Submitted:
27 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Biological Activity of BVR
2.1.1. Antiradical and Antilipooxigenase Activity of BVR
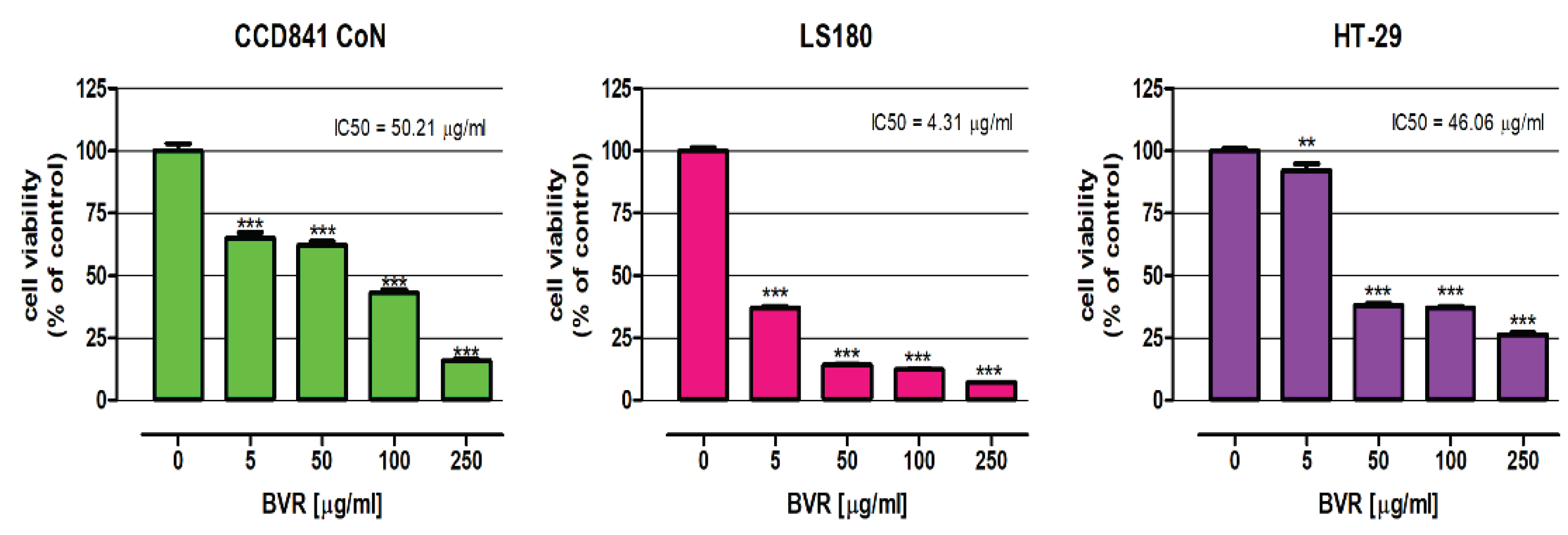
2.1.2. Influence of BVR and its Main Constituents on the Viability of CCD841 CoN, LS180, and HT-29 Cells Using the MTT Assay
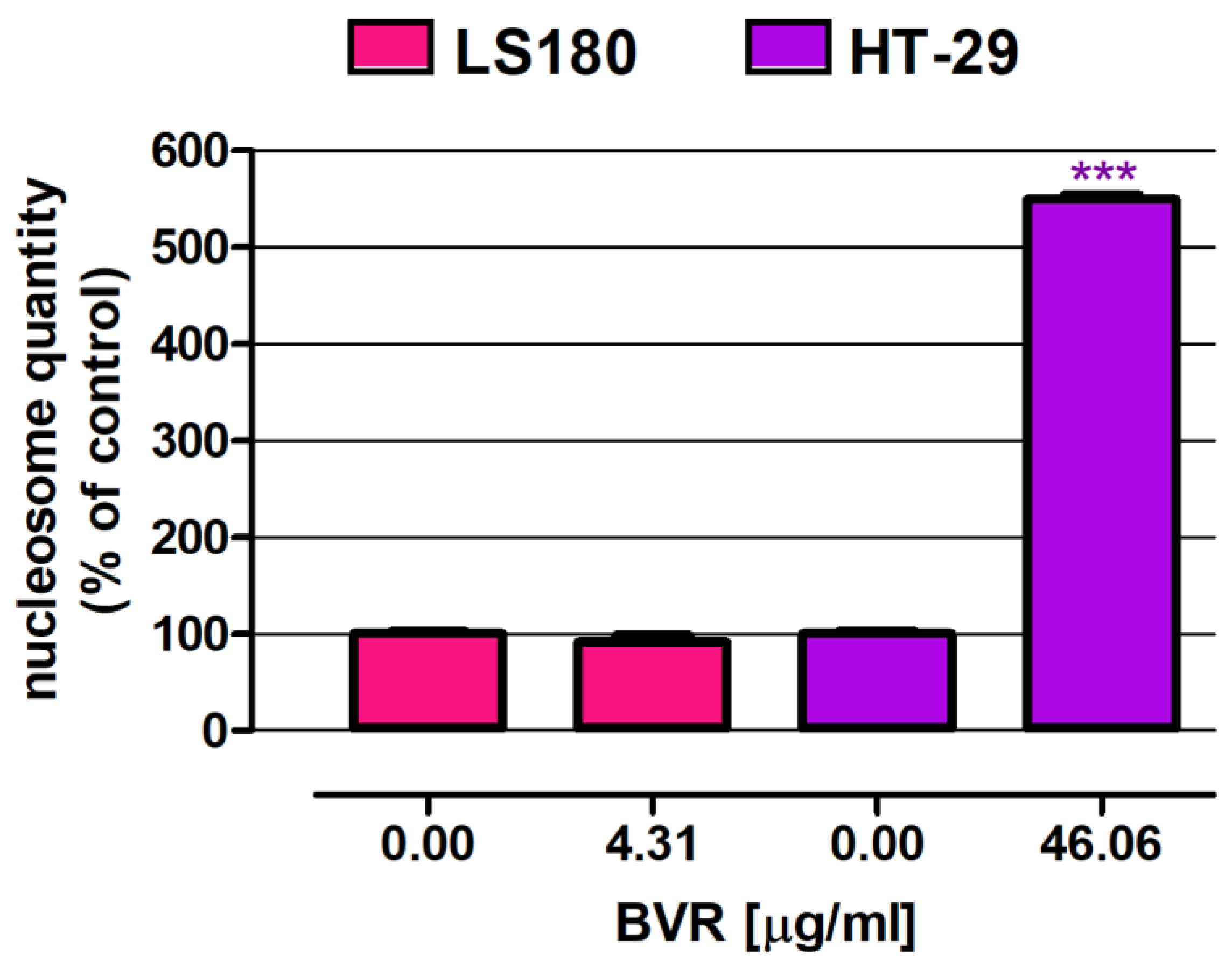
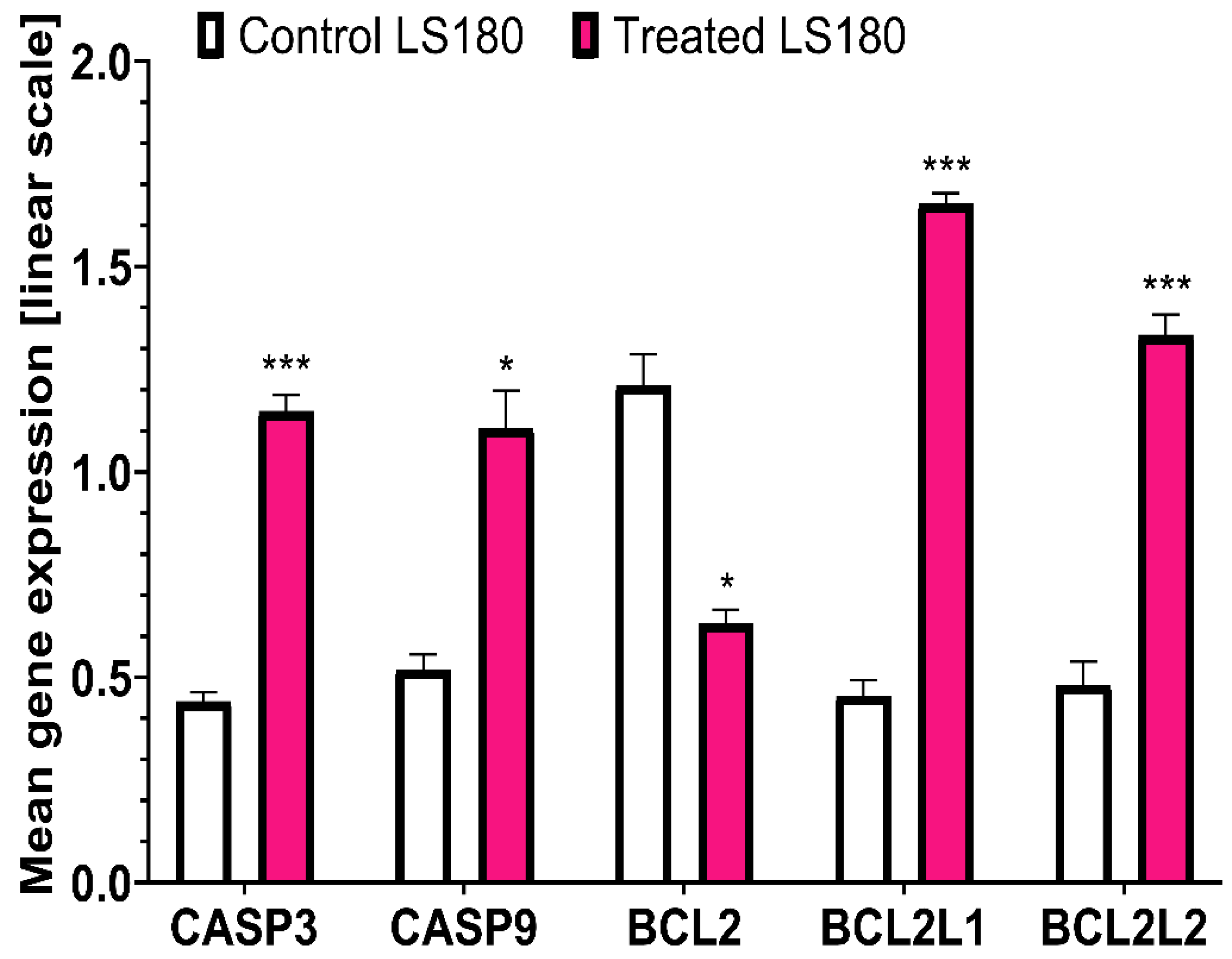
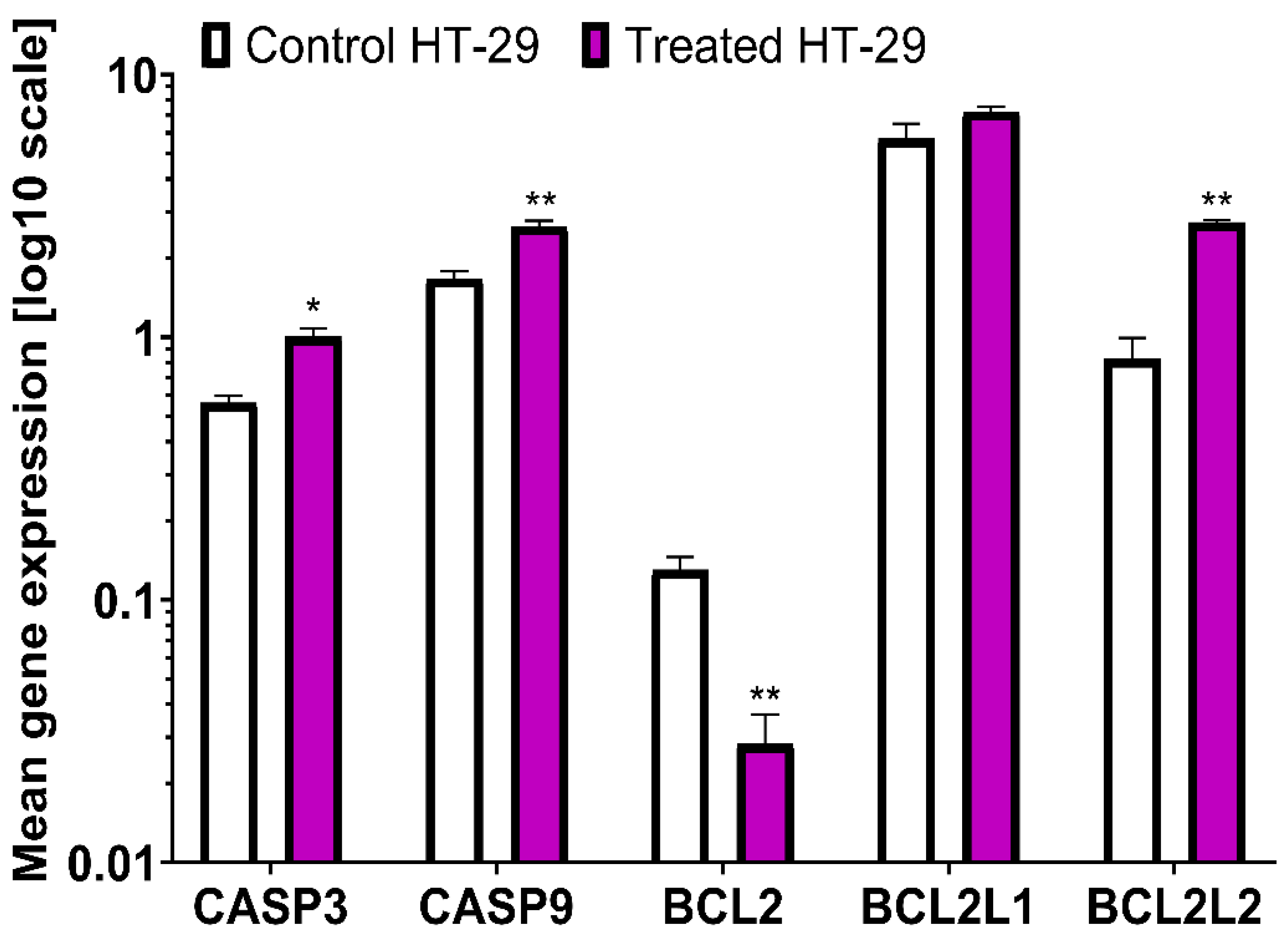
2.1.3. PCR Analysis
2.2. Phytochemical Profiling and Quantification of Major Specialised Metabolites
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of Extract
4.2. Chemicals and Reagents
4.3. Antiradical Activity Analyses
4.3.1. Determination of Antiradical Potential with the DPPH• Assay
4.3.2. Determination of Antiradical Capacity with the ABTS•+ Assay
4.3.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
4.4. Lipoxygenase (LOX) Inhibitor Screening Assay
4.5. Cell Cultures
4.6. Cell Viability Assessment – MTT Assay
4.7. Cell Death Assessment - ELISA
4.8. Gene Expression Analysis
4.9. Phytochemical Profiling and Quantification of Major Specialised Metabolites Using LC-UV- MS/MS Technique
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Cancer Research Fund International. Available online: https://www.wcrf-uk.org/. (accessed on 12 Mar 2023).
- Labianca, R.; Nordlinger, B.; Beretta, G.D.; Mosconi, S.; Mandalà, M.; Cervantes, A.; Arnold, D. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi64–vi72. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer. (accessed on 19 January 2023).
