Submitted:
19 April 2024
Posted:
19 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Molecular Mechanisms in Allergic Asthma: The Allergens Proteases
House Dust Mite
Fungi
Cockroaches
Foods
Pollen
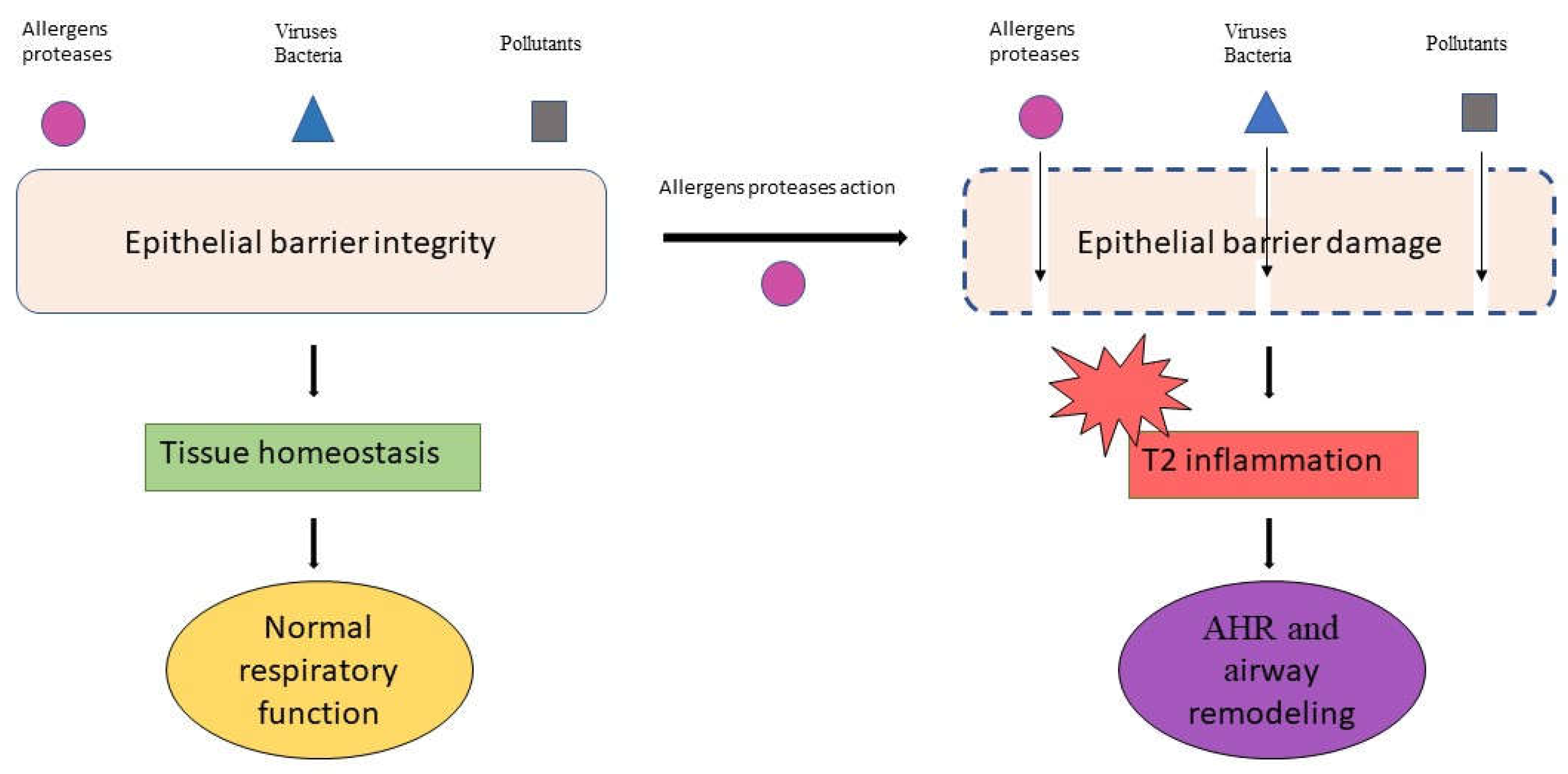
2.1. The Allergens Proteases in Epithelial Barrier Damage and Inflammatory Signals
3. Airway Remodeling and Its Role in Asthma Exacerbations
3.1. Biomarkers of Airway Remodeling
3.1.1. Epithelial Remodeling
3.1.2. Reticular Basement Membrane Thickening
3.1.3. Subepithelial Fibrosis
3.1.4. Airway Smooth Muscle
3.1.5. Mucus
3.1.6. Vasculature
3.2. Airway Remodeling: Radiological Pathways and Key Points
3.2.1. Radiological Indicators for Assessing Severity, Early Identification, and Involvement of Small Airways
3.2.2. Radiological Pathway in Patient Phenotyping
4. Relevant Therapeutic Options in Airway Remodeling
4.1. The Role of Standard Therapy in Airway Remodeling (LAMA)
4.2. The Role of Biological Drugs in Airway Remodeling
5. Discussion
6. Conclusions and Future Directions
Author Contributions
Availability statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Tight Junctions | TJs |
| Zonula Occludens | ZO |
| Chronic Rhinosinusitis with Nasal Polyps | CRSwNP |
| Eosinophilic Esophagitis | EoE |
| Atopic Dermatitis | AD |
| House-Dust Mite | HDM |
| Type 2 | T2 |
| Immunoglobulin-E | IgE |
| Toll-Like Receptor-4 | TLR4 |
| Protease-Activated Receptors | PARs |
| Dendritic Cells | DCs |
| Type 1 | T1 |
| Interferon | INF |
| myeloid Dendritic Cells | mDCs |
| Thymic Stromal Lymphopoietin | TSLP |
| type 2 Innate Lymphoid Cells | ILC2 |
| Secretory Leukocyte Proteinase Inhibitor | SLPI |
| Airway Smooth Muscle | ASM |
| Epithelial-Mesenchymal Transition | EMT |
| Transforming Growth Factor-β | TGF-β |
| Extracellular Vesicles | EVs |
| microRNA | miRNAs |
| Reticular Basement membrane | RBM |
| Bronchoalveolar Lavage Fluid | BALF |
| Follistatin-like 1 | FSTL1 |
| Platelet-Derived Growth Factor | PDGF |
| Epidermal Growth Factor | EGF |
| Heparin-Binding | EGF |
| Vascular Endothelial Growth Factor | VEGF |
| Transient Receptor Potential Vanilloid-1 | TRPV1 |
| ASM cells (ASMCs); Myosin Heavy chain 11 | MYH11 |
| Transgelin | TAGLN |
| Phosphodiesterase 4D | PDE4D |
| High-resolution Computer Tomography | HRCT |
| Wall Thickness percentage | WT% |
| Bronchial-to-Arterial | BA |
| Airway Collapsibility | AC |
| Wall Area percentage | WA% |
| Forced Expiratory Volume in 1 second | FEV1 |
| Quantitative CT | QCT |
| Bronchial WT | BWT |
| Severe Asthma Research Program | SARP |
| Functional Reserve Capacity | FRC |
| Total Lung Capacity | TLC |
| Eosinophilic Bronchitis | EB |
| Large Airway Involvement | LA |
| Small Airway Involvement | SA |
| Normal/Near-Normal | NN |
| Inhaled Corticosteroids | ICS |
| Inhaled Corticosteroids/ Long-Acting Beta2 Agonists | ICS/LABAs |
| Long-Acting Muscarinic Antagonists | LAMAs |
| Acetylcholine | Ach |
| muscarinic ACh Receptor | mAChR |
| Tiotropium | TIO |
| Glycopyrronium | GLY |
| Airway Hyperresponsiveness | AHR |
| Reactive Oxygen Species | ROS |
| Indoleamine 2,3-dioxygenase | IDO |
| Cystatin SN | CST1 |
| Toll-like Receptor | 4TLR4 |
| Ozone | O3 |
| Nitrogen dioxide | NO2 |
References
- Steelant B, Seys SF, Boeckxstaens G, Akdis CA, Ceuppens JL, Hellings PW. Restoring airway epithelial barrier dysfunction: a new therapeutic challenge in allergic airway disease. Rhinology 2016;54:195-205.
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. et Biophys. Acta (BBA) - Biomembr. 2008, 1778, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Shin, E.; Byeon, S.-J.; Hong, S.J.; Kang, S.H.; Lee, T.; Kim, T.-B.; Choi, J.-H. Serum Zonulin Is a Biomarker for Severe Asthma. Allergy, Asthma Immunol. Res. 2023, 15, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Shahana, S.; Björnsson, E.; Lúdvíksdóttir, D.; Janson, C.; Nettelbladt, O.; Venge, P.; Roomans, G. Ultrastructure of bronchial biopsies from patients with allergic and non-allergic asthma. Respir. Med. 2005, 99, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol 2011;128:549-56.e1-12.
