1. Introduction
Cardiovascular disease (CVD) is a cause of major mortality worldwide, characterized by a reduction of blood supply to the myocardium, commonly due to a narrowing or blockage of coronary arteries, causing a mismatch between myocardial oxygen supply and demand [
1,
2]. Chest pain, dyspnea, dizziness, and nausea are common symptoms that patients with a coronary artery disease (CAD) may present. Commonly, the narrowing or blockage of the coronary arteries is due to atherosclerosis, which is also responsible for CVD-related death [
3]. Atherosclerotic coronary plaques destabilization and progression are responsible for both acute and chronic coronary syndromes, as well as sudden coronary death [
4,
5].
The narrowing and blockage of coronary arteries can be diagnosed and treated with percutaneous coronary intervention (PCI) [
6,
7]. During this procedure, coronary angiography (CAG) remains the most widely used guidance modality for making decisions about the intervention of damaged blood vessels [
2]. However, there are some limitations including: projection of two dimensions for lumen artery determination, as well as limited ability to assess blood vessel wall, plaque extension and characteristics, and the degree of atherosclerosis [
8,
9].
To overcome these limitations, modern technologies have been developed, such as intravascular ultrasound (IVUS) [
10]. The large-scale prospective study for the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES), demonstrated that IVUS guidance compared to angiography guidance was associated with a reduced 2-year rates of major adverse cardiac event, including restenosis and myocardial infarction [
11]. Furthermore, a recent meta-analysis reported that in comparison with CAG, IVUS significantly reduced the development of major adverse cardiovascular events (MACE) as well as target vessel/lesion revascularization (TVR/TLR) [
10]. Despite its superiority, it´s use for guidance remains low, as an estimate from the US (United States) Medicare cohort between 2009 and 2017 described its use in up to 5.6% of PCI procedures [
12]. Which might be the result of low reimbursement for IVUS use compared to angiography for PCI guidance. However, the improvement in clinical outcomes and the prevention of complications should be emphasized.
During the assessment of affected blood vessels, the reference diameter is an important evaluated parameter for intervention during percutaneous treatment, which is obtained from an average of proximal and distal diameters [
13]. A small reference diameter may indicate a true small coronary artery, a large plaque burden, or the presence of diffuse disease [
14].
Small vessel coronary artery disease (SvCAD) is a significant risk factor for MACE in PCI; Currently there is no standardized vascular diameter to define small vessel disease, different thresholds of maximum lumen size have been described, that range from 2.25 to 3.0 mm (about 0.12 in). This lack of consensus causes heterogeneity both in the results of clinical studies and consequently discrepancies in the appropriate therapeutic approach [
15]. SvCAD is often diffuse, and revascularization should only be performed in patients with confirmed ischemia and in hemodynamically significant lesions based on functional assessment [
16].
Among the options for revascularization is contemporary second-generation Drug-eluting stents (DESs), which has been associated with superior performance in patients with small vessel CAD with the reduction in both lumen loss and (clinical) restenosis [
17]. Similarly, drug-coated balloons (DCB) provide a fast and high-dose delivery of antiproliferative drugs to the vessel wall and carries several anticipated benefits over DESs, such as the lack of a permanent scaffold and the need for only a short prescription of dual antiplatelet therapy [
17].
The primary aim of this study was to investigate the change in diameter measurement of IVUS-guided PCI as compared to angiographic measurements in patients with SvCAD. A secondary aim was to evaluate the presence of associations between clinical comorbidities with major variation in the estimation of the small blood vessel diameter.
2. Materials and Methods
This was a cross-sectional study that included patients who underwent PCI for CAD, from Jan 2021 – Dec 2022.
Study population.
Registries of patients were included from those who attended the Hemodynamic Clinic in a tertiary hospital “Hospital Regional Dr Valentín Gómez Farías del Instituto de Seguridad y Servicios Sociales para los Trabajadores del Estado (ISSSTE)” in Zapopan, Jalisco. To be included in the study patients had to fulfill inclusion criteria: age >18, any gender, CAD with affection of at least one small blood vessel, defined as a reference vessel diameter of <2.75 mm by angiographic PCI guidance.
Patients with incomplete medical chart information, severe calcified and ostial lesions or those who required revascularization surgery, valvular exchange, or aortic surgery, were excluded from the study. The institutional review board revised and approved the protocol, exemption from informed consent was granted given the retrospective design of the study.
