1. Introduction
Biological invasions are one of the most important threats to global biodiversity and a major cause of extinctions [
1,
2,
3]. The number of alien plants in each continent continues to increase, and many of them have major negative impacts on native plant species diversity [
1,
4]. The invasiveness of alien plants depends partly on many plant traits, on the range of habitats suitable for the species, and on the dispersal mechanisms that allow alien species to expand following an initial introduction [
5,
6,
7]. Dispersal syndromes defined from diaspore morphology have often been used to predict dispersal distances [
8], but these syndromes often underestimate the potential for long-distance dispersal [
9]. For example, migratory waterbirds disperse many plants by endozoochory (gut passage) which have been assigned to gravity or unassisted syndromes [
10,
11]. Field studies indicate that waterbird endozoochory is particularly likely for halotolerant plants, which have more avian vectors [
12]. They also confirm that waterbirds are frequent vectors of alien plants [
13,
14].
The buttonweed
Cotula coronopifolia (Asteraceae, formerly Compositae; also known as “brass buttons”) is native to South Africa but is a widespread alien species in Europe, North America, and Australia [
15]. In Europe it was first recorded in 1742 in the Netherlands [
16], is halotolerant and is particularly widespread in coastal areas, although it is also common in some inland wetlands [
17,
18,
19]. It occupies a wide latitudinal range in Europe of >22o spanning between southern Spain and Sweden [
17]. Rapid recent expansion has occurred along the Swedish Baltic coast, and in the UK and Ireland [
19,
20].
Buttonweed is considered invasive because it excludes native plants (
Figure S1), and is reported to promote soil salinization [
17,
20,
21]. Its potential future impact on native communities depends both on its competitive ability across a broad salinity range, and its capacity for dispersal into new habitats. Long distance seed dispersal seems more likely to occur by endozoochory, as
C. coronopifolia seeds have been repeatedly recorded in waterbird faeces and pellets [
22,
23,
24], and have also been germinated from cattle faeces [
20].
Although the species is recorded across a wide salinity range in Europe, there is a lack of previous studies concerning the plasticity of
Cotula coronopifolia germination or establishment in response to a salinity gradient, or geographical variation in halotolerance across the introduced range. Despite previous evidence that seeds can germinate after gut passage, there are no previous studies of the influence of avian ingestion on germinability in this species. [
25] found other wetland plant species to vary widely in their germination patterns in response to simulated avian digestion, with some being favoured (e.g.
Eleocharis palustris) and others hindered (e.g.
Typha latifolia). These differences can determine the establishment success after seeds are dispersed into new habitats by waterfowl or other animal vectors [
26]. Furthermore, both germination patterns, and how they are influenced by gut passage, can vary according to the salinity conditions [
27,
28]. Previous studies on the effects of gut passage on wetland plants have considered only one population per plant species (see Green et al. 2023 for review), but we might expect some populations to be more adapted to endozoochory than others (e.g. due to variation in the density of biotic vectors).
In this study we tested the germination response of
Cotula coronopifolia under a broad salinity range, and how this was influenced by simulated passage through the gut of a waterbird. We compared the response of seeds from several populations representing the broad latitudinal non-native range in Europe. We looked for interactions between population, salinity, and gut passage effects. Given results for previous studies on native species [
27,
28,
29], our initial hypotheses were that i) germination would be inhibited at higher salinities; ii) populations would differ in their germination responses; iii) there would be important interactions between salinity and the response to gut passage. We consider the implications of our results for the invasion of habitats of different salinities by
C. coronopifolia, and the potential role of long-distance dispersal via waterbirds in the future expansion of this alien plant.
