Submitted:
17 May 2024
Posted:
22 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Laboratory Colony
2.2. Larval Extraction
2.3. Larval Rearing Media Composition
2.4. Larval Development, Pupation and Adult Emergence
2.5. Statistical Analysis
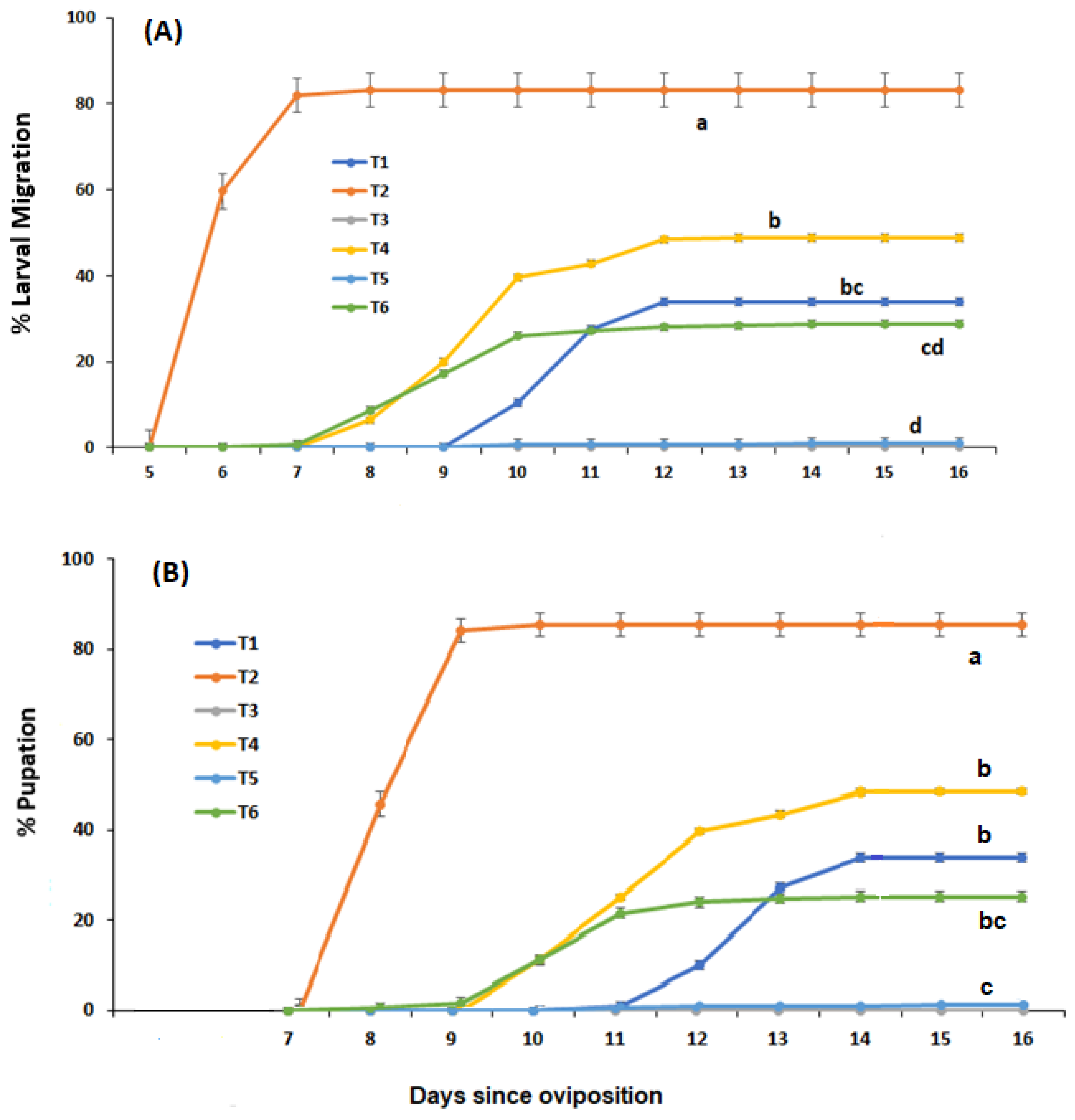
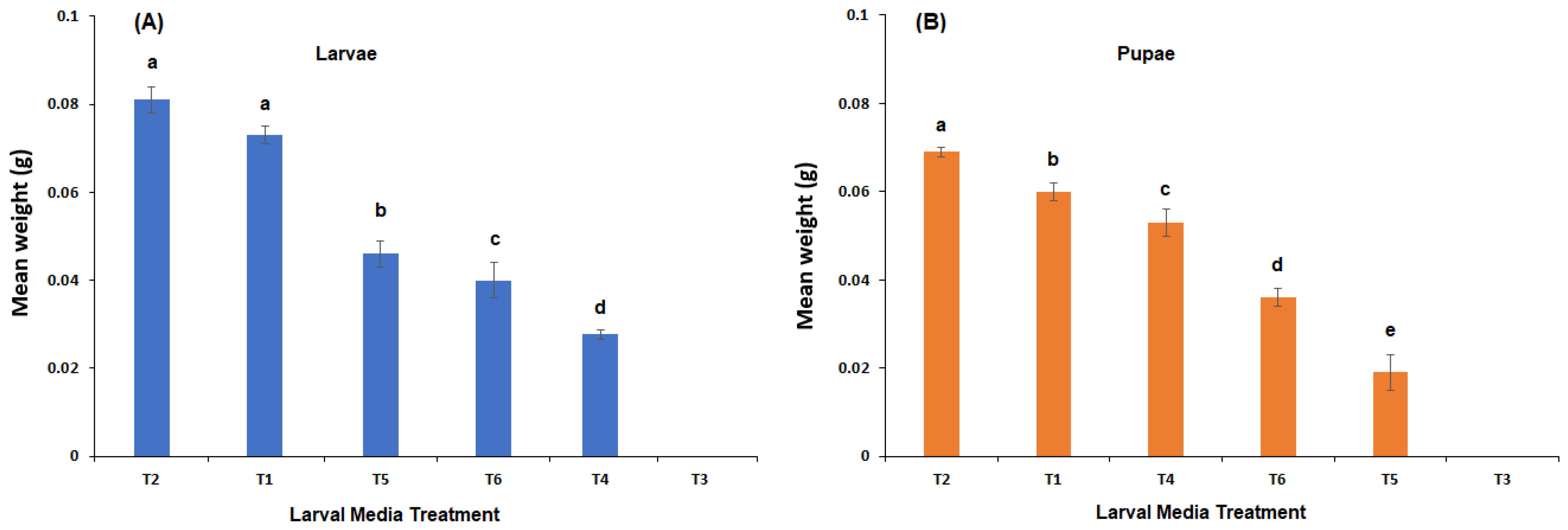
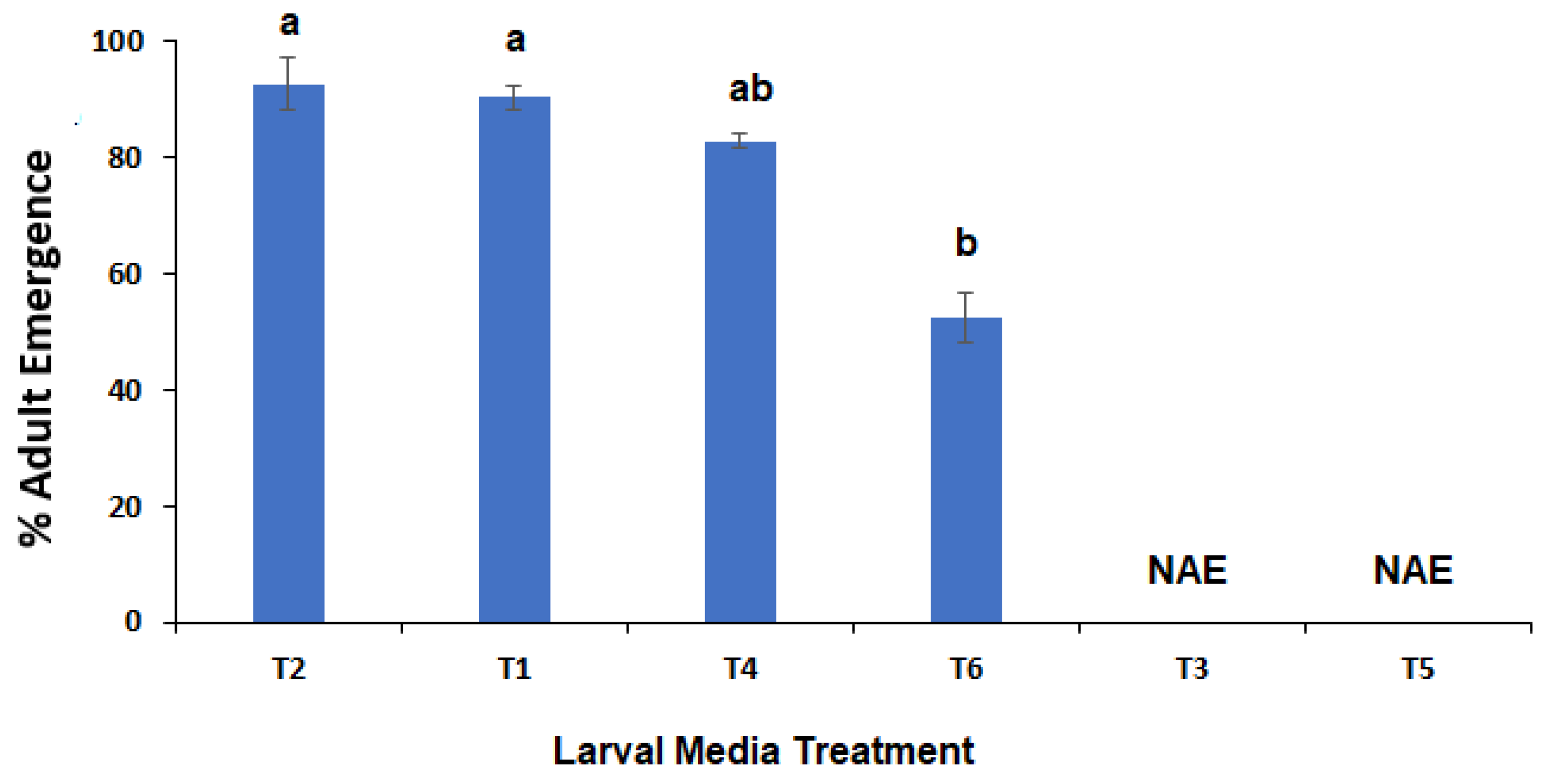
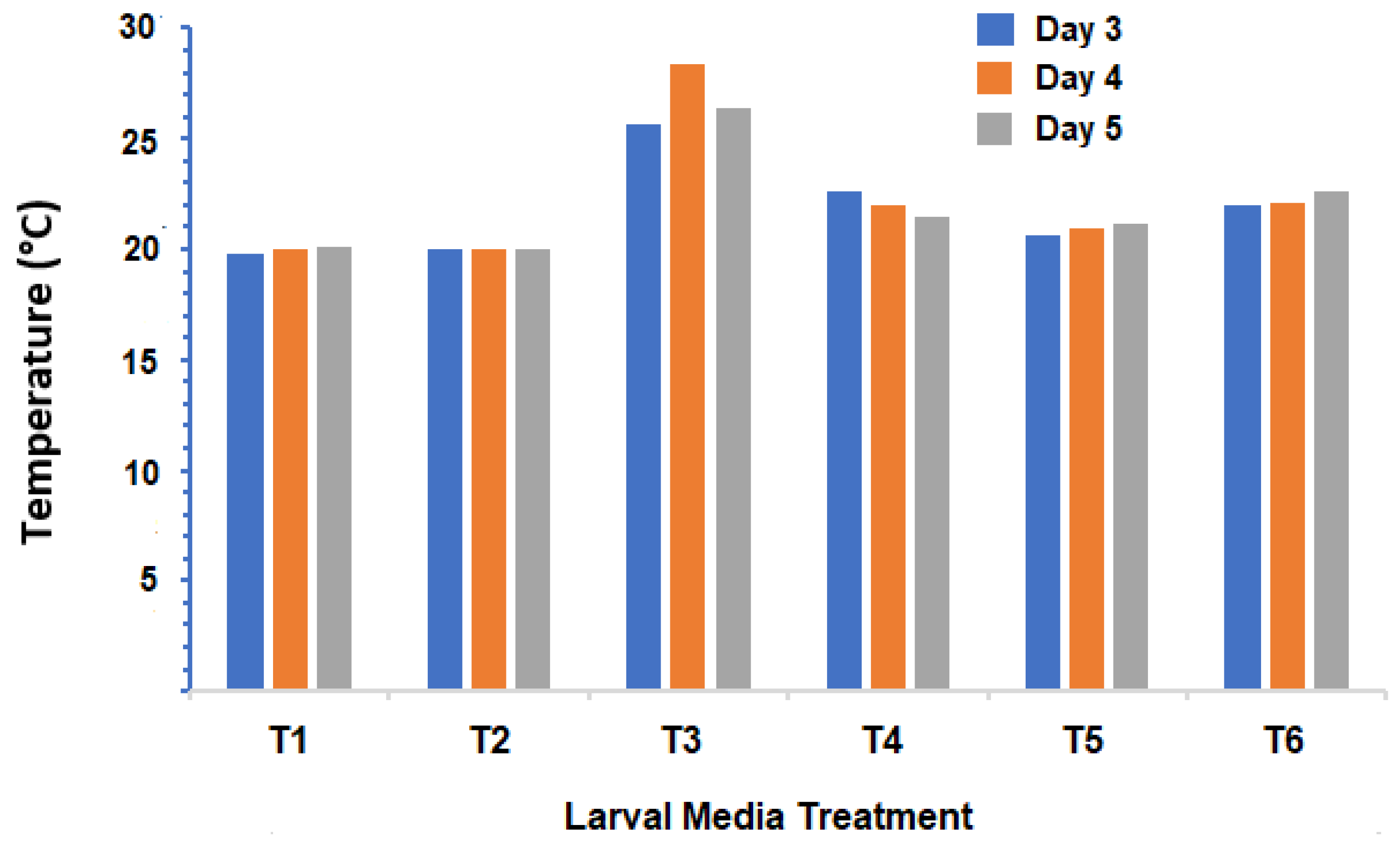
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rader, R.; Edwards, W.; Westcott, D.A.; Cunningham, S.A.; Howlett, B.G. Diurnal effectiveness of pollination by bees and flies in agricultural Brassica rapa: Implications for ecosystem resilience. Basic Appl. Ecol. 2013, 14, 20–27. [Google Scholar] [CrossRef]
- Howlett, B.G.; Gee, M. The potential management of the drone fly (Eristalis tenax) as a crop pollinator in New Zealand. N. Z. Plant Protect. 2019, 72, 221–229. [Google Scholar] [CrossRef]
- Cook, D.F.; Voss, S.C.; Finch, J.T.D.; Rader, R.; Cook, J.M.; Spurr, C.J. The role of flies as pollinators of horticultural crops: An Australian case study with worldwide relevance. Insects 2020, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.F.; Tufail, S.M.; Voss, S.C.; Deyl, R.A.; Howse, E.T.; Foley, J.; Norrish, B.; Delroy, N.; Shivananjappa, S.L. Blow flies (Diptera: Calliphoridae) ability to pollinate Hass avocado (Persea americana) trees within paired tree enclosures. J. Appl. Entomol. 2023, 147, 577–591. [Google Scholar] [CrossRef]
- Cook, D.F.; Tufail, S.M.; Voss, S.C.; Howse, E.T.; Foley, J.; Norrish, B.; Delroy, N. Blow flies (Diptera: Calliphoridae) and hover flies (Diptera: Syrphidae) ability to pollinate Hass avocado (Persea americana) trees within large, multi-tree enclosures. J. Appl. Entomol. 2024; In preparation. [Google Scholar]