Study Outcomes
Coronary angiography (CAG) was performed using a standard percutaneous approach through the femoral artery, unless this artery was unavailable, following the intracoronary administration of 100 to 200 g of nitroglycerin (NTG). A reference vessel diameter (RVD) of <2.75 mm in the targeted lesion was defined as a small coronary vessel.
The minimum lumen diameter (MLD), and lesion length were also recorded. The diameter percentage of stenosis was calculated as the ratio between the MLD and the RVD.
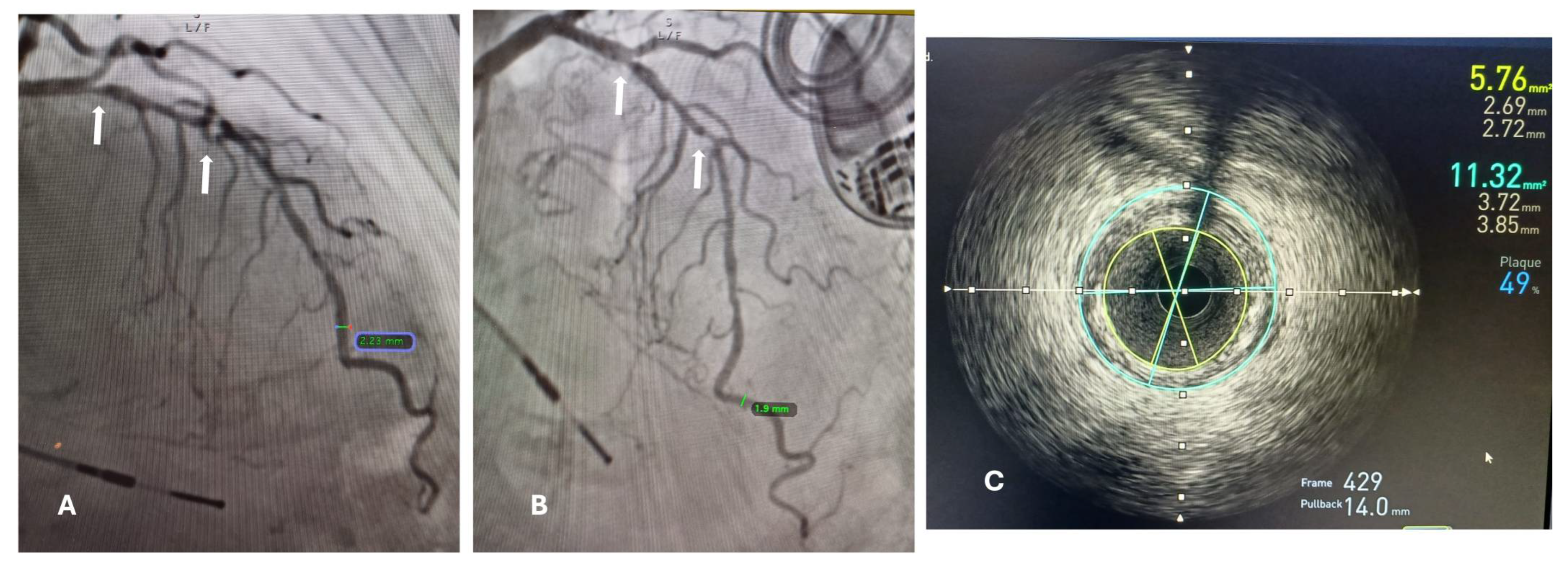
Intravascular ultrasound (IVUS) was performed immediately after the CAG in the targeted small blood vessel previously identified; by a 40 MHz IVUS coronary imaging catheter. The catheter was advanced distally in the target vessel as far as possible and then automatically pulled back, image acquisition and posterior analysis was performed using computerized planimetry for every 1mm of axial length. Lumen and external elastic membrane cross-sectional areas were measured. The lesion site was the image slice with the smallest cross sectional lumen area. The proximal and distal reference segments were the most normal-looking segments within 5 mm proximal and distal to the lesion, following the criteria of the American College of Cardiology Clinical Expert Consensus Document on IVUS [
14].
Coronary angiography and IVUS analyses were performed by two independent observers, and decisions for the selection of the stent or balloon were made in consensus. A third, physician was used in the case of differing opinions for treatment.
Following PCIs, all patients were treated with clopidogrel 75 mg/day for 12 months and aspirin 100 mg/day permanently.
Other medications were prescribed or adjusted according to the previous conditions and comorbidities.
