1. Introduction
Breast cancer is the most common cancer globally [
1]. Soft tissue reconstruction following breast cancer treatment is an important consideration as there is good evidence it improves physical and psychological well-being [
2,
3]. The most common method of breast reconstruction is the use of silicone implants [
4]. Silicone implants have been developed as a permanent prosthesis. However, this stimulates a foreign body response resulting in fibrous capsule formation, which can lead to capsular contracture causing breast deformity, pain and device failure [
5,
6]. This is a significant drawback, which essentially limits the lifespan of breast implants, often resulting in revision surgery [
7,
8], or conversion to autologous reconstruction [
9].
Autologous reconstruction with free tissue transfer offers a robust method to breast reconstruction. The most common method is an abdominal based free flap on the deep inferior epigastric artery. These flaps have higher satisfaction rates compared to silicone implants, likely because they offer a more like-for-like reconstruction, highlighting the advantage of autologous tissue [
10,
11]. However, these flaps require patients to have an adequate donor site for free flap harvest, and there is an associated donor site morbidity such as abdominal bulge or hernia [
12]. Additionally, free tissue transfer is complex and the surgery requires a high level of specialized care, which can limit patient access to this type of surgery. Autologous fat graft is an alternative method to breast reconstruction which is safe and minimizes the donor site requirement [
13,
14]. However, the variable rate of fat graft survival usually means it is limited to small volume correction or repeat procedures to reconstruct large volumes [
15]. Therefore, there are limitations with current methods of breast reconstruction largely because these techniques replace tissue rather than regenerating it.
A recent review explores the perspectives offered by bioengineering to regenerate soft tissue which holds immense promise in the field of breast reconstruction [
16]. One approach to stimulate regeneration of soft tissue has utilized additively manufactured chambers with a pedicled fat flap [
17,
18]. However, the long-term outcomes are not reported, and there has been variable success. Another approach has used folded surgical mesh [
19] at the time of implantation, however this technique has not been successfully reproduced by others likely due to is complexity [
20].
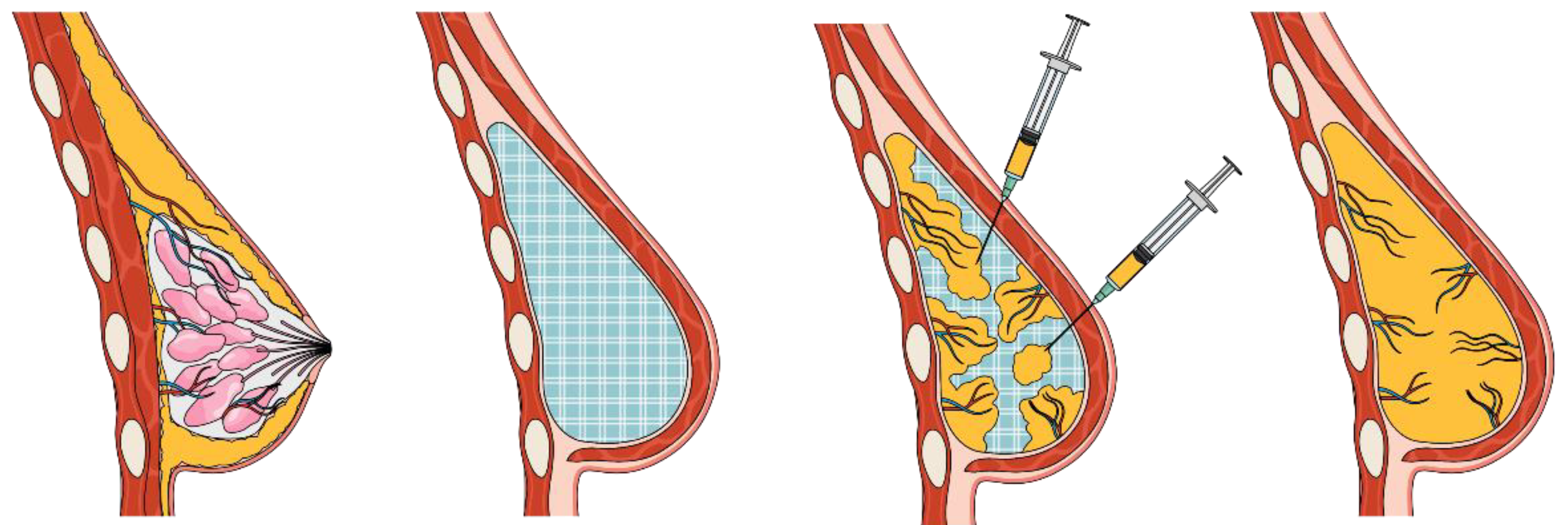
Scaffold guided breast tissue regeneration (SGBTR) utilizes additively manufactured bioresorbable breast scaffolds made from medical-grade polycaprolactone (mPCL), which are filled with autologous fat graft. Over time, the body acts as a bioreactor, which supports the regeneration of soft tissue whilst the scaffold dissolves, resulting in a tissue engineered construct of regenerated soft tissue (
Figure 1). Additive manufacturing techniques can produce patient specific implants which can have complex porous geometries with high accuracy and reproducibility. Reproducible control of the internal scaffold architecture is essential as the internal structure plays a critical role in guiding cell infiltration, tissue organization and ultimately homeostasis [
21]. Whilst, extensive control of scaffold design is currently possible, rational design that directs cell organization to achieve specific tissue structures is not yet a commonly taken approach and remains a challenge. In addition, optimal design relies on knowledge of how cells sense and respond to different scaffold morphologies, composition and surface properties during the regeneration process which is still largely unknown.
We hypothesize using bioresorbable, mPCL scaffolds which are additively manufactured results in a clinically acceptable and reproducible method to regenerate soft tissue. The aim of this study is to describe the long-term tissue regeneration outcomes and mechanical properties of SGBTR in a preclinical large animal model.
2. Materials and Methods
2.1. Scaffold Design, Manufacturing and Characterisation
Scaffolds were designed to be 100 ml approximating an A-cup sized breast. The scaffolds were additively manufactured from medical-grade polycaprolactone (mPCL) which is a bioresorbable polymer material that is FDA and CE approved for implantation [
22]. These scaffolds had 90% porosity, a pore size of 8mm, 100% pore connectivity and each strut was 350 µm in diameter. Scaffolds were pre-treated with plasma and sterilized by gamma radiation (Steritech Pty. Ltd., Australia).
