Submitted:
19 April 2024
Posted:
22 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Common Genetic Alterations in NMIBC
3. Tumorigenesis Associated with NMIBC
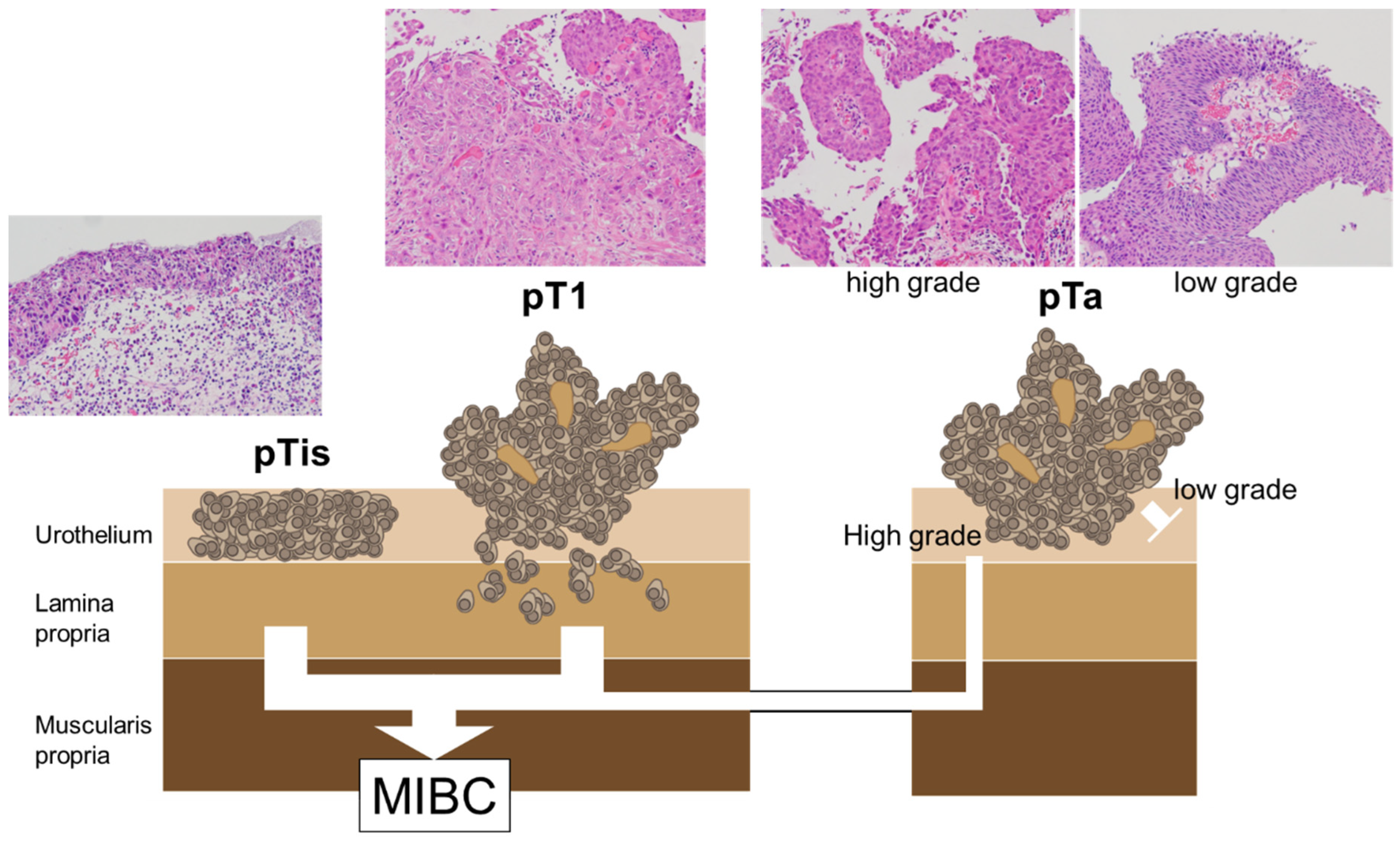
4. Pathological Diagnosis and Screening of NMIBC
4.1. Urine-Based Diagnosis
4.2. Blood-Based Diagnosis
4.3. Cystoscopic Diagnosis
4.4. Tissue-Based Diagnosis
5. Management of NMIBC
5.1. TURBT and En Bloc Resection
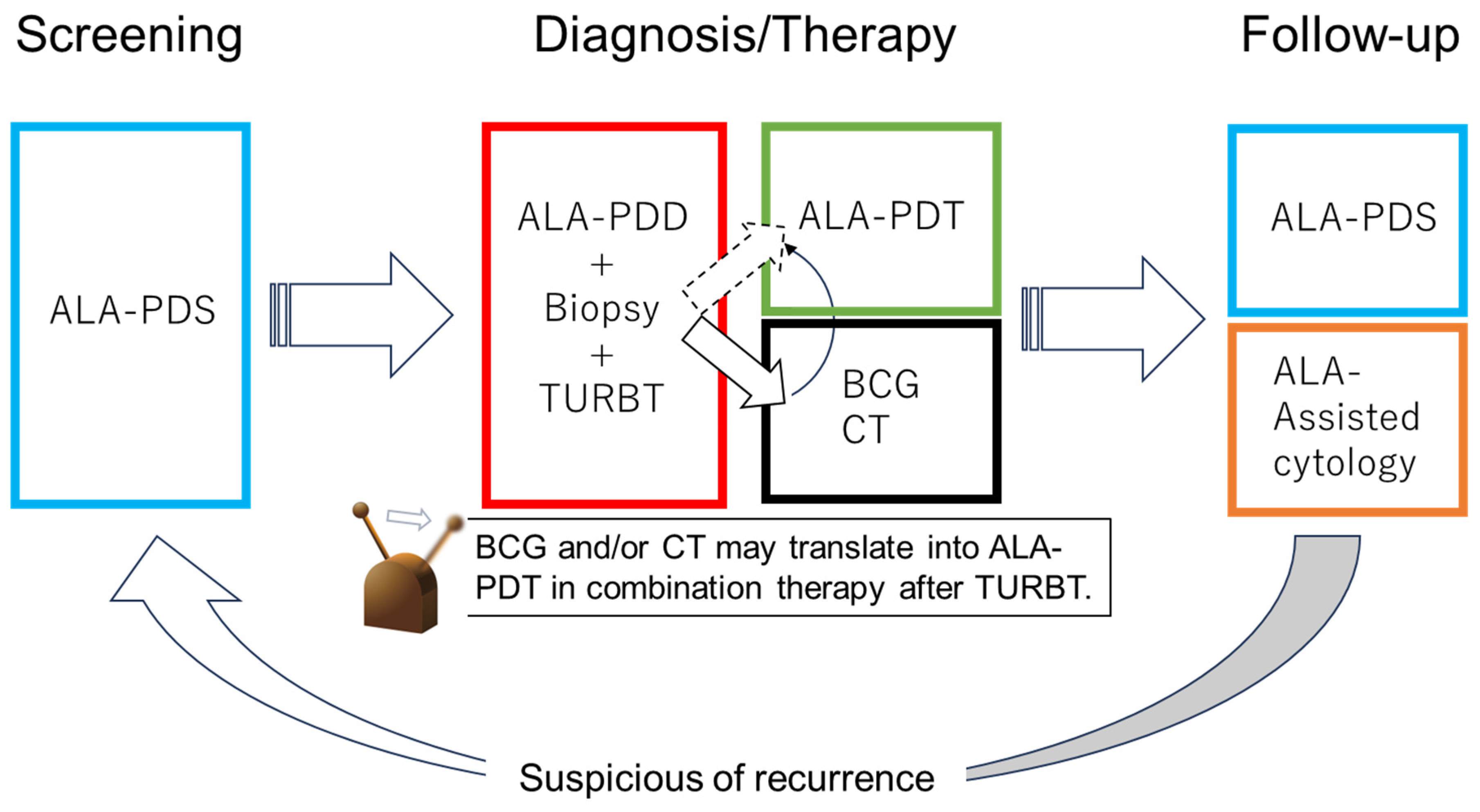
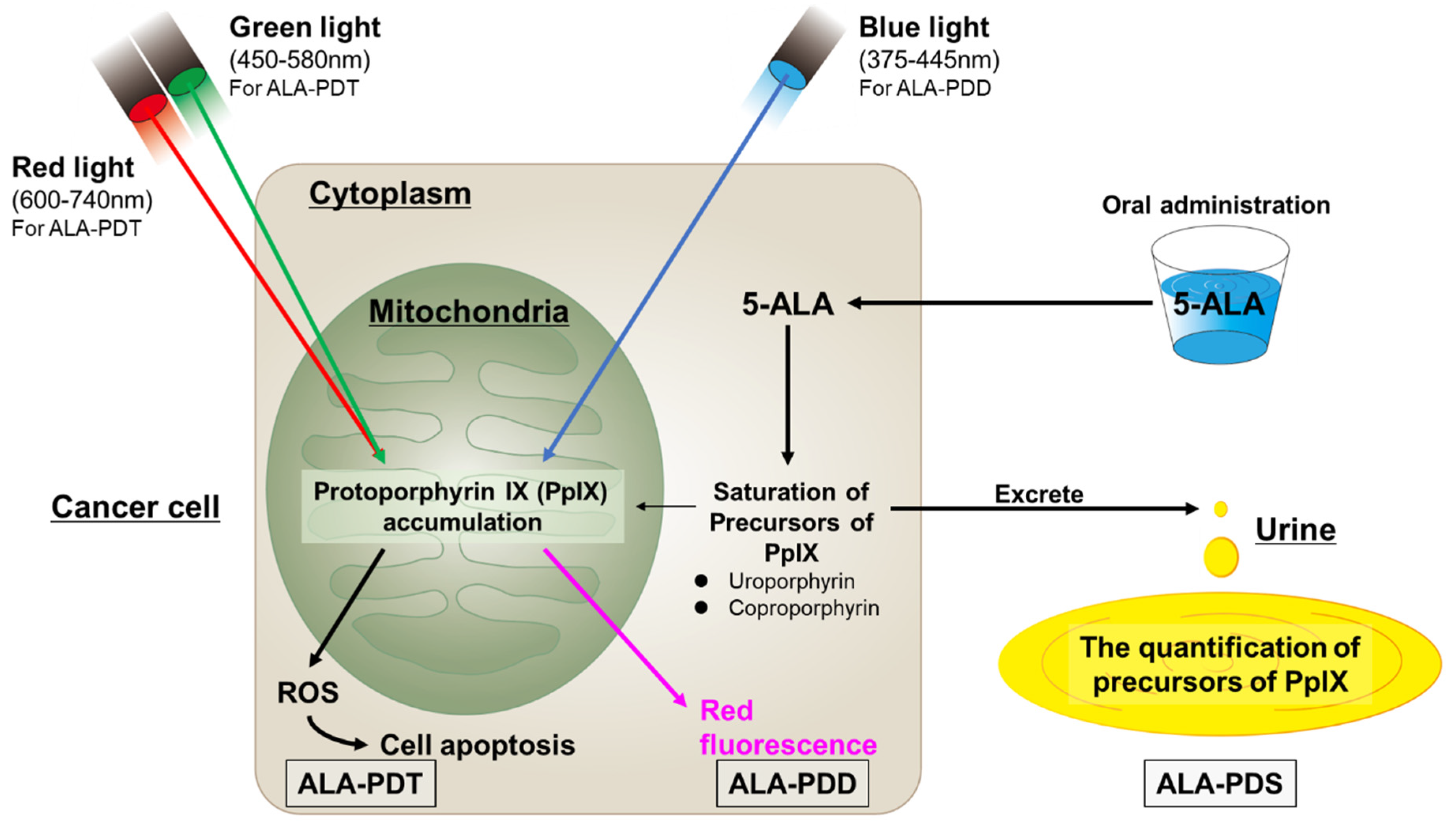
6. Photodynamic Diagnosis, Photodynamic Therapy, and Photodynamic Screening with 5-Aminolaevulinic Acid
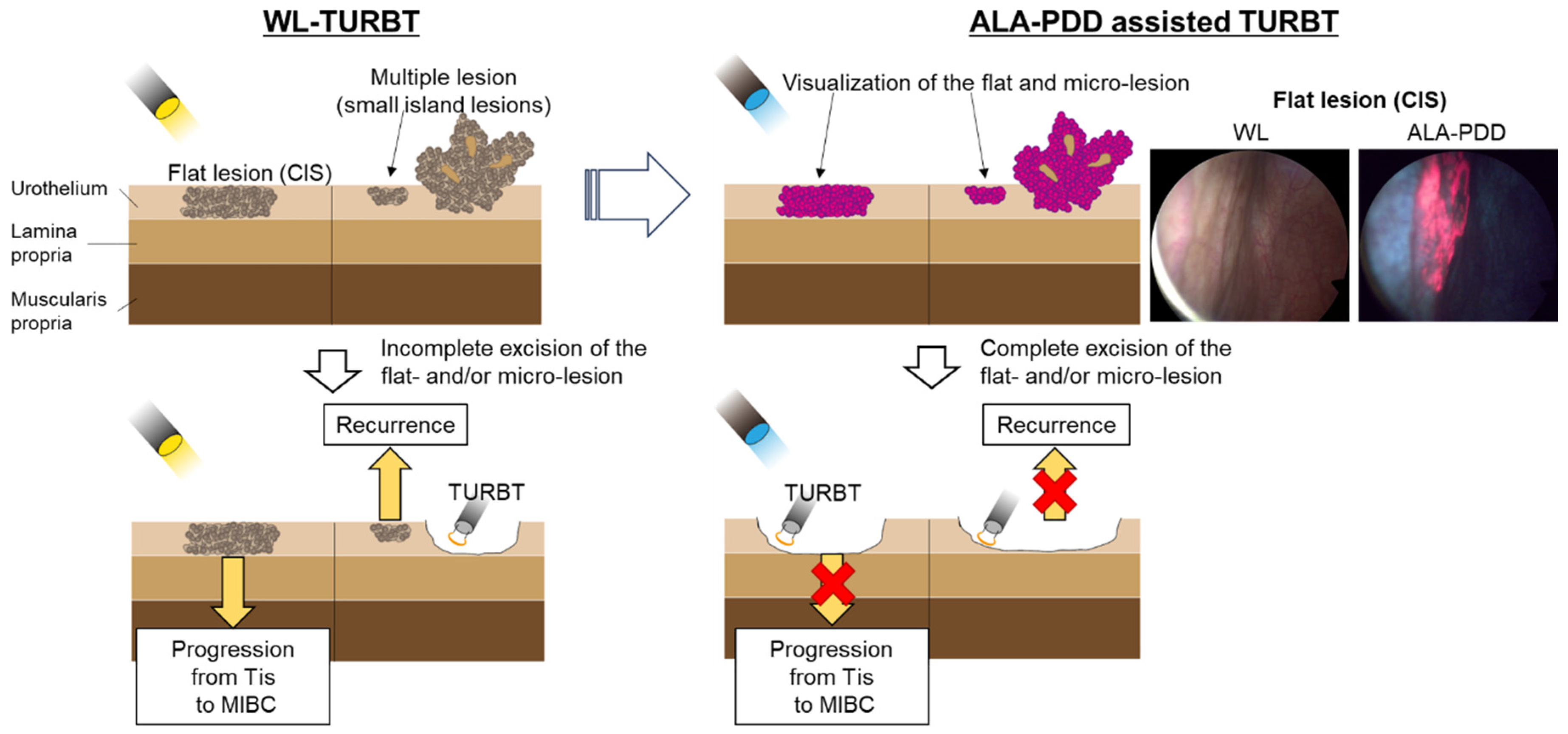
7. PDD with 5-ALA for TURBT in NMIBCs
8. PDT with 5-ALA for TURBT in NMIBCs
9. Screening Using 5-ALA after TURBT in NMIBCs
10. Conclusions and Future Perspective
Author Contributions
Conflicts of Interest
References
- Sung, H; Ferlay, J; Siegel, RL; Laversanne, M; Soerjomataram, I; Jemal, A; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Antoni, S; Ferlay, J; Soerjomataram, I; Znaor, A; Jemal, A; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef]
- Wilhelm-Benartzi, C.S.; Christensen., B.C.; Koestler, D.C.; Houseman, E.A.; Schned, A.R.; Karagas, M.R.; Kelsey, K.T.; Marsit, C.J. Association of secondhand smoke exposures with DNA methylation in bladder carcinomas. Cancer Causes Control 2011, 22, 1205–1213. [Google Scholar] [CrossRef]
- Moch, H. Urinary and Male Genital Tumours: WHO Classification of Tumours, 5th ed.; Chee, A.I., Goldman-Levy, G., Lokuhetty, D., Vishal-Rao, B., White, A.V., Eds.; IARC Publications: Lyon, France, 2022; Volume 8, 576p. [Google Scholar]
- Berdik, C. Unlocking bladder cancer. Nature 2017, 551, S34–S35. [Google Scholar] [CrossRef]
- James, A.C.; Gore, J.L. The costs of non-muscle invasive bladder cancer. Urol. Clin. North. Am 2013, 40, 261–269. [Google Scholar] [CrossRef]
- Abdollah, F.; Gandaglia, G.; Thuret, R.; Schmitges, J.; Tian, Z.; Jeldres, C.; Passoni, N.M.; Briganti, A.; Shariat, S.F.; Perrotte, P.; Montorsi, F.; Karakiewicz, P.I.; Sun, M. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol 2013, 37, 219–225. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez; Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Seisen, T.; Soukup, V.; Sylvester, R.J. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Hurst, C.D.; Alder, O.; Platt, F.M.; Droop, A.; Stead, L.F.; Burns, J.E.; Burghel, G.J.; Jain, S.; Klimczak, L.J.; Lindsay, H.; Roulson, J.A.; Taylor, C.F.; Thygesen, H.; Cameron, A.J.; Ridley, A.J.; Mott, H.R.; Gordenin, D.A.; Knowles, M.A. Genomic Subtypes of Non-invasive Bladder Cancer with Distinct Metabolic Profile and Female Gender Bias in KDM6A Mutation Frequency. Cancer Cell 2017, 32, 701–715.e7. [Google Scholar] [CrossRef]
- Ségal-Bendirdjian, E.; Geli, V. Non-canonical roles of telomerase: unraveling the imbroglio. Front. Cell Dev. Biol 2019, 7, 332. [Google Scholar] [CrossRef]
- Agarwal, N.; Rinaldetti, S.; Cheikh, B.B.; Zhou, Q.; Hass, E.P.; Jones, R.T.; Joshi, M.; LaBarbera, D.V.; Knott, S.R.V.; Cech, T.R.; Theodorescu, D. TRIM28 is a transcriptional activator of the mutant TERT promoter in human bladder cancer. Proc Natl Acad Sci USA 2021, 118, e2102423118. [Google Scholar] [CrossRef]
