Submitted:
16 May 2024
Posted:
17 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2,NB
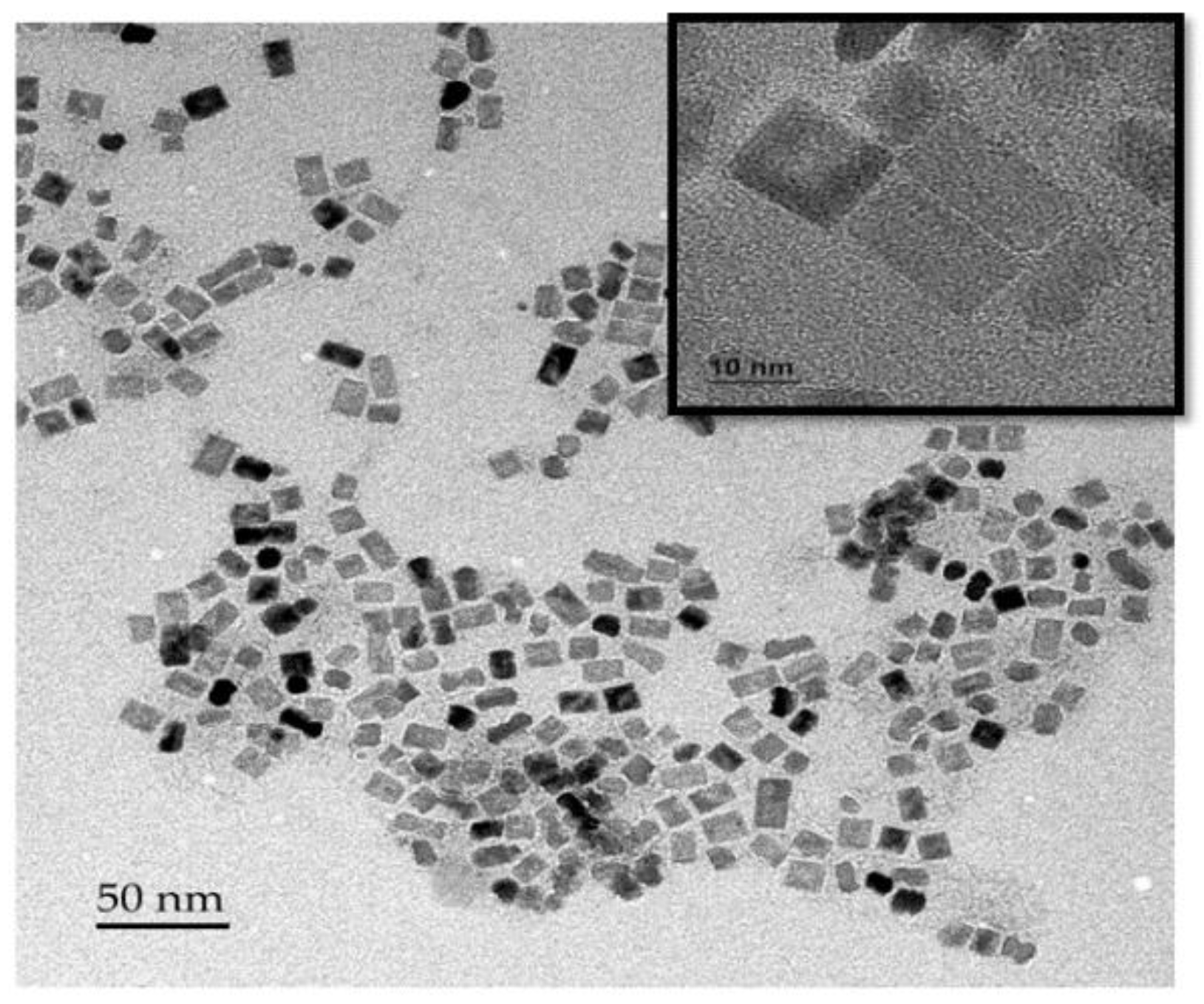
2.3. Development at Low Temperature of Composite Fiber-Based DSSC Photoanode Layer
2.4. DSSC Device Assembly
2.5. Dye Adsorption Characterization
2.6. DSSC Photovoltaic Performance Characterization
2.7. Electrochemical Impedance Spectroscopy (EIS) Characterization
3. Results
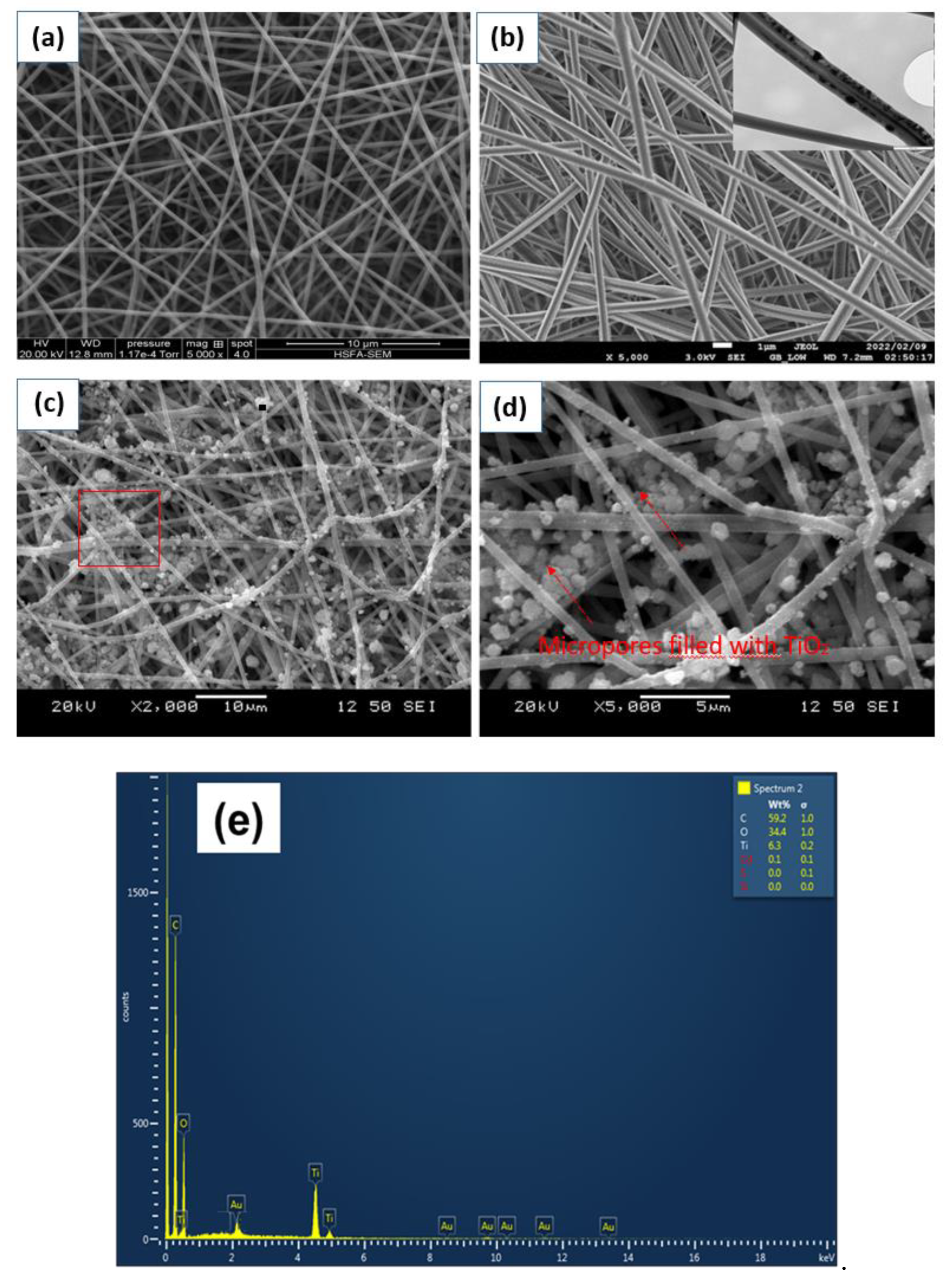
3.1. Morphology Characterization of the Developed Nanofibrous Photoanode Mats
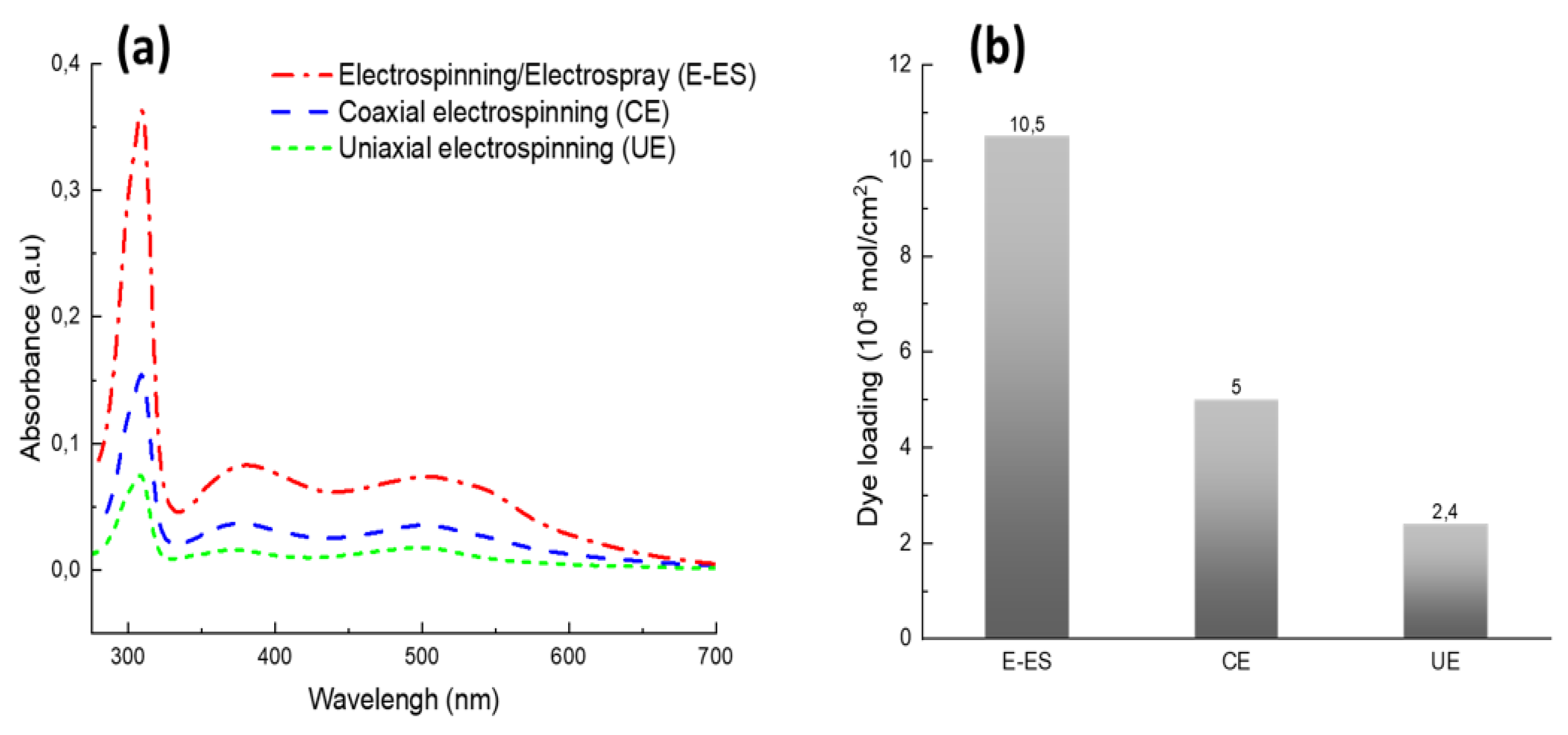
3.2. Dye Adsorption on the Surface of Nanofibrous Photoanode Mats
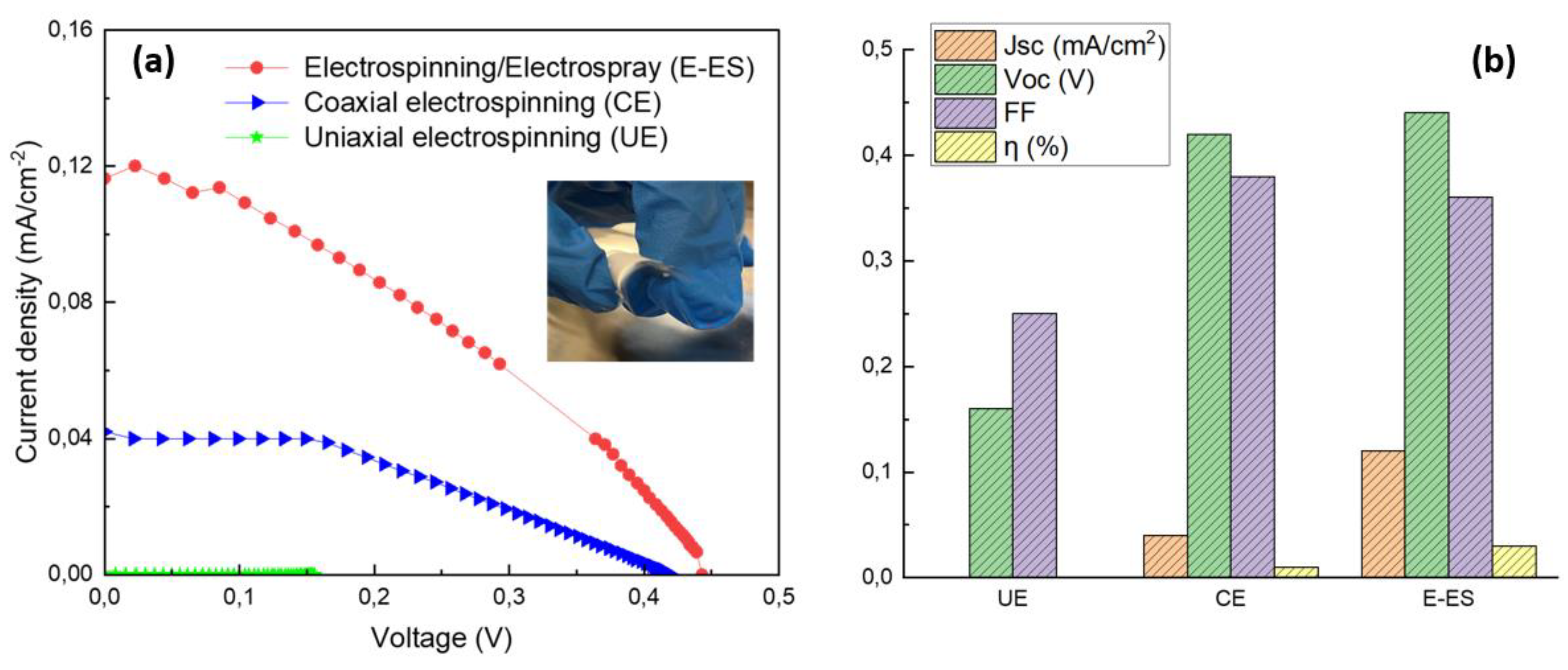
3.3. Current-Voltage (J-V) Characterization
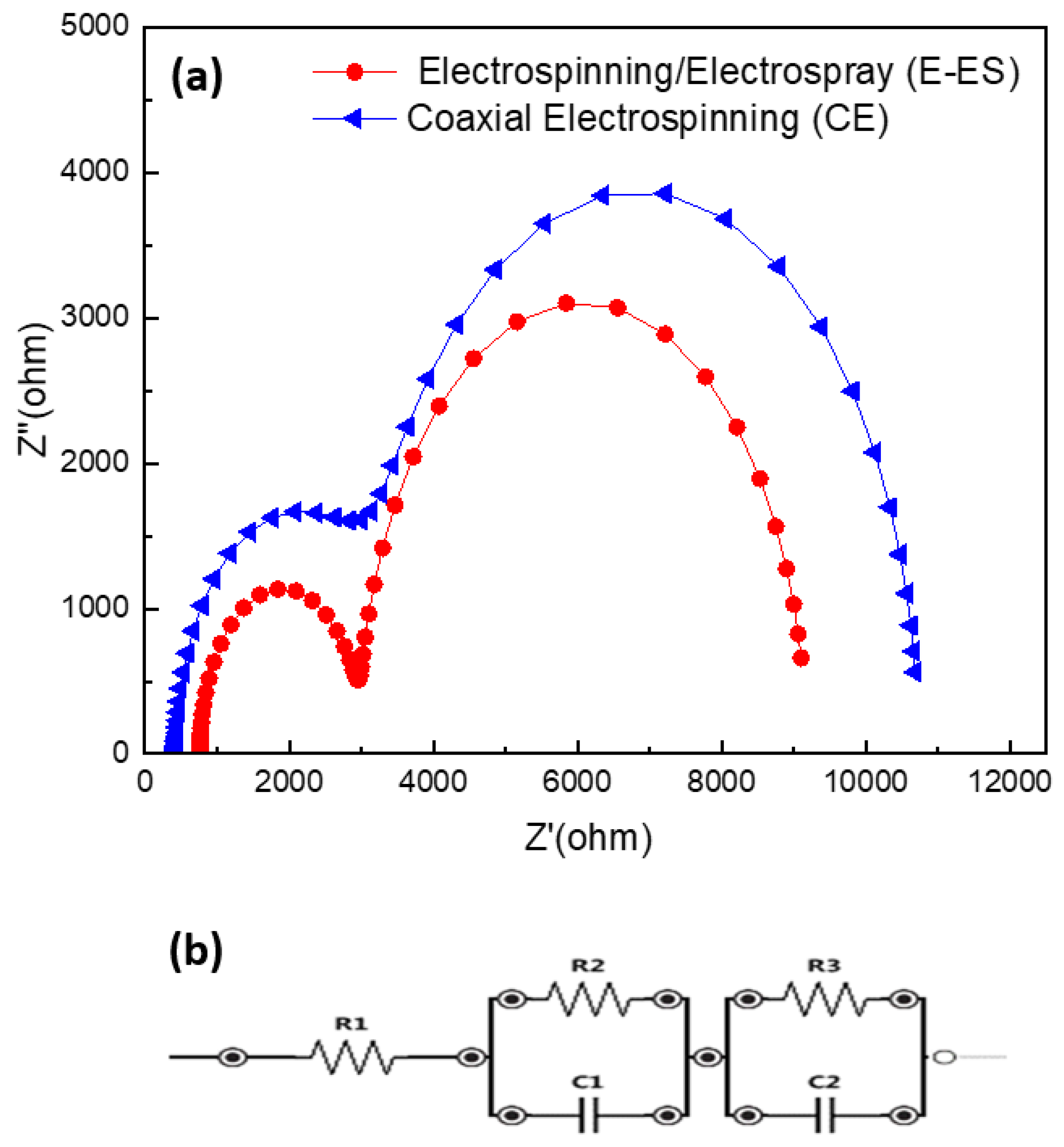
3.4. Electrochemical Impedance Spectroscopy (EIS) Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naik, P.; Abdellah, I.M.; Abdel-Shakour, M.; Su, R.; Keremane, K.S.; El-Shafei, A.; Adhikari, A.V. Improvement in performance of N3 sensitized DSSCs with structurally simple aniline based organic co-sensitizers. Sol Energy 2018, 174, 999–1007. [Google Scholar] [CrossRef]
- Aziz, N.; Rahman, M.; Umar, A. Comparative study of dye-sensitized solar cell utilizing selenium and palladium cathode. J Indian Chem Soc 2022, 99, 100289. [Google Scholar] [CrossRef]
- Zhang, P.; Chu, F.; Zhou, M.; Tao, B.; Miao, F. DSSC using natural dye sensitized and Ag/CdS/TiO2 composite structured light anode. Vacuum 2024, 219, 112763. [Google Scholar] [CrossRef]