- Caruso, C.; Giancaspro, R.; Guida, G.; Macchi, A.; Landi, M.; Heffler, E.; Gelardi, M. Nasal Cytology: A Easy Diagnostic Tool in Precision Medicine for Inflammation in Epithelial Barrier Damage in the Nose. A Perspective Mini Review. Front. Allergy 2022, 3, 768408. [Google Scholar] [CrossRef] [PubMed]
- Steelant, B. Epithelial dysfunction in chronic respiratory diseases, a shared endotype? Curr. Opin. Pulm. Med. 2020, 26, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.E. Epithelial Barrier Function and Immunity in Asthma. Ann. Am. Thorac. Soc. 2014, 11, S244–S251. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, G.H.; Meyers, D.A.; Howard, T.D.; Zheng, S.L.; Hawkins, G.A.; Ampleford, E.J.; Xu, J.; Koning, H.; Bruinenberg, M.; Nolte, I.M.; et al. Identification of PCDH1 as a Novel Susceptibility Gene for Bronchial Hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2009, 180, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Loxham, M.; Davies, D.E. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J. Allergy Clin. Immunol. 2017, 139, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- A Fedorov, I.; Wilson, S.J.; E Davies, D.; Holgate, S.T. Epithelial stress and structural remodelling in childhood asthma. Thorax 2005, 60, 389–394. [Google Scholar] [CrossRef]
- Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, et al. Early thickening of the reticular basement membrane in children with difficult asthma Am J Respir Crit Care Med 2003;167:78-82.
- Cokugras H, Akcakaya N, Seckin, Camcioglu Y, Sarimurat N, Aksoy F. Ultrastructural examination of bronchial biopsy specimens from children with moderate asthma. Thorax 2001;56:25-9.
- Teoh, K.-T.; Siu, Y.-L.; Chan, W.-L.; Schlüter, M.A.; Liu, C.-J.; Peiris, J.S.M.; Bruzzone, R.; Margolis, B.; Nal, B.; Stucke, V.M.; et al. The SARS Coronavirus E Protein Interacts with PALS1 and Alters Tight Junction Formation and Epithelial Morphogenesis. Mol. Biol. Cell 2010, 21, 3838–3852. [Google Scholar] [CrossRef]
- Lehmann, A.D.; Blank, F.; Baum, O.; Gehr, P.; Rothen-Rutishauser, B.M. Diesel exhaust particles modulate the tight junction protein occludin in lung cells in vitro. Part. Fibre Toxicol. 2009, 6, 26. [Google Scholar] [CrossRef]
- Petecchia L, Sabatini F, Varesio L, Camoirano A, Usai C, Pezzolo A, et al. Bronchial airway epithelial cell damage following exposure to cigarette smoke includes disassembly of tight junction components mediated by the extracellular signal-regulated kinase 1/2 pathway. Chest 2009;135:1502-12.
- Poto, R.; Fusco, W.; Rinninella, E.; Cintoni, M.; Kaitsas, F.; Raoul, P.; Caruso, C.; Mele, M.C.; Varricchi, G.; Gasbarrini, A.; et al. The Role of Gut Microbiota and Leaky Gut in the Pathogenesis of Food Allergy. Nutrients 2023, 16, 92. [Google Scholar] [CrossRef]
- Wan, H.; Winton, H.L.; Soeller, C.; Tovey, E.R.; Gruenert, D.C.; Thompson, P.J.; Stewart, G.A.; Taylor, G.W.; Garrod, D.R.; Cannell, M.B.; et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Investig. 1999, 104, 123–133. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. et Biophys. Acta (BBA) - Biomembr. 2009, 1788, 864–871. [Google Scholar] [CrossRef]
- Sokol, C.L.; Barton, G.M.; Farr, A.G.; Medzhitov, R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 2008, 9, 310–318. [Google Scholar] [CrossRef]
- Kheradmand, F.; Kiss, A.; Xu, J.; Lee, S.-H.; Kolattukudy, P.E.; Corry, D.B. A Protease-Activated Pathway Underlying Th Cell Type 2 Activation and Allergic Lung Disease. J. Immunol. 2002, 169, 5904–5911. [Google Scholar] [CrossRef]
- Phipps, S.; Lam, C.E.; Kaiko, G.E.; Foo, S.Y.; Collison, A.; Mattes, J.; Barry, J.; Davidson, S.; Oreo, K.; Smith, L.; et al. Toll/IL-1 Signaling Is Critical for House Dust Mite–specific Th1 and Th2 Responses. Am. J. Respir. Crit. Care Med. 2009, 179, 883–893. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Newton, G.K.; Perrior, T.R.; Robinson, C. Allergen Delivery Inhibitors: A Rationale for Targeting Sentinel Innate Immune Signaling of Group 1 House Dust Mite Allergens through Structure-Based Protease Inhibitor Design. Mol. Pharmacol. 2018, 94, 1007–1030. [Google Scholar] [CrossRef]
- Chevigné, A.; Barumandzadeh, R.; Groslambert, S.; Cloes, B.; Dehareng, D.; Filée, P.; Marx, J.-C.; Frère, J.-M.; Matagne, A.; Jacquet, A.; et al. Relationship between Propeptide pH Unfolding and Inhibitory Ability during ProDer p 1 Activation Mechanism. J. Mol. Biol. 2007, 374, 170–185. [Google Scholar] [CrossRef]
- Herman J, Thelen N, Smargiasso N, et al. Der p 1 is the primary activator of Der p 3, Der p 6 and Der p 9 the proteolytic allergens produced by the house dust mite Dermatophagoides pteronyssinus. Biochim Biophys Acta. 2014;1840(3):1117-1124. [CrossRef]
- Tovey, E.R.; Chapman, M.D.; Platts-Mills, T.A.E. Mite faeces are a major source of house dust allergens. Nature 1981, 289, 592–593. [Google Scholar] [CrossRef]
- Sudha, V.T.; Arora, N.; Gaur, S.N.; Pasha, S.; Singh, B.P. Identification of a serine protease as a major allergen (Per a 10) of Periplaneta americana. Allergy 2008, 63, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-D.; Tam, M.F.; Chou, H.; Han, S.-H. The Importance of Serine Proteinases as Aeroallergens Associated with Asthma. Int. Arch. Allergy Immunol. 1999, 119, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Bouley, J.; Groeme, R.; Le Mignon, M.; Jain, K.; Chabre, H.; Bordas-Le Floch, V.; Couret, M.-N.; Bussières, L.; Lautrette, A.; Naveau, M.; et al. Identification of the cysteine protease Amb a 11 as a novel major allergen from short ragweed. J. Allergy Clin. Immunol. 2015, 136, 1055–1064. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Finn, R. Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2016, 44, D343–D350. [Google Scholar] [CrossRef] [PubMed]
- Puente, X.S.; Sánchez, L.M.; Overall, C.M.; López-Otín, C. Human and mouse proteases: a comparative genomic approach. Nat. Rev. Genet. 2003, 4, 544–558. [Google Scholar] [CrossRef]
- Radauer, C.; Breiteneder, H. Pollen allergens are restricted to few protein families and show distinct patterns of species distribution. J. Allergy Clin. Immunol. 2006, 117, 141–147. [Google Scholar] [CrossRef]
- Gruber, A.; Manček, M.; Wagner, H.; Kirschning, C.J.; Jerala, R.; Kim, B.-C.; van Gelder, H.; Kim, T.A.; Lee, H.-J.; Baik, K.G.; et al. Structural Model of MD-2 and Functional Role of Its Basic Amino Acid Clusters Involved in Cellular Lipopolysaccharide Recognition. J. Biol. Chem. 2004, 279, 28475–28482. [Google Scholar] [CrossRef]
- Brown, A.; Farmer, K.; MacDonald, L.; Kalsheker, N.; Pritchard, D.; Haslett, C.; Lamb, J.; Sallenave, J.-M. House Dust Mite Der p 1 Downregulates Defenses of the Lung by Inactivating Elastase Inhibitors. Am. J. Respir. Cell Mol. Biol. 2003, 29, 381–389. [Google Scholar] [CrossRef]
- Deb, R.; Shakib, F.; Reid, K.; Clark, H. Major House Dust Mite Allergens Dermatophagoides pteronyssinus 1 and Dermatophagoides farinae 1 Degrade and Inactivate Lung Surfactant Proteins A and D. J. Biol. Chem. 2007, 282, 36808–36819. [Google Scholar] [CrossRef]
- Gough, L.; Schulz, O.; Sewell, H.F.; Shakib, F. The Cysteine Protease Activity of the Major Dust Mite Allergen Der P 1 Selectively Enhances the Immunoglobulin E Antibody Response. J. Exp. Med. 1999, 190, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Ghaemmaghami, A.M.; Shakib, F. Human T cells that have been conditioned by the proteolytic activity of the major dust mite allergen Der p 1 trigger enhanced immunoglobulin E synthesis by B cells. Clin. Exp. Allergy 2002, 32, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.; Tam, M.F.; Lee, L.-H.; Chiang, C.-H.; Tai, H.-Y.; Panzani, R.C.; Shen, H.-D. Vacuolar Serine Protease Is a Major Allergen of Cladosporium cladosporioides. Int. Arch. Allergy Immunol. 2008, 146, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-D.; Tam, M.F.; Tang, R.-B.; Chou, H. Aspergillus and Penicillium allergens: Focus on proteases. Curr. Allergy Asthma Rep. 2007, 7, 351–356. [Google Scholar] [CrossRef]
- Matsuwaki, Y.; Wada, K.; White, T.A.; Benson, L.M.; Charlesworth, M.C.; Checkel, J.L.; Inoue, Y.; Hotta, K.; Ponikau, J.U.; Lawrence, C.B.; et al. Recognition of Fungal Protease Activities Induces Cellular Activation and Eosinophil-Derived Neurotoxin Release in Human Eosinophils. J. Immunol. 2009, 183, 6708–6716. [Google Scholar] [CrossRef]
- Kauffman HK, Tomee JFC, Marjolein A, van de Riet A, Timmerman JB, Borger P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol. 2000;105:1185–93.