2.2. Large Animal Study
A detailed methodology on the animal model used has been published.[
23] All animal studies were approved by the Queensland University of Technology (QUT) University Animal Ethics Committee (Approval Number: 1600000282) and in accordance with the requirements on the Animal Code for the Care and Use of Animals for Scientific Purposes (8th Ed, 2013).
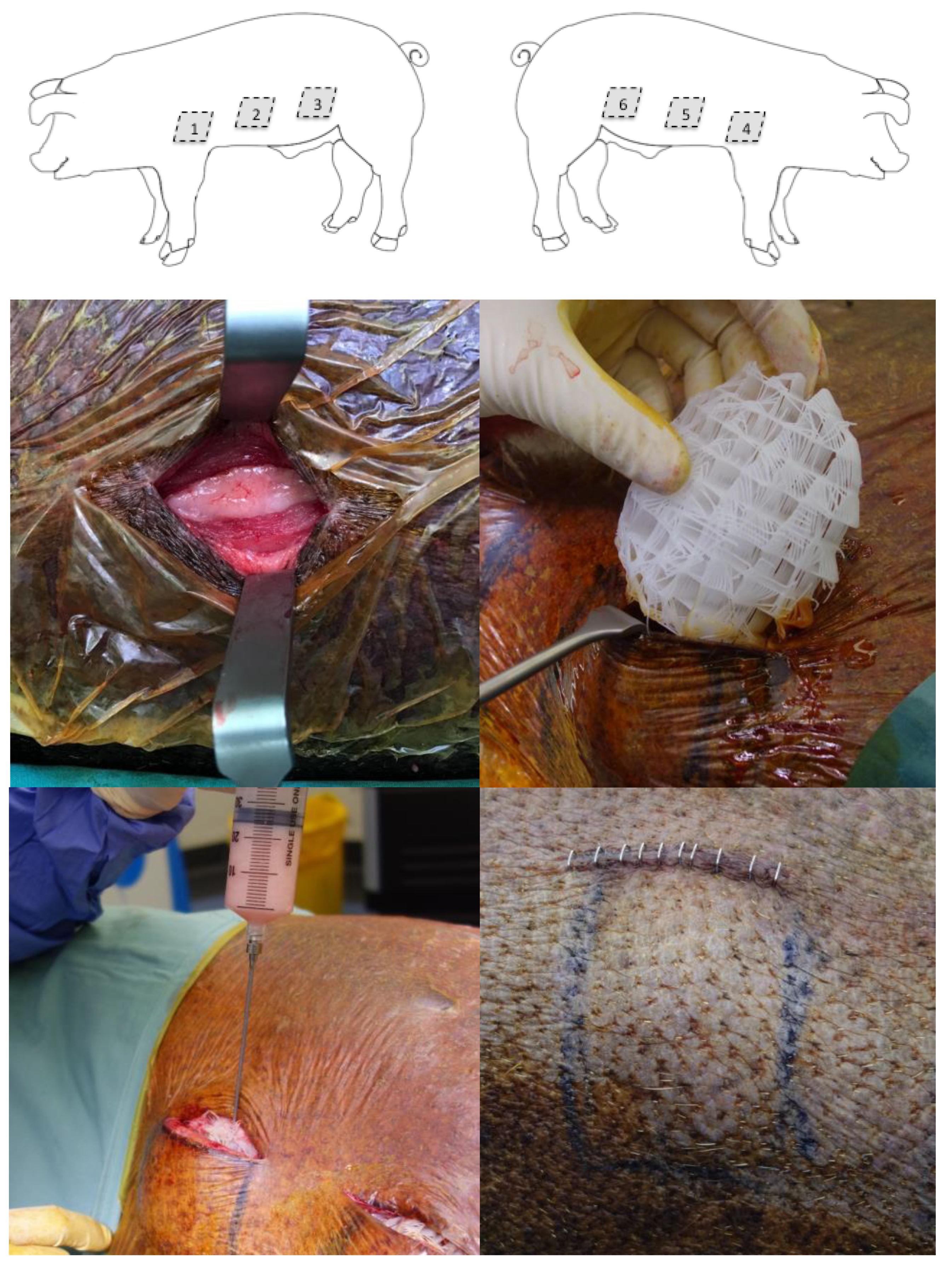
A total of 55 scaffolds which were 100 ml in volume were implanted in 11 adult female immunocompetent Australian minipigs under general anaesthesia (
Figure 2). These scaffolds were implanted in the flanks of the pig under their superficial muscle (panniculus carnosus) layer. Six treatments were performed on each animal with treatments randomised to their site. Some treatment sites required a second procedure four weeks later to fill the scaffold with autologous fat graft in a delayed fashion.
Treatment groups were:
50 ml fat graft injection only, no scaffold implanted (control);
Scaffold only implanted (control);
Scaffold implanted and filled with 50 ml immediate fat graft;
Scaffold implanted and filled with 50 ml immediate platelet rich plasma;
Scaffold implanted and filled with 50 ml delayed fat graft after four weeks;
Scaffold implanted and filled with 50 ml immediate platelet rich plasma, then 50ml delayed fat graft after four weeks.
Autologous fat graft was harvested from the animal’s abdomen using a Body-Jet Eco (Human Med AG, Schwerin, Germany) liposuction system. Platelet-rich plasma (PRP) was harvested from the animal’s whole blood using the Angel (Arthrex, Florida, USA) system and mixed with platelet poor plasma and 10 ml of 10% calcium chloride to make up a final 50 ml volume. Animals were reviewed daily for eight weeks whilst housed in an animal facility with veterinary support. They were then transferred to an agistment facility and regularly monitored until a 12-month endpoint when they were euthanised to facilitate post-humous analysis.
2.3. Post-Humous Analysis
Imaging was performed with ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) to determine volume and distribution of regenerate tissue. Immediately after euthanasia, a CT scan (Toshiba Aquilion Lightning, Tokyo, Japan) using standard helical acquisition was performed. Scaffolds were then explanted. At the fat graft only treatment site, a portion of tissue including skin, subcutaneous fat, panniculus carnosus muscle and deep muscle was excised within the boundaries marked by tattoo at time of initial surgery. A repeat CT was performed on each explanted sample with 0.5 mm slice thickness. Each sample was then scanned using a 3T-MRI (Siemens MAGNETOM Prisma, Munich, Germany) using T1 and T2 fat suppression sequences, as well as wavelength excitation curves to investigate water and fat peaks.
CT volumetric analysis was performed using a free open-source medical image reader (Horos 4.0.1). The total volume of regenerated tissue in the explanted scaffold was calculated by segmenting a region of interest (ROI) of greater than –200 Hounsfield units to capture all tissue other than air. This calculated volume was correlated with a water displacement technique of explanted samples. Volume of fat was calculated by segmenting a ROI of -50 to -200 Hounsfield units, which corresponds to the density of fat in pigs [
24].