2. Results
2.1. Effects of Salinity, Population, and Gut Passage on Germinability
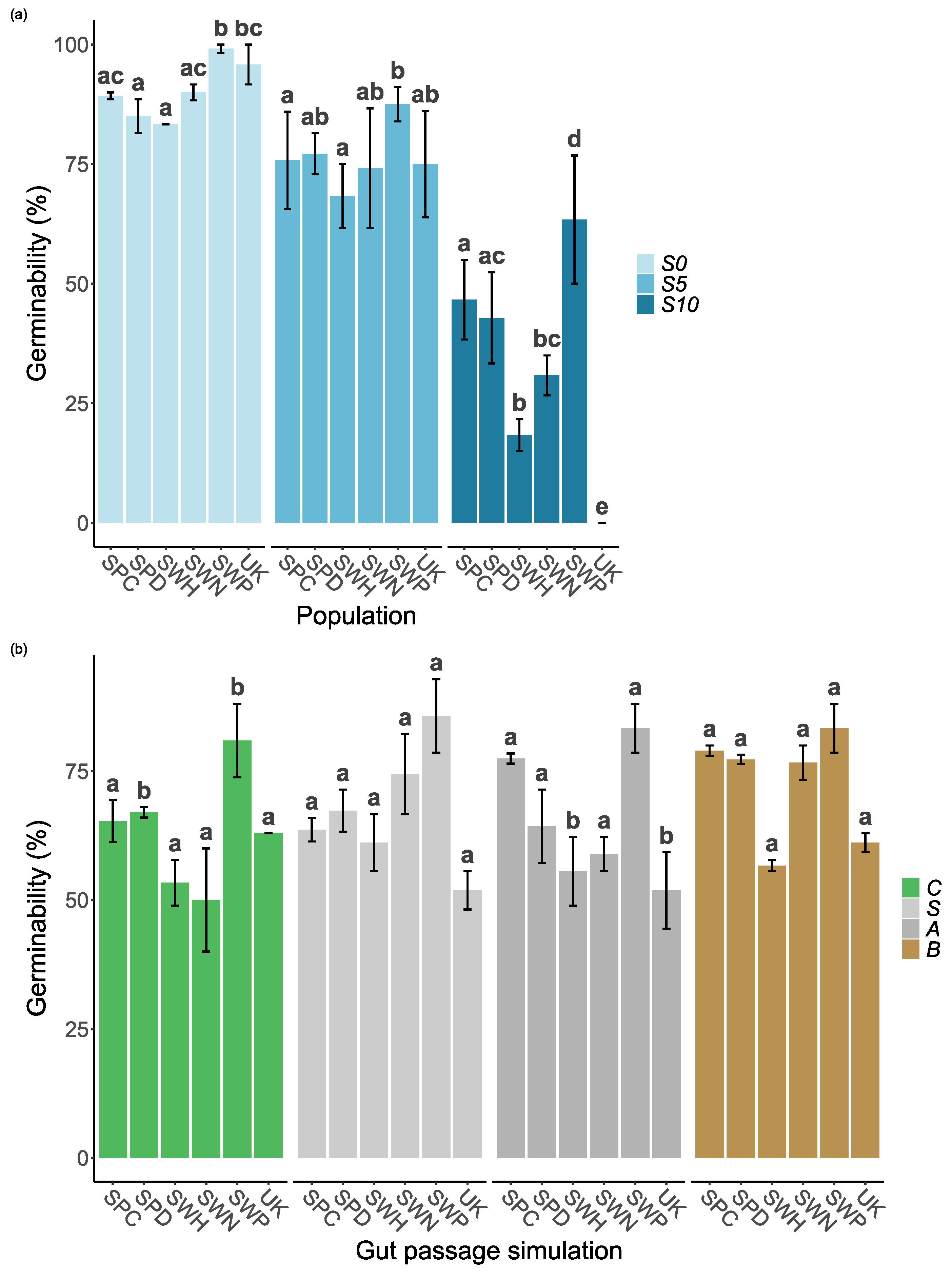
Seeds germinated on petri dishes prepared with 0, 5 or 10 g/L NaCl solutions (referred to as S0, S5 or S10 from hereon). In general, when salinity increased, it had a negative impact on germinability (
Figure 1 and
Figure 2a). No seeds germinated in S15. Salinity, gut passage treatment, and population all had highly significant effects on seed germinability (
Table 1).
The final GLM model also included highly significant interactions between the population and salinity, and between population and gut passage treatment (
Table 1). Germinability showed no clear latitudinal trend, and the extremes were represented within Sweden, with SWP having consistently higher germinability than SWH (
Figure 2a). Population differences were more pronounced at S10, at which UK had zero germinability despite having second highest germinability at S0 (
Figure 2a). Spanish populations (SPC and SPD) had intermediate germinability. The difference between these Spanish sites in soil salinity (
Table 2) was not reflected in any differences in germinability (
Figure 2a).
Germinability was consistently higher for full gut treatment (both acid and scarification) than for control seeds, and partial treatments (acid or scarification) clearly had intermediate germinability at S5 and S10 (
Figure 1). Treatment effects varied between populations, and the increase in germinability at full gut treatment compared to controls was particularly strong for SPC, SPD and SWN (
Figure 2b).
