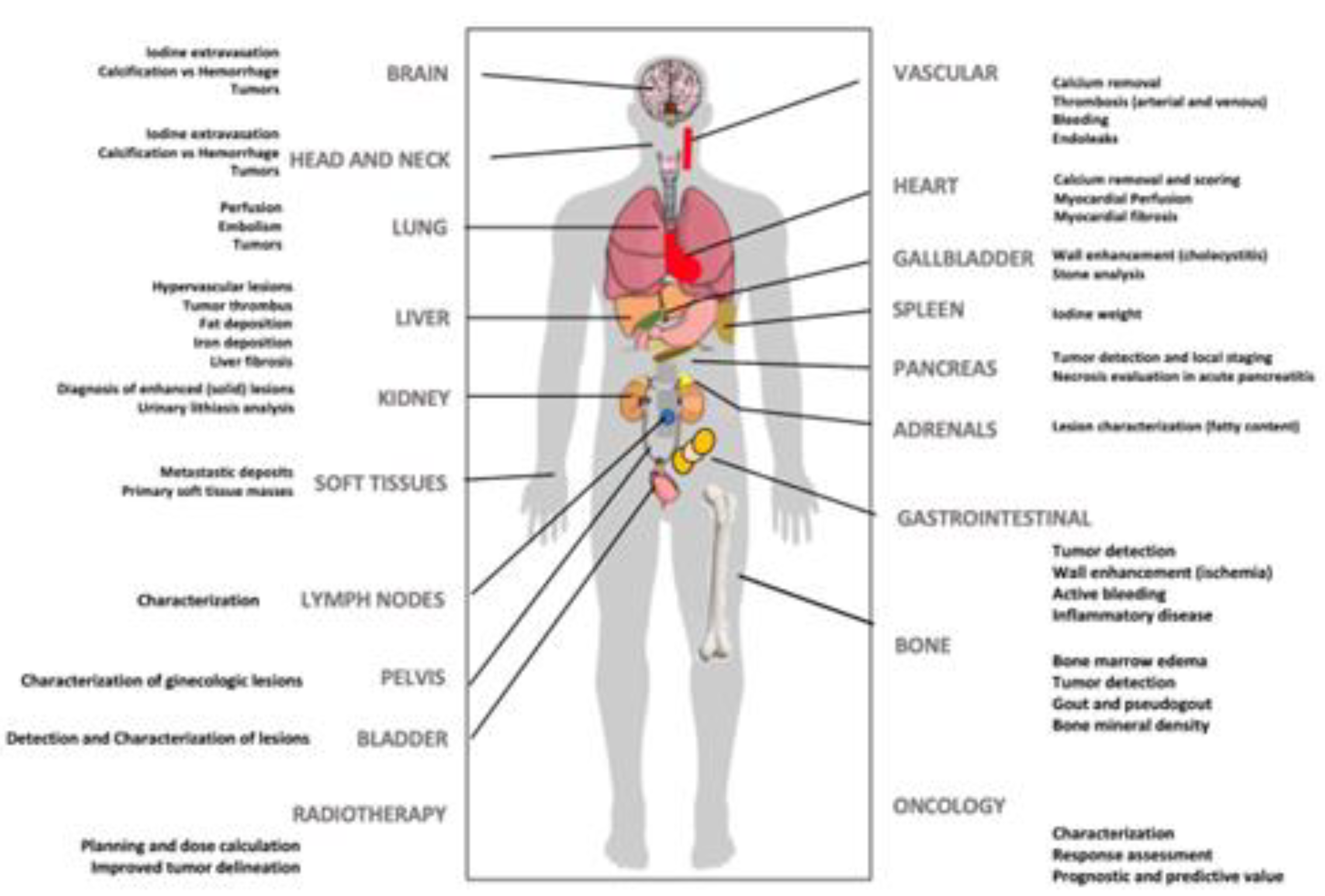
3. Clinical Applications of DECT Imaging: DOs & MAYBEs
3.1. DOs: Current Clinical Applications of DECT
3.2. MAYBEs: Advanced Applications of DECT
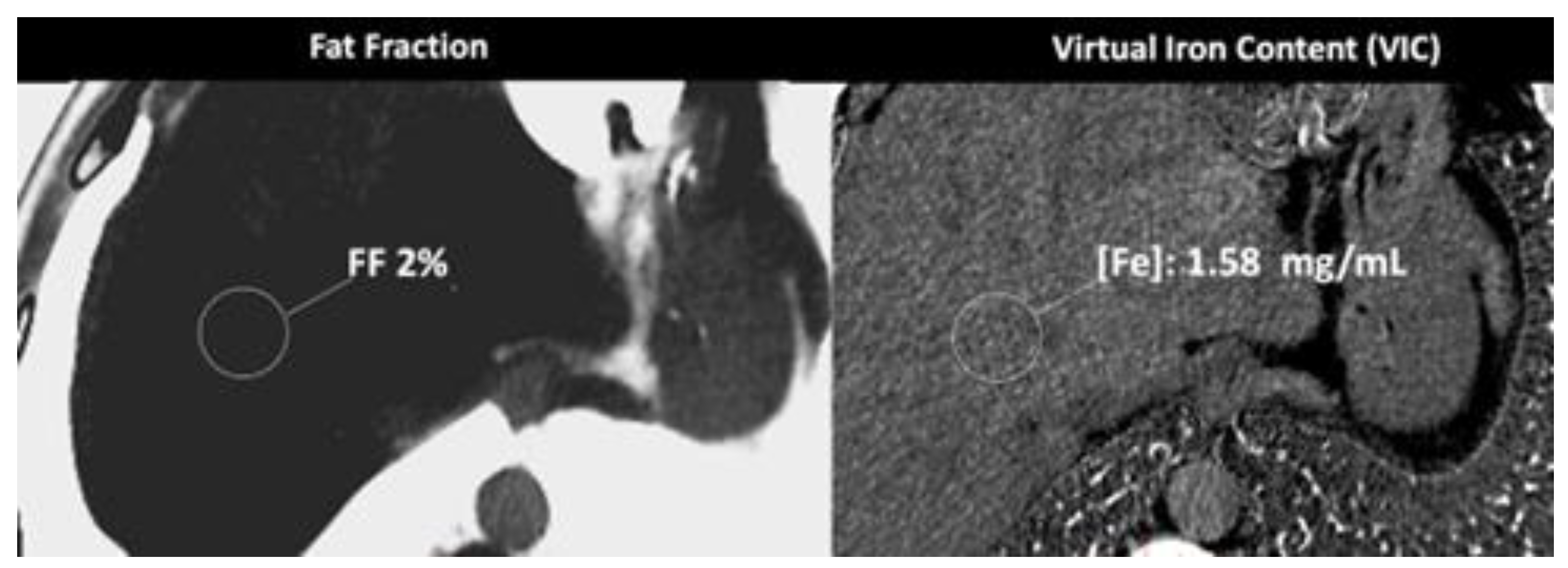
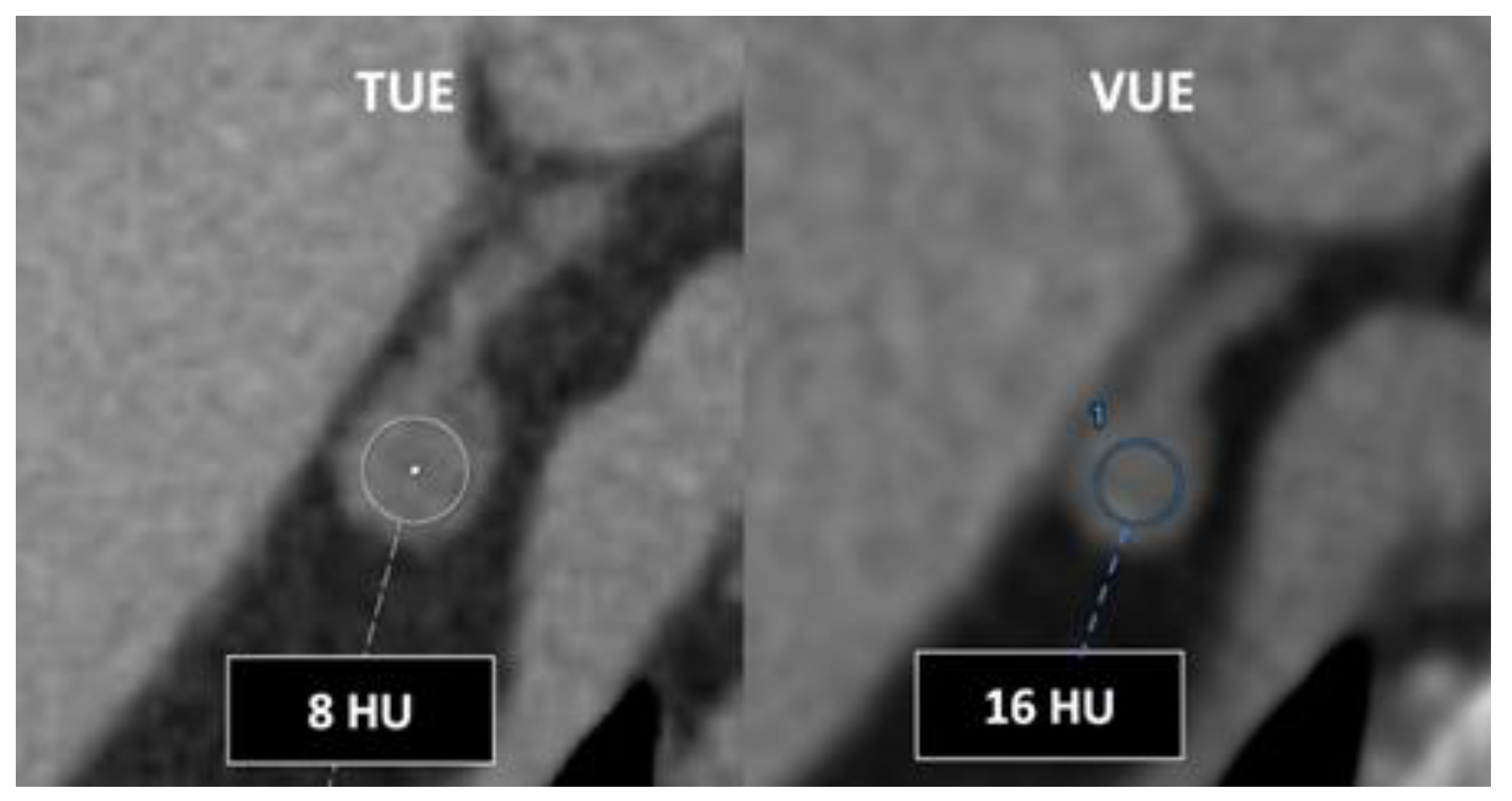
- In the case of adrenal imaging, fat fraction had higher sensitivity than VUE attenuation and the traditional threshold of 10 HU or lower for diagnosing adrenal adenomas. Loonis, et al [20] reported a threshold of fat fraction ≥ 23.8% with a 100% specificity and 59% sensitivity [Figure 11]. Besides, DECT-derived parameters can be used to differentiate adrenal adenoma from pheochromocytoma, or metastases based on the effect of lipid components on attenuation [33,34]. Finally, the iodine concentration can also be an imaging marker of dominant adrenal lesions in functional syndromes [35].