Distribution analysis was performed using a free open-source image processor (ImageJ 1.54i). Fat excitation sequences were used and a middle slice through the scaffold construct was imported. This slice was divided into three zones and converted to a binary scale. The proportion of high signal corresponding to fat and low signal corresponding to other tissue types was calculated in each zone.
Histological and immunohistochemistry studies were performed to analyse the structure and composition of the regenerate tissue. Slides were stained with Haematoxylin and Eosin (H&E) for general analysis. Immunohistochemistry was performed using Perilipin-1 (PLN1) (Anti-Perilipin-1 antibody, ab3526, Abcam) antibodies, which have been used as a marker to identify viable adipocytes [
25], Masson’s trichrome for connective tissue, and von Willebrand Factor (vWF) as a marker for vascularity.
Mechanical studies were performed to determine mechanical properties of the regenerated soft tissue construct. Explanted samples were tested using an Instron 5848 MicroTester (Instron, Massachusette, USA) at a strain rate of 0.1 mm/sec. Scaffolds were placed in a 37-degree Celsius phosphate-buffered saline (pH = 7) and compressed to 50% to calculate an approximate elastic modulus.
2.4. Statistical Analysis
Statistical analysis was performed in SPSS v26 (IBM) and Microsoft Excel. Significance was defined as p <0.05. Mean values are reported as mean ± standard errors with a 95% confidence internal.
3. Results
3.1. Macroscopic Analysis
The surgical procedure and scaffolds were well tolerated by all animals (n = 11) across the 12-month study period (
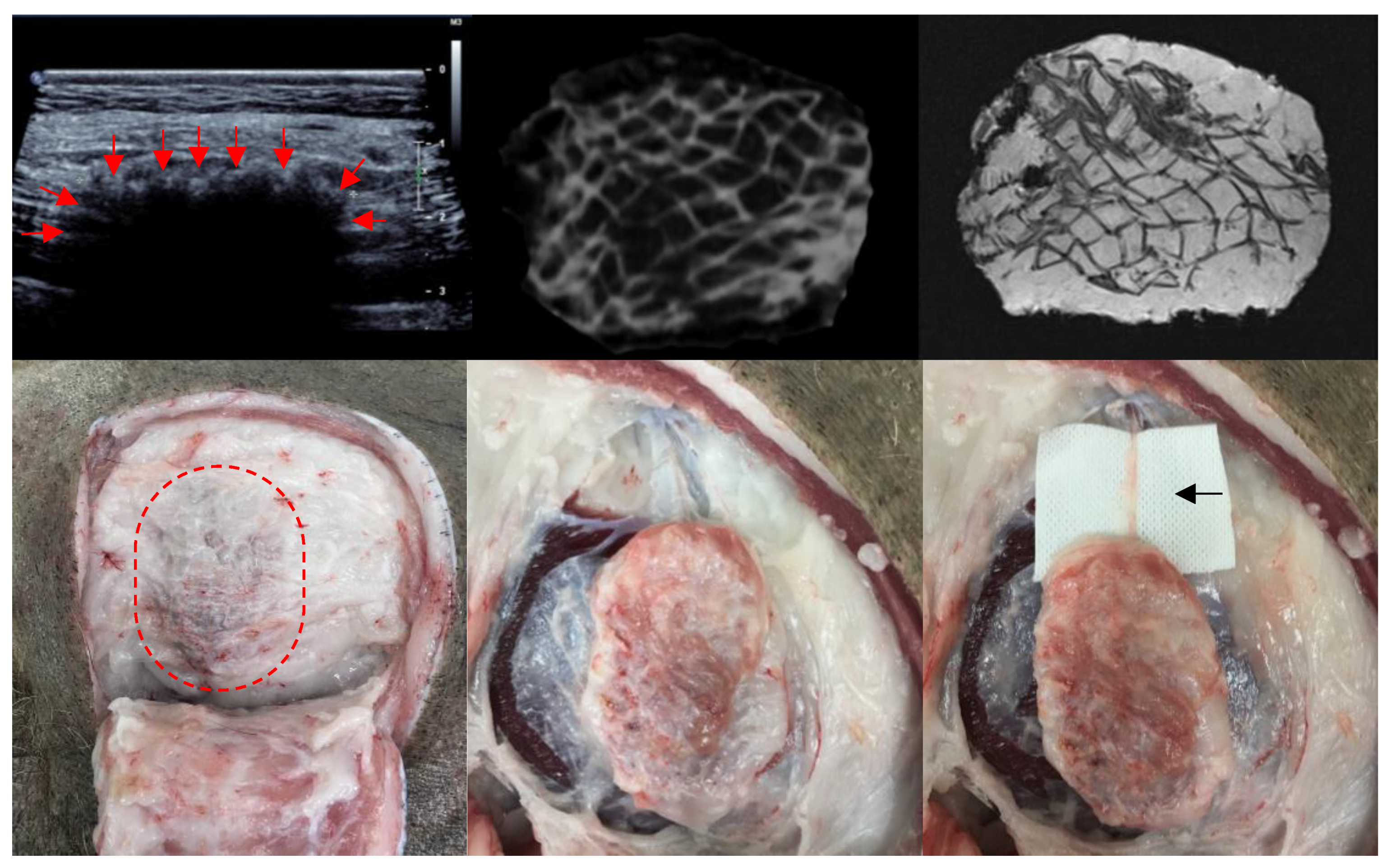
Figure 2). There were no local wounds or systemic complications across all treatment sites (n = 66). All scaffolds (n = 55) were completely filled with soft tissue macroscopically and radiologically (CT and MRI) (
Figure 3). A fibrous capsule was not identified around any scaffolds (
Figure 3). Macroscopically, all scaffolds demonstrated significant tissue integration, and this is supported by ultrasound showing tissue infiltration into the scaffold structure (
Figure 3). No scaffolds demonstrated significant rotation or migration from the implantation site. Blood vessels were identified growing into the scaffold structure from major vessels in the surrounding host tissue (
Figure 3).
