Submitted:
18 April 2024
Posted:
22 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Toxicity Assessment of Various Compounds with Different Methods.
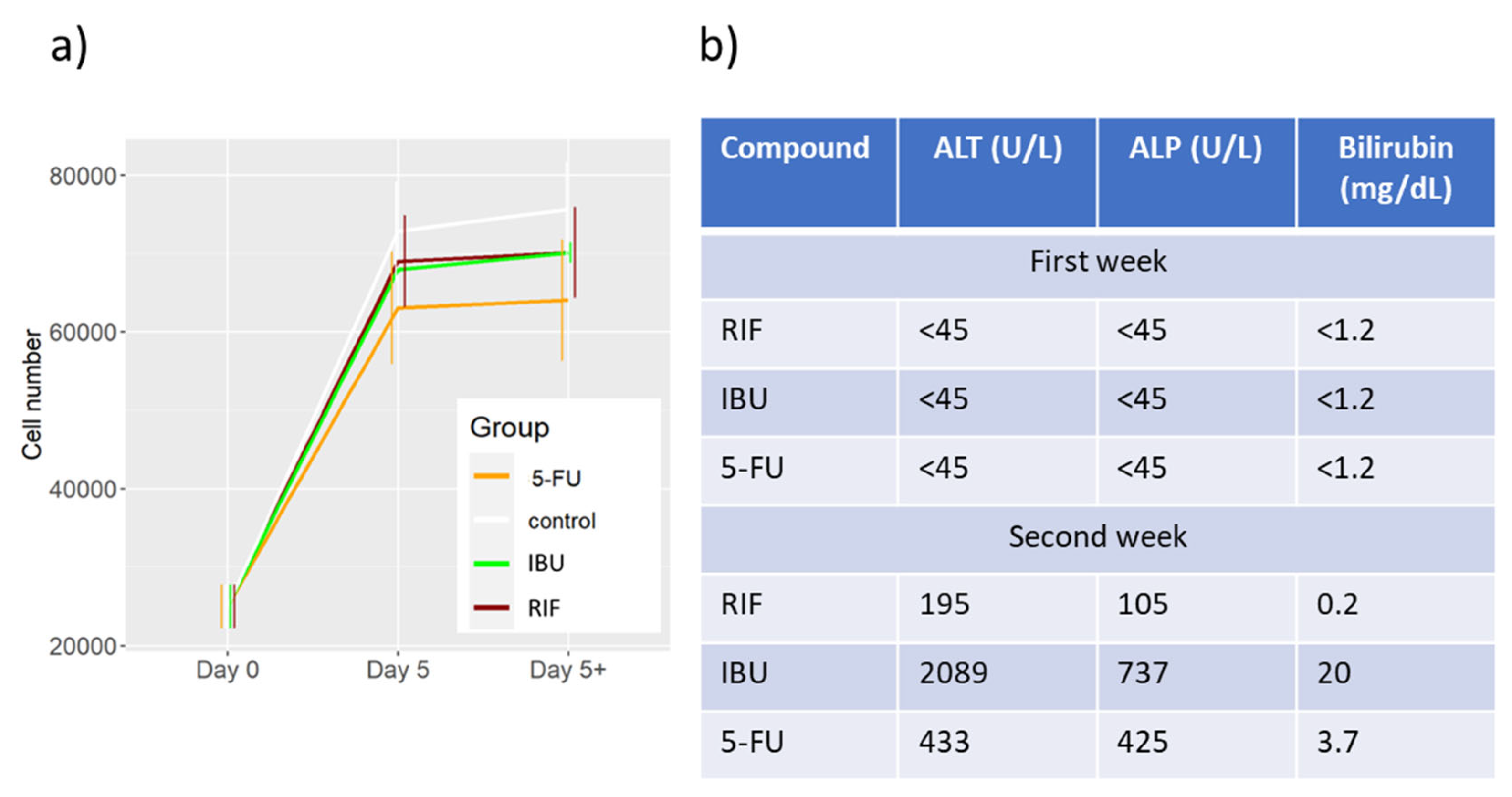
2.2. Toxicity Evaluation of DD Cell-Tox Method in Complex Cell-Based Liver Model in Comparison to Clinical Data.
3. Discussion
Highlights
- The DD Cell-Tox method directly determines the viability of cells. Therefore, it has the potential to be more accurate than methods based on an indirect determination of cell viability.