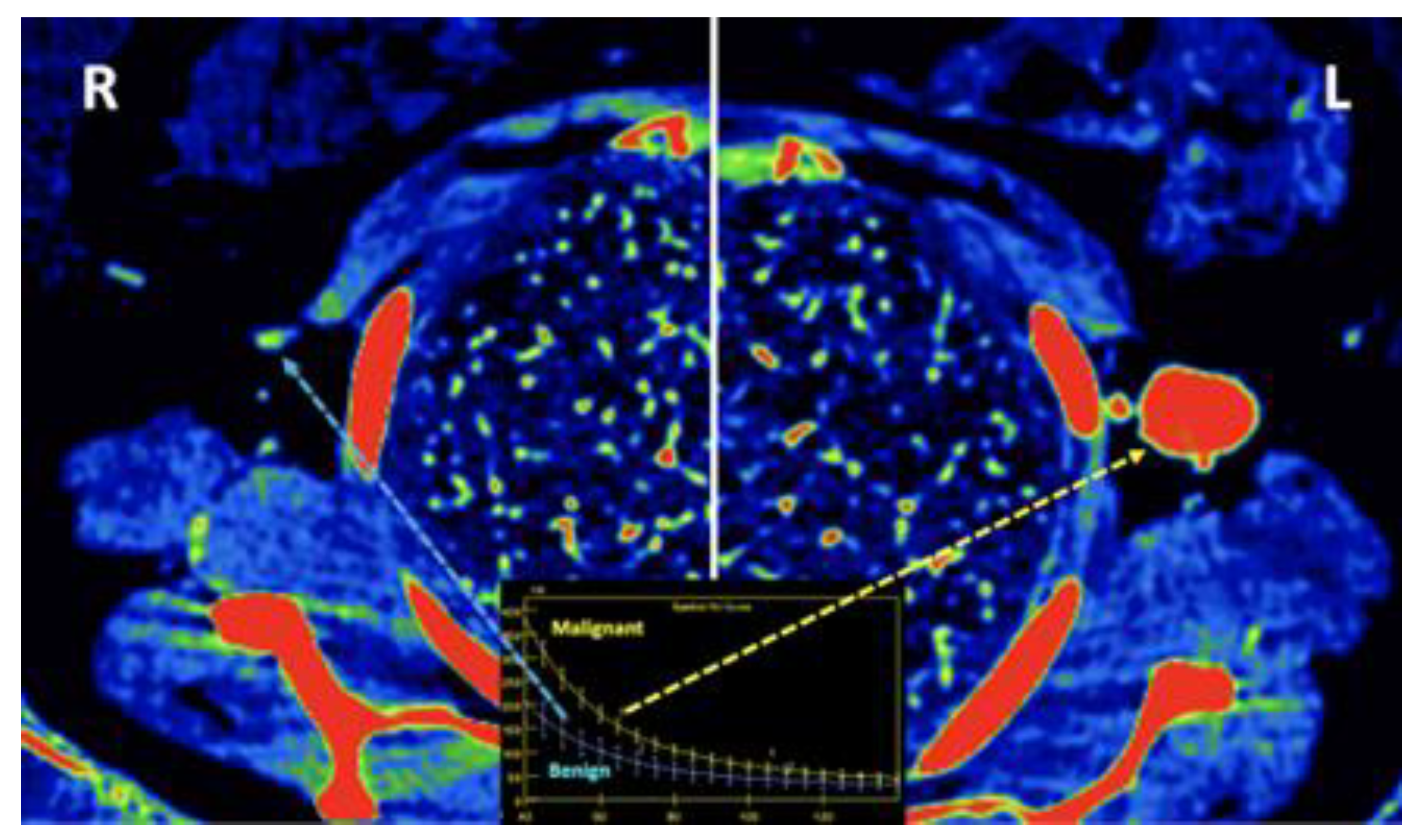
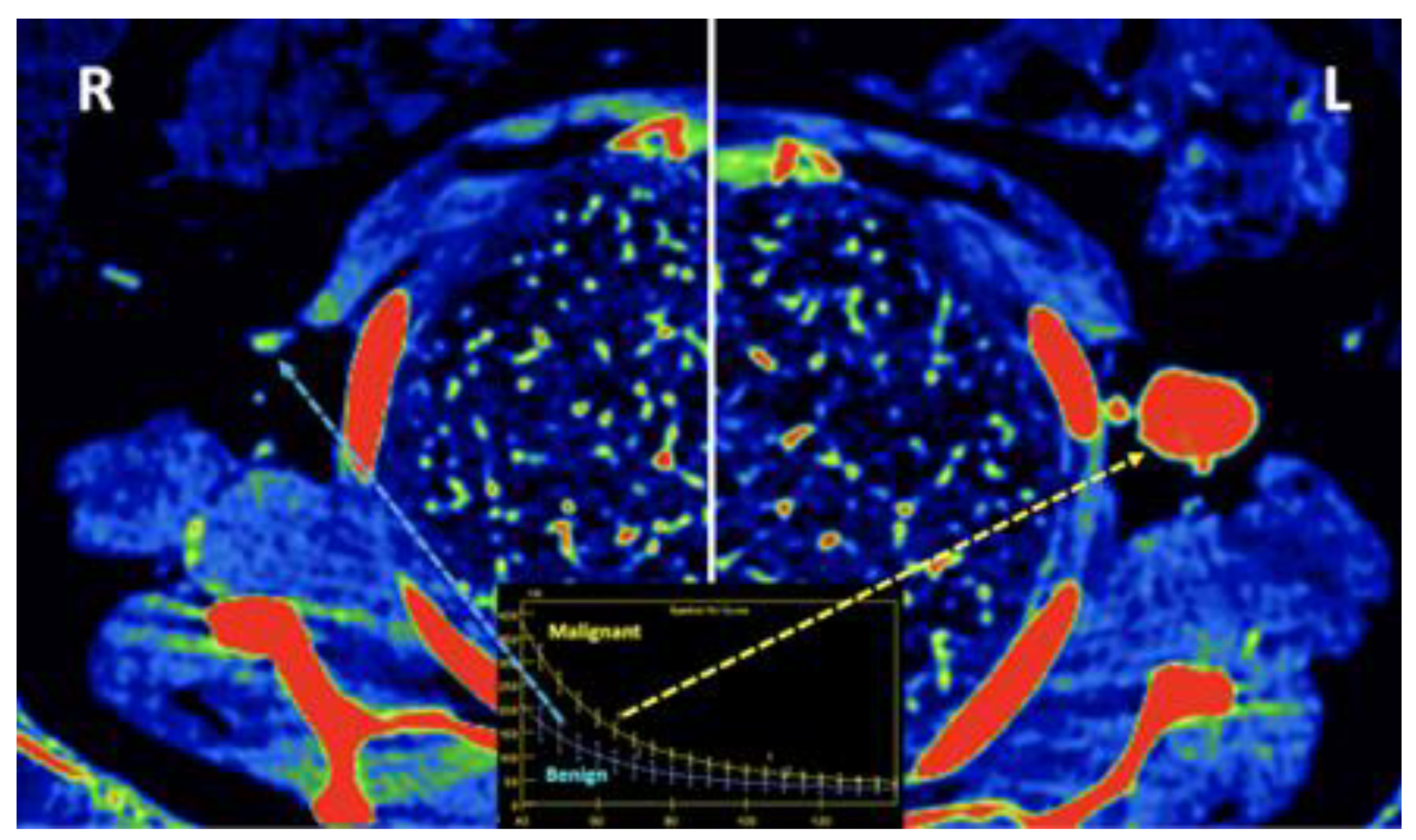
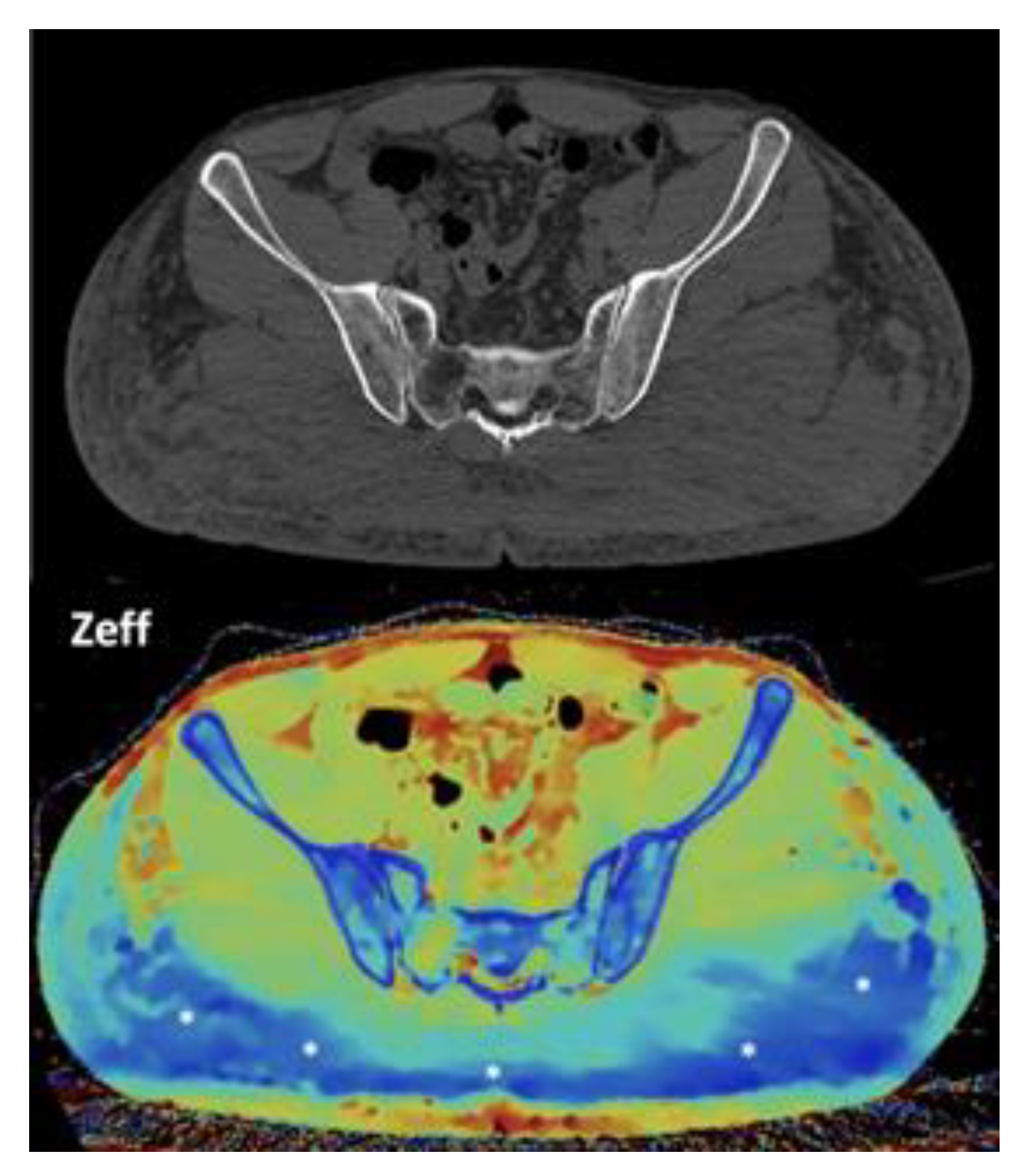
- Breast imaging. DECT seems to be a reliable tool for diagnosis and locoregional staging of breast cancer [36,37,38,39,40] [Figure 12]. Klein, et al [37] found robust cut points for the differentiation of benign and malignant lesions (Zeff < 7.7, iodine content of <0.8 mg/ml). The DECT quantitative parameters may also be useful in predicting breast cancer invasiveness and histopathological and molecular subtypes of breast tumors. In the case of node-staging, the similarity of quantitative DECT parameters between the primary lesion and axillary LNs may predict axillary metastasis in breast cancer [40,41].
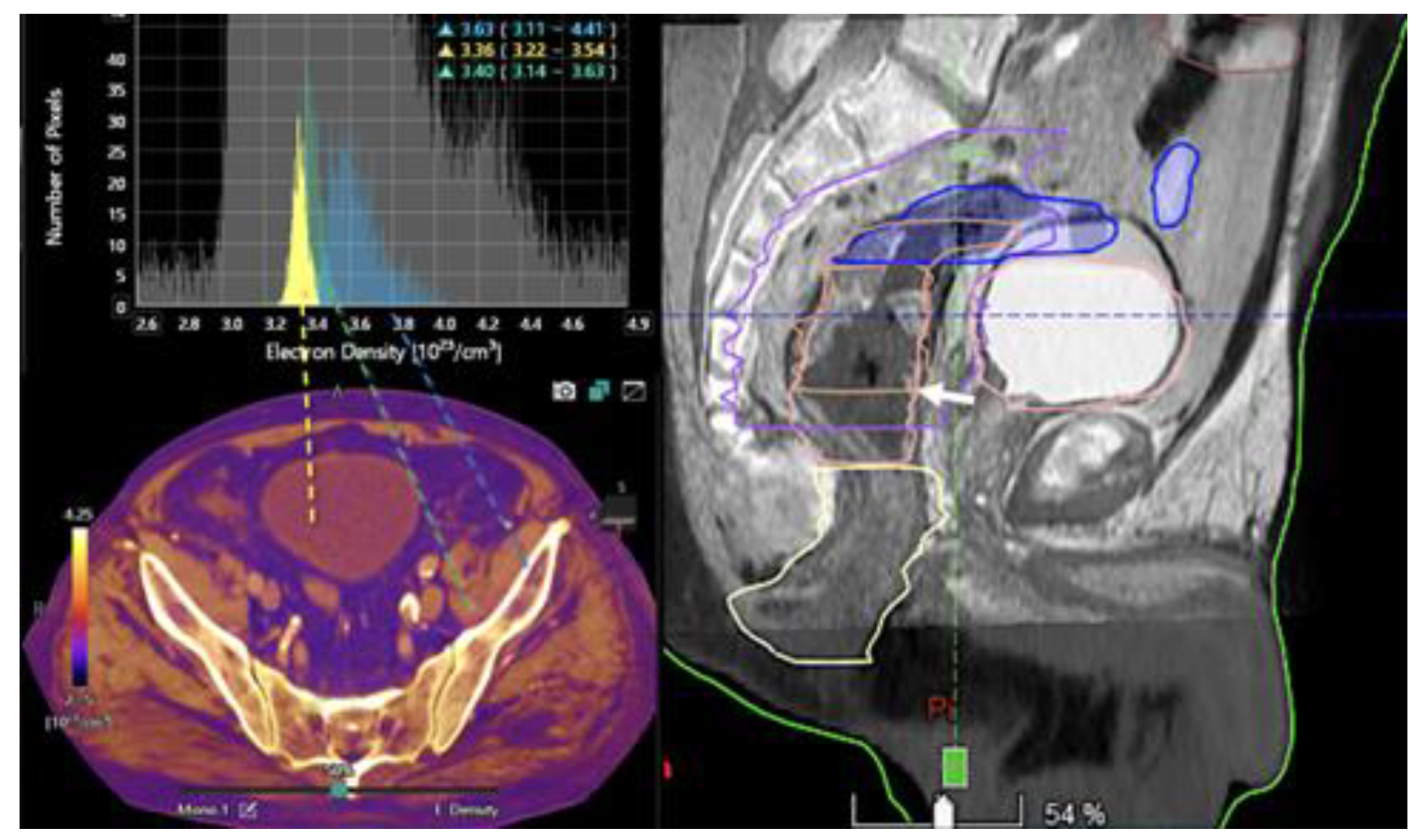
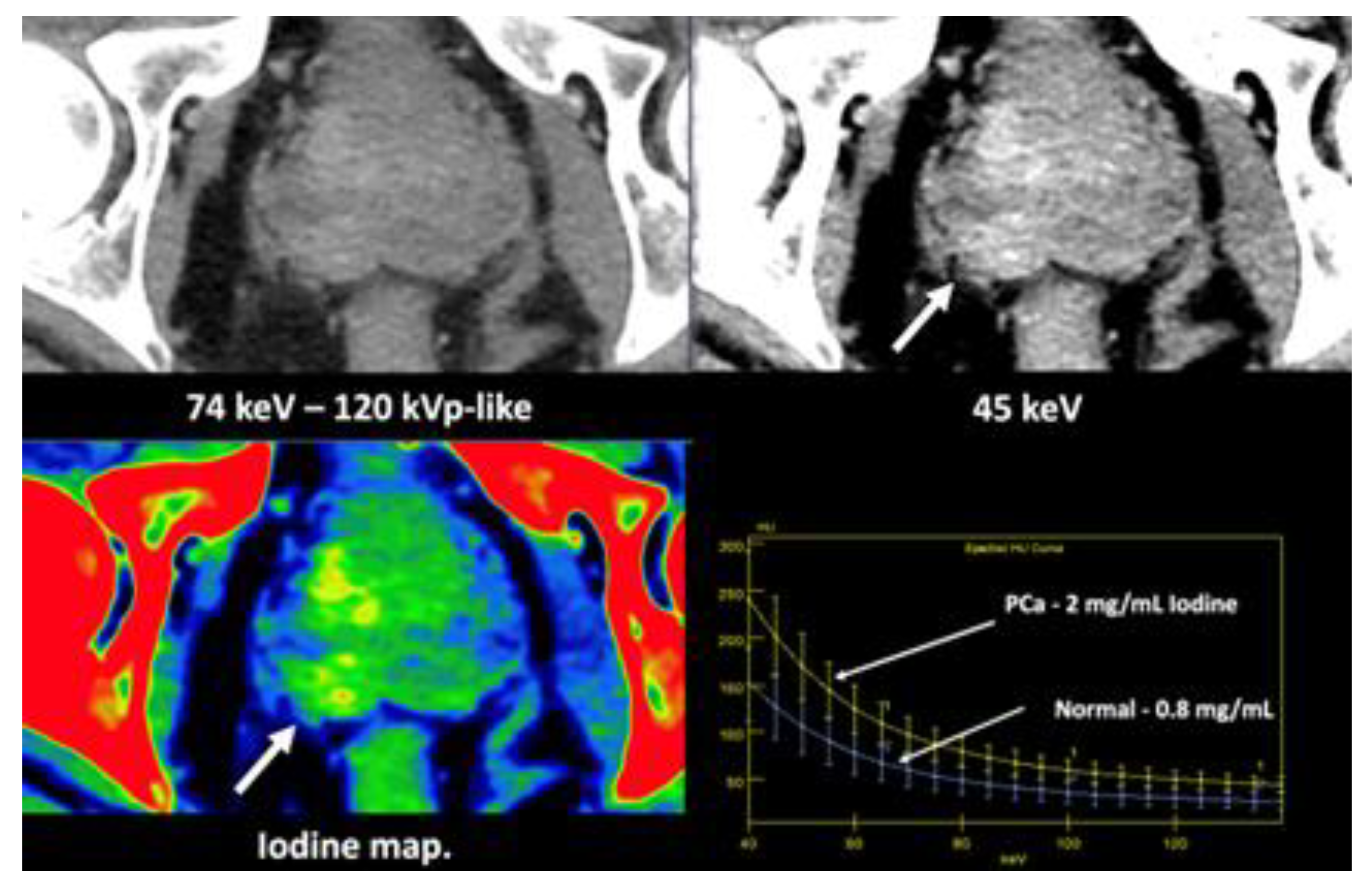
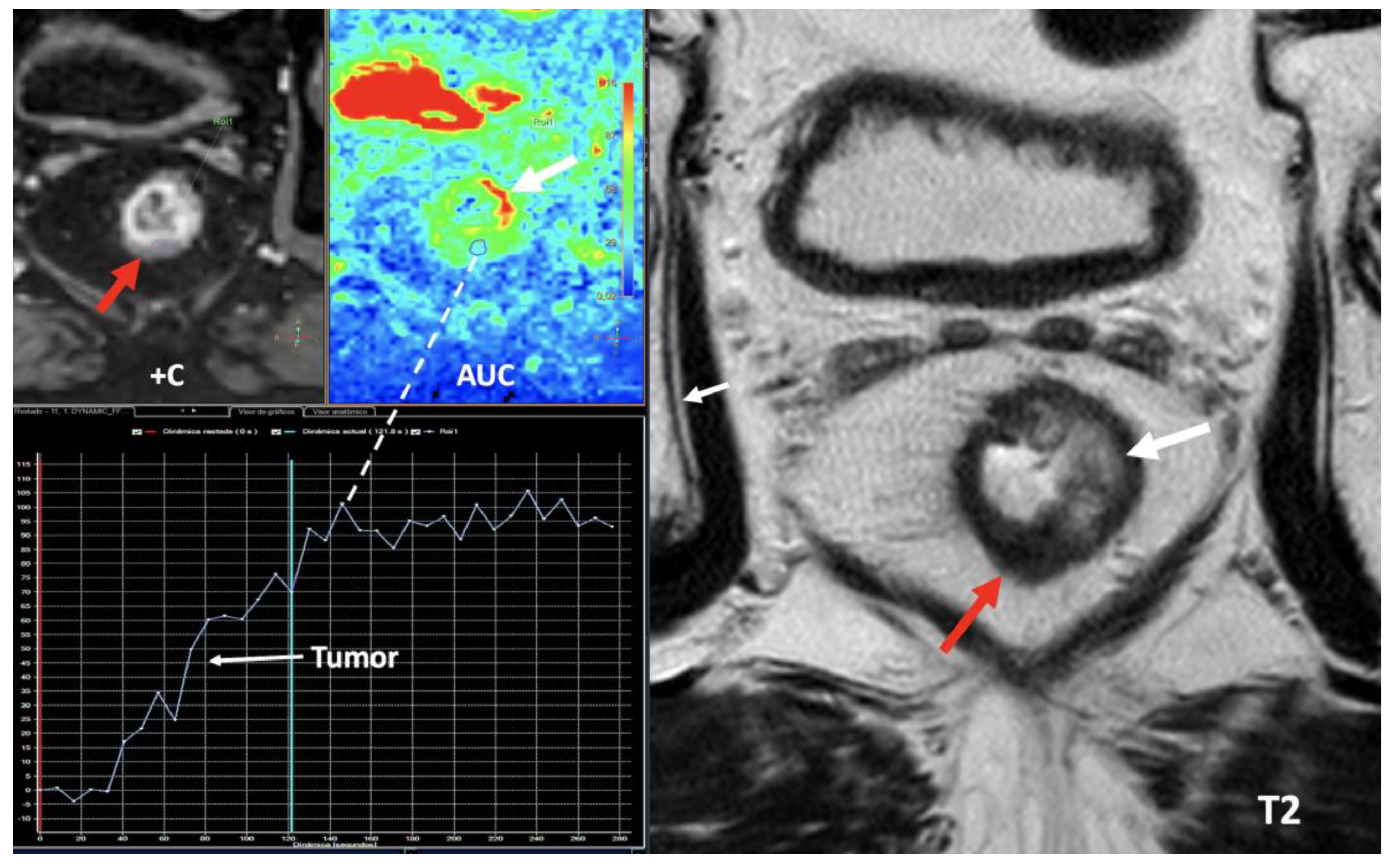
- Currently, there is not a widely reported use of DECT in clinical management of prostate cancer. However, DECT imaging may facilitate the depiction of focal areas of increased