Statistical Analysis
Categorical variables are presented as frequencies and percentages, continuous variables are expressed as mean + standard deviation (SD). Differences in diameter of vessels and minimal lumen diameter were analyzed by Man Whitney's U test. Correlation between measurements by angiography and IVUS were analyzed by Spearman test. Then, patients were grouped according to the need for blood vessel reclassification and analyzed with Chi square test, and finally to address risk factors for blood vessel reclassification we performed a multivariable logistic regression. A probability value < 0.05 was considered significant. STATA 17 was used for calculations.
3. Results
Between January to September of the year 2022, a total of 48 patients with CAD with affection of at least one small coronary blood vessel (<2.75 mm) were included in our study. The mean + SD age was 69.1 + 11.9 years and 34% were masculine gender. Clinical data from patients was analyzed and multiple comorbidities were reported, some of them even with previous acute myocardial infarctions. This information is available in
Table 1.
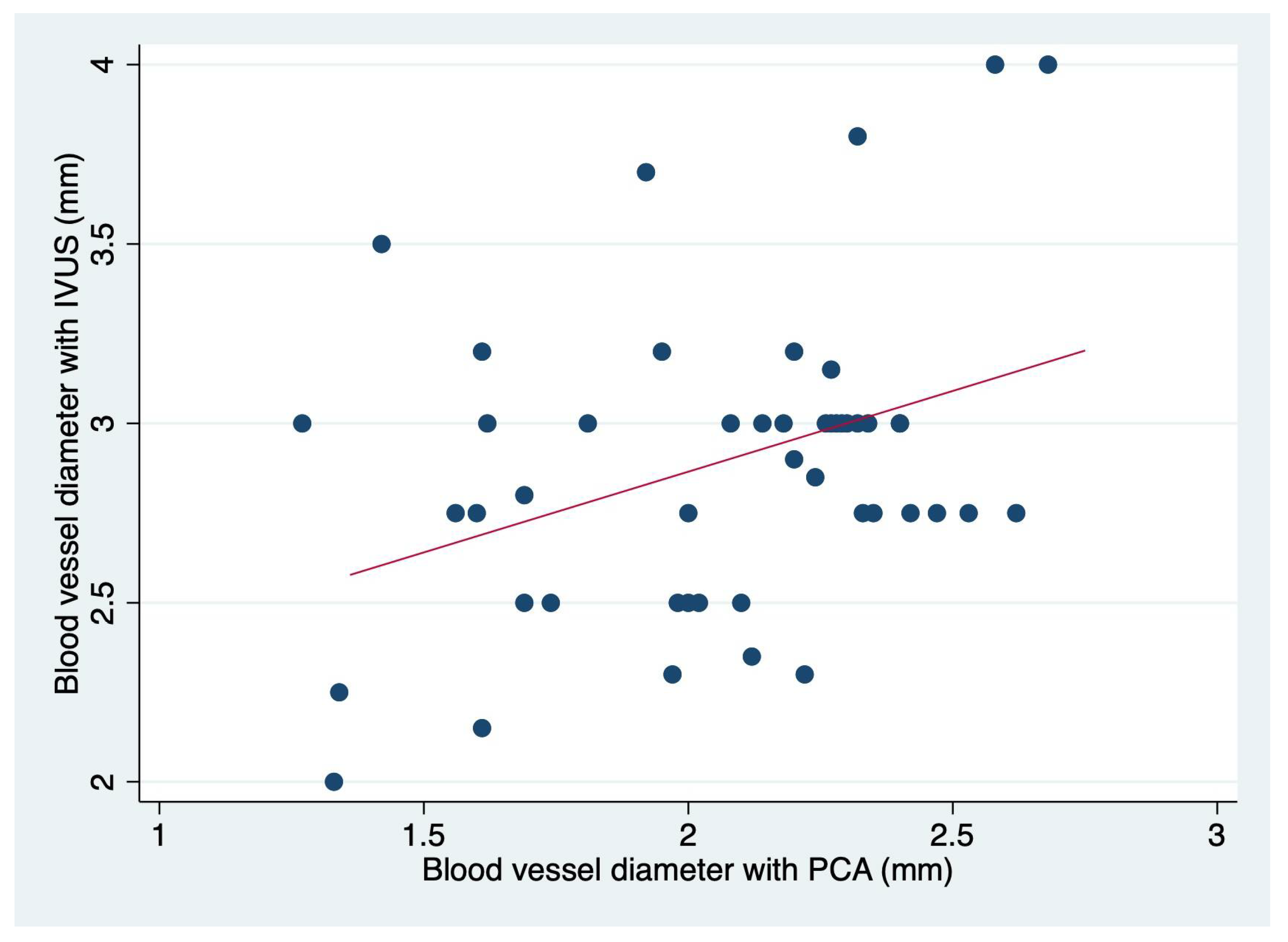
The minimal lumen diameter of affected small blood vessels was measured with PCA and IVUs (intravascular ultrasound), and we searched for a correlation among these measurements (
Figure 1).
