Submitted:
25 April 2024
Posted:
26 April 2024
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
MATERIALS AND METHOD
Data
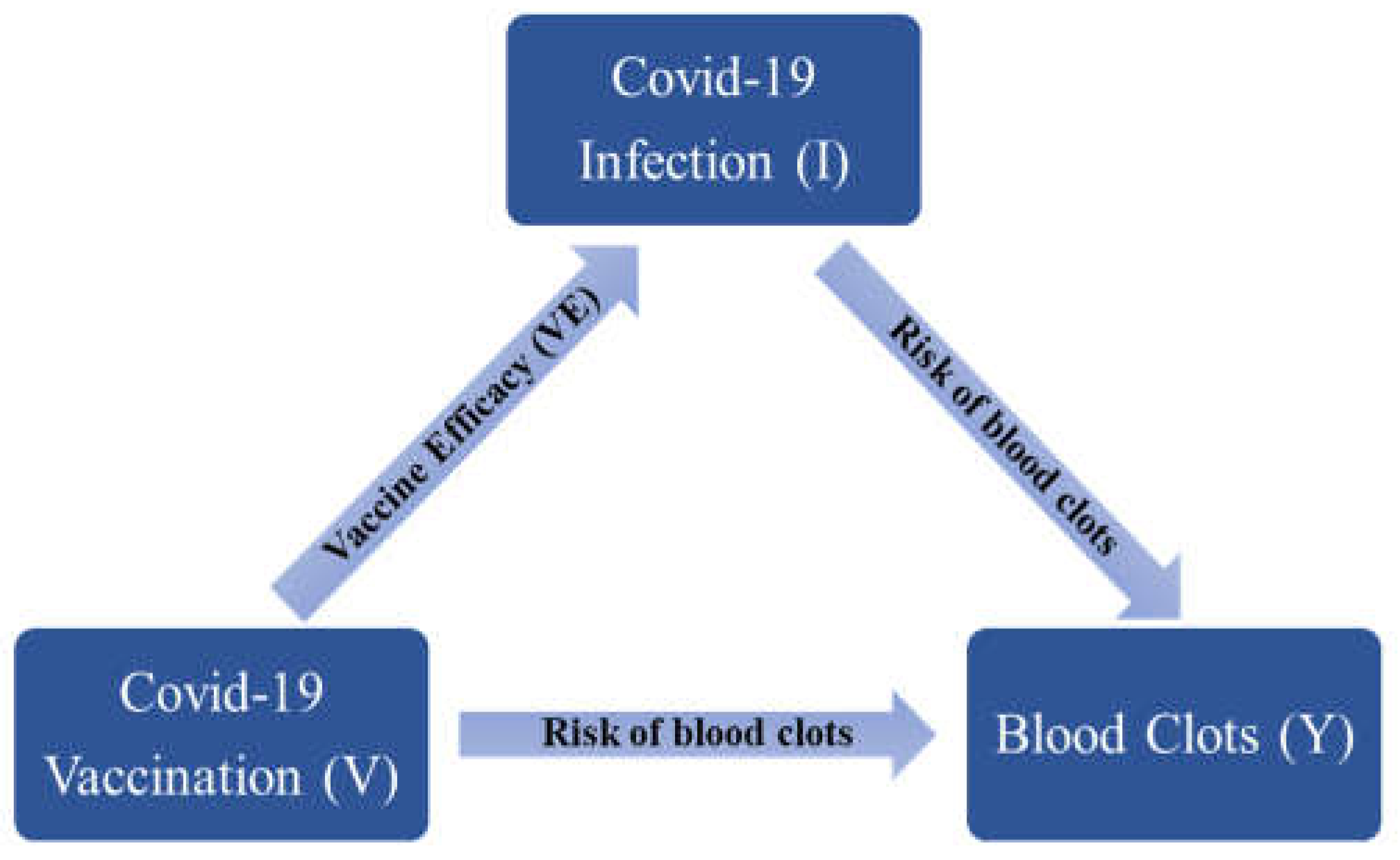
Statistical Method
RESULTS
Study Population
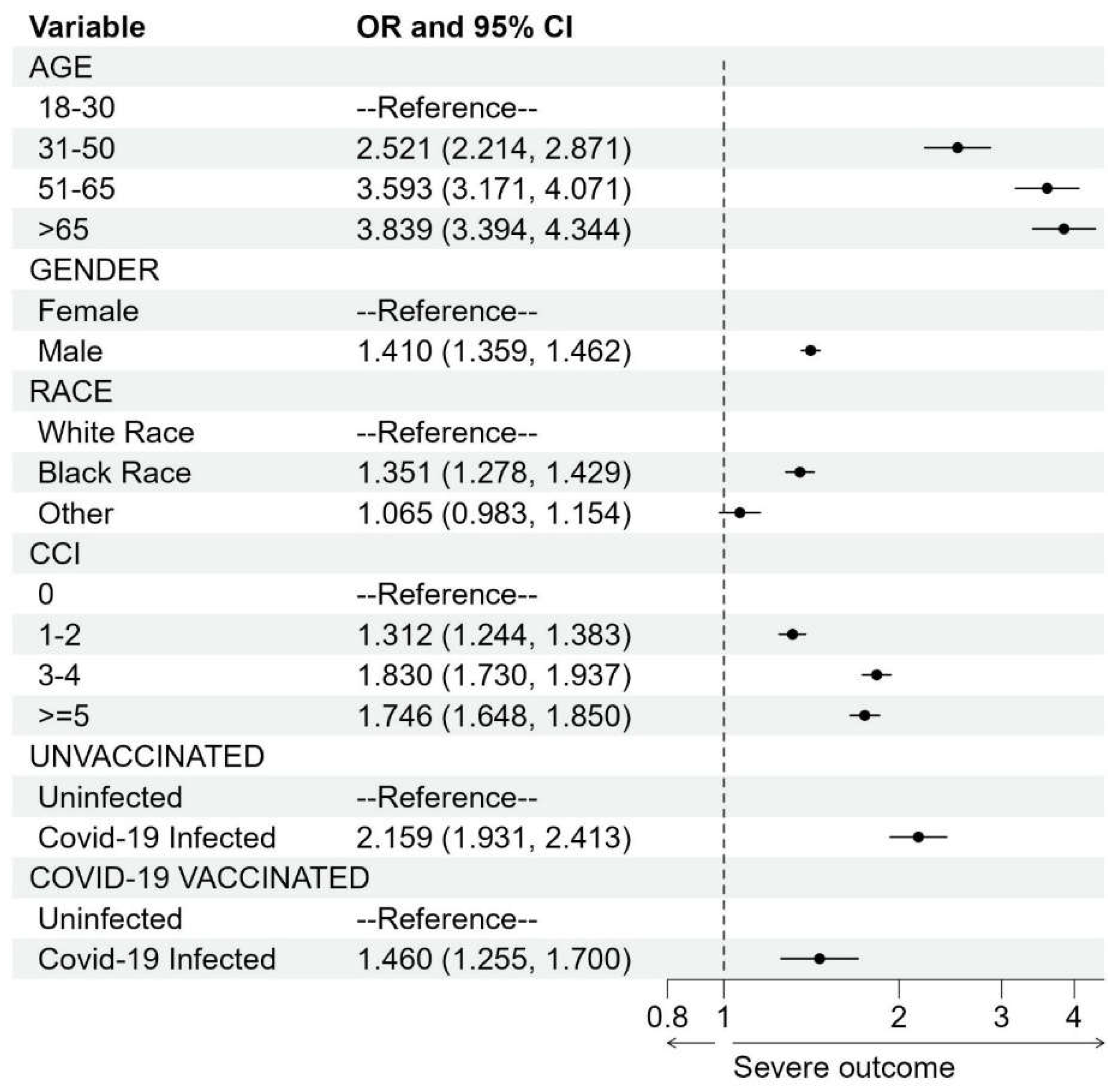
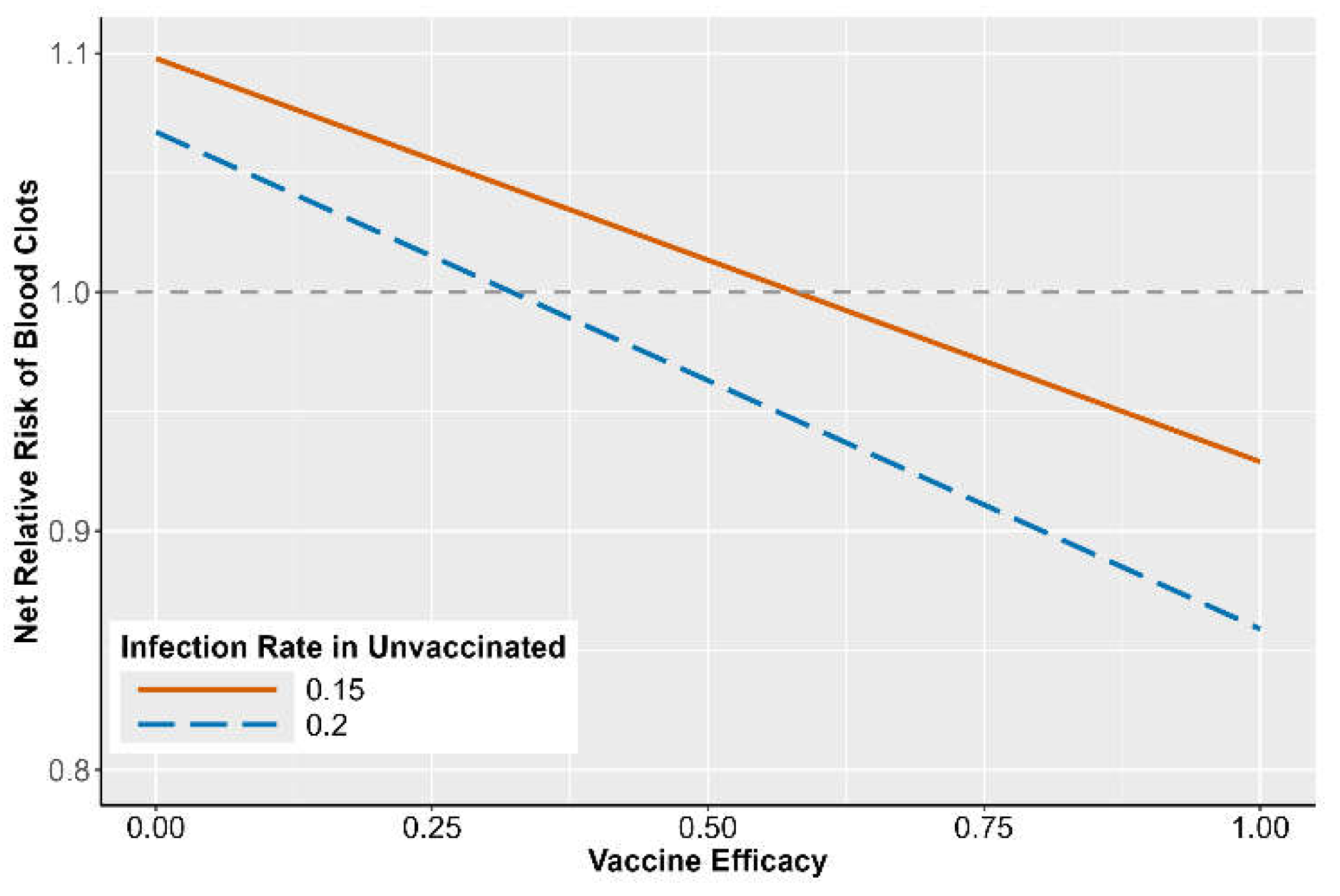
Association Studies
DISCUSSION
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. COVID Data Tracker. U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 17 November 2023. https://covid.cdc.gov/covid-data-tracker.
- Avinash Mani, Vineeta Ojha. Thromboembolism after COVID-19 Vaccination: A Systematic Review of Such Events in 286 Patients. Annals of Vascular Surgery 2022, 84, 12–20.e1. [CrossRef] [PubMed]
- Leonor Dias, Ricardo Soares-dos-Reis, Meira Joao, et al. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. Journal of Stroke and Cerebrovascular Diseases 2021, 30, 105906. [CrossRef] [PubMed]
- Elizabeth A. Andraska, Rohan Kulkarni, Mirnal Chaudhary M, Ulka Sachdev. Three cases of acute venous thromboembolism in females following vaccination for COVID-19. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2021, 10, 14–17. [CrossRef] [PubMed]
- Giuseppe Carli, Ilaria Nichele, Marco Ruggeri, Salvatore Barra, Alberto Tosetto. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med 2021, 16, 803–804. [CrossRef]
- Zaitun Zakaria, Nur Asma Sapiai, Abdul Rahman Izaini Ghani. Cerebral venous sinus thrombosis 2 weeks after the first dose of mRNA SARS-CoV-2 vaccine. Acta Neurochir 2021, 163, 2359–2362. [CrossRef] [PubMed]
- Julia Hippisley-Cox, Martina Patone, Xu W Mei, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ 2021, 374, n1931. [CrossRef]
