Submitted:
22 May 2024
Posted:
23 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Why Hydroxylapatite Coatings?
- enhanced bone apposition rate by osseoinduction, owing to preferential adsorption of bone growth-supporting factors such as bone morphogenetic proteins (BMPs) and non-collagenous proteins (NCPs) such as osteocalcin, osteonectin, silylated glycoproteins, and proteoglycans,
- enhanced bonding osteogenesis that provides a strong and continuous interface between bone tissue and implant, and thus, enables to transmit not only compressive but also (limited) tensile and shear forces,
- variable HAp coating thickness between 50 and 250 µm can be selected, dependent on application; novel deposition techniques such as suspension plasma spraying (SPS) or solution precursor plasma spraying (SPPS) allow depositing coatings with thickness << 50 µm when required,
- acceleration of the healing process when compared to implants without an osseoconductive coating,
- supporting attachment of epithelium in case of transmucosal dental implants,
- reduced risk of release of potentially toxic heavy metal ions from the implant to the periprosthetic tissue and thus, minimizing a possible cytotoxic response, and
- quality control and standards according to ASTM F1185-03 (2014), ASTM F1044-05(2017), ASTM F1160 (2014), ISO 13179-2: 2018, and others.
3. A Short History of Calcium Orthophosphate Research
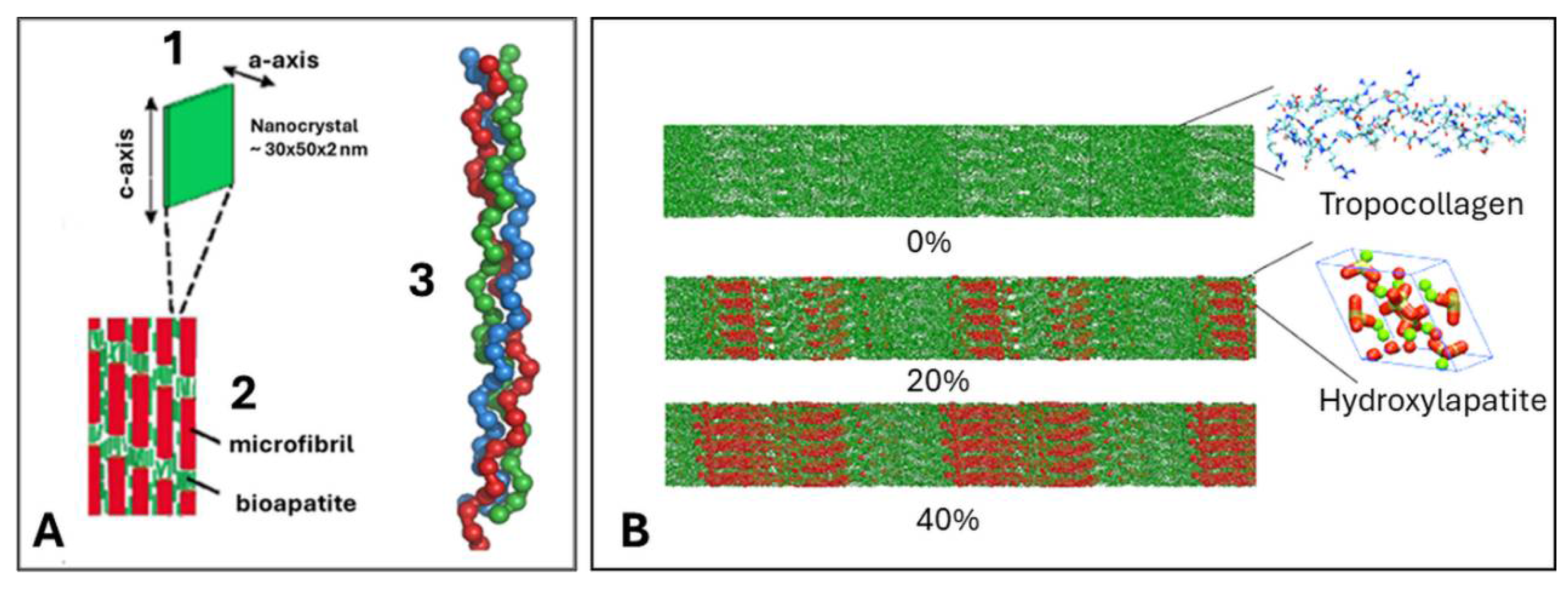
4. Hierarchical Structure of Bone
5. Osseoconductive Hydroxylapatite Coatings
5.1. Osseoconduction, Osseoinduction, and Osseointegration
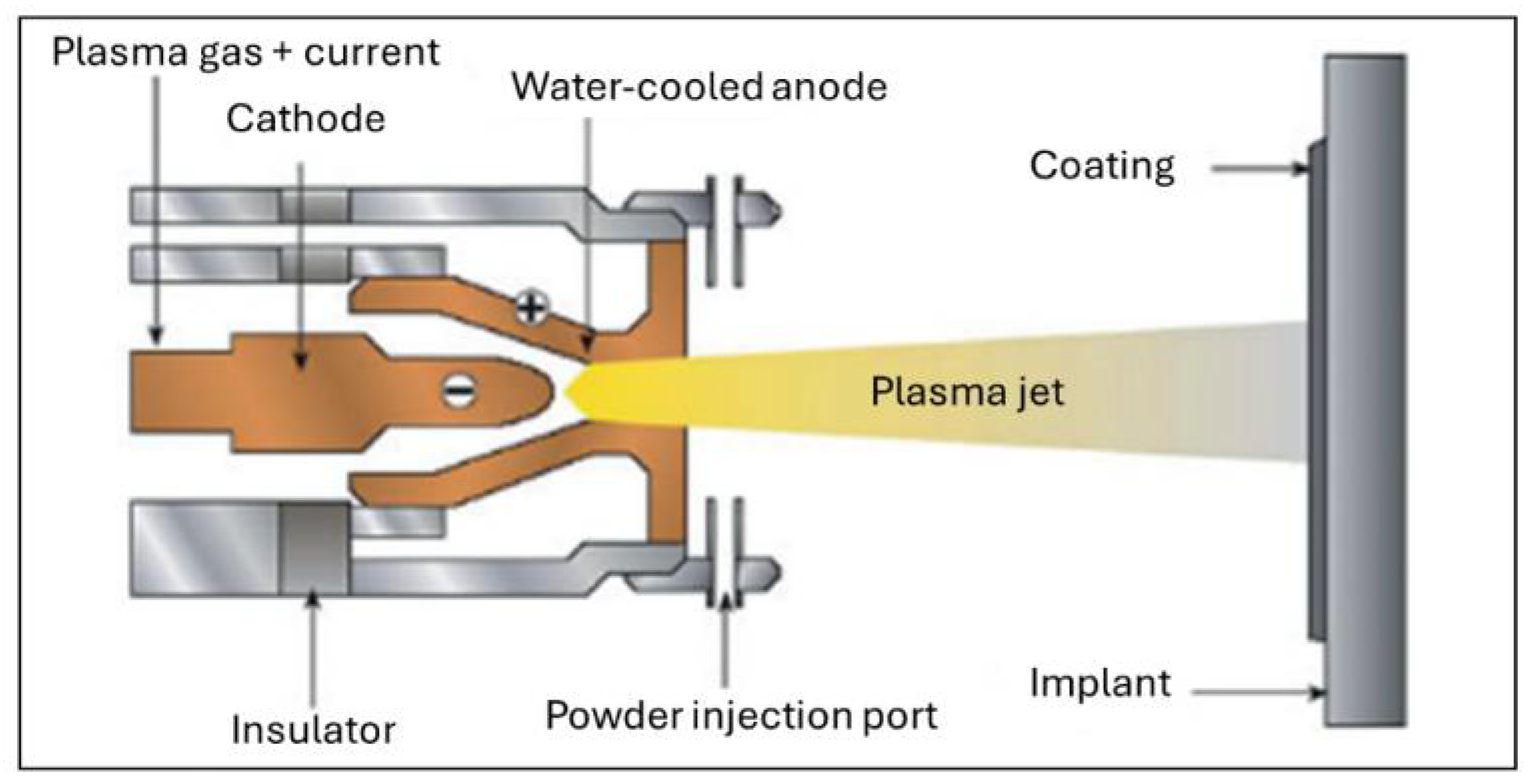
5.2. Deposition Techniques
5.3. Property Requirements and Performance Profile of Hydroxylapatite Coatings
5.3.1. Incongruent Melting and Thermal Decomposition of HAp: Phase Composition
5.3.2. Degree of Crystallinity
- Increase of adhesion to both metal and HAp. For example, a titania bond coat is thought to act as an extension of the native oxide layer on metallic titanium that may interact with HAp to form a thin reaction layer of perovskitic calcium titanate.
- Reduction of thermal decomposition of HAp by inhibiting the heat flow due to the presence of a thin titania bond coat film with low thermal conductivity (~ 1 W/mK) as opposed to a Ti6Al4V substrate (~ 7 W/mK).
- Reduction of the formation of amorphous phase that forms by a quenching contact immediately at the metal interface. Increase of crystallinity is caused by the thermal barrier function of a bond coat that prolong solidification time and thus, allowing the ACP to nucleate apatite and to crystallize. Experimental NMR results [49] show that as-sprayed coatings without a bond coat contain only 46 mass% well-ordered HAp at the free coating surface as contrasted with 62 mass% in coatings with a titania bond coat. During incubation for 12 weeks in r-SBF [50], these values increase by dissolving TCP, TTCP, CaO, and ACP phases to 74 mass% and 92 mass%, respectively.
- Reduction of residual coating stresses by reducing the gradient of the coefficient of thermal expansion between the metal substrate and the ceramic overlayer.