We found a small but significant correlation (r= 0.1242 p=0.014) depicted in
Figure 2. Importantly, the correlation is more significant in blood vessels that have a minimal lumen diameter > 2 mm (about 0.08 in) (
Table 2).
The reported mean and 95% CI for the minimal lumen diameter with PCA was 2.1 (1.9-2.2) and for IVUS 2.8 (2.8-3.0) obtaining a difference of 0.8 (0.9-0.7) p<0.0001, with an infra estimation in the real minimal lumen diameter by PCA. Out of the total sample, 37 patients’ affected blood vessels (77%) were reclassified. 21 patients (44%) affected blood vessels were reclassified as median size (2.75-3.00 mm) and 15 patients (31%) affected blood vessels, were reclassified as great caliber blood vessels (3.00 mm). Specially this last group had the biggest change in terms of the treatment. Either by the decision to use a different instrument or for the selection of the proper stent.
After PCIs there was no coronary perforation, serious aortic dissection, or acute target blood vessel occlusion in any of the analyzed cases. A stent was collocated in 46 cases, and the use of a medicated balloon was done in two cases. The final selection of stent diameter and high-pressure balloons used during post-dilation or medicated balloons was made according to vessel diameter obtained by IVUS. The stent (mean SD) diameter was of 2.7 + 0.5 able 3 enlist the characteristics of treatment for the population.
After the observation of a minimal correlation, blood vessels were reclassified according to IVUS minimal lumen diameter. Afterwards, we searched for variables that could be associated with a change in the classification of the affected vessel, based on the measurement of the minimal lumen diameter by IVUS.
Table 2 represents the frequency of variables previously attributed to restenosis or small vessel coronary artery disease. Variables were analyzed separately, and the only variable with a significant difference in the distribution associated with a reclassification in the affected blood vessel was type 2 diabetes.
We then performed a multivariate analysis, to test a model with a combination of variables to better explain the reclassification, or if the strength of association with T2D (Type 2 Diabetes) could be changed.
Table 3 depicts the result of this multivariate analysis.
4. Discussion
We confirmed that PCIs for patients with affection of a small blood vessel (<2.75 mm) should be guided by IVUs mostly in the presence of diabetes, since other previously attributed risk factors for PCI were not significantly associated with the reclassification of blood vessels. This observation was found in two previous studies [
18,
19]. However, these studies only included patients with T2D, and other risk factors were not evaluated.
The superiority of IVUS compared to angiography has been well documented in the past, after the conduction of both observational and randomized controlled trials, where IVUS has been associated with better clinical outcomes including decreased incidence of target-vessel failure after 12 months, and mitigation of target-lesion revascularization and stent thrombosis [
20,
21].
Despite the clinical benefits associated to the use of intravascular ultrasound (IVUS) guidance for PCI [
13], still most patients with coronary artery disease undergo guidance by percutaneous angiography in the real-world setting, majorly due to a low reimbursement of IVUS use compared to PCI [
12]. Given this reluctance in the guidance for PCI by IVUS an important consideration should be made for diabetic patients, irrespective of the type of plaque or the presence of additional comorbid or clinical characteristics.
The association between T2D and coronary artery disease is non-debatable and has been widely demonstrated over the last two decades, importantly in population-based studies, where the 7-year incidence of first myocardial infarction or death was 20% for diabetics, but only 3.5% for non-diabetics [
22]. However, this association still needs clarification on the physio pathological pathways, and importantly those associated to therapeutic decisions in the CAD scenario.
Previous studies in small arteries and arterioles of diabetic subjects have demonstrated that morphological changes are preceded by vasomotor dysfunction, which is the result of the affection of both smooth muscle and endothelium mediated regulatory mechanism that include an abnormal nitric oxide (NO) production [
23].
When analyzing the endothelium damage related to diabetes, it has been described that hyperglycemia suppresses flow-mediated endothelial dependent vasodilation, which has been demonstrated in both diabetics and healthy subjects with induced hyperglycemia [
24].
Furthermore, chronic hyperglycemia induces, and inflammatory cascade mainly characterized by the activation of several pathways including diacylglycerol (DAG)-protein kinase C (PCK), which later can be associated to an increase in the oxidative stress associated with endothelial dysfunction [
25], and cumulative long-term changes in the structure and function of macromolecules through formation of advanced glycation end products (AGEs) [
26].
