Submitted:
22 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
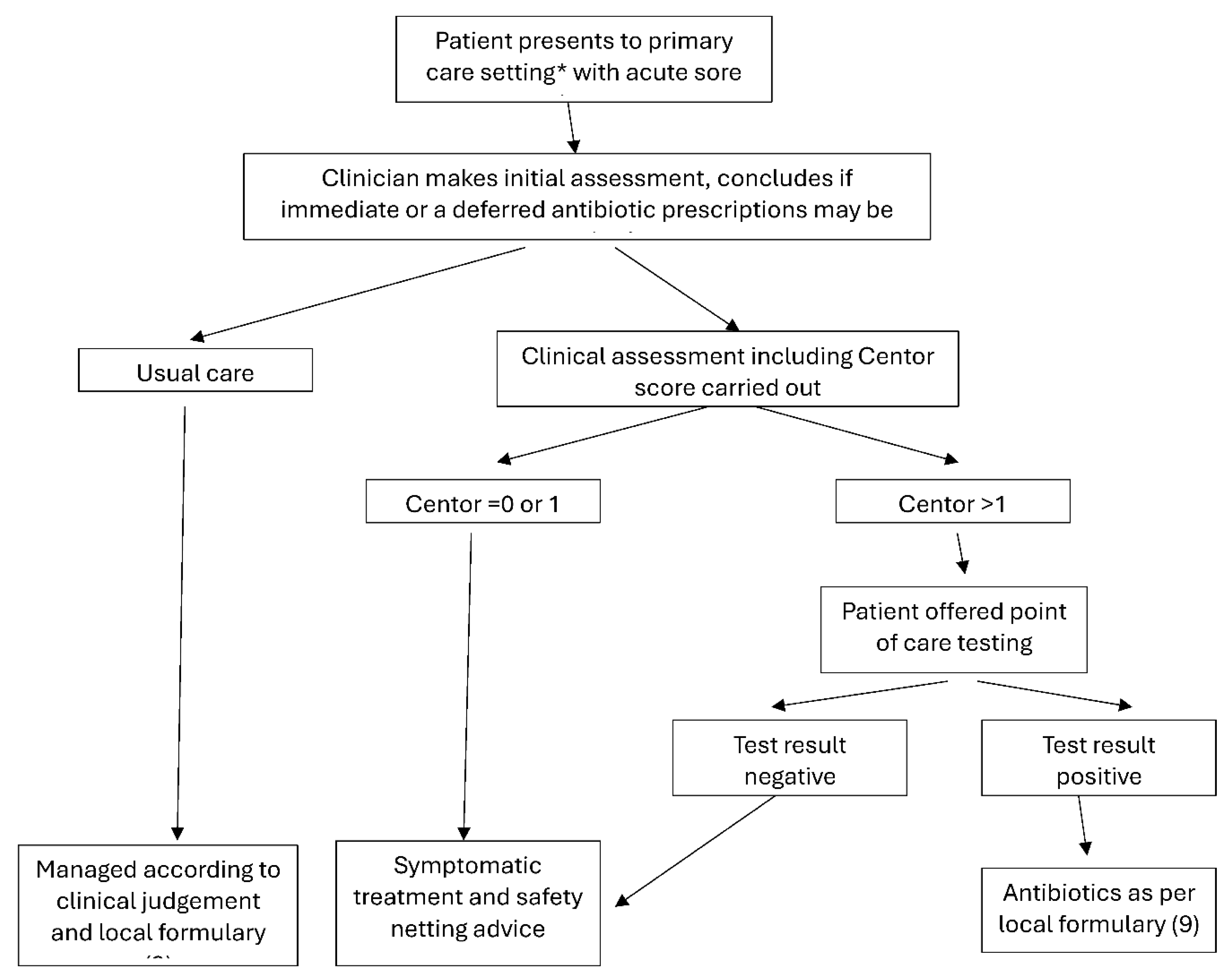
2.1. Study Design
2.2. Clinical Assessment of Patients
2.3. Point-of-Care Testing Molecular Diagnostics
2.4. Study Population
2.5. Data Collection
2.6. Questionnaire
2.7. Statistical Analysis
3. Results
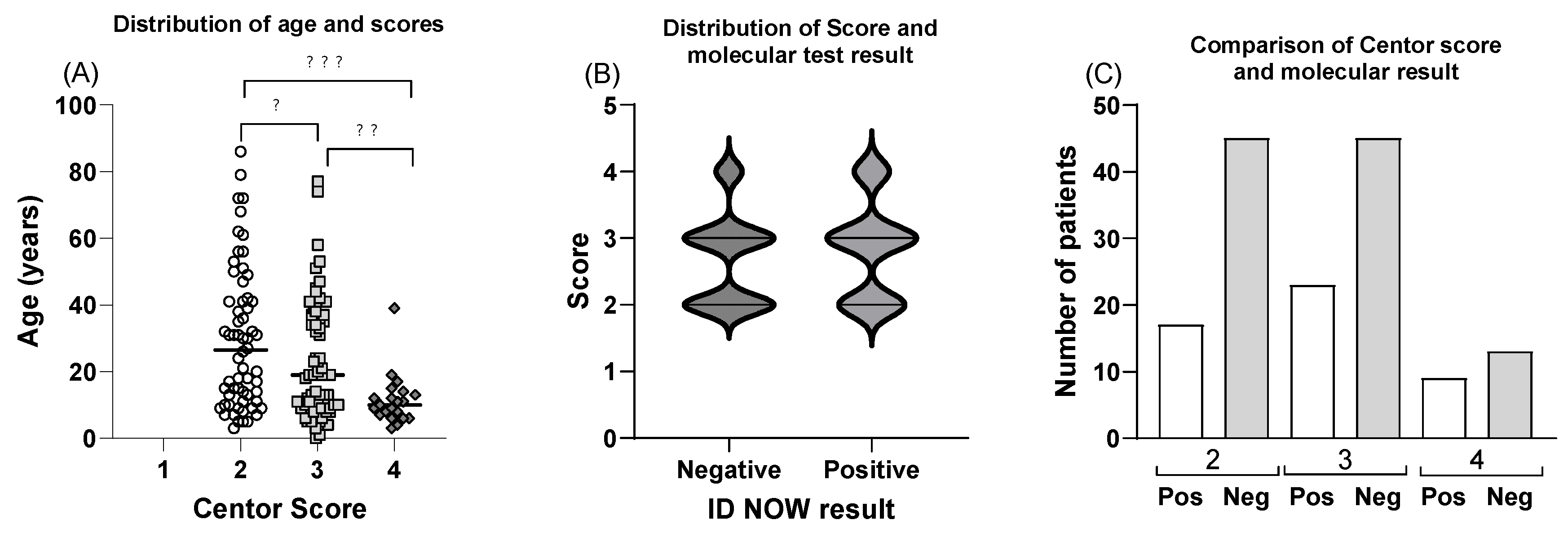
3.1. Molecular POCT Increases the Diagnostic Accuracy of Clinical Evaluation of Strep A Diagnosis
3.2. Longer Consultation Times for POCT Result in Higher Accuracy of Diagnosis
