1. Introduction
Cancer is a disease of uncontrolled cell growth or proliferation. Cancer is caused by tobacco, unhealthy diet, inherited genetic mutations, hormones and immune conditions. Colorectal cancer is the third leading cause of death in both men and women [
1,
2]. Apoptosis, or programmed cell death, is essential for cell mechanism and plays an important role in cancer treatment.
Apoptosis is induced via two main signaling pathways, the extrinsic and intrinsic pathways [
3]. The extrinsic pathway, which is called the death receptor pathway, is triggered by ligation of death receptors that are located on the cell membrane. Activation of death receptors induces caspase-3, 6 and 7 that are activated by caspase-8 and 10 [
4,
5]. The intrinsic pathway, which is called the mitochondrial pathway, is regulated by the Bcl-2 family and mediated through the release of proteins, such as cytochrome c, because of the process of mitochondrial outer membrane permeability (MOMP) [
6,
7].
The tumor suppressor, p53, contributes to the transcriptional activation of a number of genes, including the pro-apoptotic protein Bax [
8]. Bax and Bak undergo extensive conformational changes to form homo- and hetero-oligomeric pores, which leads to the release of cytochrome c from mitochondria into cytosol. The release of cytochrome c enhances caspase activation and induces apoptosis [
9,
10]. p53 can also translocate into the mitochondria during apoptosis. Localization of p53 in the mitochondria promotes permeabilization of the outer mitochondrial membrane, cytochrome c release and caspase activation [
11,
12].
Akt, also known as protein kinase B, is a serine/threonine-specific protein kinase that plays a major role in various cellular processes such as cell proliferation, survival/apoptosis, angiogenesis and metabolism [
13,
14]. Previous studies have shown that activated Akt can adjust the phosphorylation and movement of Murine Double Minute 2 (MDM2) into the nucleus where it binds to p53. MDM2 is a principal mediator of cell proliferation and apoptosis by inhibiting the p53 tumor suppressor [
15,
16,
17]. The inducible isoenzyme cyclooxygenase-2 (COX-2) is important in inflammation, angiogenesis and tumorigenesis [
18]. The overexpression of COX-2 has been associated with resistance to apoptosis in many different cell types [
19,
20]. Some cancer treatments involve surgery, radiotherapy and chemotherapy, but cause problems. These treatments can lead to cytotoxicity, as well as side effects for normal cells, and can cause tolerance to anticancer drugs because of continuous use [
21,
22].
Artemisia annua L.
, known as sweet wormwood, belongs to the plant family of
Asteraceae and has been used in traditional Chinese herbal medicine to treat malaria and fever. The ethanolic extracts of
A. annua is known to have effects on anticancer activity [
23,
24].
In the present study, we investigated the effects of Artemisia annua extracts (AAE) on apoptosis in LS174T colon cancer cells in vitro and in vivo. Our results indicate that AAE induces apoptosis-mediated p53-independent manner, as well as p53-dependent manner through p53's translocation to mitochondria.
3. Discussion
Cancer cells undergo apoptosis, known as programmed cell death, which involves the determined elimination of unnecessary cells. Apoptosis normally takes place during development, as well as aging, and removes damaged cells [
25,
26]. When apoptosis is induced, it is caused by pro-apoptotic proteins. Pro-apoptotic proteins such as Bax and Bak are translocated to the mitochondria. These proteins can be activated by important mitochondrial changes including alternation in the mitochondrial membrane potential [
8,
27]. Activated Bax and Bak lead to the formation of pores in the outer mitochondrial membrane [
28]. Thus, apoptosis through the mitochondrial pathway plays a critical role in cell death for cancer development and treatment.
As shown in previous studies, various plant extracts have anti-cancer effects and induce apoptotic effects in a variety of cancer cells [
29,
30,
31,
32,
33].
Artemisia annua L. (AAE) contains phytochemical ingredients that induce cell cycle arrest, as well as apoptotic cell death, in a number of cell types [
34,
35]. In this study, we focused on the effects of AAE on mitochondria-mediated apoptotic proteins and apoptosis in LS174T colon cancer cells.
Furthermore, LY294002, Nutlin-3, pifithrin-α and celecoxib were employed to assess whether the mechanism of AAE induces via a p53-dependent or p53-independent manner. First, we confirmed that AAE decreased the cell viability in a dose and time-dependent manner. Also, AAE modulates MOMP to induce apoptotic effects through regulation of apoptosis-related proteins and translocation of p53.
Akt is a serine/threonine kinase and the primary mediator of PI3K-initiated signaling. Akt controls a variety of cellular events, such as apoptosis, cell cycle and transcription [
36,
37]. Akt, which blocks apoptosis, phosphorylates MDM2. The phosphorylated MDM2 inhibits the transcriptional activity of p53 and more importantly, promotes its degradation by proteasome [
38,
39].
Previous studies have shown that natural extracts inhibit p-Akt/p-MDM2 and induce apoptosis through the mitochondrial pathway [
40]. Akt inhibitor LY294002 inhibits phosphorylation at Ser473 of Akt and Nutlin-3, a MDM2 inhibitor, inhibits interaction with p53 [
41,
42,
43]. We found that the inhibition of p-Akt and p-MDM2 induced apoptosis when using LY294002 and Nutlin-3. As with the inhibitor treatment, it was confirmed that the apoptotic effects were induced by inhibiting the expression of p-Akt and p-MDM2 when AAE was treated. By this process, we ascertained that p53, by inhibiting p-MDM2, is transmitted to mitochondria. According to previous studies, Bcl-2 and Bcl- X
L bind to BH3 peptides of the pro-apoptotic proteins, like Bax and Bak, and inhibit their function [
44,
45,
46]. However, it has been reported that translocated p53 promotes MOMP by forming a complex with Bcl-xL and Bcl-2 [
47].
Our results, therefore, show that AAE can causes p53 translocation and induces apoptosis through the mitochondrial pathway. Another previous study reported that apoptosis may occur, even in the absence of p53, in the treatment of extracts [
48,
49]. We used pifithrin-α and celecoxib to determine whether AAE induces apoptosis in the p53-independent, as well as in the p53-dependent manner. An inhibitor of p53, pifithrin-α, prevents transcriptional activation and inhibits p53-dependent apoptosis [
50,
51]. Celecoxib is aselective inhibitor of COX-2 and has potent anti-tumor activity in a variety of tumor types, such as colorectal, breast and lung cancer [
52,
53,
54].
Several studies have shown that the co-treatment group with a pifithrin-α and AAE is increased when compared with the pifithrin-α alone treatment group, indicating that apoptosis can occur even in a p53-independent manner. Moreover, we found that COX-2 inhibition was effective for anti-cancer effects when celecoxib was administered alone or in combination with AAE. AAE also induced apoptosis in a mouse xenograft model, as shown in our in vitro studies. AAE treatment resulted in inhibition of tumor growth, increased expression of apoptosis-related proteins and migration to mitochondria.
