Submitted:
14 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
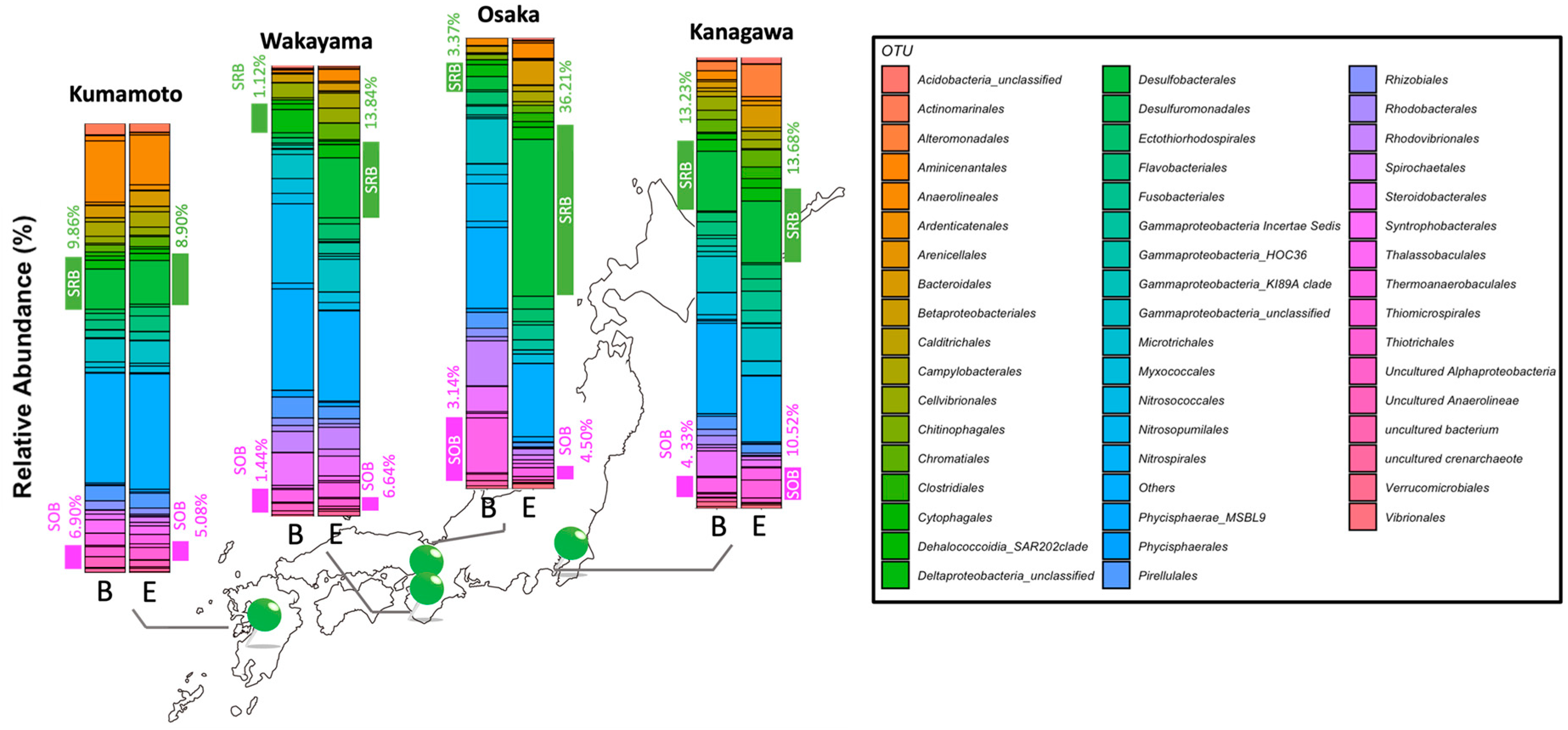
