Introduction
Cervical cancer is the fourth most common cancer among women, with an estimated 604,000 new cases and 342,000 deaths worldwide in 2020 [
1]. The burden of disease is disproportionately high in low- and middle-income countries (LMIC), which account for 85% of cases and nearly 90% of cervical cancer deaths worldwide [
2]. Cervical cancer is the leading cause of female cancer in Tanzania, with 9,772 new cases and 6,695 deaths each year [
3]. Sporadic uptake cervical cancer screening (CCS), prevalence of high-risk oncogenic human papillomavirus (HPV) subtypes, and relatively high rates of human immunodeficiency virus (HIV) co-infection compound risks of infection [
4]. Survival rates are low, with more than half of women diagnosed in Tanzania dying of the disease, as they receive their diagnosis at an advanced stage, when curable treatment options are limited [
5]. In a 2015 study from the Kilimanjaro region of Tanzania, 82% of women reported they had knowledge of cervical cancer, although only 6% had ever been screened [
6].
It is well documented that in order to avoid progression to later stages of disease, effective prevention strategies must be employed widely and routinely. Screening and treatment for pre-cancer of the cervix is a secondary prevention strategy used globally in many low-resource health care settings to prevent cervical cancer. In these contexts, the main method of secondary prevention for cervical cancer is visual inspection of the cervix with the naked eye after application of 3% to 5% acetic acid solution. Visual inspection with acetic acid (VIA) is regarded as the best approach in most low and some middle-income income countries and has been endorsed by the World Health Organization (WHO) when combined with ablative ‘see and treat’ approaches at the time that a woman receives VIA [
7,
8]. VIA can be performed by nurses and other skilled health practitioners and is inexpensive and non-invasive. It can be practiced broadly in lower-level health facilities as well as in HIV care and treatment programs, as part of regular screening services for HIV-related malignancies. More importantly, VIA provides instant results, and those eligible for treatment can receive treatment with ablative methods (thermal ablation or cryotherapy) on the very same day in the same health facility. This “see and treat” method promotes adherence to treatment, as it’s offered immediately after diagnosis, thus minimizing the likelihood of loss to follow-up associated with patient referrals for treatment at an alternate facility or higher level of care [
9]. Since 2020, the WHO has recommended HPV DNA testing as a primary screening method in preference to cytology and visual inspection with acetic acid (VIA). Absence of carcinogenic HPV types indicates an extremely low immediate risk of precancer/cancer and a reassuring low risk of cervical cancer for longer subsequent periods [
10]. The superior sensitivity of HPV testing as compared to cytology and VIA and the reassurance against cervical cancer following a negative HPV test result is increasingly leading to its adoption as the main primary screening method in many countries worldwide [
10]. VIA still plays a role as triage step among HPV positive women, but efforts to improve its performance are needed. In fact, as the interpretation of VIA is highly subjective, quality control has proven to be highly variable, impacting profoundly on both the sensitivity and specificity [11-13]. A triage strategy is important to reduce the number of women referred to colposcopic biopsy in search of precancer or treatment. In many settings, the HPV prevalence is too high and the healthcare capacity too low to refer all HPV positive women to colposcopic biopsy or to treat all.
Informed by the 2020 WHO cervical cancer prevention guidelines, Tanzania’s national cervical cancer prevention program (CECAP) is piloting the use of HPV DNA testing as a primary screening tool across 4 regional sites in 2024. Additional research programs [
14] have been implemented in several sites to evaluate effective strategies for larger scale implementation of HPV DNA testing through self-sampling in health facilities and in rural and urban communities. At present, and while further research to inform the implementation and scale up of HPV DNA testing as a primary screening strategy is completed, the CECAP program continues to deliver screening services that are underpinned by the previous WHO guidelines with the use of VIA and the “see and treat” method, and utilizes nurses and other clinicians providing frontline, non-specialist care. The program consists of six days of competency-based training, and continued mentorship by experienced senior CCS trainers to complete screening with VIA in the field under supervision, as well as routine follow-ups. Due to resource constraints, available training staff, and geographic logistics, follow-up training and supportive supervision opportunities do not always occur, and maintaining competency and quality of screening among CCS providers in the CECAP program has been a notable challenge. The resultant gaps in post-training mentorship and ongoing technical supportive supervision of CCS providers combined with a high turnover of trained providers, has resulted in poor retention of skills and quality of VIA [
15,
16].