3.2. Volume and Distribution
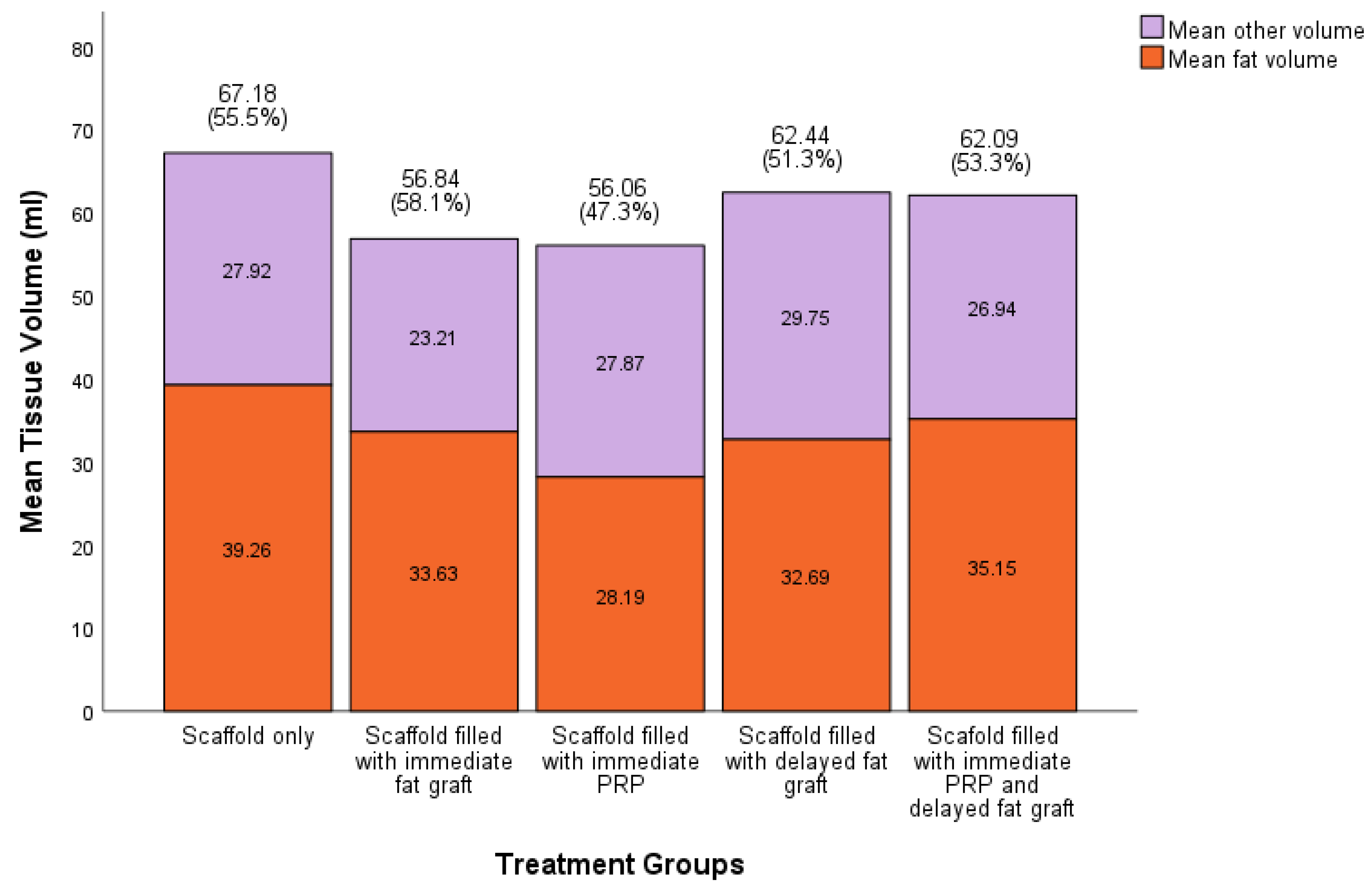
We were able to regenerate and sustain a mean soft tissue volume of 60.9 ± 4.5 ml (95% CI) at 12 months across all scaffolds. There was no statistically significant difference between total volume of soft tissue sustained between treatment groups (
Figure 4). Across all groups, the soft tissue was primarily composed of adipose tissue compared to other soft tissue types, with the highest proportion of adipose tissue regenerated in the scaffolds filled with immediate fat graft group and the lowest in the scaffold filled with immediate PRP. In scaffolds filled with fat graft the there was no statistical difference in volume of soft tissue sustained compared to scaffolds not filled with fat graft (60.46 ± 5.6 mL (95% CI), 61.62 ± 7.8 mL (95% CI) respectively, p>0.05).
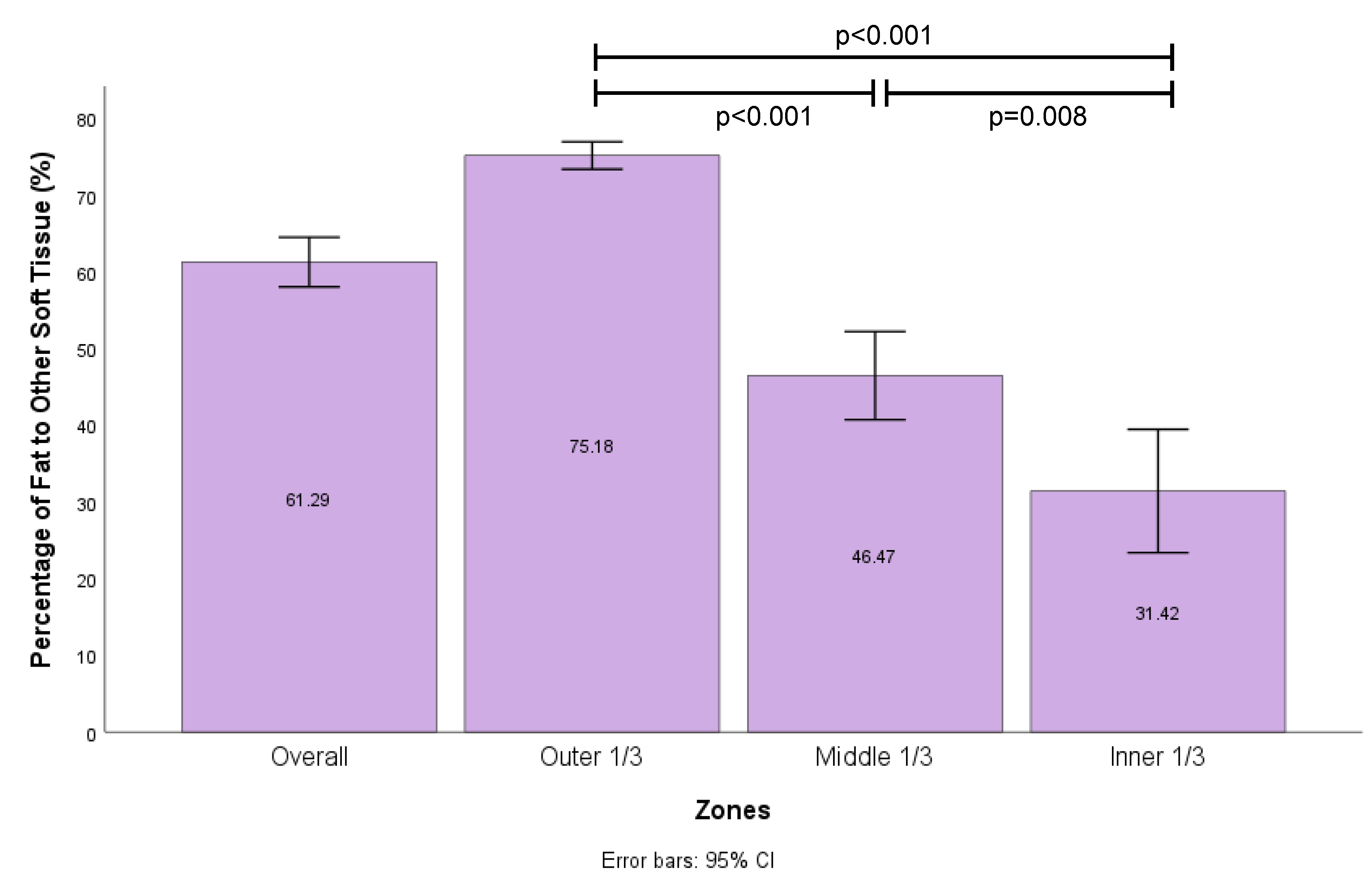
MRI analysis was performed to study the distribution of soft tissue regeneration throughout the scaffold structure at 12 months (
Figure 5)
