Submitted:
21 May 2024
Posted:
22 May 2024
You are already at the latest version
Abstract
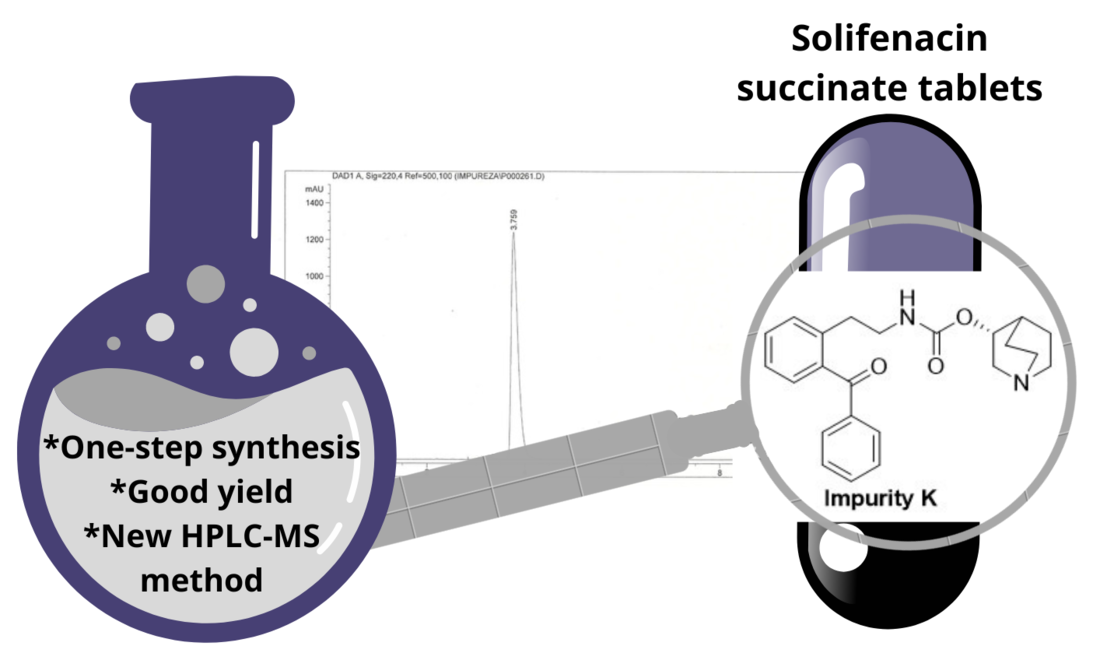
Keywords:
1. Introduction
2. Materials and Methods
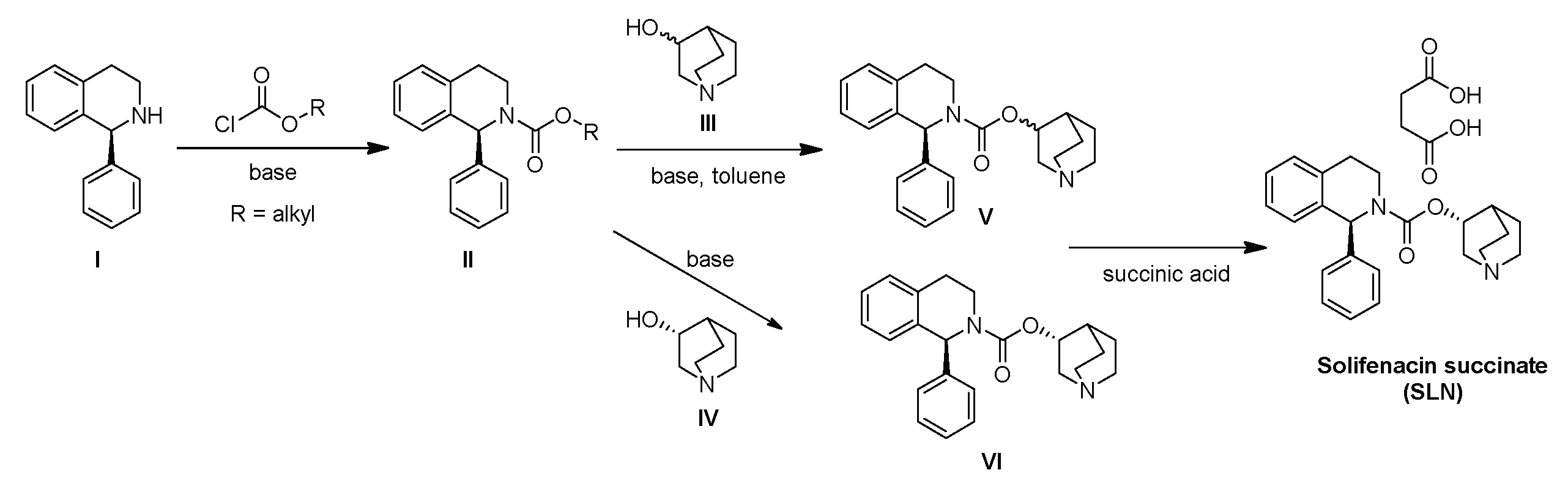
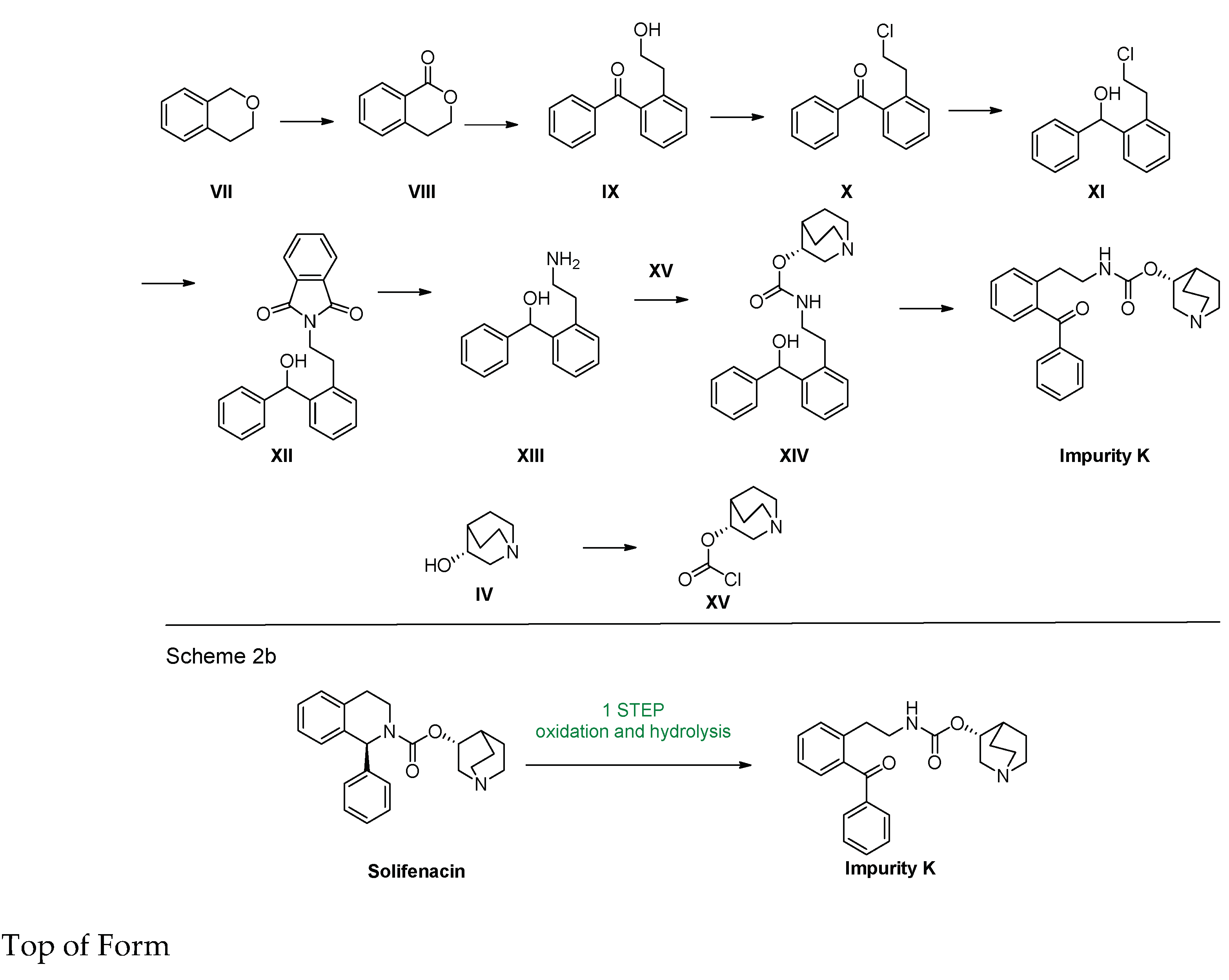
2.1. Chemical Synthesis
2.1.1. General Methods
2.1.2. Synthesis and Characterization Data of Impurity K