enhancement in the periphery of the prostate at contrast-enhanced CT that may represent a clinically significant cancer and deserve further workup [42] [Figure 13].
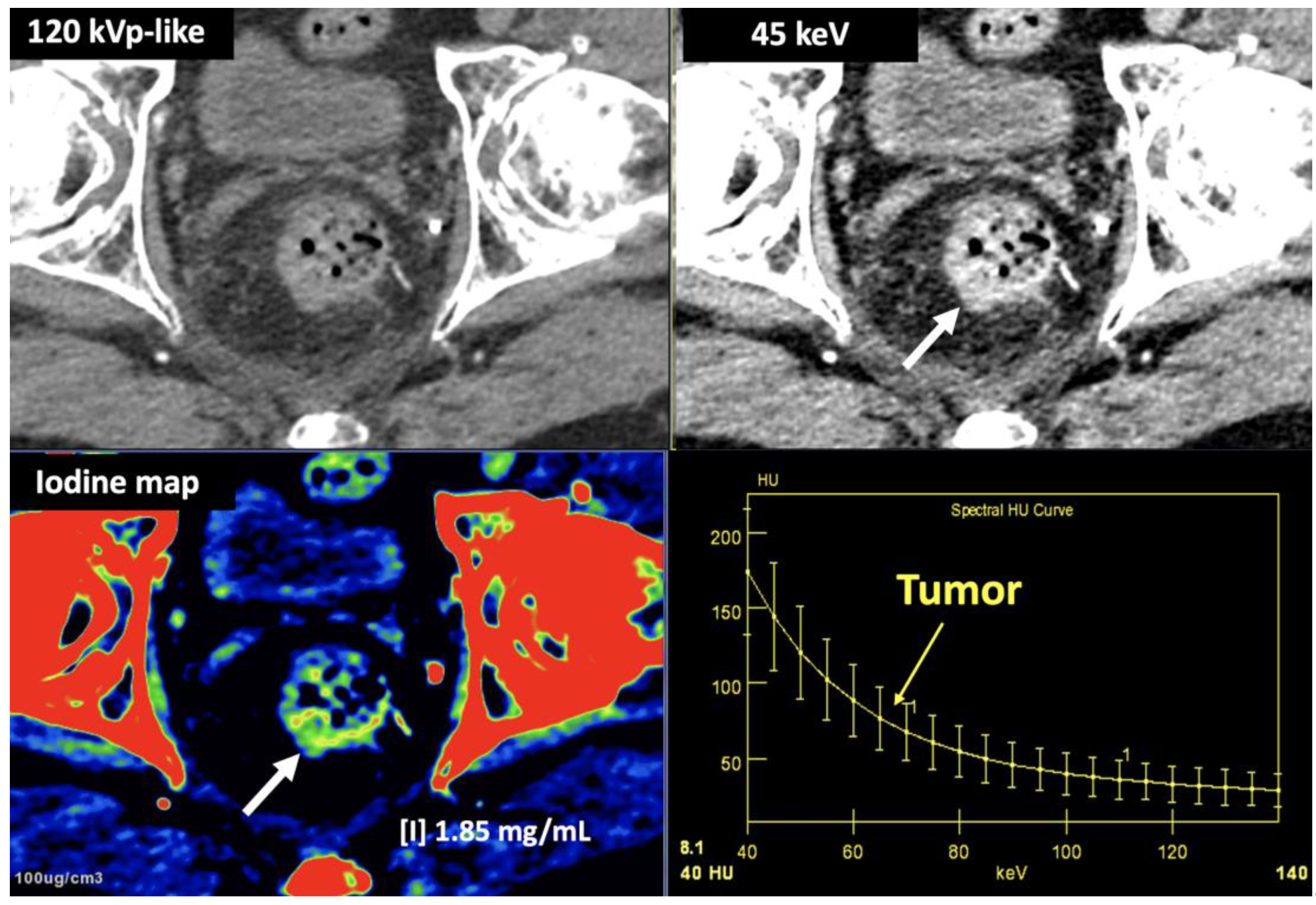
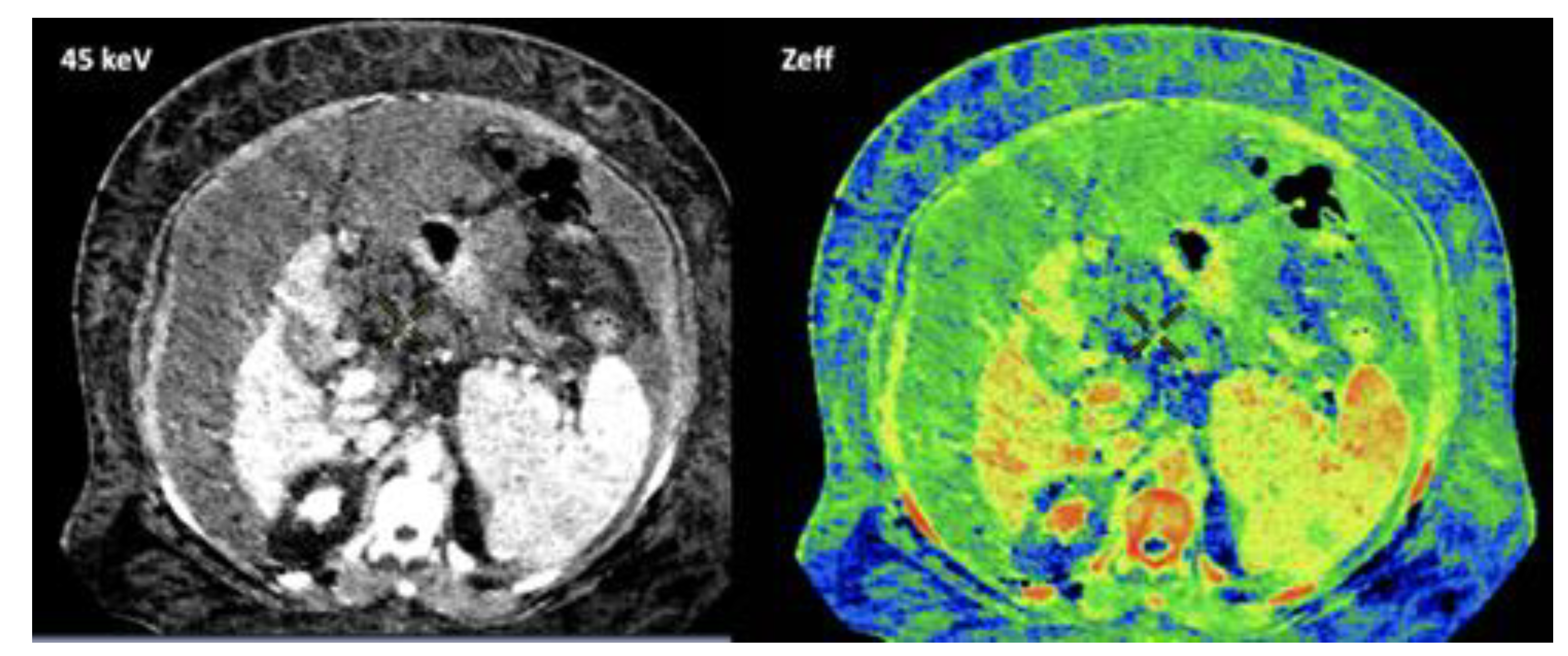
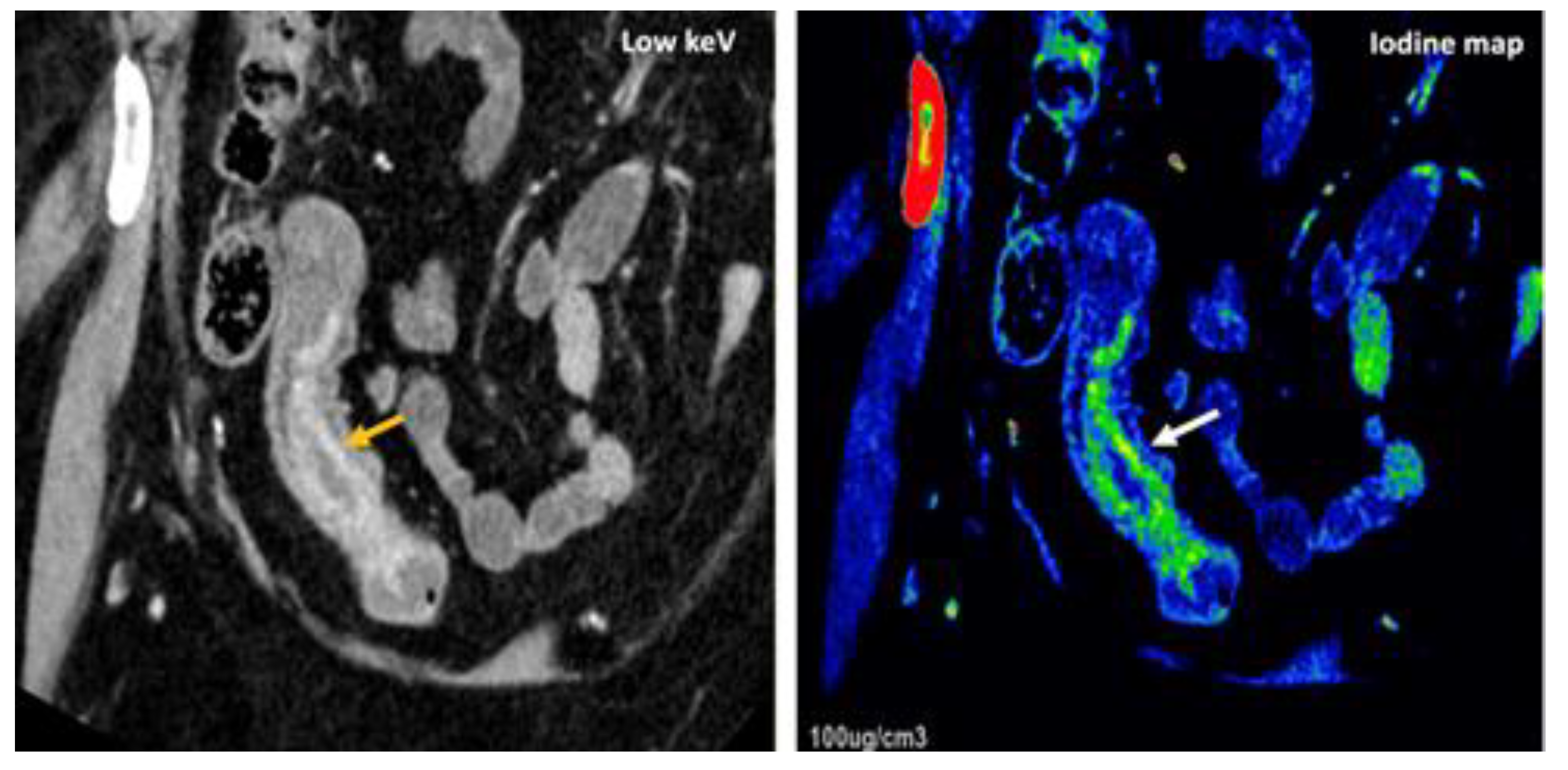
- LNs characterization is challenging in oncologic imaging. Apart of morphologic criteria, different DECT parameters have been used including iodine concentration, fat fraction, and similarity to primary tumor [41,43]. Sauter et al [44] have evaluated standard values for of iodine concentration for healthy LNs in different anatomic areas that could be used to differentiate between healthy and pathological LNs. Recent studies have suggested lower iodine concentration in metastatic LNs compared to benign LNs [45]. However, the value of DECT imaging in differentiating malignant from non-malignant LNs seems to be limited and depends on tumor type and technical features such as the used protocols of acquisition and contrast injection [Figure 14].