- Borah, S.; Xi, L.; Zaug, A.J.; Powell, N.M.; Dancik, G.M.; Cohen, S.B.; Costello, J.C.; Theodorescu, D.; Cech, T.R. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science, 2015; 347, 1006–1010. [Google Scholar]
- Nickerson, M.L.; Witte, N.; Im, K.M.; Turan, S.; Owens, C.; Misner, K.; Tsang, S.X.; Cai, Z.; Wu, S.; Dean, M.; Costello, J.C.; Theodorescu, D. Molecular analysis of urothelial cancer cell lines for modeling tumor biology and drug response. Oncogene 2017, 36, 35–46. [Google Scholar] [CrossRef]
- Nickerson, M.L.; Dancik, G.M.; Im, K.M.; Edwards, M.G.; Turan, S.; Brown, J.; Ruiz-Rodriguez, C.; Owens., C.; Costello, J.C.; Guo, G.; Tsang, S.X.; Li, Y.; Zhou, Q.; Cai, Z.; Moore, L.E.; Lucia, M.S.; Dean, M.; Theodorescu, D. Concurrent alterations in TERT, KDM6A, and the BRCA pathway in bladder cancer. Clin Cancer Res 2014, 20, 4935–4948. [Google Scholar] [CrossRef]
- Rachakonda, P.S.; Hosen, I.; de Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; Kumar, R. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef]
- Leão, R.; Lee, D.; Figueiredo, A.; Hermanns, T.; Wild, P.; Komosa, M.; Lau, I.; Mistry, M.; Nunes, N.M.; Price, A.J.; Zhang, C.; Lipman, T.; Poyet, C.; Valtcheva, N.; Oehl, K.; Coelho, H.; Sayyid, R.; Gomes, A.M.; Prado, E.; Castro, L.; Sweet, J.; Vinagre, J.; Apolónio, J.; Stephens, D.; Faleiro, I.; Fadaak, K.; Richard, P.O.; Kulkarni, G.; Zlotta, A.R.; Hamilton, R.J.; Castelo-Branco, P.; Tabori, U. Combined genetic and epigenetic alterations of the TERT promoter affect clinical and biological behavior of bladder cancer. Int J Cancer 2019, 144, 1676–1684. [Google Scholar] [CrossRef]
- Kurtis, B.; Zhuge, J.; Ojaimi, C.; Ye, F.; Cai, D.; Zhang, D.; Fallon, J.T.; Zhong, M. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol 2016, 21, 7–11. [Google Scholar] [CrossRef]
- Allory, Y.; Beukers, W.; Sagrera, A.; Flández, M.; Marqués, M.; Márquez, M.; van der Keur, K.A.; Dyrskjot, L.; Lurkin, I.; Vermeij, M.; Carrato, A.; Lloreta, J.; Lorente, J.A.; Carrillo-de Santa; Pau, E.; Masius, R.G.; Kogevinas, M.; Steyerberg, E.W.; van Tilborg, A.A.; Abas, C.; Orntoft, T.F.; Zuiverloon, T.C.; Malats, N.; Zwarthoff, E.C.; Real, F.X. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol 2014, 65, 360–366. [Google Scholar] [CrossRef]
- Lindskrog, S.V.; Prip, F.; Lamy, P.; Taber, A.; Groeneveld, C.S.; Birkenkamp-Demtröder, K.; Jensen, J.B.; Strandgaard, T.; Nordentoft, I.; Christensen, E.; Sokac, M.; Birkbak, N.J.; Maretty, L.; Hermann, G.G.; Petersen, A.C.; Weyerer, V.; Grimm, M.O.; Horstmann, M.; Sjödahl, G.; Höglund, M.; Steiniche, T.; Mogensen, K.; de Reyniès, A.; Nawroth, R.; Jordan, B.; Lin, X.; Dragicevic, D.; Ward, D.G.; Goel, A.; Hurst, C.D.; Raman, J.D.; Warrick, J.I.; Segersten, U.; Sikic, D.; van Kessel, K.E.M.; Maurer, T.; Meeks, J.J.; DeGraff, D.J.; Bryan, R.T.; Knowles, M.A.; Simic, T.; Hartmann, A.; Zwarthoff, E.C.; Malmström, P.U.; Malats, N.; Real, F.X.; Dyrskjøt, L. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat Commun 2021, 2, 2301. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; Castro, M.A.A.; Gibb, E.A.; Kanchi, R.S.; Gordenin, D.A.; Shukla, S.A.; Sanchez-Vega, F.; Hansel, D.E.; Czerniak, B.A.; Reuter, V.E.; Su, X.; de Sa Carvalho, B.; Chagas, V.S.; Mungall, K.L.; Sadeghi, S.; Pedamallu, C.S.; Lu, Y.; Klimczak, L.J.; Zhang, J.; Choo, C.; Ojesina, A.I.; Bullman, S.; Leraas, K.M.; Lichtenberg, T.M.; Wu, C.J.; Schultz, N.; Getz, G.; Meyerson, M.; Mills, G.B.; McConkey, D.J.; TCGA Research Network; Weinstein, J.N.; Kwiatkowski, D.J.; Lerner, S.P. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef]