Even more, endothelial cells express insulin receptors, which can trigger NO-dependent vasodilation by increasing its production as well as the production of endothelium-derived relaxing factors (PGI2) [
27].
Endothelial dysfunction in T2D patients seems to be multifactorial, additional molecules described in association with this phenomenon includes the inflammatory cytokines tumor necrosis factor-alpha (TNF-a) and IL-6 [
28,
29], the peroxisome proliferator-activated receptor-g (PPAR-g) [
30], an increased generation of oxygen free radicals by NAD(P)H-dependent oxidases, xanthine oxidase, lipoxygenase, mitochondrial oxidase and NOS, as well as the diacylglycerol-PKC (DAG-PKC) pathway [
31].
Furthermore, T2D has been associated with decreased stiffness of coronary resistance micro vessels (CRM), as a result of reduced stiffness in vascular smooth muscle cells (VSMC), determined by a computational model, with the analysis of male T2DM homozygous db/db mice and heterozygous non-diabetic control Db/db mice, where vascular smooth muscle cells (VSMC) and coronary resistance micro vessels (CRM) were collected, cultured and studied by atomic force microscopy and state-of-the-art traction force microscopy which showed that diabetic coronary VSMCs had reduced stiffness, decreased adhesion to fibronectin and increased tensile force properties which together are mechanistically revealing the underlying causes of the altered mechanical and contractile properties of intact diabetic CRM [
32].
The mechanisms involved in the etiology of microvascular complications associated with T2D have been described in isolation, and it rather seems that a combination of several of the previous mechanisms described is responsible for the endothelial dysfunction, the common final pathway seems to be an increased in the oxidative stress with impaired vasomotion.
Another important previously attributable risk factor for small blood vessel reclassification is active smoking, since in 0.3-5.3% of cases, have been associated with coronary ectasia, which consists of a pathological remodeling characterized by a diffuse dilation of the coronary artery diameter of > 1.5 times [
33]. However, in our study, active smoking was not associated with vascular reclassification.
Hypertension has been associated with vascular reclassification as well, majorly due to a phenomenon termed early vascular aging (EVA), which has been described as an arterial stiffening in the middle layer of the large elastic arteries, a process that can be measured by pulse wave velocity [
34]; factors that can trigger such stiffness include the hypertension per se, but also chronic inflammatory conditions such as rheumatoid arthritis or inflammatory bowel disease. Although, hypertension would be mostly associated with rigidity rather than endothelium elasticity. In our study hypertension was not associated with an increased risk for reclassification, however 64.6% of our studied population had hypertension and the reason why we did not find an association could be due to high numbers of patients with hypertension in both groups. A study with more patients could clarify a real association or an additive behavior along with T2D.
The exact dimension of the lumen diameter of the affected vessel is a key factor to determine the size of the stent and balloons utilized during the procedure, this ensures appropriate treatment of small-vessel coronary lesions, as well as, a better evaluation of the atherosclerotic plaque evaluation, it is for this main reason that IVUs guidance has been associated with positive clinical outcomes, with declined incidence in major adverse cardiac event (MACE) and in-stent restenosis (ISR) in T2D patients with CAD [
19].
Importantly, we recognized that when angiography is used alone for the guidance of PCI, the residual clearance between the mesh stent and the intima, after the plaque rupture results in the expansion of the balloon, and this space can be filled with the contrast agent, resulting in a false impression of completion, which is avoided with IVUs [
35]. Even more, the failure of coverage of the full length affected segment with the stent, has been associated with an increased risk of subacute stent thrombosis and late restenosis [
36].
5. Conclusions
PCI treatment for small coronary vessels remains debatable, based on the absence of an agreement to define a true small blood vessel, the fact that small blood coronary vessels are often considered not treatable with a stent because of no clinically relevant outcomes, high restenosis rate, and difficulties associated to implement a stent in a small vessel. The IVUS-guidance for PCI seems to be of upmost importance in T2D patients and should be considered whenever available. his section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
Author Contributions
Conceptualization, SAZ, JBG and MGZC; methodology, SAZ, JJBO and MGZC; formal analysis, MGZC.; investigation, SAZ and EZD; resources, JJBO; data curation, SAZ and EZD; writing—original draft preparation, SAZ, EZD, VAV, AASV, JJBO, MGZC; writing—review and editing, MGZC; supervision, MGZC and JJBO. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Upon acceptance, APC will be paid by “Fondo Semilla” of Universidad Autonoma de Guadalajara.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board from Hospital Regional Valentín Gómez Farias, ISSSTE in Zapopan Jalisco for studies involving humans.