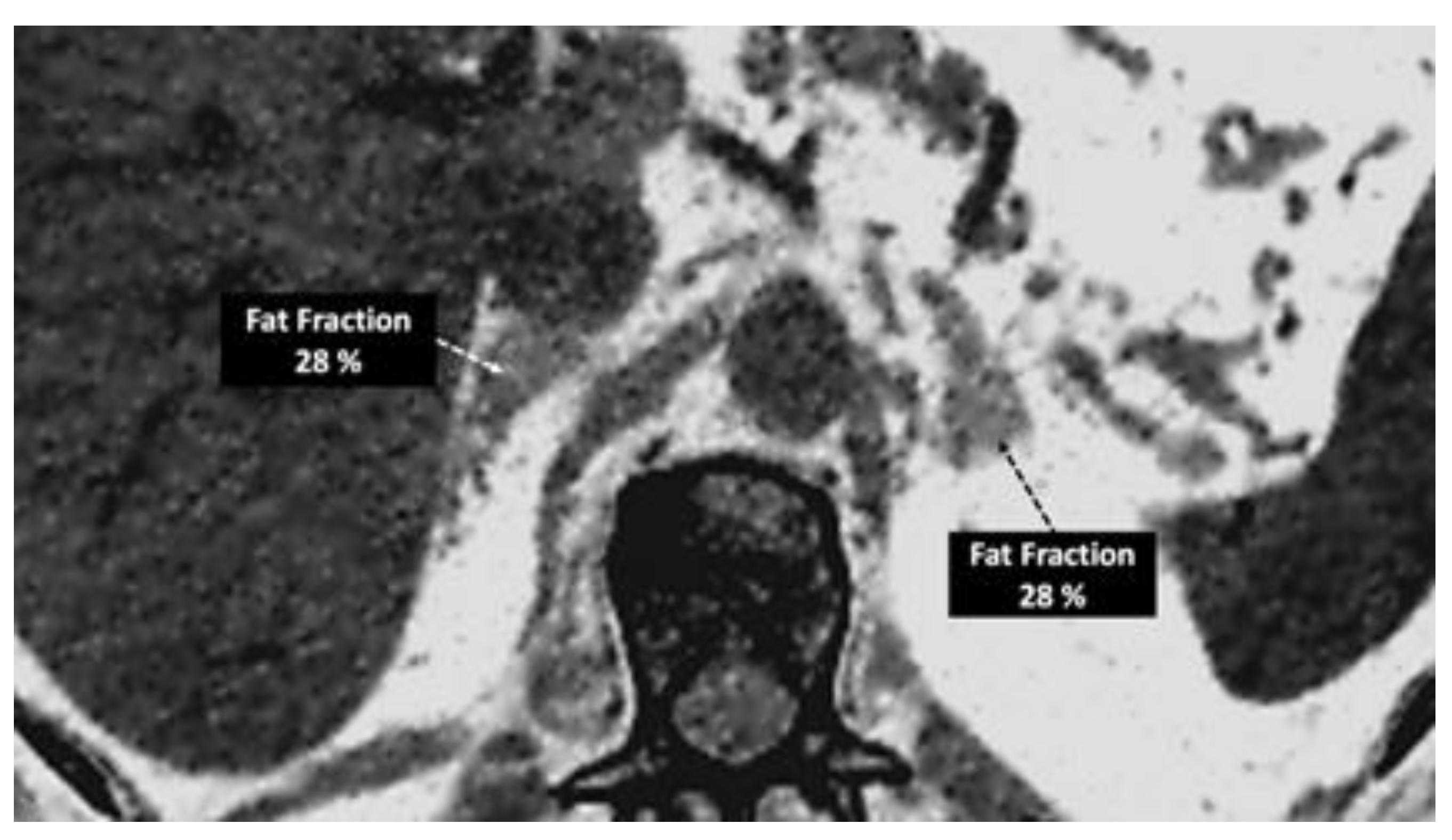
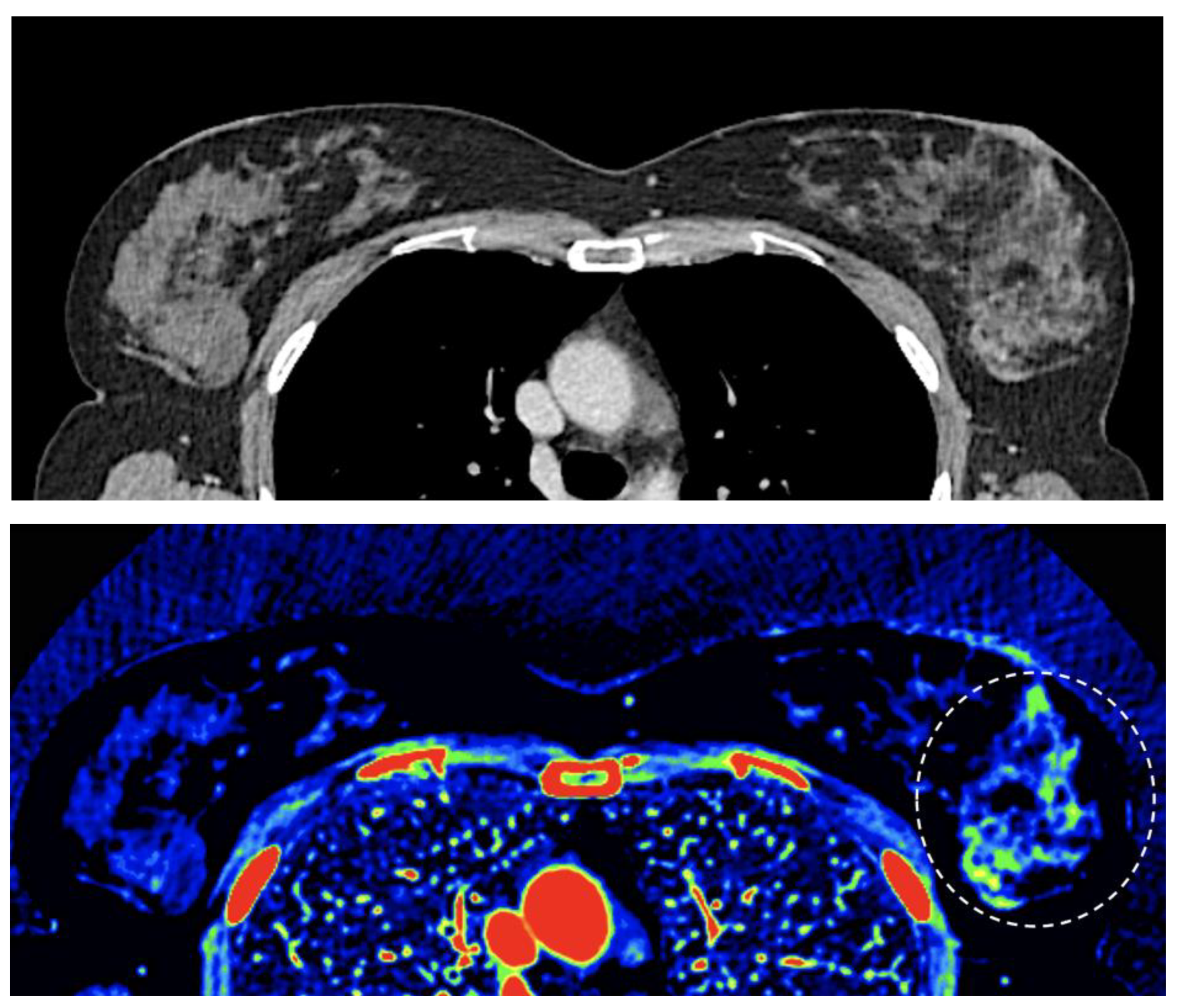
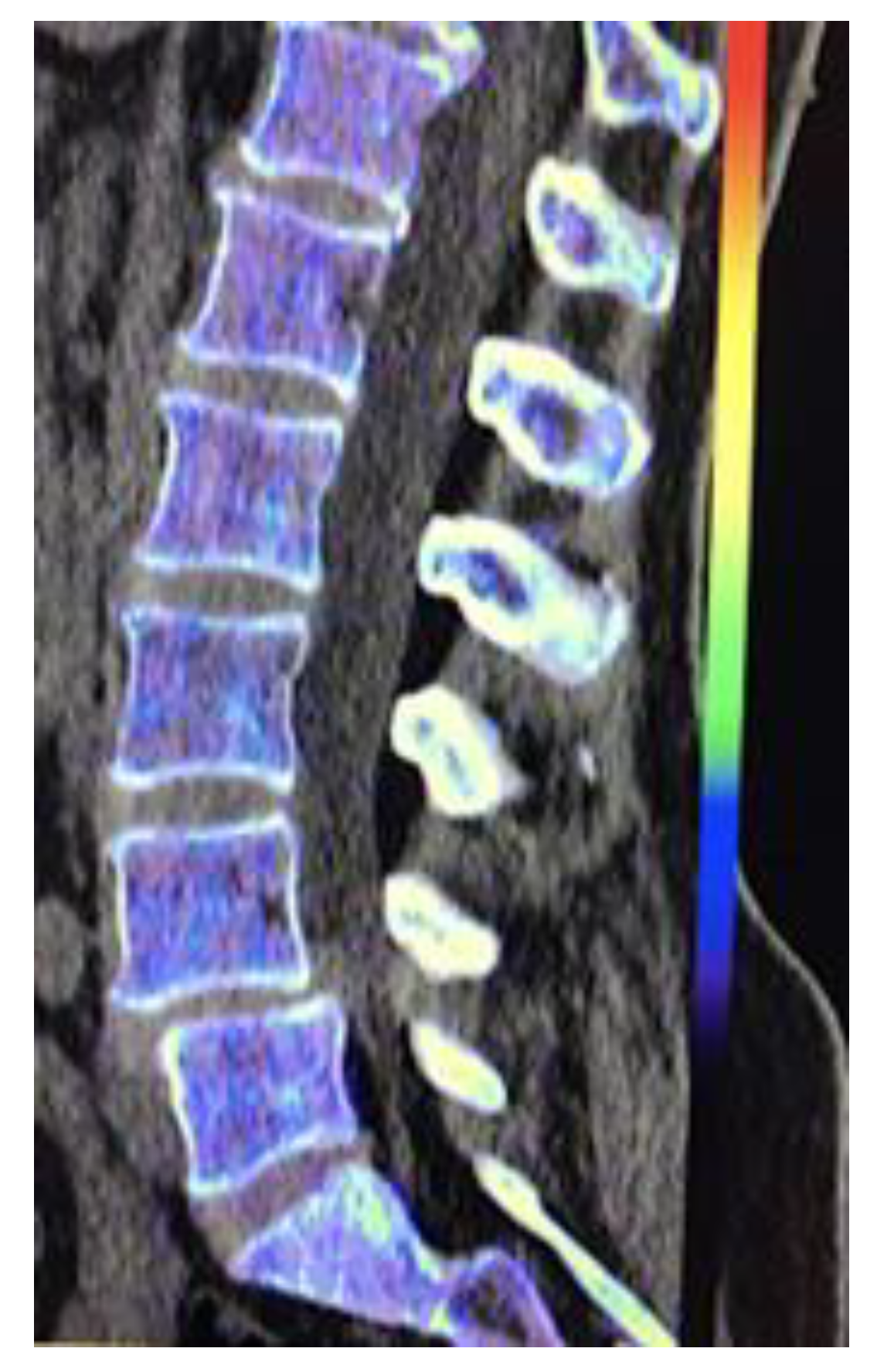
- Imaging of body composition is another growing application of DECT imaging that can be used to improve the evaluation of muscle tissue, visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT) compartments. SAT and VAT assessment is of special interest in diseases related to metabolic syndrome and critically ill patients [46]. Moreover, sarcopenia is associated with a poorer prognosis in cancer patients [47]. Measuring fat fraction of the skeletal muscle by DECT is a new approach for the determination of muscle quality, an important parameter for the diagnostic confirmation of sarcopenia [48]. In the case of bone mineral density analysis, DECT can provide a more detailed analysis when compared with dual x-ray absorptiometry [49] [Figure 15]. Finally, DECT can also be a useful tool for evaluating silicone implants [Figure 16]. Silicone contains the heavier element silicon (Z value=14), whereas soft tissue predominantly comprises lighter elements, depicting the presence of silicone within the soft tissues in cases of silicone gel breast implant rupture and LNs silicone spread [50].