In conclusion, the present study indicated that AAE, a natural compound, led to the down-regulation of Akt, leading to the suppression of MDM2/COX-2, the activation of p53 translocation and then to apoptosis through a p53-independent manner.
4. Materials and Methods
4.1. Methods of Extraction
Artemisia annua L. was purchased from a medical herb market (Daejeon, Korea). The plant material was ground using a blender. The obtained powder (100 g) was extracted with 95 % ethanol (200 mL) at room temperature for 3 days and was filtered through filter paper. The filtered solvent was evaporated to dryness with a rotary evaporator to eliminate ethanol. A stock solution of the extract was dissolved in DMSO (Dimethyl sulfoxide; Samchun, Korea; stock solution, 100 mg/mL) and stored at -20 ℃.
4.2. Reagent
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), pifithrin-α (p53 inhibitor), celecoxib (COX-2 inhibitor), Nutlin-3 (p-MDM2 inhibitor) and LY294002 (PI3K/Akt inhibitor) were purchased from Sigma Aldrich (Sigma Aldrich; St. Louis, MO, USA). The Pierce Lactate Dehydrogenase (LDH) Cytotoxicity Assay Kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA). MitoTracker was purchased from Molecular Probes (Eugene, OR, USA). Specific antibodies such as p-Akt (Ser473), (t)Akt, COX-2, procaspase-3, Bcl-2, Bcl-XL, COX-4, Bax, Bak, Bim, PARP and β-actin were obtained from Cell Signaling Technology (Beverly, MA, USA). p53 and cytochrome c were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). (t)MDM2 was purchased from Novus Biologicals (Littleton, CO, USA) and p-MDM2 was purchased from Abcam (Cambridge, MA, USA). Muse™ Annexin V and Dead Cell Assay kit (MCH100105), Muse™ MitoPotential Kit (MCH100110), Muse™ Caspase-3/7 Kit (MCH100108) and Muse™ Cell Analyzer (PB4455ENEU) were purchased from Millipore (EMD Millipore Corporation, Darmstadt, Germany).
4.3. Cell Culture
LS174T and fibroblast cells were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). LS174T cells were grown in RPMI-1640 medium (Hyclone; Waltham, MA, USA) and fibroblast cells were grown in DMEM medium (Hyclone) containing 10 % fetal bovine serum (Hyclone) and 1 % antibiotics (100 mg/streptomycin, 100 U/ml penicillin) at 37 ℃ in a 5 % CO2 atmosphere.
4.4. Cell Proliferation Assay (MTT Assay)
Cells were seeded at 1×104 cells/ml in a 12-well plate for 24 h and were incubated with various concentrations of AAE (10-100 μg/ml) for 12-48 h. Certain samples were pre-treated with the inhibitors (pifithrin-α, celecoxib, Nutlin-3 and LY294002) for 30 min prior to treatment with AAE (60 μg/ml). Following incubation with the test compounds, the cells were incubated with a 30 μl MTT solution (5 mg/ml) for 30 min. Subsequently, 200 μl of dimethyl sulfoxide (DMSO, Sigma) was added to dissolve the purple formazan crystals. The optical densities of the solutions were quantified at a 595 nm wavelength by using a Microplate reader (BIO-RAD Laboratories, Inc.; Tokyo, Japan).
4.5. LDH Release Assay
Cells were seeded at 1×104 cells/ml in a 12-well plate for 24 h and were incubated with various concentrations of AAE (10-100 μg/ml) for 12-48 h. Certain samples were pre-treated with the inhibitors (pifithrin-α, celecoxib, Nutlin-3 and LY294002) for 30 min prior to treatment with AAE (60 μg/ml). After 24 h, the LDH cytotoxicity assay kit was used according to the protocol and the absorbance was determined by using a microplate reader at 490 and 644 nm wavelengths. These results were calculated as a percentage of released LDH compared to the total LDH activity.
4.6. Morphology Analysis (Observation of Cellular Morphology)
Cells were seeded at 1×105 cells/ml in a 6-well plate for 24 h and were incubated with various concentrations of AAE (40-80 μg/ml) for 12-48 h. The cellular morphology was photographed under a microscope (Carl Zeiss; Thornwood, NY, USA). The photographs were taken at a magnification of × 200.
4.7. Determination of Apoptosis by Annexin V/PI Staining
Cells were seeded at 1×105 cells/ml in a 6-well plate. After a 24 h incubation, cells were treated with various concentrations of AAE (40-80 μg/ml) for 24 h. Certain samples were pre-treated with the inhibitors (pifithrin-α, celecoxib, Nutlin-3 and LY294002) for 30 min prior to treatment with AAE (60 μg/ml). The Muse™ Annexin V & Dead Cell Kit (Merck Millipore Co.) was used according to the protocol and the analysis was analyzed in the Muse™ Cell Analyzer (Merck Millipore Co.).
4.8. Determination of Apoptosis by Hoechst 33342 Staining
Cells were seeded at 1×104 cells/ml in a 12-well plate with cover glasses and incubated for 24 h. Following treatment with various concentrations of AAE (40-80 μg/ml), the cells were stained with 0.7 μM Hoechst 33342 for 30 min and fixed with 3.5 % formaldehyde for 20 min. Then the cells were washed with PBS twice and the coverslips were mounted for fluorescence microscope observation. Subsequently, the cells were observed using a fluorescence microscope (Carl Zeiss).
4.9. Measurement of Mitochondrial Membrane Potential
Cells were seeded at 1×105 cells/ml in a 6-well plate. After a 24 h incubation, cells were treated with various concentrations of AAE (40-80 μg/ml) for 24 h. Certain samples were pre-treated with the inhibitors (pifithrin-α, celecoxib, Nutlin-3 and LY294002) for 30 min prior to treatment with AAE (60 μg/ml). The Muse™ MitoPotential Kit (Merck Millipore Co.) was used according to the protocol and the analysis was analyzed in the Muse™ Cell Analyzer (Merck Millipore Co.).
4.10. Caspase-3/7 Activity Assay
Cells were seeded at 1×105 cells/ml in a 6-well plate. After a 24 h incubation, cells were treated with various concentrations of AAE (40-80 μg/ml) for 24 h. Certain samples were pre-treated with the inhibitors (pifithrin-α, celecoxib, Nutlin-3 and LY294002) for 30 min prior to treatment with AAE (60 μg/ml). The Muse™ Caspase-3/7 Assay Kit (Merck Millipore Co.) was used according to the protocol and analysis was analyzed in the Muse™ Cell Analyzer (Merck Millipore Co.). The activity of caspase-3 was determined using a caspase-3 assay kit (Abcam PLC; Cambridge, UK). Cells were harvested by trypsinization, collected by centrifugation and washed with PBS. Resuspended cells were added to a lysis buffer and mixed with a reaction buffer. After addition of DEVD-p-NA, cells were incubated at 37 ℃ in a 5 % CO2 atmosphere for 2 h. The optical densities of solutions were quantified using a Microplate reader (BIO-RAD Laboratories, Inc.) at a 405 nm wavelength.