Digital cervicography is one method known to improve quality of VIA, which uses a digital camera to transmit an image of the cervix to a television screen/computer monitor, allowing the image to be reviewed at higher clarity and resolution than is possible with the naked eye [
17,
18]. This method has been well established in a number of countries, including Zambia (14), where images are transmitted to experts at coordinating sites for review [
20,
21]. While effective, the program requires a digital camera (oftentimes large and expensive), laptop and/or television monitor, and a consistent electricity source, making it less feasible in many low-income countries with limited health resources, including Tanzania. At the time of study, there was one site in the northern area of the country that had trialed a digital cervicography program, but the program never successfully scaled to other sites due to lack of resources. The widespread use of smartphones and increasingly reliable mobile telephone networks in Tanzania offered a promising solution to challenges observed in the attempted scale-up of digital cervicography. With the knowledge that one of the greatest challenges in creating quality and sustainable VIA programs was ensuring CCS providers had acquired (and retained) the necessary clinical skills in VIA, program designers hypothesized that smartphone cameras could offer a viable option to improve mentorship, oversight, and measurable quality assurance, particularly in remote locations [
15].
The Smartphone-Enhanced Visual Inspection with Acetic acid (SEVIA) program included the dissemination of smartphones to CCS providers, together with a smartphone application permitting real-time, secure sharing of de-identified VIA cervical images and relevant clinical information by health providers to expert ‘reviewers’. Image reviewers are senior VIA providers (i.e. gynecologists, and other skilled physician/non-physician VIA trainers within Tanzania’s CECAP program who review images in real-time, providing supervision and mentorship to nurses and non-physician clinicians [
15]. When there is discordance between the health provider and the reviewer, the application sends a notification, allowing the reviewer to provide secure in-app feedback and recommendations on the diagnosis and treatment plan. This is meant to ensure the most accurate diagnosis and treatment plan for the client, while also promoting continued learning and high-quality supervision for the provider [
15].
Program efficacy of the SEVIA concept was tested in a pilot study in the Kilimanjaro region from June 2014 – March 2015 [
16] and transitioned to scale in a pre-post study to evaluate effectiveness of the intervention at existing CECAP sites using VIA. The program was delivered intensively for 6 months at 24 health facilities, followed by a 6-month maintenance phase. This comprehensive implementation evaluation was completed to evaluate client and health provider-related implementation outcomes, and reports on experiences, perspectives, and general acceptance of the program by both groups.
Methods
The study was conducted from September to December 2016 in 5 regions of Northern Tanzania (Kilimanjaro, Arusha, Kigoma, Bukoba, and Tanga). Measures included a thorough evaluation completed during the implementation phase, in order to understand barriers and enablers of the intervention, and early learnings from program consumers, providers, and higher-level stakeholders. An external consultant completed observational activities and open-ended interviews with image reviewers (n= 5), providers (n=17), community mobilizers (n=14), patients (n=21), supervisors (n=4) and implementation partners (n=5) involved with the SEVIA program. Facilities were selected for assessment based on proportional calculations of regional representation in the program, and by inclusion of rural/urban and higher/lower performing facilities. A total of 66 interviews were conducted with program informants at 14 facilities, in all 5 of the program regions (see
Table 1). Additional interviews conducted at program sites run by an international nongovernmental organization were excluded from this analysis, as these clinics functioned on a private-for profit model, and the patient population was of a higher socioeconomic status.
The Reach, Efficacy, Adoption, Implementation, and Maintenance (RE-AIM) Framework [
22,
23] and the Consolidated Framework for Implementation Research (CFIR) [
24,
25] are implementation science frameworks that were developed to guide systematic assessment of multilevel implementation contexts and assist in translating research to evidence-based practice. Drawing on the CFIR and RE-AIM frameworks, a customized logic model (Appendix 1) was created to visually demonstrate the inputs, resources, and outputs required for program success. An implementation evaluation framework was designed by adopting similar implementation outcome variables [
31]. Implementation outcome measures included acceptability, adoption, appropriateness, feasibility, fidelity, implementation costs, coverage, and sustainability. Data collection was done iteratively, with continual refinement of the protocol.
One researcher trained in qualitative research methods conducted, transcribed, and coded all participant comments and interviews, allowing for data immersion and obtaining an overall sense of the data. Using the outcome variable framework [
26], content inductive analysis was used for each variable [
27]. An open coding approach was adopted, forming a general description of the research topic by generating categories and subcategories as they emerged [
27]. This systematic approach was appropriate for open-ended interviews to determine trends and patterns. Discussion with a second researcher familiar with the data, confirmed emerging categories. Lastly, to minimize confirmation bias, two external researchers unfamiliar with the data and preliminary findings, independently coded all participant interviews inductively with QSR-NVivo [
28]. Secondary researchers undertook a thematic analysis, established consensus on emerging themes, and resolved conflicts to determine final results. Findings were confirmed with the preliminary researcher, and all three collaborated to determine the final themes presented.