- Yang, H.; Liu, W.; Xu, C.; Fan, D.; Cao, Y.; Xue, W. Laser sintering of TiO2 films for flexible dye-sensitized solar cells. Appl. Sci. 2019, 9, 823. [Google Scholar] [CrossRef]
- Baiju, K.G.; Murali, B.; Rao, R.S.; Jayanarayanan, K.; Kumaresan, D. Heat sink assisted elevated temperature sintering process of TiO2 on polymer substrates for producing high performance flexible dye-sensitized solar cells. Chem. Eng. Process 2020, 149, 107817. [Google Scholar] [CrossRef]
- Sabet, M.; Jahangiri, H. Using a low temperature method to fabrication of flexible dye sensitized solar cells with three different counter electrodes. J.Mater. Sci.: Mater.Electron 2018, 29, 778–783. [Google Scholar] [CrossRef]
- Li, B.; Huang, F.; Zhong, J.; Xie, J.; Wen, M.; Peng, Y. Fabrication of Flexible Dye-Sensitized Solar Cell Modules using Commercially Available Materials. Energy Technol 2016, 4, 536–542. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, C.; Yin, X.; Xiong, Y.; Xu, S.; Yan, X.; Deng, F. Novel flexible photoanode based on Ag nanowire/polymer composite electrode. J.Mater. Sci: Mater.Electron 2017, 28, 10092–10097. [Google Scholar] [CrossRef]
- Noorasid, N.; Arith, F.; Mustafa, A.; Azam, M.; Mahalingam, S.; Chelvanathan, P.; Amin, N. Current advancement of flexible dye sensitized solar cell: A review. Optik 2022, 254, 168089. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Pandey, A.K.; Abd Rahim, N.; Shahabuddin, S.; Tyagi, S.K. Chemical sintering of TiO2 based photoanode for efficient dye sensitized solar cells using Zn nanoparticles. Ceram. Int. 2018, 44, 18444–18449. [Google Scholar] [CrossRef]