- Free, J.B. Insect Pollination of Crops; Academic Press: London, UK, 1993. [Google Scholar]
- Larson, B.M.H.; Kevan, P.G.; Inouye, D.W. Flies and flowers: Taxonomic diversity of anthophiles and pollinators. Can. Entomol. 2001, 133, 439–465. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Rader, R.; Cunningham, S.A.; Howlett, B.G.; Inouye, D.W. Non-bee insects as visitors and pollinators of crops: Biology, ecology, and management. Annu. Rev. Entomol. 2020, 65, 391–407. [Google Scholar] [CrossRef]
- Pascacio-Villafán, C.; Cohen, A.C. How rearing systems for various species of flies benefit humanity. Insects 2023, 14, 553. [Google Scholar] [CrossRef]
- Hardy, G.H. Notes on the genus Calliphora (Diptera). Classification, synonymy, distribution and phylogeny. Proc. Linn. Soc. N.S.W. 1937, 62, 17–26. [Google Scholar]
- Pérez, C.; Segura, N.A.; Patarroyo, M.A.; Bello, F.J. Evaluating the biological cycle and reproductive and population parameters of Calliphora vicina (Diptera: Calliphoridae) reared on three different diets. J. Med. Entomol. 2016, 53, 1268–1275. [Google Scholar] [CrossRef]
- Day, D.M.; Wallman, J.F. Influence of substrate tissue type on larval growth in Calliphora augur and Lucilia cuprina (Diptera: Calliphoridae). J. Forensic Sci. 2006, 51, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Rabêlo, K.C.N.; Thyssen, P.J.; Salgado, R.L.; Araújo, M.S.C.; Vasconcelos, S.D. Bionomics of two forensically important blowfly species Chrysomya megacephala and Chrysomya putoria (Diptera: Calliphoridae) reared on four types of diet. Forensic Sci. Int. 2011, 210, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Longnecker, M.; Tomberlin, J.K. Effects of temperature and tissue type on Chrysomya rufifacies (Diptera: Calliphoridae) (Macquart) development. Forensic Sci. Int. 2014, 245, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Li, G.; Li, H.; Wang, Q.; Wan, L. The effect of dietary fat levels on the size and development of Chrysomya megacephala (Diptera: Calliphoridae). J. Insect Sci. 2014, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Niederegger, S.; Wartenberg, N.; Spiess, R.; Mall, G. Influence of food substrates on the development of the blowflies Calliphora vicina and Calliphora vomitoria (Diptera, Calliphoridae). Parasitol. Res. 2013, 112, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Leal, T.T.; Prado, A.P.D.; Antunes, A.J. Rearing the larvae of the blowfly Chrysomya chloropyga (Wiedemann) (Diptera, Calliphoridae) on oligidic diets. Rev. Bras. Zool. 1982, 1, 41–44. [Google Scholar] [CrossRef]
- Taylor, D.B. Response of screwworms (Diptera: Calliphoridae) to changes in the concentration of blood, egg, and milk in the larval diet. J. Econ. Entomol. 1988, 81, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.R.; Rajan, V.V.; Verghese, A. A non-meat-based artificial diet and protocol for mass rearing of Chrysomya megacephala (Fab.) (Diptera: Calliphoridae), an important pollinator of mango. Curr. Sci. 2015, 108, 17–19. [Google Scholar]
- Green, P.W.C.; Simmonds, M.S.J.; Blaney, W.M. Diet nutriment and rearing density affect the growth of black blowfly larvae, Phormia regina (Diptera: Calliphoridae). Eur. J. Entomol. 2003, 100, 39–42. [Google Scholar] [CrossRef]
- Yan, G.; Schlink, A.C.; Brodie, B.S.; Hu, J.; Martin, G.B. The effects of diets and long-term laboratory rearing on reproduction, behavior, and morphology of Lucilia cuprina (Diptera: Calliphoridae). J. Med. Entomol. 2019, 56, 665–670. [Google Scholar] [CrossRef]