Results
A full extrapolation of results by implementation outcome are provided in
Table 2. Overall, findings indicated that the intervention was trusted by both patients and providers, was easily implemented within regular/routine practice and care and assisted in both the quality of diagnosis/care offered to patients, and in the oversight and ongoing training of providers. Women appreciated seeing an image of their cervix as it provided immediate understanding of their health and body, and providers expressed increased confidence in the care they were able to provide. Community level knowledge or trust of CCS in general was seen as a barrier impacting overall acceptability of the program’s desired outcome. Most common misconceptions of screening were that the procedure would be intrusive and painful/uncomfortable, and that the reproductive parts would be removed for examination. Women additionally expressed a reluctance to screen in fear of receiving a positive result or prognosis. The SEVIA application was seen to be quickly understood by providers, but issues related to mobile network coverage impacted the speed and regularity with which providers could share images with reviewers in real-time. While the intervention appeared to reach both women of middle and lower socioeconomic statuses, it was difficult to determine the true reach of the program, as interviews were generally conducted with respondents who had already reached the centre. Community mobilizers did provide some useful information in regard to coverage/reach of the program, but their position was inherently biased as they were tasked with mobilizing within a particular community. The extent to which more rural communities not encompassed within the study had an understanding of screening or cervical cancer in general could not be established. Motivations for participation were unclear, as providers and image reviewers were provided small per diems to see patients and review images. In the absence of compensatory incentives in the long-term, program sustainability could not be sufficiently determined.
Discussion
A number of key findings were highlighted across all settings and by numerous respondent types. A major challenge was network connectivity issues and interruptions especially in rural health facility settings, which was discussed by providers, reviewers, and other program stakeholders. While Tanzania has seen significant improvements in the speed and reliability of mobile telephone networks in recent years, coverage issues persist in many, primarily rural areas. Certain cellular providers have strong networks in particular geographic regions but not in others. As the program spans the country, program implementers were challenged to select a carrier to provide reliable coverage at all sites, and some sites struggled more than others. A more comprehensive coverage plan with multi-providers may be required to ensure every facility is equipped with a phone with sufficient network coverage. This would dramatically decrease the necessity of providers saving images, reduce any delays of image reviewers responding to providers, and allow the intervention to function in real time as intended.
An overwhelming majority of providers and reviewers reported that SEVIA integrated seamlessly into their general practice of screening, giving it high feasibility and probability of long-term integration in standard practice. However, it was unclear whether study participants would be equally motivated to continue using SEVIA once study per diems ceased. A significant portion of the clients who were screened during the intervention phase of the study were acquired at outreach campaigns (small mobilization efforts conducted at facilities in rural villages) where providers were given per diems. Campaigns were funded by the research study in order for newly trained providers to reach their minimum number of screens for certification, as well as to increase the number of study participants observed in a short period of time. It was later observed that a consistently low number of women reported to facilities for screening after the intervention phase of the program.
The intervention was also found to increase knowledge and skills of providers with limited training. SEVIA permitted providers to better visualize the cervix, and consequently increased confidence in their diagnosis and role as screeners in general. These findings are supported by other mHealth interventions in resource-limited countries which demonstrate the value of mobile phones in tackling barriers to service provision and improving both the range and quality of services offered by community level health providers [29-31]. For example, in a qualitative study evaluating the acceptability and usability of a mobile phone–based ophthalmic testing system to perform comprehensive eye examinations in Nakuru, Kenya, healthcare providers reported that the tool aided them in detection and diagnosis, provided decision support, improved communication among providers, and assisted in education and training [
32].
Improved health education and enhanced health literacy are essential for SEVIA or similar mobile health programs and/or applications supporting cervical cancer screening services to be broadly accepted and sustainably implemented at the community level, especially in rural and remote communities. While there appeared to be widespread acceptability for use of the smartphone to capture images of the cervix by women who had already agreed to screen, there appeared to be low acceptability at the community level of cervical cancer screening in general. For example, a number of patients and providers commented there was a misconception that the reproductive parts would be removed for examination during the procedure. Others expressed fears that screening would be painful and uncomfortable, and feared receiving a positive result (especially among HIV+ women). As CCS is paired with HIV testing in Tanzania, this may especially deter women most at-risk of receiving a positive HIV diagnosis. Additionally, results indicated that the majority of women were not aware that early-stage detection and treatment by ablative methods could be done on-site. These beliefs are aligned with other studies reporting barriers to cervical cancer screening uptake in the region. For example, a qualitative cross-sectional study in Lilongwe, Malawi, found barriers to CCS with VIA uptake to include misconceptions of screening procedures and fatalistic views on cancer in general [
33]. In this study, most participants reported that prior to undergoing cervical cancer screening they had limited understanding of the process. Myths and misconceptions of the screening process included expectations that the exam would be painful, fear of receiving a positive screening result, distrust in healthcare workers and suspicion of specimen collection and removal of the uterus [
33]. In another cross-sectional study assessing factors associated with cervical screening uptake among HIV infected women at Mildmay, Uganda, where CCS with VIA was integrated into HIV care, respondents reported similar misconceptions related to screening, such as removal of their ovaries and/or uterus and “cutting off of flesh” [
34]. While our study did not specifically seek to ascertain the acceptability of CCS in the general sense, these findings have implications for the potential impact SEVIA on target populations in future. The distinct lack of knowledge around cervical cancer at the community level and primarily at rural community sites where our evaluation took place signals the need for more widespread education on cervical cancer, screening, and treatment. Our findings, in addition to others [
11] demonstrate that much of the fear, mistrust, and misconceptions can be alleviated with targeted health education. Results suggest that in order for SEVIA to reverse the trajectory of cancer diagnoses in the country, efforts must also be placed on community education, increasing health literacy, and general promotion of CCS.