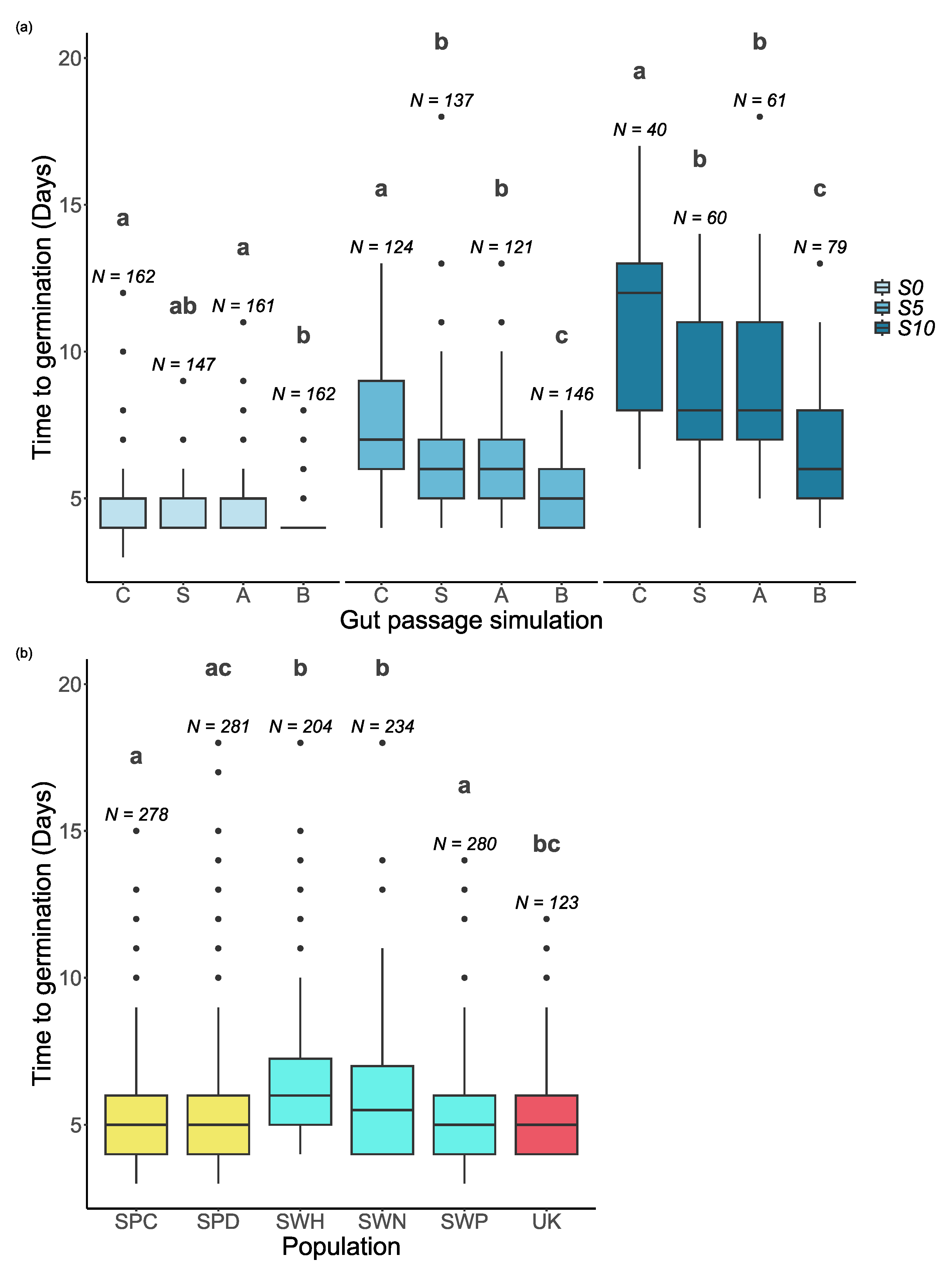
2.2. Effects of Gut Passage and Salinity on Time to Germination
Salinity, gut passage treatment and population all had significant effects on the time to germination for seeds that germinated within 20 days (
Table 1). The final model also included a significant interaction between salinity and gut passage treatment (
Table 1).
Overall, the time to germination increased when the salinity increased (
Figure 1 and
Figure 3a). Seeds subjected to the full gut passage treatment germinated consistently faster than control seeds, and partial gut passage had clear intermediate effects at S5 and S10 (
Figure 3a). As reflected by the salinity x gut passage interaction, at S0 partial digestion did not have a significant effect on time to germination compared with controls (
Figure 3a). There were differences in time to germination between populations within Sweden, and no evidence of a latitudinal trend across the six populations (
Figure 3b). The difference between Spanish sites in soil salinity was not reflected in any differences in time to germination (
Figure 3b).
3. Discussion
We found experimentally that buttonweed C. coronopifolia germinates readily across a broad salinity range, responds positively to simulated gut passage (suggesting adaptation or pre-adaptation to endozoochory), and shows broadly similar germination responses from alien populations across a wide latitudinal range. Significant interactions revealed some variation between populations in how germinability was influenced by salinity and by gut passage. Likewise, the effect of gut passage on the time to germination changed significantly with salinity. Our results provide evidence that dispersal by endozoochory plays an important role in the expansion of this invasive plant.
3.1. Effect of Gut Passage on Germination Patterns
The passage of diaspores through avian digestion has a variable effect on the germination of different angiosperm species, including both fleshy- and dry-fruited species [
25,
30,
31]. Even within other Asteraceae found in European wetlands, the response of germinability or germination time to simulated gut passage was positive in some species (e.g.
Eupatorium cannibinum) but negative in others (e.g.
Tragopogon pratensis, [
25]). Diaspore morphology is diverse, but there is no evidence that seeds from fleshy-fruited angiosperms dispersed by frugivores have an architecture better adapted to resist gut passage than those from dry-fruited plants dispersed by waterbirds [
32]. Our results show an overall increase in germinability and an acceleration in the time to germination for
Cotula coronopifolia in response to gut passage. This is particularly striking given that our seeds were cold-stored for months prior to the experiment, and that this stratification could be expected to increase germination of control seeds by breaking physiological dormancy [
28]. If seeds had been stored at room temperature, the positive effects of gut passage on germination may have been even stronger.