- Shefa, K.; Hossain, M.M.; Islam, M.H.; Islam, A.T.M.F.; Saifullah, A.S.M. An artificial larval diet for blowfly, Lucilia cuprina (Diptera Calliphoridae). J. Entomol. Zool. Stud. 2013, 1, 99–102. [Google Scholar]
- Chaudhury, M.F.; Chen, H.; Sagel, A.; Skoda, S.R. Effects of new dietary ingredients used in artificial diet for screwworm larvae (Diptera: Calliphoridae). J. Econ. Entomol. 2015, 108, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Levot, G.W.; Brown, K.R.; Shipp, E. Larval growth of some calliphorid and sarcophagid larvae. Bull. Entomol. Res. 1979, 69, 469–475. [Google Scholar] [CrossRef]
- Saunders, D.S. Maternal influence on the incidence and duration of larval diapause in Calliphora vicina. Physiol. Entomol. 1987, 12, 331–338. [Google Scholar] [CrossRef]
- Davies, L.; Ratcliffe, G.G. Development rates of some pre-adult stages in blowflies with reference to low temperatures. Med. Vet. Entomol. 1994, 8, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ames, C.; Turner, B. Low temperature episodes in development of blowflies: Implications for postmortem interval estimation. Med. Vet. Entomol. 2003, 17, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Arnott, S.; Turner, B. Post-feeding larval behaviour in the blowfly, Calliphora vicina: Effects on post-mortem interval estimates. Forensic Sci. Int. 2008, 177, 162–167. [Google Scholar] [CrossRef]
- Bernhardt, V.; Schomerus, C.; Verhoff, M.A.; Amendt, J. Of pigs and men—Comparing the development of Calliphora vicina (Diptera: Calliphoridae) on human and porcine tissue. Int. J. Legal Med. 2017, 131, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.F.; Dadour, I.R.; Keals, N.J. Stable fly, house fly (Diptera: Muscidae), and other nuisance fly development in poultry litter associated with horticultural crop production. J. Econ. Entomol. 1999, 92, 1352–1357. [Google Scholar] [CrossRef]