2.2. HPLC Equipment and Methods
2.2.1. HPLC Equipment
2.2.2. HPLC Method for the Identification of Impurity K
3. Results and Discussion
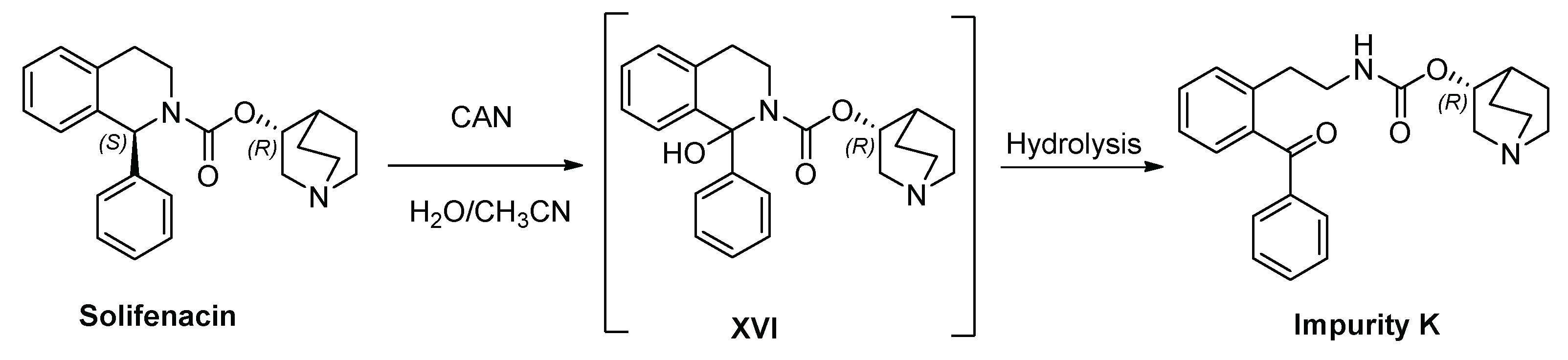
3.1. Synthesis of Impurity K
3.2. HPLC-MS Method for the Analysis of Impurity K
3.3. Characterization of Impurity K
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Astellas Pharma US, Inc. and GlaxoSmithKline. Vesicare® (solifenacin succinate tablets): US prescribing information. Available online: http://www.astellas.us/docs/vesicare.pdf (accessed on 8 April 2024).
- Drug.com. Vesicare® FDA Approval History. Available online: https://www.drugs.com/history/vesicare.html (accessed on 8 April 2024).