4. Limitations of DECT Imaging: DON´Ts
- VNCa improves CT sensitivity and specificity to assess bone marrow disorders. On VNCa imaging, the bone marrow attenuation mainly reflect the water and fat content on it. However, the optimal cutoff value for discrimination between infiltrated and normal bone marrow (ranging between -80 and 6 HU in the literature) and calcium suppression indices need to be defined [Figure 22]. VNCa imaging also shows limitations in evaluating bone marrow alterations in areas of sclerotic bone (e.g., close to the cortical bone) [22]. Apart of this, any bone marrow process (focal red marrow hyperplasia, malignant infiltrative lesions, etc.) that increases its attenuation can be misinterpreted as edema.
- DECT-derived fat fraction, a quantitative marker of fat content in the liver, correlates with histopathological exam, the reference standard for steatosis. Pathology assessesment is based on the fraction of hepatocytes containing fatty vesicles: grade 0 (healthy, <5%), grade 1 (mild, 5–33%), grade 2 (moderate, 34–66%), and grade 3 (severe, >66%); while DECT evidences a substantially lower fatty liver content due to the simultaneous presence of fat, water, and soft-tissue in the voxel. Pathologic data can be correlated with DECT-derived fat quantification and a conversion factor may aid in the prediction of the histopathological fat fraction based on fat quantification using DECT [30]. Patients with coexisting hepatic fat and iron overload represent a clinical challenge. In the presence of multiple material elements in the same voxel, it is still not clear whether the presence of fat and iron in the same voxel results in reduced performance of DECT [27].
- In the case of urates, monosodium urate foci may be either undetectable or underestimated by DECT with low urate burden. This phenomenum has been reported in dense liquid tophi and calcified tophi due to subthreshold CT attenuation and obscuration of urate by calcium [59]. Concerning kidney lithiasis evaluation, inconsistent characterization may occur in tiny stones, as a result of decreased signal from the stone which approaches the level of background noise. Besides, drainage devices composition can also create stone mimics [18,60].
5. The Future of DECT Imaging
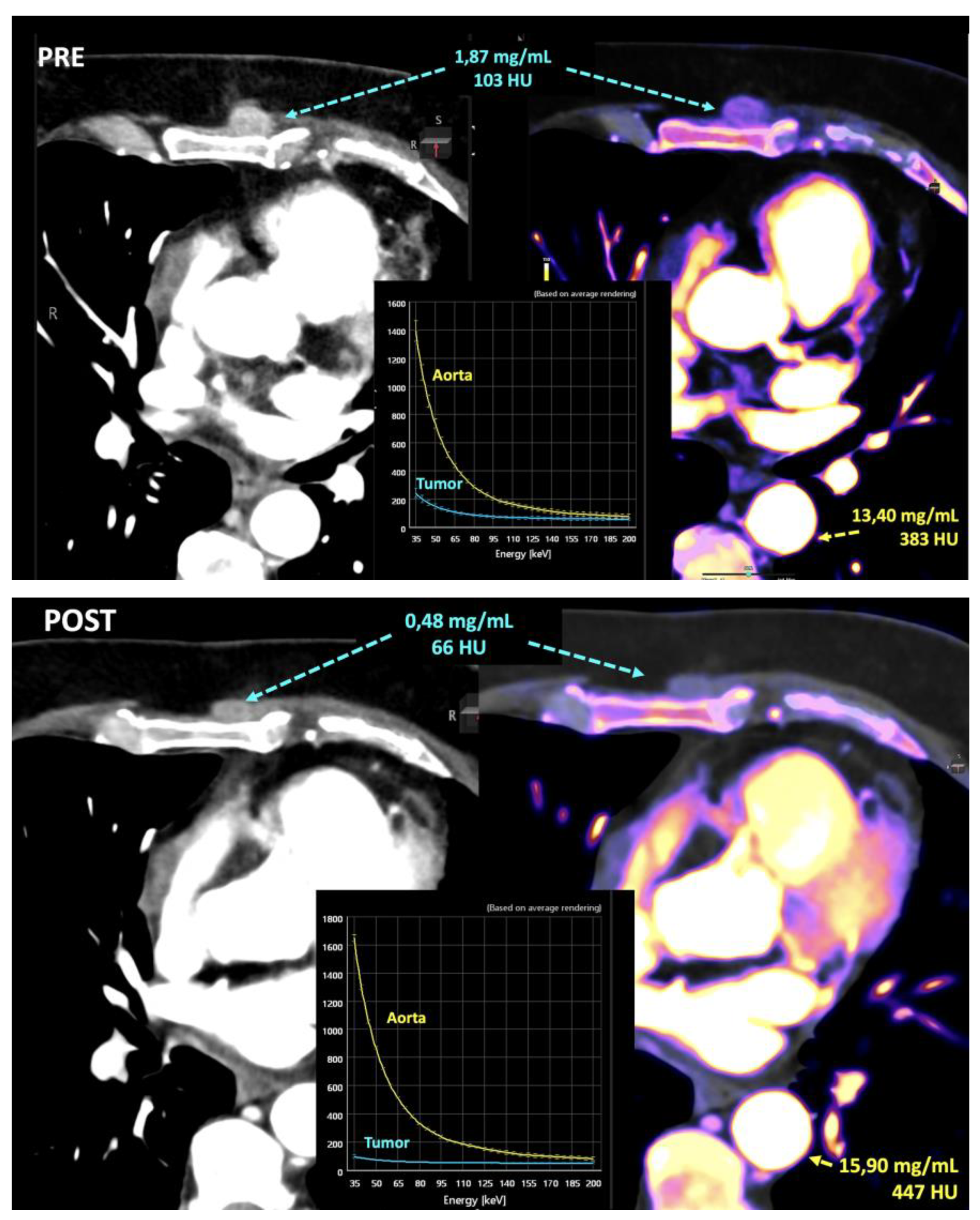
- Iodine concentration may be a surrogate marker of changes in tumor perfusion due to therapy [79]. Different iodine-related parameters have been proposed such as concentration of intralesional iodine, vital iodine tumor burden, and (lesion volume × iodine concentration) may be more sensitive than the evaluation criteria based on maximum diameter or change of CT value.
- Zeff is also a quantitative index for characterization of composition of a voxel, although a biological correlation of these changes to tumor microenvironment is challenging.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chung, R.; Dane, B.; Yeh, B.M.; Morgan, D.E.; Sahani, D.V.; Kambadakone, A. Dual-Energy Computed Tomography: Technological Considerations. Radiol. Clin. North Am. 2023, 61, 945–961. [Google Scholar] [CrossRef] [PubMed]
- Forghani, R.; De Man, B.; Gupta, R. Dual-Energy Computed Tomography: Physical Principles, Approaches to Scanning, Usage, and Implementation: Part F. Neuroimaging Clin. North Am. 2017, 27, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Forghani, R.; De Man, B.; Gupta, R. Dual-Energy Computed Tomography: Physical Principles, Approaches to Scanning, Usage, and Implementation: Part. Neuroimaging Clin. North Am. 2017, 27, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.P.; Antunes, C.; Curvo-Semedo, L. Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”. Tomography 2023, 9, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Borgheresi, A.; Mari, A.; Floridi, C.; Bruno, F.; Carotti, M.; Schicchi, N.; Barile, A.; Maggi, S.; Giovagnoni, A. Dual-energy CT: theoretical principles and clinical applications. La Radiol. medica 2019, 124, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Tatsugami, F.; Higaki, T.; Nakamura, Y.; Honda, Y.; Awai, K. Dual-energy CT: minimal essentials for radiologists. Jpn. J. Radiol. 2022, 40, 547–559. [Google Scholar] [CrossRef] [PubMed]
- So, A.; Nicolaou, S. Spectral Computed Tomography: Fundamental Principles and Recent Developments. Korean J. Radiol. 2021, 22, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.W.; Goo, J.M. Dual-Energy CT: New Horizon in Medical Imaging. Korean J. Radiol. 2017, 18, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Parakh, A.; Lennartz, S.; An, C.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Dual-Energy CT Images: Pearls and Pitfalls. RadioGraphics 2021, 41, 98–119. [Google Scholar] [CrossRef] [PubMed]
- Parakh, A.; An, C.; Lennartz, S.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Recognizing and Minimizing Artifacts at Dual-Energy CT. RadioGraphics 2021, 41, 509–523. [Google Scholar] [CrossRef]
- Patino, M.; Prochowski, A.; Agrawal, M.D.; Simeone, F.J.; Gupta, R.; Hahn, P.F.; Sahani, D.V. Material Separation Using Dual-Energy CT: Current and Emerging Applications. RadioGraphics 2016, 36, 1087–1105. [Google Scholar] [CrossRef] [PubMed]
- Krauss, B.; Grant, K.L.; Schmidt, B.T.; Flohr, T.G. The Importance of Spectral Separation: an assessment of dual-energy spectral separation for quantitative ability and dose efficiency. Investig. Radiol. 2015, 50, 114–118. [Google Scholar] [CrossRef]
- Sodickson, A.D.; Keraliya, A.; Czakowski, B.; Primak, A.; Wortman, J.; Uyeda, J.W. Dual energy CT in clinical routine: how it works and how it adds value. Emerg. Radiol. 2021, 28, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Parakh, A.; Kay, F.; Baruah, D.; Kambadakone, A.R.; Leng, S. Update on Multienergy CT: Physics, Principles, and Applications. RadioGraphics 2020, 40, 1284–1308. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, M.C.; Thrower, S.L.; Ger, R.B.; Leng, S.; Court, L.E.; Brock, K.K.; Tamm, E.P.; Cressman, E.N.; Cody, D.D.; Layman, R.R. Multi-energy computed tomography and material quantification: Current barriers and opportunities for advancement. Med Phys. 2020, 47, 3752–3771. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, M.C.; Cressman, E.N.K.; Tamm, E.P.; Baluya, D.L.; Duan, X.; Cody, D.D.; Schellingerhout, D.; Layman, R.R. Dual-Energy CT: Lower Limits of Iodine Detection and Quantification. Radiology 2019, 292, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Molwitz, I.; Leiderer, M.; Özden, C.; Yamamura, J. Dual-Energy Computed Tomography for Fat Quantification in the Liver and Bone Marrow: A Literature Review. Rofo-Fortschritte Auf Dem Geb. Der Rontgenstrahlen Und Der Bild. Verfahr. 2020, 192, 1137–1153. [Google Scholar] [CrossRef] [PubMed]
- Nourian, A.; Ghiraldi, E.; Friedlander, J.I. Dual-Energy CT for Urinary Stone Evaluation. Curr. Urol. Rep. 2020, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gosangi, B.; Mandell, J.C.; Weaver, M.J.; Uyeda, J.W.; Smith, S.E.; Sodickson, A.D.; Khurana, B. Bone Marrow Edema at Dual-Energy CT: A Game Changer in the Emergency Department. RadioGraphics 2020, 40, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Loonis, A.-S.T.; Yu, H.; Glazer, D.I.; Bay, C.P.; Sodickson, A.D. Dual Energy–Derived Metrics for Differentiating Adrenal Adenomas From Nonadenomas on Single-Phase Contrast-Enhanced CT. Am. J. Roentgenol. 2023, 220, 693–704. [Google Scholar] [CrossRef]
- Ananthakrishnan, L.; Rajiah, P.; Ahn, R.; Rassouli, N.; Xi, Y.; Soesbe, T.C.; Lewis, M.A.; Lenkinski, R.E.; Leyendecker, J.R.; Abbara, S. Spectral detector CT-derived virtual non-contrast images: comparison of attenuation values with unenhanced CT. Abdom. Imaging 2017, 42, 702–709. [Google Scholar] [CrossRef] [PubMed]
- D’angelo, T.; Albrecht, M.H.; Caudo, D.; Mazziotti, S.; Vogl, T.J.; Wichmann, J.L.; Martin, S.; Yel, I.; Ascenti, G.; Koch, V.; et al. Virtual non-calcium dual-energy CT: clinical applications. Eur. Radiol. Exp. 2021, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
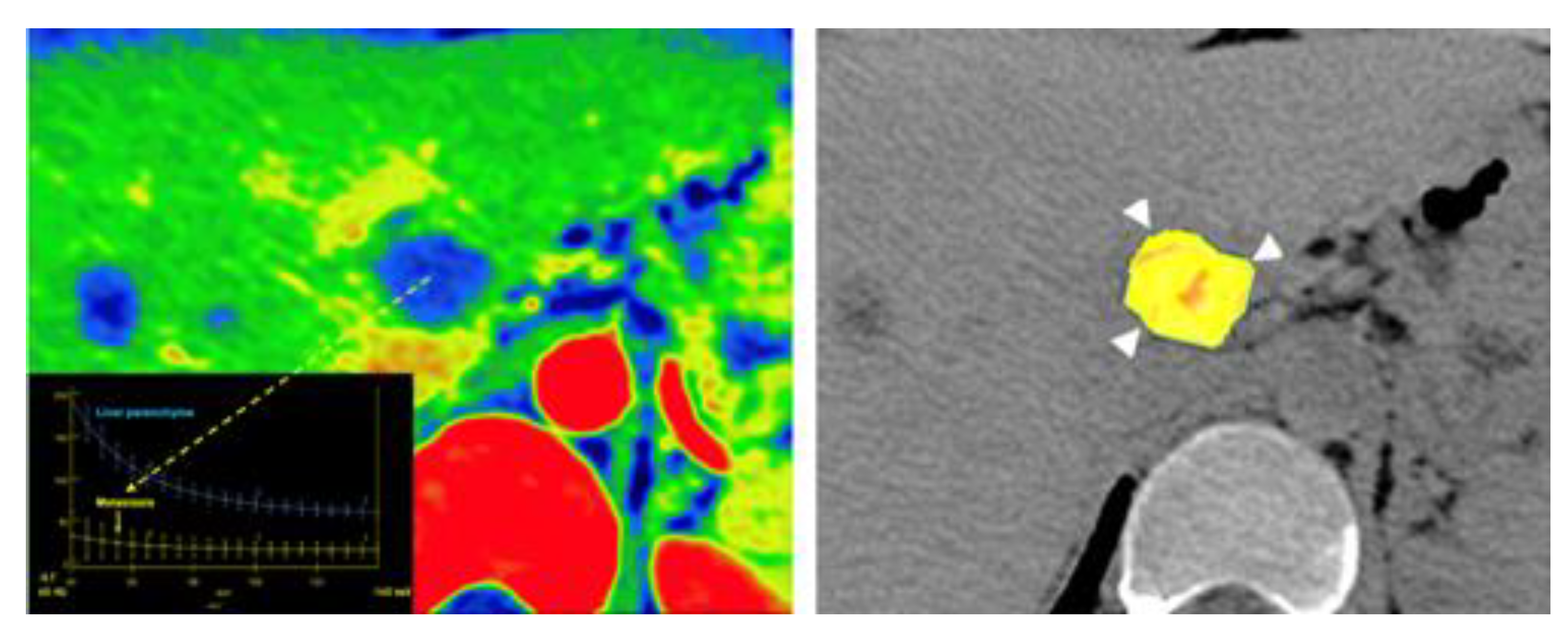
- Mileto, A.; Allen, B.C.; Pietryga, J.A.; Farjat, A.E.; Zarzour, J.G.; Bellini, D.; Ebner, L.; Morgan, D.E. Characterization of Incidental Renal Mass With Dual-Energy CT: Diagnostic Accuracy of Effective Atomic Number Maps for Discriminating Nonenhancing Cysts From Enhancing Masses. Am. J. Roentgenol. 2017, 209, W221–W230. [Google Scholar] [CrossRef] [PubMed]
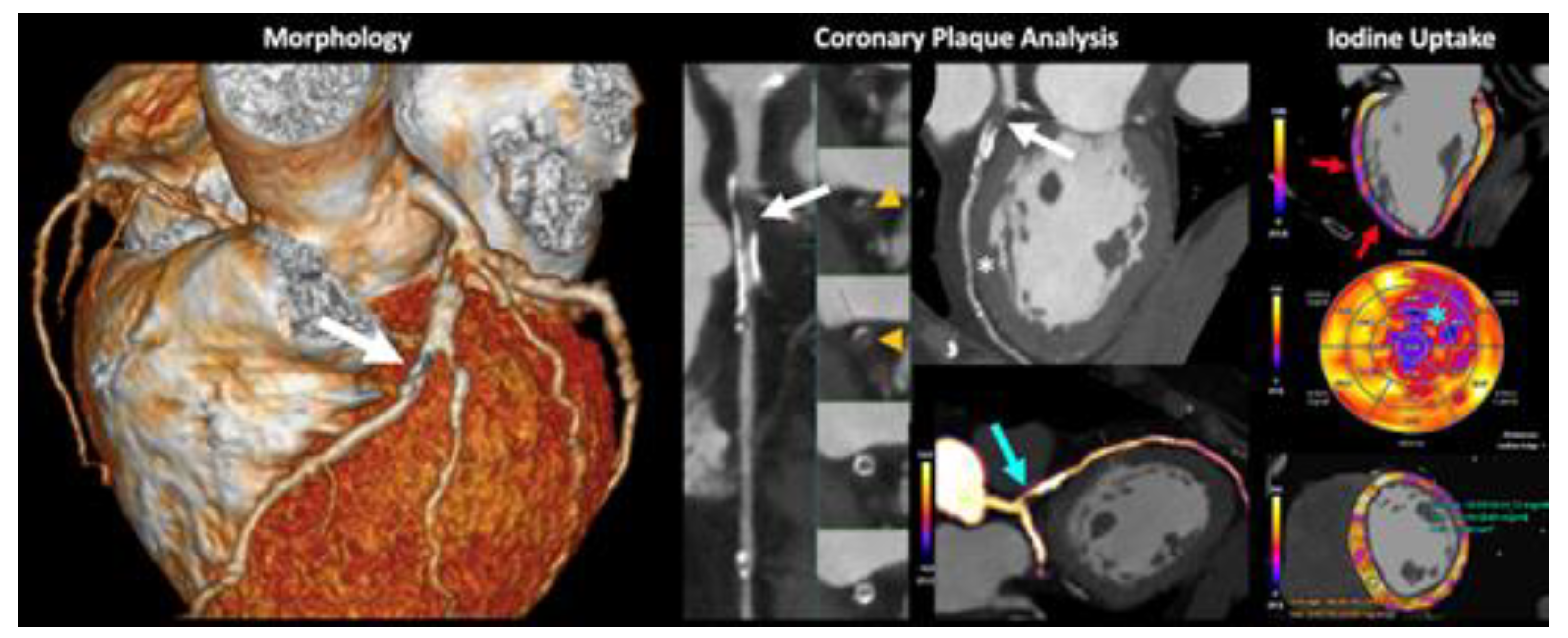
- Danad, I.; Fayad, Z.A.; Willemink, M.J.; Min, J.K. New Applications of Cardiac Computed Tomography: Dual-Energy, Spectral, and Molecular CT Imaging. JACC: Cardiovasc. Imaging 2015, 8, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Dell’aversana, S.; Ascione, R.; De Giorgi, M.; De Lucia, D.R.; Cuocolo, R.; Boccalatte, M.; Sibilio, G.; Napolitano, G.; Muscogiuri, G.; Sironi, S.; et al. Dual-Energy CT of the Heart: A Review. J. Imaging 2022, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- De Santis, D.; Eid, M.; De Cecco, C.N.; Jacobs, B.E.; Albrecht, M.H.; Varga-Szemes, A.; Tesche, C.; Caruso, D.; Laghi, A.; Schoepf, U.J. Dual-Energy Computed Tomography in Cardiothoracic Vascular Imaging. Radiol. Clin. North Am. 2018, 56, 521–534. [Google Scholar] [CrossRef]
- Marri, U.K.; Madhusudhan, K.S. Dual-Energy Computed Tomography in Diffuse Liver Diseases. J. Gastrointest. Abdom. Radiol. 2022, 05, 094–106. [Google Scholar] [CrossRef]
- Elbanna, K.Y.; Mansoori, B.; Mileto, A.; Rogalla, P.; Guimaraes, L.S. Dual-energy CT in diffuse liver disease: is there a role? Abdom. Imaging 2020, 45, 3413–3424. [Google Scholar] [CrossRef] [PubMed]