Figure 5. Proportion of fat per zone in each scaffold based on MRI analysis with error bars (n = 43 in each group). Significant statistical difference between outer 1/3, middle 1/3 and inner 1/3 zones on Welch’s ANOVA and Games-Howell post-hoc test.. The largest percentage of adipose tissue was identified in the outer 1/3 (75%) compared to the middle 1/3 (46%) and inner 1/3 (31%) of the scaffold which were all statistically different (p <0.001 and p = 0.008).
3.3. Microscopic Analysis
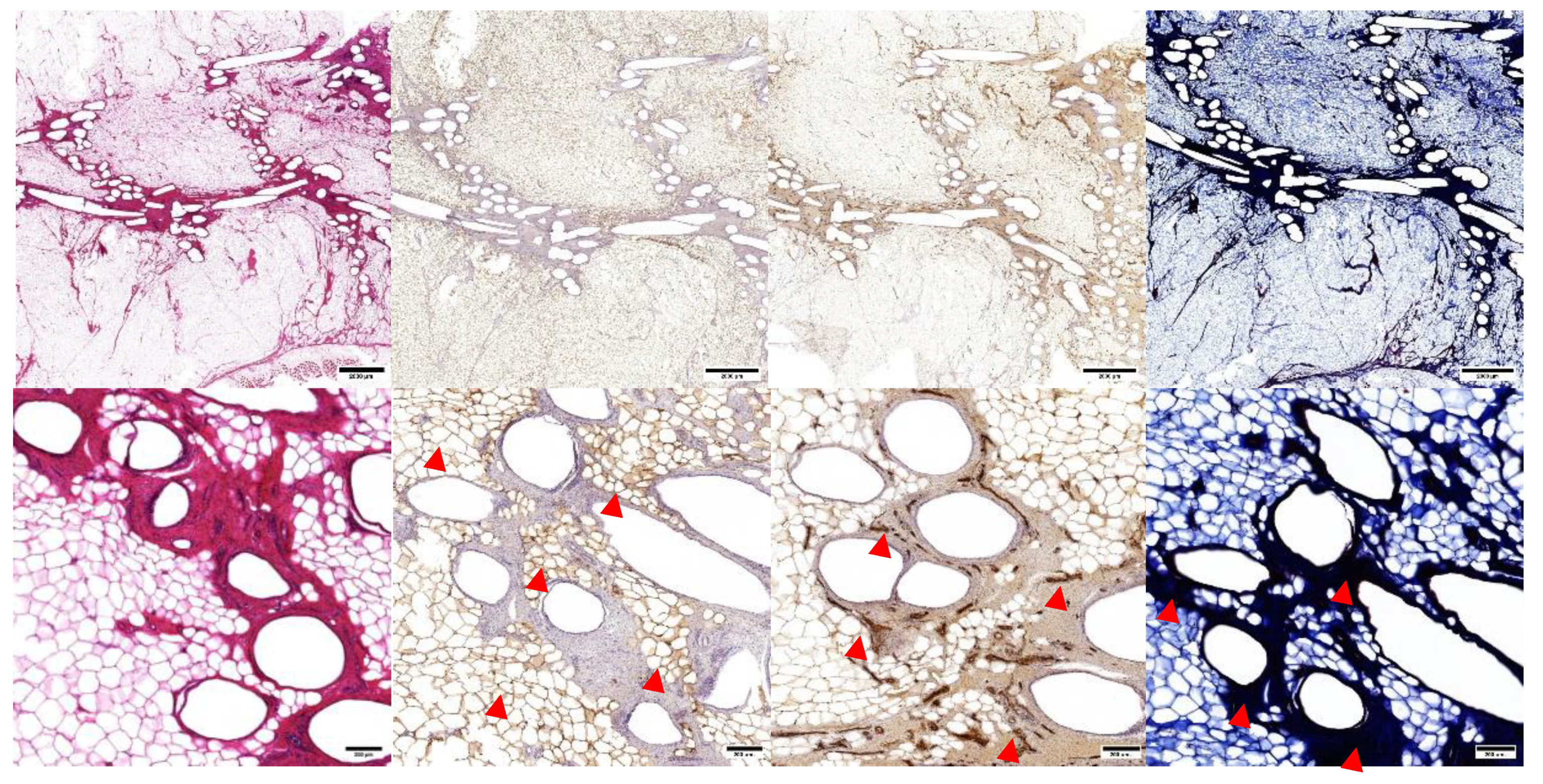
Tissue structure and composition were assessed with histology and immunohistochemistry (
Figure 6). H&E overview demonstrated an arrangement of circular mPCL bars and struts, surrounded by an adjacent rich extracellular matrix (ECM), and an outer zone of regenerate adipocytes. The presence of adipocytes was confirmed using PLN1 stain. There was rich vascularity in the ECM zone and around adipocytes on vWF analysis. Masson’s trichrome demonstrated collagen staining around scaffold struts. There was no evidence of a microscopic confluent fibrous capsule at the periphery of the scaffold.