- Cook, D.F.; Dadour, I.R.; Voss, S.C. Management of stable fly and other nuisance flies breeding in rotting vegetable matter associated with horticultural crop production. Int. J. Pest Manag. 2011, 57, 315–320. [Google Scholar] [CrossRef]
- Cook, D.F.; Telfer, D.V.; Lindsey, J.B.; Deyl, R.A. Substrates across horticultural and livestock industries that support the development of stable fly, Stomoxys calcitrans (Diptera: Muscidae). Austral Entomol. 2018, 57, 344–348. [Google Scholar] [CrossRef]
- Cook, D.F. Influence of temperature on copula duration and mating propensity in Lucilia cuprina Wiedemann (Diptera: Calliphoridae). J. Aust. Entomol. Soc. 1994, 33, 5–8. [Google Scholar] [CrossRef]
- Cook, D.F. Influence of previous mating experience on future mating success in male Lucilia cuprina (Diptera: Calliphoridae). J. Insect Behav. 1994, 8, 207–217. [Google Scholar] [CrossRef]
- Voss, S.C.; Cook, D.F.; Hung, W.-F.; Dadour, I.R. Survival and development of the forensically important blow fly, Calliphora varifrons (Diptera: Calliphoridae) at constant temperatures. Forensic Sci. Med. Pathol. 2014, 10, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.C.; Magni, P.; Dadour, I.; Nansen, C. Reflectance-based determination of age and species of blowfly puparia. Int. J. Legal Med. 2017, 131, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.F.; Tufail, S.M.; Howse, E.T.; Voss, S.C. Manipulating larval rearing media to optimise mass production of the blow fly Calliphora vicina (Diptera: Calliphoridae). Austral Entomol. 2024, 63, 95–108. [Google Scholar] [CrossRef]