- Shi, M.J.; Meng, X.Y.; Lamy, P.; Banday, A.R.; Yang, J.; Moreno-Vega, A.; Chen, C.L.; Dyrskjøt, L.; Bernard-Pierrot, I.; Prokunina-Olsson, L.; Radvanyi, F. APOBEC-mediated Mutagenesis as a Likely Cause of FGFR3 S249C Mutation Over-representation in Bladder Cancer. Eur Urol 2019, 76, 9–13. [Google Scholar] [CrossRef]
- van Rhijn, B.W.; Vis, A.N.; van der Kwast, T.H.; Kirkels, W.J.; Radvanyi, F.; Ooms, E.C.; Chopin, D.K.; Boevé, E.R.; Jöbsis, A.C.; Zwarthoff, E.C. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol 2003, 21, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- López-Knowles, E.; Hernández, S.; Malats, N.; Kogevinas, M.; Lloreta, J.; Carrato, A.; Tardón, A.; Serra, C.; Real, F.X. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res 2006, 66, 7401–7404. [Google Scholar] [PubMed]
- Hernández, S.; López-Knowles, E.; Lloreta, J.; Kogevinas, M.; Amorós, A.; Tardón, A.; Carrato, A.; Serra, C.; Malats, N.; Real, F.X. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol 2006, 24, 3664–3671. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Moser, K.; Kriegmair, M.; Hofstetter, A.; Hofstaedter, F.; Knuechel, R. Frequent genetic alterations in simple urothelial hyperplasias of the bladder in patients with papillary urothelial carcinoma. Am J Pathol 1999, 154, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.D.; Cheng, G.; Platt, F.M.; Castro, M.A.A.; Marzouka, N.S.; Eriksson, P.; Black, E.V.I.; Alder, O.; Lawson, A.R.J.; Lindskrog, S.V.; Burns, J.E.; Jain, S.; Roulson, J.A.; Brown, J.C.; Koster, J.; Robertson, A.G.; Martincorena, I.; Dyrskjøt, L.; Höglund, M.; Knowles, M.A. Stage-stratified molecular profiling of non-muscle-invasive bladder cancer enhances biological, clinical, and therapeutic insight. Cell Rep Med 2021, 2, 100472. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; Boyault, S.; Burkhardt, B.; Butler, A.P.; Caldas, C.; Davies, H.R.; Desmedt, C.; Eils, R.; Eyfjörd, J.E.; Foekens, J.A.; Greaves, M.; Hosoda, F.; Hutter, B.; Ilicic, T.; Imbeaud, S.; Imielinski, M.; Jäger, N.; Jones, D.T.; Jones, D.; Knappskog, S.; Kool, M.; Lakhani, S.R.; López-Otín, C.; Martin, S.; Munshi, N.C.; Nakamura, H.; Northcott, P.A.; Pajic, M.; Papaemmanuil, E.; Paradiso, A.; Pearson, J.V.; Puente, X.S.; Raine, K.; Ramakrishna, M.; Richardson, A.L.; Richter, J.; Rosenstiel, P.; Schlesner, M.; Schumacher, T.N.; Span, P.N.; Teague, J.W.; Totoki, Y.; Tutt, A.N.; Valdés-Mas, R.; van Buuren, M.M.; van ‘t Veer, L.; Vincent-Salomon, A.; Waddell, N.; Yates, L.R.; Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain; Zucman-Rossi, J.; Futreal, P.A.; McDermott, U.; Lichter, P.; Meyerson, M.; Grimmond, S.M.; Siebert, R.; Campo, E.; Shibata, T.; Pfister, S.M.; Campbell, P.J.; Stratton, M.R. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar]