Patients generally expressed positive experiences with SEVIA and appreciated the addition of smartphone technology into screening. The data suggests SEVIA may even serve to empower patients with respect to health education and individual/personal health literacy. Being able to directly visualize one’s own cervix and any associated lesions provides immediate reassurance and information about one’s health, thereby providing opportunity for improved individual health literacy and understanding of self. Increased health literacy has been shown to improve one’s knowledge and self-care behaviours among individuals with various health conditions across socioeconomic and cultural settings [35-37]. Desire to know one’s health status was reported as a key outcome in the same study of VIA clients in Lilongwe noted above, “I did that [screening] because I wanted to know the condition of my body, you can just be staying and never be certain you are okay or not. So, this time I thought it wise to go get screened” [
33].
As Tanzania moves towards more widespread implementation of HPV DNA testing as a primary screening strategy that can provide more broad population coverage (e.g., women can perform a vaginal self-swab in a rural community with the guidance of a trained community health worker and results can be quickly delivered back to a local screening nurse to communicate with the woman about the test result and next steps for follow-up screening and directing the woman to a local screening site for visual assessment for treatment if they are HPV DNA positive. The role of a mobile health platform such as SEVIA has potential for supporting rapid roll out and scale up of new strategies that utilize HPV self-sampling.
Limitations
As this study employed a cross-sectional design, temporality cannot be inferred. Additionally, given the challenges of sampling hard-to-reach populations, the present results from patients could underrepresent more marginalized women. All of the patient testimonies were obtained from women who reached health facilities in the program and agreed to be screened, thus a great deal of information is missing on the challenges of increasing uptake of CCS more generally. Finally, the variables in this analysis by all respondent types were self-report, and thus may be subject to social desirability bias.
Conclusion
Findings from this semi-structured qualitative implementation evaluation indicated that the SEVIA program was a highly regarded tool for the enhancement of CCS services in Northern Tanzania. Acceptability, adoption, appropriateness, and feasibility of the intervention were highly recognized. It proved an effective means of improving good clinical practice among providers and fit seamlessly into existing roles and processes. While technical restraints caused adaptations to the intervention protocol, these are to be expected in the preliminary development of a technology. Alterations to the App were ongoing at the time of study, and usability of the tool was increasing. Network connectivity issues were a persistent challenge to program adherence in a number of locations and will need to be overcome for the program to be effective in future. Allocation of permanent funds for outreach activities and more comprehensive community education and mobilization approaches are recommended, in order to increase the regularity of screening in general and access the most vulnerable women. In 2018, as a consequence of SEVIA supporting the development of good clinical practice among CCS providers through oversight and continuous training, Tanzania’s CECAP program integrated the SEVIA model into their Cervical Cancer Prevention Strategic Plan for 2019 to 2024 [
38]. Funding restrictions and resource shortages have remained intermittent challenges, as has the COVID-19 pandemic, with health system resources re-directed to public health. At the time of writing, the SEVIA program was undergoing further scale up and evaluation of a new version of the SEVIA mobile App that includes integration of HPV DNA test results and additional tracking functions and follow-up indicators to reduce loss to follow-up and support navigation of women to improved linkage to follow-up screening services.
Author Contributions
Conceptualization, K.Y., A.F., Methodology, K.Y., A.F., Formal Analysis, A.F., N.S., S.C., Writing – Original Draft Preparation, K.Y., A.F., N.S., S.C., M.C., O.G., E.E., Writing – Review & Editing, K.Y., A.F., N.S., S.C., M.C., N.W., L.A., G.M., S.Y., O.O., O.G., J.S., E.E., Visualization, A.F., Supervision, K.Y., Project Administration, N.W., K.Y., Funding Administration, K.Y.,. The authors have reviewed and approved the final manuscript.
Funding
This research was funded by Grand Challenges Canada: [Grant Number 0774-05].
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the National Institute of Medical Research.
Acknowledgments
We thank all those who contributed their time and expertise to this project, particularly participants and country staff and partners who supported project implementation and helped enable this evaluation. Disclosure statement: No potential conflict of interest was reported by the author(s).
Conflicts of Interest
The authors declare no conflict of interest.