- Simon, K.; Balchen, V. Colorectal cancer development and advances in screening. Clin. Interv. Aging 2016, 11, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Maresso, K.C.; Tsai, K.Y.; Brown, P.H.; Szabo, E.; Lippman, S.; Hawk, E.T. Molecular cancer prevention: Current status and future directions. CA: A Cancer J. Clin. 2015, 65, 345–383. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yan-ze, L.; Yong, P.E.; Zhong-zhi, Q.I; . Pei-gen, X.I. New collection of crude drugs in Chinese pharmacopoeia 2010 II. Sankezhen (Berberis spp.). Chin. Herbal Med. 2011, 3, 272–88. [Google Scholar]
- Henriette’s Herbal Homepage. Available online: https://www.henriettes-herb.com. 2020.
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytotherapy Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, P.; Chai, M.-J.; Yang, F.; Li, H.-S.; Zhao, J.; Wang, H.; Lu, D.-D. [Effects of berberine on serum inflammatory factors and carotid atherosclerotic plaques in patients with acute cerebral ischemic stroke]. China J. Chin. Mater. Medica 2016, 41, 4066–4071. [Google Scholar] [CrossRef]
- Meng, S.; Wang, L.; Huang, Z.; Zhou, Q.; Sun, Y.; Cao, J.; Li, Y.; Wang, C. Berberine ameliorates inflammation in patients with acute coronary syndrome following percutaneous coronary intervention. Clin. Exp. Pharmacol. Physiol. 2012, 39, 406–411. [Google Scholar] [CrossRef]
- Kiasalari, Z.; Khalili, M.; Ahmadi, P. Effect of alcoholic extract of berberis vulgaris fruit on acute and chronic inflammation in male rats. Journal of Babol University of Medical Sciences 2011, 13(1).
- Minaiyan, M.; Ghannadi, A.; Mahzouni, P.; Jaffari-Shirazi, E. Comparative Study of Berberis vulgaris Fruit Extract and Berberine Chloride Effects on Acetic Acid-Induced Colitis in Rats. 2011, 10, 97–104.
- Majeed, W.; Aslam, B.; Javed, I.; Khaliq, T.; Muhammad, F.; Ali, A.; Raza, A. Histopathological evaluation of gastro protective effect of Berberis vulgaris (Zereshk) seeds against aspirin induced ulcer in albino mice. . 2015, 28, 1953–8. [Google Scholar]
- Mohebali, S.; Nasri, S.; Kamalinejhad, M.; Noori, A.S. Antinociceptive & anti-inflammatory effects of Berberis vulgaris L. root’s hydroalcoholic extract and determination of it’s possible antinociceptive mechanism in male mice. Journal of Paramedical Sciences (JPS) 2011, 2(4), 12-18.
- Ivanovska, N.; Philipov, S. Study on the anti-inflammatory action of Berberis vulgaris root extract, alkaloid fractions and pure alkaloids. Int. J. Immunopharmacol. 1996, 18, 553–561. [Google Scholar] [CrossRef] [PubMed]
- NIH. Clinical Trials.gov. Available online: https://clinicaltrials.gov/ (accessed on 26 April 2023).
- El-Wahab, A.E.A.; A Ghareeb, D.; Sarhan, E.E.; Abu-Serie, M.M.; A El Demellawy, M. In vitro biological assessment of berberis vulgaris and its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement. Altern. Med. 2013, 13, 218–218. [Google Scholar] [CrossRef] [PubMed]
- Gird, C.E.; Ligiaelena, D.U. ; Costea,T. ; Nencu,I.; Popescu, M.; Balaci, T.; Olaru, O. Research regarding obtaining herbal extracts with antitumor activity. note ii. phytochemical analysis, antioxidant activity and cytotoxic effects of Chelidonium majus L., Medicago sativa L. and Berberis vulgaris L. dry extracts. Medicago sativa L. and Berberis vulgaris L. dry extracts. Farmacia 2017, 65, 703–708. [Google Scholar]
- Ghafourian, E.; Sadeghifard, N.; Pakzad, I.; Valizadeh, N.; Maleki, A.; Jafari, F.; Ghiasvand, N.; Abdi, J.; Shokoohinia, Y.; Ghafourian, S. Ethanolic Extract of Berberis Vulgaris Fruits Inhibits the Proliferation of MCF-7 Breast Cancer Cell Line Through Induction of Apoptosis. Infect. Disord. - Drug Targets 2017, 17, 192–198. [Google Scholar] [CrossRef] [PubMed]