- Ching, C.B.; Amin, M.B.; Tubbs, R.R.; Elson, P.; Platt, E.; Dreicer, R.; Fergany, A.; Hansel, D.E. HER2 gene amplification occurs frequently in the micropapillary variant of urothelial carcinoma: analysis by dual-color in situ hybridization. Mod Pathol. 2011, 24, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Willis, D.L.; Flaig, T.W.; Hansel, D.E.; Milowsky, M.I.; Grubb, R.L.; Al-Ahmadie, H.A.; Plimack, E.R.; Koppie, T.M.; McConkey, D.J.; Dinney, C.P.; Hoffman, V.A.; Droller, M.J.; Messing, E.; Kamat, A.M. Micropapillary bladder cancer: current treatment patterns and review of the literature. Urol Oncol 2014, 32, 826–832. [Google Scholar] [CrossRef]
- Isharwal, S.; Huang, H.; Nanjangud, G.; Audenet, F.; Chen, Y.B.; Gopalan, A.; Fine, S.W.; Tickoo, S.K.; Lee, B.H.; Iyer, G.; Chadalavada, K.; Rosenberg, J.E.; Bajorin, D.F.; Herr, H.W.; Donat, S.M.; Dalbagni, G.; Bochner, B.H.; Solit, D.B.; Reuter, V.E.; Al-Ahmadie, H.A. Intratumoral heterogeneity of ERBB2 amplification and HER2 expression in micropapillary urothelial carcinoma. Hum Pathol 2018, 77, 63–69. [Google Scholar] [CrossRef]
- Teo, M.Y.; Al-Ahmadie, H.; Seier, K.; Tully, C.; Regazzi, A.M.; Pietzak, E.; Solit, D.B.; : Tickoo, S.; Reuter, V.; Cha, E.K.; Herr, H.; Donahue, T.; Donat, S.M.; Dalbagni, G.; Bochner, B.H.; Funt, S.; Iyer, G.V.; Bajorin, D.F.; Ostrovnaya, I.; Rosenberg, J.E. Natural history, response to systemic therapy, and genomic landscape of plasmacytoid urothelial carcinoma. Br J Cancer 2021, 124, 1214–1221. [Google Scholar] [CrossRef]
- Taylor, C.F.; Platt, F.M.; Hurst, C.D.; Thygesen, H.H.; Knowles, M.A. Frequent inactivating mutations of STAG2 in bladder cancer are associated with low tumour grade and stage and inversely related to chromosomal copy number changes. Hum Mol Genet 2014, 23, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.S.; Humayun-Zakaria, N.; Goel, A.; Abbotts, B.; Zeegers, M.P.; Cheng, K.K.; James, N.D.; Arnold, R.; Bryan, R.T.; Ward, D.G. STAG2 Protein Expression in Non-muscle-invasive Bladder Cancer: Associations with Sex, Genomic and Transcriptomic Changes, and Clinical Outcomes. Eur Urol Open Sci 2022, 38, 88–95. [Google Scholar] [CrossRef] [PubMed]
- di Martino, E.; L’Hôte, C.G.; Kennedy, W.; Tomlinson, D.C.; Knowles, M.A. Mutant fibroblast growth factor receptor 3 induces intracellular signaling and cellular transformation in a cell type- and mutation-specific manner. Oncogene 2009, 28, 4306–4316. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; van Tilborg, A.A.; Zwarthoff, E.C. DNA methylation-based biomarkers in bladder cancer. Nat Rev Urol 2013, 10, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Berlin, Germany, 2017; 1049p. [Google Scholar]
- Leivo, M.Z.; Sahoo, D.; Hamilton, Z.; Mirsadraei, L.; Shabaik, A.; Parsons, J.K.; Kader, A.K.; Derweesh, I.; Kane, C.; Hansel, D.E. Analysis of T1 Bladder Cancer on Biopsy and Transurethral Resection Specimens: Comparison and Ranking of T1 Quantification Approaches to Predict Progression to Muscularis Propria Invasion. Am J Surg Pathol 2018, 42, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.; Dutto, D.; Gontero, P. Clinical and biological markers for risk-stratification of T1 high-grade non-muscle invasive bladder cancer. Curr Opin Urol 2022, 32, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, P.R.; Theodorescu, D.; Rosser, C.J.; Ahdoot, M. Identifying novel biomarkers associated with bladder cancer treatment outcomes. Front Oncol 2023, 13, 1114203. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017, 71, 96–108. [Google Scholar] [CrossRef]
- IARC. Global Cancer Observatory: Cancer Tomorrow. WHO (2023).