- Chen, L.-C.; Ke, C.-R.; Hon, M.-H.; Ting, J.-M. Electrophoretic deposition of TiO2 coatings for use in all-plastic flexible dye-sensitized solar cells. Surf Coat Tech 2015, 284, 51–56. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Tobe, N.; Matsumoto, D.; Arakawa, H. Highly efficient plastic substrate dye-sensitized solar cells using a compression method for preparation of TiO 2 photoelectrodes. Chem Comm 2007, 4767–4769. [Google Scholar] [CrossRef] [PubMed]
- Khir, H.; Pandey, A.; Saidur, R.; Ahmad, M.S.; Abd Rahim, N.; Dewika, M.; Samykano, M. Recent advancements and challenges in flexible low temperature dye sensitised solar cells. Sustain. Energy Technol. Assessments 2022, 53, 102745. [Google Scholar] [CrossRef]
- Sun, L.; Chen, C.; Hao, L.; Wang, W.; Zhao, Y.; Ye, Y. Antimony incorporated flexible Cu2ZnSn (S, Se) 4 solar cell for enhanced mechanical endurance and efficiency. Vacuum 2024, 221, 112902. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Deng, C. Modification of TiO 2 electrode films in dye-sensitized solar cells with PMMA. J sol-gel sci techn 2011, 57, 128–131. [Google Scholar] [CrossRef]
- Li, Y.; Lee, D.-K.; Kim, J.Y.; Kim, B.; Park, N.-G.; Kim, K.; Shin, J.-H.; Choi, I.-S.; Ko, M.J. Highly durable and flexible dye-sensitized solar cells fabricated on plastic substrates: PVDF-nanofiber-reinforced TiO 2 photoelectrodes. Energy Environ. Sci 2012, 5, 8950–8957. [Google Scholar] [CrossRef]