- El Khalki, L.; Tilaoui, M.; Jaafari, A.; Mouse, H.A.; Zyad, A. Studies on the Dual Cytotoxicity and Antioxidant Properties of Berberis vulgaris Extracts and Its Main Constituent Berberine. Adv. Pharmacol. Sci. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Ng, L.T.; Hsu, F.-F.; Shieh, D.-E.; Chiang, L.-C. Cytotoxic effects of Coptis chinensis and Epimedium sagittatum extracts and their major constituents (berberine, coptisine and icariin) on hepatoma and leukaemia cell growth. Clin. Exp. Pharmacol. Physiol. 2004, 31, 65–69. [Google Scholar] [CrossRef]
- Kalmarzi, R.N.; Naleini, S.N.; Ashtary-Larky, D.; Peluso, I.; Jouybari, L.; Rafi, A.; Ghorat, F.; Heidari, N.; Sharifian, F.; Mardaneh, J.; et al. Anti-Inflammatory and Immunomodulatory Effects of Barberry (Berberis vulgaris) and Its Main Compounds. Oxidative Med. Cell. Longev. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Villinski, J.; Dumas, E.; Chai, H.B.; Pezzuto, J.; Angerhofer, C.; Gafner, S. Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and Hydrastis canadensis. Pharm. Biol. 2003, 41, 551–557. [Google Scholar] [CrossRef]
- Saeidnia, S.; Gohari, A.; Kurepaz-Mahmoodabadi, M.; Mokhber-Dezfuli, N. Phytochemistry and Pharmacology of Berberis Species. Pharmacogn. Rev. 2014, 8, 8–15. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Kaushik, N. Phytochemical and pharmacological studies in genus Berberis. Phytochem. Rev. 2012, 11, 523–542. [Google Scholar] [CrossRef]
- Och, A.; Olech, M.; Bąk, K.; Kanak, S.; Cwener, A.; Cieśla, M.; Nowak, R. Evaluation of the Antioxidant and Anti-Lipoxygenase Activity of Berberis vulgaris L. Leaves, Fruits, and Stem and Their LC MS/MS Polyphenolic Profile. Antioxidants 2023, 12, 1467. [Google Scholar] [CrossRef] [PubMed]
- Gorizpa, M.; Bahmanyar, F.; Mirmoghtadaie, L.; Shafaei, F. Evaluation of Antioxidant and Antimicrobial Properties of Root and Stem Bark Extracts of Three Species of Barberry in Bread. Research and Innovation in Food Science and Technology 2022, 10(4), 413–426. [Google Scholar] [CrossRef]
- Luo, A.; Fan, Y. Antioxidant activities of berberine hydrochloride. J Med Plants Res 2011, 5(16), 3702–7. [Google Scholar]
- Chaves, S.K.M.; Afzal, M.I.; Islam, M.T.; Hameed, A.; Da Mata, A.M.O.F.; Araújo, L.D.S.; Ali, S.W.; Rolim, H.M.L.; Medeiros, M.d.G.F.D.; Costa, E.V.; et al. Palmatine antioxidant and anti-acetylcholinesterase activities: A pre-clinical assessment. Cell. Mol. Biol. 2020, 66, 54–59. [Google Scholar] [CrossRef]
- Xu, T.; Kuang, T.; Du, H.; Li, Q.; Feng, T.; Zhang, Y.; Fan, G. Magnoflorine: A review of its pharmacology, pharmacokinetics and toxicity. Pharmacol. Res. 2020, 152, 104632. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Babaei, A.B.; Naji-Tabasi, S. Investigating functional properties of barberry species: an overview. J. Sci. Food Agric. 2019, 99, 5255–5269. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jiang, K.; Wu, H.; Yang, C.; Zhao, G.; Deng, G. Magnoflorine ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Frontiers in pharmacology 2018, 30, 9:393441. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, H.; Chen, W.; Huang, P. Berberine induces apoptosis and arrests the cell cycle in multiple cancer cell lines. Arch. Med Sci. 2021, 19, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Och, A.; Zalewski, D.; Komsta. ; Kołodziej, P.; Kocki, J.; Bogucka-Kocka, A. Cytotoxic and Proapoptotic Activity of Sanguinarine, Berberine, and Extracts of Chelidonium majus L. and Berberis thunbergii DC. toward Hematopoietic Cancer Cell Lines. Toxins 2019, 11, 485. [Google Scholar] [CrossRef]
- Bala, M.; Pratap, K.; Verma, P.K.; Singh, B.; Padwad, Y. Validation of ethnomedicinal potential of Tinospora cordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC. J. Ethnopharmacol. 2015, 175, 131–137. [Google Scholar] [CrossRef]
- Mou, L.; Liang, B.; Liu, G.; Jiang, J.; Liu, J.; Zhou, B.; Huang, J.; Zang, N.; Liao, Y.; Ye, L.; Liang, H. ; Berbamine exerts anticancer effects on human colon cancer cells via induction of autophagy and apoptosis.; inhibition of cell migration and MEK/ERK signalling pathway. J BUON 2019, 24(5), 1870-1875.