4.11. Fractionation of Mitochondria and Cytosol Proteins
Mitochondrial fractions were analyzed by using a Mitochondria/Cytosol Fraction Kit (Abcam, PLC, Cambridge, UK). Cells were seeded at 1×106 cells/ml in a 100 mm plate and incubated for 24 h with AAE (40-80 μg/ml). After a 24 h incubation, cells were harvested by trypsinization, collected by centrifugation and washed with PBS. The washed cellular pellet was homogenized in an ice-cold cytosol extraction buffer using a sonicator and centrifuged at 3,000 rpm for 10 min at 4 ℃. The supernatant liquids were transferred into fresh tubes and centrifuged at 13,000 rpm for 20 min at 4 ℃. The collected supernatant has a cytosolic fraction. The pellets were resuspended with an ice-cold mitochondria extraction buffer.
4.12. Western Blotting
Cells were seeded at 1×105 cells/ml in a 6-well plate. After a 24 h incubation, cells were treated with various concentrations of AAE (40-80 μg/ml) for 6 h. Certain samples were pre-treated with the inhibitors (pifithrin-α, celecoxib, Nutlin-3 and LY294002) for 30 min prior to treatment with AAE (60 μg/ml). After a 6 h, cells were rinsed twice with ice-cold PBS, scraped with a lysis buffer [50 mM Tris-HCl (pH 8.0, 150 mM NaCl, 1 % NP40, 0.5 % sodium deoxycholate, 1 mM PMSF)] and subjected to the western blot analysis. Protein quantification was performed using a Bradford assay and 30 μg of protein were loaded per lane. Primary antibodies reacted overnight at 4 ℃ and secondary antibodies reacted for 90 min at room temperature with gentle agitation.
4.13. Immunofluorescence (IF) Staining
Cells were seeded at 1×104 cells/ml in a 12-well plate with cover glasses and incubated for 24 h. After treatment with the indicated dose for 24 h, cells were stained with MitoTracker for 30 min at 37 ℃ in a 5 % CO2 atmosphere. Cells were fixed with 4 % formaldehyde for 20 min, prior to permeabilization with 0.2 % Triton X-100 and blocking in 2 % bovine serum albumin. Cells were then incubated overnight at 4 ℃ with cytochrome c, p53 primary antibodies. On the second day, cells were washed with PBS and reacted with a secondary antibody for 1 h. The coverslips were mounted for fluorescence microscope observation. Subsequently, the cells were observed using a confocal microscope (Olympus; Tokyo, Japan).
4.14. Xenograft Model
Male 4-week-old Balb/c nu/nu mice were obtained from SLC (Tokyo, Japan) and housed in sterile, filter-topped cages. For tumor induction, LS174T colon cancer cells (2×105 cells/0.1 ml) were subcutaneously injected into the left flank of the mice (n = 5/group). One week after the injection of cells, they were co-treated with 20-40 mg/kg/day for 21 days. The tumor size was measured using calipers at 2 day intervals and the tumor volume was calculated using the modified formula V = 1/2 (length × width)2. The body weight was measured at a set time, once per week. All animal experiments were approved by the Ethics Committee for Animal Experimentation, Hannam University (Daejeon, Korea, HNU 2016-8).
4.15. Immunohistochemistry
The tumor specimens from mice were fixed in 10 % formaldehyde, embedded in paraffin and sectioned into 5 μM thick slices. Consecutive thin cryosections (5 μM) of optimum cutting temperature compound (Sakura Finetek; Torrance, CA, USA) embedded tumor tissues were fixed in acetone at 4 ˚C for 10 min. Following washing in PBS, sections were treated with 3 % H2O2 for 10 min to block endogenous peroxidase activity, and the sections were inhibited with normal rabbit serum. Then, the sections were blocked and washed in PBS and incubated with a specific antibody overnight at 4 ˚C. Negative controls were incubated with the primary normal serum immunoglobulin G (IgG) for the species from which the primary antibody was obtained.
4.16. TUNEL Assay
Levels of apoptosis in distal colon tissue were determined by using the TdT-mediated dUTP nick-end labeling (TUNEL) method. The tumor tissues were fixed in 10% formaldehyde, embedded in paraffin and sectioned into 5 μM thick slices. Tissue sections were processed for the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Vector Laboratories; Burlingame, CA, USA) according to manufacturer’s instructions.
4.17. Statistical Analysis
All the experiments were repeated at least three times and analyzed using t-tests (SPSS 20.0, Chicago, IL, USA). p<0.05 was considered to indicate a statistically significant difference.
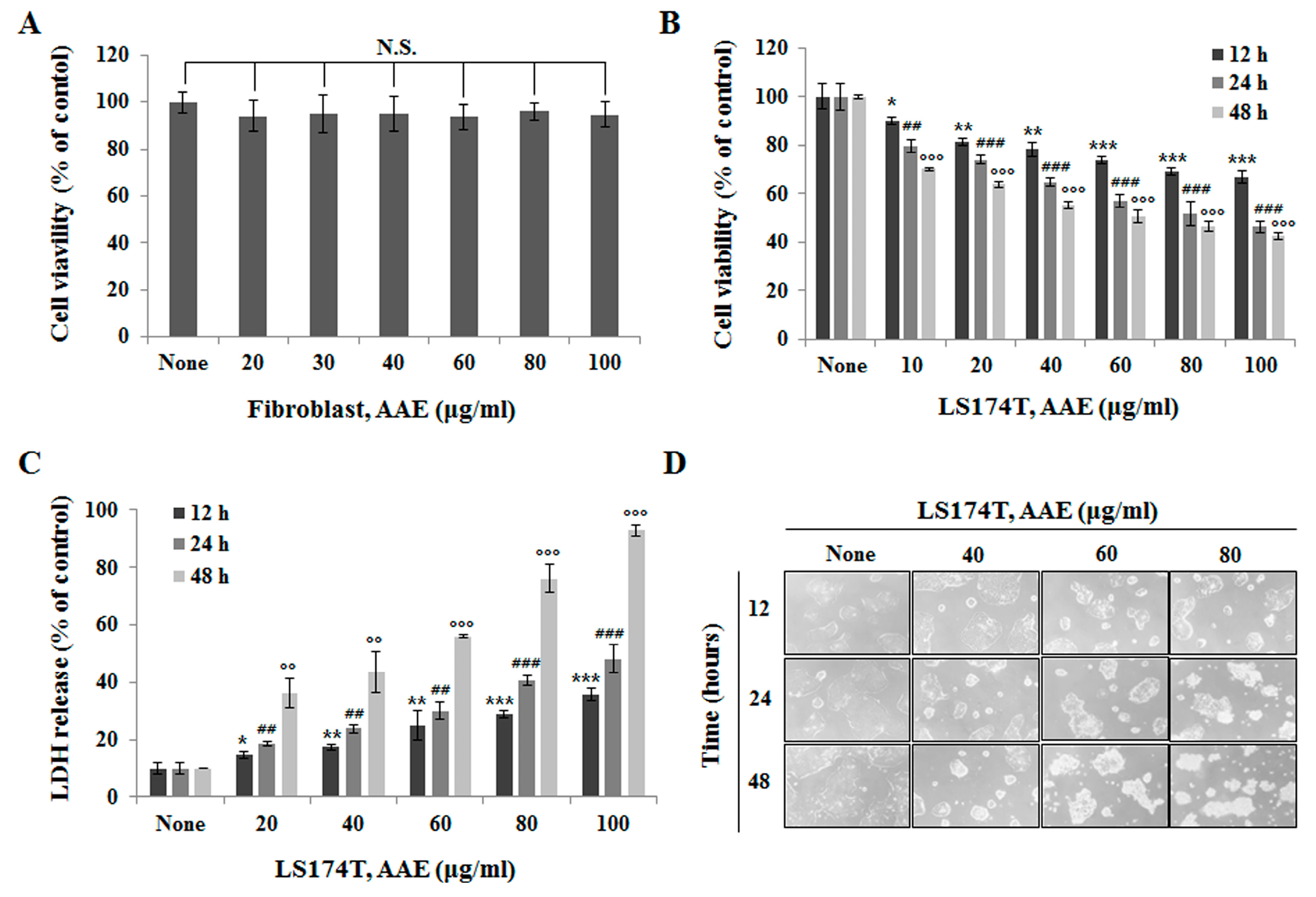
Figure 1.