Previously, positive germination responses to gut passage were recorded in non-saline conditions for some other dry-fruited plant species from wet habitats, but many other species showed negative effects [
25,
28]. We found that this positive effect of gut passage also occurs for
C. coronopifolia in saline conditions, although there was a salinity x gut passage interaction for time to germination. Halotolerance also increases the number of waterbird species that are likely to disperse a given plant species [
12], because migratory waterbirds concentrate in coastal wetlands.
3.2. Implications for Long-Distance Dispersal
Seeds of
Cotula coronopifolia were previously recovered from waterbird egesta and then germinated [
23,
24]. Our experiment confirms that the diaspores of this species can resist the passage through the gut of a waterbird, and that gut passage can facilitate germination in saline conditions with a maximum tolerance of ≥10 <15 g/L NaCl.
Our full gut passage treatment lasted a total of 4 hours, which is comparable to the mean retention times of seeds in the gut of a waterbird [
23,
33]. However, maximum gut retention times can exceed several days, and so maximum seed dispersal distances by endozoochory can be several hundred or >1000 km [
23,
34,
35]. Even outside migratory periods, seeds can be dispersed >100km by waterbirds, greatly exceeding expectations for wind dispersal of
Cotula coronopifolia [
36]. Alien plant species can gain an advantage over native species by showing higher seed survival and germinability, and lower time to germination, in response to gut passage [
26,
37].
Our results suggest that germination and establishment are likely to be favoured at salinities of 0-10 g/L after endozoochory events, favouring establishment of new Cotula coronopifolia populations, as well as gene flow between existing populations. The relative lack of differences in germination response we found between populations is consistent with latitudinal connectivity and gene flow over recent decades and centuries since this alien species colonized Europe. This would be expected if there is a major role for waterbird dispersal vectors. Population effects were generally much weaker than the effects of salinity and gut passage, and we found no evidence of local adaptation when comparing nearby populations living in different soil salinities.
3.3. The Importance of Halotolerance
Germination of
Cotula coronopifolia remained high at 10 g/L NaCL in all but one population (UK). This exceptional population may have a unique origin, as there was a direct introduction reported into nearby gardens by 1886 [
38]. The halotolerance we observed in all other populations exceeds that of many native mudflat species in Europe [
39,
40] and helps explain how this alien species has occupied both fresh and brackish habitats, including coastal marshes and wetlands in the Mediterranean climatic zone that vary in salinity over the year (see [
41] for SPD). Hydrological changes due to climate change and water extraction for irrigation and other human activities are causing wetland salinization in many areas [
42], and this halotolerance may allow
C. coronopifolia to colonise further habitats in future and to outcompete native species less adapted to salt stress. Invasion may itself promote further salinization [
17]. The observed expansion in the species’ distribution over recent decades in Europe is consistent with these expectations, and with an important role for dispersal by waterbird vectors (e.g. to isolated, inland wetlands, [
43]).
4. Materials and Methods
4.1. Study Species
Buttonweed
Cotula coronopifolia (Asteraceae) is a decumbent and stoloniferous herb which can spread vegetatively by growing roots on the stem nodes. It is an annual or short-lived perennial plant [
18,
20] which inhabits wetlands, salt and freshwater marshes and temporary pools. It is considered as a pioneer species with an ability to self-fertilise, high seed production and efficient seed dispersal [
15]. It can be considered a halotolerant mudflat species (Ellenberg F = 7, Ellenberg S = 3, [
44]).
The plant present heads with yellow disk flowers, the outer ones being female and the others hermaphrodite, with plano-convex achenes (cypsella) with marginal thickening [
45]. Van Der Toorn et al. (1980) estimated seed production at 20,000-50,000 diaspores per individual, considered cross-pollination to occur by wind and insects, and seeds to float for <10 minutes. However, more recently floating time was estimated at 29-40h (L. Tomasson, unpublished data). Like many Asteraceae, it has been assigned an anemochory dispersal syndrome [
44], but it is debatable whether the achenes have traits that may be adaptations for wind dispersal (
Figure S2). Although
C. coronopifolia may perhaps have initially reached Europe from South Africa through boat traffic, its spread may also be partly driven by its use as an ornamental plant, dating back at least to the 19th century [
38]. It is currently traded legally in Europe as a pond plant.
4.2. Study Sites and Plant Material
We collected mature flower heads from
Cotula coronopifolia individuals with mature achenes containing the seeds at six different sampling sites (
Table 1). Heads from about 20 individuals were collected from each population, in 3 different countries: Spain, Sweden and the United Kingdom. Four populations were essentially coastal, including the Atlantic coast in Spain (SPC in
Table 1), the Irish Sea in the UK (UK), and the Baltic Sea and Kattegat in Sweden (SWN and SWH). SPD and SWP were populations from inland wetlands with low salinities. The sites sampled in Sweden have a low salinity compared to the Atlantic and Irish Sea. The area adjacent to SWN has a lower and less fluctuating salinity than SWH which is located at Kattegat. The area around SWH has a variable surface salinity due to the outlet between the Baltic and North Sea.
The samples were collected from April to August in 2022 and stored in darkness at 4 ° C until the experiment started in February 2023. Seeds were separated from each of the six populations, with an even representation of available flower heads (
Table 2).