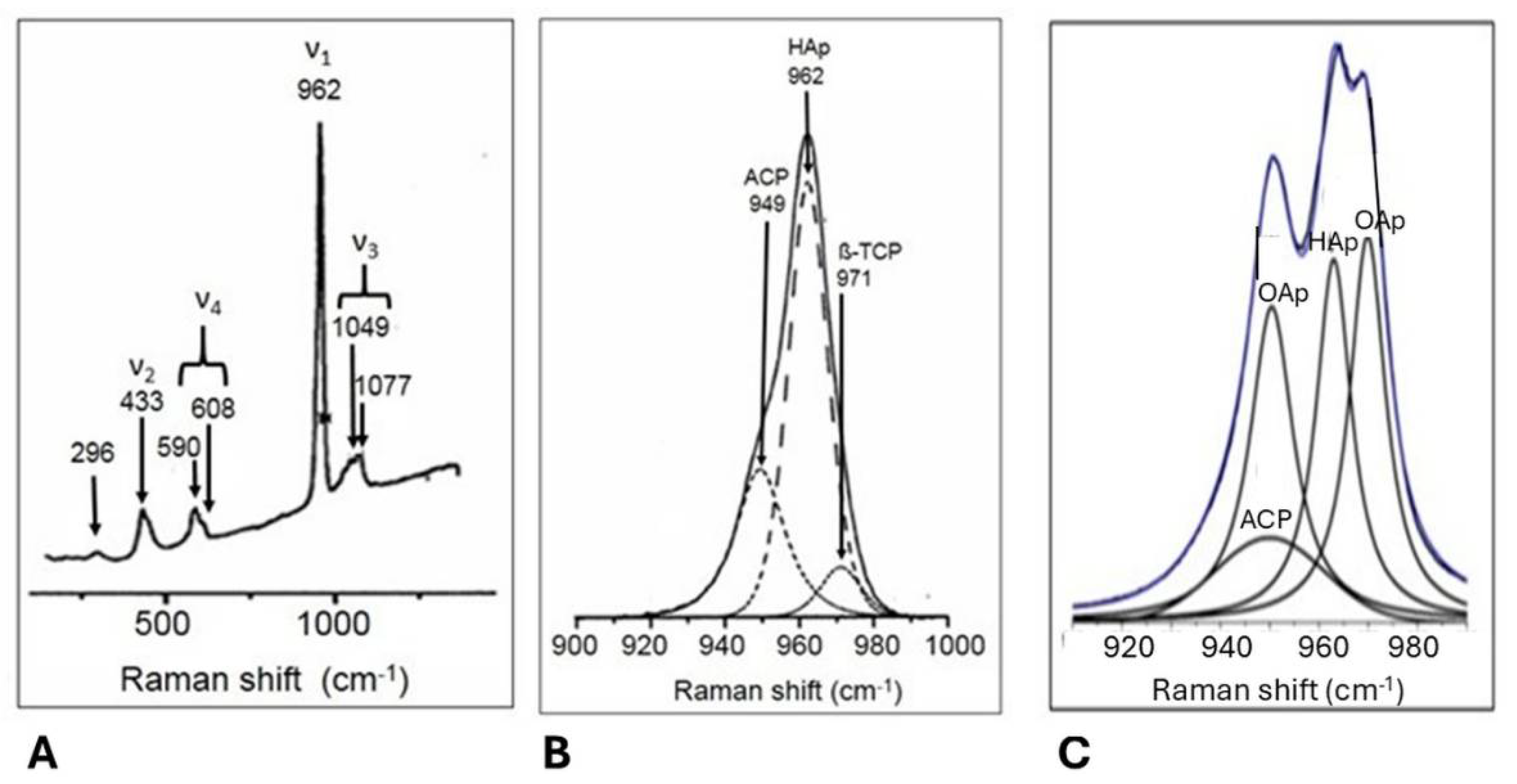
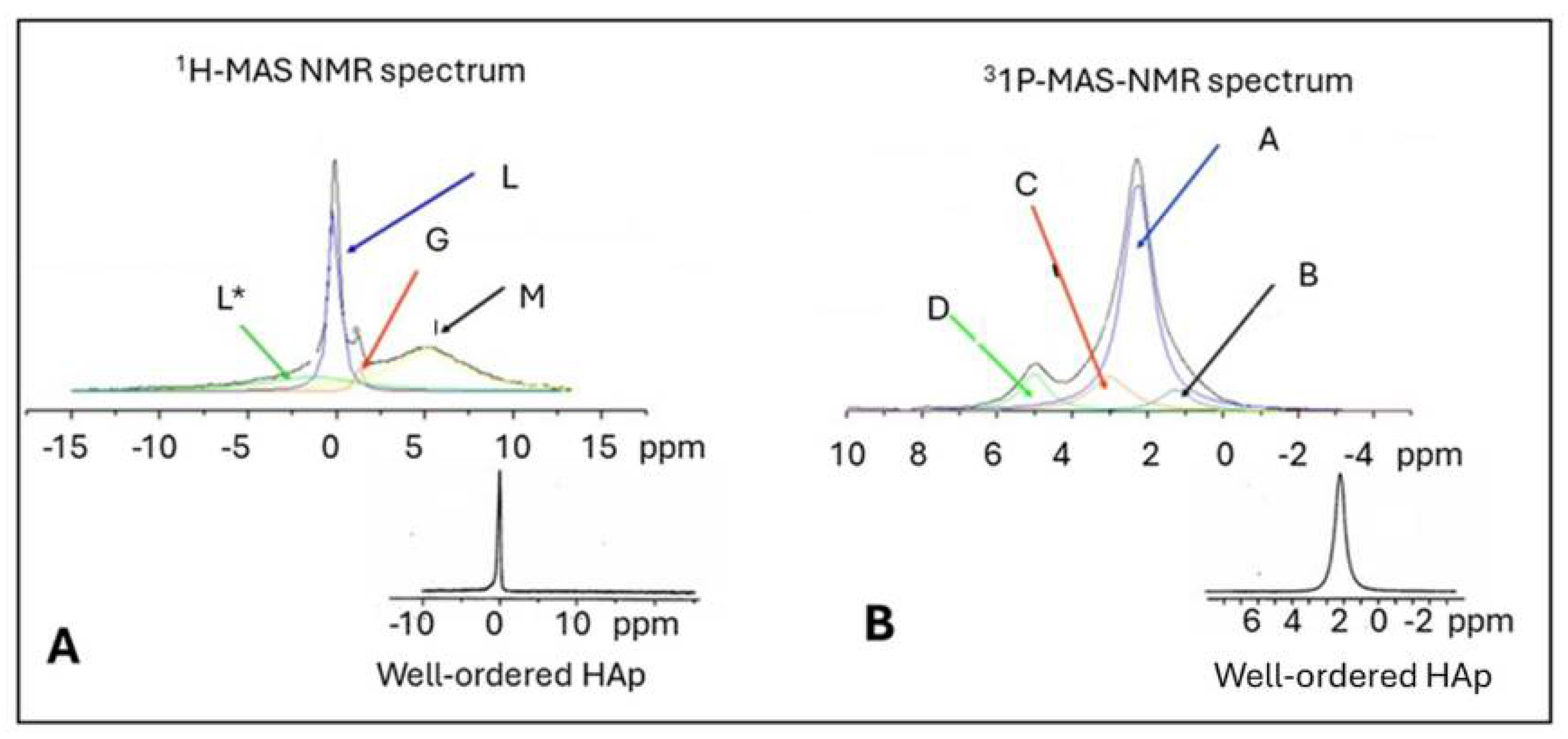
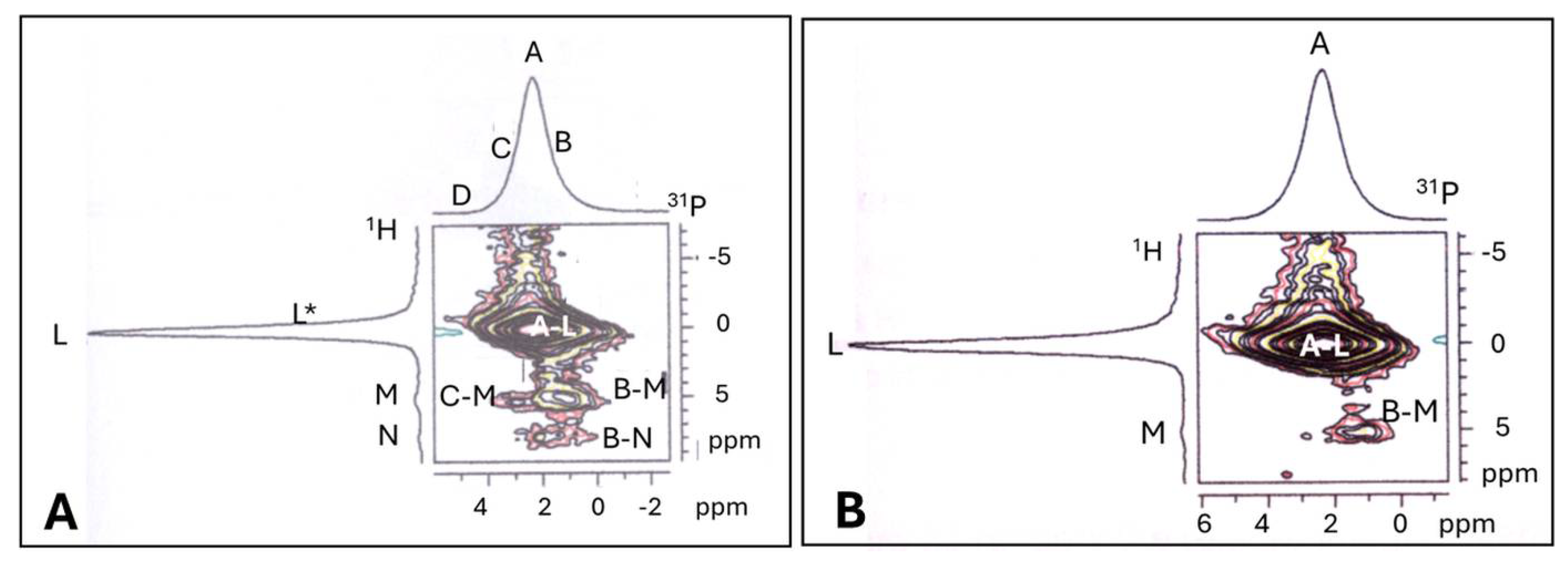
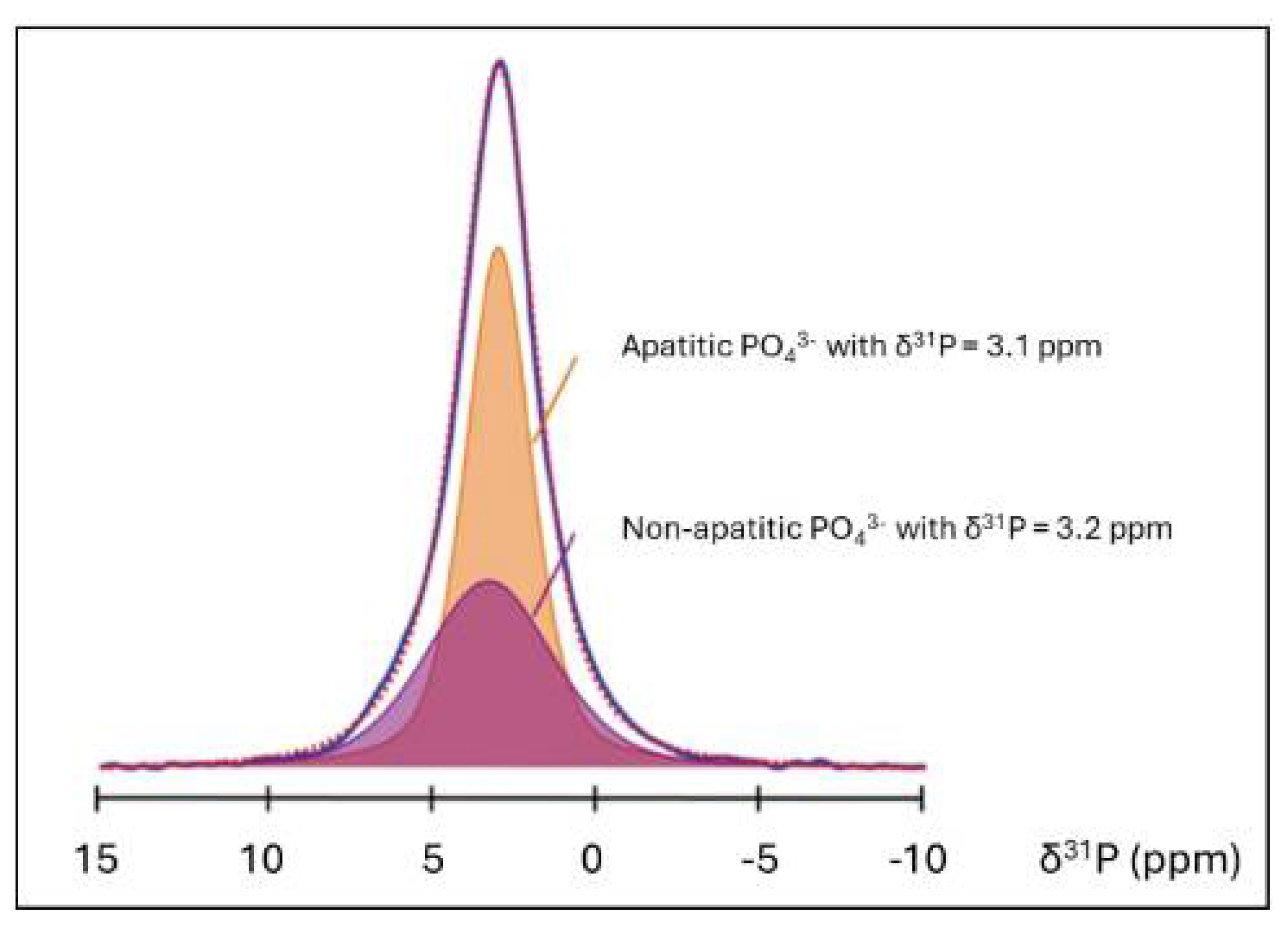
5.3.3. Assessment of Structural Order in Hydroxylapatite Coatings: Raman and NMR Studies
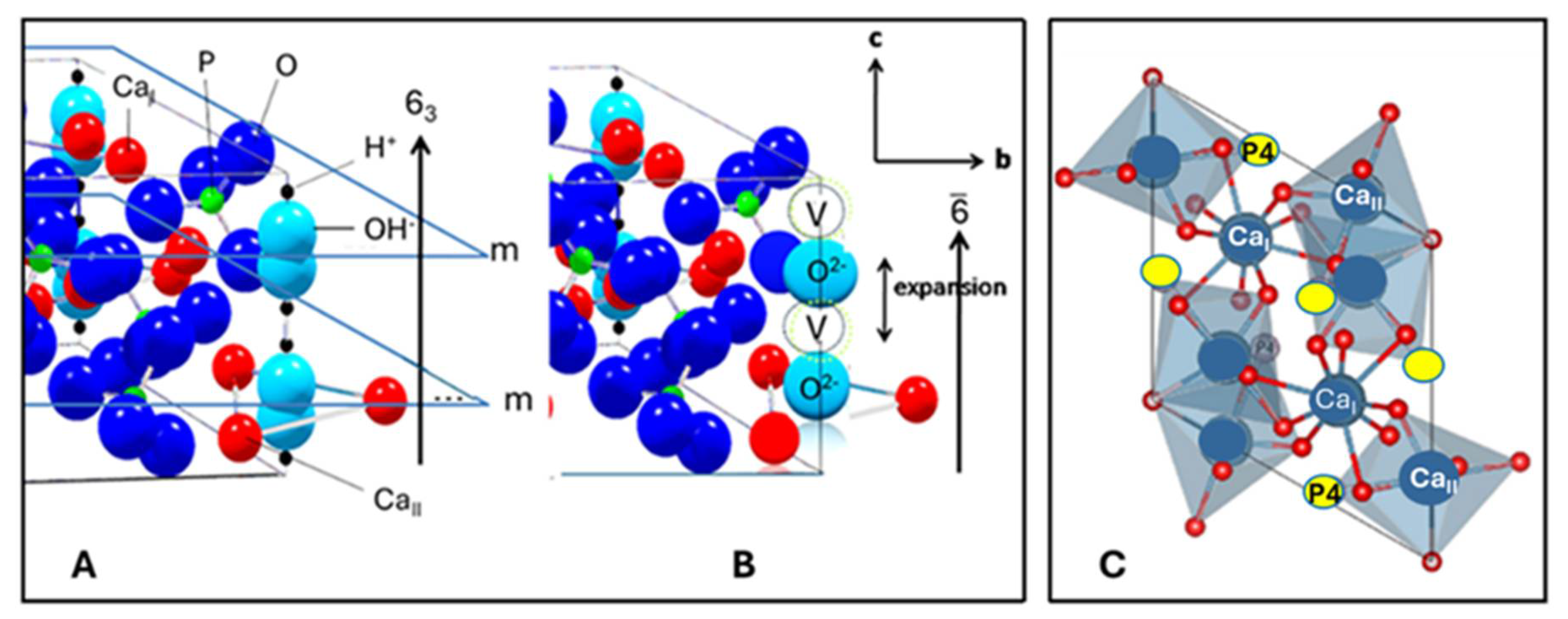
5.3.4. Crystallographic Structure of Hydroxylapatite
5.3.5. Oxyapatite: Fact or Fiction?
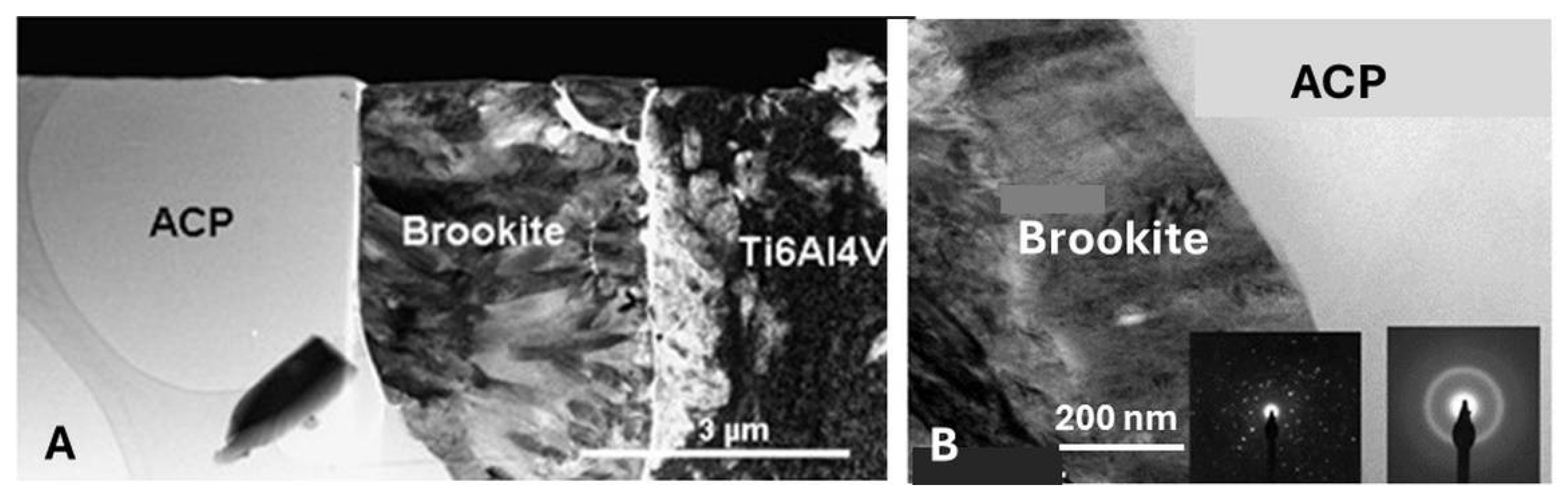
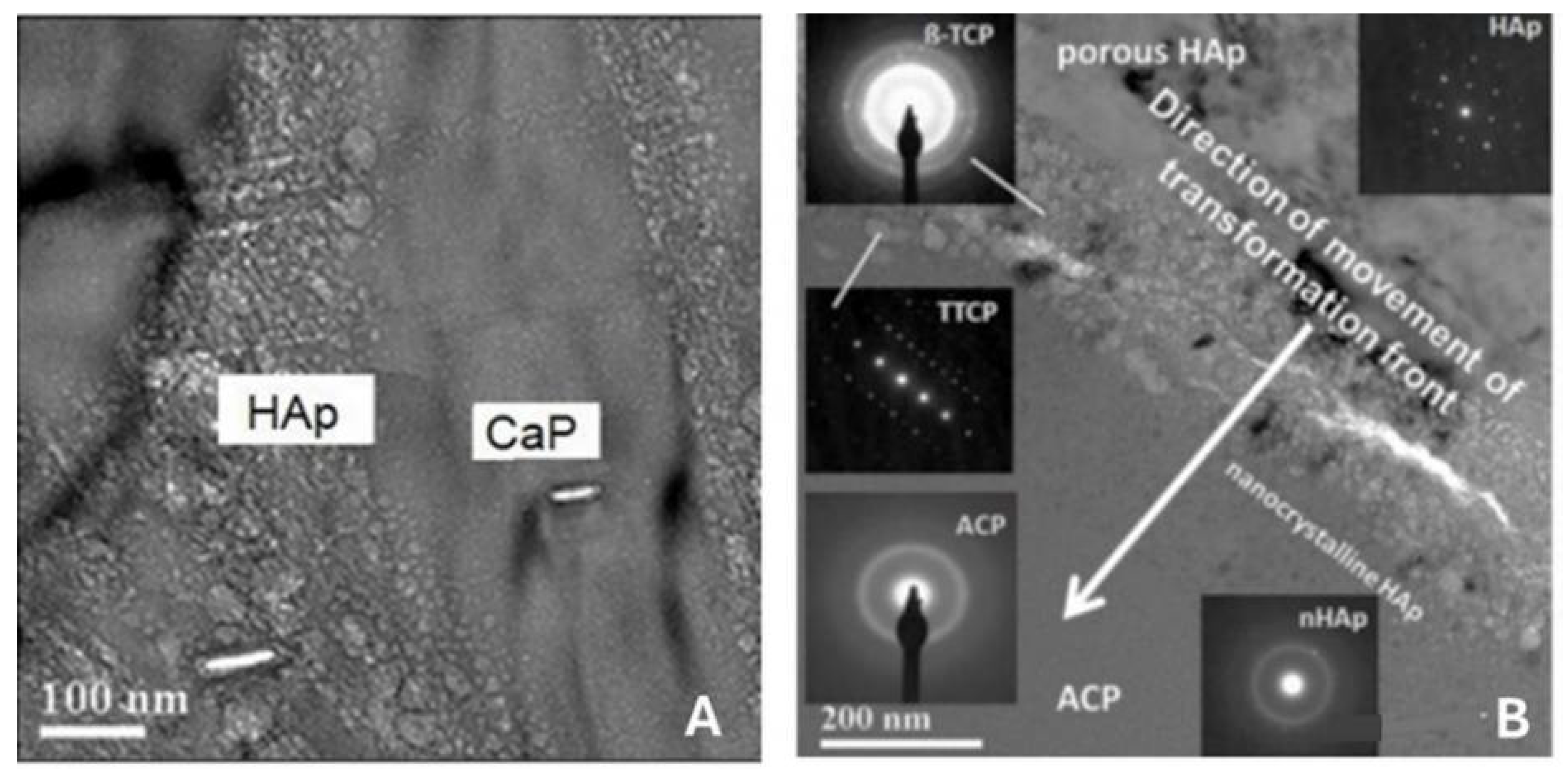
5.3.6. Transformation of Amorphous Calcium Phosphate (ACP)
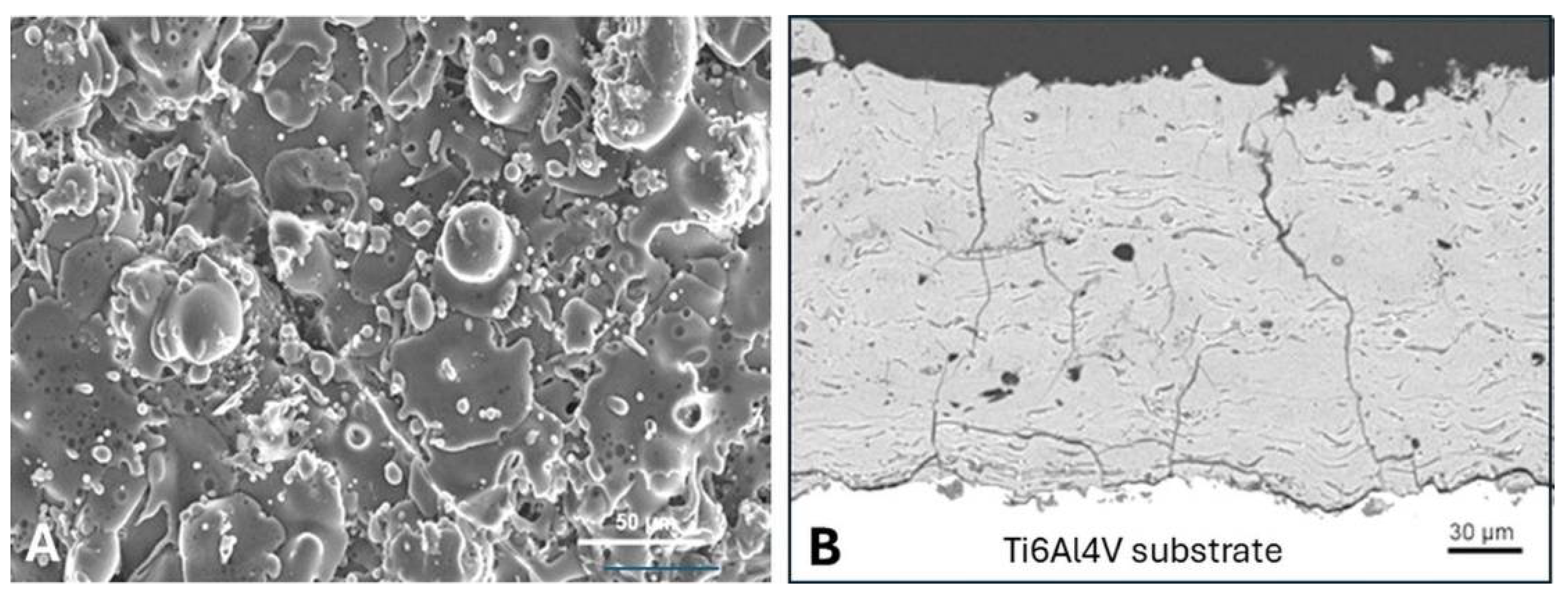
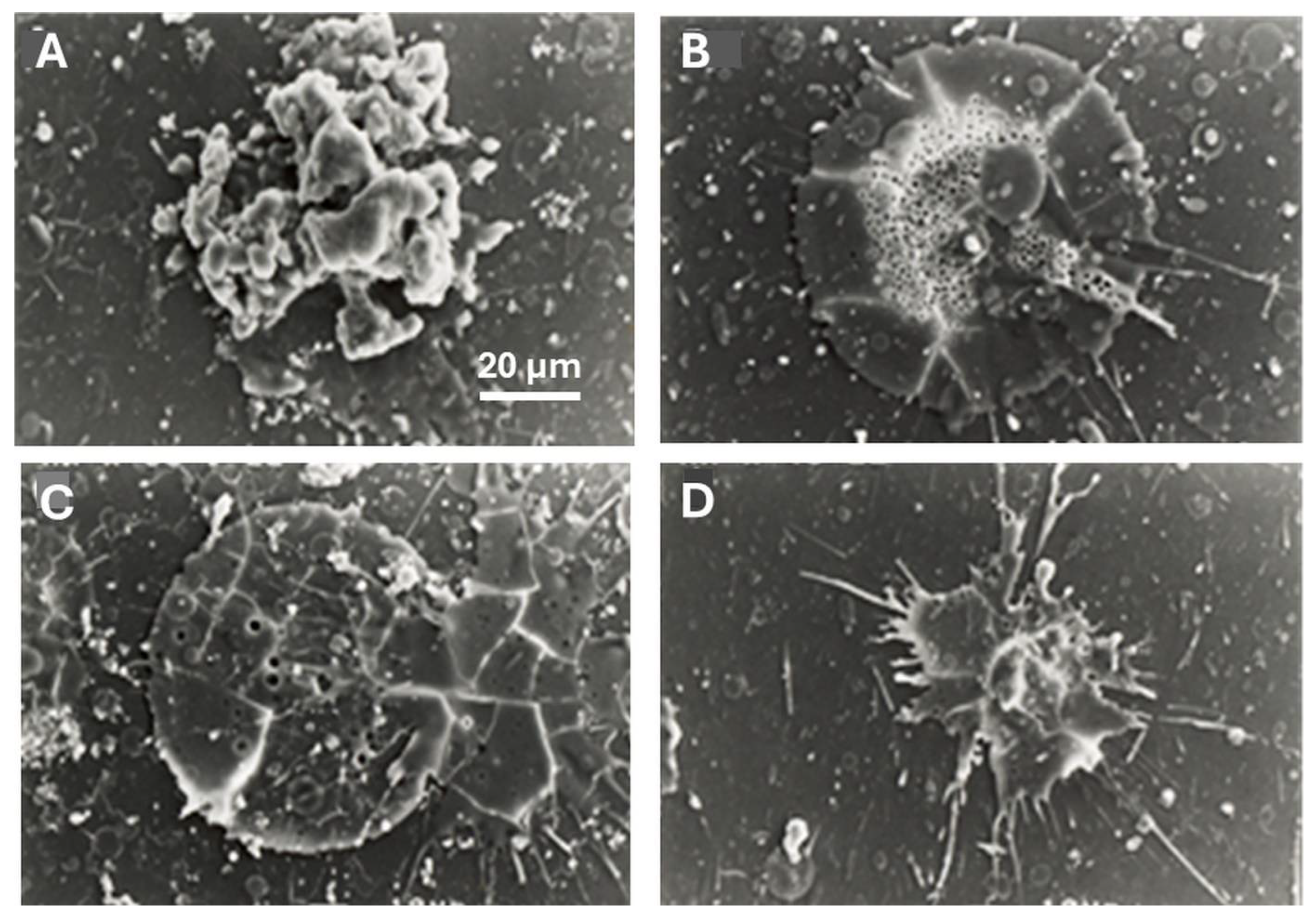
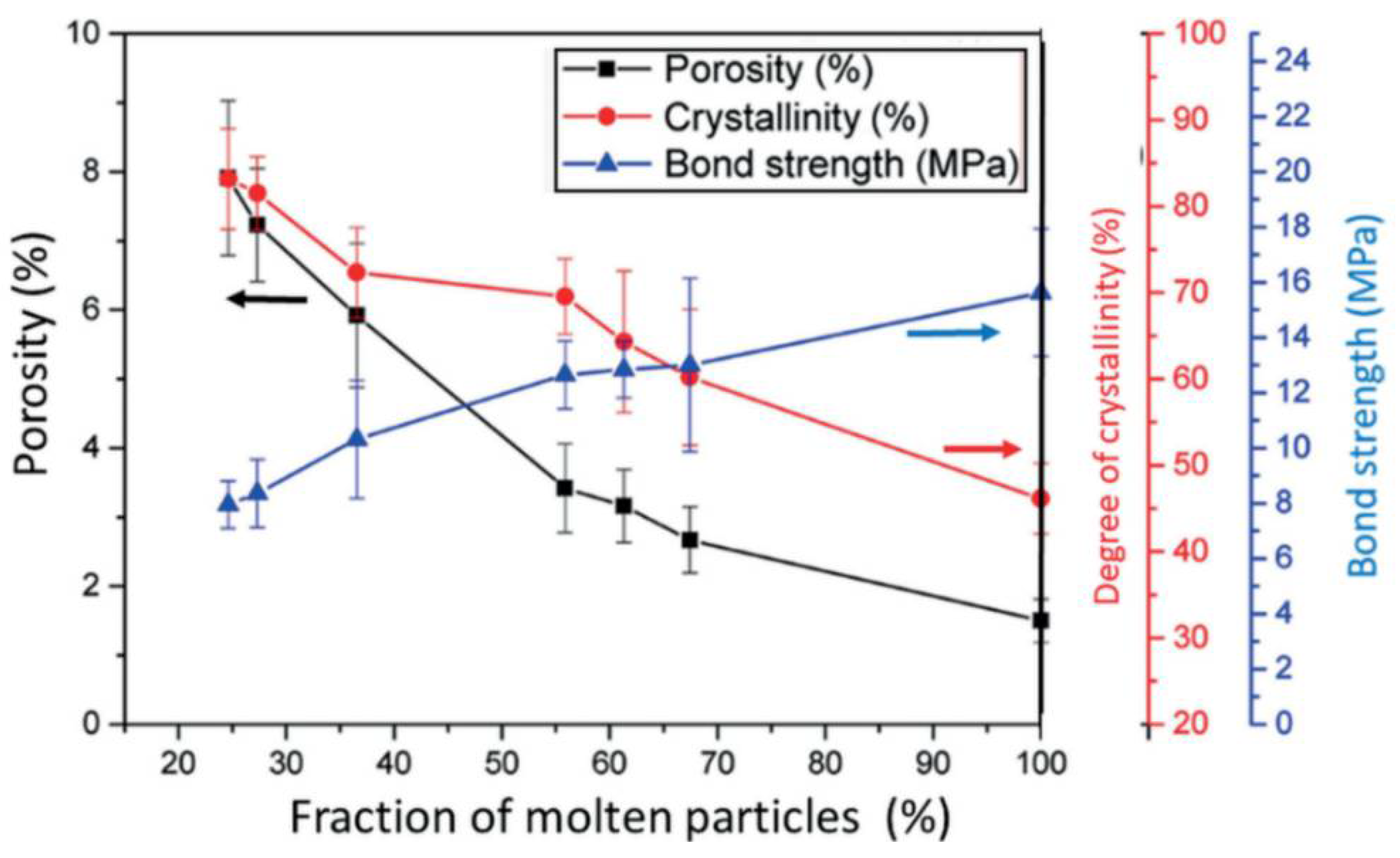
5.3.7. Coating Porosity, Surface Roughness, and Surface Nanotopography
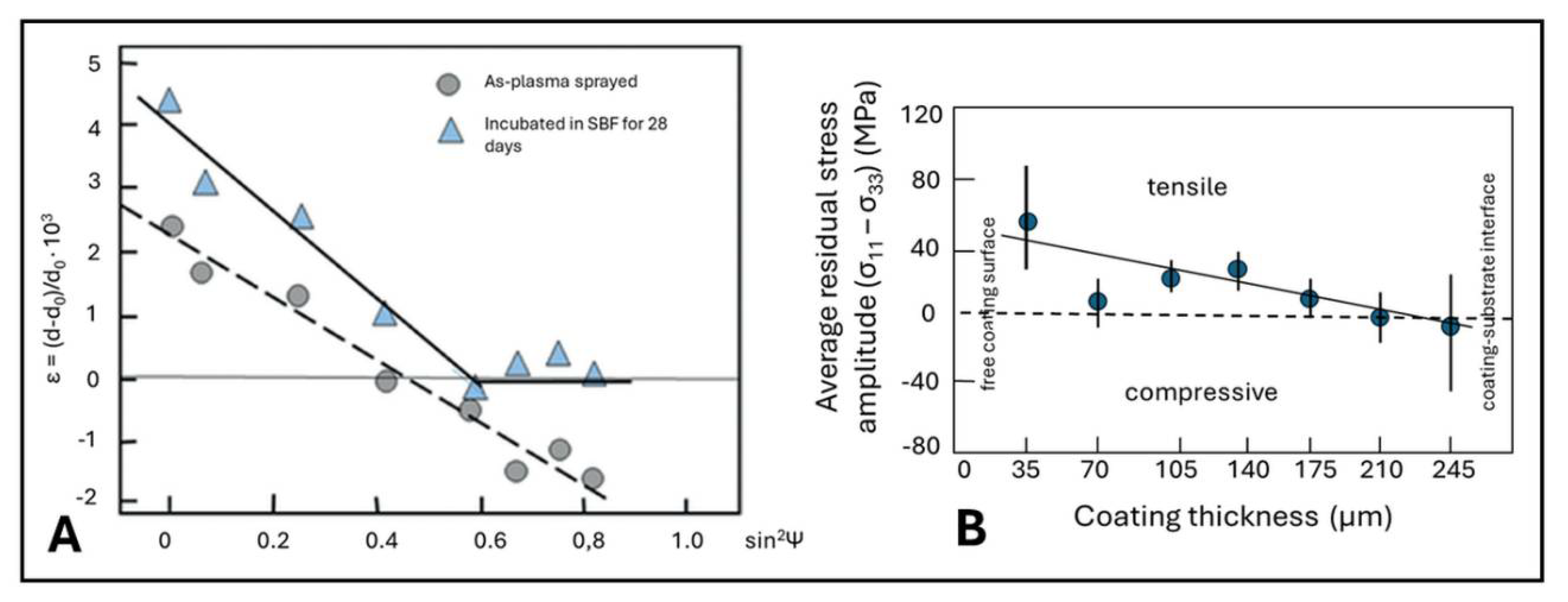
5.3.8. Residual Coating Stresses
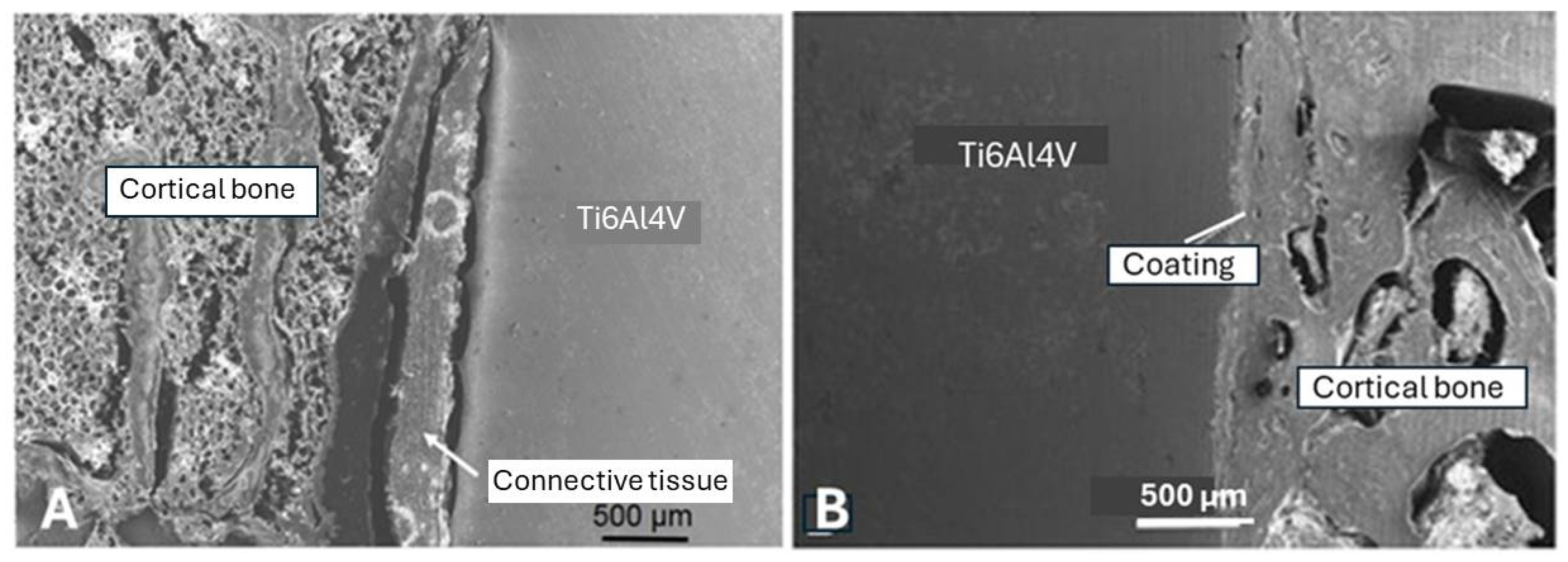
5.3.9. Adhesion of Plasma-Sprayed Hydroxylapatite Coatings
6. Concluding Remarks
References
- Merfort, R.; Maffulli, N.; Hofmann, U.K.; Hildebrand, F.; Simeone, F.; Eschweiler, J.; Migliorini, F. Head, acetabular liner composition, and rate of revision and wear in total hip arthroplasty: a Bayesian network meta-analysis. Sci. Rep. 2023, 13, 1–23. [Google Scholar] [CrossRef] [PubMed]
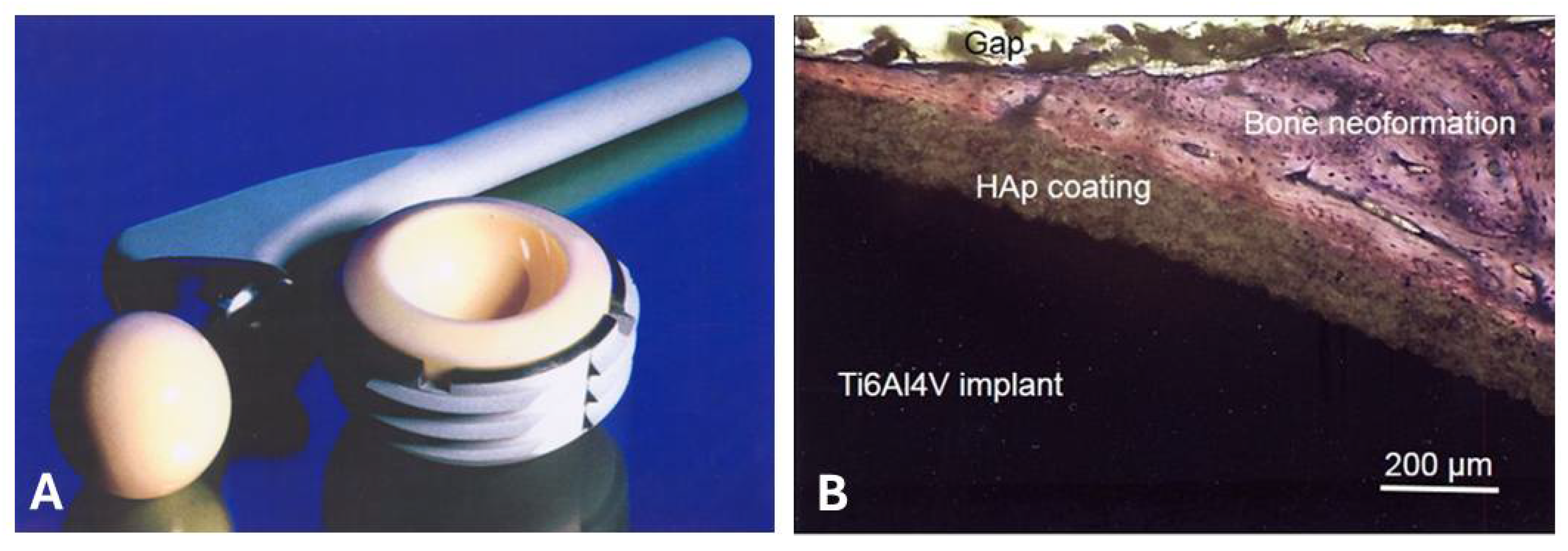
- Heimann, R.B.; Schürmann, N.; Müller, R.T. In vitro and in vivo performance of Ti6Al4V implants with plasma-sprayed osteoconductive hydroxylapatite–bioinert titania bond coat “duplex” systems: an experimental study in sheep. J. Mater. Sci. Mater. Med. 2004, 15, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Stambough, J.B.; Levine, B.R.; Springer, B.D. Highlights of the American Joint Replacement Registry Annual Report. Arthroplast. Today 2023, 21, 101137. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A. , Lützner, J., Melsheimer, O., Morlock, M., Steinbrück, A. (2023). EPDR Annual Report 2023. EPDR Deutsche Endoprothesenregister gGmbH, Berlin. https://www.epdr.de/fileadmin/.
- Global Hip Replacement Implants Market Report and Forecast 2023-2031. https://www.researchandmarkets.com/reports/5797997.
- Itiravivong, P.; Promasa, A.; Laiprasert, T.; Techapongworachai, T.; Kuptnitazsaikul, S.; Thanakit, V.; Heimann, R.B. Comparison of tissue reaction and osteointegration of metal implants between hydroxyapatite/Ti alloy coat: An animal experimental study. J. Medical Assoc. Thailand 2003, 86, S422–S430. [Google Scholar]
- Zhang, D.H.; Chen, Q.; Shi, C.; et al. Dealing with the foreign body response to implanted biomaterials: Strategies and applications of new materials. Adv. Funct. Mater. 2021, 31, 2007226. [Google Scholar] [CrossRef]
- Heimann, R.B., Lehmann, H.D. (2015). Bioceramic Coatings for Medical Implants. Trends and Techniques. Wiley-VCH, Weinheim. 467 pp. ISBN 978-3-527-334743-9.
- Brande, W.T. , Taylor, A.S. (1863): Chemistry. Blanchard and Lea, Philadelphia. 696 pp.
- Warington, R. Jr. Researches on the phosphates of calcium, and upon the solubility of tricalcic phosphate. J. Chem. Soc. 1866, 19, 296–318. [Google Scholar] [CrossRef]