Informed Consent Statement
“Not applicable.”
Data Availability Statement
All relevant data is included in the manuscript.
Conflicts of Interest
“The authors declare no conflicts of interest.
References
- Shahjehan, R. D., and B. S. Bhutta. "Coronary Artery Disease." In Statpearls. Treasure Island (FL) with ineligible companies. Disclosure: Beenish Bhutta declares no relevant financial relationships with ineligible companies., 2024.
- Wolk, M. J., S. R. Bailey, J. U. Doherty, P. S. Douglas, R. C. Hendel, C. M. Kramer, J. K. Min, M. R. Patel, L. Rosenbaum, L. J. Shaw, R. F. Stainback, J. M. Allen, and Force American College of Cardiology Foundation Appropriate Use Criteria Task. "Accf/Aha/Ase/Asnc/Hfsa/Hrs/Scai/Scct/Scmr/Sts 2013 Multimodality Appropriate Use Criteria for the Detection and Risk Assessment of Stable Ischemic Heart Disease: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons." J Am Coll Cardiol 63, no. 4 (2014): 380-406.
- Frak, W., A. Wojtasinska, W. Lisinska, E. Mlynarska, B. Franczyk, and J. Rysz. "Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease." Biomedicines 10, no. 8 (2022).
- Collet, J. P., H. Thiele, E. Barbato, O. Barthelemy, J. Bauersachs, D. L. Bhatt, P. Dendale, M. Dorobantu, T. Edvardsen, T. Folliguet, C. P. Gale, M. Gilard, A. Jobs, P. Juni, E. Lambrinou, B. S. Lewis, J. Mehilli, E. Meliga, B. Merkely, C. Mueller, M. Roffi, F. H. Rutten, D. Sibbing, G. C. M. Siontis, and E. S. C. Scientific Document Group. "2020 Esc Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent St-Segment Elevation." Eur Heart J 42, no. 14 (2021): 1289-367.
- Virmani, R., F. D. Kolodgie, A. P. Burke, A. Farb, and S. M. Schwartz. "Lessons from Sudden Coronary Death: A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions." Arterioscler Thromb Vasc Biol 20, no. 5 (2000): 1262-75.
- Orbach, D. B., B. K. Pramanik, J. Lee, T. S. Maldonado, T. Riles, and R. I. Grossman. "Carotid Artery Stent Implantation: Evaluation with Multi-Detector Row Ct Angiography and Virtual Angioscopy--Initial Experience." Radiology 238, no. 1 (2006): 309-20. [CrossRef]
- Malik, P. "Grossman’s Cardiac Catheterization, Angiography, and Intervention, 7th Edn (2005)." Can J Cardiol 23, no. 7 (2007): 602.
- Topol, E. J., and S. E. Nissen. "Our Preoccupation with Coronary Luminology. The Dissociation between Clinical and Angiographic Findings in Ischemic Heart Disease." Circulation 92, no. 8 (1995): 2333-42.
- Kip, K. E., K. Hollabaugh, O. C. Marroquin, and D. O. Williams. "The Problem with Composite End Points in Cardiovascular Studies: The Story of Major Adverse Cardiac Events and Percutaneous Coronary Intervention." J Am Coll Cardiol 51, no. 7 (2008): 701-7. [CrossRef]
- Hu, M., J. Tan, and Y. Yang. "Comparison of Six Different Percutaneous Coronary Intervention Guidance Modalities." J Cardiovasc Dev Dis 9, no. 10 (2022). [CrossRef]
- Maehara, A., G. S. Mintz, B. Witzenbichler, G. Weisz, F. J. Neumann, M. J. Rinaldi, D. C. Metzger, T. D. Henry, D. A. Cox, P. L. Duffy, B. R. Brodie, T. D. Stuckey, E. L. Mazzaferri, Jr., T. McAndrew, P. Généreux, R. Mehran, A. J. Kirtane, and G. W. Stone. "Relationship between Intravascular Ultrasound Guidance and Clinical Outcomes after Drug-Eluting Stents." Circ Cardiovasc Interv 11, no. 11 (2018): e006243. [CrossRef]
- Mentias, A., A. Aminian, D. Youssef, A. Pandey, V. Menon, L. Cho, S. E. Nissen, and M. Y. Desai. "Long-Term Cardiovascular Outcomes after Bariatric Surgery in the Medicare Population." J Am Coll Cardiol 79, no. 15 (2022): 1429-37. [CrossRef]