3.4. Mechanical Testing
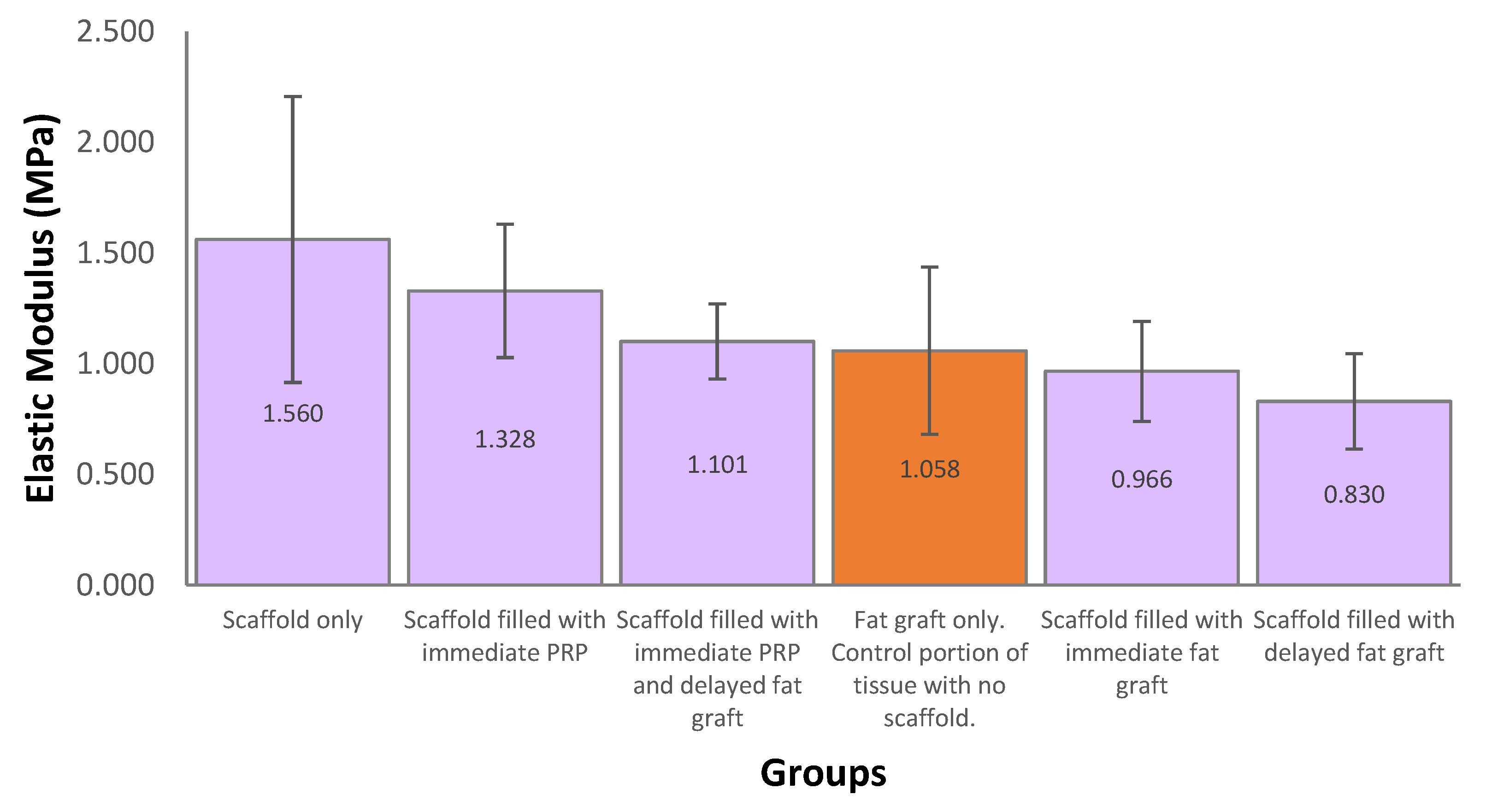
The mean elastic modulus for explanted scaffolds was 1.157 MPa which was similar to a control portion of tissue excised at the fat graft only treatment site which was 1.058 MPa (
Figure 7).
4. Discussion
The ability to regenerate soft tissue through SGBTR is an emerging field of research. This study was able to regenerate and sustain a mean soft tissue volume of 60.9 ± 4.5 mL (95% CI) over 12 months. Importantly, in the treatment groups where scaffolds were not filled with autologous fat graft, this study regenerated 66.1 ± 9.7 ml (95% CI), representing de-novo regeneration of soft tissue. In contrast, scaffolds filled with 50 ml of autologous fat graft regenerated 59.5 ± 4.6 mL (95% CI), representing a modest 20% increase in soft tissue, but this did not account for potential fat graft resorption which may increase that gain. In other large animal studies, Shim et al.[
26] were also able to regenerate de-novo soft tissue utilizing empty PCL scaffolds designed in a ball-like shape in four pigs over three months. However, their scaffolds were significantly smaller (3cm in diameter equating to 14 ml volume) compared to our volumes (100 ml), limiting their clinical applicability. Chhaya et al. [
27] were able to achieve a 6.1-fold and 4.95-fold increase in soft tissue in 75 ml mPCL scaffolds which were filled with immediate and delayed fat graft respectively over three months. Our study supports the previous work in achieving soft tissue regeneration utilizing mPCL scaffolds and to date is the largest and longest pre-clinical large animal study highlighting the safety and efficacy of SGBTR.
The composition of regenerated tissue is an important consideration. In the context of breast reconstruction, the ability to regenerate fat is critical as the breast is predominantly composed of adipose tissue.[
28] However, the breast is also composed of other soft tissue such as glandular and connective tissue.[
28,
29] In our study, the soft tissue regenerated was largely composed of adipose tissue across all treatment groups. This was confirmed with immunohistochemistry using PLN1. We also regenerated other soft tissue types including fibrotic and vascular tissue, confirmed on Masson’s trichrome and vWF stains respectively. The ideal proportion of adipose tissue to other tissue types is largely unknown, but the ability to modulate the type and proportion of regenerated soft tissue will be key for clinical application.