- Zhang, L.-L.; Ma, L.-N.; Yan, D.; Zhang, C.-E.; Gao, D.; Xiong, Y.; Sheng, F.-Y.; Dong, X.-P.; Xiao, X.-H. Dynamic monitoring of the cytotoxic effects of protoberberine alkaloids from Rhizoma Coptidis on HepG2 cells using the xCELLigence system. Chin. J. Nat. Med. 2014, 12, 428–435. [Google Scholar] [CrossRef]
- Kalaiarasi, A.; Anusha, C.; Sankar, R.; Rajasekaran, S.; John Marshal, J.; Muthusamy, K.; Ravikumar, V. Plant Isoquinoline Alkaloid Berberine Exhibits Chromatin Remodeling by Modulation of Histone Deacetylase To Induce Growth Arrest and Apoptosis in the A549 Cell Line. J. Agric. Food Chem. 2016, 64, 9542–9550. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-H.; Tang, W.-C.; Cheng, Y.-W.; Sia, P.; Huang, C.-C.; Lee, Y.-C.; Jiang, H.-Y.; Wu, M.-H.; Lai, I.-L.; Lee, J.-W.; et al. Targeting of multiple oncogenic signaling pathways by Hsp90 inhibitor alone or in combination with berberine for treatment of colorectal cancer. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2015, 1853, 2261–2272. [Google Scholar] [CrossRef]
- He, B.; Wu, K.; Yang, Q.; Mu, Y.; Zhou, L.; Liu, Y.; Zhou, Q. Berberine inhibits the proliferation of colon cancer cells by inactivating Wnt/β-catenin signaling. Int. J. Oncol. 2012, 41, 292–298. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Patil, B.S. The natural alkaloid berberine targets multiple pathways to induce cell death in cultured human colon cancer cells. Eur. J. Pharmacol. 2012, 688, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Mu, L.; Cui, Y.; Li, Y.; Chen, P.; Xie, H.; Wang, X. Berberine Promotes Apoptosis of Colorectal Cancer via Regulation of the Long Non-Coding RNA (lncRNA) Cancer Susceptibility Candidate 2 (CASC2)/AU-Binding Factor 1 (AUF1)/B-Cell CLL/Lymphoma 2 (Bcl-2) Axis. J. Pharmacol. Exp. Ther. 2019, 25, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Chakraborty, S.; Singh, S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs 2010, 19, 1297–1307. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, X.; Wu, P.; Wink, M.; Li, J.; Dian, L.; Liang, Z. Jatrorrhizine inhibits mammary carcinoma cells by targeting TNIK mediated Wnt/β-catenin signalling and epithelial-mesenchymal transition (EMT). Phytomedicine 2019, 63, 153015. [Google Scholar] [CrossRef]
- Wang, P.; Gao, X.-Y.; Yang, S.-Q.; Sun, Z.-X.; Dian, L.-L.; Qasim, M.; Phyo, A.T.; Liang, Z.-S.; Sun, Y.-F. Jatrorrhizine inhibits colorectal carcinoma proliferation and metastasis through Wnt/β-catenin signaling pathway and epithelial–mesenchymal transition. Drug Des. Dev. Ther. 2019, ume 13, 2235–2247. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, H.; Zhang, W.W.; Huang, J.T.; Song, E.L.; Chen, S.G.; He, F.; Xu, J.; Wang, H.Q. Neuroprotective effect of Jatrorrhizine on hydrogen peroxide-induced cell injury and its potential mechanisms in PC12 cells. Neuroscience Letters 2011, 498, 227–231. [CrossRef]
- Kumari, S.; Kaladhar, D.; Solmon, K.S.; Malla, R.; Kishore, G. Anti-proliferative and metastatic protease inhibitory activities of protoberberines: An in silico and in vitro approaches. Process. Biochem. 2013, 48, 1565–1571. [Google Scholar] [CrossRef]