AAE inhibits cell proliferation in LS174T colon cancer cells. (A) Cell proliferation rate was measured by MTT assay. Fibroblast cells were treated with the indicated concentrations of AAE for 24 h. (B) LS174T cells were treated with the indicated concentrations of AAE for 12-48 h. (C) AAE increased the LDH release in cells. LS174T cells were treated with the indicated concentrations of AAE for 12-48 h. The statistical analysis of the data was carried out by use of a t-test. *P < 0.05, **,##,ooP < 0.01,***,###,oooP < 0.001 compared to control. N.S.;not significant (each experiment, n=3). (D) Phase contrast image-based monitoring of apoptosis induction. 40x phase contrast images LS174T cells after 24 h incubation.
Figure 1.
AAE inhibits cell proliferation in LS174T colon cancer cells. (A) Cell proliferation rate was measured by MTT assay. Fibroblast cells were treated with the indicated concentrations of AAE for 24 h. (B) LS174T cells were treated with the indicated concentrations of AAE for 12-48 h. (C) AAE increased the LDH release in cells. LS174T cells were treated with the indicated concentrations of AAE for 12-48 h. The statistical analysis of the data was carried out by use of a t-test. *P < 0.05, **,##,ooP < 0.01,***,###,oooP < 0.001 compared to control. N.S.;not significant (each experiment, n=3). (D) Phase contrast image-based monitoring of apoptosis induction. 40x phase contrast images LS174T cells after 24 h incubation.
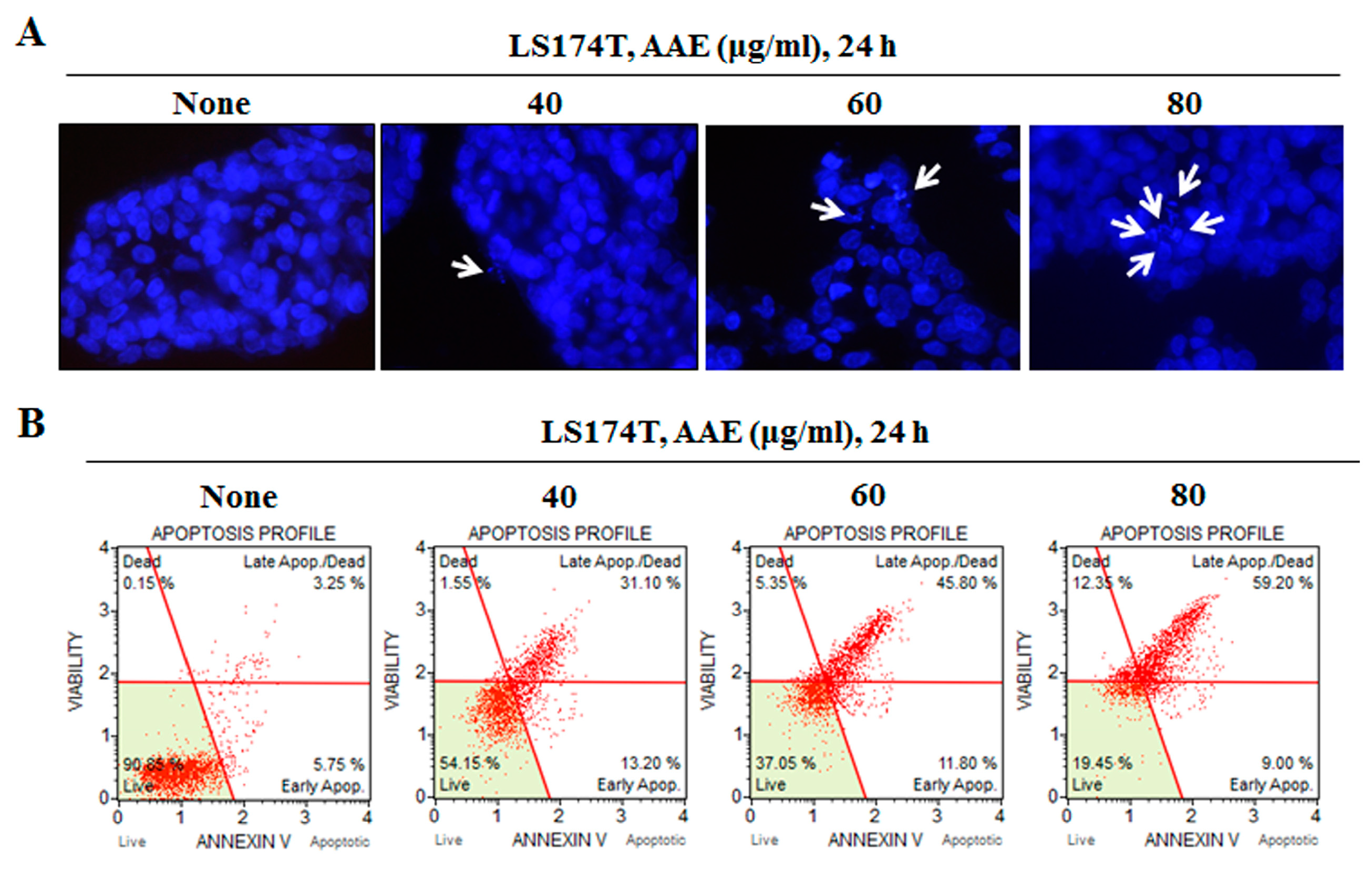
Figure 2.