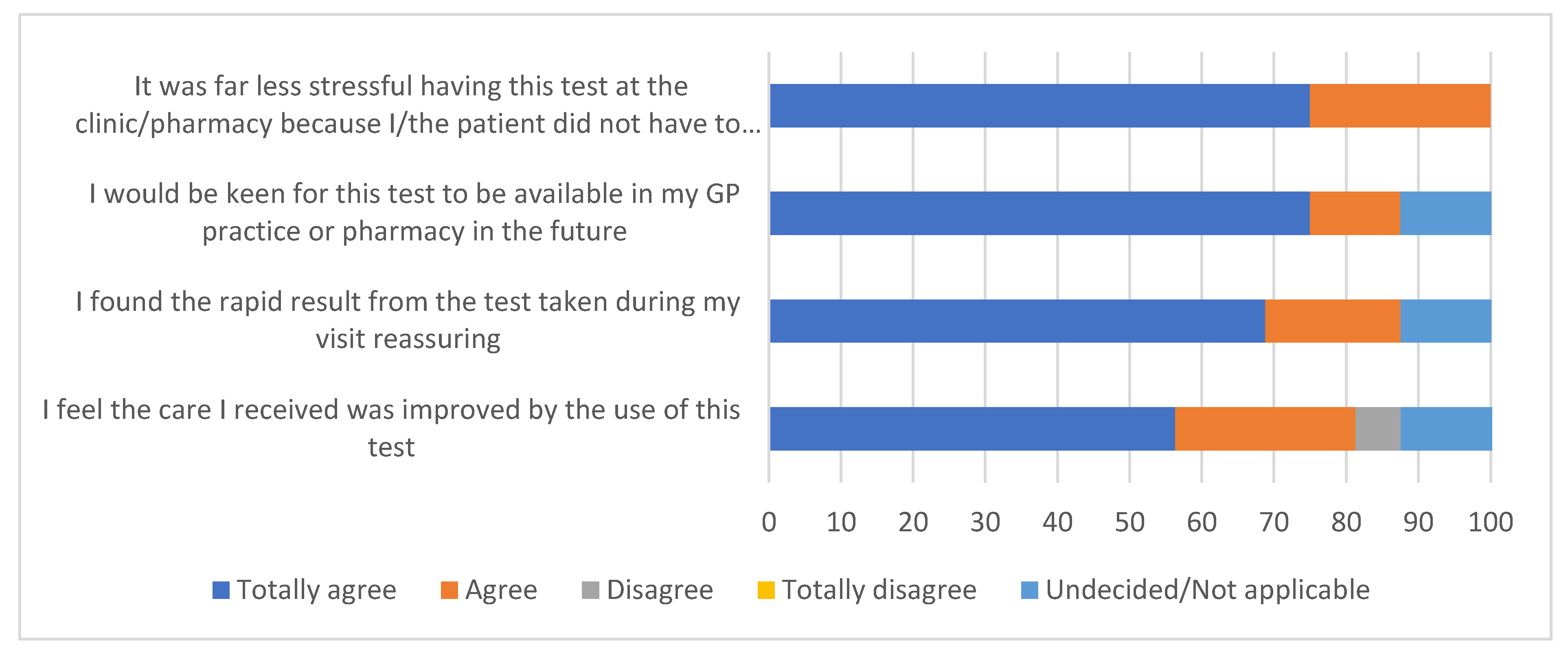
3.3. Patient Responses Following the Availability of Immediate Test Results
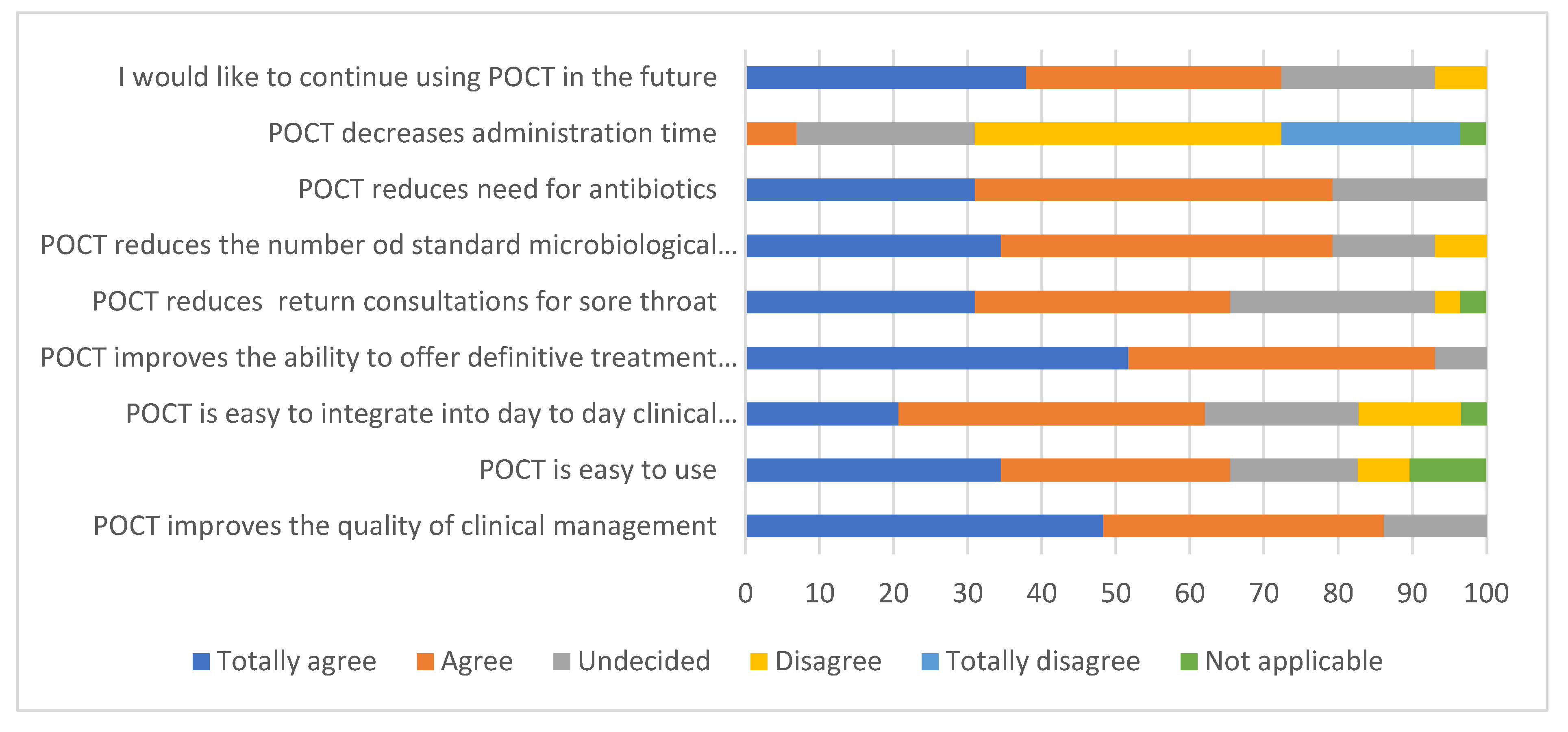
3.4. Staff Survey
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Rights retention
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantzourani, E., Wasag, D., Cannings-John, R., Ahmed, H., & Evans, A. (2022). Characteristics of the sore throat test and treat service in community pharmacies (STREP) in Wales: cross-sectional analysis of 11 304 consultations using anonymized electronic pharmacy records. The Journal of antimicrobial chemotherapy, 78(1), 84–92. [CrossRef]
- Worrall G. J. (2007). Acute sore throat. Canadian family physician Medecin de famille canadien, 53(11), 1961–1962.
- Bower J. R. (2012), Pharyngitis. Netter’s Infectious Diseases. 177–182. [CrossRef]
- Overview: Sore Throat (acute): Antimicrobial prescribing: Guidance NICE. Available at: https://www.nice.org.uk/guidance/ng84 (Accessed: 01 March 2024).
- Management-of-contacts-of-invasive-group-A- ... Available at: https://assets.publishing.service.gov.uk/media/64071ec5d3bf7f25fa417a91/Management-of-contacts-of-invasive-group-a-streptococcus.pdf (Accessed: 09 March 2024).
- Group A streptococccus in children. NICE Guidelines 2022. https://www.england.nhs.uk/wp-content/uploads/2022/12/PRN00080-Interim-clinical-guidance-summary-for-case-management-for-prophylaxis-please-refer-to-community-conta.pdf.
- Oliver J, Malliya Wadu E, Pierse N, Moreland NJ, Williamson DA, Baker MG. Group A Streptococcus pharyngitis and pharyngeal carriage: a meta-analysis. PLoS neglected tropical diseases. 2018 Mar 19;12(3):e0006335.
