1. Introduction
Carotenoids are common and diverse organic compounds [
1]. Some carotenoids are known to have essential functional roles in animals [
2], such as in the visual and immune systems, diapause-related processes, protection against oxidative stress, ornament-based signaling, etc. [
3,
4,
5,
6]. However, the specific carotenoid composition in most animals and the function and metabolism of most carotenoids are largely unknown. Biosynthesis of carotenoids largely occurs in photosynthetic plants, algae, and some bacteria, archaea, and fungi [
7], but is limited in Animalia to certain aphids, mites, and gall midges [
8]. Most animals acquire necessary carotenoids from their diet and use them as gained from the feed or modified [
9,
10].
Both diet and genetic background likely affect carotenoid composition in insects' bodies [
11,
12]. Holometabolous insects acquire most building materials of the adult body from the larval feed, during the larval stages. Hence, adult carotenoid body composition and related functions may largely reflect the larvae's available feed resources [
11,
12,
13]. However, knowledge of carotenoid pathways between larval feed and adult carotenoid composition and functionality is scant.
The House fly is a cosmopolitan pest insect that poses public health hazards [
14], but also a growing economic importance in the animal feed industry [
15]. The maggot develops in and feeds upon rotting organic substrates. The carotenoid composition of these substrates may differ substantially between different sources. [
16] report that adult House flies contained the carotenoids Lutein and Zeaxanthin, but not β-carotene. However, our knowledge of this important issue is lacking beyond the scope of the visual system.
We hypothesized that the carotenoid composition in the adult House fly’s body would reflect its composition in the larval feed (substrate). Hence, the carotenoid composition of adult flies is expected to vary according to differences in the carotenoid composition of the larval feed. Alternatively, if only specific carotenoids can be used for specific biological functions, then unsuitable carotenoids should be either modified into the specific suitable ones or excreted from the body. The carotenoid composition of adult flies is then expected to be homogenous, even if carotenoid composition differs among larval feeds.
2. Materials and Methods
To examine our hypothesis that the carotenoid composition of the adult fly will reflect the carotenoid composition in the maggot's substrate, we simultaneously reared four groups of House fly maggots, originating from the same laboratory colony, each group on a different substrate. Rearing substrates were cucumber, red tomato, yellow tomato, and control. The latter, contained bran and powdered rodent chaw, and lacked carotenoids. We placed, open petri dishes, 60 mm diameter, in a cage containing a colony of 5-6 days old House flies, for 24 hours, to allow oviposition in the dishes. Each petri dish was filled with one of the four substrates, two dishes of each substrate. We then transferred the substrate containing the eggs into a rearing cup (90 mm diameter×65 mm high), containing 300 ml of the same substrate, and placed the cups in a growing chamber (29±2°C, 70% humidity, 14:10 dark:light regime) for 10 days. We then sampled the aged substrates; 3 samples of 50 mg from each substrate type, for carotenoid composition analysis, and added a layer of dry coarse sawdust, to allow suitable environment for pupation. Pupa were then collected during the next five days and hatched in separate, empty containers. Substrate samples, and five hatched adult females, from each substrate type, were then analyzed using untargeted HPLC with photodiode array detector, as described in [
17], to quantify the carotenoid composition in each sample.