- Lee, S. Y., J. J. Zhang, G. S. Mintz, S. J. Hong, C. M. Ahn, J. S. Kim, B. K. Kim, Y. G. Ko, D. Choi, Y. Jang, J. Kan, T. Pan, X. Gao, Z. Ge, S. L. Chen, and M. K. Hong. "Procedural Characteristics of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention and Their Clinical Implications." J Am Heart Assoc 11, no. 14 (2022): e025258. [CrossRef]
- Mintz, G. S., J. J. Popma, A. D. Pichard, K. M. Kent, L. F. Salter, Y. C. Chuang, J. Griffin, and M. B. Leon. "Intravascular Ultrasound Predictors of Restenosis after Percutaneous Transcatheter Coronary Revascularization." J Am Coll Cardiol 27, no. 7 (1996): 1678-87. [CrossRef]
- Murphy, G., A. Naughton, R. Durand, E. Heron, C. McCaughey, R. T. Murphy, and I. Pearson. "Long-Term Outcomes for Drug-Eluting Balloons Versus Drug-Eluting Stents in the Treatment of Small Vessel Coronary Artery Disease: A Systematic Review and Meta-Analysis." Interv Cardiol 18 (2023): e14. [CrossRef]
- Wybraniec, M. T., P. Banka, T. Bochenek, T. Roleder, and K. Mizia-Stec. "Small Vessel Coronary Artery Disease: How Small Can We Go with Myocardial Revascularization?" Cardiol J 28, no. 5 (2021): 767-78.
- Sanz-Sánchez, Jorge, Mauro Chiarito, Gauravpal S. Gill, Liefke C. van der Heijden, Yigal Piña, Bernardo Cortese, Fernando Alfonso, Clemens von Birgelen, Jose Luis Diez Gil, Ron Waksman, and Hector M. Garcia-Garcia. "Small Vessel Coronary Artery Disease: Rationale for Standardized Definition and Critical Appraisal of the Literature." Journal of the Society for Cardiovascular Angiography & Interventions 1, no. 5 (2022): 100403. [CrossRef]
- Jensen, L. O., P. Thayssen, G. S. Mintz, R. Egede, M. Maeng, A. Junker, A. Galloee, E. H. Christiansen, K. E. Pedersen, H. S. Hansen, and K. N. Hansen. "Comparison of Intravascular Ultrasound and Angiographic Assessment of Coronary Reference Segment Size in Patients with Type 2 Diabetes Mellitus." Am J Cardiol 101, no. 5 (2008): 590-5. [CrossRef]
- Li, L., L. Wang, C. J. Zhai, Y. R. Mou, J. H. Wang, and L. Q. Cui. "Clinical Utility of Intravascular Ultrasonography-Guided Therapy in a Small-Vessel Coronary Lesion Associated with Type 2 Diabetes Mellitus." Anatol J Cardiol 22, no. 2 (2019): 68-76.
- Zhang, J., X. Gao, J. Kan, Z. Ge, L. Han, S. Lu, N. Tian, S. Lin, Q. Lu, X. Wu, Q. Li, Z. Liu, Y. Chen, X. Qian, J. Wang, D. Chai, C. Chen, X. Li, B. D. Gogas, T. Pan, S. Shan, F. Ye, and S. L. Chen. "Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The Ultimate Trial." J Am Coll Cardiol 72, no. 24 (2018): 3126-37. [CrossRef]
- Gao, X. F., J. Kan, Y. J. Zhang, J. J. Zhang, N. L. Tian, F. Ye, Z. Ge, P. X. Xiao, F. Chen, G. Mintz, and S. L. Chen. "Comparison of One-Year Clinical Outcomes between Intravascular Ultrasound-Guided Versus Angiography-Guided Implantation of Drug-Eluting Stents for Left Main Lesions: A Single-Center Analysis of a 1,016-Patient Cohort." Patient Prefer Adherence 8 (2014): 1299-309. [CrossRef]
- Haffner, S. M., S. Lehto, T. Ronnemaa, K. Pyorala, and M. Laakso. "Mortality from Coronary Heart Disease in Subjects with Type 2 Diabetes and in Nondiabetic Subjects with and without Prior Myocardial Infarction." N Engl J Med 339, no. 4 (1998): 229-34. [CrossRef]
- De Vriese, A. S., T. J. Verbeuren, J. Van de Voorde, N. H. Lameire, and P. M. Vanhoutte. "Endothelial Dysfunction in Diabetes." Br J Pharmacol 130, no. 5 (2000): 963-74.