- Cook, D.F.; Tufail, S.M.; Voss, S.C.; Rogers, E.K.; Shivananjappa, S. Maggots cannot live on meatmeal alone: Production parameters for mass rearing of the ovoviviparous blowfly Calliphora dubia (Diptera: Calliphoridae). J. Econ. Entomol. 2024, toae043. [Google Scholar] [CrossRef]
- Kökdener, M.; Kiper, F. Effects of larval population density and food type on the life cycle of Musca domestica (Diptera: Muscidae). Environ. Entomol. 2021, 50, 324–329. [Google Scholar] [CrossRef]
- Donovan, S.E.; Hall, M.J.R.; Turner, B.D.; Moncrieff, C.B. Larval growth rates of the blowfly, Calliphora vicina, over a range of temperatures. Med. Vet. Entomol. 2006, 20, 106–114. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Scott, M.J.; Concha, C.; Welch, J.B.; Phillips, P.L.; Skoda, S.R. Review of research advances in the screwworm eradication program over the past 25 years. Entomol. Exp. Appl. 2017, 164, 226–236. [Google Scholar] [CrossRef]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O. Technological and bioactive functionalities of canola meal proteins and hydrolysates. Food Rev. Int. 2013, 29, 231–260. [Google Scholar] [CrossRef]
- Kudełka. W.; Kowalska, M.; Popis, M. Quality of soybean products in terms of essential amino acids composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef] [PubMed]
- Spragg, J.C.; Mailer, R.J.; Canola Meal Value Chain Quality Improvement. A Final Report for AOF and Pork CRC. 2008. Available online: www.porkcrc.com.au/1B-106_Final_Project_Report.pdf (accessed on 15 January 2024).
- Seberry, D.; Mailer, R.J.; Parker, P. The Quality of Australian Canola; Australian Oilseed Federation and NSW Department of Primary Industries Publication: www.australianoilseeds.com/__data/assets/pdf_file/0008/5849/2008_Quality_of_Australian_Canola_Book.pdf (accessed on 11 January, 2024).