- Millien, V.O.; Lu, W.; Shaw, J.; Yuan, X.; Mak, G.; Roberts, L.; Song, L.-Z.; Knight, J.M.; Creighton, C.J.; Luong, A.; et al. Cleavage of Fibrinogen by Proteinases Elicits Allergic Responses Through Toll-Like Receptor 4. Science 2013, 341, 792–796. [Google Scholar] [CrossRef]
- Landers, C.T.; Tung, H.-Y.; Knight, J.M.; Madison, M.C.; Wu, Y.; Zeng, Z.; Porter, P.C.; Rodriguez, A.; Flick, M.J.; Kheradmand, F.; et al. Selective cleavage of fibrinogen by diverse proteinases initiates innate allergic and antifungal immunity through CD11b. J. Biol. Chem. 2019, 294, 8834–8847. [Google Scholar] [CrossRef]
- Burzynski, L.C.; Humphry, M.; Pyrillou, K.; Wiggins, K.A.; Chan, J.N.; Figg, N.; Kitt, L.L.; Summers, C.; Tatham, K.C.; Martin, P.B.; et al. The Coagulation and Immune Systems Are Directly Linked through the Activation of Interleukin-1α by Thrombin. Immunity 2019, 50, 1033–1042. [Google Scholar] [CrossRef]
- Sudha, V.T.; Arora, N.; Singh, B.P. Serine protease activity of Per a 10 augments allergen-induced airway inflammation in a mouse model. Eur. J. Clin. Investig. 2009, 39, 507–516. [Google Scholar] [CrossRef]
- Pomes A, Chapman MD, Vailes LD, et al. Cockroach allergen Bla g 2; structure, function, and implications for allergic sensitization. Am J Respir Crit Care Med. 2002;165:391–7.
- Campbell, E.M.; Kunkel, S.L.; Strieter, R.M.; Lukacs, N.W. Temporal Role of Chemokines in a Murine Model of Cockroach Allergen-Induced Airway Hyperreactivity and Eosinophilia. J. Immunol. 1998, 161, 7047–7053. [Google Scholar] [CrossRef]
- Papouchado, B.G.; Chapoval, S.P.; Marietta, E.V.; Weiler, C.R.; David, C.S. Cockroach Allergen-Induced Eosinophilic Airway Inflammation in HLA-DQ/Human CD4+ Transgenic Mice. J. Immunol. 2001, 167, 4627–4634. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Cao, W.; Kasturi, S.P.; Ravindran, R.; I Nakaya, H.; Kundu, K.; Murthy, N.; Kepler, T.B.; Malissen, B.; Pulendran, B. The T helper type 2 response to cysteine proteases requires dendritic cell–basophil cooperation via ROS-mediated signaling. Nat. Immunol. 2010, 11, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Kouzaki, H.; O’Grady, S.M.; Lawrence, C.B.; Kita, H. Proteases Induce Production of Thymic Stromal Lymphopoietin by Airway Epithelial Cells through Protease-Activated Receptor-2. J. Immunol. 2009, 183, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; Angkasekwinai, P.; Chang, S.H.; Chung, Y.; Dong, C. Protease Allergens Induce the Expression of IL-25 via Erk and p38 MAPK Pathway. J. Korean Med Sci. 2010, 25, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Sokol C. L., Chu N. Q., Yu S., Nish S. A., Laufer T. M., Medzhitov R.. 2009. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 10: 713–720.
- Oboki, K.; Ohno, T.; Kajiwara, N.; Arae, K.; Morita, H.; Ishii, A.; Nambu, A.; Abe, T.; Kiyonari, H.; Matsumoto, K.; et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 18581–18586. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, S.; Takeda, H.; Tokura, T.; Suzuki, M.; Inui, K.; Hara, M.; Matsuda, H.; Matsuda, A.; Oboki, K.; Ohno, T.; et al. IL-33–Mediated Innate Response and Adaptive Immune Cells Contribute to Maximum Responses of Protease Allergen–Induced Allergic Airway Inflammation. J. Immunol. 2013, 190, 4489–4499. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; de Matos, M.R.; Cortes, L.; Nunes-Correia, I.; Todo-Bom, A.; Pires, E.; Veríssimo, P. Pollen Proteases Play Multiple Roles in Allergic Disorders. Int. J. Mol. Sci. 2020, 21, 3578. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.E.H.; Ward, J.M.; Cummings, M.; Karrar, E.E.; Root, M.; Mohamed, A.B.A.; Naclerio, R.M.; Preuss, D. Dual Function of Novel Pollen Coat (Surface) Proteins: IgE-binding Capacity and Proteolytic Activity Disrupting the Airway Epithelial Barrier. PLOS ONE 2013, 8, e53337. [Google Scholar] [CrossRef] [PubMed]
- Höllbacher, B.; Schmitt, A.O.; Hofer, H.; Ferreira, F.; Lackner, P. Identification of Proteases and Protease Inhibitors in Allergenic and Non-Allergenic Pollen. Int. J. Mol. Sci. 2017, 18, 1199. [Google Scholar] [CrossRef]
- Tulic, M.K.; Vivinus-Nébot, M.; Rekima, A.; Medeiros, S.R.; Bonnart, C.; Shi, H.; Walker, A.; Dainese, R.; Boyer, J.; Vergnolle, N.; et al. Presence of commensal house dust mite allergen in human gastrointestinal tract: a potential contributor to intestinal barrier dysfunction. Gut 2016, 65, 757–766. [Google Scholar] [CrossRef]
- Baglivo, I.; Colantuono, S.; Lumaca, A.; Papa, A.; Gasbarrini, A.; Caruso, C. The last step to achieve barrier damage control. Front. Immunol. 2024, 15, 1354556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Zuo, J.; Newton, G.K.; Stewart, M.R.; Perrior, T.R.; Garrod, D.R.; Robinson, C. Allergen Delivery Inhibitors: Characterisation of Potent and Selective Inhibitors of Der p 1 and Their Attenuation of Airway Responses to House Dust Mite Allergens. Int. J. Mol. Sci. 2018, 19, 3166. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Robinson, C. Cellular and Molecular Events in the Airway Epithelium Defining the Interaction Between House Dust Mite Group 1 Allergens and Innate Defences. Int. J. Mol. Sci. 2018, 19, 3549. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yu, S.-J.; Tsai, J.-J.; Yu, C.-H.; Liao, E.-C. Antagonism of Protease Activated Receptor-2 by GB88 Reduces Inflammation Triggered by Protease Allergen Tyr-p3. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Wan, H.; Winton, H.L.; Soeller, C.; Taylor, G.W.; Gruenert, D.C.; Thompson, P.J.; Cannell, M.B.; Stewart, G.A.; Garrod, D.R.; Robinson, C. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin. Exp. Allergy 2001, 31, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Tomee JF, van Weissenbruch R, de Monchy JG, Kauffman HF: Interactions between inhalant allergen extracts and airway epithelial cells: effect on cytokine production and cell detachment. J Allergy Clin Immunol 1998, 102:75–85.
- Schwab, C.J.; Cooley, J.D.; Brasel, T.; Jumper, C.A.; Graham, S.C.; Straus, D.C. Characterization of Exposure to Low Levels of Viable Penicillium chrysogenum Conidia and Allergic Sensitization Induced by a Protease Allergen Extract from Viable P. chrysogenum Conidia in Mice. Int. Arch. Allergy Immunol. 2003, 130, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kurup, V.; Xia, J.-Q.; Crameri, R.; Rickaby, D.; Choi, H.; Flückiger, S.; Blaser, K.; Dawson, C.; Kelly, K. Purified Recombinant A. fumigatus Allergens Induce Different Responses in Mice. Clin. Immunol. 2001, 98, 327–336. [Google Scholar] [CrossRef]
- Tai, H.-Y.; Tam, M.F.; Chou, H.; Perng, D.-W.; Shen, H.-D. Pen ch 13 Major Fungal Allergen Decreases CD44 Expression in Human Bronchial Epithelial Cells. Int. Arch. Allergy Immunol. 2010, 153, 367–371. [Google Scholar] [CrossRef]
- Grozdanovic, M.M.; Čavić, M.; Nešić, A.; Andjelković, U.; Akbari, P.; Smit, J.J.; Gavrović-Jankulović, M. Kiwifruit cysteine protease actinidin compromises the intestinal barrier by disrupting tight junctions. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2016, 1860, 516–526. [Google Scholar] [CrossRef]
- Ghaemmaghami, A.M.; Gough, L.; Sewell, H.F.; Shakib, F. The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin. Exp. Allergy 2002, 32, 1468–1475. [Google Scholar] [CrossRef]
- Furmonaviciene, R.; Ghaemmaghami, A.M.; Boyd, S.E.; Jones, N.S.; Bailey, K.; Willis, A.C.; Sewell, H.F.; Mitchell, D.A.; Shakib, F. The protease allergen Der p 1 cleaves cell surface DC-SIGN and DC-SIGNR: experimental analysis of in silico substrate identification and implications in allergic responses. Clin. Exp. Allergy 2007, 37, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.; Matos, I.; Choi, J.-H.; Dandamudi, D.B.; Shrestha, E.; Longhi, M.P.; Jeffrey, K.L.; Anthony, R.M.; Kluger, C.; Nchinda, G.; et al. Microbial Stimulation Fully Differentiates Monocytes to DC-SIGN/CD209+ Dendritic Cells for Immune T Cell Areas. Cell 2010, 143, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Smits, H.H.; Ratajczak, C.; Nithiananthan, A.; A Wierenga, E.; A Stewart, G.; Jacquet, A.; Tonnel, A.-B.; Pestel, J. Monocyte-derived dendritic cells exposed to Der p 1 allergen enhance the recruitment of Th2 cells: major involvement of the chemokines TARC/CCL17 and MDC/CCL22. . 2003, 14, 219–28. [Google Scholar]
- Takai, T.; Kato, T.; Ota, M.; Yasueda, H.; Kuhara, T.; Okumura, K.; Ogawa, H. Recombinant Der p 1 and Der f 1 with in vitro Enzymatic Activity to Cleave Human CD23, CD25 and α1-Antitrypsin, and in vivo IgE-Eliciting Activity in Mice. Int. Arch. Allergy Immunol. 2005, 137, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Engeroff, P.; Vogel, M. The role of CD23 in the regulation of allergic responses. Allergy 2021, 76, 1981–1989. [Google Scholar] [CrossRef]
- Reed CE, Kita H: The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol 2004;114:997–1008.