- Sa'adah, U.; Himmah, S.W.; Suprayogi, T.; Diantoro, M.; Sujito, S.; Nasikhudin, N. The effect of time deposition of PAN/TiO2 electrospun on photocurrent performance of dye-sensitized solar cell. Mater. Today: Proc 2019, 13, 175–180. [Google Scholar] [CrossRef]
- Zohrevand, A.; Ajji, A.; Mighri, F. Microstructure and properties of porous nanocomposite films: effects of composition and process parameters. Polym. int 2014, 63, 2052–2060. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Lin, T. Functional applications of electrospun nanofibers. In Nanofibers-production, properties and functional applications, Lin,T.;Publisher: InTech 2011, Croatia, pp.287-302.
- Gallah, H.; Mighri, F.; Ajji, A.; Bandyopadhyay, J. Flexible PET/(PET-TiO2) core/shell nanofibrous mats as potential photoanode layer for dye-sensitized solar cells, DSSCs. Mater.Chem.Phys 2023, 305, 127911. [Google Scholar] [CrossRef]
- Gallah, H.; Mighri, F.; Ajji, A.; Bandyopadhyay, J. Flexible electrospun PET/TiO2 nanofibrous structures: Morphology, thermal and mechanical properties. Polym. Adv. Technol 2020, 31, 1612–1623. [Google Scholar] [CrossRef]
- Wali, Q.; Bakr, Z.H.; Manshor, N.A.; Fakharuddin, A.; Jose, R. SnO2–TiO2 hybrid nanofibers for efficient dye-sensitized solar cells. Sol Energy 2016, 132, 395–404. [Google Scholar] [CrossRef]