4.3. Gut Passage Simulation
To test how passage through the gut of a waterbird modifies seed germination patterns at different salinities, we performed a gut passage simulation, following the methodology of [
46] and [
47]. Seeds of each population were randomly divided into four groups (40 seeds per group), including a control group. The other groups were subjected to three digestion treatments: seed scarification simulating grit in the waterbird gizzard, chemical treatment with hydrochloric acid, or a combination of both (scarification followed by acid). For the scarification step, we added the seeds to a 150 mL plastic flask with 10 g of grit (2-4 mm size) and 4 mL of water. The flasks were then placed in an incubator (New Brunswick Classic® C24 Incubator Shaker) for 2 hr at 42 ° C and 300 shakes/min. Before continuing with the chemical step, we separated the seeds from the grit using a stack of two sieves, one with a 2 mm mesh to retain grit and another with a 300 µm mesh to retain seeds. Then, seeds were immersed in 5 ml of a HCl solution at pH = 2.5 for 2 h at 42 ° C without shaking. After this second step, we retrieved the seeds and rinsed them with distilled water.
4.4. Germination Experiment
Seeds from gut treatments and controls (
Figure S2) were placed on 90 x 15 mm Petri dishes (with four compartments) with agar (0.8%) mixed with 0, 5, 10 or 15 g/L NaCl solutions. A total of 96 Petri dishes were used as the resulting combination of 4 salinities, 4 treatments, and 6 populations, with 25-35 seeds per dish. Two populations were sown in each dish, in different compartments, and each population was divided between two separate dishes.
The Petri dishes with seeds were placed in a germination chamber (25/20 °C, 70–80% relative humidity, and a 16/8 h light/dark period) for 20 days (6 to 26 February 2023), considered long enough due to the generally rapid germination of plants in the family Asteraceae. Germination data were recorded daily from day 3 onwards, counting as germinated those that were showing a hypocotyl of 4-6 mm and visible cotyledons.
4.5. Statistical Analyses
No seeds germinated in the most saline treatment (NaCl = 15 g/L), so this treatment was excluded from statistical analyses. The influence of the different treatments for the gut passage simulation (factor of 4 levels), salinity (factor of 3 levels) and population (factor of 6 levels) on the germination pattern was modelled with generalised linear models (GLMs). We analysed the germination response (Yes = 1, or No = 0) with a binomial error distribution and logit function. We analysed the time to germination (i.e. days taken to germinate) with a negative binomial error distribution and log-link function, excluding seeds that failed to germinate. This error distribution was chosen because a Poisson distribution was found to be inappropriate, owing to overdispersion.
Global models with all covariates (salinity, population, and gut passage simulation) and their first order interactions were used for both dependent variables, germinability and time to germination. Final model selection was based on the lowest value of the corrected Akaike information criteria [AICc]. Statistical significance and contribution to variability was tested for the fixed terms and their interactions. Differences between the factor levels in the fixed terms were further tested using False Discovery Rate (FDR) post hoc tests. Generalised linear model analyses were performed and checked using the packages DHARMa and lmer under R version 4.3.2 for Windows (R Core Team 2015).
5. Conclusions
Our experimental study complements previous field studies and provides strong evidence for the importance of avian endozoochory in the invasion of a dry-fruited Asteraceae. In contrast to the extensive literature on dispersal of native plants by waterbirds [
48], ours represents one of the best examples to date of how non-classical endozoochory can spread alien plants assigned to other dispersal syndromes (see [
13,
36,
49] for other examples).
To understand the connectivity between European populations of this alien species, and the relative importance of alternative dispersal mechanisms (including hydrochory and human vectors), genetic studies are required that also relate population differences to waterbird movement patterns. The potential for hydrochory of
C. coronopifolia should be assessed in detail with buoyancy experiments (e.g. [
49]). These studies would help to evaluate the importance of long-distance endozoochory for
Cotula coronopifolia compared to other dispersal mechanisms such as wind, water, or anthropic activity.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Figure S1: Cotula coronopifolia (yellow flowers) growing in Nedra Sandby on the east coast of Öland, Sweden where it has displaced native vegetation. This area is grazed by cattle and used by migratory waterbirds a) Photo taken in 2019 (by LT) and b) Drone photo taken in 2017 (photo credit: Thomas Gunnarsson).; Figure S2: Cotula coronopifolia seeds before (top three seeds) and after (bottom three seeds) simulated gut passage by scarification and acid treatment. Photo credit: Iciar Jiménez Martín.
Author Contributions
Conceptualization, Raúl Sánchez-García and Andy Green; Data curation, Raúl Sánchez-García; Formal analysis, Raúl Sánchez-García and Andy Green; Funding acquisition, Andy Green, Francisco Hortas and Maria Ortiz; Investigation, Raúl Sánchez-García and Maria Ortiz; Methodology, Raúl Sánchez-García and Maria Ortiz; Project administration, Andy Green; Resources, Andy Green, Lina Tomasson, Francisco Hortas and Maria Ortiz; Software, Raúl Sánchez-García; Supervision, Andy Green and Maria Ortiz; Validation, Andy Green; Visualization, Raúl Sánchez-García; Writing – original draft, Raúl Sánchez-García and Andy Green; Writing – review & editing, Raúl Sánchez-García, Andy Green, Lina Tomasson, Francisco Hortas and Maria Ortiz.