AAE induces apoptosis in LS174T colon cancer cells. (A) Cell apoptosis observed using Hoechst 33342 staining. Cells were treated with the indicated concentrations of AAE for 24 h. Fluorescence was detected using a fluorescence microscope. Arrows indicate apoptotic bodies, which were DNA fragments produced when apoptosis occurred. (B) Cells were treated with the indicated concentrations of AAE for 24 h. Cells stained with Muse™ Annexin V and Dead Cell Assay kit and analyzed by Muse™ Cell Analyzer. Data shows four cell populations – Live, Dead, Late Apop./Dead, Early Apop.
Figure 2.
AAE induces apoptosis in LS174T colon cancer cells. (A) Cell apoptosis observed using Hoechst 33342 staining. Cells were treated with the indicated concentrations of AAE for 24 h. Fluorescence was detected using a fluorescence microscope. Arrows indicate apoptotic bodies, which were DNA fragments produced when apoptosis occurred. (B) Cells were treated with the indicated concentrations of AAE for 24 h. Cells stained with Muse™ Annexin V and Dead Cell Assay kit and analyzed by Muse™ Cell Analyzer. Data shows four cell populations – Live, Dead, Late Apop./Dead, Early Apop.
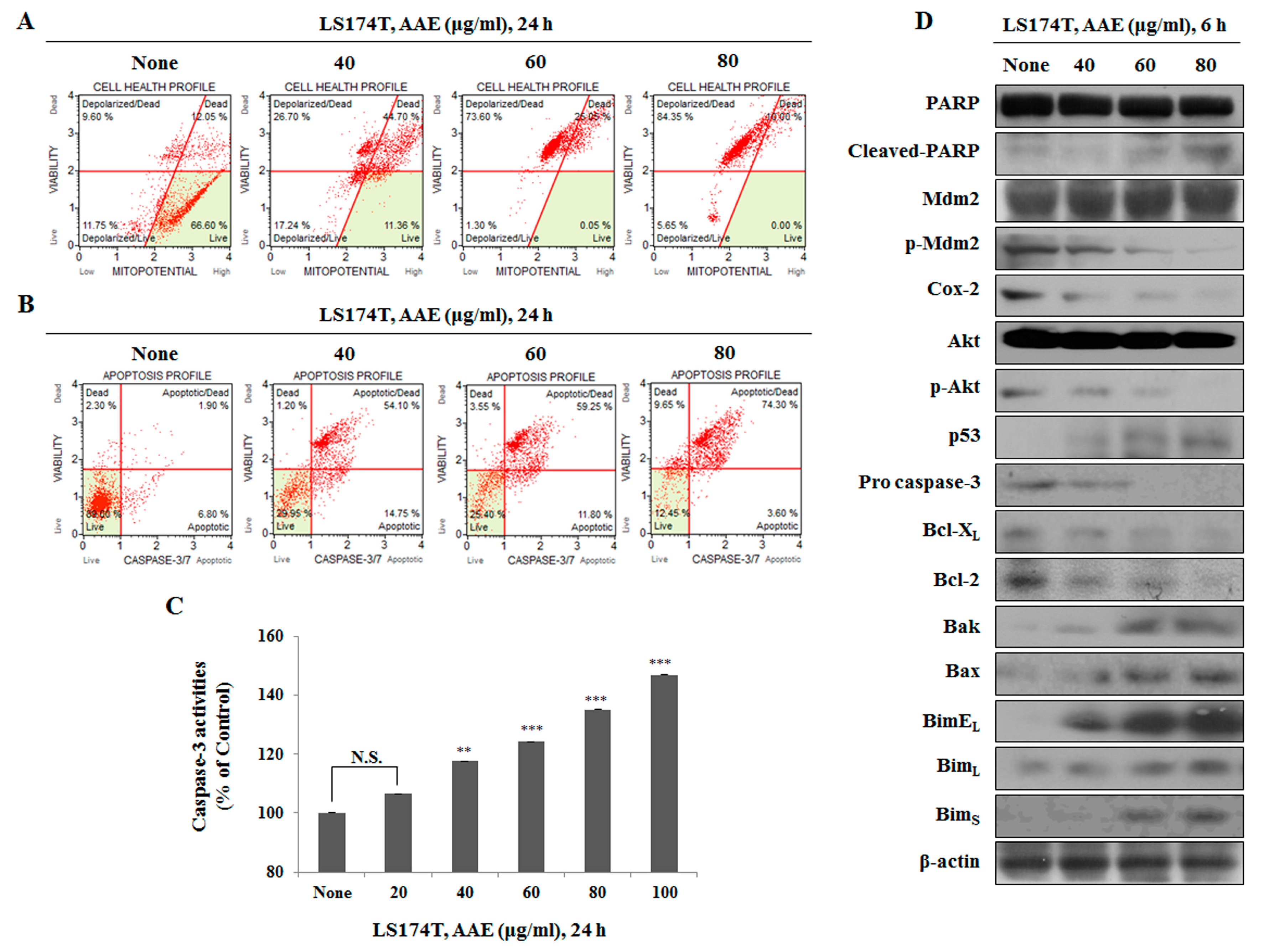
Figure 3.
Evaluation of the effect of AAE on the apoptosis through the mitochondrial signaling pathway. (A) Cells were treated with the indicated concentrations of AAE for 24 h. Cells stained with Muse™ Mitopotential Kit and analyzed by Muse™ Cell Analyzer. Data shows four cell populations - Live, Depolarized/Live, Depolarized/Dead, and Dead cells. (B) Cells stained with Muse™ Caspase-3/7kit and analyzed by Muse™ Cell Analyzer. Data shows four cell populations – Live, Dead, Apoptotic, Apoptotic/Dead. (C) AAE induces caspase-3 activation . The statistical analysis of the data was carried out by use of a t-test. **P < 0.01, ***P < 0.001 compared to control. N.S.; not significant (each experiment, n=3). (D) Cells were treated with the indicated concentrations of AAE for 24 h. Protein level was measured by Western blotting. The β-actin probe served as protein-loading control.
Figure 3.
Evaluation of the effect of AAE on the apoptosis through the mitochondrial signaling pathway. (A) Cells were treated with the indicated concentrations of AAE for 24 h. Cells stained with Muse™ Mitopotential Kit and analyzed by Muse™ Cell Analyzer. Data shows four cell populations - Live, Depolarized/Live, Depolarized/Dead, and Dead cells. (B) Cells stained with Muse™ Caspase-3/7kit and analyzed by Muse™ Cell Analyzer. Data shows four cell populations – Live, Dead, Apoptotic, Apoptotic/Dead. (C) AAE induces caspase-3 activation . The statistical analysis of the data was carried out by use of a t-test. **P < 0.01, ***P < 0.001 compared to control. N.S.; not significant (each experiment, n=3). (D) Cells were treated with the indicated concentrations of AAE for 24 h. Protein level was measured by Western blotting. The β-actin probe served as protein-loading control.