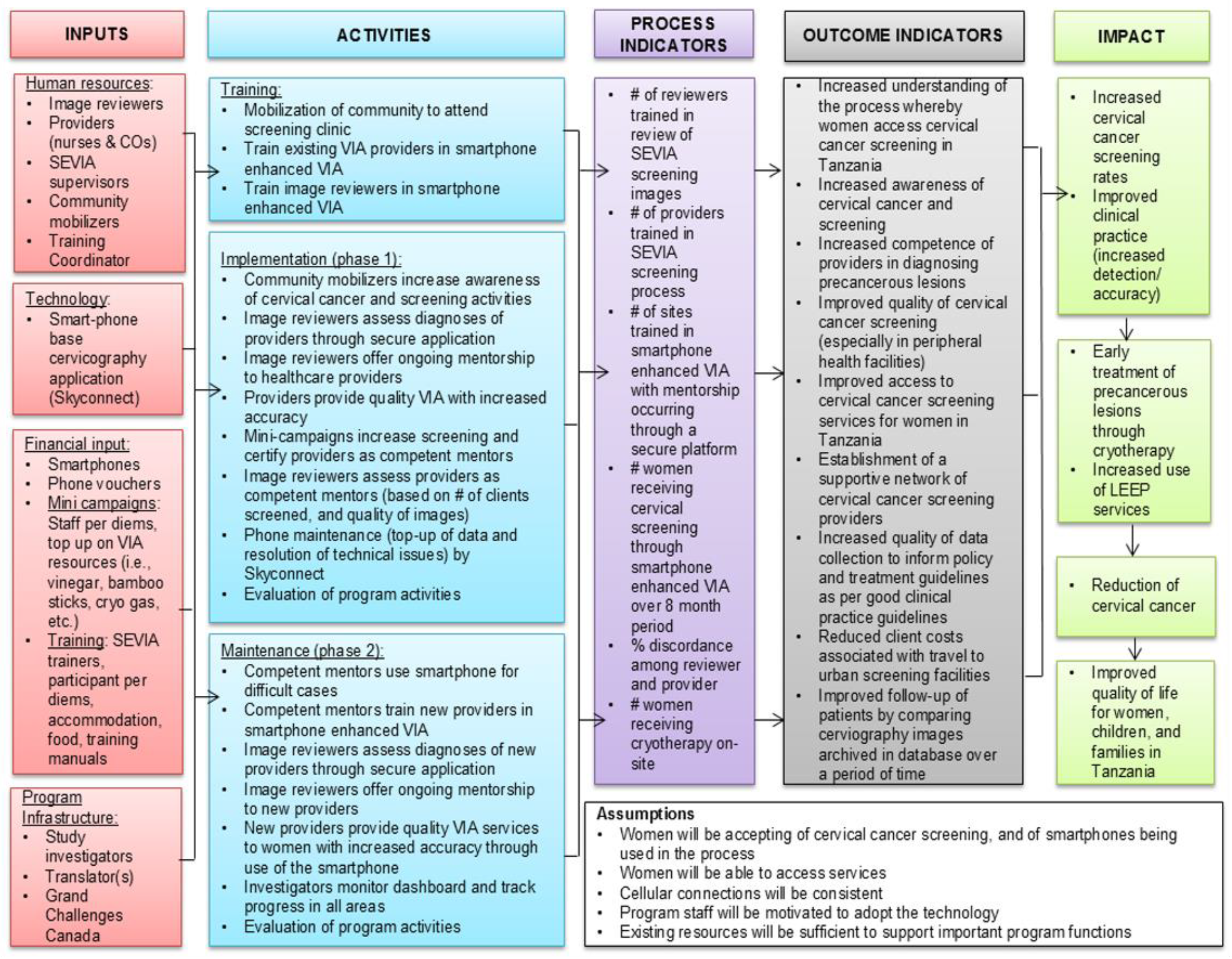
Appendix A. SEVIA Logic Model
References
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians, 71(3), 209–249. [CrossRef]
- Bray F., Ferlay Jacques, Soerjomataram Isabelle, Siegel R.L., Torre L.A., Jemal A., Global Cancer Statistics GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;2018.
- Bruni L., Diaz M., Castellsagué X., Ferrer E., Bosch F.X., de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010.
- Runge, Ava S., Megan E. Bernstein, Alexa N. Lucas, and Krishnansu S. Tewari. “Cervical Cancer in Tanzania: A Systematic Review of Current Challenges in Six Domains.” Gynecologic Oncology Reports 29 (August 1, 2019): 40–47. [CrossRef]
- Denny L. Control of cancer of the cervix in low- and middle-income countries. Ann Surg Oncol. 2015;22:728–733.
- Cunningham MS, Skrastins E, Fitzpatrick R, et al. Cervical cancer screening and HPV vaccine acceptability among rural and urban women in Kilimanjaro Region, Tanzania. BMJ Open. 2015;5:e005828.
- Cervical Cancer Common amongst African Women. WHO | Regional Office for Africa, n.d. https://www.afro.who.int/news/cervical-cancer-common-amongst-african-women.
- World Health Organization WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. http://apps.who.int/iris/bitstream/10665/94830/1/9789241548694_eng.pdf.