- Tran, L.; Xiao, J.F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in bladder cancer biology and therapy. Nat Rev Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef]
- Matuszczak, M.; Salagierski, M. Diagnostic and Prognostic Potential of Biomarkers CYFRA 21.1, ERCC1, p53, FGFR3 and TATI in Bladder Cancers. Int J Mol Sci 2020, 21, 3360. [Google Scholar] [CrossRef]
- Mertens, L.S.; Claps, F.; Mayr, R.; Bostrom, P.J.; Shariat, S.F.; Zwarthoff, E.C.; Boormans, J.L.; Abas, C.; van Leenders, G.J.L.H.; Götz, S.; Hippe, K.; Bertz, S.; Neuzillet, Y.; Sanders, J.; Broeks, A.; Peters, D.; van der Heijden, M.S.; Jewett, M.A.S.; Stöhr, R.; Zlotta, A.R.; Eckstein, M.; Soorojebally, Y.; van der Schoot, D.K.E.; Wullich, B.; Burger, M.; Otto, W.; Radvanyi, F.; Sirab, N.; Pouessel, D.; van der Kwast, T.H.; Hartmann, A.; Lotan, Y.; Allory, Y.; Zuiverloon, T.C.M.; van Rhijn, B.W.G. Prognostic markers in invasive bladder cancer: FGFR3 mutation status versus P53 and KI-67 expression: a multi-center, multi-laboratory analysis in 1058 radical cystectomy patients. Urol Oncol 2022, 40, 110.e1–110.e9. [Google Scholar] [CrossRef] [PubMed]
- Cummings, K.B.; Barone, J.G.; Ward, W.S. Diagnosis and staging of bladder cancer. Urol Clin North Am 1992, 19, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Khadhouri, S.; Gallagher, K.M.; MacKenzie, K.R.; Shah, T.T.; Gao, C.; Moore, S.; Zimmermann, E.F.; Edison, E.; Jefferies, M.; Nambiar, A.; Mannas, M.P.; Lee, T.; Marra, G.; Lillaz, B.; Gómez; Rivas, J.; Olivier, J.; Assmus, M.A.; Uçar, T.; Claps, F.; Boltri, M.; Burnhope, T.; Nkwam, N.; Tanasescu, G.; Boxall, N.E.; Downey, A.P.; Lal, A.A.; Antón-Juanilla, M.; Clarke, H.; Lau, D.H.W.; Gillams., K.; Crockett, M.; Nielsen, M.; Takwoingi, Y.; Chuchu, N.; O’Rourke, J.; MacLennan, G.; McGrath, J.S.; Kasivisvanathan, V.; IDENTIFY Study group. The IDENTIFY study: the investigation and detection of urological neoplasia in patients referred with suspected urinary tract cancer - a multicentre observational study. BJU Int 2021, 128, 440–450. [Google Scholar] [CrossRef]
- Xing, J.; Reynolds, J.P. Diagnostic Advances in Urine Cytology. Surg Pathol Clin 2018, 11, 601–610. [Google Scholar] [CrossRef]
- Barkan, G.A.; Wojcik, E.M.; Nayar, R.; Savic-Prince, S.; Quek, M.L.; Kurtycz, D.F.; Rosenthal, D.L. The Paris System for Reporting Urinary Cytology: The Quest to Develop a Standardized Terminology. Adv Anat Pathol 2016, 23, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Nikas, I.P.; Seide, S.; Proctor, T.; Kleinaki, Z.; Kleinaki, M.; Reynolds, J.P. The Paris System for Reporting Urinary Cytology: A Meta-Analysis. J Pers Med 2022, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Wang, X.; Gao, L.; Shi, J.; Sun, C.; Wan, Z. Bladder tumour antigen (BTA stat) test compared to the urine cytology in the diagnosis of bladder cancer: A meta-analysis. Can Urol Assoc J 2014, 8, E347–E352. [Google Scholar] [CrossRef] [PubMed]
- Dimashkieh, H.; Wolff, D.J.; Smith, T.M.; Houser, P.M.; Nietert, P.J.; Yang, J. Evaluation of urovysion and cytology for bladder cancer detection: a study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol 2013, 121, 591–597. [Google Scholar] [CrossRef]
- Zippe, C.; Pandrangi, L.; Agarwal, A. NMP22 is a sensitive, cost-effective test in patients at risk for bladder cancer. J Urol 1999, 161, 62–65. [Google Scholar] [CrossRef]
- He, H.; Han, C.; Hao, L.; Zang, G. ImmunoCyt test compared to cytology in the diagnosis of bladder cancer: A meta-analysis. Oncol Lett 2016, 12, 83–88. [Google Scholar] [CrossRef]
- Wang, Z.; Que, H.; Suo, C.; Han, Z.; Tao, J.; Huang, Z.; Ju, X.; Tan, R.; Gu, M. Evaluation of the NMP22 BladderChek test for detecting bladder cancer: a systematic review and meta-analysis. Oncotarget 2017, 8, 100648–100656. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Auinger, L.; Speicher, M.R. Cell-Free DNA and Apoptosis: How Dead Cells Inform About the Living. Trends Mol Med 2020, 26, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Khier, S.; Lohan, L. Kinetics of circulating cell-free DNA for biomedical applications: critical appraisal of the literature. Future Sci OA 2018, 4, FSO295. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Birkenkamp-Demtröder, K.; Sethi, H.; Shchegrova, S.; Salari, R.; Nordentoft, I.; Wu, H.T.; Knudsen, M.; Lamy, P.; Lindskrog, S.V.; Taber, A.; Balcioglu, M.; Vang, S.; Assaf, Z.; Sharma, S.; Tin, A.S.; Srinivasan, R.; Hafez, D.; Reinert, T.; Navarro, S.; Olson, A.; Ram, R.; Dashner, S.; Rabinowitz, M.; Billings, P.; Sigurjonsson, S.; Andersen, C.L.; Swenerton, R.; Aleshin, A.; Zimmermann, B.; Agerbæk, M.; Lin, C.J.; Jensen, J.B.; Dyrskjøt, L. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. J Clin Oncol 2019, 37, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Ploeg, M.; Kiemeney, L.A.; Smits, G.A.; Vergunst, H.; Viddeleer, A.C.; Geboers, A.D.; van Berkel, H.; van Boven, E.; Aben, K.K.; Witjes, J.A. Discrepancy between clinical staging through bimanual palpation and pathological staging after cystectomy. Urol Oncol 2012, 30, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Pasin, E.; Josephson, D.Y.; Mitra, A.P.; Cote, R.J.; Stein, J.P. Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev Urol 2008, 10, 31–43. [Google Scholar] [PubMed]
- Edgerton, N.; Sirintrapun, S.J.; Munoz, M.; Chen, Z.; Osunkoya, A.O. Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 24 cases. Int J Urol 2011, 18, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.I.; Williams, S.B.; Willis, D.L.; Slack, R.S.; Dickstein, R.J.; Parikh, S.; Chiong, E.; Siefker-Radtke, A.O.; Guo, C.C.; Czerniak, B.A.; McConkey, D.J.; Shah, J.B.; Pisters, L.L.; Grossman, H.B.; Dinney, C.P.; Kamat, A.M. Clinical risk stratification in patients with surgically resectable micropapillary bladder cancer. BJU Int 2017, 119, 684–691. [Google Scholar] [CrossRef]