- Bragg, W.H. Application of the ionisation spectrometer to the determination of the structure of minute crystals. Proc. Phys. Soc. (London) 1921, 33, 222–224. [Google Scholar] [CrossRef]
- Hendricks, S.B.; Hill, W.L.; Jacob, K.D.; Jefferson, M.E. Structural Characteristics of Apatite-Like Substances and Composition of Phosphate Rock and Bone as Determined from Microscopical and X-Ray Diffraction Examinations1. Ind. Eng. Chem. 1931, 23, 1413–1418. [Google Scholar] [CrossRef]
- Roseberry, H.H.; Hastings, A.B.; Morse, J.K. X-ray analysis of bone and teeth. J. Biol. Chem. 1931, 90, 395–407. [Google Scholar] [CrossRef]
- Trömel, G. Untersuchungen über die Bildung eines halogenfreien Apatits aus basischen Calciumphosphaten. Z. Phys. Chem. A 1932, 158, 422–432. [Google Scholar] [CrossRef]
- Bredig, M.A. Zur Apatitstruktur der anorganischen Knochen- und Zahnsubstanz. Hoppe-Seyler’s Z. Physiol. Chem. 1933, 216, 239–243. [Google Scholar] [CrossRef]
- Bredig, M.A.; Franck, H.H.; Füldner, H. Beiträge zur Kenntnis der Kalk-Phosphorsäure-Verbindungen II. Z. Elektrochem. 1933, 39, 959–969. [Google Scholar] [CrossRef]
- De Jong, W.F. La substance minerale dans les os. Recl. Trav. Chim. Pays-Bas Belg. 1926, 45, 445–448. [Google Scholar] [CrossRef]
- De Leeuw, N.H.; Bowe, J.R.; Rabone, J.A.L. A computational investigation of stoichiometric and calcium-deficient oxy- and hydroxyapatites. Faraday Discuss 2007, 134, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Alberius Henning, P.; Landa-Canovas, A.; Larsson, A.K.; Lidin, S. The structure of oxyapatite solved by HREM. Acta Cryst. 1999, B55, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Calderin, L.; Stott, M.J.; Rubio, A. Electronic and crystallographic structure of apatites. Phys. Rev. 2003, B67, 134106–134112. [Google Scholar] [CrossRef]