- Kamat, A.G.; Koilpillai, J.P.; Ganala, N.T.; Upputuri, V.L.; Boddu, V.B.; Meenakshisunderam, S. Process for the preparation of solifenacin succinate. WO2012/001481A1, 28 June 2010.
- Yusuke, I; Kouji, T.; Masatoshi, I.; Shuichi, N.; Koji, N.; Naoki, Y.; Makoto, T.; Yasuhiro, Y. Composition containing solifenacin succinate. WO2005/075474A1, 18 August 2005.
- Solifenacin succinate. European Pharmacopoeia 11.5. Available online: https://pheur.edqm.eu/app/11-5/content/11-5/2779E.htm?highlight=on&terms=solifenacin%20succinate&terms=solifenacin&terms=succinate&terms=5. (accessed on 8 May 2024).
- Jadhav, R. A.; Sanil, Y. M.; Shankarwar, S. G.; Shankarwar, A. G.; Pawar, R. P.; Bembalkar, S. R. Development and Validation of Rapid Stability-Indicating RP-HPLC Method for Assay and Related Substances of Solifenacin Succinate. Chromatographia 2020, 83, 1107–1119. [CrossRef]
- Chavakula, R.; Rao, M. N.; Raju, M.V.; Nageswara Rao, R.V. Synthesis and spectral characterization of potential impurities of solifenacin succinate. Org. Chem.: Indian J. 2014, 10, 152-156.
- Masatoshi, I.; Yusuke, I. Solifenacin-containing composition. WO2005/087232, 22 September 2005.
- Reddy, B.V.R.; Reddy, B.S.; Raman, N.V.V.S.S.; Reddy, K.S.; Rambabu, C. Development and Validation of a Specific Stability Indicating High Performance Liquid Chromatographic Methods for Related Compounds and Assay of Solifenacin Succinate. J. Chem., 2013, 412353. [CrossRef]
- Singh, D.; Kurmi, M.; Handa, T.; Singh, S. LC–MS/TOF, LC–MSn and H/D Exchange Studies on Solifenacin Succinate Targeted to Characterize its Forced Degradation Products. Chromatographia, 2016, 79, 159-168.
- Desai, D.; Patel, G.; Shukla, N.; Rajput, S. Development and Validation of Stability-Indicating HPLC Method for Solifenacin Succinate: Isolation and Identification of Major Base Degradation Product. Acta Chromatogr., 2012, 3, 399-418. [CrossRef]
- Desheng, W.; Jihan, L.; Chi, Z.; Chun, L.; Zhongyi, W. Method for preparation of Solifenacin Impurity. CN 107011338A, 04 August 2017.
- Ganthi, H.K.; Reddy, R.; Park, Y.J.; Bapatu, H.R.; Park, S.J.; Cho, W.H. Stability Indicating HPLC Method for Quantification of Solifenacin Succinate & Tamsulosin Hydrochloride along with Its Impurities in Tablet Dosage Form. Am. J. Anal. Chem., 2016, 7, 840-862. [CrossRef]
- Yusuke, I.; Kouji, T.; Masatoshi, I.; Shuichi, N.; Koji, N.; Naoki, Y.; Makoto, T.; Yasuhiro, Y. Composition containing solifenacin succinate. WO2005/075474, 07 February 2005.
- Kim, D.H.; Ho, M.J.; Jeong, C.K.; Kang, M.J. Novel Bioequivalent Tablet of Solifenacin Succinate Prepared Using Direct Compression Technique for Improved Chemical Stability. Pharmaceutics 2023, 15, 1723. [CrossRef]
- ICH Guideline, Impurities in new drug products Q3B (R2) Current Step 4 version dated 2 June 2006. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://database.ich.org/sites/default/files/Q3B%28R2%29%20Guideline.pdf. (accessed on 25 April 2024).