- Yu CK, Chen CL: Activation of mast cells is essential for development of house dust mite Dermatophagoides farinae-induced allergic airway inflammation in mice. J Immunol 2003;171: 3808–3815.
- Asokananthan N, Graham PT, Stewart DJ, et al.: House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol 2002, 169:4572–4578.
- Sun, G.; Stacey, M.A.; Schmidt, M.; Mori, L.; Mattoli, S. Interaction of Mite Allergens Der P3 and Der P9 with Protease-Activated Receptor-2 Expressed by Lung Epithelial Cells. J. Immunol. 2001, 167, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.; Reiser, G. A novel therapeutic target in various lung diseases: Airway proteases and protease-activated receptors. Pharmacol. Ther. 2007, 115, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease activated receptors in human airways: upregulation of PAR-2 in respiratory epithelial cells from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803.
- Lewkowich, I.P.; Day, S.B.; Ledford, J.R.; Zhou, P.; Dienger, K.; Wills-Karp, M.; Page, K. Protease-activated receptor 2 activation of myeloid dendritic cells regulates allergic airway inflammation. Respir. Res. 2011, 12, 122–122. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Lee, Y.-H.; Jeon, C.-H. Protease-dependent activation of nasal polyp epithelial cells by airborne fungi leads to migration of eosinophils and neutrophils. Acta Oto-Laryngologica 2006, 126, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Day, S.B.; Zhou, P.; Ledford, J.R.; Page, K. German Cockroach Frass Proteases Modulate the Innate Immune Response via Activation of Protease-Activated Receptor-2. J. Innate Immun. 2010, 2, 495–504. [Google Scholar] [CrossRef]
- Miike, S.; Kita, H. Human eosinophils are activated by cysteine proteases and release inflammatory mediators. J. Allergy Clin. Immunol. 2003, 111, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Coward, W.R.; I Pritchard, D.; A Hewitt, C.R.; Pritchard, D.I.; Hewitt, C.R.A. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J. Leukoc. Biol. 2003, 73, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Serhan N, Cenac N, Basso L, Gaudenzio N. Mas-related G proteincoupled receptors (Mrgprs) – key regulators of neuroimmune interactions. Neurosci Lett. 2021;749:135724. [CrossRef]
- Soh, W.T.; Zhang, J.; Hollenberg, M.D.; Vliagoftis, H.; Rothenberg, M.E.; Sokol, C.L.; Robinson, C.; Jacquet, A. Protease allergens as initiators–regulators of allergic inflammation. Allergy 2023, 78, 1148–1168. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.; Steer, C.A.; Mathä, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.J.; Takei, F. Group 2 Innate Lymphoid Cells Are Critical for the Initiation of Adaptive T Helper 2 Cell-Mediated Allergic Lung Inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef]
- Azouz, N.P.; Ynga-Durand, M.A.; Caldwell, J.M.; Jain, A.; Rochman, M.; Fischesser, D.M.; Ray, L.M.; Bedard, M.C.; Mingler, M.K.; Forney, C.; et al. The antiprotease SPINK7 serves as an inhibitory checkpoint for esophageal epithelial inflammatory responses. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Azouz NP, Klingler AM, Pathre P, et al. Functional role of kallikrein 5 and proteinase-activated receptor 2 in eosinophilic esophagitis. Sci Transl Med. 2020;12(545):eaaz7773. [CrossRef]
- Kozlik, P.; Zuk, J.; Bartyzel, S.; Zarychta, J.; Okon, K.; Zareba, L.; Bazan, J.G.; Kosalka, J.; Soja, J.; Musial, J.; et al. The relationship of airway structural changes to blood and bronchoalveolar lavage biomarkers, and lung function abnormalities in asthma. Clin. Exp. Allergy 2019, 50, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Aysola, R.S.; Hoffman, E.A.; Gierada, D.; Wenzel, S.; Cook-Granroth, J.; Tarsi, J.; Zheng, J.; Schechtman, K.B.; Ramkumar, T.P.; Cochran, R.; et al. Airway Remodeling Measured by Multidetector CT Is Increased in Severe Asthma and Correlates With Pathology. Chest 2008, 134, 1183–1191. [Google Scholar] [CrossRef]
- Lezmi, G.; Gosset, P.; Deschildre, A.; Abou-Taam, R.; Mahut, B.; Beydon, N.; de Blic, J. Airway Remodeling in Preschool Children with Severe Recurrent Wheeze. Am. J. Respir. Crit. Care Med. 2015, 192, 164–171. [Google Scholar] [CrossRef]
- Bahmer, T.; Sand, J.M.B.; Weckmann, M. Lost in transition: biomarkers of remodeling in patients with asthma. Curr. Opin. Pulm. Med. 2020, 26, 40–46. [Google Scholar] [CrossRef]
- Yang, Z.; Qu, Z.; Yi, M.; Shan, Y.; Ran, N.; Xu, L.; Liu, X. MiR-448-5p inhibits TGF-β1-induced epithelial-mesenchymal transition and pulmonary fibrosis by targeting Six1 in asthma. J. Cell. Physiol. 2018, 234, 8804–8814. [Google Scholar] [CrossRef] [PubMed]
- Rout-Pitt, N.; Farrow, N.; Parsons, D.; Donnelley, M. Epithelial mesenchymal transition (EMT): a universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir. Res. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Sun, Z.; Ji, N.; Ma, Q.; Zhu, R.; Chen, Z.; Wang, Z.; Qian, Y.; Wu, C.; Hu, F.; Huang, M.; et al. Epithelial-Mesenchymal Transition in Asthma Airway Remodeling Is Regulated by the IL-33/CD146 Axis. Front. Immunol. 2020, 11, 1598. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, B.; Kowal, E.J.K.; Van Balkom, B.W.M.; Bartel, S.; Bhattacharyya, S.N.; Buzás, E.I.; Buck, A.H.; de Candia, P.; Chow, F.W.N.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef]
- Milger, K.; Götschke, J.; Krause, L.; Nathan, P.; Alessandrini, F.; Tufman, A.; Fischer, R.; Bartel, S.; Theis, F.J.; Behr, J.; et al. Identification of a plasma miRNA biomarker signature for allergic asthma: A translational approach. Allergy 2017, 72, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, M.; Ahmad, T.; Mabalirajan, U.; Aich, J.; Agrawal, A.; Ghosh, B. Antagonism of mmu-mir-106a attenuates asthma features in allergic murine model. J. Appl. Physiol. 2012, 113, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; Horvat, J.C.; Pinkerton, J.W.; Starkey, M.R.; Essilfie, A.T.; Mayall, J.R.; Nair, P.M.; Hansbro, N.G.; Jones, B.; Haw, T.J.; et al. MicroRNA-21 drives severe, steroid-insensitive experimental asthma by amplifying phosphoinositide 3-kinase–mediated suppression of histone deacetylase 2. J. Allergy Clin. Immunol. 2016, 139, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Levänen, B.; Bhakta, N.R.; Paredes, P.T.; Barbeau, R.; Hiltbrunner, S.; Pollack, J.L.; Sköld, C.M.; Svartengren, M.; Grunewald, J.; Gabrielsson, S.; et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J. Allergy Clin. Immunol. 2013, 131, 894–903. [Google Scholar] [CrossRef]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development. Allergy 2019, 75, 346–356. [Google Scholar] [CrossRef]
- Bazan-Socha, S.; Buregwa-Czuma, S.; Jakiela, B.; Zareba, L.; Zawlik, I.; Myszka, A.; Soja, J.; Okon, K.; Zarychta, J.; Kozlik, P.; et al. Reticular Basement Membrane Thickness Is Associated with Growth- and Fibrosis-Promoting Airway Transcriptome Profile-Study in Asthma Patients. Int. J. Mol. Sci. 2021, 22, 998. [Google Scholar] [CrossRef]
- Nihlberg, K.; Larsen, K.; Hultgårdh-Nilsson, A.; Malmström, A.; Bjermer, L.; Westergren-Thorsson, G. Tissue fibrocytes in patients with mild asthma: A possible link to thickness of reticular basement membrane? Respir. Res. 2006, 7, 50–50. [Google Scholar] [CrossRef] [PubMed]
- Mauri, P.; Riccio, A.M.; Rossi, R.; Di Silvestre, D.; Benazzi, L.; De Ferrari, L.; Negro, R.W.D.; Holgate, S.T.; Canonica, G.W. Proteomics of bronchial biopsies: Galectin-3 as a predictive biomarker of airway remodelling modulation in omalizumab-treated severe asthma patients. Immunol. Lett. 2014, 162, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.S.; Yuan, S.; Innes, A.L.; Kerr, S.; Woodruff, P.G.; Hou, L.; Muller, S.J.; Fahy, J.V. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc. Natl. Acad. Sci. 2010, 107, 14170–14175. [Google Scholar] [CrossRef]
- Vignola, A.M.; Chanez, P.; Chiappara, G.; Merendino, A.; Pace, E.; Rizzo, A.; la Rocca, A.M.; Bellia, V.; Bonsignore, G.; Bousquet, J. Transforming Growth Factor- β Expression in Mucosal Biopsies in Asthma and Chronic Bronchitis. Am. J. Respir. Crit. Care Med. 1997, 156, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.-M.; Sutanto, E.N.; Iosifidis, T.; Kicic-Starcevich, E.; Looi, K.; Garratt, L.W.; Martinovich, K.M.; Lannigan, F.J.; Knight, D.A.; Stick, S.M.; et al. Reduced transforming growth factor β1 (TGF-β1) in the repair of airway epithelial cells of children with asthma. Respirology 2016, 21, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Izuhara, K.; Arima, K.; Ohta, S.; Suzuki, S.; Inamitsu, M.; Yamamoto, K.-I. Periostin in Allergic Inflammation. Allergol. Int. 2014, 63, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Y.; Miller, M.; Cao, L.; Zhao, J.; Wu, J.; Wang, J.; Liu, L.; Li, S.; Zou, M.; et al. Autophagy plays a role in FSTL1-induced epithelial mesenchymal transition and airway remodeling in asthma. Am. J. Physiol. Cell. Mol. Physiol. 2017, 313, L27–L40. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Tatler, A. Pathobiology of Airway Remodeling in Asthma: The Emerging Role of Integrins. J. Asthma Allergy 2022, ume 15, 595–610. [Google Scholar] [CrossRef]