- The DD Cell-Tox method provides a more comprehensive assessment of toxic compound-induced cell outcomes.
- The application of the DD Cell-Tox method can be of particular importance in toxicological studies where compensatory proliferation is expected.
Limitations
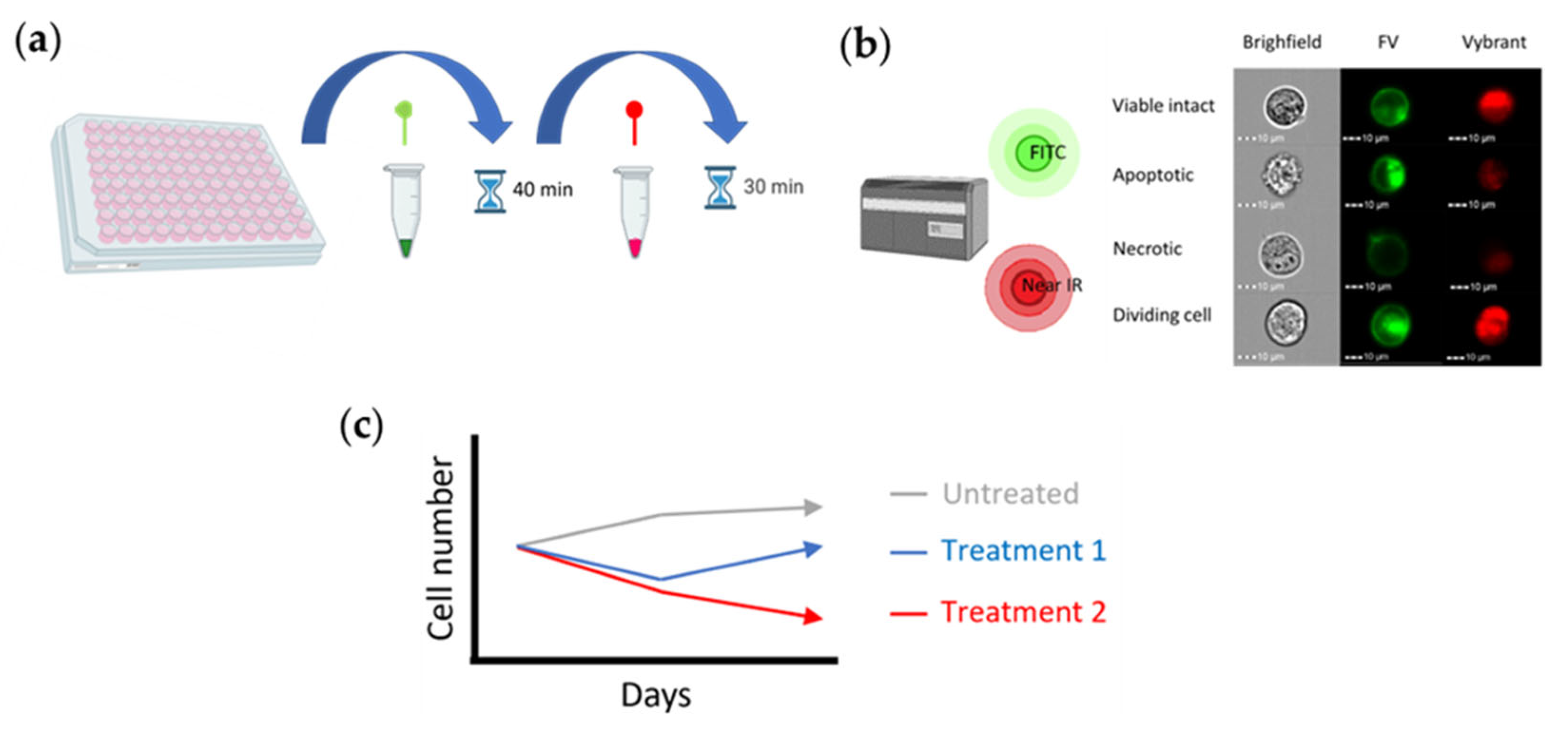
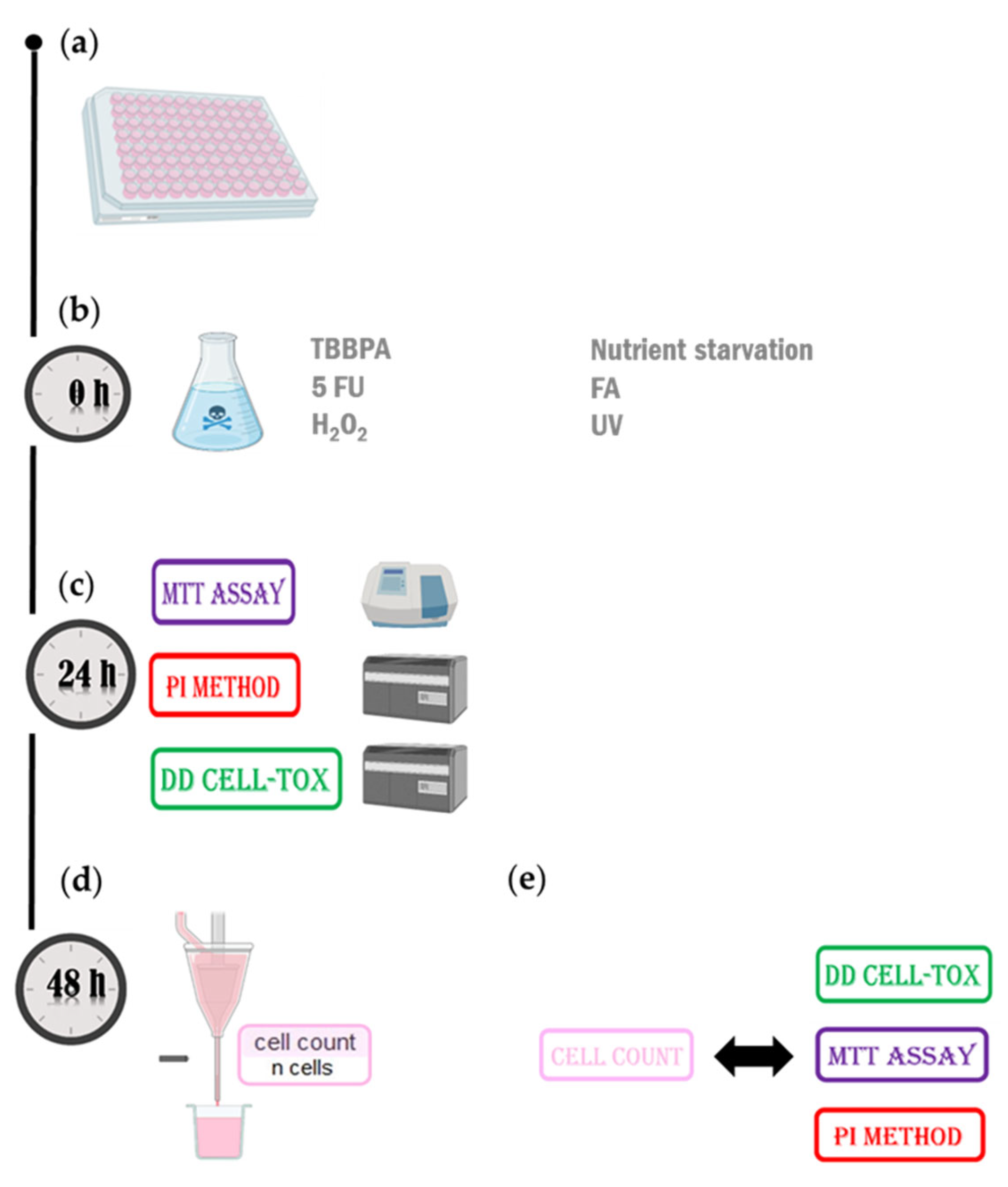
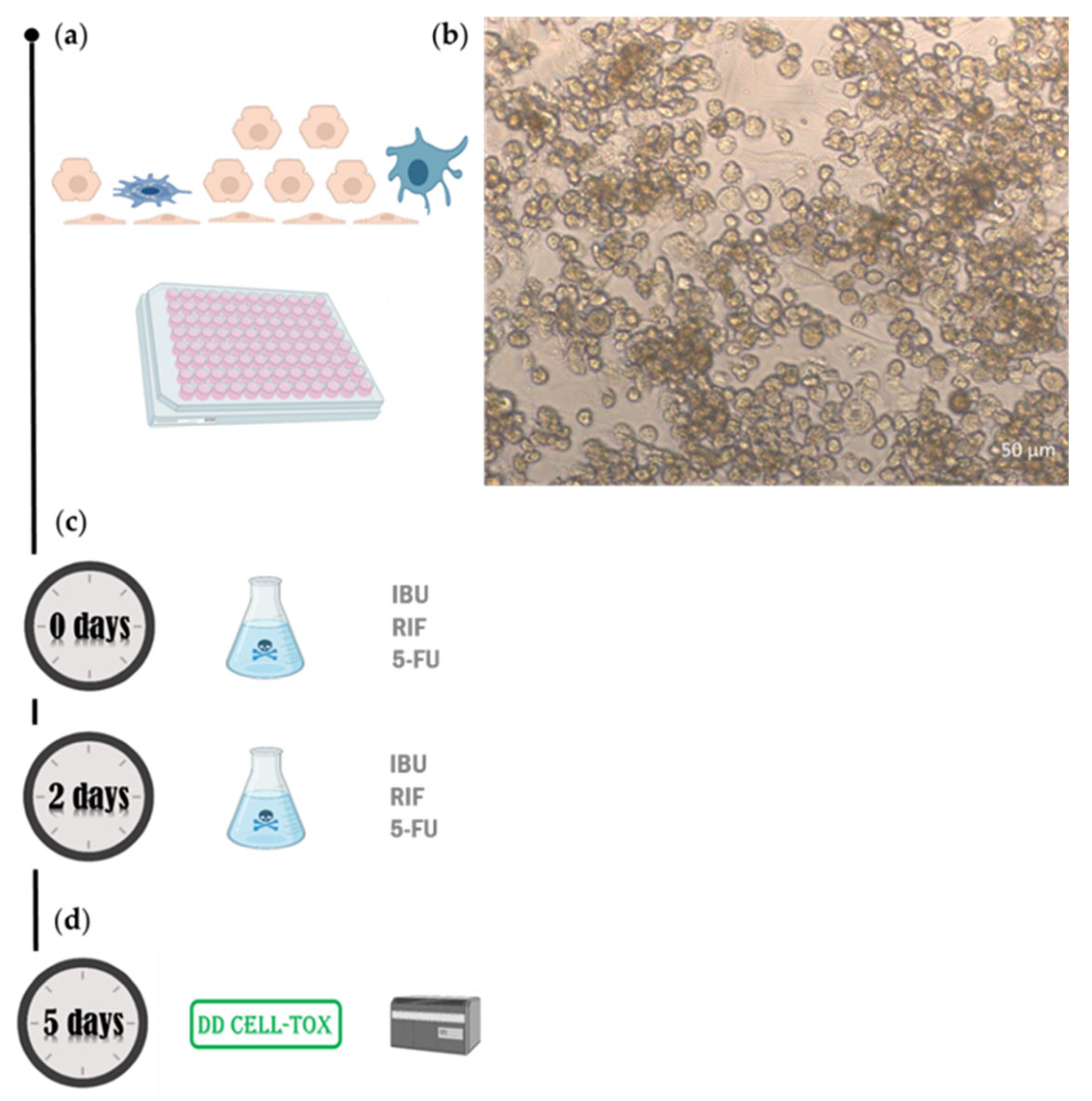
4. Materials and Methods
4.1. Cells
4.2. Chemicals
4.2. Protocol
4.3. Testing the Method in the First Configuration (Monoculture)