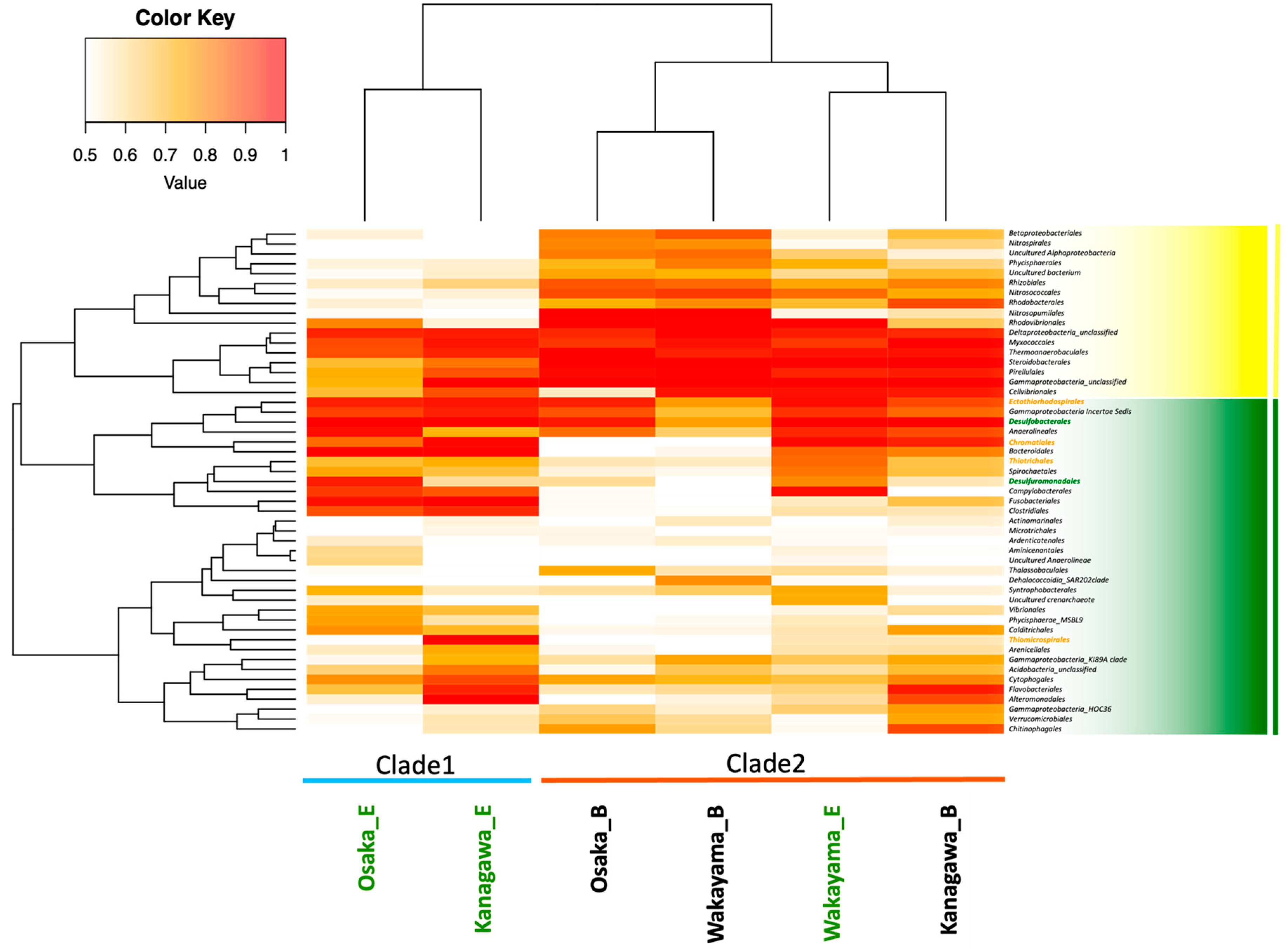
2.1. Microbial Composition in Eelgrass Meadows
2.2. Determination of Total Bacteria and Chromatiales Population