- Sha, J.; Rorke, S.; Langton, D. Airway smooth muscle as an underutilised biomarker: a case report. BMC Pulm. Med. 2019, 19, 1–4. [Google Scholar] [CrossRef]
- Oenema, T.A.; Maarsingh, H.; Smit, M.; Groothuis, G.M.M.; Meurs, H.; Gosens, R. Bronchoconstriction Induces TGF-β Release and Airway Remodelling in Guinea Pig Lung Slices. PLOS ONE 2013, 8, e65580. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.Y.; Hur, J.; Kim, K.H.; Kang, J.Y.; Rhee, C.K.; Lee, S.Y. TRPV1 Blocking Alleviates Airway Inflammation and Remodeling in a Chronic Asthma Murine Model. Allergy, Asthma Immunol. Res. 2018, 10, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Qiu, C.; Chen, R. A narrative review of research advances in the study of molecular markers of airway smooth muscle cells. Ann. Transl. Med. 2021, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.H.Y.; Shang, Y.; Mitzner, W.; Sham, J.S.K.; Tang, W.-Y. Aberrant DNA Methylation of Phosphodiestarase 4D Alters Airway Smooth Muscle Cell Phenotypes. Am. J. Respir. Cell Mol. Biol. 2016, 54, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.M.; Lavender, P.; Kuo, C.-H.S.; Galea, F.; Michaeloudes, C.; Flanagan, J.M.; Chung, K.F.; Adcock, I.M. DNA methylation modules in airway smooth muscle are associated with asthma severity. Eur. Respir. J. 2018, 51, 1701068. [Google Scholar] [CrossRef] [PubMed]
- Bahmer, T.; Sand, J.M.B.; Weckmann, M. Lost in transition: biomarkers of remodeling in patients with asthma. Curr. Opin. Pulm. Med. 2020, 26, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.Y.; Rogers, D.F. Mucus hypersecretion in asthma: intracellular signalling pathways as targets for pharmacotherapy. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Harkness, L.M.; Ashton, A.W.; Burgess, J.K. Asthma is not only an airway disease, but also a vascular disease. Pharmacol. Ther. 2015, 148, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.H.; Mitzner, W. Invited Review: Understanding airway pathophysiology with computed tomograpy. J. Appl. Physiol. 2003, 95, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.I.S.; Colby, T.V.; Müller, N.L. Asthma and Associated Conditions: High-Resolution CT and Pathologic Findings. Am. J. Roentgenol. 2004, 183, 817–824. [Google Scholar] [CrossRef]
- Chae, E.J.; Kim, T.-B.; Cho, Y.S.; Park, C.-S.; Seo, J.B.; Kim, N.; Moon, H.-B. Airway Measurement for Airway Remodeling Defined by Post-Bronchodilator FEV1/FVC in Asthma: Investigation Using Inspiration-Expiration Computed Tomography. Allergy, Asthma Immunol. Res. 2011, 3, 111–117. [Google Scholar] [CrossRef]
- Gupta, S.; Siddiqui, S.; Haldar, P.; Raj, J.V.; Entwisle, J.J.; Wardlaw, A.J.; Bradding, P.; Pavord, I.D.; Green, R.H.; Brightling, C.E. Qualitative Analysis of High-Resolution CT Scans in Severe Asthma. Chest 2009, 136, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Siddiqui, S.; Haldar, P.; Raj, J.V.; Entwisle, J.J.; Wardlaw, A.J.; Bradding, P.; Pavord, I.D.; Green, R.H.; Brightling, C.E. Qualitative Analysis of High-Resolution CT Scans in Severe Asthma. Chest 2009, 136, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Gupta, S.; Hartley, R.; Brightling, C.E. Computed tomography scans in severe asthma. Curr. Opin. Pulm. Med. 2012, 18, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Hall, C.; Hoffman, E.A.; Woods, J.C.; Gierada, D.S.; Castro, M. Using imaging as a biomarker for asthma. J. Allergy Clin. Immunol. 2017, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.E. Quantitative computed tomography. Eur. J. Radiol. 2009, 71, 415–424. [Google Scholar] [CrossRef] [PubMed]
- A Little, S.; Sproule, M.W.; Cowan, M.D.; Macleod, K.J.; Robertson, M.; Love, J.G.; Chalmers, G.W.; McSharry, C.P.; Thomson, N.C. High resolution computed tomographic assessment of airway wall thickness in chronic asthma: reproducibility and relationship with lung function and severity. Thorax 2002, 57, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Bumbacea, D.; Campbell, D.; Nguyen, L.; Carr, D.; Barnes, P.; Robinson, D.; Chung, K. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur. Respir. J. 2004, 24, 122–128. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, E.M.; Spielberg, D.R.; Brody, A.S. Clinical potential for imaging in patients with asthma and other lung disorders. J. Allergy Clin. Immunol. 2017, 139, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Harmanci, E.; Kebapci, M.; Metintas, M.; Ozkan, R. High-Resolution Computed Tomography Findings Are Correlated with Disease Severity in Asthma. Respiration 2002, 69, 420–426. [Google Scholar] [CrossRef]
- Gupta, S.; Hartley, R.; Khan, U.T.; Singapuri, A.; Hargadon, B.; Monteiro, W.; Pavord, I.D.; Sousa, A.R.; Marshall, R.P.; Subramanian, D.; et al. Quantitative computed tomography–derived clusters: Redefining airway remodeling in asthmatic patients. J. Allergy Clin. Immunol. 2014, 133, 729–738. [Google Scholar] [CrossRef]
- Wang, D.; Luo, J.; Du, W.; Zhang, L.-L.; He, L.-X.; Liu, C.-T. A morphologic study of the airway structure abnormalities in patients with asthma by high-resolution computed tomography. J. Thorac. Dis. 2016, 8, 2697–2708. [Google Scholar] [CrossRef]
- Paganin, F.; Trussard, V.; Seneterre, E.; Chanez, P.; Giron, J.; Godard, P.; Sénac, J.P.; Michel, F.B.; Bousquet, J. Chest Radiography and High Resolution Computed Tomography of the Lungs in Asthma. Am. Rev. Respir. Dis. 1992, 146, 1084–1087. [Google Scholar] [CrossRef]
- Obojski, A.; Patyk, M.; Zaleska-Dorobisz, U. Airway remodeling assessed by quantitative computed tomography at various stages of asthma severity defined according to Global Initiative for Asthma report: a single-center study. Pol. Arch. Intern. Med. 2022, 132. [Google Scholar] [CrossRef]
- Krings, J.G.; Goss, C.W.; Lew, D.; Samant, M.; McGregor, M.C.; Boomer, J.; Bacharier, L.B.; Sheshadri, A.; Hall, C.; Brownell, J.; et al. Quantitative CT metrics are associated with longitudinal lung function decline and future asthma exacerbations: Results from SARP-3. J. Allergy Clin. Immunol. 2021, 148, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Shin, K.E.; Chang, H.S.; Lee, J.-U.; Park, S.-L.; Park, J.S.; Park, J.S.; Park, C.-S. Relationships Between High-Resolution Computed Tomographic Features and Lung Function Trajectory in Patients With Asthma. Allergy, Asthma Immunol. Res. 2023, 15, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Tonga, K.O.; Chapman, D.G.; Farah, C.S.; Oliver, B.G.; Zimmermann, S.C.; Milne, S.; Sanai, F.; Jetmalani, K.; Berend, N.; Thamrin, C.; et al. Reduced lung elastic recoil and fixed airflow obstruction in asthma. Respirology 2019, 25, 613–619. [Google Scholar] [CrossRef]
- Shimizu, K.; Tanabe, N.; Oguma, A.; Kimura, H.; Suzuki, M.; Yokota, I.; Makita, H.; Sato, S.; Hirai, T.; Nishimura, M.; et al. Parenchymal destruction in asthma: Fixed airflow obstruction and lung function trajectory. J. Allergy Clin. Immunol. 2022, 149, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Gelb, A.F.; Yamamoto, A.; Verbeken, E.K.; Nadel, J.A. Unraveling the Pathophysiology of the Asthma-COPD Overlap Syndrome. Chest 2015, 148, 313–320. [Google Scholar] [CrossRef]
- Busacker, A.; Newell, J.D.; Keefe, T.; Hoffman, E.A.; Granroth, J.C.; Castro, M.; Fain, S.; Wenzel, S. A Multivariate Analysis of Risk Factors for the Air-Trapping Asthmatic Phenotype as Measured by Quantitative CT Analysis. Chest 2009, 135, 48–56. [Google Scholar] [CrossRef]
- Tunon-De-Lara, J.-M.; Laurent, F.; Giraud, V.; Perez, T.; Aguilaniu, B.; Meziane, H.; Basset-Merle, A.; Chanez, P. Air trapping in mild and moderate asthma: Effect of inhaled corticosteroids. J. Allergy Clin. Immunol. 2007, 119, 583–590. [Google Scholar] [CrossRef]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; et al. Mepolizumab and Exacerbations of Refractory Eosinophilic Asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef]