4.4. Testing the Method in the Second Arrangement (Coculture Model)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Gupta, K.H.; Goldufsky, J.W.; Wood, S.J.; Tardi, N.J.; Moorthy, G.S.; Gilbert, D.Z.; Zayas, J.P.; Hahm, E.; Altintas, M.M.; Reiser, J.; Shafikhani, S.H. , Apoptosis and Compensatory Proliferation Signaling Are Coupled by CrkI-Containing Microvesicles. Dev Cell 2017, 41, 674–684e5. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, S.A.; Conner, C.M.; Rosenblatt, J.; Montell, D.J. Unconventional Ways to Live and Die: Cell Death and Survival in Development, Homeostasis, and Disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Alenzi, F.Q. , Links between apoptosis, proliferation and the cell cycle. Br J Biomed Sci 2004, 61, 99–102. [Google Scholar] [CrossRef]
- Krafts, K. , Tissue Repair. In Encyclopedia of Toxicology (Third Edition), Wexler, P., Ed. Academic Press: Oxford, 2014; pp 577-583.
- Cusack, R.; Chawke, L.; O’brien, D.; O’connor, B.; O’connor, T. Predictors of hepatotoxicity among patients treated with antituberculous medication. Qjm: Int. J. Med. [CrossRef]
- Vincenzi, B.; Imperatori, M.; Picardi, A.; Gentilucci, U.V.; Gallo, P.; Fausti, V.; Ceruso, M.S.; Santini, D.; Tonini, G. Liver toxicity in colorectal cancer patients treated with first-line FOLFIRI-containing regimen: a single institution experience. Expert Rev. Anticancer. Ther. 2015, 15, 971–976. [Google Scholar] [CrossRef]
- Diseases, B.M.N.I. o. D. a. D. a. K. , LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]-fluorouracil. In livertox.nih.gov: 2018.
- Diseases, B.M.N.I. o. D. a. D. a. K. , LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]- rifampicin. In livertox.nih.gov: 2018.
- Diseases, B.M.N.I. o. D. a. D. a. K. , LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]- ibuprofen. In livertox.nih.gov: 2018.