- Maxime Taquet, Masud Husain, John R Geddes, Sierra Luciano, Paul J Harrison. Cerebral venous thrombosis and portal vein thrombosis: A retrospective cohort study of 537,913 COVID-19 cases. eClinicalMedicine 2021, 39, 101061. [CrossRef]
- Farah Yasmin, Hala Najeeb, Unaiza Naeem, et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immunity, Inflammation and Disease 2023, 11, e807. [CrossRef]
- Frederick K. Ho, Kenneth K.C. Man, Mark Toshner, et al. Thromboembolic Risk in Hospitalized and Nonhospitalized COVID-19 Patients: A Self-Controlled Case Series Analysis of a Nationwide Cohort. Mayo Clinic Proceedings 2021, 96, 2587–2597. [CrossRef]
- Xiaoming Xiong, Jianhua Chi, and Qinglei Gao. Prevalence and risk factors of thrombotic events on patients with COVID-19: a systematic review and meta-analysis. Thrombosis Journal 2021, 19, 1–9. [CrossRef]
- Sam Schulman, Yu Hu, and Stavros Konstantinides. Venous Thromboembolism in COVID-19. Thrombosis and Haemostasis 2020, 120, 1642–1653. [CrossRef] [PubMed]
- Katsoularis I, Fonseca-Rodríguez O, Farrington P, et al. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. The Lancet 2021, 398, 599–607. [CrossRef] [PubMed]
- Mahmoud B. Malas, Isaac N. Naazie, Nadin Elsayed, et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. eClinicalMedicine 2020, 29, 100639. [CrossRef]
- C. R. Simpson, T. Shi, E. Vasileiou, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Natural Medicine 2021, 29, 1290–1297. [CrossRef] [PubMed]
- Marie Joelle Jabagi, Jérémie Botton, Marion Bertrand, et al. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2022, 327, 80–82. [CrossRef]
- Jacob Dag Berild, Vilde Bergstad Larsen, Emilia Myrup Thiesson, et al. Analysis of Thromboembolic and Thrombocytopenic Events After the AZD1222, BNT162b2, and MRNA-1273 COVID-19 Vaccines in 3 Nordic Countries. JAMA Netw Open 2022, 5, e2217375. [CrossRef]