3. Results
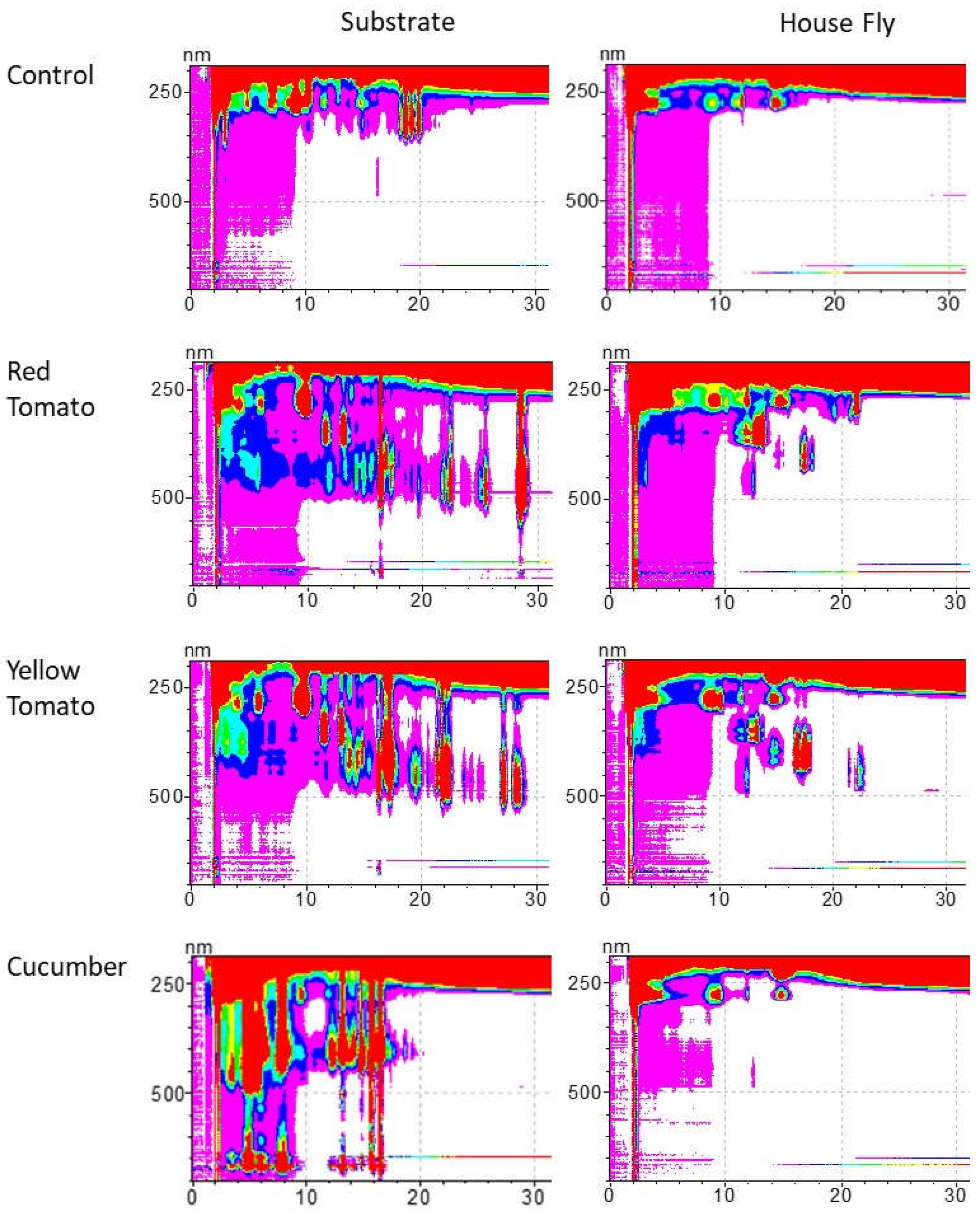
We found that the four different substrates differed substantially in their carotenoid composition (
Table 1). The carotenoid composition analysis of the respective adult House flies indicates that adult House flies, bred on different larval substrate types, differed in their carotenoid composition. However, carotenoid composition in the adult flies was not identical to the carotenoid composition in the substrate. Adult flies only contained carotenoids that were present in the substrate, but not all carotenoids present in the substrate were found in the adults. Moreover, Lycopene and Prolycopene, the commonest carotenoids in the red and yellow tomato substrates, respectively, were completely absent from the adult flies (
Table 1,
Figure 1). Specifically,
Control: As expected, the Control substrate did not contain carotenoids. Accordingly, we did not detect carotenoids in the emerging adult flies.