Chhaya et al. [
27] identified the positive influence of autologous fat graft on increasing adipose tissue regeneration in a pilot study. We similarly identified that the highest proportion of adipose tissue regenerated was in the treatment group filled with immediate fat graft, but this did not reach statistical significance. In fact, we identified that there was largely no effect of various treatments such as fat graft, timing and PRP on tissue regeneration. The difference may be due to the larger scaffold sizes investigated in our study, meaning a longer distance from the injected fat graft to peripheral vascularized tissue. On MRI analysis, we identified the greatest proportion of adipose tissue sustained was in the outer 1/3 of the scaffold, closest to the peripheral vascularized tissue, and the least was in the inner 1/3, which is furthest from vascularized tissue. This closely follows a model on fat graft survival by Eto et al. [
25] where there is an outer zone of surviving adult adipocytes and adipose-derived stromal cells (ASCs), an intermediate zone of necrotic adipocytes but surviving ASCs where regeneration occurs, and an inner zone of necrotic adipocytes and ASCs where no regeneration occurs. This highlights that there may be a role for using autologous fat graft to guide tissue regeneration towards forming more adipose tissue, but vascularity remains is a critical consideration.
A different approach to improving adipose tissue vascularization is by transferring vascularized tissue as a fat flap. Findlay et al. [
17] investigated a pedicled fat flap in a 78.5 ml tissue engineered chambers to regenerate adipose tissue and achieved a 5-fold increase. This was scaled up in a human trial of 5 patients [
18]. However, there was limited success where one patient formed 210 ml of new tissue, but 3 patients failed to develop any soft tissue enlargement and the device was encased in a thick fibrous capsule. Faglin et al. [
30] investigated tissue engineered chambers in rats and pigs, and discussed the importance of modulating the fibrous encapsulation around the implants as it influences the extent of tissue regeneration. This highlights the importance of considering the foreign body response (FBR) to implanted biomaterials and its influence on tissue regeneration.
A FBR is universal to any implanted biomaterial, and usually results in fibrous capsule formation to wall the implant off from the host [
31]. In the context of breast reconstruction, a thick fibrous capsule is undesirable as it can result in capsular contracture causing breast deformity, pain and implant rupture [
5,
6]. We identified no macroscopic evidence of a fibrous encapsulation around our scaffolds at 12 months. This is largely due to the porous structure of our scaffold which was large enough to encourage tissue integration, rather than peripheral fibrous capsule formation [
32]. This contrasts with other studies using implants with smaller pores resulting in peripheral fibrous encapsulation [
17,
18,
26]. In addition, we identified the scaffold FBR resulted in a rich ECM network containing blood vessels, essentially creating an internal vascularized structure around mPCL struts allowing for tissue regeneration. The use of mPCL is an important factor in creating a favorable FBR for tissue regeneration due to its slow degradation profile. This has led to the ability to regenerate clinically relevant large volumes of soft tissue without a vascularized fat flap.
This study did not cause any significant wound issues or discomfort for the pigs over the 12-month study period suggesting that mPCL scaffolds are well tolerated. The mechanical properties of the regenerated soft tissue construct are an important factor in this. Mechanical testing on our samples demonstrated a similar elasticity to the soft tissue dissected from the pig. We also identified, the soft tissue constructs were significantly stiffer than human breast tissue [
33]. However, this may be desirable in the context of breast reconstruction to maintain shape and projection over time, negating the effect of gravity and breast ptosis [
34,
35]. Scaffolds also provide mechanical protection against external forces, which improves fat graft survival [
36]. Conscious design and additive manufacturing is critical to engineer a reliable and reproducible scaffold to protect tissue regeneration. It overcomes repeatability issues reported [
20] when using an alternative method of folding surgical mesh to create a scaffold [
19].
This study did not identify any treatment effects of fat grafting, timing or PRP on tissue regeneration. This may be due to an inadequate sample size to identify a small effect. The generalizability of this study is limited to smaller clinically relevant volumes approximating an A-cup breast size. The ability to scale this approach to larger volumes may influence the proportion of soft tissue regeneration due to limitations in vascularity. However, this approach has been successfully applied to reconstruct a large volume pectus excavatum deformity in a human [
37].
5. Conclusions
This study was able to regenerate clinically relevant volumes of soft tissue in the pre-clinical long-term large animal model utilizing SGBTR principles. This provides safety and efficacy data for future clinical trials.
Author Contributions
Conceptualization, animal model development and design, M.C. J.J. S.S. O.U. M.W. D.W.H.; Ethics application and performing surgical procedure M.C. S.S. M.W. D.W.H. Imaging analysis, M.C. J.J.; Tissue analysis, J.J. M.C.; Mechanical analysis, M.M. D.W.H.; Clinical analysis, M.C. J.J. O.U. M.W.; Writing—original draft preparation, M.C.; Manuscript review and editing, M.C. J.J. M.M. R.F. S.S. O.U. M.W. D.W.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Health Innovation, Investment and Research Office, Queensland Health – Junior Doctor Research Fellowship.
Institutional Review Board Statement
All animal studies were approved by the Queensland University of Technology (QUT) University Animal Ethics Committee (Approval Number: 1600000282) and in accordance with the requirements on the Animal Code for the Care and Use of Animals for Scientific Purposes (8th Ed, 2013).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is not publicly available due to ethical reasons.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15-23.
- Rowland JH, Desmond KA, Meyerowitz BE, Belin TR, Wyatt GE, Ganz PA. Role of breast reconstructive surgery in physical and emotional outcomes among breast cancer survivors. Journal of the National Cancer Institute. 2000;92(17):1422-9.
- Lovelace DL, McDaniel LR, Golden D. Long-term effects of breast cancer surgery, treatment, and survivor care. Journal of midwifery & women's health. 2019;64(6):713-24.
- Panchal H, Matros E. Current Trends in Postmastectomy Breast Reconstruction. Plast Reconstr Surg. 2017;140(5S Advances in Breast Reconstruction):7s-13s.
- Headon H, Kasem A, Mokbel K. Capsular Contracture after Breast Augmentation: An Update for Clinical Practice. Arch Plast Surg. 2015;42(5):532-43.
- Handel N, Cordray T, Gutierrez J, Jensen JA. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg. 2006;117(3):757-67; discussion 68-72.
- Coroneos CJ, Selber JC, Offodile ACI, Butler CE, Clemens MW. US FDA Breast Implant Postapproval Studies: Long-term Outcomes in 99,993 Patients. Annals of Surgery. 2019;269(1):30-6.
- Finlay B, Kollias V, Hall KA, Clement Z, Bingham J, Whitfield R, et al. Long-term outcomes of breast reconstruction and the need for revision surgery. ANZ Journal of Surgery. 2021;91(9):1751-8.