- Arifin, Z.; Suyitno, S.; Hadi, S.; Sutanto, B. Improved performance of dye-sensitized solar cells with TiO2 nanoparticles/Zn-doped TiO2 hollow fiber photoanodes. Energies 2018, 11, 2922. [Google Scholar] [CrossRef]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Electrospinning/electrospraying vs. electrospinning: A comparative study on the design of poly (l-lactide)/zinc oxide non-woven textile. Appl. surf. sci 2014, 311, 842–850. [Google Scholar]
- Ahmad, S.; Al-Ahmed, A.; Hakeem, A.S.; Alshahrani, T.; Mahmood, Q.; Mehmood, U.; Qayyum, H.; Younas, M.; Illyas, M.; Dafalla, H. Enhancing the performance of dye-sensitized solar cell using nano-sized erbium oxide on titanium oxide photoanode by impregnation route. J. Photochem.Photobiol 2021, 7, 100047. [Google Scholar] [CrossRef]
- Charbonneau, C.; Tanner, T.; Davies, M.L.; Watson, T.M.; Worsley, D.A. Effect of TiO 2 Photoanode Porosity on Dye Diffusion Kinetics and Performance of Standard Dye-Sensitized Solar Cells. J. Nanomater 2016, 2016. [Google Scholar] [CrossRef]
- Roji, M.A.M.; Kumar, P.R.; Shajan, X.S.; Raj, T.A.B. Silver doped ZnSnO3/SnO hybrid nanostructures as DSSC photoanodes: charge injection dynamics, slow recombination kinetics and simulation studies. Opt. Mater 2023, 138, 113696. [Google Scholar] [CrossRef]
- Joshi, D.N.; Dutta, V. Tandem DSSC fabrication by controlled infiltration of organic dyes in mesoporous electrode using electric-field assisted spray technique. Sol Energy 2021, 223, 318–325. [Google Scholar]