Cucumber: In the Cucumber substrate we detected only chlorophylls, but not carotenoids. Here too, we did not detect carotenoids in the emerging adult flies.
Red tomato: In the red tomato substrate, we detected several carotenoids: Phytoene, two isomers of Phytofluene, β-carotene, Lutein, asymmetric ζ-carotene, 3 isomers of ζ-carotene, and as expected, several isomers of Lycopene, which was the dominant carotenoid. Surprisingly, however, the adult House flies, emerging from this substrate, did not contain Lycopene at all. Phytoene, β-carotene and Lutein were also lacking in the adults. While so, adult flies did contain Phytofluene, asymmetric ζ-carotene and ζ-carotene, of similar isomers as in the substrate.
Yellow tomato: The yellow tomato substrate contained Phytoene, two isomers of Phytofluene, β-carotene, asymmetric ζ-carotene, 3 isomers of ζ-carotene and several isomers of Lycopene. Unlike the red tomato substrate, the primary carotenoids were Prolycopene and Neurosporene. Interestingly, the adult House flies, emerging from this substrate did not contain Prolycopene and Lycopene, but similarly to adult flies from the red tomato substrate, contained isomers of Phytofluene and ζ-carotene. In addition, adult flies also contained Neurosporene.
4. Discussion
We used HPLC to characterize the carotenoid composition in the body of adult House flies and in the respective larval feed. We found that carotenoid composition in adult House flies can be diverse and that it is related to the carotenoid composition in the larval feed. These findings are in support of our primary hypothesis and in agreement with previous findings, e.g., [
11] on silkworms and [
12] on dragonflies. However, unlike our hypothesis, carotenoid composition in the adult flies did not directly reflect carotenoid identity in the respective substrate feed of the larvae. Interestingly, Phytoene, β-carotene, Lutein, Prolycopene, and Lycopene were absent from adult flies that originated from substrates that contained fair amounts of these carotenoids. As far as we know, we describe here for the first time the presence of the carotenoids Phytofluene, ζ-carotene, asymmetric ζ-carotene, and Neurosporene in House flies. These findings strongly suggest that House flies, and likely also other insects, can metabolize or selectively absorb and accumulate carotenoids from their feed [
11].
In agreement with [
16], we did not find β-carotene in the adult flies. However, [
16] reports also Lutein and Zeaxanthin, which we did not find in our House flies, even though Lutein was present in the red tomato substrate. Indeed, Zeaxanthin was absent from all our substrates. [
16] did not report the substrate carotenoid content, hence we can only speculate that β-carotene may have been missing from the substrates, and hence from the flies.
The specific function of the carotenoids we detected in the House flies and their transition and metabolic pathways are largely unknown. Further work is required to unravel the role of these carotenoids in the biology and ecology of House flies and insects in general, the consequences of habitat variability in carotenoid composition, and the biochemical pathways of carotenoid absorption and metabolism. Interestingly, our findings indicate that the carotenoid composition of adult House flies, and possibly other insects, reflect substrate-specific carotenoid fingerprints. This relation could potentially be used to relate between free-ranging adult House flies and their natal habitat, possibly aiding in identifying and locating House fly infestation sources. The use of insects, including House fly larvae, is increasingly promoted as a replacement for other animal feed sources, especially for fish and poultry [
18,
19]. Considering our findings, the specific composition of House fly feed should be considered not only with respect to protein and fat content but also to other essential compounds, such as vitamins and carotenoids.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, Aviv Shaish, Ido Tsurim. and Li-Or Lahmi.; methodology, Aviv Shaish, Ido Tsurim, Ayelet Harari and Li-Or Lahmi.; formal analysis, Ido Tsurim, Aviv Shaish and Ayelet Harari.; investigation, Li-Or Lahmi, Ido Tsurim.; resources, Aviv Shaish and Ido Tsurim; writing—original draft preparation, Aviv Shaish and Ido Tsurim.; writing—review and editing, Aviv Shaish, Ido Tsurim, Ayelet Harari and Li-Or Lahmi..; visualization, Aviv Shaish and Ayelet Harari.; supervision – Ido Tsurim and Aviv Shaish; project administration, Li-Or Lahmi and Ido Tsurim. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Britton, G.; Liaanen-Jensen, S.; Pfander, H. Carotenoids. Handbook; 2004; ISBN 978-3-7643-6180-8.