- Hedegaard, J.; Lamy, P.; Nordentoft, I.; Algaba, F.; Høyer, S.; Ulhøi, B.P.; Vang, S.; Reinert, T.; Hermann, G.G.; Mogensen, K.; Thomsen, M.B.H.; Nielsen, M.M.; Marquez, M.; Segersten, U.; Aine, M.; Höglund, M.; Birkenkamp-Demtröder, K.; Fristrup, N.; Borre, M.; Hartmann, A.; Stöhr, R.; Wach, S.; Keck, B.; Seitz, A.K.; Nawroth, R.; Maurer, T.; Tulic, C.; Simic, T.; Junker, K.; Horstmann, M.; Harving, N.; Petersen, A.C.; Calle, M.L.; Steyerberg, E.W.; Beukers, W.; van Kessel, K.E.M.; Jensen, J.B.; Pedersen, J.S.; Malmström, P.U.; Malats, N.; Real, F.X.; Zwarthoff, E.C.; Ørntoft, T.F.; Dyrskjøt, L. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell 2016, 30, 27–42. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder cancer. Nat Rev Dis Primers 2023, 9, 58. [Google Scholar] [CrossRef]
- Gallioli, A.; Diana, P.; Fontana, M.; Territo, A.; Rodriguez-Faba, Ó.; Gaya, J.M.; Sanguedolce, F.; Huguet, J.; Mercade, A.; Piana, A.; Aumatell, J.; Bravo-Balado, A.; Algaba, F.; Palou, J.; Breda, A. En Bloc Versus Conventional Transurethral Resection of Bladder Tumors: A Single-center Prospective Randomized Noninferiority Trial. Eur Urol Oncol 2022, 5, 440–448. [Google Scholar] [CrossRef]
- D’Andrea, D.; Soria, F.; Hurle, R.; Enikeev, D.; Kotov, S.; Régnier, S.; Xylinas, E.; Lusuardi, L.; Heidenreich, A.; Cai, C.; Frego, N.; Taraktin, M.; Ryabov, M.; Gontero, P.; Compérat, E.; Shariat, S.F.; eBLOC Study Team. En Bloc Versus Conventional Resection of Primary Bladder Tumor (eBLOC): A Prospective, Multicenter, Open-label, Phase 3 Randomized Controlled Trial. Eur Urol Oncol, 2023; 6, 508–515. [Google Scholar]
- Teoh, Y.C.J.; Chan, T.; Tsang, C.; Li, K.; Cheng, K.-C.; Cho, C.; Chan, H.-C.; Chiu, Y.; Ho., B.; Li, T.; Law, M.; Lee, Y.; Cheng, C.; Lo, K.; Lam, K.; Chan, K; So, H.-S.; Leung, C.; Chan, C.; Yiu, M.; Ng, C.; Poon, V.; Leung, C.; Chi-Fai, N.; The EB-StaR Study Group. A0707 - Transurethral en bloc resection versus standard resection of bladder tumour: a multi-center randomized trial (EB-StaR Study). Eur Urol 2023 83, S997–S998.Babjuk, M.; Burger, M.; Compérat, E.M,, Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; Zigeuner, R.; Capoun, O.; Cohen, D.; Escrig, J.L.D.; Hernández, V.; Peyronnet, B.; Seisen, T.; Soukup, V. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol 2019, 76, 639–657. [Google Scholar]
- Messing, E.M.; Tangen, C.M.; Lerner, S.P.; Sahasrabudhe, D.M.; Koppie, T.M.; Wood, D.P., Jr.; Mack, P.C.; Svatek, R.S.; Evans, C.P.; Hafez, K.S.; Culkin, D.J.; Brand, T.C.; Karsh, L.I.; Holzbeierlein, J.M.; Wilson, S.S.; Wu, G.; Plets, M.; Vogelzang, N.J.; Thompson, I.M., Jr. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. JAMA 2018, 319, 1880–1888. [Google Scholar] [CrossRef]
- Höltl, L.; Eder, I.E.; Klocker, H.; Hobisch, A.; Bartsch, G.; Stenzl, A. Photodynamic diagnosis with 5-aminolevulinic acid in the treatment of secondary urethral tumors: first in vitro and in vivo results. Eur. Urol 2001, 39, 178–182. [Google Scholar]
- Nakai, Y.; Tatsumi, Y.; Miyake, M.; Anai, S.; Kuwada, M.; Onishi, S.; Chihara, Y.; Tanaka, N.; Hirao, Y.; Fujimoto, K. Expression of ferrochelatase has a strong correlation in protoporphyrin IX accumulation with photodynamic detection of bladder cancer. Photodiagn Photodyn Ther, 2016; 13, 225–232. [Google Scholar]
- Inoue, K.; Fukuhara, H.; Yamamoto, S.; Karashima, T.; Kurabayashi, A.; Furihata, M.; Hanazaki, K.; Lai, H.W.; Ogura, S.I. Current status of photodynamic technology for urothelial cancer. Cancer Sci 2022, 113, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, Y.; Endo, Y.; Yonemura, Y.; Takahashi, K.; Ishizuka, M.; Abe, F.; Tanaka, T.; Okura, I.; Nakajima, M.; Ishikawa, T.; Ogura, S. Pivotal roles of peptide transporter PEPT1 and ATP-binding cassette (ABC) transporter ABCG2 in 5-aminolevulinic acid (ALA)-based photocytotoxicity of gastric cancer cells in vitro. Photodiagnosis Photodyn Ther 2012, 9, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, Y.; Fukuhara, H.; Matsumoto, K.; Endo, Y.; Nakajima, M.; Tanaka, T.; Okura, I.; Kurabayashi, A.; Furihata, M.; Inoue, K.; Shuin, T.; Ogura, S. Expression levels of PEPT1 and ABCG2 play key roles in 5-aminolevulinic acid (ALA)-induced tumor-specific protoporphyrin IX (PpIX) accumulation in bladder cancer. Photodiagnosis Photodyn Ther 2013, 10, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, M.; Hagiya, Y.; Mizokami, Y.; Honda, K.; Tabata, K.; Kamachi, T.; Takahashi, K.; Abe, F.; Tanaka, T.; Nakajima, M.; Ogura, S.; Okura, I. Porphyrins in urine after administration of 5-aminolevulinic acid as a potential tumor marker. Photodiagnosis Photodyn Ther 2011, 8, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Fukuhara, H.; Karashima, T.; Inoue, K. Real-world experience with 5-aminolevulinic acid for the photodynamic diagnosis of bladder cancer: Diagnostic accuracy and safety. Photodiagnosis Photodyn Ther 2020, 32, 101999. [Google Scholar] [CrossRef]
- Yatabe, T.; Marie, S.L.; Fukuhara, H.; Karashima, T.; Inoue, K.; Yokoyama, M. 5-Aminolevulinic acid-induced severe hypotension during transurethral resection of a bladder tumor: a case report. J Anesth Clin Rep 2019, 5, 58. [Google Scholar] [CrossRef]