- ICH Guideline, Impurities in new drug products Q3A (R2) Current Step 4 version dated 25 October 2006. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://database.ich.org/sites/default/files/Q3A%28R2%29%20Guideline.pdf. (accessed on 25 April 2024).
- Isobe, K.; Mohri, K.; Takeda, N.; Hosoi, S.; Tsuda, Y. Stereoselective methoxylation at the 11β-position of the erythrinan skeleton: total synthesis of (±)-erythristemine J. Chem. Soc., Perkin Trans 1 1989, 1357-1358.
- Isobe, K.; Mohri, K.; Suzuki, k.; Haruna, M.; Ito, K.; Hosoi, S.; Tsuda, Y. Synthesis of 11 beta hydroxy erythrina alkaloid erythrartine and its o acetate erythrascine. Heterocycles 1991, 32, 1195-1198.
- Yamanouchi Pharmaceutical Co., Ltd. Novel quinuclidine derivatives and medicinal composition thereof. WO1996/020194, 04 July 1996.
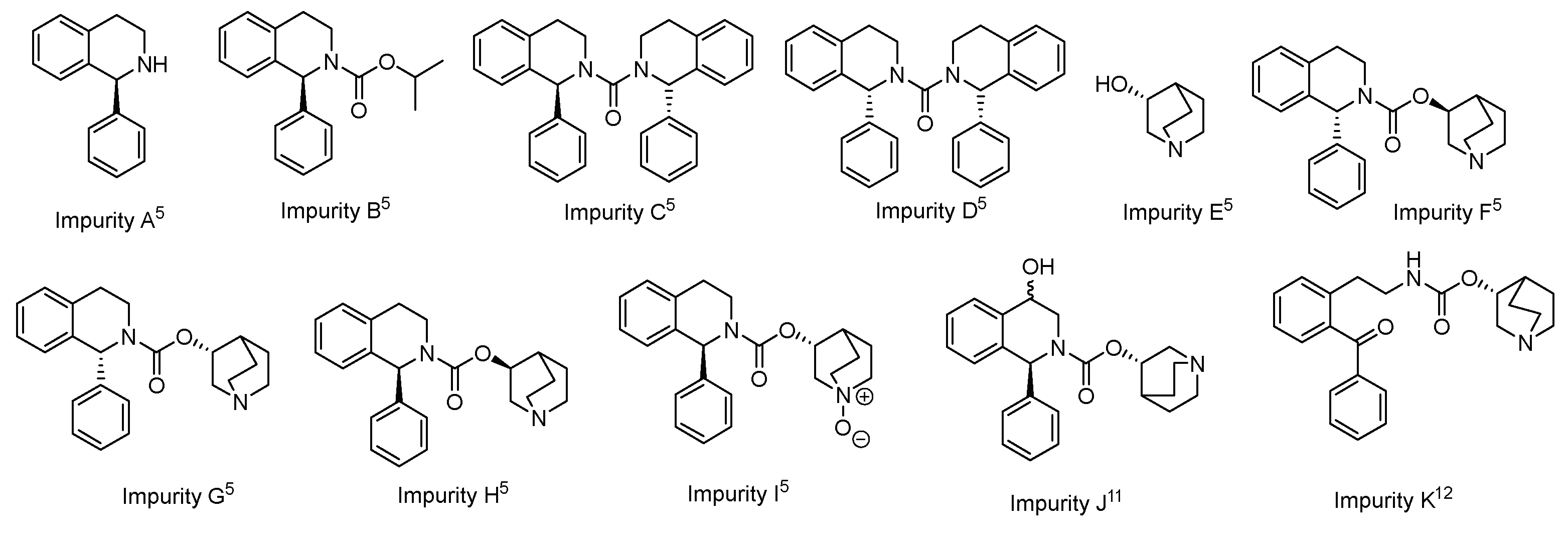
| Compound | Retention time (min) |
|---|---|
| SFC | 5.2 |
| Impurity I | 3.6 |
| Impurity K | 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