- Vershinin, A. Biological Functions of Carotenoids - Diversity and Evolution. BioFactors 1999, 10, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, A.D.; Chittka, L. The Evolution of Color Vision in Insects. Annu Rev Entomol 2001, 46, 471–510. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M. Oxidative Stress in Marine Environments: Biochemistry and Physiological Ecology. Annual review of physiology 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Svensson, P.A.; Wong, B.B.M. Carotenoid-Based Signals in Behavioural Ecology: A Review. Behaviour 2011, 148, 131–189. [Google Scholar] [CrossRef]
- Heath, J.; Cipollini, D.; Stireman, J. The Role of Carotenoids and Their Derivatives in Mediating Interactions between Insects and Their Environment. Arthropod-Plant Interactions 2013, 7. [Google Scholar] [CrossRef]
- Cazzonelli, C.I. Carotenoids in Nature: Insights from Plants and Beyond. Funct Plant Biol 2011, 38, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Takemura, M.; Maoka, T. Carotenoid Biosynthesis in Animals: Case of Arthropods. Adv Exp Med Biol 2021, 1261, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Pfander, H.; Liaaen-Jensen, S. Carotenoids. Vol. 3, Biosynthesis and Metabolism; Birkhäuser Verlag: Basel [etc.], 1998; ISBN 978-0-8176-5829-8. [Google Scholar]
- Maoka, T. Carotenoids in Marine Animals. Mar Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Chieco, C.; Morrone, L.; Bertazza, G.; Cappellozza, S.; Saviane, A.; Gai, F.; Di Virgilio, N.; Rossi, F. The Effect of Strain and Rearing Medium on the Chemical Composition, Fatty Acid Profile and Carotenoid Content in Silkworm (Bombyx Mori) Pupae. Animals 2019, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Kawase, N.; Ueda, T.; Nishida, R. Carotenoids of Dragonflies, from the Perspective of Comparative Biochemical and Chemical Ecological Studies. Biochemical Systematics and Ecology 2020, 89, 104001. [Google Scholar] [CrossRef]
- Goldsmith, T.H.; Barker, R.J.; Cohen, C.F. Sensitivity of Visual Receptors of Carotenoid-Depleted Flies: A Vitamin A Deficiency in an Invertebrate. Science 1964, 146, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Nayduch, D.; Neupane, S.; Pickens, V.; Purvis, T.; Olds, C. House Flies Are Underappreciated Yet Important Reservoirs and Vectors of Microbial Threats to Animal and Human Health. Microorganisms 2023, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Gadzama, I.U.; Amodu, J.T. House Fly (Musca Domestica) Larvae as Protein Source for Livestock Production - a Review. Journal of Animal Production Research 2018, 30, 91–96. [Google Scholar]
- Finke, M.D. Complete Nutrient Content of Four Species of Feeder Insects. Zoo Biol 2013, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Coster, A.C.F.; Jenkins, A.; Xu, A.; Greenfield, J.R.; Harats, D.; Shaish, A.; Samocha-Bonet, D. Obesity and Insulin Resistance Are Inversely Associated with Serum and Adipose Tissue Carotenoid Concentrations in Adults. J Nutr 2020, 150, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the Use of Insects in the Diet of Farmed Fish: Past and Future. Animal Feed Science and Technology 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Kone, N.; Sylla, M.; Nacambo, S.; Kenis, M. Production of House Fly Larvae for Animal Feed through Natural Oviposition. Journal of Insects as Food and Feed 2017, 3, 1–10. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).