- World Health Organization. Prevention of cervical cancer through screening using visual inspection with acetic acid (VIA) and treatment with cryotherapy. A demonstration project in six African countries: Malawi, Madagascar, Nigeria, Uganda, the United Republic of Tanzania, and Zambia. World Health Organization. 2012.
- Gage JC, Schiffman M, Katki HA, Castle PE, Fetterman B, Wentzensen N, et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst. 2014 Aug;106(8).
- Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TCJ. Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005 Nov;294(17):2173–81.
- Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009 Apr;360(14):1385–94.
- Catarino R, Schäfer S, Vassilakos P, Petignat P, Arbyn M. Accuracy of combinations of visual inspection using acetic acid or lugol iodine to detect cervical precancer: a meta-analysis. BJOG. 2018 Apr;125(5):545–53.
- de Sanjosé S, Perkins RB, Campos N, Inturrisi F, Egemen D, Befano B, Rodriguez AC, Jerónimo J, Cheung LC, Desai K, Han P. Design of the HPV-automated visual evaluation (PAVE) study: Validating a novel cervical screening strategy. Elife. 2024 Jan 15;12:RP91469.
- Yeates K, Erwin E, Mtema Z, Magoti F, Nkumbugwa S, Yuma S, Hopman WM, Ferguson A, Oneko O, Macheku G, Mtei AF. Smartphone-Enhanced Training, QA, Monitoring, and Evaluation of a Platform for Secondary Prevention of Cervical Cancer: Opportunities and Challenges to Implementation in Tanzania. JCO Global Oncology. 2020 Jul;6:1114-23.
- Yeates KE, Sleeth J, Hopman W, Ginsburg O, Heus K, Andrews L, et al. Evaluation of a Smartphone-Based Training Strategy Among Health Care Workers Screening for Cervical Cancer in Northern Tanzania: The Kilimanjaro Method. Journal of Global Oncology. 2016;2(6):356-64.
- Bomfim-Hyppólito S, Franco ES, Franco RG, et al. Cervicography as an adjunctive test to visual inspection with acetic acid in cervical cancer detection screening. Int J Gynaecol Obstet. 2006;92:58– 63.
- Bae SN, Kim JH, Lee CW, et al. Correlation between the digital cervicography and pathological diagnosis performed at private clinics in Korea. Int J Med Sci. 2012;9:698–703.
- Mwanahamuntu MH, Sahasrabuddhe VV, Blevins M, et al. Utilization of cervical cancer screening services and trends in screening positivity rates in a ‘screen-and-treat’ program integrated with HIV/AIDS care in Zambia. PLoS One. 2013;8:e74607.
- Parham GP, Mwanahamuntu MH, Kapambwe S, Muwonge R, Bateman AC, Blevins M, et al. Population-Level Scale-Up of Cervical Cancer Prevention Services in a Low-Resource Setting: Development, Implementation, and Evaluation of the Cervical Cancer Prevention Program in Zambia. PLOS ONE. 2015;10(4):e0122169.
- Parham GP, Mwanahamuntu MH, Pfaendler KS, Sahasrabuddhe VV, Myung D, Mkumba G, et al. eC3--a modern telecommunications matrix for cervical cancer prevention in Zambia. Journal of lower genital tract disease. 2010;14(3):167-73.
- Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013 Jun;103(6):e38-46. Epub 2013 Apr 18. PMID: 23597377; PMCID: PMC3698732. [CrossRef]
- Harden SM, Gaglio B, Shoup JA, Kinney KA, Johnson SB, Brito F, Blackman KC, Zoellner JM, Hill JL, Almeida FA, Glasgow RE, Estabrooks PA. Fidelity to and comparative results across behavioral interventions evaluated through the RE-AIM framework: a systematic review. Syst Rev. 2015 Nov 8;4:155. PMID: 26547687; PMCID: PMC4637141. [CrossRef]
- Damschroder, L., Hall, C., Gillon, L. et al. The Consolidated Framework for Implementation Research (CFIR): progress to date, tools and resources, and plans for the future. Implementation Sci 10, A12 (2015). [CrossRef]
- Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016 May 17;11:72. PMID: 27189233; PMCID: PMC4869309. [CrossRef]
- Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what is it and how to do it. BMJ Research Methods & Reporting. 2013;347:f6753.
- Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs.2008;62(1):107–15. [CrossRef]
- Godau R. Qualitative Data Analysis Software: NVivo. Qual Res J. 2004.
- Arul C, Cheong Y, Lee L. The hope of mobile phones in Indian rural healthcare. Journal of Health Communication 2012;6(1):406-421.
- Kay M. World Health Organization. 2011. mHealth: New horizons for health through mobile technologies URL: http://www.who.int/goe/publications/goe_mhealth_web.pdf.
- Braun R, Catalani C, Wimbush J, Israelski D. Community health workers and mobile technology: A systematic review of the literature. PLoS One 2013;8(6):e65772.