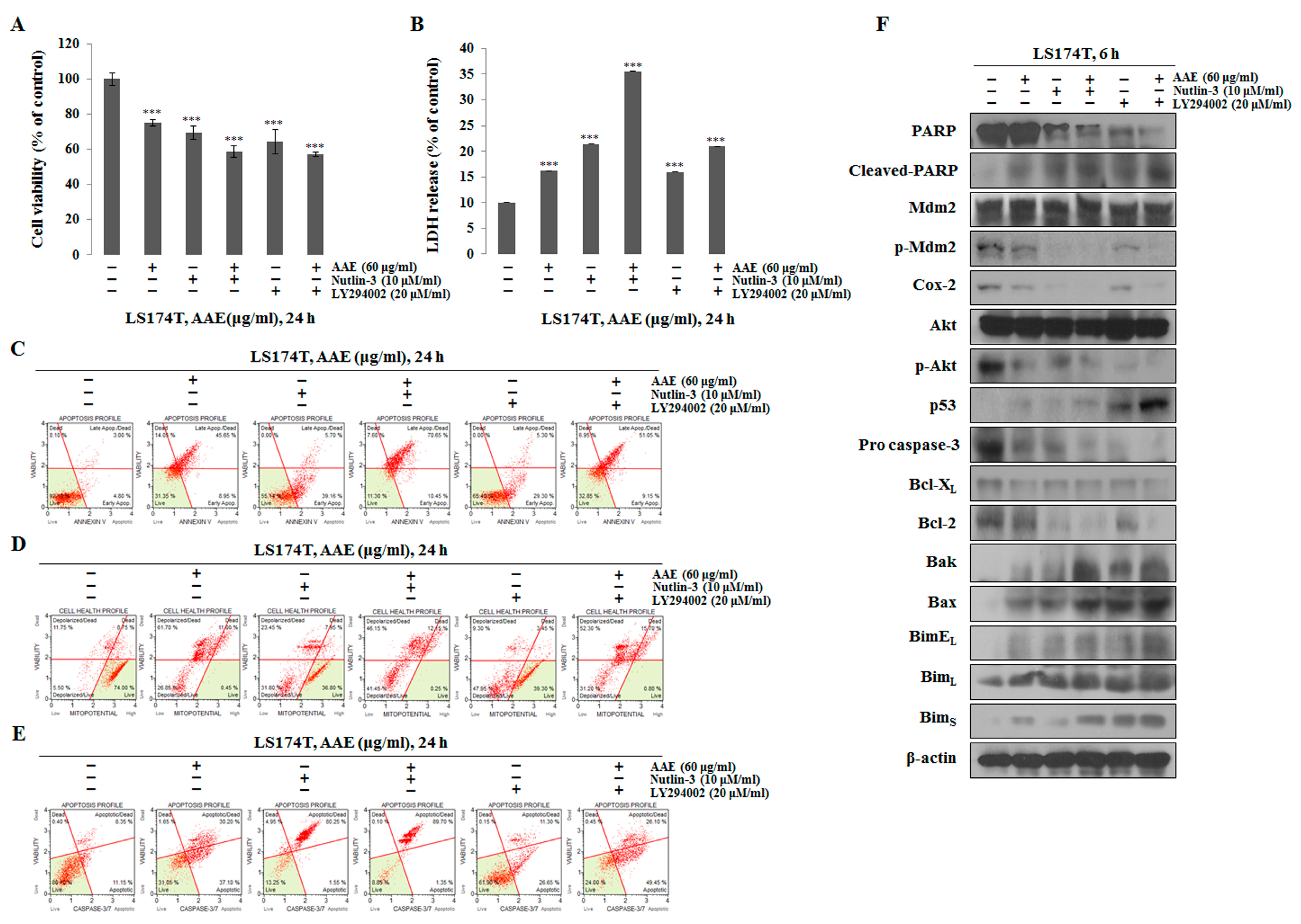
Figure 4.
AAE exerts apoptotic effects via p53-dependent manner. (A) Cells were pre-treated with 20 μM LY294002 or 10 μM Nutlin-3 for 30 min and co-treated with 60 μg/ml AAE 24 h. The statistical analysis of the data was carried out by use of a t-test. ***P<0.001 compared to control (each experiment, n=3). (B) AAE increased the LDH release in LS174T cells. The statistical analysis of the data was carried out by use of a t-test. ***P<0.001 compared to control (each experiment, n=3). (C) Cells stained with Muse™ Annexin V and Dead Cell Assay kit and analyzed by Muse™ Cell Analyzer. (D) Cells stained with Muse™ Mitopotential Kit and analyzed by Muse™ Cell Analyzer. (E) Cells stained with Muse™ Caspase-3/7kit and analyzed by Muse™ Cell Analyzer. (F) Protein level was measured by Western blotting. The β-actin probe served as protein-loading control.
Figure 4.
AAE exerts apoptotic effects via p53-dependent manner. (A) Cells were pre-treated with 20 μM LY294002 or 10 μM Nutlin-3 for 30 min and co-treated with 60 μg/ml AAE 24 h. The statistical analysis of the data was carried out by use of a t-test. ***P<0.001 compared to control (each experiment, n=3). (B) AAE increased the LDH release in LS174T cells. The statistical analysis of the data was carried out by use of a t-test. ***P<0.001 compared to control (each experiment, n=3). (C) Cells stained with Muse™ Annexin V and Dead Cell Assay kit and analyzed by Muse™ Cell Analyzer. (D) Cells stained with Muse™ Mitopotential Kit and analyzed by Muse™ Cell Analyzer. (E) Cells stained with Muse™ Caspase-3/7kit and analyzed by Muse™ Cell Analyzer. (F) Protein level was measured by Western blotting. The β-actin probe served as protein-loading control.