- Abrari, M.; Ahmadi, M.; Chenari, H.M.; Ghanaatshoar, M. Investigating the effect of ZrO2 nanofibers in ZnO-based photoanodes to increase dye-sensitized solar cells (DSSC) efficiency: Inspecting the porosity and charge transfer properties in ZnO/ZrO2 nanocomposite photoanode. Opt. Mater 2024, 147, 114690. [Google Scholar] [CrossRef]
- Sufyan, M.; Mehmood, U.; Gill, Y.Q.; Nazar, R.; Khan, A.U.H. Hydrothermally synthesize zinc oxide (ZnO) nanorods as an effective photoanode material for third-generation Dye-sensitized solar cells (DSSCs). Mater. Lett 2021, 297, 130017. [Google Scholar] [CrossRef]
- Pourandarjani, A.; Nasirpouri, F. A new approach to understanding the deficiency of backside illuminated dye-sensitized solar cells’ fill factor as a result of cracking of the TNAs. Mater. Today Proc 2019, 18, 501–509. [Google Scholar] [CrossRef]
- Li, Y.; Yoo, K.; Lee, D.-K.; Kim, J.H.; Park, N.-G.; Kim, K.; Ko, M.J. Highly bendable composite photoelectrode prepared from TiO2/polymer blend for low temperature fabricated dye-sensitized solar cells. Curr.Appl.Phys. 2010, 10, e171–e175. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, C.; Han, Y.; Jin, T.; Chi, B.; Pu, J.; Jian, L. Low-Temperature Preparation of Hierarchical Structure TiO2 for Flexible Dye-Sensitized Solar Cell. J Am Ceram Soc 2012, 95, 1372–1377. [Google Scholar] [CrossRef]
- Zohrevand, A. Devlopement of polymer nanocomposites films and their potential for photovoltaic cell applications, Ph.D. thesis, Laval University, Quebec, QC, Canada, 2014. 161.