Funding
This research was funded by Ministerio de Ciencia e Innovacion, project number PID2020–112774GB-I00/AEI/10.13039/501100011033 and RSG was funded by an FPI grant from the Spanish Ministerio de Ciencia, Innovacion y Universidades (PRE2021-099466).
Data Availability Statement
Acknowledgments
We thank Ángel Huesca Burguillos, Billy James Williams Marland, Alba Mata González, and Esther Mª Martín Carretié for assistance with the experiment. We also thank the staff of The Greenhouse Service of CITIUS, ICTS-Doñana, LEA-EBD and LEM-EBD. Plants were collected under permit from Natural England and the Junta de Andalucía. We are grateful to Salinas de Cetina for the permission to enter the study area.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- van der Velde, G.; Rajagopal, S.; Kuyper-Kollenaar, M.; Bij de Vaate, A.; Thieltges, D.W.; MacIsaac, H.J. Biological Invasions: Concepts to Understand and Predict a Global Threat. In Ecological Studies, Wetlands: Functioning, Biodiversity Conservation, and Restoration; Bobbink, R., Beltman, B., Verhoeven, J.T.A., Whigham, D.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 191, pp. 61–85. [Google Scholar] [CrossRef]
- Downey, P.O.; Richardson, D.M. Alien plant invasions and native plant extinctions: a six-threshold framework. AoB Plants 2016, 8, plw047. [Google Scholar] [CrossRef] [PubMed]
- Foxcroft, L.C.; Pyšek, P.; Richardson, D.M.; Genovesi, P.; MacFadyen, S. Plant invasion science in protected areas: progress and priorities. Biol Invasions 2017, 19, 1353–1378. [Google Scholar] [CrossRef]
- van Kleunen, M.; Pyšek, P.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Weigelt, P.; Stein, A.; Dullinger, S.; König, C.; et al. The Global Naturalized Alien Flora (GloNAF) database. Ecology 2019, 100, e02542. [Google Scholar] [CrossRef] [PubMed]
- Hovick, S.M.; Peterson, C.J.; Carson, W.P. Predicting invasiveness and range size in wetland plants using biological traits: a multivariate experimental approach. Santamaria L (Ed.). J. Ecol. 2012, 100, 1373–1382. [Google Scholar] [CrossRef]
- Palma, E.; Vesk, P.A.; White, M.; Baumgartner, J.B.; Catford, J.A. Plant functional traits reflect different dimensions of species invasiveness. Ecology 2021, 102, e03317. [Google Scholar] [CrossRef] [PubMed]
- Moyano, J.; Essl, F.; Heleno, R.; Vargas, P.; Nuñez, M.A.; Rodriguez-Cabal, M.A. Diaspore traits specialized to animal adhesion and sea current dispersal are positively associated with the naturalization of European plants across the world. Ecography 2022, 2022, e06423. [Google Scholar] [CrossRef]
- Lososová, Z.; Axmanová, I.; Chytrý, M.; Midolo, G.; Abdulhak, S.; Karger, D.N.; Renaud, J.; Van Es, J.; Vittoz, P.; Thuiller, W. Seed dispersal distance classes and dispersal modes for the European flora. Glob. Ecol. Biogeogr. 2023, 32, 1485–1494. [Google Scholar] [CrossRef]
- González-Varo, J.P.; Rumeu, B.; Bracho-Estévanez, C.A.; Acevedo-Limón, L.; Baltzinger, C.; Lovas-Kiss, Á.; Green, A.J. Overlooked seed-dispersal modes and underestimated distances. Glob Ecol Biogeogr 2024, e13835. [Google Scholar] [CrossRef]
- Green, A.J.; Baltzinger, C.; Lovas-Kiss, Á. Plant dispersal syndromes are unreliable, especially for predicting zoochory and long-distance dispersal. Oikos 2021, 2022, e08327. [Google Scholar] [CrossRef]
- Navarro-Ramos, M.J.; Van Leeuwen, C.H.A.; Olsson, C.; Elmberg, J.; Månsson, J.; Martín-Vélez, V.; Lovas-Kiss, Á.; Green, A.J. Seed dispersal between aquatic and agricultural habitats by greylag geese. Agric Ecosyst Environ 2024, 359, 108741. [Google Scholar] [CrossRef]
- Almeida, B.A.; Lukács, B.A.; Lovas-Kiss, Á.; Reynolds, C.; Green, A.J. Functional Traits Drive Dispersal Interactions Between European Waterfowl and Seeds. Front. Plant Sci. 2022, 12, 795288. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vélez, V.; van Leeuwen, C.H.A.; Sánchez, M.I.; Hortas, F.; Shamoun-Baranes, J.; Thaxter, C.B.; Lens, L.; Camphuysen, C.J.; Green, A.J. Spatial patterns of weed dispersal by wintering gulls within and beyond an agricultural landscape. J. Ecol. 2021, 109, 1947–1958. [Google Scholar] [CrossRef]
- Green, A.J.; Lovas-Kiss, Á.; Reynolds, C.; Sebastián-González, E.; Silva, G.G.; Van Leeuwen, C.H.A.; Wilkinson, D.M. Dispersal of aquatic and terrestrial organisms by waterbirds: A review of current knowledge and future priorities. Freshw Biol 2023, 68, 173–190. [Google Scholar] [CrossRef]
- Vandertoorn, J. On the Ecology of Cotula-Coronopifolia L and Ranunculus-Sceleratus L.1. Geographic-Distribution, Habitat, and Field Observations. Acta Botanica Neerlandica 1980, 29, 385–396. [Google Scholar] [CrossRef]
- Ridley, H.N. The dispersal of plants throughout the world. L. Reeve and Co. 1930.