- Molwitz, I.; Campbell, G.M.; Yamamura, J.; Knopp, T.; Toedter, K.D.-C.; Fischer, R.; Wang, Z.J.; Busch, A.; Ozga, A.-K.; Zhang, S.; et al. Fat Quantification in Dual-Layer Detector Spectral Computed Tomography: Experimental Development and First In-Patient Validation. Investig. Radiol. 2022, 57, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Boesen, M.R.; Hansen, S.L.; Ulriksen, P.S.; Holm, S.; Lönn, L.; Hansen, K.L. Assessment of Liver Fat: Dual-Energy CT versus Conventional CT with and without Contrast. Diagnostics 2022, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- Marri, U.K.; Das, P.; Shalimar; Kalaivani, M. ; Srivastava, D.N.; Madhusudhan, K.S. Noninvasive Staging of Liver Fibrosis Using 5-Minute Delayed Dual-Energy CT: Comparison with US Elastography and Correlation with Histologic Findings. Radiology 2021, 298, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Kruis, M.F. Improving radiation physics, tumor visualisation, and treatment quantification in radiotherapy with spectral or dual-energy CT. J. Appl. Clin. Med Phys. 2021, 23, e13468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Liu, X.-L.; Liao, Z.-B.; Lu, X.-M.; Chen, L.-L.; Lei, Y.; Zhang, H.-W.; Lin, F. Dual-energy spectral detector computed tomography differential diagnosis of adrenal adenoma and pheochromocytoma: Changes in the energy level curve, a phenomenon caused by lipid components? Front. Endocrinol. 2023, 13, 998154. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, M.T.; Gassenmaier, S.; Walter, S.S.; Artzner, C.; Lades, F.; Faby, S.; Nikolaou, K.; Bongers, M.N. Differentiation of adrenal adenomas from adrenal metastases in single-phased staging dual-energy CT and radiomics. Diagn. Interv. Radiol. 2022, 28, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Yang, D.; Zhang, Y.; Zhang, Y.; Mu, Y. The value of CT-based energy imaging to discriminate dominant side lesions in primary aldosteronism. Front. Endocrinol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Schafigh, D.G.; Wallis, M.G.; Campbell, G.M.; Malter, W.; Schömig-Markiefka, B.; Maintz, D.; Hellmich, M.; Krug, K.B. Assignment of the biological value of solid breast masses based on quantitative evaluations of spectral CT examinations using electron density mapping, Zeffective mapping and iodine mapping. Eur. J. Radiol. 2024, 171, 111280. [Google Scholar] [CrossRef] [PubMed]
- Zopfs, D.; Graffe, J.; Reimer, R.P.; Schaefer, S.; Persigehl, T.; Maintz, D.; Borggrefe, J.; Haneder, S.; Lennartz, S.; Hokamp, N.G. Quantitative distribution of iodinated contrast media in body computed tomography: data from a large reference cohort. Eur. Radiol. 2021, 31, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, D.; Zeng, X.; Jiang, S.; Li, L.; Yu, T.; Zhang, J. Dual-energy CT quantitative parameters for the differentiation of benign from malignant lesions and the prediction of histopathological and molecular subtypes in breast cancer. Quant. Imaging Med. Surg. 2021, 11, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Metin, N.O.; Balcı, S.; Metin, Y.; Taşçı, F.; Gözükara, M.G. Correlation between Quantitative Parameters Obtained by Dual Energy Spectral CT and Prognostic Histopathological Factors and Biomarkers in Breast Cancer. Clin. Breast Cancer 2024. [Google Scholar] [CrossRef] [PubMed]
- Volterrani, L.; Gentili, F.; Fausto, A.; Pelini, V.; Megha, T.; Sardanelli, F.; Mazzei, M.A. Dual-Energy CT for Locoregional Staging of Breast Cancer: Preliminary Results. Am. J. Roentgenol. 2020, 214, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Terada, K.; Kawashima, H.; Yoneda, N.; Toshima, F.; Hirata, M.; Kobayashi, S.; Gabata, T. Predicting axillary lymph node metastasis in breast cancer using the similarity of quantitative dual-energy CT parameters between the primary lesion and axillary lymph node. Jpn. J. Radiol. 2022, 40, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Lebovic, G.; Vlachou, P.A. Diagnostic Value of CT in Detecting Peripheral Zone Prostate Cancer. Am. J. Roentgenol. 2019, 213, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Yel, I.; D’angelo, T.; Gruenewald, L.D.; Koch, V.; Golbach, R.; Mahmoudi, S.; Ascenti, G.; Blandino, A.; Vogl, T.J.; Booz, C.; et al. Dual-Energy CT Material Decomposition: The Value in the Detection of Lymph Node Metastasis from Breast Cancer. Diagnostics 2024, 14, 466. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.P.; Ostmeier, S.; Nadjiri, J.; Deniffel, D.; Rummeny, E.J.; Pfeiffer, D. Iodine concentration of healthy lymph nodes of neck, axilla, and groin in dual-energy computed tomography. Acta Radiol. 2020, 61, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Radice, D.; Femia, M.; De Marco, P.; Origgi, D.; Preda, L.; Barberis, M.; Vigorito, R.; Mauri, G.; Mauro, A.; et al. Metastatic and non-metastatic lymph nodes: quantification and different distribution of iodine uptake assessed by dual-energy CT. Eur. Radiol. 2017, 28, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, A.; Pakarinen, T.; Sassi, A.; Kyttä, J.; Cancino, W.; Rinta-Kiikka, I.; Pertuz, S.; Arponen, O. Methodology, clinical applications, and future directions of body composition analysis using computed tomography (CT) images: A review. Eur. J. Radiol. 2021, 145, 109943. [Google Scholar] [CrossRef] [PubMed]
- Chianca, V.; Albano, D.; Messina, C.; Gitto, S.; Ruffo, G.; Guarino, S.; Del Grande, F.; Sconfienza, L.M. Sarcopenia: imaging assessment and clinical application. Abdom. Imaging 2021, 47, 3205–3216. [Google Scholar] [CrossRef] [PubMed]
- Molwitz, I.; Leiderer, M.; McDonough, R.; Fischer, R.; Ozga, A.-K.; Ozden, C.; Tahir, E.; Koehler, D.; Adam, G.; Yamamura, J. Skeletal muscle fat quantification by dual-energy computed tomography in comparison with 3T MR imaging. Eur. Radiol. 2021, 31, 7529–7539. [Google Scholar] [CrossRef]
- Mallinson, P.I.; Coupal, T.M.; McLaughlin, P.D.; Nicolaou, S.; Munk, P.L.; Ouellette, H.A. Dual-Energy CT for the Musculoskeletal System. Radiology 2016, 281, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, K.N.; Doerge, S.; Leng, S.; Drees, T.A.; Hunt, K.N.; Zingula, S.N.; Pruthi, S.; Geske, J.R.; Carter, R.E.; McCollough, C.H.; et al. Ability of Dual-Energy CT to Detect Silicone Gel Breast Implant Rupture and Nodal Silicone Spread. Am. J. Roentgenol. 2019, 212, 933–942. [Google Scholar] [CrossRef]
- Lennartz, S.; Parakh, A.; Cao, J.; Kambadakone, A. Longitudinal reproducibility of attenuation measurements on virtual unenhanced images: multivendor dual-energy CT evaluation. Eur. Radiol. 2021, 31, 9240–9249. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.E.; Mager, P.; Yu, N.C.; Katz, D.P.; Brady, J.R.; Gupta, N. Iodine quantification and detectability thresholds among major dual-energy CT platforms. Br. J. Radiol. 2019, 92, 20190530. [Google Scholar] [CrossRef] [PubMed]
- Hindman, N.M. How Low Can We Go? The Very Low Limits of Iodine Detection and Quantification in Dual-Energy CT. Radiology 2019, 292, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.E. The Role of Dual-Energy Computed Tomography in Assessment of Abdominal Oncology and Beyond. Radiol. Clin. North Am. 2018, 56, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Vernuccio, F.; Meyer, M.; Godwin, B.; Rosenberg, M.; Rudnick, N.; Harring, S.; Nelson, R.; Ramirez-Giraldo, J.C.; Farjat, A.; et al. Dual-Energy CT Material Density Iodine Quantification for Distinguishing Vascular From Nonvascular Renal Lesions: Normalization Reduces Intermanufacturer Threshold Variability. Am. J. Roentgenol. 2019, 212, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, S.; Cao, J.; Pisuchpen, N.; Srinivas-Rao, S.; Locascio, J.J.; Parakh, A.; Hahn, P.F.; Mileto, A.; Sahani, D.; Kambadakone, A. Intra-patient variability of iodine quantification across different dual-energy CT platforms: assessment of normalization techniques. Eur. Radiol. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Coupal, T.M.; Mallinson, P.I.; Gershony, S.L.; McLaughlin, P.D.; Munk, P.L.; Nicolaou, S.; Ouellette, H.A. Getting the Most From Your Dual-Energy Scanner: Recognizing, Reducing, and Eliminating Artifacts. Am. J. Roentgenol. 2016, 206, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Soesbe, T.C.; Ananthakrishnan, L.; Lewis, M.A.; Duan, X.; Nasr, K.; Xi, Y.; Abbara, S.; Leyendecker, J.R.; Lenkinski, R.E. Pseudoenhancement effects on iodine quantification from dual-energy spectral CT systems: A multi-vendor phantom study regarding renal lesion characterization. Eur. J. Radiol. 2018, 105, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Zhang, D.; Levine, B.D.; Dalbeth, N.; Pool, B.; Ranganath, V.K.; Benhaim, P.; Nelson, S.D.; Hsieh, S.S.; FitzGerald, J.D. Limitations of dual-energy CT in the detection of monosodium urate deposition in dense liquid tophi and calcified tophi. Skelet. Radiol. 2021, 50, 1667–1675. [Google Scholar] [CrossRef]
- Jepperson, M.; Cernigliaro, J.; Sella, D.; Ibrahim, E.; Thiel, D.; Leng, S.; Haley, W. Dual-energy CT for the evaluation of urinary calculi: Image interpretation, pitfalls and stone mimics. Clin. Radiol. 2013, 68, e707–e714. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Rajiah, P.S. Milestones in CT: Past, Present, and Future. Radiology 2023, 309, e230803. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.M.; Hippe, D.S.; Zamora, D.A.; Cao, J.; Parakh, A.; Kambadakone, A.R.; Xiao, J.M.; Wang, S.S.; Toia, G.V.; Gunn, M.L.; et al. A Method for Reducing Variability Across Dual-Energy CT Manufacturers in Quantification of Low Iodine Content Levels. Am. J. Roentgenol. 2022, 218, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Ascenti, G.; Agostini, A.; Longo, C.; Lombardo, F.; Inno, A.; Modena, A.; Gori, S. Dual-Energy CT in Oncologic Imaging. Tomography 2024, 10, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, S.; Singh, R.; Netaji, A.; Madhusudhan, K.S.; Homayounieh, F.; Primak, A.; Lades, F.; Saini, S.; Kalra, M.K.; Sharma, S. Characterization of Benign and Malignant Pancreatic Lesions with DECT Quantitative Metrics and Radiomics. Acad. Radiol. 2022, 29, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Yu, W.; Liu, S.-Q.; Xie, M.-G.; Liu, M. The value of radiomics based on dual-energy CT for differentiating benign from malignant solitary pulmonary nodules. BMC Med Imaging 2022, 22, 1–7. [Google Scholar] [CrossRef]