- Hoseinzadeh, T.; Solaymani, S.; Kulesza, S.; Achour, A.; Ghorannevis, Z.; Ţălu, Ş.; Bramowicz, M.; Ghoranneviss, M.; Rezaee, S.; Boochani, A. Microstructure, fractal geometry and dye-sensitized solar cells performance of CdS/TiO2 nanostructures. J Electroanal Chem 2018, 830, 80–87. [Google Scholar] [CrossRef]
- Qi, L.; Wang, Q.; Wang, T.; Li, C.; Ouyang, Q.; Chen, Y. Dye-sensitized solar cells based on ZnO nanoneedle/TiO2 nanoparticle composite photoelectrodes with controllable weight ratio. J Mater Res 2012, 27, 2982–2987. [Google Scholar] [CrossRef]
- Mehmood, U.; Aslam, H.Z.; Al-Sulaiman, F.A.; Al-Ahmed, A.; Ahmed, S.; Malik, M.I.; Younas, M. Electrochemical impedance spectroscopy and photovoltaic analyses of dye-sensitized solar cells based on carbon/TiO2 composite counter electrode. J. Electrochem. Soc 2016, 163, H339. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G.; Lin, Y.; Xie, Y.; Wei, Y. Counter electrodes in dye-sensitized solar cells. Chem Soc Rev 2017, 46, 5975–6023. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Hung, I.-M. Effect of different concentration Li-doping on the morphology, defect and photovoltaic performance of Li–ZnO nanofibers in the dye-sensitized solar cells. Mater.Chem.Phys 2014, 143, 693–701. [Google Scholar] [CrossRef]
- Oktaviani, E.; Nursam, N.M.; Hidayat, J.; Pranoto, L.M.; Rosa, E.S.; Prastomo, N.; Timuda, G.E. Electrical and Electrochemical Properties of Sandwich-and Monolithic-Structured Dye-Sensitized Solar Cells with Various Counter Electrode Materials. Int.J.Electrochem.Sci. 2021, 16, 210922. [Google Scholar] [CrossRef]