- Gulliford, M. C. , Moore, M. V., Little, P., Hay, A. D., Fox, R., Prevost, A. T., Juszczyk, D., Charlton, J., & Ashworth, M. (2016). Safety of reduced antibiotic prescribing for self-limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ (Clinical research ed.), 354, i3410. [CrossRef]
- Thompson TZ, McMullen AR. Group A Streptococcus Testing in Pediatrics: the Move to Point-of-Care Molecular Testing. J Clin Microbiol. 2020 May 26;58(6):e01494-19. [CrossRef] [PubMed] [PubMed Central]
- Centor RM; Witherspoon JM; Dalton HP; Brody CE; Link K (1981). "The diagnosis of strep throat in adults in the emergency room". Medical Decision Making. 1 (3): 239–246. [CrossRef]
- Willis, B. H., Coomar, D., & Baragilly, M. (2020). Comparison of Centor and McIsaac scores in primary care: a meta-analysis over multiple thresholds. The British journal of general practice: the journal of the Royal College of General Practitioners, 70(693), e245–e254. [CrossRef]
- Nardi, S, Carolis L, Iannini R, De Sandro MV, Solito G, Calafatti M, Gizzi C. (2022). Usefulness of rapid molecular tests in pediatric respiratory tract infections Ital J Pediatr. 48(1):21. [CrossRef]
- Varzgalien, L, Heerey A, Cox C, McGuinness T, McGuire G, Cals JW, O'Shea E, Kelly M. (2017) Point-of-care testing in primary care: needs and attitudes of Irish GPs. BJGP Open. 15;1(4):bjgpopen17X101229. [CrossRef]
- Wolf F, Matthes A, Markwart R, Bleidorn J. 2022. Perspectives of physicians and medical assistants on the implementation of NAAT-based point-of-care testing for SARS-CoV-2 in primary care in Germany. Z Evid Fortbild Qual Gesundhwes. 2022 Dec;175:43-49. [CrossRef]
- Matthes A, Wolf F, Bleidorn J, Markwart R. 2022. "It Was Very Comforting to Find Out Right Away." - Patient Perspectives on Point-of-Care Molecular SARS-CoV-2 Testing in Primary Care. Patient Prefer Adherence. 10;16:2031-2039. [CrossRef]
- Daniels R., Cottin J., Khanafer N. (2023). Point-of-Care Testing for SARS-CoV-2: A Prospective Study in a Primary Health Centre. Diagnostics (Basel) 28;13(11):1888. [CrossRef]
- NHS England. (2023). Group A Streptococcus: reinstatement of NICE sore throat guidance for children and young people and withdrawal of NHS England interim guidance. Publication reference: PR00247. https://www.england.nhs.uk/wp-content/uploads/2022/12/PRN00247_Group-A-Streptococcus-reinstatement-of-NICE-sore-throat-guidance-for-children-and-young-people-and-wi.pdf.
- North & East Decon Formulary and Referral. Upper respiratory tract infections. NICE CG69: Respiratory tract infections (self-limiting): prescribing antibiotics. https://northeast.devonformularyguidance.nhs.uk/formulary/chapters/5-infections/upper-respiratory-tract-infections.
- ID NOWTM Strep A2. Available at: https://dam.abbott.com/en-gb/panbio/ID-NOW-Strep-A%202-Product-Sheet-EME-English.pdf.
- Thompson T.Z and A.R. McMullen. (2020). Group A Streptococcus Testing in Pediatrics: the Move to Point-of-Care Molecular Testing. J. Clin Microbiology. 58:6. [CrossRef]
- Online surveys. Available at: https://www.onlinesurveys.ac.uk/ (Accessed: 06 April 2024).