- Shim, S.S.; Schiebler, M.L.; Evans, M.D.; Jarjour, N.; Sorkness, R.L.; Denlinger, L.C.; Rodriguez, A.; Wenzel, S.; Hoffman, E.A.; Lin, C.-L.; et al. Lumen area change (Delta Lumen) between inspiratory and expiratory multidetector computed tomography as a measure of severe outcomes in asthmatic patients. J. Allergy Clin. Immunol. 2018, 142, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, Z.; Shen, C.; Jin, C.; Yu, N.; Wang, J.; Yin, N.; Guo, Y. Small airway dysfunction may be an indicator of early asthma: findings from high-resolution CT. Ann. Allergy, Asthma Immunol. 2019, 122, 498–501. [Google Scholar] [CrossRef]
- Chan, R.; Duraikannu, C.; Thouseef, M.J.; Lipworth, B. Impaired Respiratory System Resistance and Reactance Are Associated With Bronchial Wall Thickening in Persistent Asthma. J. Allergy Clin. Immunol. Pr. 2023, 11, 1459–1462. [Google Scholar] [CrossRef]
- Patyk, M.; Obojski, A.; Sokołowska-Dąbek, D.; Parkitna-Patyk, M.; Zaleska-Dorobisz, U. Airway wall thickness and airflow limitations in asthma assessed in quantitative computed tomography. Ther. Adv. Respir. Dis. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Park, J.-S.; Lee, Y.-M.; Lee, J.-H.; Jang, A.-S.; Kim, D.-J.; Hwangbo, Y.; Uh, S.-T.; Kim, Y.-H.; Park, C.-S. Differences in radiological/HRCT findings in eosinophilic bronchitis and asthma: implication for bronchial responsiveness. Thorax 2006, 61, 41–47. [Google Scholar] [CrossRef]
- Gupta, S.; Siddiqui, S.; Haldar, P.; Entwisle, J.J.; Mawby, D.; Wardlaw, A.J.; Bradding, P.; Pavord, I.D.; Green, R.H.; Brightling, C.E. Quantitative analysis of high-resolution computed tomography scans in severe asthma subphenotypes. Thorax 2010, 65, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hartley, R.; Khan, U.T.; Singapuri, A.; Hargadon, B.; Monteiro, W.; Pavord, I.D.; Sousa, A.R.; Marshall, R.P.; Subramanian, D.; et al. Quantitative computed tomography–derived clusters: Redefining airway remodeling in asthmatic patients. J. Allergy Clin. Immunol. 2014, 133, 729–738. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, L.; Qiao, J.; Cheng, C.; Liu, Y.; Zhang, S.; Fang, X.; Li, Z.; Renz, H.; Liu, X.; et al. Novel imaging phenotypes of naïve asthma patients with distinctive clinical characteristics and T2 inflammation traits. Ther. Adv. Chronic Dis. 2022, 13. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.; Kim, T.; Jin, K.N.; Cho, S.-H.; Lee, C.H.; Kang, H.-R. Phenotypic clusters on computed tomography reflects asthma heterogeneity and severity. World Allergy Organ. J. 2022, 15, 100628. [Google Scholar] [CrossRef]
- Kim, S.; Lee, C.; Jin, K.; Cho, S.; Kang, H. Severe Asthma Phenotypes Classified by Site of Airway Involvement and Remodeling via Chest CT Scan. J. Investig. Allergol. Clin. Immunol. 2018, 28, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Nakamura, Y.; Sim, J.J.; Yamashiro, Y.; Uchida, K.; Hosaka, K.; Isogai, S. Inhaled corticosteroid reduced lamina reticularis of the basement membrane by modulation of insulin-like growth factor (IGF)-I expression in bronchial asthma. Clin. Exp. Allergy 1998, 28, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, D.; Chetta, A.; Del Donno, M.; Bertorelli, G.; Casalini, A.; Pesci, A.; Testi, R.; Foresi, A. Effect of short-term treatment with low-dose inhaled fluticasone propionate on airway inflammation and remodeling in mild asthma: a placebo-controlled study. Am. J. Respir. Crit. Care Med. 1997, 155, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-R.; Liang, Y.-R.; Tzeng, I.-S.; Tzeng, I.-S.; Hsieh, P.-C.; Hsieh, P.-C.; Kuo, C.-Y.; Kuo, C.-Y.; Huang, S.-Y.; Huang, S.-Y.; et al. Transcriptome analysis in patients with asthma after inhaled combination therapy with long-acting β2-agonists and corticosteroids. Int. J. Med Sci. 2022, 19, 1770–1778. [Google Scholar] [CrossRef]
- Koarai, A.; Ichinose, M. Possible involvement of acetylcholine-mediated inflammation in airway diseases. Allergol. Int. 2018, 67, 460–466. [Google Scholar] [CrossRef]
- Cazzola, M.; Ora, J.; Rogliani, P.; Matera, M.G. Role of muscarinic antagonists in asthma therapy. Expert Rev. Respir. Med. 2017, 11, 239–253. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Matera, M.G. Long-acting muscarinic antagonists and small airways in asthma: Which link? Allergy 2021, 76, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Ora, J.; Calzetta, L.; Ritondo, B.L.; Matera, M.G.; Rogliani, P. Current long-acting muscarinic antagonists for the treatment of asthma. Expert Opin. Pharmacother. 2021, 22, 2343–2357. [Google Scholar] [CrossRef]
- Muiser, S.; Gosens, R.; Berge, M.v.D.; Kerstjens, H.A. Understanding the role of long-acting muscarinic antagonists in asthma treatment. Ann. Allergy, Asthma Immunol. 2022, 128, 352–360. [Google Scholar] [CrossRef]
- Varricchi, G.; Ferri, S.; Pepys, J.; Poto, R.; Spadaro, G.; Nappi, E.; Paoletti, G.; Virchow, J.C.; Heffler, E.; Canonica, W.G. Biologics and airway remodeling in severe asthma. Allergy 2022, 77, 3538–3552. [Google Scholar] [CrossRef]
- Schulman, E.S. Development of a Monoclonal Anti-Immunoglobulin E Antibody (Omalizumab) for the Treatment of Allergic Respiratory Disorders. Am. J. Respir. Crit. Care Med. 2001, 164, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Zastrzeżyńska, W.; Przybyszowski, M.; Bazan-Socha, S.; Gawlewicz-Mroczka, A.; Sadowski, P.; Okoń, K.; Jakieła, B.; Plutecka, H.; Ćmiel, A.; Sładek, K.; et al. Omalizumab may decrease the thickness of the reticular basement membrane and fibronectin deposit in the bronchial mucosa of severe allergic asthmatics. J. Asthma 2020, 57, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Flood-Page, P.; Menzies-Gow, A.; Phipps, S.; Ying, S.; Wangoo, A.; Ludwig, M.S.; Barnes, N.; Robinson, D.; Kay, A.B. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J. Clin. Investig. 2003, 112, 1029–1036. [Google Scholar] [CrossRef]
- Chachi, L.; Diver, S.; Kaul, H.; Rebelatto, M.C.; Boutrin, A.; Nisa, P.; Newbold, P.; Brightling, C. Computational modelling prediction and clinical validation of impact of benralizumab on airway smooth muscle mass in asthma. Eur. Respir. J. 2019, 54, 1900930. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, M.J.; Kooner, H.K.; Eddy, R.L.; Jeimy, S.; Licskai, C.; Mackenzie, C.A.; Svenningsen, S.; Nair, P.; Yamashita, C.; Parraga, G. Asthma Control, Airway Mucus, and 129Xe MRI Ventilation After a Single Benralizumab Dose. Chest 2022, 162, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef]
- Scott, G.; Asrat, S.; Allinne, J.; Lim, W.K.; Nagashima, K.; Birchard, D.; Srivatsan, S.; Ajithdoss, D.K.; Oyejide, A.; Ben, L.-H.; et al. IL-4 and IL-13, not eosinophils, drive type 2 airway inflammation, remodeling and lung function decline. Cytokine 2023, 162, 156091. [Google Scholar] [CrossRef]
- Cao, L.; Liu, F.; Liu, Y.; Liu, T.; Wu, J.; Zhao, J.; Wang, J.; Li, S.; Xu, J.; Dong, L. TSLP promotes asthmatic airway remodeling via p38-STAT3 signaling pathway in human lung fibroblast. Exp. Lung Res. 2018, 44, 288–301. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Zhang, T.-T.; Li, H.-T.; Chen, F.-H.; Zou, X.-L.; Ji, J.-Z.; Chen, H. Neutralization of TSLP Inhibits Airway Remodeling in a Murine Model of Allergic Asthma Induced by Chronic Exposure to House Dust Mite. PLOS ONE 2013, 8, e51268. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chou, H.-C.; Chen, C.-M.; Chiang, B.-L. Anti-thymic stromal lymphopoietin antibody suppresses airway remodeling in asthma through reduction of MMP and CTGF. Pediatr. Res. 2019, 86, 181–187. [Google Scholar] [CrossRef]
- Diver, S.; Khalfaoui, L.; Emson, C.; Wenzel, S.E.; Menzies-Gow, A.; Wechsler, M.E.; Johnston, J.; Molfino, N.; Parnes, J.R.; Megally, A.; et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 1299–1312. [Google Scholar] [CrossRef]
- Austin, C.D.; Edick, M.G.; Ferrando, R.E.; Solon, M.; Baca, M.; Mesh, K.; Bradding, P.; Gauvreau, G.M.; Sumino, K.; FitzGerald, J.M.; et al. A randomized, placebo-controlled trial evaluating effects of lebrikizumab on airway eosinophilic inflammation and remodelling in uncontrolled asthma (CLAVIER). Clin. Exp. Allergy 2020, 50, 1342–1351. [Google Scholar] [CrossRef]
- Hackett, T.-L.; Singhera, G.K.; Shaheen, F.; Hayden, P.; Jackson, G.R.; Hegele, R.G.; Van Eeden, S.; Bai, T.R.; Dorscheid, D.R.; Knight, D.A. Intrinsic Phenotypic Differences of Asthmatic Epithelium and Its Inflammatory Responses to Respiratory Syncytial Virus and Air Pollution. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1090–1100. [Google Scholar] [CrossRef]
- Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFNgamma and IL-4. J Allergy Clin Immunol 2012;130:1087-96.e1010.