- Hoshikawa, T.; Yamada, M.; Kikuchi, R.; Eguchi, K. Impedance analysis of internal resistance affecting the photoelectrochemical performance of dye-sensitized solar cells. J. Electrochem.Soc 2005, 152, E68. [Google Scholar] [CrossRef]
- Longo, C.; Nogueira, F.; Cachet, H.; De Paoli, M.-A. Solid-state and flexible solar cells based on dye-sensitized TiO2: study by electrochemical impedance spectroscopy. J.Phys.Chem.B 2002, 106, 5925–5930. [Google Scholar] [CrossRef]
- Castillo-Rodriguez, J.; Ortiz, P.D.; Mahmood, R.; Gossage, R.A.; Llanos, J.; Espinoza, D.; Zarate, X.; Koivisto, B.D.; Schott, E. The development of Au-titania photoanode composites toward semiflexible dye-sensitized solar cells. Sol Energy 2023, 263, 111955. [Google Scholar] [CrossRef]
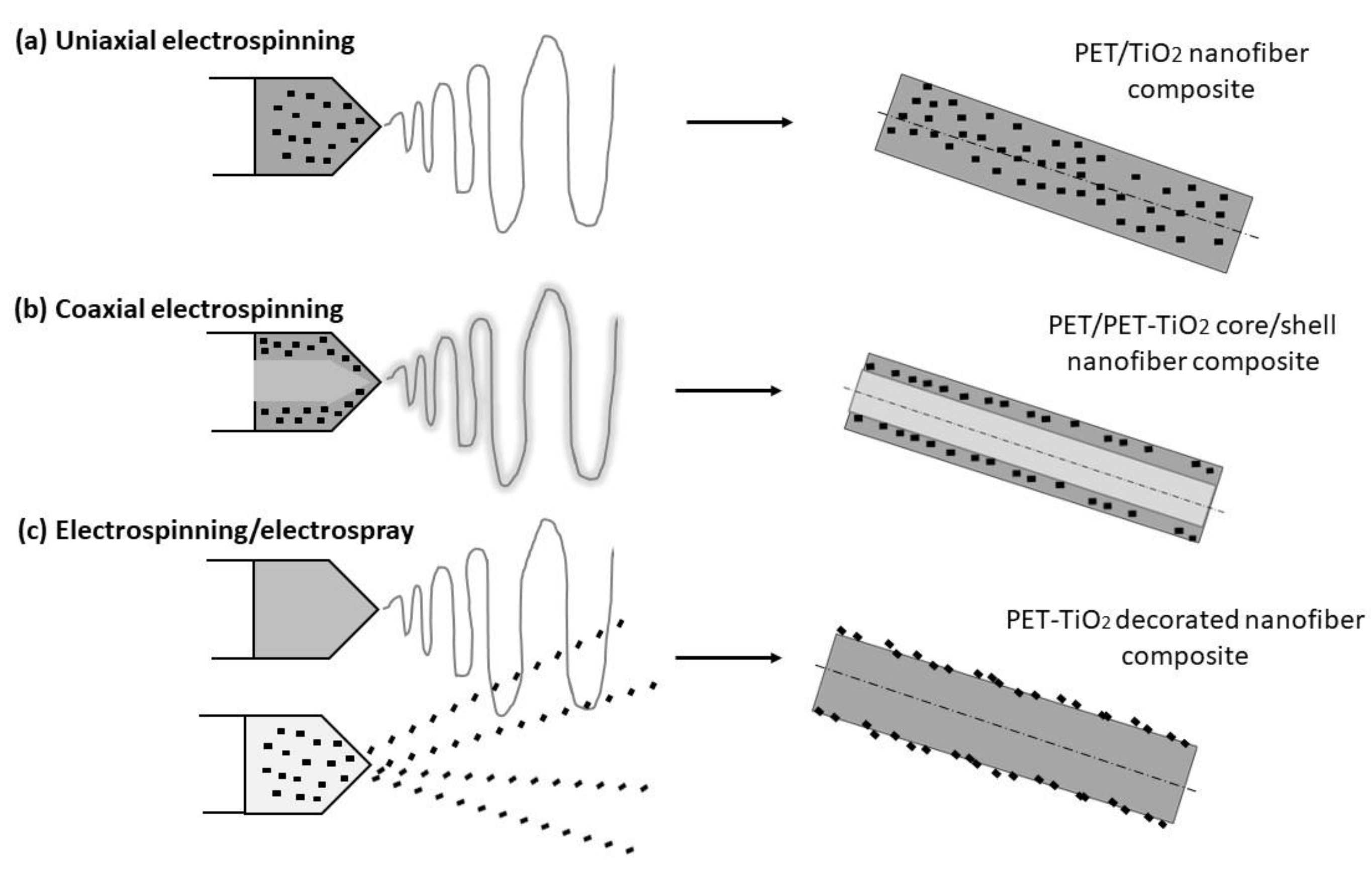
| Process | Uniaxial electrospinning (UE) |
Coelectrospinning (CE) |
Electrospinning-Electrospraying (E-ES) |
|---|---|---|---|
| Flow rate (ml/h) | 1.0 | Shell: 0.6 Core: 0.7 |
E: 1.0 ES: 0.5 |
| Voltage (kV) | 22.0 | 23.4 | E: 23.4 ES: 18.0 |
| Tip-to-collectordistance (cm) | 15.0 | 14.0 | E: 14.0 ES: 10.0 |
| Electrospinning method used | Jsc (mA/cm2) | Voc (V) | FF(%) | η (%) | Rs(Ω) | Rct (Ω) |
|---|---|---|---|---|---|---|
| UE | 0.0003 | 0.16 | 0.25 | 1.8*10-3 | - | - |
| CE | 0.04 | 0.42 | 0.38 | 0.01 | 431 | 2741 |
| E-ES | 0.12 | 0.44 | 0.36 | 0.03 | 762 | 2214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