- Choi, H.; Won, C.S.; Kim, B.G. Protein and energy concentrations of meat meal and meat and bone meal fed to pigs based on in vitro assays. Anim. Nutr. 2021, 7, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J.R. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.S.; Crous, K.L.; Villet, M.H. Models of development for blowfly sister species Chrysomya chloropyga and Chrysomya putoria. Med. Vet. Entomol. 2009, 23, 56–61. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60, 3367–3386. [Google Scholar] [CrossRef] [PubMed]
- Brinker, A.; Reiter, R. Fish meal replacement by plant protein substitution and guar gum addition in trout feed, Part 1: Effects on feed utilization and fish quality. Aquaculture 2010, 310, 350–360. [Google Scholar] [CrossRef]
- Millecam, J.; Khan, D.R.; Dedeurwaerder, A.; Saremi, B. Optimal methionine plus cystine requirements in diets supplemented with L-methionine in starter, grower, and finisher broilers. Poult. Sci. 2021, 100, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Parr, C.; Utterback, P.; Parsons, C.M. Nutritional evaluation of canola meals produced from new varieties of canola seeds for poultry. Poult. Sci. 2015, 94, 984–991. [Google Scholar] [CrossRef]
- Newkirk, R. Meal nutrient composition. In Canola Composition, Production, Processing and Utilisation; Daun, J.K., Eskin, M.N.A., Hickling, D., Eds.; AOCS Press: Urbana, IL, USA, 2011; pp. 229–244. [Google Scholar]
- Mogilnicka, I.; Bogucki, P.; Ufnal, M. Microbiota and malodor-etiology and management. Int. J. Mol. Sci. 2020, 21, 2886. [Google Scholar] [CrossRef]
- Rhinesmith-Carranza, J.; Liu, W.; Tomberlin, J.K.; Longnecker, M.; Tarone, A.M. Impacts of dietary amino acid composition and microbial presence on preference and performance of immature Lucilia sericata (Diptera: Calliphoridae). Ecol. Entomol. 2018, 43, 612–620. [Google Scholar] [CrossRef]
- El-Moaty, Z.A.; Kheirallah, A.M. Developmental variation of the blow fly Lucilia sericata (Meigen, 1826) (Diptera: Calliphoridae) by different substrate tissue types. J. Asia Pac. Entomol. 2013, 16, 297–300. [Google Scholar] [CrossRef]
- Omotoso, A.E.; Muse, W.A. Survival, fecundity and life cycle of the blowfly, Chrysomya chloropyga (Wied.) Diptera: Calliphoridae) fed with cow lung, beef and liver in the laboratory. IOSR J. Agric. Vet. Sci. 2020, 13, 1–5. [Google Scholar] [CrossRef]
- Yan, G. Diet affects the temperature-size relationship in the blowfly Aldrichina grahami. Insects 2024, 15, 246. [Google Scholar] [CrossRef]
- Miranda, C.D.; Cammack, J.A.; Tomberlin, J.K. Large-scale production of house fly, Musca domestica (Diptera: Muscidae), larvae fed 3 manure types. J. Econ. Entomol. 2023, 116, 1102–1109. [Google Scholar] [CrossRef]
| Livestock Meatmeal | Soyabean Meal | Canola Meal | |
|---|---|---|---|
| Essential amino acids | |||
| Arginine | 4.80 | 7.20 | 5.80 |
| Histidine | 1.44 | 2.60 | 2.70 |
| Iso-leucine | 1.87 | 4.00 | 4.00 |
| Leucine | 4.16 | 7.80 | 7.00 |
| Lysine | 3.64 | 6.40 | 5.80 |
| Methionine | 1.11 | 1.30 | 1.90 |
| Phenylalanine | 2.29 | 5.00 | 3.80 |
| Threonine | 2.31 | 4.00 | 4.50 |
| Valine | 2.69 | 4.80 | 5.00 |
| Non-essential amino acids | |||
| Alanine | 5.16 | 4.30 | 4.30 |
| Aspartic acid | 5.18 | 11.70 | 7.00 |
| Cystine | 1.03 | 0.64 | |
| Glutamic acid | 8.83 | 18.70 | 17.50 |
| Glycine | 9.33 | 4.20 | 4.90 |
| Proline | 6.04 | 5.10 | 6.00 |
| Serine | 2.66 | 5.10 | 4.60 |
| Tyrosine | 1.57 | 3.20 | 3.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