- Rolle, J.; O Asante, D.; Kok-Fong, L.L.; Boucetta, H.; A Seidu, T.; Tai, L.L.K.; Alolga, R.N. Jatrorrhizine: a review of its pharmacological effects. J. Pharm. Pharmacol. 2021, 73, 709–719. [Google Scholar] [CrossRef]
- Ali, D.; Ali, H. Assessment of DNA damage and cytotoxicity of palmatine on human skin epithelial carcinoma cells. Toxicol. Environ. Chem. 2014, 96, 941–950. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, X.F.; Tang, Y.L.; Xiang, J.F.; Tian, M.Y. Studies of the interactions between three protoberberine alkaloids and Bcl-2 by fluorescence spectroscopy. Acta Chimica Sinica 2011, 69(02), 247. [Google Scholar]
- Wu, J.; Xiao, O.; Zhang, N. ; Xue. , C.; Leung, A.W.; Zhang, H.; Xu, C.; Tang, Q. Photodynamic action of palmatine hydrochlo-ride on colon adenocarcinoma HT-29 cells. Photodiagnosis and Photodynamic Therapy 2016, 15, 53–58. [Google Scholar] [CrossRef]
- Inbaraj, J.J.; Kukielczak, B.M.; Bilski, P.; He, Y.-Y.; Sik, R.H.; Chignell, C.F. Photochemistry and Photocytotoxicity of Alkaloids from Goldenseal (Hydrastis canadensisL.). 2. Palmatine, Hydrastine, Canadine, and Hydrastinine. Chem. Res. Toxicol. 2006, 19, 739–744. [Google Scholar] [CrossRef]
- Hirakawa, K.; Kawanishi, S.; Hirano, T. The Mechanism of Guanine Specific Photooxidation in the Presence of Berberine and Palmatine: Activation of Photosensitized Singlet Oxygen Generation through DNA-Binding Interaction. Chem. Res. Toxicol. 2005, 18, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-K.; Li, H.; Dong, C.-L.; He, X.; Guo, C.-R.; Zhang, C.-F.; Yu, C.-H.; Wang, C.-Z.; Yuan, C.-S. Palmatine from Mahonia bealei attenuates gut tumorigenesis in ApcMin/+ mice via inhibition of inflammatory cytokines. Mol. Med. Rep. 2016, 14, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Yao, Y.; Shen, B.; Liu, J.; Pan, Q.; Liu, N.; Li, L.; Huang, J.; Long, Z.; Shao, L. Columbamine suppresses the proliferation and malignization of colon cancer cells via abolishing Wnt/β-catenin signaling pathway. Cancer Manag. Res. 2019, ume 11, 8635–8645. [Google Scholar] [CrossRef]
- Sun, X.-L.; Zhang, X.-W.; Zhai, H.-J.; Zhang, D.; Ma, S.-Y. Magnoflorine inhibits human gastric cancer progression by inducing autophagy, apoptosis and cell cycle arrest by JNK activation regulated by ROS. Biomed. Pharmacother. 2020, 125, 109118. [Google Scholar] [CrossRef]
- Mohamed, S.; Hassan, E.M.; Ibrahim, N.; Mohamed, S.; Hassan, E.M.; Ibrahim, N. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. Nat. Prod. Res. 2010, 24, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, H.; Liu, Q.; Zhao, Y.; Cui, X.; Guo, S.; Zhang, L.; Ho, C.-T.; Bai, N. Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba. Food Funct. 2016, 7, 2870–2877. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Olech, M.; Łyko, L.; Nowak, R. Influence of Accelerated Solvent Extraction Conditions on the LC-ESI-MS/MS Polyphenolic Profile, Triterpenoid Content, and Antioxidant and Anti-lipoxygenase Activity of Rhododendron luteum Sweet Leaves. Antioxidants 2020, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Nowak, R.; Pielczyk, J.; Łamacz, K. Optimization of Extraction Conditions for Determination of Tiliroside in Tilia L. Flowers Using an LC-ESI-MS/MS Method. J. Anal. Methods Chem. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Baraniak, B.; Szymanowska, U. Lipooxygenase in food of plant origin. Żywność Nauka Technologia Jakość 2006, 2, 29–45. [Google Scholar]