- Gross, K.A. , Pluduma, L. (2012). Putting oxyhydroxyapatite into perspective. A pathway to oxyapatite and its application. In: R. B. Heimann (ed.), Calcium Phosphate. Structure, Synthesis, Properties, and Applications. Biochemistry Research Trends Ser., Nova Science Publ., New York, pp. 95-120. ISBN 978-1-62257-299-1.
- Heimann, R.B. Characterisation of as-sprayed and incubated hydroxyapatite coatings with high resolution techniques. Mater.-wiss. Werkstofftechn. 2009, 40, 21–30. [Google Scholar]
- Hattori, T.; Iwadate, Y. Hydrothermal preparation of calcium hydroxylapatite powders. J. Am. Ceram. Soc. 1990, 73, 1803–1807. [Google Scholar] [CrossRef]
- Liu, C.; Huang, A.; Shen, W.; Cui, J. Kinetics of hydroxyapatite precipitation at pH 10 and 11. Biomater. 2001, 23, 301–306. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: update on chemical composition and structure. Osteopor. Int. 2001, 20, 1013–1021. [Google Scholar] [CrossRef]
- Pasteris, J.D. (2012). Structurally incorporated water in bone apatite: A cautionary tale. In: R. B. Heimann (ed.), Calcium Phosphate. Structure, Synthesis, Properties, and Applications. Biochemistry Research Trends Ser., Nova Science Publ., New York, pp. 63-94.
- Heimann, R.B. (2020). Materials for Medical Application. Walter de Gruyter GmbH, Berlin, Boston, 615 pp, ISBN 978-3-11-061919-5.