- Messam, C.A.; Pittman, R.N. Asynchrony and Commitment to Die during Apoptosis. Exp. Cell Res. 1998, 238, 389–398. [Google Scholar] [CrossRef]
- Aragane, Y.; Kulms, D.; Metze, D.; Wilkes, G.; Pöppelmann, B.; Luger, T.A.; Schwarz, T. Ultraviolet Light Induces Apoptosis via Direct Activation of CD95 (Fas/APO-1) Independently of Its Ligand CD95L. J. Cell Biol. 1998, 140, 171–182. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Bernard, S.; Herzel, H. , Why do cells cycle with a 24 hour period? Genome Inform 2006, 17, 72–79. [Google Scholar]
- Nishikawa, S.; Takamatsu, A. Effects of cell death-induced proliferation on a cell competition system. Math. Biosci. 2019, 316, 108241. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, H.D.; Bergmann, A. The Role of Apoptosis-Induced Proliferation for Regeneration and Cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008797. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Li, J.; Wei, Y.; Lu, J.; Dong, R. Hepatic Stellate Cell: A Double-Edged Sword in the Liver. Physiol. Res. 2021, 70, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Yang, C.; Martino, M.; Duncan, M.B.; Rieder, F.; Tanjore, H.; Kalluri, R. Fibroblasts Derive from Hepatocytes in Liver Fibrosis via Epithelial to Mesenchymal Transition. J. Biol. Chem. 2007, 282, 23337–23347. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wan, C.; Guo, R.; Guo, D. , Is Hydrogen Peroxide a Suitable Apoptosis Inducer for All Cell Types? Biomed Res Int 2016, 2016, 7343965. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Chen, C.; An, J.; Shang, Y.; Li, H.; Xia, H.; Yu, J.; Wang, C.; Liu, Y.; et al. Regulation of TBBPA-induced oxidative stress on mitochondrial apoptosis in L02 cells through the Nrf2 signaling pathway. Chemosphere 2019, 226, 463–471. [Google Scholar] [CrossRef]
- Cho, K.-H.; Choi, S.-M.; Kim, B.-C.; Lee, S.-H.; Park, M.-S.; Kim, M.-K.; Kim, J.-K. 5-fluorouracil-induced oligodendrocyte death and inhibitory effect of cycloheximide, trolox, and Z-VAD-FMK in murine cortical culture. Cancer 2004, 100, 1484–1490. [Google Scholar] [CrossRef]
- Noguchi, M.; Hirata, N.; Tanaka, T.; Suizu, F.; Nakajima, H.; Chiorini, J.A. Autophagy as a modulator of cell death machinery. Cell Death Dis. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. , Ferroptosis: past, present and future. Cell Death Dis 2020, 11, 88. [Google Scholar] [CrossRef]
- Salucci, S.; Burattini, S.; Battistelli, M.; Baldassarri, V.; Maltarello, M.C.; Falcieri, E. Ultraviolet B (UVB) Irradiation-Induced Apoptosis in Various Cell Lineages in Vitro. Int. J. Mol. Sci. 2012, 14, 532–546. [Google Scholar] [CrossRef]
- Aldewachi, H.S.; A Wright, N.; Appleton, D.R.; Watson, A.J. The effect of starvation and refeeding on cell population kinetics in the rat small bowel mucosa. J. Anat. 1975, 119, 105–121. [Google Scholar] [PubMed]
- Goodlad, R.A.; Wright, N.A. The effects of starvation and refeeding on intestinal cell proliferation in the mouse. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1984, 45, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, L. Apoptosis and Cell Proliferation Are Involved in the Initiation of Liver Carcinogenesis by a Subnecrogenic Dose of Diethylnitrosamine in Refed Rats. J. Nutr. 2000, 130, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, K.; Jacob, S.T. An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug. Biochem. Pharmacol. 1997, 53, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Granada, A.E.; Jiménez, A.; Stewart-Ornstein, J.; Blüthgen, N.; Reber, S.; Jambhekar, A.; Lahav, G. The effects of proliferation status and cell cycle phase on the responses of single cells to chemotherapy. Mol. Biol. Cell 2020, 31, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Goldbeter, A. , The balance between cell cycle arrest and cell proliferation: control by the extracellular matrix and by contact inhibition. Interface Focus 2014, 4, 20130075. [Google Scholar] [CrossRef] [PubMed]