- Nohara, T.; Kato, Y.; Nakano, T.; Nakagawa, T.; Iwamoto, H.; Yaegashi, H.; Nakashima, K.; Iijima, M.; Kawaguchi, S.; Shigehara, K.; Izumi, K.; Kadono, Y.; Mizokami, A. Intraoperative hypotension caused by oral administration of 5-aminolevulinic acid for photodynamic diagnosis in patients with bladder cancer. Int J Urol 2019, 26, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, T.; Karashima, T.; Kume, M.; Kawanishi, Y.; Fukuhara, H.; Ueba, T.; Inoue, K.; Okuhara, Y.; Yokoyama, M. Identification of risk factors for post-induction hypotension in patients receiving 5-aminolevulinic acid: a single-center retrospective study. J Anesth Clin Rep 2020, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Nohara, T.; Nishimoto, K.; Hatakeyama, Y.; Hyodo, Y.; Okuhara, Y.; Oyama, M.; Mizokami, A.; Inoue, K.; Matsuyama, H. Japan Urological Photodynamic Society. Identification of risk factors associated with oral 5-aminolevulinic acid-induced hypotension in photodynamic diagnosis for non-muscle invasive bladder cancer: a multicenter retrospective study. BMC Cancer 2021, 21, 1223. [Google Scholar] [CrossRef] [PubMed]
- Kriegmair, M.; Baumgartner, R.; Knüchel, R.; Stepp, H.; Hofstädter, F.; Hofstetter, A. Detection of early bladder cancer by 5-aminolevulinic acid induced porphyrin fluorescence. J Urol 1996, 155, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Filbeck, T.; Roessler, W.; Knuechel, R.; Straub, M.; Kiel, H.J.; Wieland, W.F. Clinical results of the transurethreal resection and evaluation of superficial bladder carcinomas by means of fluorescence diagnosis after intravesical instillation of 5-aminolevulinic acid. J Endourol 1999, 13, 117–121. [Google Scholar] [CrossRef]
- De Dominicis, C.; Liberti, M.; Perugia, G.; De Nunzio, C.; Sciobica, F.; Zuccalà, A.; Sarkozy, A.; Iori, F. Role of 5-aminolevulinic acid in the diagnosis and treatment of superficial bladder cancer: improvement in diagnostic sensitivity. Urology 2001, 57, 1059–1062. [Google Scholar] [CrossRef]
- Zaak, D.; Kriegmair, M.; Stepp, H.; Stepp, H.; Baumgartner, R.; Oberneder, R.; Schneede, P.; Corvin, S.; Frimberger, D.; Knüchel, R.; Hofstetter, A. Endoscopic detection of transitional cell carcinoma with 5-aminolevulinic acid: results of 1012 fluorescence endoscopies. Urology 2001, 57, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, H.; Zhao, Y.; Liu, H.; Luo, Y.; Sylvester, R.J.; Li, J.P.; Lam, T.B.; Lin, T.; Huang, J. Diagnostic accuracy of photodynamic diagnosis with 5-aminolevulinic acid, hexaminolevulinate and narrow band imaging for non-muscle invasive bladder cancer. J Cancer 2020, 11, 1082–1093. [Google Scholar] [CrossRef]
- Hagimoto, H.; Makita, N.; Mine, Y.; Kokubun, H.; Murata, S.; Abe, Y.; Kubota, M.; Tsutsumi, N.; Yamasaki, T.; Kawakita, M. Comparison between 5-aminolevulinic acid photodynamic diagnosis and narrow-band imaging for bladder cancer detection. BMC Urol 2021, 21, 180. [Google Scholar] [CrossRef]
- Naito, S.; Algaba, F.; Babjuk, M.; Bryan, R.T.; Sun, Y.H.; Valiquette, L.; de la Rosette, J. The Clinical Research Office of the Endourological Society (CROES) multicentre randomised trial of narrow band imaging-assisted Transurethral Resection of Bladder Tumour (TURBT) versus conventional white light imaging-assisted TURBT in primary non-muscle-invasive bladder cancer patients: trial protocol and 1-year results. Eur Urol 2016, 70, 506–515. [Google Scholar]
- Matsumoto, H.; Shiraishi, K.; Azuma, H.; Inoue, K.; Uemura, H.; Eto, M.; Ohyama, C.; Ogawa, O.; Kikuchi, E.; Kitamura, H.; Shinohara, N.; Takahashi, S.; Tsuzuki, T.; Nakagawa, M.; Narumi, Y.; Nishiyama, H.; Habuchi, T.; Hinotsu, S.; Fujii, Y.; Fujimoto, K.; Fujimoto, H.; Mizowaki, T.; Matsuyama, H. Clinical Practice Guidelines for Bladder Cancer 2019 update by the Japanese Urological Association: Summary of the revision. Int J Urol 2020, 27, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Maesaka, F.; Marugami, N.; Miyamoto, T.; Nakai, Y.; Ohnishi, S.; Gotoh, D.; Owari, T.; Hori, S.; Morizawa, Y.; Itami, Y.; Inoue, T.; Anai, S.; Torimoto, K.; Fujii, T.; Shimada, K.; Tanaka, N.; Fujimoto, K. A Potential Application of Dynamic Contrast-Enhanced Magnetic Resonance Imaging Combined with Photodynamic Diagnosis for the Detection of Bladder Carcinoma in Situ: Toward the Future ‘MRI-PDD Fusion TURBT’. Diagnostics (Basel) 2019, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Babjuk, M.; Gontero, P.; Jacqmin, D.; Karl, A.; Kruck, S.; Mariappan, P.; Redorta, J.P.; Stenzl, A.; Velthoven van, R.; Zaak, D. Clinical and cost effectiveness of hexaminolevulinate-guided blue-light cystoscopy: evidence review and updated expert recommendations. Eur Urol 2014, 66, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Hungerhuber, E.; Stepp, H.; Kriegmair, M.; Stief, C.; Hofstetter, A.; Hartman, A.; Knuechel, R.; Karl, A.; Trischler, S.; Zaak, D. Seven years’ experience with 5-aminolevulinic acid in detection of transitional cell carcinoma of the bladder. Urology 2007, 69, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Rink, M.; Babjuk, M.; Catto, J.W.; Jichlinski, P.; Shariat, S.F.; Stenzl, A.; Stepp, H.; Zaak, D.; Witjes, J.A. Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: a critical review of the current literature. Eur Urol 2013, 64, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Veeratterapillay, R.; Gravestock, P.; Nambiar, A.; Gupta, A.; Aboumarzouk, O.; Rai, B.; Vale, L.; Heer, R. Time to turn on the blue lights: a systematic review and meta-analysis of photodynamic diagnosis for bladder cancer. Eur Urol Open Sci 2021, 31, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Miyake, M.; Nishimura, N.; Nishimoto, K.; Fukuhara, H.; Kobayashi, K.; Oyama, M.; Inoue, K.; Matsuyama, H.; Fujimoto, K.; Miyake, H. Comparative assessment of disease recurrence after transurethral resection of non-muscle-invasive bladder cancer with and without a photodynamic diagnosis using 5-aminolevulinic acid: a propensity score-matching analysis. Int J Clin Oncol 2024, 29, 205–212. [Google Scholar] [CrossRef]