- Chui Celine Sze Ling, Min Fan, Eric Yuk Fai Wan, et al. Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: A self-controlled case series study. eClinicalMedicine 2022, 50, 101504. [CrossRef] [PubMed]
- Gareth J. Griffith, Tim T. Morris, Matthew J. Tudball, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nature communications 2020, 11, 1–12. [CrossRef]
- Bu Fan, Martijn J. Schuemie, Akihiko Nishimura, et al. Bayesian safety surveillance with adaptive bias correction. Statistics in Medicine 2023, 43, 395–418. [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Gasparini, Alessandro. An R package for computing comorbidity scores. Journal of Open Source Software 2018, 3, 648. [CrossRef]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; et al. Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. JAMA 2022, 327, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Katherine E. Fleming-Dutra, Allison Avrich Ciesla, Lauren E. Roper, et al. Preliminary Estimates of Effectiveness of Monovalent mRNA Vaccines in Preventing Symptomatic SARS-CoV-2 Infection Among Children Aged 3–5 Years—Increasing Community Access to Testing Program, United States, July 2022–February 2023. Morbidity and Mortality Weekly Report 2023, 72, 177–182. [CrossRef] [PubMed]
- Kelly M Hatfield, James Baggs, Hannah Wolford, et al. Effectiveness of Coronavirus Disease 2019 (COVID-19) Vaccination Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection Among Residents of US Nursing Homes Before and During the Delta Variant Predominance. Clinical Infectious Diseases 2022, 75 (Suppl. 