- Chitu, V.; Yeung, Y.; Yu, W.; Nandi, S.; Stanley, E.R. Measurement of Macrophage Growth and Differentiation. Curr. Protoc. Immunol. 2011, 92, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mazaleuskaya, L.L.; Theken, K.N.; Gong, L.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. , PharmGKB summary: ibuprofen pathways. Pharmacogenet Genomics 2015, 25, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.; Backus, H.; Freemantle, S.; van Triest, B.; Codacci-Pisanelli, G.; van der Wilt, C.; Smid, K.; Lunec, J.; Calvert, A.; Marsh, S.; et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2002, 1587, 194–205. [Google Scholar] [CrossRef]
- Advani, P.P.; Fakih, M.G. 5-FU-induced hyperammonemic encephalopathy in a case of metastatic rectal adenocarcinoid successfully rechallenged with the fluoropyrimidine analog, capecitabine. Anticancer Res. 2011, 31, 335–338. [Google Scholar]
- Zhang, C.; Wang, H.; Ning, Z.; Xu, L.; Zhuang, L.; Wang, P.; Meng, Z. Serum liver enzymes serve as prognostic factors in patients with intrahepatic cholangiocarcinoma. OncoTargets Ther. 2017, ume 10, 1441–1449. [Google Scholar] [CrossRef]
- Barguilla, I.; Maguer-Satta, V.; Guyot, B.; Pastor, S.; Marcos, R.; Hernández, A. In Vitro Approaches to Determine the Potential Carcinogenic Risk of Environmental Pollutants. Int. J. Mol. Sci. 2023, 24, 7851. [Google Scholar] [CrossRef] [PubMed]
- Audebert, M.; Assmann, A.-S.; Azqueta, A.; Babica, P.; Benfenati, E.; Bortoli, S.; Bouwman, P.; Braeuning, A.; Burgdorf, T.; Coumoul, X.; et al. New approach methodologies to facilitate and improve the hazard assessment of non-genotoxic carcinogens—a PARC project. Front. Toxicol. 2023, 5, 1220998. [Google Scholar] [CrossRef] [PubMed]
- Loftus, L.V.; Amend, S.R.; Pienta, K.J. Interplay between Cell Death and Cell Proliferation Reveals New Strategies for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 4723. [Google Scholar] [CrossRef] [PubMed]
- Madorran, E.; Stožer, A.; Arsov, Z.; Maver, U.; Rožanc, J. A Promising Method for the Determination of Cell Viability: The Membrane Potential Cell Viability Assay. Cells 2022, 11, 2314. [Google Scholar] [CrossRef] [PubMed]
- Madorran, E.; Stožer, A.; Arsov, Z.; Maver, U.; Rožanc, J. A Promising Method for the Determination of Cell Viability: The Membrane Potential Cell Viability Assay. Cells 2022, 11, 2314. [Google Scholar] [CrossRef] [PubMed]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. , Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. Journal of visualized experiments : JoVE 2011.
- Van Merloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Luk, H.-Y.; McFarlin, B.K.; Vingren, J.L. Using image-based flow cytometry to monitor satellite cells proliferation and differentiation in vitro. Methods 2017, 112, 175–181. [Google Scholar] [CrossRef]
- Madorran, E.; Kocbek Šaherl, L.; Rakuša, M.; Munda, M. , In Vitro Human Liver Model for Toxicity Assessment with Clinical and Preclinical Instrumentation. In Preprints, Preprints: 2024.