- Title, L. M., P. M. Cummings, K. Giddens, and B. A. Nassar. "Oral Glucose Loading Acutely Attenuates Endothelium-Dependent Vasodilation in Healthy Adults without Diabetes: An Effect Prevented by Vitamins C and E." J Am Coll Cardiol 36, no. 7 (2000): 2185-91. [CrossRef]
- Schalkwijk, C. G., and C. D. Stehouwer. "Vascular Complications in Diabetes Mellitus: The Role of Endothelial Dysfunction." Clin Sci (Lond) 109, no. 2 (2005): 143-59. [CrossRef]
- Weidig, P., D. McMaster, and U. Bayraktutan. "High Glucose Mediates Pro-Oxidant and Antioxidant Enzyme Activities in Coronary Endothelial Cells." Diabetes Obes Metab 6, no. 6 (2004): 432-41. [CrossRef]
- Baron, A. D. "Hemodynamic Actions of Insulin." Am J Physiol 267, no. 2 Pt 1 (1994): E187-202. [CrossRef]
- Kim, F., B. Gallis, and M. A. Corson. "Tnf-Alpha Inhibits Flow and Insulin Signaling Leading to No Production in Aortic Endothelial Cells." Am J Physiol Cell Physiol 280, no. 5 (2001): C1057-65.
- Omori, K., K. Naruishi, F. Nishimura, H. Yamada-Naruishi, and S. Takashiba. "High Glucose Enhances Interleukin-6-Induced Vascular Endothelial Growth Factor 165 Expression Via Activation of Gp130-Mediated P44/42 Mapk-Ccaat/Enhancer Binding Protein Signaling in Gingival Fibroblasts." J Biol Chem 279, no. 8 (2004): 6643-9.
- Quinones, M. J., M. Hernandez-Pampaloni, H. Schelbert, I. Bulnes-Enriquez, X. Jimenez, G. Hernandez, R. De La Rosa, Y. Chon, H. Yang, S. B. Nicholas, T. Modilevsky, K. Yu, K. Van Herle, L. W. Castellani, R. Elashoff, and W. A. Hsueh. "Coronary Vasomotor Abnormalities in Insulin-Resistant Individuals." Ann Intern Med 140, no. 9 (2004): 700-8. [CrossRef]
- Picchi, A., S. Capobianco, T. Qiu, M. Focardi, X. Zou, J. M. Cao, and C. Zhang. "Coronary Microvascular Dysfunction in Diabetes Mellitus: A Review." World J Cardiol 2, no. 11 (2010): 377-90.
- McCallinhart, P. E., Y. Cho, Z. Sun, S. Ghadiali, G. A. Meininger, and A. J. Trask. "Reduced Stiffness and Augmented Traction Force in Type 2 Diabetic Coronary Microvascular Smooth Muscle." Am J Physiol Heart Circ Physiol 318, no. 6 (2020): H1410-H19. [CrossRef]
- Vieyra-Herrera, G., M. G. Garcia-Navarrete, C. A. Damazo-Escobedo, H. Gonzalez-Pacheco, L. L. Rodriguez-Chavez, and C. Silva-Ruz. "Outlook of Coronary Ectasia at the National Institute of Cardiology Ignacio Chavez: A Cross-Sectional Study." Arch Cardiol Mex 93, no. 2 (2023): 197-202. [CrossRef]
- Nilsson, P. M. "Early Vascular Aging in Hypertension." Front Cardiovasc Med 7 (2020): 6. [CrossRef]
- Ahn, J. M., S. J. Kang, S. H. Yoon, H. W. Park, S. M. Kang, J. Y. Lee, S. W. Lee, Y. H. Kim, C. W. Lee, S. W. Park, G. S. Mintz, and S. J. Park. "Meta-Analysis of Outcomes after Intravascular Ultrasound-Guided Versus Angiography-Guided Drug-Eluting Stent Implantation in 26,503 Patients Enrolled in Three Randomized Trials and 14 Observational Studies." Am J Cardiol 113, no. 8 (2014): 1338-47.
- Costa, M. A., D. J. Angiolillo, M. Tannenbaum, M. Driesman, A. Chu, J. Patterson, W. Kuehl, J. Battaglia, S. Dabbons, F. Shamoon, B. Flieshman, A. Niederman, T. A. Bass, and Stllr Investigators. "Impact of Stent Deployment Procedural Factors on Long-Term Effectiveness and Safety of Sirolimus-Eluting Stents (Final Results of the Multicenter Prospective Stllr Trial)." Am J Cardiol 101, no. 12 (2008): 1704-11. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).