2), S147–S154. [CrossRef]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O'Fallon, W.M.; Melton, L.J., 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1999, 158, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Chen Shen, Malcolm Risk, Elena Schiopu, et al. Efficacy of COVID-19 vaccines in patients taking immunosuppressants. Ann Rheum Dis 2022, 81, 875–880. [CrossRef] [PubMed]
- Malcolm Risk, Chen Shen, Salim S Hayek, et al. Comparative Effectiveness of Coronavirus Disease 2019 (COVID-19) Vaccines Against the Delta Variant. Clin Infect Dis 2022, 75, e623–e629. [CrossRef] [PubMed]
- Malcolm Risk, Salim S Hayek, Elena Schiopu, et al. COVID-19 vaccine effectiveness against omicron (B.1.1.529) variant infection and hospitalisation in patients taking immunosuppressive medications: a retrospective cohort study. The Lancet Rheumatology 2022, 4, e775–e784. [CrossRef] [PubMed]
- Martian Patone, Xu W Mei, Lahiru Handunnetthi, et al. Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex. Circulation 2022, 146, 743–754. [CrossRef]
- Huiting Luo, Xiaolin Li, Qidong Ren, et al. Acute kidney injury after COVID-19 vaccines: a real-world study. Renal Failure 2022, 44, 958–965. [CrossRef]
- Fabrizi Fabrizio, Carlo M. Alfieri, Roberta Cerutti, et al. COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Pathogens 2020, 9, 1052. [CrossRef] [PubMed]
| Patient Characteristics in SCCS Study | |||
|
Dose 1 (N = 18,466) |
Dose 2 (N = 17,523) |
||
| AGE | |||
| 18-30 | 161 (0.87%) | 142 (0.81%) | |
| 31-50 | 1,416 (7.67%) | 1,304 (7.44%) | |
| 51-65 | 4,422 (23.95%) | 4,166 (23.77%) | |
| >65 | 12,467 (67.51%) | 11,911 (68.97%) | |
| GENDER | |||
| Female | 8,691 (47.06%) | 8,247 (47.06%) | |
| Male | 9,775 (52.94%) | 9,276 (52.94%) | |
| RACE | |||
| White Race | 14,803 (80.16%) | 14,089 (80.40%) | |
| Black Race | 2,697 (14.61%) | 2,514 (14.35%) | |