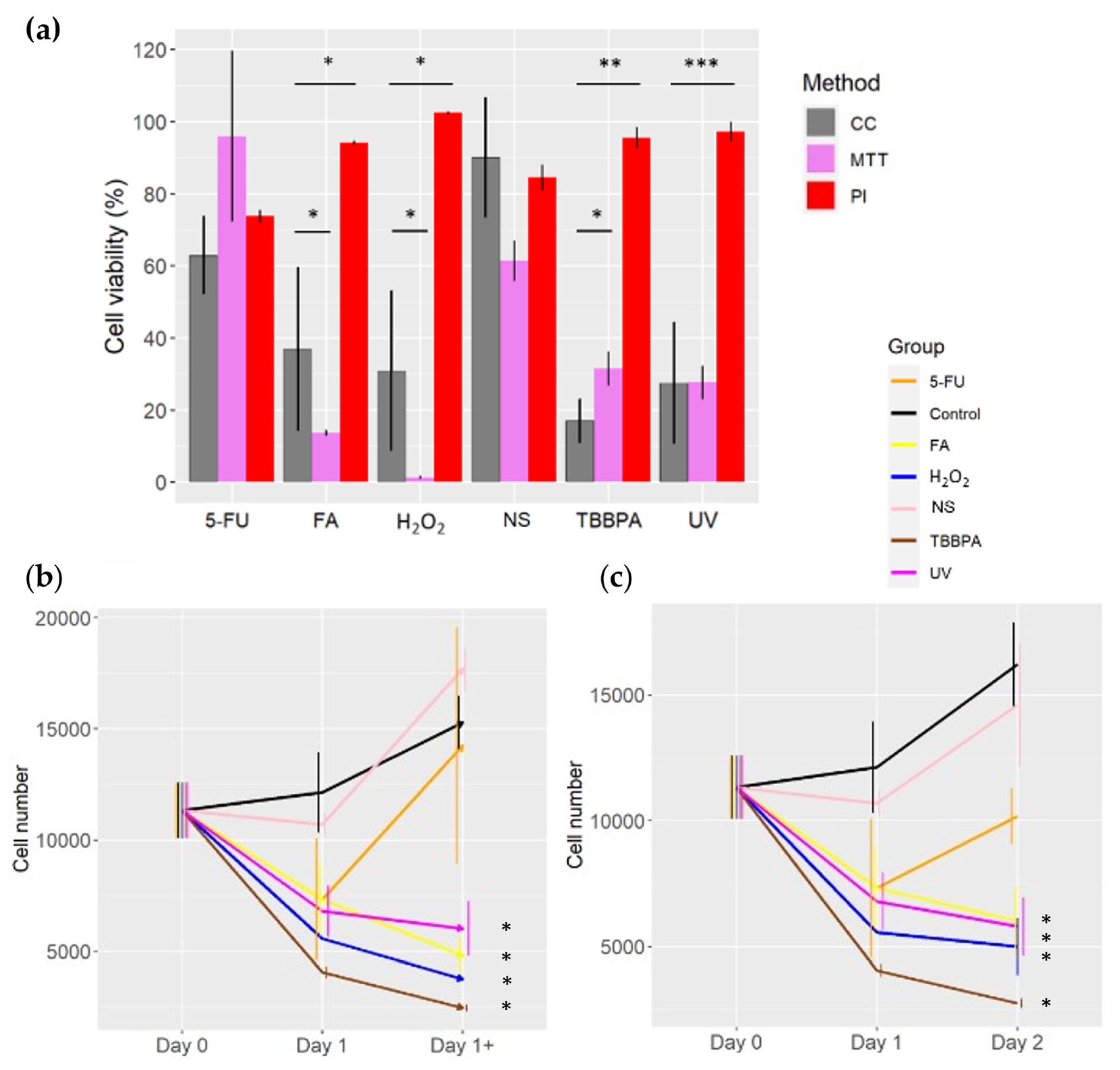
| Group | Number of initial cells | Number of final cells | Dead cells (%) | Division cells (%) |
|---|---|---|---|---|
| Control | 11329 | 12130 | 12 | 20 |
| 5-FU | 11329 | 7327 | 7 | 26 |
| FA | 11329 | 7318 | 35 | 0 |
| H2O2 | 11329 | 5564 | 33 | 0 |
| NS | 11329 | 10694 | 12 | 39 |
| TBBPA | 11329 | 4064 | 45 | 3 |
| UV | 11329 | 6797 | 26 | 7 |
| Group | Number of initial cells | Number of final cells | Cell number increase (%) | Dead cells (%) | Division cells (%) |
|---|---|---|---|---|---|
| Control | 25000 | 72797 | 291 | 3 | 2 |
| 5-FU | 25000 | 63056 | 252 | 4 | 3 |
| IBU | 25000 | 67919 | 272 | 3 | 4 |
| RIF | 25000 | 68995 | 276 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