- Lodhia V, Karanja S, Lees S, Bastawrous A. Acceptability, Usability, and Views on Deployment of Peek, a Mobile Phone mHealth Intervention for Eye Care in Kenya: Qualitative Study JMIR Mhealth Uhealth 2016;4(2):e30. [CrossRef]
- Bula AK, Lee F, Chapola J, Mapanje C, Tsidya M, Thom A, et al. (2022) Perceptions of cervical cancer and motivation for screening among women in Rural Lilongwe, Malawi: A qualitative study. PLoS ONE 17(2): e0262590. [CrossRef]
- Bukirwa A, Mutyoba JN, Mukasa BN, Karamagi Y, Odiit M, Kawuma E, Wanyenze RK. Motivations and barriers to cervical cancer screening among HIV infected women in HIV care: a qualitative study. BMC Womens Health. 2015 Oct 12;15:82. PMID: 26458898; PMCID: PMC4603977. [CrossRef]
- Stormacq, Coraline1,2,3; Wosinski, Jacqueline2,3,4; Boillat, Evelyne2; Van den Broucke, Stephan5 Effects of health literacy interventions on health-related outcomes in socioeconomically disadvantaged adults living in the community: a systematic review, JBI Evidence Synthesis: July 2020 - Volume 18 - Issue 7 - p 1389-1469. [CrossRef]
- Dennison CR, McEntee ML, Samuel L, Johnson BJ, Rotman S, Kielty A, Russell SD. Adequate health literacy is associated with higher heart failure knowledge and self care confidence in hospitalized patients. The Journal of cardiovascular nursing. 2011 Sep;26(5):359.
- Heine M, Lategan F, Erasmus M, Lombaard CM, Mc Carthy N, Olivier J, van Niekerk M, Hanekom S. Health education interventions to promote health literacy in adults with selected non-communicable diseases living in low-to-middle income countries: A systematic review and meta-analysis. J Eval Clin Pract. 2021 Dec;27(6):1417-1428. Epub 2021 Mar 22. PMID: 33749092. [CrossRef]
- United Republic of Tanzania, Ministry of Health, Community Development, Gender, Elders and Children (2019). Tanzania Cervical Cancer Prevention and Control Strategic Plan 2020 – 2024.
Table 1.
Provides a description of the participants recruited with their affiliated role and geographic region.
Table 1.
Provides a description of the participants recruited with their affiliated role and geographic region.
| Region |
Number of Sites |
Image Reviewers |
Mobilizers |
Patients |
Providers |
Supervisors |
Implementation Partners |
Total |
| Arusha |
4 |
1 |
4 |
3 |
3 |
1 |
|
12 |
| Kagera |
2 |
1 |
4 |
4 |
4 |
1 |
|
14 |
| Kigoma |
2 |
1 |
3 |
4 |
4 |
1 |
|
13 |
| Kilimanjaro |
5 |
1 |
3 |
8 |
6 |
1 |
|
19 |
| |
|
|
|
|
|
|
|
|
| Tanga |
1 |
|
|
2 |
1 |
|
|
3 |
| N/A |
|
|
|
|
|
|
5 |
|
| Total |
16 |
4 |
16 |
25 |
22 |
4 |
2 |
73 |
Table 2.
Results by Implementation Outcome Variable.
Table 2.
Results by Implementation Outcome Variable.
| Implementation Outcome |
Working Definition |
Related Terms |
Results |
| Acceptability |
The perception among stakeholders (for example patients, providers, managers, policy makers) that the intervention is agreeable |
Comfort, relative advantage, credibility |
Trust was highlighted by mobilizers, providers, and image reviewers, as a key requirement for buy-in from the community. Respondents reported general trust among the community towards providers and emphasized the use of trusted community leaders as mobilizers. Knowledge that reviewers were specialists was also seen to instill trust among patients. For those who remained untrusting, dominant beliefs included: (1) that the reproductive parts would be removed during the screening process for examination; (2) the intimate/intrusive nature of the procedure; (3) fear of pain from speculum; (3) fear of receiving a positive result (especially for HIV+ women); and (4) uncomfortableness with male providers. While some women feared screening services and were initially hesitant, they were quite willing to attend screening services once educated by community mobilizers. For example, one patient indicated that they were “scared at first, but once provided with health education felt totally fine.” Most women did not appear to have preliminary knowledge of cervical cancer or available screening services until program education was offered, and many were unaware that pre-cancerous treatment was available on-site. SEVIA campaigns (concentrated outreach activities) appeared to have increased awareness of cervical cancer, in addition to screening services available in the community. At a few sites, it was noted that clients may have preferred to receive screening services from a provider that they did not know (i.e., a foreigner or someone from the referral hospital) while others did not indicate a preference. Acceptability of the use of smartphones was widespread when proper pre-counselling was provided, and the cervix was shown post-procedure. |
| Adoption |
The intention or action to carry out the program |
Uptake, utilization, intention to try |
Program staff appeared highly motivated to implement the smartphone-based and mobile App supported program. For providers in particular, adoption was high, as the smartphone was an add-on to their existing VIA practices, and a tool which simplified their roles. One provider stated, “The addition of the phone has simplified my work because I can see the cervix from a different angle.” While other program staff (image reviewers, community mobilizers, supervisors, etc.) also appeared motivated to employ SEVIA, it was unclear whether this motivation was driven by true readiness to adopt the intervention, or by implementation phase compensation. For example, one mobilizer explained that their motivation to continue their work was that “many women need help,” while another mobilizer indicated that they had concerns about a “gap in funding” which would influence their ability to continue with program implementation. |
| Appropriateness |
The perceived fit of the program in the setting |
Relevance, perceived fit, compatibility, perceived usefulness or suitability |