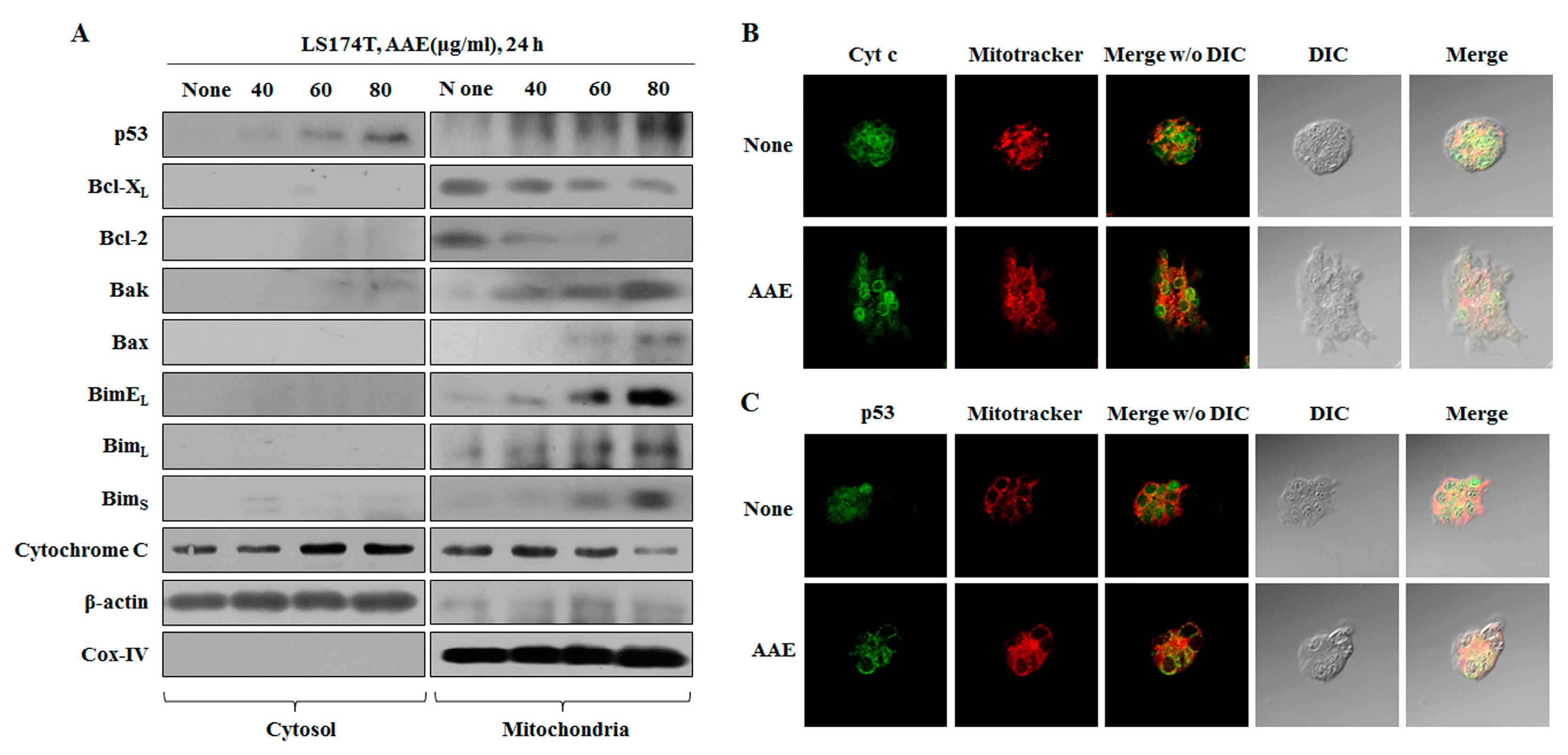
Figure 5.
AAE regulates mitochondrial membrane potential, leading to the secretion of cytochrome c and p53’s translocation to mitochondria. (A) Cells were treated with the indicated concentrations of AAE for 24 h. Fraction of mitochondria/cytosol proteins were analyzed by western blotting. (B), (C) Cells were treated with 60 μg/ml AAE 24 h, pre-stained with mitotracker before fixiation and permeabilization of cells and reacted with specific antibody. Florescence detected by confocal microscope.
Figure 5.
AAE regulates mitochondrial membrane potential, leading to the secretion of cytochrome c and p53’s translocation to mitochondria. (A) Cells were treated with the indicated concentrations of AAE for 24 h. Fraction of mitochondria/cytosol proteins were analyzed by western blotting. (B), (C) Cells were treated with 60 μg/ml AAE 24 h, pre-stained with mitotracker before fixiation and permeabilization of cells and reacted with specific antibody. Florescence detected by confocal microscope.
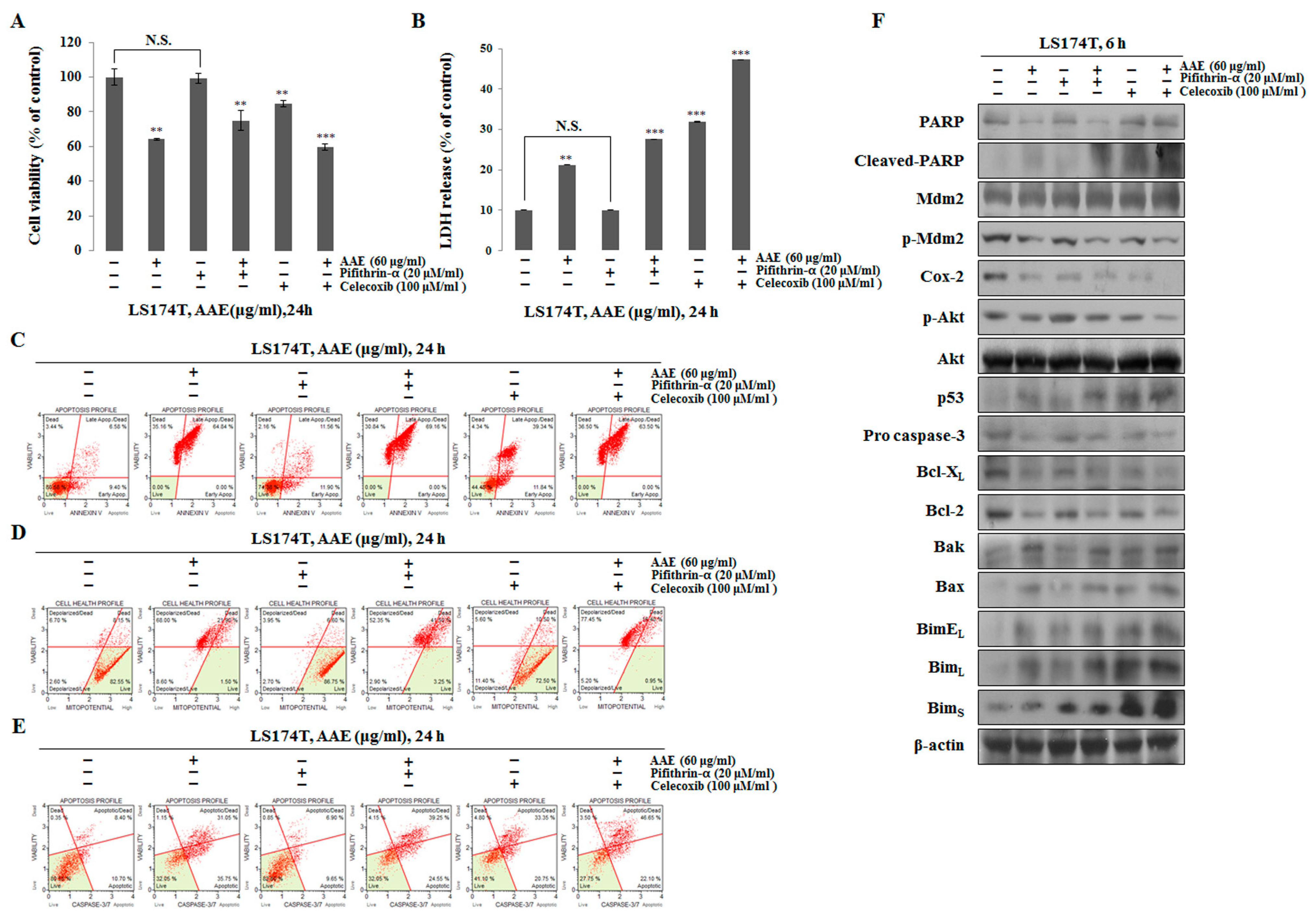
Figure 6.