- Visser NJ, Damen THC, Timman R, Hofer SOP, Mureau MAM. Surgical Results, Aesthetic Outcome, and Patient Satisfaction after Microsurgical Autologous Breast Reconstruction following Failed Implant Reconstruction. Plastic and Reconstructive Surgery. 2010;126(1):26-36.
- Eltahir Y, Werners LLCH, Dreise MM, Zeijlmans van Emmichoven IA, Werker PMN, de Bock GH. Which Breast Is the Best? Successful Autologous or Alloplastic Breast Reconstruction: Patient-Reported Quality-of-Life Outcomes. Plastic and Reconstructive Surgery. 2015;135(1):43-50.
- Fracon S, Renzi N, Manara M, Ramella V, Papa G, Arnež Z. Patient satisfaction after breast reconstruction: implants vs. autologous tissues. Acta Chir Plast. 2018;59(3-4):120-8.
- Chang EI, Chang EI, Soto-Miranda MA, Zhang H, Nosrati N, Robb GL, et al. Comprehensive analysis of donor-site morbidity in abdominally based free flap breast reconstruction. Plastic and reconstructive surgery. 2013;132(6):1383-91.
- Agha RA, Fowler AJ, Herlin C, Goodacre TEE, Orgill DP. Use of autologous fat grafting for breast reconstruction: A systematic review with meta-analysis of oncological outcomes. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2015;68(2):143-61.
- Coleman SR, Saboeiro AP. Fat Grafting to the Breast Revisited: Safety and Efficacy. Plastic and Reconstructive Surgery. 2007;119(3):775-85.
- Herly M, Ørholt M, Larsen A, Pipper CB, Bredgaard R, Gramkow CS, et al. Efficacy of breast reconstruction with fat grafting: A systematic review and meta-analysis. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2018;71(12):1740-50.
- Berkane Y, Oubari H, van Dieren L, Charlès L, Lupon E, McCarthy M, et al. Tissue engineering strategies for breast reconstruction: a literature review of current advances and future directions. Ann Transl Med. 2024;12(1):15.
- Findlay MW, Dolderer JH, Trost N, Craft RO, Cao Y, Cooper-White J, et al. Tissue-engineered breast reconstruction: bridging the gap toward large-volume tissue engineering in humans. Plastic and reconstructive surgery. 2011;128(6):1206-15.
- Morrison WA, Marre D, Grinsell D, Batty A, Trost N, O'Connor AJ. Creation of a large adipose tissue construct in humans using a tissue-engineering chamber: a step forward in the clinical application of soft tissue engineering. EBioMedicine. 2016;6:238-45.
- Rehnke RD, M Asher Schusterman I, Clarke JM, Price BC, Waheed U, Debski RE, et al. Breast reconstruction using a three-dimensional absorbable mesh scaffold and autologous fat grafting: a composite strategy based on tissue-engineering principles. Plastic and reconstructive surgery. 2020;146(4):409e-13e.
- Hwang K, Wu X. Breast Reconstruction Using a Three-Dimensional Absorbable Mesh Scaffold and Autologous Fat Grafting: A Composite Strategy Based on Tissue-Engineering Principles. Plastic and Reconstructive Surgery. 2021;148(4):660e-1e.
- Mohseni M, Bas O, Castro NJ, Schmutz B, Hutmacher DW. Additive biomanufacturing of scaffolds for breast reconstruction. Additive Manufacturing. 2019;30:100845.
- Guarino V, Gentile G, Sorrentino L, Ambrosio L. Polycaprolactone: synthesis, properties, and applications. Encyclopedia of polymer science and technology. 2002:1-36.
- Cheng M, Janzekovic J, Mohseni M, Medeiros Savi F, McGovern J, Galloway G, et al. A preclinical animal model for the study of scaffold-guided breast tissue engineering. Tissue Engineering Part C: Methods. 2021;27(6):366-77.
- McEvoy FJ, Madsen MT, Strathe AB, Svalastoga E. Hounsfield Unit dynamics of adipose tissue and non-adipose soft tissues in growing pigs. Research in veterinary science. 2008;84(2):300-4.
- Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno S, et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plastic and reconstructive surgery. 2012;129(5):1081-92.
- Shim K-S, Ryu DH, Jo H-S, Kim K-B, Kim D-H, Park Y-K, et al. Breast tissue reconstruction using polycaprolactone ball scaffolds in a partial mastectomy pig model. Tissue Engineering and Regenerative Medicine. 2023:1-13.
- Chhaya MP, Balmayor ER, Hutmacher DW, Schantz JT. Transformation of Breast Reconstruction via Additive Biomanufacturing. Sci Rep. 2016;6:28030.
- Vandeweyer E, Hertens D. Quantification of glands and fat in breast tissue: an experimental determination. Annals of Anatomy-Anatomischer Anzeiger. 2002;184(2):181-4.
- Gaskin KM, Peoples GE, McGhee DE. The fibro-adipose structure of the female breast: A dissection study. Clinical Anatomy. 2020;33(1):146-55.
- Faglin P, Gradwohl M, Depoortere C, Germain N, Drucbert A-S, Brun S, et al. Rationale for the design of 3D-printable bioresorbable tissue-engineering chambers to promote the growth of adipose tissue. Scientific Reports. 2020;10(1):11779.
- Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. The AAPS journal. 2010;12:188-96.
- Jordan SW, Fligor JE, Janes LE, Dumanian GA. Implant porosity and the foreign body response. Plastic and reconstructive surgery. 2018;141(1):103e-12e.
- Ramiao NG, Martins PS, Rynkevic R, Fernandes AA, Barroso M, Santos DC. Biomechanical properties of breast tissue, a state-of-the-art review. Biomechanics and modeling in mechanobiology. 2016;15:1307-23.
- Janzekovic J, Hunt J, Peltz T, Wagels M, Brown T, Hutmacher DW. Biomechanical principles of breast implants and current state of research in soft tissue engineering for cosmetic breast augmentation. Aesthetic Plastic Surgery. 2022:1-10.
- Rinker B, Veneracion M, Walsh CP. Breast ptosis: causes and cure. Annals of plastic surgery. 2010;64(5):579-84.
- Bao W, Cao L, Wei H, Zhu D, Zhou G, Wang J, et al. Effect of 3D printed polycaprolactone scaffold with a bionic structure on the early stage of fat grafting. Materials Science and Engineering: C. 2021;123:111973.
- Cheng ME, Janzekovic J, Theile HJ, Rutherford-Heard C, Wille M-L, Cole C, et al. Pectus excavatum camouflage: a new technique using a tissue engineered scaffold. European Journal of Plastic Surgery. 2022;45(1):177-82.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).