- Burdino E, Cerutti F, Milia MG, Allice T, Gregori G, Aprà F, De Iaco F, Aluffi E, Micca G, Ghisetti V. 2022. Fast and reliable real life data on COVID-19 triaging with ID NOW. J Clin Virol Plus. 2(1):100065. [CrossRef]
- Barnacle JR, Houston H, Baltas I, Takata J, Kavallieros K, Vaughan N, Amin AK, Aali SA, Moore K, Milner P, Gupta Wright A, John L. 2022. Diagnostic accuracy of the Abbott ID NOW SARS-CoV-2 rapid test for the triage of acute medical admissions. J Hosp Infect. 123:92-99. [CrossRef]
- Trabattoni E, Le V, Pilmis B, Pean de Ponfilly G, Caisso C, Couzigou C, Vidal B, Mizrahi A, Ganansia O, Le Monnier A, Lina B, Nguyen Van JC. Implementation of Alere i Influenza A & B point of care test for the diagnosis of influenza in an ED. Am J Emerg Med. 2018 Jun;36(6):916-921. Epub 2017 Oct 18. [CrossRef]
- O'Connell S, Conlan C, Reidy M, Stack C, Mulgrew A, Baruah J. The impact of point-of-care testing for influenza A and B on patient flow and management in a medical assessment unit of a general hospital. BMC Res Notes. 2020 Mar 10;13(1):143. [CrossRef]
- Wächtler H, Kaduszkiewicz H, Kuhnert O, Malottki KA, Maaß S, Hedderich J, Wiese B, Donner-Banzhoff N, Hansmann-Wiest J (2023). Influence of a guideline or an additional rapid strep test on antibiotic prescriptions for sore throat: the cluster randomized controlled trial of HALS (Hals und Antibiotika Leitlinien Strategien). BMC Prim Care. 24(1):75. [CrossRef]
- Guy, R. et al. (2023) ‘Increase in invasive group a streptococcal infection notifications, England, 2022’, Eurosurveillance, 28(1). [CrossRef]
- Allen T, Gyrd-Hansen D, Kristensen SR, Oxholm AS, Pedersen LB, Pezzino M. Physicians under Pressure: Evidence from Antibiotics Prescribing in England. Med Decis Making. 2022 Apr;42(3):303-312. Epub 2022 Jan 12. [CrossRef] [PubMed] [PubMed Central]
- Group A streptococcal infections: 15th update on seasonal activity in England (no date) GOV.UK. Available at: https://www.gov.uk/government/publications/group-a-streptococcal-infections-activity-during-the-2022-to-2023-season/group-a-streptococcal-infections-15th-update-on-seasonal-activity-in-england (Accessed: 09 March 2024).
- Group A streptococcus in children. Available at: https://www.england.nhs.uk/wp-content/uploads/2022/12/PRN00058-group-a-streptococcus-in-children-december-2022.pdf (Accessed: 09 March 2024).
- Espaur-Report-2022-TO-2023.PDF. Available at: https://assets.publishing.service.gov.uk/media/65cf498ee1bdec001132225c/ESPAUR-report-2022-to-2023.pdf (Accessed: 09 March 2024).
- Further 5 serious shortage protocols (ssps) phenoxymethylpenicillin products to enable the supply of alternative antibiotics, RPS brandmark. Available at: https://www.rpharms.com/publications/pharmacy-alerts/details/Further-5-Serious-Shortage-Protocols-SSPs-phenoxymethylpenicillin-products-to-enable-the-supply-of-alternative-antibiotics (Accessed: 09 March 2024).
- Gunnarsson RK, Orda U, Elliott B, Heal C, Gorges H, Glasziou P, Del Mar C. (2021). Improving antibiotics targeting using PCR point-of-care testing for group A streptococci in patients with uncomplicated acute sore throat. Aust J Gen Pract. 50(1-2):76-83. [CrossRef]
- NIHR. Available at: https://www.nihr.ac.uk/search-results.htm?search=tariff (Accessed: 04 March 2024).
- Mikulic, M. (2023) Top antibacterial drugs dispensed in England by items 2022, Statista. Available at: https://www.statista.com/statistics/377978/top-ten-antibacterial-drugs-dispensed-by-item-in-england/ (Accessed: 09 March 2024.
- NHS choices. Available at: https://www.england.nhs.uk/primary-care/pharmacy/pharmacy-first/ (Accessed: 01 March 2024).
- Seeley, A., Fanshawe, T., Voysey, M., Hay, A., Moore, M., & Hayward, G. (2021). Diagnostic accuracy of Fever-PAIN and Centor criteria for bacterial throat infection in adults with sore throat: a secondary analysis of a randomised controlled trial. BJGP open, 5(6), BJGPO.2021.0122. [CrossRef]
| Centor Score | POCT Results: Positive | Negative | % Positive (95% confidence interval) |
|---|---|---|---|
| 2 | 15 | 51 | 22.73 (14.18 to 34.28) |
| 3 | 20 | 33 | 37.74 (25.91 to 51.22) |
| 4 | 15 | 10 | 60.00 (40.70 to 76.64) |
| 3 or 4 | 35 | 43 | 44.87 (34.33 to 55.89) |
| Total | 50 | 94 | 34.72 (27.42 to 42.81) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