AAE exerts apoptotic effects via p53-independent manner. (A) Cells were pre-treated with 20 μM pifithrin-α or 100 μM celecoxib for 30 min and co-treated with 60 μg/ml AAE 24 h. (B) AAE increased the LDH release in LS174T cells. The statistical analysis of the data was carried out by use of a t-test. **P < 0.01, ***P < 0.001 compared to control. N.S.;not significant (each experiment, n=3). (C) Cells stained with Muse™ Annexin V and Dead Cell Assay kit and analyzed by Muse™ Cell Analyzer. (D) Cells stained with Muse™ Mitopotential Kit and analyzed by Muse™ Cell Analyzer. (E) Cells stained with™ Caspase-3/7kit and analyzed by Muse™ Cell Analyzer. (F) Protein level was measured by Western blotting. The β-actin probe served as protein-loading control.
Figure 6.
AAE exerts apoptotic effects via p53-independent manner. (A) Cells were pre-treated with 20 μM pifithrin-α or 100 μM celecoxib for 30 min and co-treated with 60 μg/ml AAE 24 h. (B) AAE increased the LDH release in LS174T cells. The statistical analysis of the data was carried out by use of a t-test. **P < 0.01, ***P < 0.001 compared to control. N.S.;not significant (each experiment, n=3). (C) Cells stained with Muse™ Annexin V and Dead Cell Assay kit and analyzed by Muse™ Cell Analyzer. (D) Cells stained with Muse™ Mitopotential Kit and analyzed by Muse™ Cell Analyzer. (E) Cells stained with™ Caspase-3/7kit and analyzed by Muse™ Cell Analyzer. (F) Protein level was measured by Western blotting. The β-actin probe served as protein-loading control.
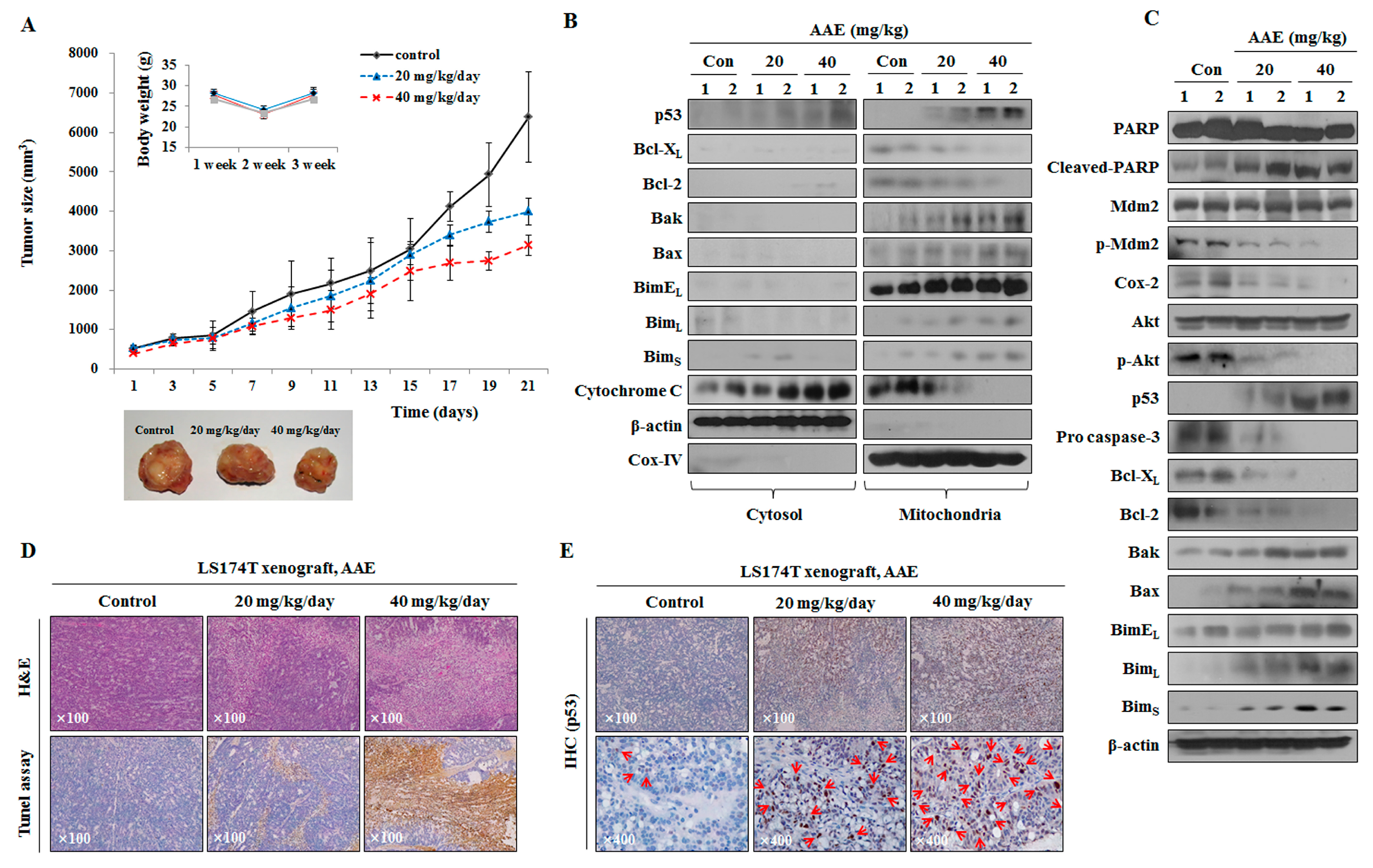
Figure 7.
AAE induces cell death and suppresses cell growth in LS174T xenograft model. (A) Measurement of tumor size and mouse weight. AAE groups reduced tumor growth compared with the control group. (B), (C) Analyzed protein levels by western blotting. Tissue samples were homogenized in RIPA lysis buffer. Mitochondria/cytosol protein fraction executed like an in vitro experiment. (D) H&E and TUNEL assay. Magnification, x100. (E) Specific proteins immunohistochemical staining assay. Arrows indicate positive reaction to specific proteins.
Figure 7.
AAE induces cell death and suppresses cell growth in LS174T xenograft model. (A) Measurement of tumor size and mouse weight. AAE groups reduced tumor growth compared with the control group. (B), (C) Analyzed protein levels by western blotting. Tissue samples were homogenized in RIPA lysis buffer. Mitochondria/cytosol protein fraction executed like an in vitro experiment. (D) H&E and TUNEL assay. Magnification, x100. (E) Specific proteins immunohistochemical staining assay. Arrows indicate positive reaction to specific proteins.