- Sanz Elorza, M.; Sánchez, E.D.; Vesperinas, E.S. Las especies invasoras, Cotula coronopifolia. In Atlas de las plantas alóctonas invasoras en España. Dirección General para la Biodiversidad, Ministerio de Medio Ambiente: Madrid, Spain, 2004; pp. 130-132. https://www.gisandbeers.com/GeoBazar/Libros/Atlas%20biodiversidad/Atlas%20de%20las%20Plantas%20Aloctonas%20Invasoras%20de%20Espana.
- Costea, M.; El Miari, H.; Laczkó, L.; Fekete, R.; Molnár, A.V.; Lovas-Kiss, Á.; Green, A.J. The effect of gut passage by waterbirds on the seed coat and pericarp of diaspores lacking “external flesh”: Evidence for widespread adaptation to endozoochory in angiosperms. PLoS ONE 2019, 14, e0226551. [Google Scholar] [CrossRef]
- Killick, H.J. Cotula coronopifolia L. In BSBI Online Plant Atlas 2020; Stroh, P.A., Walker, K.J., Humphrey, T.A., Pescott, O.L., Burkmar, R.J., Eds.; Botanical Society of Britain and Ireland & Princeton University Press: Princeton, UK, 2023; Volume 2, https://plantatlas2020.org/atlas. [Google Scholar]
- Tomasson, L. Four years with Cotula coronopifolia: Monitoring and climate suitability modelling. Dissertation, Umeå University, Sweden, 2022. https://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-192974.
- Tomasson, L. Cotula coronopifolia – invasive or just another alien species? Master’s thesis, Department of ecology, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2020.
- Raulings, E.; Morris, K.; Thompson, R.; Nally, R.M. Do birds of a feather disperse plants together?: Plant dispersal by ducks. Freshw Biol 2011, 56, 1390–1402. [Google Scholar] [CrossRef]
- Lovas-Kiss, Á.; Sánchez, M.I.; Wilkinson, D.M.; Coughlan, N.E.; Alves, J.A.; Green, A.J. Shorebirds as important vectors for plant dispersal in Europe. Ecography 2019, 42, 956–967. [Google Scholar] [CrossRef]
- Navarro-Ramos, M.J.; Green, A.J.; Lovas-Kiss, A.; Roman, J.; Brides, K.; van Leeuwen, C.H.A. A predatory waterbird as a vector of plant seeds and aquatic invertebrates. Freshw Biol 2022, 67, 657–671. [Google Scholar] [CrossRef]
- van Leeuwen, C.H.A.; Soons, M.B.; Vandionant, L.G.V.T.I.; Green, A.J.; Bakker, E.S. Seed dispersal by waterbirds: a mechanistic understanding by simulating avian digestion. Ecography 2023, 2023, e06470. [Google Scholar] [CrossRef]
- Lovas-Kiss, Á.; Martín-Vélez, V.; Brides, K.; Wilkinson, D.M.; Griffin, L.R.; Green, A.J. Migratory geese allow plants to disperse to cooler latitudes across the ocean. J Biogeogr 2023, 50, 1602–1614. [Google Scholar] [CrossRef]
- Espinar, J.L.; García, L.V.; Figuerola, J.; Green, A.J.; Clemente, L. Helophyte germination in a Mediterranean salt marsh: Gut-passage by ducks changes seed response to salinity. J Veg Sci 2004, 15, 315–322. [Google Scholar] [CrossRef]
- Espinar, J.L.; Figuerola, J.; Green, A.J. Long term impacts of endozoochory and salinity on germination of wetland plants after entering simulated seed banks. Front. Plant Sci. 2023, 14, 1275622. [Google Scholar] [CrossRef]
- Castillo, J.M.; Curado, G.; Muñoz-Rodríguez, A.F.; Grewell, B.J. Germination syndrome divergence among pairs of sympatric sister species along an estuarine salinity gradient. Environ Exp Bot 2021, 181, 104274. [Google Scholar] [CrossRef]
- Traveset, A.; Robertson, A.W.; Rodríguez-Pérez, J. A review on the role of endozoochory on seed germination. In Seed Dispersal: Theory and Its Application in a Changing World; CABI Publising: Spain, 2007; pp. 78–103. [Google Scholar]
- Soltani, E.; Baskin, C.C.; Baskin, J.M.; Heshmati, S.; Mirfazeli, M.S. A meta-analysis of the effects of frugivory (endozoochory) on seed germination: role of seed size and kind of dormancy. Plant Ecol 2018, 219, 1283–1294. [Google Scholar] [CrossRef]