- Krug, K.B.; Schömig-Markiefka, B.; Campbell, G.M.; Püsken, M.; Maintz, D.; Schlamann, M.; Klein, K.; Schafigh, D.G.; Malter, W.; Hellmich, M. Correlation of CT-data derived from multiparametric dual-layer CT-maps with immunohistochemical biomarkers in invasive breast carcinomas. Eur. J. Radiol. 2022, 156, 110544. [Google Scholar] [CrossRef]
- Azour, L.; Ko, J.P.; O’donnell, T.; Patel, N.; Bhattacharji, P.; Moore, W.H. Combined whole-lesion radiomic and iodine analysis for differentiation of pulmonary tumors. Sci. Rep. 2022, 12, 11813. [Google Scholar] [CrossRef]
- Jia, Y.; Xiao, X.; Sun, Q.; Jiang, H. CT spectral parameters and serum tumour markers to differentiate histological types of cancer histology. Clin. Radiol. 2018, 73, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, D.; Netaji, A.; Diwan, K.; Sharma, S. Normalized Dual-Energy Iodine Ratio Best Differentiates Renal Cell Carcinoma Subtypes Among Quantitative Imaging Biomarkers From Perfusion CT and Dual-Energy CT. Am. J. Roentgenol. 2020, 215, 1389–1397. [Google Scholar] [CrossRef]
- Shi, C.; Yu, Y.; Yan, J.; Hu, C. The added value of radiomics from dual-energy spectral CT derived iodine-based material decomposition images in predicting histological grade of gastric cancer. BMC Med Imaging 2022, 22, 173. [Google Scholar] [CrossRef]
- Fan, S.; Li, X.; Zheng, L.; Hu, D.; Ren, X.; Ye, Z. Correlations between the iodine concentrations from dual energy computed tomography and molecular markers Ki-67 and HIF-1α in rectal cancer: A preliminary study. Eur. J. Radiol. 2017, 96, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Koch, V.; Dos Santos, D.P.; Ackermann, J.; Grünewald, L.D.; Weitkamp, I.; Yel, I.; Martin, S.S.; Albrecht, M.H.; Scholtz, J.-E.; et al. Imaging biomarkers to stratify lymph node metastases in abdominal CT – Is radiomics superior to dual-energy material decomposition? Eur. J. Radiol. Open 2022, 10, 100459. [Google Scholar] [CrossRef] [PubMed]
- Schramm, N.; Schlemmer, M.; Englhart, E.; Hittinger, M.; Becker, C.; Reiser, M.; Berger, F. Dual Energy CT for Monitoring Targeted Therapies in Patients with Advanced Gastrointestinal Stromal Tumor: Initial Results. Curr. Pharm. Biotechnol. 2011, 12, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Drljevic-Nielsen, A.; Mains, J.R.; Thorup, K.; Andersen, M.B.; Rasmussen, F.; Donskov, F. Early reduction in spectral dual-layer detector CT parameters as favorable imaging biomarkers in patients with metastatic renal cell carcinoma. Eur. Radiol. 2022, 32, 7323–7334. [Google Scholar] [CrossRef]
- Kang, H.-J.; Kim, S.H.; Bae, J.S.; Jeon, S.K.; Han, J.K. Can quantitative iodine parameters on DECT replace perfusion CT parameters in colorectal cancers? Eur. Radiol. 2018, 28, 4775–4782. [Google Scholar] [CrossRef] [PubMed]
- Mulé, S.; Pigneur, F.; Quelever, R.; Tenenhaus, A.; Baranes, L.; Richard, P.; Tacher, V.; Herin, E.; Pasquier, H.; Ronot, M.; et al. Can dual-energy CT replace perfusion CT for the functional evaluation of advanced hepatocellular carcinoma? Eur. Radiol. 2017, 28, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Skornitzke, S.; Fritz, F.; Mayer, P.; Koell, M.; Hansen, J.; Pahn, G.; Hackert, T.; Kauczor, H.-U.; Stiller, W. Dual-energy CT iodine maps as an alternative quantitative imaging biomarker to abdominal CT perfusion: determination of appropriate trigger delays for acquisition using bolus tracking. Br. J. Radiol. 2018, 91, 20170351. [Google Scholar] [CrossRef] [PubMed]
- Yel, I.; Bucolo, G.M.; Mahmoudi, S.; Koch, V.; Gökduman, A.; D′Angelo, T.; Grünewald, L.D.; Dimitrova, M.; Eichler, K.; Vogl, T.J.; et al. Dual-Energy CT Iodine Uptake of Head and Neck: Definition of Reference Values in a Big Data Cohort. Diagnostics 2024, 14, 496. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Del Canto, M.; Clemente, A.; Gragnano, E.; Cioce, F.; Urraro, F.; Martinelli, E.; Cappabianca, S. The Role of Dual-Energy CT for the Assessment of Liver Metastasis Response to Treatment: Above the RECIST 1.1 Criteria. J. Clin. Med. 2023, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Lafata, K.J.; Wang, Y.; Konkel, B.; Yin, F.-F.; Bashir, M.R. Radiomics: a primer on high-throughput image phenotyping. Abdom. Imaging 2022, 47, 2986–3002. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, S.; Mager, A.; Hokamp, N.G.; Schäfer, S.; Zopfs, D.; Maintz, D.; Reinhardt, H.C.; Thomas, R.K.; Caldeira, L.; Persigehl, T. Texture analysis of iodine maps and conventional images for k-nearest neighbor classification of benign and metastatic lung nodules. Cancer Imaging 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Han, X.; Jia, X.; Ding, C.; Zhang, K.; Li, H.; Cao, X.; Zhang, X.; Zhang, X.; Shi, H. Dual-energy CT-based radiomics for predicting invasiveness of lung adenocarcinoma appearing as ground-glass nodules. Front. Oncol. 2023, 13, 1208758. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yu, Y.; He, T.; Yu, N.; Dang, S.; Wu, H.; Ren, J.; Duan, X. Effect of radiomics from different virtual monochromatic images in dual-energy spectral CT on the WHO/ISUP classification of clear cell renal cell carcinoma. Clin. Radiol. 2021, 76, 627–e23. [Google Scholar] [CrossRef] [PubMed]
- Reinert, C.P.; Krieg, E.; Esser, M.; Nikolaou, K.; Bösmüller, H.; Horger, M. Role of computed tomography texture analysis using dual-energy-based bone marrow imaging for multiple myeloma characterization: comparison with histology and established serologic parameters. Eur. Radiol. 2020, 31, 2357–2367. [Google Scholar] [CrossRef]
- Lenga, L.; Bernatz, S.; Martin, S.S.; Booz, C.; Solbach, C.; Mulert-Ernst, R.; Vogl, T.J.; Leithner, D. Iodine Map Radiomics in Breast Cancer: Prediction of Metastatic Status. Cancers 2021, 13, 2431. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, F.; Wang, L.; Li, E.; Xu, Z.; Wels, M.; Yao, W.; Zhang, H. Evaluation of dual-energy CT derived radiomics signatures in predicting outcomes in patients with advanced gastric cancer after neoadjuvant chemotherapy. Eur. J. Surg. Oncol. (EJSO) 2021, 48, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Brendlin, A.S.; Peisen, F.; Almansour, H.; Afat, S.; Eigentler, T.; Amaral, T.; Faby, S.; Calvarons, A.F.; Nikolaou, K.; E Othman, A. A Machine learning model trained on dual-energy CT radiomics significantly improves immunotherapy response prediction for patients with stage IV melanoma. J. Immunother. Cancer 2021, 9, e003261. [Google Scholar] [CrossRef] [PubMed]
- Foncubierta-Rodriguez, A.; del Toro, O.A.J.; Platon, A.; Poletti, P.-A.; Muller, H.; Depeursinge, A. Benefits of texture analysis of dual energy CT for Computer-Aided pulmonary embolism detection. 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). LOCATION OF CONFERENCE, JapanDATE OF CONFERENCE; pp. 3973–3976.
- Jiang, W.; Pan, X.; Luo, Q.; Huang, S.; Liang, Y.; Zhong, X.; Zhang, X.; Deng, W.; Lv, Y.; Chen, L. Radiomics analysis of pancreas based on dual-energy computed tomography for the detection of type 2 diabetes mellitus. Front. Med. 2024, 11, 1328687. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, S.; Chen, S.; He, Y.; Gao, H.; Yan, L.; Hu, X.; Li, P.; Shen, H.; Luo, M.; et al. Prediction of osteoporosis using radiomics analysis derived from single source dual energy CT. BMC Musculoskelet. Disord. 2023, 24, 100. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, S.; Homayounieh, F.; Singh, R.; Primak, A.; Kalra, M.K.; Romero, J.M.; Inc. , M.S.H.U. Spectral segmentation and radiomic features predict carotid stenosis and ipsilateral ischemic burden from DECT angiography. Diagn. Interv. Radiol. 2022, 28, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Choi, I.Y.; Cha, S.H.; Yeom, S.K.; Chung, H.H.; Lee, S.H.; Cha, J.; Lee, J.-H. Feasibility of computed tomography texture analysis of hepatic fibrosis using dual-energy spectral detector computed tomography. Jpn. J. Radiol. 2020, 38, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.W.; Bae, J.P.; Lee, H.Y.; Kim, N.; Chung, M.P.; Park, H.Y.; Chang, Y.; Seo, J.B.; Lee, K.S. Perfusion- and pattern-based quantitative CT indexes using contrast-enhanced dual-energy computed tomography in diffuse interstitial lung disease: relationships with physiologic impairment and prediction of prognosis. Eur. Radiol. 2015, 26, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, S.H.; Ryu, H.S.; Han, J.K. Iodine Quantification on Spectral Detector-Based Dual-Energy CT Enterography: Correlation with Crohn's Disease Activity Index and External Validation. Korean J. Radiol. 2018, 19, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Martin, S.; Koch, V.; Gruenewald, L.D.; Bernatz, S.; D’angelo, T.; Vogl, T.J.; Booz, C.; Yel, I. Value of Dual-Energy CT Perfusion Analysis in Patients with Acute Pancreatitis: Correlation and Discriminative Diagnostic Accuracy with Varying Disease Severity. Diagnostics 2022, 12, 2601. [Google Scholar] [CrossRef] [PubMed]
- Huang J, Hou J, Yang W, et al Automatic Kidney Stone Composition Analysis Method Based on Dual-energy CT. Curr Med Imaging 2023.