- Balenga, N.A.; Klichinsky, M.; Xie, Z.; Chan, E.C.; Zhao, M.; Jude, J.; Laviolette, M.; Panettieri, R.A., Jr.; Druey, K.M. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 2015, 6, 6763. [Google Scholar] [CrossRef] [PubMed]
- Grunstein, M.M.; Veler, H.; Shan, X.; Larson, J.; Grunstein, J.S.; Chuang, S. Proasthmatic effects and mechanisms of action of the dust mite allergen, Der p 1, in airway smooth muscle. J. Allergy Clin. Immunol. 2005, 116, 94–101. [Google Scholar] [CrossRef] [PubMed]
- John RJ, Rusznak C, Ramjee M, Lamont AG, Abrahamson M, Hewitt EL: Functional effects of the inhibition of the cysteine protease activity of the major house dust mite allergen Der p 1 by a novel peptide-based inhibitor. Clin Exp Allergy 2000;30:784–793.
- López-Rodríguez, J.C.; Manosalva, J.; Cabrera-García, J.D.; Escribese, M.M.; Villalba, M.; Barber, D.; Martínez-Ruiz, A.; Batanero, E. Human glutathione-S-transferase pi potentiates the cysteine-protease activity of the Der p 1 allergen from house dust mite through a cysteine redox mechanism. Redox Biol. 2019, 26, 101256. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, M.C.; Brown, R.; Ryan, S.; Mall, M.A.; Weldon, S.; Taggart, C.C. Proteases, Mucus, and Mucosal Immunity in Chronic Lung Disease. Int. J. Mol. Sci. 2021, 22, 5018. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, S.-H. TGF-β/SMAD4 mediated UCP2 downregulation contributes to Aspergillus protease-induced inflammation in primary bronchial epithelial cells. Redox Biol. 2018, 18, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Aldajani, W.A.; Salazar, F.; Sewell, H.F.; Knox, A.; Ghaemmaghami, A.M. Expression and regulation of immune-modulatory enzyme indoleamine 2,3-dioxygenase (IDO) by human airway epithelial cells and its effect on T cell activation. Oncotarget 2016, 7, 57606–57617. [Google Scholar] [CrossRef]
- Yao, L.; Yuan, X.; Fu, H.; Guo, Q.; Wu, Y.; Xuan, S.; Kermani, N.Z.; Adcock, I.M.; Zeng, X.; Liu, Y.; et al. Epithelium-derived cystatin SN inhibits house dust mite protease activity in allergic asthma. Allergy 2023, 78, 1507–1523. [Google Scholar] [CrossRef]
- Rappaport, S.M.; Smith, M.T. Environment and Disease Risks. Science 2010, 330, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Sozener, Z.C.; Ozturk, B.O.; Cerci, P.; Turk, M.; Akin, B.G.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef] [PubMed]
- López-Rodríguez, J.C.; Rodríguez-Coira, J.; Benedé, S.; Barbas, C.; Barber, D.; Villalba, M.T.; Escribese, M.M.; Villaseñor, A.; Batanero, E. Comparative metabolomics analysis of bronchial epithelium during barrier establishment after allergen exposure. Clin. Transl. Allergy 2021, 11, e12051. [Google Scholar] [CrossRef] [PubMed]
- Parrón-Ballesteros, J.; Gordo, R.G.; López-Rodríguez, J.C.; Olmo, N.; Villalba, M.; Batanero, E.; Turnay, J. Beyond allergic progression: From molecules to microbes as barrier modulators in the gut-lung axis functionality. Front. Allergy 2023, 4. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie KJ, Anderton SM, Schwarze J. Viral respiratory tract infections and asthma in early life: cause and effect? Clin Exp Allergy. (2014) 44(1):9–19. Clin. Exp. Allergy. [CrossRef]
- Caruso, C.; Colantuono, S.; Ciasca, G.; Basile, U.; Di Santo, R.; Bagnasco, D.; Passalacqua, G.; Caminati, M.; Michele, S.; Senna, G.; et al. Different aspects of severe asthma in real life: Role of Staphylococcus aureus enterotoxins and correlation to comorbidities and disease severity. Allergy 2023, 78, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Martens, K.; Seys, S.F.; Alpizar, Y.A.; Schrijvers, R.; Bullens, D.M.A.; Breynaert, C.; Lebeer, S.; Steelant, B. Staphylococcus aureus enterotoxin B disrupts nasal epithelial barrier integrity. Clin. Exp. Allergy 2021, 51, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Kline, S.N.; Orlando, N.A.; Lee, A.J.; Wu, M.-J.; Zhang, J.; Youn, C.; Feller, L.E.; Pontaza, C.; Dikeman, D.; Limjunyawong, N.; et al. Staphylococcus aureus proteases trigger eosinophil-mediated skin inflammation. Proc. Natl. Acad. Sci. 2024, 121. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Seiber, E.E.; Ferketich, A.K. Secondhand smoke and asthma: What are the effects on healthcare utilization among children? Prev. Med. 2013, 57, 125–128. [Google Scholar] [CrossRef]
- E Baena-Cagnani, C.; Gómez, R.M.; Baena-Cagnani, R.; Canonica, G.W. Impact of environmental tobacco smoke and active tobacco smoking on the development and outcomes of asthma and rhinitis. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 136–140. [Google Scholar] [CrossRef]
- Sun, Y.-B.; Liu, M.; Fan, X.-S.; Zhou, L.-P.; Li, M.-W.; Hu, F.-Y.; Yue, Q.-F.; Zhang, Y.-M. Effects of cigarette smoke on the aggravation of ovalbumin-induced asthma and the expressions of TRPA1 and tight junctions in mice. Mol. Immunol. 2021, 135, 62–72. [Google Scholar] [CrossRef]
- Wu, C.-C.; Hsu, T.-Y.; Chang, J.-C.; Ou, C.-Y.; Kuo, H.-C.; Liu, C.-A.; Wang, C.-L.; Chuang, H.; Chen, C.-P.; Yang, K.D. Paternal Tobacco Smoke Correlated to Offspring Asthma and Prenatal Epigenetic Programming. Front. Genet. 2019, 10, 471. [Google Scholar] [CrossRef]
- Christensen, S.; Jaffar, Z.; Cole, E.; Porter, V.; Ferrini, M.; Postma, B.; Pinkerton, K.E.; Yang, M.; Kim, Y.J.; Montrose, L.; et al. Prenatal environmental tobacco smoke exposure increases allergic asthma risk with methylation changes in mice. Environ. Mol. Mutagen. 2017, 58, 423–433. [Google Scholar] [CrossRef]
- Lu, K.; Lai, K.P.; Stoeger, T.; Ji, S.; Lin, Z.; Lin, X.; Chan, T.F.; Fang, J.K.-H.; Lo, M.; Gao, L.; et al. Detrimental effects of microplastic exposure on normal and asthmatic pulmonary physiology. J. Hazard. Mater. 2021, 416, 126069. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xu, Z.; Fan, E.; Li, Y.; Zhang, L. Effect of nitrogen dioxide and sulfur dioxide on viability and morphology of oak pollen. Int. Forum Allergy Rhinol. 2016, 6, 95–100. [Google Scholar] [CrossRef]
- Motta, A.; Marliere, M.; Peltre, G.; Sterenberg, P.; Lacroix, G. Traffic-Related Air Pollutants Induce the Release of Allergen-Containing Cytoplasmic Granules from Grass Pollen. Int. Arch. Allergy Immunol. 2006, 139, 294–298. [Google Scholar] [CrossRef]
- Reinmuth-Selzle, K.; Ackaert, C.; Kampf, C.J.; Samonig, M.; Shiraiwa, M.; Kofler, S.; Yang, H.; Gadermaier, G.; Brandstetter, H.; Huber, C.G.; et al. Nitration of the Birch Pollen Allergen Bet v 1.0101: Efficiency and Site-Selectivity of Liquid and Gaseous Nitrating Agents. J. Proteome Res. 2014, 13, 1570–1577. [Google Scholar] [CrossRef]
- Backes, A.T.; Reinmuth-Selzle, K.; Leifke, A.L.; Ziegler, K.; Krevert, C.S.; Tscheuschner, G.; Lucas, K.; Weller, M.G.; Berkemeier, T.; Pöschl, U.; et al. Oligomerization and Nitration of the Grass Pollen Allergen Phl p 5 by Ozone, Nitrogen Dioxide, and Peroxynitrite: Reaction Products, Kinetics, and Health Effects. Int. J. Mol. Sci. 2021, 22, 7616. [Google Scholar] [CrossRef]