| Other | 966 (5.23%) | 920 (5.25%) | |
| CHARLSON COMORBIDITY INDEX (CCI) | |||
| 0 | 2,938 (15.91%) | 2,801 (15.98%) | |
| 1-2 | 5,153 (27.91%) | 4,910 (28.02%) | |
| 3-4 | 5,326 (28.84%) | 5,061 (28.88%) | |
| >=5 | 5,049 (27.34%) | 4,751 (27.11%) | |
| NUM. BLOOD CLOTS IN RISK PERIODS | |||
| 763 | 894 | ||
| Patient Characteristics in Case-control Study | |||
|
Blood clots N = 28,304 (57.47%) |
Physical injury N = 20,943 (42.53%) |
Overall N = 49,247 |
|
| AGE | |||
| 18-30 | 378 (1.34%) | 1,118 (5.33%) | 1,496 (3.04%) |
| 31-50 | 2,686 (9.49%) | 2,902 (13.86%) | 5,588 (11.35%) |
| 51-65 | 7,280 (25.72%) | 5,184 (24.75%) | 12,464 (25.31%) |
| >65 | 17,960 (63.45%) | 11,739 (56.05%) | 29,699 (60.31%) |
| GENDER | |||
| Female | 13,207 (46.66%) | 11,588 (55.34%) | 24,795 (50.35%) |
| Male | 15,097 (53.34%) | 9,355 (44.67%) | 24,452 (49.65%) |
| RACE | |||
| White Race | 22,568 (79.73%) | 17,135 (81.8%) | 39,703 (80.62%) |
| Black Race | 4,175 (14.75%) | 2,577 (12.30%) | 6,752 (13.71%) |
| Other | 1,561 (5.52%) | 1,231 (5.88%) | 2,792 (5.67%) |
| CHARLSON COMORBIDITY INDEX (CCI) | |||
| 0 | 4,722 (16.68%) | 5,389 (25.73%) | 10,111 (20.53%) |
| 1-2 | 7,957 (28.11%) | 6,469 (30.89%) | 14,426 (29.29%) |
| 3-4 | 8,047 (28.43%) | 4,535 (21.65%) | 12,582 (25.55%) |
| >=5 | 7,578 (26.77%) | 4,550 (21.73%) | 12,128 (24.63%) |
| COVID-19 VACCINATION BEFORE INFECTION | |||
| NO | 12,313 (43.50%) | 9,341 (44.60%) | 21,654 (43.97%) |
| YES | 15,991 (56.50%) | 11,602 (55.40%) | 27,593 (56.03%) |
| INFECTION WITHIN 28 DAYS PRIOR TO AT THE DATE OF EVENT | |||
| NO | 26,510 (93.66%) | 20,200 (96.45%) | 46,710 (94.85%) |
| YES | 1,794 (6.34%) | 743 (3.55%) | 2,537 (5.15%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