- Euler, A.; Laqua, F.C.; Cester, D.; Lohaus, N.; Sartoretti, T.; dos Santos, D.P.; Alkadhi, H.; Baessler, B. Virtual Monoenergetic Images of Dual-Energy CT—Impact on Repeatability, Reproducibility, and Classification in Radiomics. Cancers 2021, 13, 4710. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Pan, Z.; Chen, Y.; Wang, L.; Xia, Y.; Wang, L.; Li, J.; Lu, W.; Shi, X.; Feng, J.; et al. Robustness of radiomics features of virtual unenhanced and virtual monoenergetic images in dual-energy CT among different imaging platforms and potential role of CT number variability. Insights into Imaging 2023, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, S.; O’shea, A.; Parakh, A.; Persigehl, T.; Baessler, B.; Kambadakone, A. Robustness of dual-energy CT-derived radiomic features across three different scanner types. Eur. Radiol. 2022, 32, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhong, J.; Wang, L.; Shi, X.; Lu, W.; Li, J.; Feng, J.; Xia, Y.; Chang, R.; Fan, J.; et al. Robustness of CT radiomics features: consistency within and between single-energy CT and dual-energy CT. Eur. Radiol. 2022, 32, 5480–5490. [Google Scholar] [CrossRef]
- Douek, P.C.; Boccalini, S.; Oei, E.H.G.; Cormode, D.P.; Pourmorteza, A.; Boussel, L.; Si-Mohamed, S.A.; Budde, R.P.J. Clinical Applications of Photon-counting CT: A Review of Pioneer Studies and a Glimpse into the Future. Radiology 2023, 309, e222432. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Rajendran, K.; Baffour, F.I.; Diehn, F.E.; Ferrero, A.; Glazebrook, K.N.; Horst, K.K.; Johnson, T.F.; Leng, S.; Mileto, A.; et al. Clinical applications of photon counting detector CT. Eur. Radiol. 2023, 33, 5309–5320. [Google Scholar] [CrossRef]
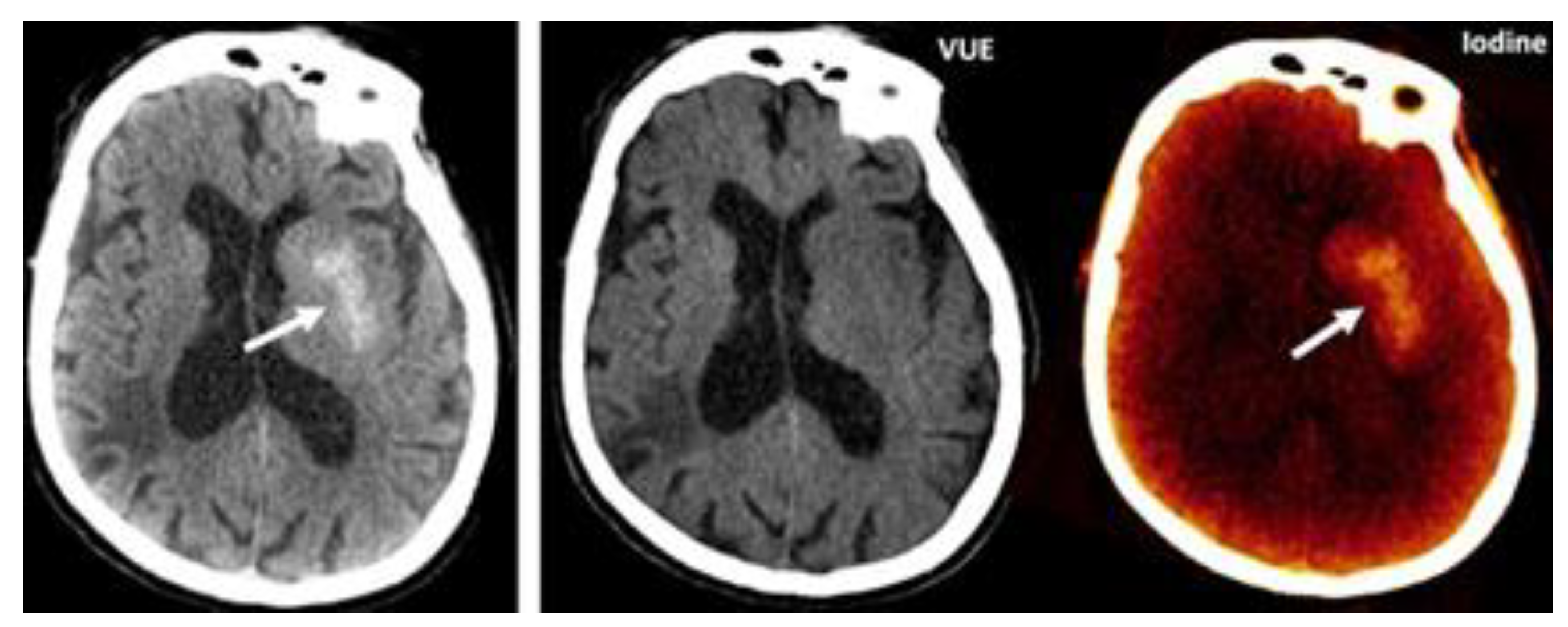
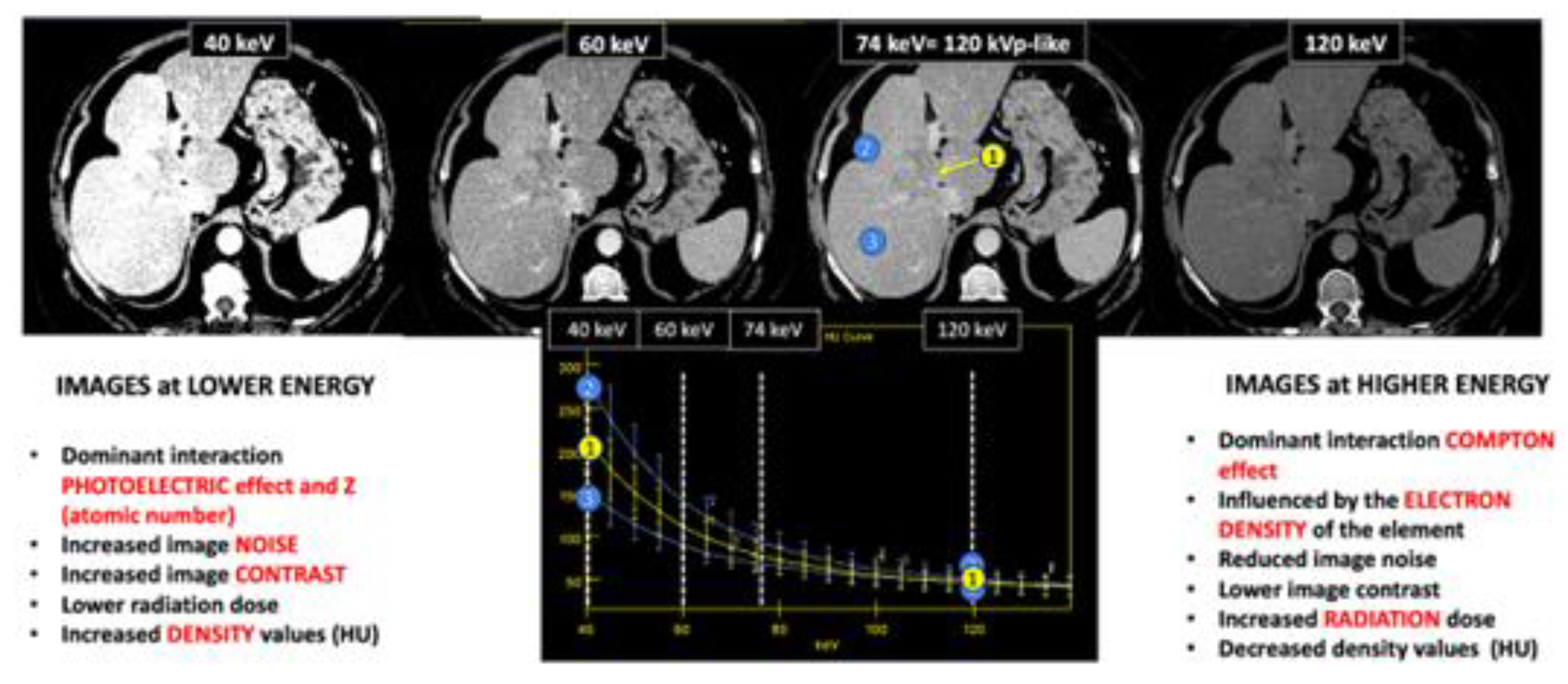
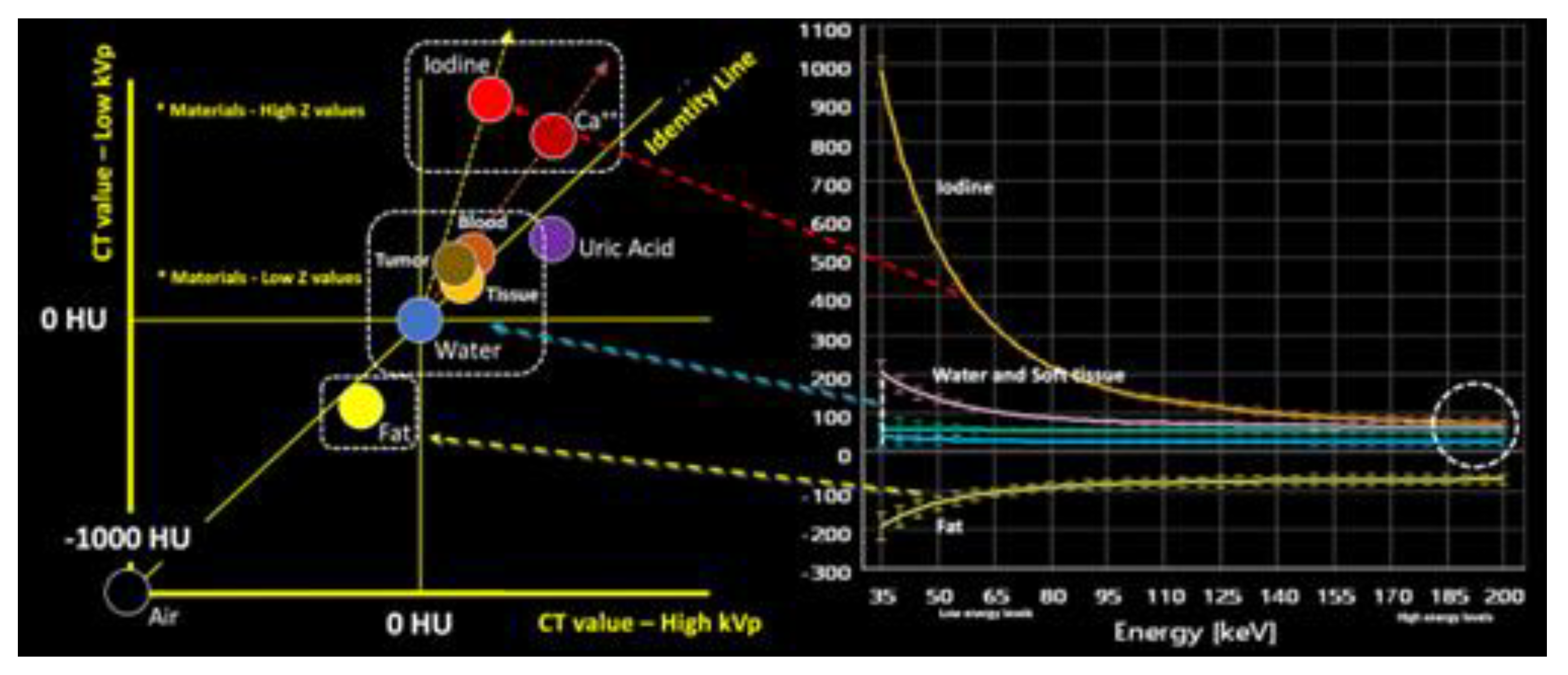
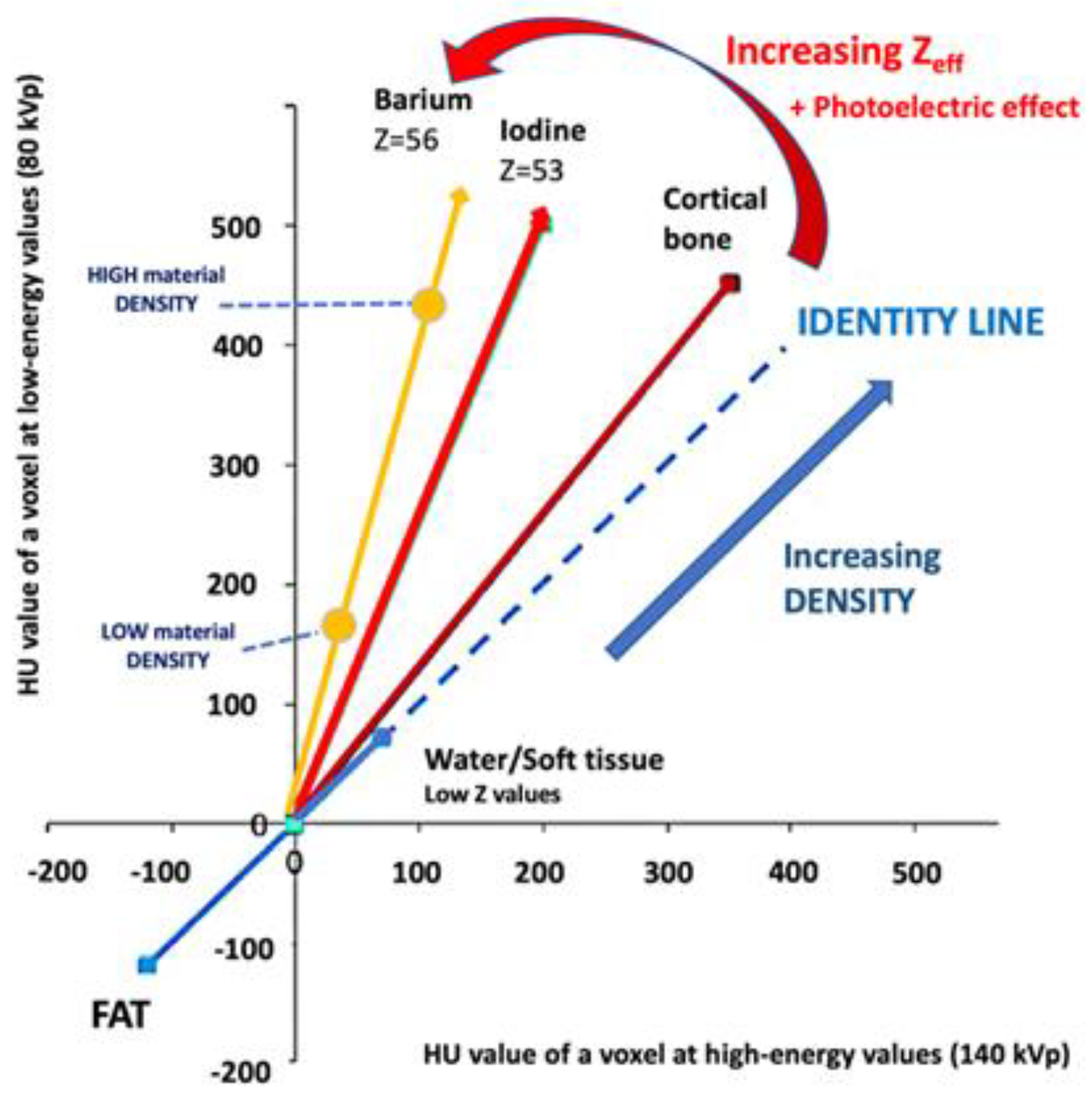
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