- Glimcher, M.J. A basic architectural principle in the organisation of mineralized tissue. Clin. Orthop. 1968, 61, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Rodrigues da Silva Sasso, G.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Allo, B.A.; Costa, D.O.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactive and biodegradable nanocomposites and hybrid biomaterials for bone regeneration. J. Funct. Mater. 2012, 5, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.K.; Gautieri, A.; Chang, S.W.; Buehler, M.J. Molecular mechanics of mineralized collagen fibrils in bone. Nature Comm. 2013, 4, 1724. [Google Scholar] [CrossRef] [PubMed]
- Jarcho, M. , Bolen, C.M., Thomas, M.B. et al. (1976). Hydroxylapatite synthesis and characterization in dense polycrystalline form. J. Mater. Sci,m 11, 2027-2035; also Jarcho, M. (1981). Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Rel. Res.,157, 259-278.
- Ducheyne, P.; Hench, L.L.; Kagan, A.; Martens, M.; Mulier, J.C.; Burssens, A. The effect of hydroxyapatite impregnation on bonding of porous coated implants. J. Biomed. Mater. Res. 1980, 14, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osseoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. 2), S96–S101. [Google Scholar] [PubMed]
- León, B., Jansen, J.A. (2009). Thin Calcium Phosphate Coatings for Medical Implants. Springer, New York. 328 pp. ISBN 978-0-387-77718-4.
- Heimann, R.B. (2008). Plasma Spray Coating. Principles and Applications. 2nd. edn., Wiley-VCH, Weinheim. ISBN 978-3-527-32050-9.