Appropriateness was deemed very high in all program regions, and by all stakeholders involved. All providers indicated that they liked using the technology. Reasons included: (1) taking a picture of the cervix with the smartphone allowed them to visualize it better and make a more accurate assessment which enhanced confidence in their role as a provider; (2) they appreciated having another specialist available to corroborate their diagnosis; and (3) they were receiving critical ongoing training/education from their image reviewer. Both image reviewers and providers noted that the addition of the smartphone did not interfere with their existing processes or outstanding responsibilities. However, a desire for ongoing mentorship in the form of refresher training was consistently recommended among providers, mobilizers, and image reviewers. Despite many providers feeling unsatisfied with the duration of training and/or lack of refresher training, the majority still reported feeling comfortable training others. The majority of patients indicated they had an overall positive experience with SEVIA and were comfortable with the addition of the smartphone to the screening process. Reasons included: (1) they liked being able to see a picture of their cervix after the screening – it helped provide them reassurance about their health status; and (2) they liked that the provider could double check the diagnosis with a specialist. |
| Feasibility |
The practicality of the program being carried out in the setting |
Practicality, fit, utility, trialability |
While many providers noted challenges learning the technology in the early stages of implementation, all expressed mastery of the application within 4-5 months of using it. Overall, SEVIA appeared to assist providers in their roles, and helped streamline processes rather than creating additional work. Image reviewers did not report difficulty learning the technology or express any challenges in reviewing images on top of their pre-existing responsibilities. Apart from the addition of the smartphone, all program resources were covered in existing VIA programs, although these resources did not account for an increase in patients due to SEVIA efforts. This may be a barrier to program implementation in settings where many women are being screened, or where supply chain issues are a challenge. Network connectivity was seen as the biggest challenge at almost all of the facilities visited, which created barriers to implementation in many settings. Other challenges included sharing phones among providers at a given site, and delays in reviewer responses. The former speaks to a challenge of limited program resources (i.e. phones) and the latter due to network connectivity issues. |
| Fidelity |
the degree to which the program is carried out as intentionally planned |
Adherence, delivery as intended, integrity, quality of programme delivery, intensity of dosage of delivery |
A number of adaptions were made throughout the lifecycle of the program, most notably to the application. While the program initially intended for providers to send patient files and receive a response from reviewers within a 5-minute timeframe, this was not the case in all instances. In many cases, files were being saved within the application, and sent when network connectivity returned. Protocol for follow-up differed by facility (the patient returns for results in person, or is called if treatment is required), but in all cases loss to follow-up was not seen as a concern (all women returned or were easily contacted by phone). While it was intended that all providers offer extensive group education sessions to clients, one-on-one counseling on the purpose of the phone being used and explanation of where the image is being sent, as well as showing the woman a picture of her cervix, it was noted that not all these practices were being employed by providers in every setting and there was limited oversight to ensure adherence to desired standards. |
| Implementation costs |
the incremental costs of carrying out the program |
Marginal cost, total cost |
Initial implementation costs were higher during the implementation stage, which included training expenses, purchasing of phones, and mass screening campaign expenses, however, once this phase was completed, program maintenance fees were quite low. |
| Reach |
The ability of intervention to reach target population/s |
Coverage, range, accessibility |
In most regions and program sites, respondents reported that women of all economic positions were accessing screening services, but more so among middle- and lower-income women. In many of the rural settings, transport issues were noted as a barrier, especially for women of the lowest income bracket. It was also noted that women of the highest income bracket may have been receiving screening elsewhere (i.e., private facilities), or may not have recognized the need to access screening services altogether. For example, a patient from the Kilimanjaro region noted the perception that, “higher class go to town, while the lower class go to the village health care centres.” In terms of geographic accessibility, the majority of respondents discussed inadequate program reach to rural areas where screening is more difficult to access and expressed the need for increased mobilization to villages and rural areas. For example, one patient expressed there was a need for “more education to women in the villages: in the interior. They don’t get information, so it is not easy to convince them to come [for screening].” |
| Sustainability |
The ability of the program to continue independent of research implementation |
Longevity, maintainability, support |
Sufficient infrastructure existed to sustain the program long-term - reliable technology, widespread adoption, motivated stakeholders, and alignment with the local policy climate. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).