- Costa, J.C.; Neto, C.; Arsenio, P.; Capelo, J. Geographic variation among Iberian communities of the exotic halophyte Cotula coronopifolia. Alp Bot 2009, 119, 53–61. [Google Scholar] [CrossRef]
- Peralta-Sánchez, J.M.; Ansotegui, A.; Hortas, F.; Redón, S.; Martín-Vélez, V.; Green, A.J.; Navarro-Ramos, M.J.; Lovas-Kiss, A.; Sánchez, M.I. Seed Size, Not Dispersal Syndrome, Determines Potential for Spread of Ricefield Weeds by Gulls. Plants 2023, 12, 1470. [Google Scholar] [CrossRef] [PubMed]
- Lovas-Kiss, Á.; Navarro-Ramos, M.J.; Vincze, O.; Löki, V.; Urgyán, R.; Pallér-Kapusi, F.; van Leeuwen, C.H.A.; Green, A.J.; Lukács, B.A. Traits for transport: alien wetland plants gain an advantage during endozoochorous seed dispersal by waterfowl. Freshw Biol 2023, 68, 1703–1715. [Google Scholar] [CrossRef]
- Urgyán, R.; Lukács, B.A.; Fekete, R.; Molnár, V.A.; Nagy, A.; Vincze, O.; Green, A.J.; Lovas-Kiss, Á. Plants dispersed by a non-frugivorous migrant change throughout the annual cycle. Glob Ecol Biogeogr 2023, 32, 70–82. [Google Scholar] [CrossRef]
- Martín-Vélez, V.; Lovas-Kiss, Á.; Sánchez, M.I.; Green, A.J. Endozoochory of the same community of plants lacking fleshy fruits by storks and gulls. J Veg Sci 2021, 32. [Google Scholar] [CrossRef]
- Reynolds, C.; Cumming, G.S.; Vilà, M.; Green, A.J. Birds as key vectors for the dispersal of some alien species: Further thoughts. Divers Distrib 2017, 23, 577–580. [Google Scholar] [CrossRef]
- Wilkinson, D.M. Plants on the wing: waterbirds and plant dispersal in Britain. British Wildlife 2023, 35, 167–171. [Google Scholar]
- Noe, G.B.; Zedler, J.B. Differential effects of four abiotic factors on the germination of salt marsh annuals. Am J Bot 2000, 87, 1679–1692. [Google Scholar] [CrossRef]
- Goodman, A.M.; Ganf, G.G.; Dandy, G.C.; Maier, H.R.; Gibbs, M.S. The response of freshwater plants to salinity pulses. Aquat Bot 2010, 93, 59–67. [Google Scholar] [CrossRef]
- Lopez-Archilla, A.I.; Coleto, M.; Montes, C.; Penin, I.; Guerrero, M.C. Temporal variation of phytoplankton in two neighbouring Mediterranean shallow lakes in Doñana National Park (Spain). Limnetica 2012, 31, 289–304. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci Total Environ 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Gassó, N.; Thuiller, W.; Pino, J.; Vilà, M. Potential distribution range of invasive plant species in Spain. NeoBiota 2012, 12, 25–40. [Google Scholar] [CrossRef]
- Julve, P. Index botanique, écologique et chorologique de la flore de France. Baseflor 1998. http://perso.wanadoo.fr/philippe.julve/catminat.htm.
- Benedí, C.; Cotula, L. Flora Iberica; Benedí, C., Buira, A., Rico, E., Crespo, M.B., Quintanar, A., Aedo, C., Eds.; Real Jardín Botánico CSIC: Madrid, Spain, 2019; Volume XVI(III), pp. 275–279. [Google Scholar]
- Carbonell, J.A.; Céspedes, V.; Green, A.J. Is the spread of the alien water boatman Trichocorixa verticalis verticalis (Hemiptera, Corixidae) aided by zoochory and drought resistant eggs? Freshw Biol 2021, 66, 409–420. [Google Scholar] [CrossRef]
- Dawson, W.R.; Whittow, G.C. Regulation of body temperature. In Sturkie's Avian Physiology, 5th ed.; Whittow, G.C., Ed.; Academic Press: Honolulu, Hawaii, 2000; pp. 343–390. [Google Scholar] [CrossRef]
- Green, A.J.; Lovas-Kiss, Á.; Reynolds, C.; Sebastián-González, E.; Silva, G.G.; Van Leeuwen, C.H.A.; Wilkinson, D.M. Dispersal of aquatic and terrestrial organisms by waterbirds: A review of current knowledge and future priorities. Freshw Biol 2023, 68, 173–190. [Google Scholar] [CrossRef]
- Navarro-Ramos, M.J.; Green, A.J.; de Vries, R.; van Leeuwen, C.H.A. Float, fly, then sink: wetland plant seed buoyancy is lost after internal dispersal by waterbirds. Hydrobiologia 2024, in press. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).