- Heimann, R.B. (2018). Hydroxylapatite coatings: Applied mineralogy research in the bioceramics field. In: S. Heuss-Aßbichler, G. Amthauer, M. John (eds), Highlights in Applied Mineralogy, Walter de Gruyter GmbH Berlin, Boston, pp. 301-316. ISBN 978-3-11-049122-7.
- Sun, L. Thermal spray coatings on orthopedic devices: When and how the FDA reviews your coatings. J. Thermal Spray Technol. 2018, 27, 1280–1290. [Google Scholar] [CrossRef]
- Graßmann, O.; Heimann, R.B. Compositional and microstructural changes of engineered plasma-sprayed hydroxyapatite coatings on Ti6Al4V substrates during incubation in protein-free simulated body fluid. J. Biomed. Mater. Res. 2000, 53, 685–693. [Google Scholar] [CrossRef]
- Heimann, R.B.; Graßmann, O.; Zumbrink, T.; Jennissen, H.P. Biomimetic processes during in vitro leaching of plasma-sprayed hydroxyapatite coatings for endoprosthetic application. Mater.-wiss. Werkstofftechn. 2001, 32, 913–921. [Google Scholar] [CrossRef]
- Dyshlovenko, S.; Pateyron, B.; Pawlowski, L.; Murano, D. Numerical simulation of hydroxyapatite powder behaviour in plasma jet. Surf. Coat. Technol. 2004, 179, 110–117. [Google Scholar] [CrossRef]
- Riboud, P.V. Composition et stabilité des phases a structure d’apatite dans le systeme CaO-P2O5-oxide de Fer-H2O a haute temperature. Ann. Chim. 1973, 8, 381–390. [Google Scholar]
- Frayssinet, P.; Tourenne, F.; Roquet, N.; Conte, P.; Delga, C.; Bonel, G. Comparative biological properties of HA plasma-sprayed coatings having different crystallinities. J. Mater. Sci. Mater. Med. 1994, 5, 11–17. [Google Scholar] [CrossRef]
- De Santis, D.; Guerriero, C.; Nocini, P.F.; Ungersbock, A.; Richards, G.; Gotte, P.; Armato, U. Adult human bone cells from jaw bones cultured on plasma-sprayed or polished surfaces of titanium or hydroxyapatite discs. J. Mater. Sci. Mater. Med. 1996, 7, 21–28. [Google Scholar] [CrossRef]
- Ntsoane, T.P.; Topić, M.; Härting, M.; Heimann, R.B.; Theron, C. Spatial and depth-resolved studies of air plasma-sprayed hydroxyapatite coatings by means of diffraction techniques: Part 1. Surf. Coat. Technol. 2016, 294, 153–163. [Google Scholar] [CrossRef]
- Hesse, C.; Hengst, M.; Kleeberg, R.; Götze, J. Influence of experimental parameters on spatial phase distribution in as-sprayed and incubated hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 2008, 19, 3225–3241. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; A Condrate, R.; Lee, D. Infrared spectral investigation of plasma spray coated hydroxyapatite. Mater. Lett. 1998, 36, 38–43. [Google Scholar] [CrossRef]
- Heimann, R.B. (2007). Novel approaches towards design and biofunctionality of plasma-sprayed osteoconductive calcium phosphate coatings for biomedical implants: The concept of bond coats. In: P. J. Pannone (ed.), Trends in Biomaterials Research, Nova Science Publishers Inc., USA, pp. 1-80. ISBN 978-1-60021-361-8.
- Heimann, R.B.; Tran, H.V.; Hartmann, P. Laser-Raman and nuclear magnetic resonance (NMR) studies on plasma-sprayed hydroxyapatite coatings: Influence of bioinert bond coats on phase composition and resorption kinetics in simulated body fluid. Mater.-wiss. Werkstofftechn. 2003, 34, 1163–1169. [Google Scholar] [CrossRef]
- Kim, H.M. , Miyazaki, M., Kokubo, T., Nakamura, T. (2001). Revised simulated body fluid. Key Eng. Mater., 192/195, 47-50.
- Park, E.; Sr. , R.A.C.; Lee, D.; Kociba, K.; Gallagher, P.K. Characterization of hydroxyapatite: Before and after plasma spraying. J. Mater. Sci. Mater. Med. 2002, 13, 211–218. [Google Scholar] [CrossRef]
- Demnati, I.; Parco, M.; Grossin, D.; Fagoaga, I.; Drouet, C.; Barykin, G.; Combes, C.; Braceras, I.; Goncalves, S.; Rey, C. Hydroxyapatite coating on titanium by a low energy plasma spraying mini-gun. Surf. Coatings Technol. 2012, 206, 2346–2353. [Google Scholar] [CrossRef]
- Demnati, I. , Grossin, D., Combes, C., Rey, C. (2014). Plasma-sprayed apatite coatings: Review of physical-chemical aspects and their biological consequences. J. Med. Biol. Eng., 34(1), 1-7.
- Heimann, R.B., Vu, T.A. (1996). Improvement of adhesion of bioceramic coatings on jaw and bone implants made from titanium alloy. Second Interim Report, SMWK-Projekt No. 7541.82-0390/414. February 15, 1996.
- Cusco, R. , Guitian, F., de Aza, S., Artus, L. (1998). Differentiation between hydroxyapatite and ß-tricalcium phosphate by means of µ-Raman spectroscopy. J. Eur. Ceram. Soc., 18, 1301-1305.
- Shamray, V.; Sirotinkin, V.; Kalita, V.; Komlev, V.; Barinov, S.; Fedotov, A.; Gordeev, A. Study of the crystal structure of hydroxyapatite in plasma coating. Surf. Coatings Technol. 2019, 372, 201–208. [Google Scholar] [CrossRef]
- Hartmann, P. , Jäger, C., Barth, S., Vogel, J., Meyer, K. (2001). Solid state NMR, X-ray diffraction, and infrared characterization of local structure in heat-treated oxyhydroxylapatite microcrystals: An analogy of the thermal deposition of hydroxyapatite during plasma-spray procedure. J. Solid State Chem., 160, 460-468.
- Jäger, C.; Welzel, T.; Meyer-Zaika, W.; Epple, M. A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn. Reson. Chem. 2006, 44, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.V. (2004). Investigation into the thermal dehydroxylation and decomposition during atmospheric plasma spraying: NMR and Raman spectroscopic study of as-sprayed coatings and coatings incubated in simulated body fluid. Ph.D. Thesis, Dept. of Mineralogy, Technische Universität Bergakademie Freiberg, Germany.
- Hartmann, P. , Barth, S., Vogel, J., Jäger, C. (2000). Investigation of structural changes in plasma-sprayed hydroxy/apatite coatings. In: Rammlmair, D. et al. (eds)., Applied Mineralogy in Research, Economy, Technology, Ecology and Culture. A.A. Balkema, Rotterdam, Brookfield. vol.1, pp. 147-150. ISBN 90-5809-164-3.
- LeGeros, R. Formation and transformation of calcium phosphates: relevance to vascular calcification. Clin. Res. Cardiol. 2001, 90, III116–III124. [Google Scholar] [CrossRef]
- Von Euw, S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneau, F.; Nassif, N.; Azaïs, T. Bone mineral: new insights into its chemical composition. Sci. Rep. 2019, 9, 8456. [Google Scholar] [CrossRef] [PubMed]
- Posner, A.S.; Perloff, A.; Diorio, A.F. Refinement of the hydroxyapatite structure. Acta Crystallogr. 1958, 11, 308–309. [Google Scholar] [CrossRef]
- Heimann, R.B. (2018). Plasma-sprayed hydroxylapatite coatings as biocompatible intermediaries between inorganic implant surfaces and living tissue. J. Thermal Spray Technol., 27(8), 1212-1237.
- Laskus, A.; Kolmas, J. Ionic Substitutions in Non-Apatitic Calcium Phosphates. Int. J. Mol. Sci. 2017, 18, 2542. [Google Scholar] [CrossRef]
- Graziani, G., Boi, M., Bianchi, M. (2018). A review on ionic substitution in hydroxyapatite thin films. Coatings, 8(8), 269.
- Trombe, J.C. , Montel, C. (1971). Sur la préparation del’oxyapatite phospho-calcique, C. R. Acad. Sci. Paris, 273, 462-465.
- Takahashi, T.; Tanase, S.; Yamamoto, O. Electrical conductivity of some hydroxyapatites. Electrochimica Acta 1978, 23, 369–373. [Google Scholar] [CrossRef]
- Heimann, R.; Wirth, R. Formation and transformation of amorphous calcium phosphates on titanium alloy surfaces during atmospheric plasma spraying and their subsequent in vitro performance. Biomaterials 2006, 27, 823–831. [Google Scholar] [CrossRef]
- Weng, J.; Liu, Q.; Wolke, J.G.C.; Zhang, D.; DE Groot, K. The role of amorphous phase in nucleating bone-like apatite on plasma-sprayed hydroxyapatite coatings in simulated body fluid. J. Mater. Sci. Lett. 1997, 16, 335–337. [Google Scholar] [CrossRef]
- Cook, S.D., Thomas, K.A., Kay, J.F., Jarcho, M. (1988) Hydroxyapatite coated titanium for orthopaedic implant applications. Clin. Orthop., 232, 225-243.
- Yang, C.Y. , Wang, B.C., Chang, E., Wu, J.D. (1995). Bond degradation at the plasma-sprayed HA coating/Ti-6Al-4V alloy interface: An in vitro study. J. Mater. Sci. Mater. Med., 6, 258-265.
- Lemons, J.E. (1994). Biodegradation and wear of total joint replacements. In: H.U. Cameron (ed.). Bone Implant Interface, Mosby: St. Louis, Baltimore, Boston, pp. 307-317.
- Wintermantel, E., Ha, S.W. (1996). Biokompatible Werkstoffe und Bauweisen. Implantate für Medizin und Umwelt. Springer-Verlag: Berlin, Heidelberg, Tokio, p. 225.