- Khreis, H.; Kelly, C.; Tate, J.; Parslow, R.; Lucas, K.; Nieuwenhuijsen, M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 2017, 100, 1–31. [Google Scholar] [CrossRef]
- Yue, H.; Yan, W.; Ji, X.; Zhang, Y.; Li, G.; Sang, N. Maternal exposure to NO2 enhances airway sensitivity to allergens in BALB/c mice through the JAK-STAT6 pathway. Chemosphere 2018, 200, 455–463. [Google Scholar] [CrossRef]
- De Grove, K.C.; Provoost, S.; Hendriks, R.W.; McKenzie, A.N.; Seys, L.J.; Kumar, S.; Maes, T.; Brusselle, G.G.; Joos, G.F. Dysregulation of type 2 innate lymphoid cells and T H 2 cells impairs pollutant-induced allergic airway responses. J. Allergy Clin. Immunol. 2017, 139, 246–257. [Google Scholar] [CrossRef]
- Mirowsky, J.E.; Dailey, L.A.; Devlin, R.B. Differential expression of pro-inflammatory and oxidative stress mediators induced by nitrogen dioxide and ozone in primary human bronchial epithelial cells. Inhal. Toxicol. 2016, 28, 374–382. [Google Scholar] [CrossRef]
- Bayram, H.; Sapsford, R.J.; Abdelaziz, M.M.; Khair, O.A. Effect of ozone and nitrogen dioxide on the release of proinflammatory mediators from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients in vitro. J. Allergy Clin. Immunol. 2001, 107, 287–294. [Google Scholar] [CrossRef]
- Reinmuth-Selzle, K.; Bellinghausen, I.; Leifke, A.L.; Backes, A.T.; Bothen, N.; Ziegler, K.; Weller, M.G.; Saloga, J.; Schuppan, D.; Lucas, K.; et al. Chemical modification by peroxynitrite enhances TLR4 activation of the grass pollen allergen Phl p 5. Front. Allergy 2023, 4, 1066392. [Google Scholar] [CrossRef]
- Loxham, M.; E Smart, D.; Bedke, N.J.; Smithers, N.P.; Filippi, I.; Blume, C.; Swindle, E.J.; Tariq, K.; Howarth, P.H.; Holgate, S.T.; et al. Allergenic proteases cleave the chemokine CX3CL1 directly from the surface of airway epithelium and augment the effect of rhinovirus. Mucosal Immunol. 2018, 11, 404–414. [Google Scholar] [CrossRef]
- Perros, F.; Dorfmuller, P.; Souza, R.; Durand-Gasselin, I.; Godot, V.; Capel, F.; Adnot, S.; Eddahibi, S.; Mazmanian, M.; Fadel, E.; et al. Fractalkine-induced smooth muscle cell proliferation in pulmonary hypertension. Eur. Respir. J. 2007, 29, 937–943. [Google Scholar] [CrossRef]
- Heijink, I.H.; Postma, D.S.; Noordhoek, J.A.; Broekema, M.; Kapus, A. House Dust Mite–Promoted Epithelial-to-Mesenchymal Transition in Human Bronchial Epithelium. Am. J. Respir. Cell Mol. Biol. 2010, 42, 69–79. [Google Scholar] [CrossRef]
- Gao, F.; Qiao, J.; Zhang, Y.; Jin, X. Chronic intranasal administration of Aspergillus fumigatus spores leads to aggravation of airway inflammation and remodelling in asthmatic rats. Respirology 2009, 14, 360–370. [Google Scholar] [CrossRef]
- Kurup, V.P.; Grunig, G. Animal models of allergic bronchopulmonary aspergillosis. Mycopathologia 2002, 153, 165–177. [Google Scholar] [CrossRef]
- Caruso, C.; Ciasca, G.; Baglivo, I.; Di Santo, R.; Gasbarrini, A.; Firinu, D.; Bagnasco, D.; Passalacqua, G.; Schiappoli, M.; Caminati, M.; et al. Immunoglobulin free light chains in severe asthma patient: Could they be a new biomarker? Allergy 2024. [Google Scholar] [CrossRef]
| Marker | Description | Reference |
|---|---|---|
| High-resolution CT (HRCT) | HRCT is crucial for identifying static and dynamic airway changes in asthma, revealing details as small as 1 mm in diameter. | Brown RH, Mitzner W, 1985; Silva CI, Colby TV, 2004 |
| Bronchial Wall Thickness (% WT) | % WT, the bronchial-to-arterial diameter ratio (BA ratio), and the level of airway collapsibility (AC) are acknowledged as efficient measurements for assessing airway remodeling in CT scans. | Chae EJ, Kim TB, Cho YS, et al., 2010 |
| Wall Area Percentage (WA%) | WA% is a crucial marker for assessing airway remodeling in severe asthma, with a negative correlation between WA% and FEV1 observed, indicating the relationship between airway wall thickness and lung function impairment. | Hartley RA, Barker BL, Newby, 2016 |
| Quantitative CT (qCT) scans | QCT scans serve as effective biomarkers for airway remodeling, enhancing the precise analysis of severe asthma. Biomarkers such as wall thickness percentage (WT%), wall area percentage (WA%), and air trapping are higher in asthma patients and particularly elevated in severe cases. | Walker C, Gupta S, Hartley et al., 2012; Aysola RS, Hoffman EA, et al., 2008; Trivedi A, Hall C, Hoffman EA, 2017; Adams JE, 2009 |
| Bronchial Wall Thickness (BWT) and Emphysema | BWT and emphysema are more prevalent in patients with severe asthma, indicating their role as radiological markers for lung function changes in asthma. | Gupta S, Siddiqui, 2009; Harmanci E, Kebapci M, 2002; Gupta S, Hartley R, Khan UT, et al., 2014; Wang D, Luo J, Du W, Zhang LL, 2016 |
| Biomarker | Description | Reference |
|---|---|---|
| Sub-epithelial fibrosis | Characterized by thicker airway smooth muscle, mucous gland hyperplasia, angiogenesis, and damaged epithelial layers, contributing to stiffer airway walls. | Kozlik P, 2020; Aysola RS, chest 2008 |
| Epithelial remodeling | Involves deterioration of epithelial cells, loss of ciliated cells, and an increase in goblet cells. The epithelial-mesenchymal transition (EMT) driven by TGF-β is a key process, with markers like reduced E-cadherin and increased N-cadherin. | Bahmer T, Sand JMB, 2020; Yang ZC, Qu ZH, Yi MJ, 2019; Rout-Pitt N, Farrow N, 2018 |
| Reticular Basement Membrane (RBM) thickening | Linked to gene expressions affecting airway growth and fibrosis. Identifying specific fibrocytes in BALF as markers suggests a role in airway remodeling. | Bazan-Socha S, Buregwa-Czuma, 2021; Nihlberg K, Larsen K, e coll., 2006 |
| Subepithelial Fibrosis | TGFβ’s role in transforming airway fibroblasts into myofibroblasts leading to subepithelial fibrosis. The severity of fibrosis correlates with TGFB1 mRNA levels, and periostin’s association with IL-4 and IL-13 impacts fibrosis and inflammation. | Sidhu SS, Yuan S, Innes AL, 2010; Vignola AM, Chanez P, 1997; Izuhara K, Arima K, Ohta, 2014 |
| Airway Smooth Muscle (ASM) | ASM cell mitogens, such as PDGF, TGFβ, EGF, are involved in asthma. Histology assessed through endobronchial biopsies serves as a valuable biomarker. | Joseph C, Tatler AL, 2022; Sha J, Rorke S, Langton D, et al., 2019 |
| Mucus | Hypersecretion of mucins MUC5AC and MUC5B by goblet cells contributes to airway remodeling, with targeting MUC5AC secretion as a potential therapeutic strategy. | Bahmer T, Sand JMB, Weckmann, 2016; Lai HY, Rogers DF, 2010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