- Miyake, M.; Nishimura, N.; Nakahama, T.; Nishimoto, K.; Oyama, M.; Matsushita, Y.; Miyake, H.; Fukuhara, H.; Inoue, K.; Kobayashi, K.; Matsumoto, H.; Matsuyama, H.; Fujii, T.; Hirao, Y.; Fujimoto, K. Additional oncological benefit of photodynamic diagnosis with blue light cystoscopy in transurethral resection for primary non-muscle-invasive bladder cancer: A comparative study from experienced institutes. BJUI Compass 2023, 4, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Matsuyama, H.; Oka, S.; Nakamura, K.; Misumi, T.; Hiroyoshi, T.; Ito, H.; Isoyama, N.; Hirata, H.; Matsumoto, H.; Shiraishi, K. Risks and benefits of transurethral resection of the bladder tumor using photodynamic diagnosis with oral 5-aminolevulinic acid hydrochloride according to age and history of recurrence in patients with non-muscle invasive bladder cancer. Photodiagnosis Photodyn Ther 2023, 41, 103294. [Google Scholar] [CrossRef]
- Yamashita, T.; Kinoshita, H.; Sakaguchi, T.; Isomoto, H. Objective tumor distinction in 5-aminolevulinic acid-based endoscopic photodynamic diagnosis, using a spectrometer with a liquid crystal tunable filter. Ann Transl Med 2020, 8, 178. [Google Scholar] [CrossRef]
- Brandau, S.; Suttmann, H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed Pharmacother 2007, 61, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; van der MEIJDEN, A.P.; Lamm, D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 2002, 168, 1964–1970. [Google Scholar] [CrossRef]
- Draga, R.O.; Grimbergen, M.C.; Kok, E.T.; Jonges, T.N.; van Swol, C.F.; Bosch, J.L. Photodynamic diagnosis (5-aminolevulinic acid) of transitional cell carcinoma after bacillus Calmette-Guerin immunotherapy and mitomycin C intravesical therapy. Eur Urol 2010, 57, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, R.; Nohara, T.; Naito, R.; Kadomoto, S.; Iwamoto, H.; Yaegashi, H.; Kawaguchi, S.; Shigehara, K.; Izumi, K.; Kadono, Y.; Mizokami, A. Intravesical BCG therapy with photodynamic diagnosis-guided transurethral resection of bladder tumors improves recurrence-free survival. Photodiagnosis Photodyn Ther 2023, 42, 103574. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Fritz, J.; Zavadil, C.; Schäfer, G.; Culig, Z.; Brunner, A. Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical Bacillus Calmette-Guérin therapy in bladder cancer. Oncotarget 2016, 7, 39916–39930. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer. Int J Urol 2017, 24, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Ofude, M.; Kawahara, T.; Hori, T.; Urata, S.; Miyagi, T. Poor Prognosis With Intravesical Bacillus Calmette-Guerin History After Photodynamic Diagnosis-assisted Transurethral Resection Using 5-Aminolevulinic Acid. In Vivo 2022, 36, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.M.; Giram, P.; Foster, B.A.; You, Y. Photodynamic Therapy for Bladder Cancers, A Focused Review. Photochem Photobiol 2023, 99, 420–436. [Google Scholar] [CrossRef]
- Zein, T.A.; Friedberg, N.; Kim, H. Bone marrow suppression after intravesical mitomycin C treatment. J Urol 1986, 136, 459–460. [Google Scholar] [CrossRef]
- Wittes, R.; Klotz, L.; Kosecka, U. Severe bacillus Calmette-Guerin cystitis responds to systemic steroids when antituberculous drugs and local steroids fail. J Urol 1999, 161, 1568–1569. [Google Scholar] [CrossRef]
- Ishizuka, M.; Abe, F.; Sano, Y.; Takahashi, K.; Inoue, K.; Nakajima, M.; Kohda, T.; Komatsu, N.; Ogura, S.; Tanaka, T. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int Immunopharmacol 2011, 11, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Kriegmair, M.; Baumgartner, R.; Lumper, W.; Waidelich, R.; Hofstetter, A. Early clinical experience with 5-aminolevulinic acid for the photodynamic therapy of superficial bladder cancer. Br J Urol 1996, 77, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Waidelich, R.; Stepp, H.; Baumgartner, R.; Weninger, E.; Hofstetter, A.; Kriegmair, M. Clinical experience with 5-aminolevulinic acid and photodynamic therapy for refractory superficial bladder cancer. J Urol 2001, 165, 1904–1907. [Google Scholar] [CrossRef] [PubMed]
- Shackley, D.C.; Briggs, C.; Gilhooley, A.; Whitehurst, C.; O’Flynn, K.J.; Betts, C.D.; Moore, J.V.; Clarke, N.W. Photodynamic therapy for superficial bladder cancer under local anaesthetic. BJU Int 2002, 89, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.P.; Steiner, H.; Stenzl, A.; Akkad, T.; Bartsch, G.; Holtl, L. Photodynamic therapy with intravesical instillation of 5-aminolevulinic acid for patients with recurrent superficial bladder cancer, a single-center study. Urology 2003, 61, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Waidelich, R.; Beyer, W.; Knüchel, R.; Stepp, H.; Baumgartner, R.; Schröder, J.; Hofstetter, A.; Kriegmair, M. Whole bladder photodynamic therapy with 5-aminolevulinic acid using a white light source. Urology 2003, 61, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Skyrme, R.J.; French, A.J.; Datta, S.N.; Allman, R.; Mason, M.D.; Matthews, P.N. A phase-1 study of sequential mitomycin C and 5-aminolaevulinic acid-mediated photodynamic therapy in recurrent superficial bladder carcinoma. BJU Int 2005, 95, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Filonenko, E.V.; Kaprin, A.D.; Alekseev, B.Y.; Apolikhin, O.I.; Slovokhodov, E.K.; Ivanova-Radkevich, V.I.; Urlova, A.N. 5-Aminolevulinic acid in intraoperative photodynamic therapy of bladder cancer (results of multicenter trial). Photodiagnosis Photodyn Ther 2016, 16, 106–109. [Google Scholar] [CrossRef]
- Yamamichi, G.; Nakata, W.; Yoshimura, A.; Tsujimura, G.; Tsujimoto, Y.; Nin, M.; Mimura, A.; Miwa, H.; Tsujihata, M. High performance of 5-aminolevulinic acid-induced fluorescent selective upper tract urinary cytology. Int J Urol 2020, 27, 213–218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