- Li, H.; Ma, Y.; Zhao, Z.; Tian, Y. Fatigue behavior of plasma sprayed structural-grade hydroxyapatite coating under simulated body fluids. Surf. Coatings Technol. 2019, 368, 110–118. [Google Scholar] [CrossRef]
- Reisel, G.; Heimann, R.B. Correlation between surface roughness of plasma-sprayed chromium oxide coatings and powder grain size distribution: a fractal approach. Surf. Coatings Technol. 2004, 185, 215–221. [Google Scholar] [CrossRef]
- Heimann, R.B. On the Self-Affine Fractal Geometry of Plasma-Sprayed Surfaces. J. Therm. Spray Technol. 2011, 20, 898–908. [Google Scholar] [CrossRef]
- Gentile, F.; Tirinato, L.; Battista, E.; Causa, F.; Liberale, C.; di Fabrizio, E.M.; Decuzzi, P. Cells preferentially grow on rough substrates. Biomaterials 2010, 31, 7205–7212. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Heimann, R.B., Kleiman, J.I. (1988). Shock-induced growth of superhard materials. In: H.C. Freyhardt (ed.). Crystals, Growth, Properties, and Applications, vol. 11. Springer, Berlin, Heidelberg, New York, London, Paris, Tokyo. pp. 1-73. ISBN 3-540-18602-6.
- Gill, S.C.; Clyne, T.W. Stress distributions and material response in thermal spraying of metallic and ceramic deposits. Met. Trans. B 1990, 21, 377–385. [Google Scholar] [CrossRef]
- Kuroda, S.; Clyne, T. The quenching stress in thermally sprayed coatings. Thin Solid Films 1991, 200, 49–66. [Google Scholar] [CrossRef]
- Kuroda, S.; Dendo, T.; Kitahara, S. Quenching stress in plasma sprayed coatings and its correlation with the deposit microstructure. J. Therm. Spray Technol. 1995, 4, 75–84. [Google Scholar] [CrossRef]
- Matejicek, J.; Sampath, S. Intrinsic residual stresses in single splats produced by thermal spray processes. Acta Mater. 2001, 49, 1993–1999. [Google Scholar] [CrossRef]
- Matejicek, J.; Sampath, S. In situ measurement of residual stresses and elastic moduli in thermal sprayed coatings: Part 1: apparatus and analysis. Acta Mater. 2003, 51, 863–872. [Google Scholar] [CrossRef]
- Stammeier, J.A.; Purgstaller, B.; Hippler, D.; Mavromatis, V.; Dietzel, M. In-situ Raman spectroscopy of amorphous calcium phosphate to crystalline hydroxyapatite transformation. MethodsX 2018, 5, 1241–1250. [Google Scholar] [CrossRef]
- Topić, M.; Ntsoane, T.; Heimann, R. Microstructural characterisation and stress determination in as-plasma sprayed and incubated bioconductive hydroxyapatite coatings. Surf. Coatings Technol. 2006, 201, 3633–3641. [Google Scholar] [CrossRef]
- Heimann, R.B.; Ntsoane, T.P.; Pineda-Vargas, C.A.; Przybylowicz, W.J.; Topić, M. Biomimetic formation of hydroxyapatite investigated by analytical techniques with high resolution. J. Mater. Sci. Mater. Med. 2008, 19, 3295–3302. [Google Scholar] [CrossRef]
- Heimann, R.B. , Graßmann, O., Hempel, M., Bucher, R., Härting, M. (2000). Phase content, resorption resistance and residual stresses of bioceramic coatings. In: Rammlmair, D. et al. (eds)., Applied Mineralogy in Research, Economy, Technology, Ecology and Culture. A.A. Balkema, Rotterdam, Brookfield. vol.1, pp. 155-158. ISBN 90-5809-164-3.
- Geesink, R.G. (1990). Hydroxyapatite-coated total hip prostheses two-year clinical and roentgenographic results of 100 cases. Clin. Orthop. Relat, Res., 261, 39-58.
- ISO 12891-2 (2020). Retrieval and Analysis of Surgical Implants. Part 2: Analysis of Retrieved Surgical Implants. Int. Org. Stand., Geneva, Switzerland.
- ISO 13779-2 (2008). Implants for Surgery-Hydroxyapatite. Part 2: Coatings of Hydroxyapatite. Int. Org. Stand., Geneva, Switzerland.
- Heimann, R.B. (2016). Plasma-sprayed hydroxyapatite-based coatings: Chemical, mechanical, microstructural, and biomedical properties. J. Thermal Spray Technol., 25(5), 827-850.
- Lacombe, R. (2006). Adhesion Measurement Methods: Theory and Practice, CRC Taylor & Francis, Boca Raton, USA.
- Filiaggi, M.J. , Coombs, N.A., and Pilliar, R.M. (1991). Characterization of the interface in the plasma-sprayed HApcCoating/Ti-6Al-4V implant system, J. Biomed. Mater. Res., 25, 1211-1229.
- Webster, T.J. , Ergun, C., Doremus, R.H., and W.A. Lanford, W.A. (2003). Increased osteoblast adhesion on titanium-coated hydroxylapatite that forms CaTiO3, J. Biomed. Mater. Res. A, 67(3), 975-980.
- Wei, D.; Zhou, Y.; Jia, D.; Wang, Y. Structure of calcium titanate/titania bioceramic composite coatings on titanium alloy and apatite deposition on their surfaces in a simulated body fluid. Surf. Coatings Technol. 2007, 201, 8715–8722. [Google Scholar] [CrossRef]
- Joshi, M.U.; Kulkarni, S.P.; Choppadandi, M.; Keerthana, M.; Kapusetti, G. Current state of art smart coatings for orthopedic implants: A comprehensive review. Smart Mater. Med. 2023, 4, 661–679. [Google Scholar] [CrossRef]
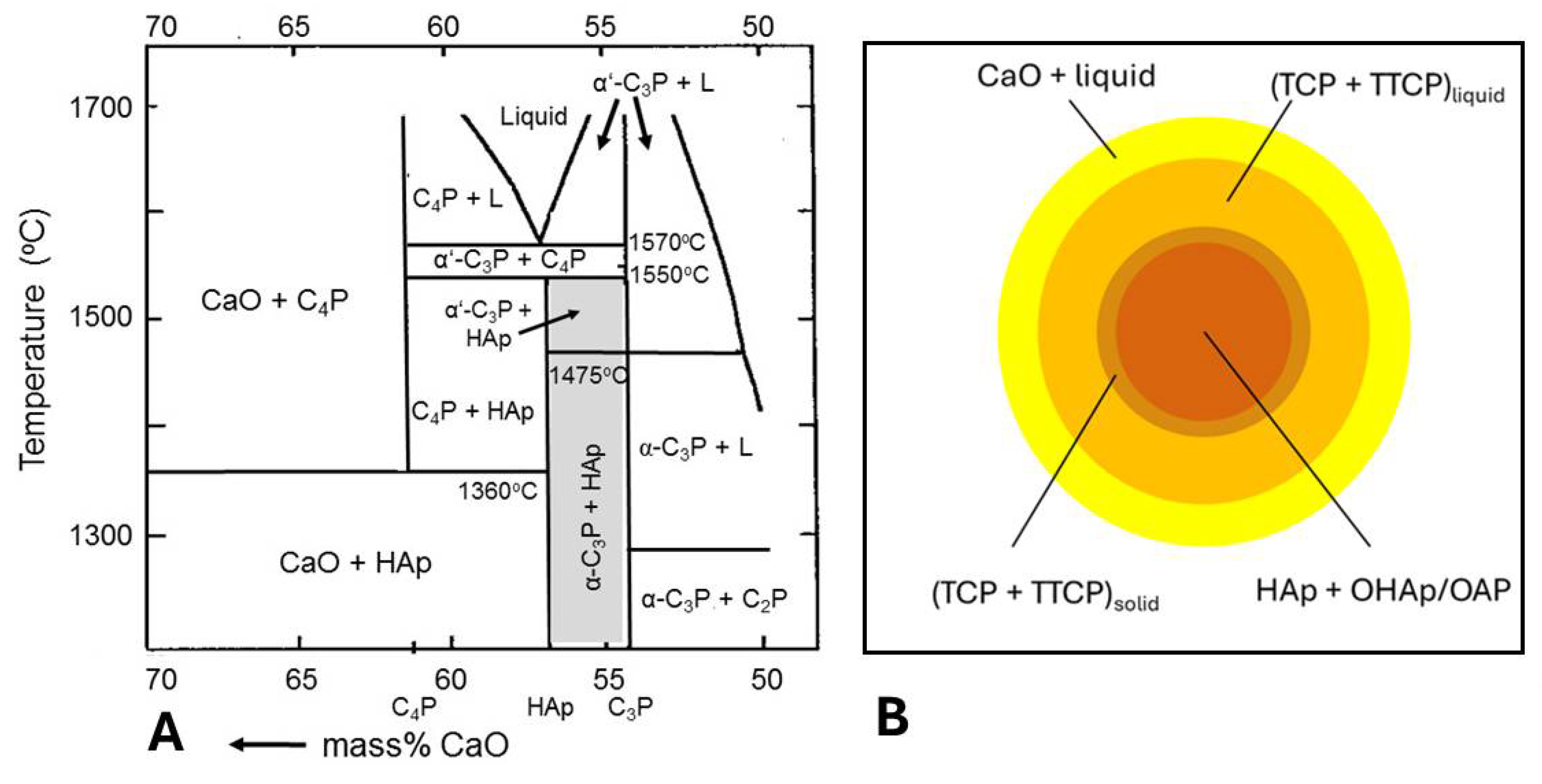


| Step 1: | Ca10(PO4)6(OH)2 | → | Ca10(PO4)6(OH)2-xOx□x + xH2O | Oxyhydroxylapatite (OHAp) |
| Step 2: | Ca10(PO4)6(OH)2-xOx□x | → | Ca10(PO4)6Ox□x + (1-x)H2O | Oxyapatite (OAp) |
| Step 3: | Ca10(PO4)6Ox□x | → | 2 Ca3(PO4)2 + Ca4O(PO4)2 | TCP + TTCP (C3P + C4P) |
| Step 4a: | Ca3(PO4)2 | → | 3 CaO + P2O5 | Stepwise decomposition of TCP and TTCP |
| Step 4b: | Ca4O(PO4)2 | → | 4 CaO + P2O5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