- Podgórski, R.; Cieśla, M.; Podgórska, D.; Bajorek, W.; Płonka, A.; Czarny, W.; Trybulski, R.; Król, P. Plasma microRNA-320a as a Potential Biomarker of Physiological Changes during Training in Professional Volleyball Players. J. Clin. Med. 2022, 11, 263. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134–134. [Google Scholar] [CrossRef]
| ABTS•+ [mgTE/g] |
ORAC [mgTE/g] |
DPPH• [mgTE/g] |
LOX inhibition [%] |
|---|---|---|---|
| 122.92±0.01 | 220.29±0.02 | 63.93±0.01 | 62.60±0.87 |
| CCD841 CoN | LS180 | HT-29 | |
| µg/mL | µg/mL | µg/mL | |
| BVR | 50.21 ± 1.22 | 4.31 ± 1.19 | 46.06 ± 1.11 |
| Berberine | 254.8 ± 2.05 | 0.45 ± 1.25 | 15.92 ± 1.08 |
| Palmatine | 179.8 ± 2.24 | 12.92 ± 1.10 | 29.34 ± 1.08 |
| Berbamine | 13.63 ± 1.02 | 37.62 ± 1.02 | 8.77 ± 1.07 |
| Gene | LS180 | HT29 | |||||
|---|---|---|---|---|---|---|---|
| Control | Exposed | Change of expression (%) | Control | Exposed | Change of expression (%) | ||
| CASP3 | 0.44±0.04 | 1.15±0.07 | 261.36 ↑ | 0.56±0.04 | 1.01±0.08 | 180.36 ↑ | |
| CASP9 | 0.52±0.06 | 1.11±0.16 | 213.46 ↑ | 1.67±0.12 | 2.64±0.14 | 158.08 ↑ | |
| BCL2 | 1.21±0.13 | 0.63±0.06 | 52.06 ↓ | 0.13±0.02 | 0.03±0.01 | 23.08 ↓ | |
| BCL2L1 | 0.45±0.07 | 1.65±0.05 | 366.66 ↑ | 5.7±0.81 | 7.23±0.31 | 126.84 ↑ | |
| BCL2L2 | 0.48±0.1 | 1.33±0.09 | 277.08 ↑ | 0.83±0.17 | 2.74±0.06 | 330.12 ↑ | |
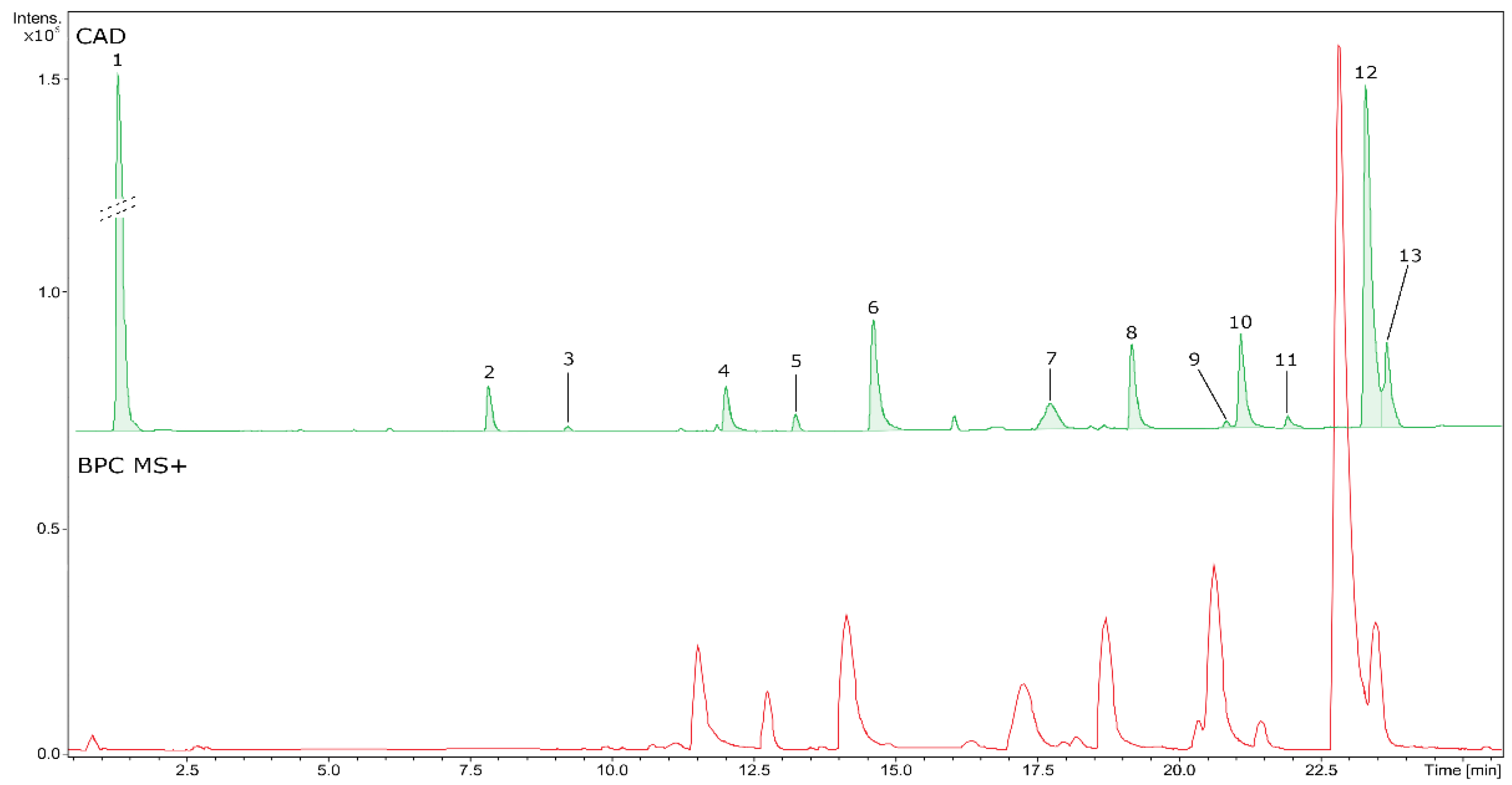
| No | RT (min) | Formula | Error (ppm) | Measured m/z | MS/MS fragments | CAD area (%) | Identity | Content [mg eq/g of dry BVR] ± SD |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.81 | - | 0.0 | 341.1089a | 179.0580,119.0366 | 54.62 | - | uic |
| 2 | 7.28 | C16H19O9 | -0.4 | 355.1036a | 193.0514, 78.0270, 134.0379 | 1.81 | Feruloyl-hexoside | 7.10d ± 0.03 |
| 3 | 8.67 | C17H22O10 | -1.3 | 385.1145a | 223.0624, 79.0699, 164.0471 | 0.22 | Sinapoyl-hexoside | 0.66d ± 0.00 |
| 4 | 11.46 | C20H24NO4 | 1.1 | 342.1695b | 265.0854, 97.1117, 282.0882, 65.0694 | 1.89 | Magnoflorine* | 11.63e ± 0.12 |
| 5 | 12.69 | C19H24NO3 | 0.4 | 314.1749b | 269.1169,209.0957, 237.0907, 165.0697 | 0.64 | Unidentified alkaloid | < LLOQ |
| 6 | 14.06 | C36H39N2O6 | 0.6 | 595.2799b | 595.2798,564.2381, 552.2376, 367.1648 | 6.04 | Aromoline | 7.11e ± 0.22 |
| 7 | 17.16 | C37H40N2O6 | 0.9 | 609.2954b | 609.2955,578.2539, 566.2538, 381.1804 | 2.46 | Berbamine* | 3.19e ± 0.04 |
| 8 | 18.61 | C37H40N2O6 | 0.4 | 609.2957b | 609.2955,381.1807, 174.0913, 578.2532 | 3.85 | Oxycanthine | 5.48e ± 0.10 |
| 9 | 20.27 | C20H20NO4 | -0.2 | 338.1381b | 322.1077,308.0919, 294.1126, 236.0709 | 0.44 | Columbamine | 3.32e ± 0.08 |
| 10 | 20.53 | C20H20NO4 | -0.5 | 338.1389b | 322.1079,308.0920, 294.1128, 236.0708 | 4.4 | Jatrorrhizine* | 23.32e ± 0.11 |
| 11 | 21.35 | C38H43N2O6 | 0.9 | 623.3110b | 623.3108,381.1810, 174.0912, 592.2681 | 0.22 | Rodiasine | < LLOQ |
| 12 | 22.73 | C20H18NO4 | 0.3 | 336.1229b | 320.0916,292.0966, 278.0809, 306.0760 | 19.34 | Berberine* | 70.27 ± 0.48 |
| 13 | 22.97 | C21H22NO4 | 1.4 | 352.1537b | 336.1226,278.0807, 322.1073, 308.1278 | 4.07 | Palmatine* | 7.57e ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