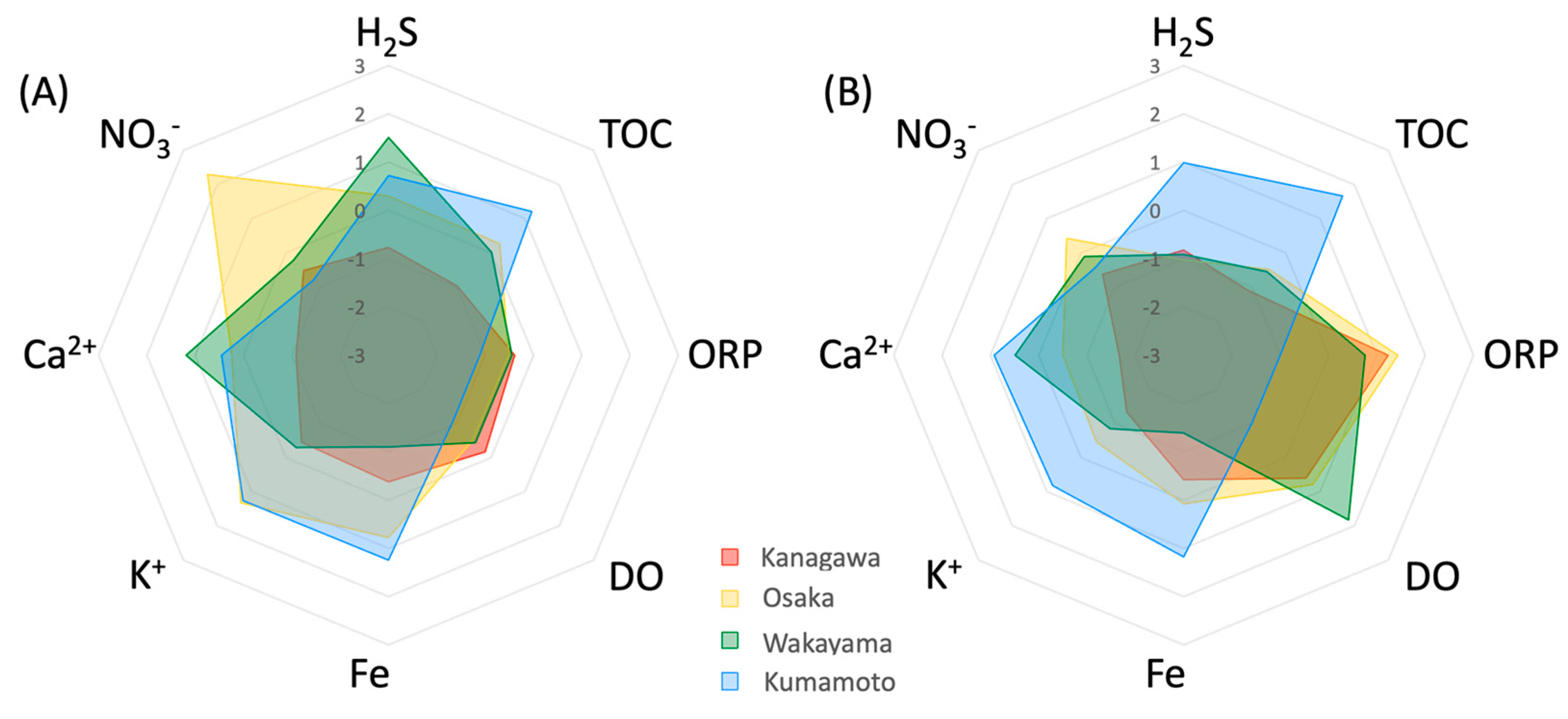
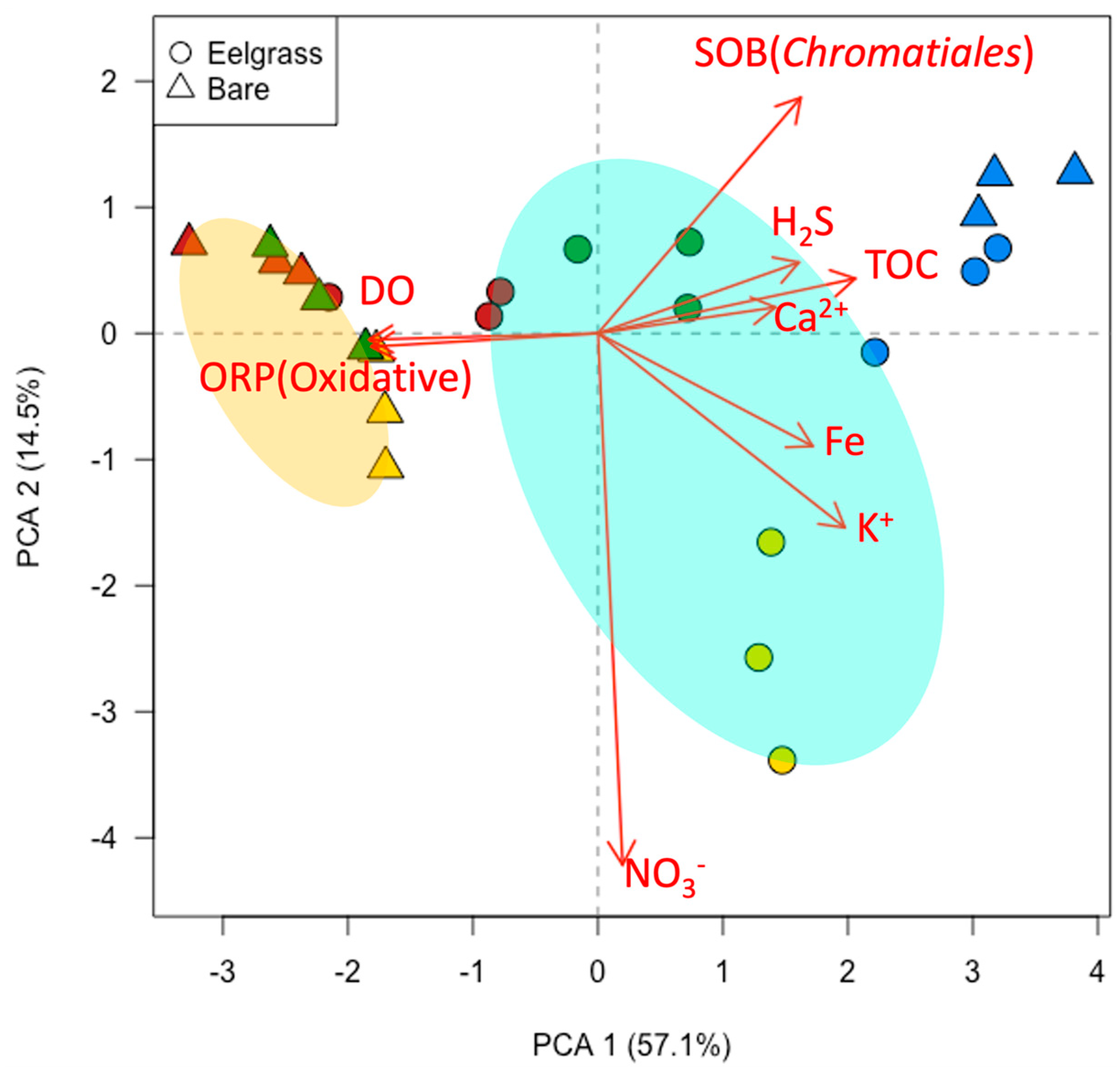
2.3. Determination of Chemical Component in Eelgrass Sediment
3. Discussion
3.1. Characteristics of Bacterial Composition in Eelgrass Sediments
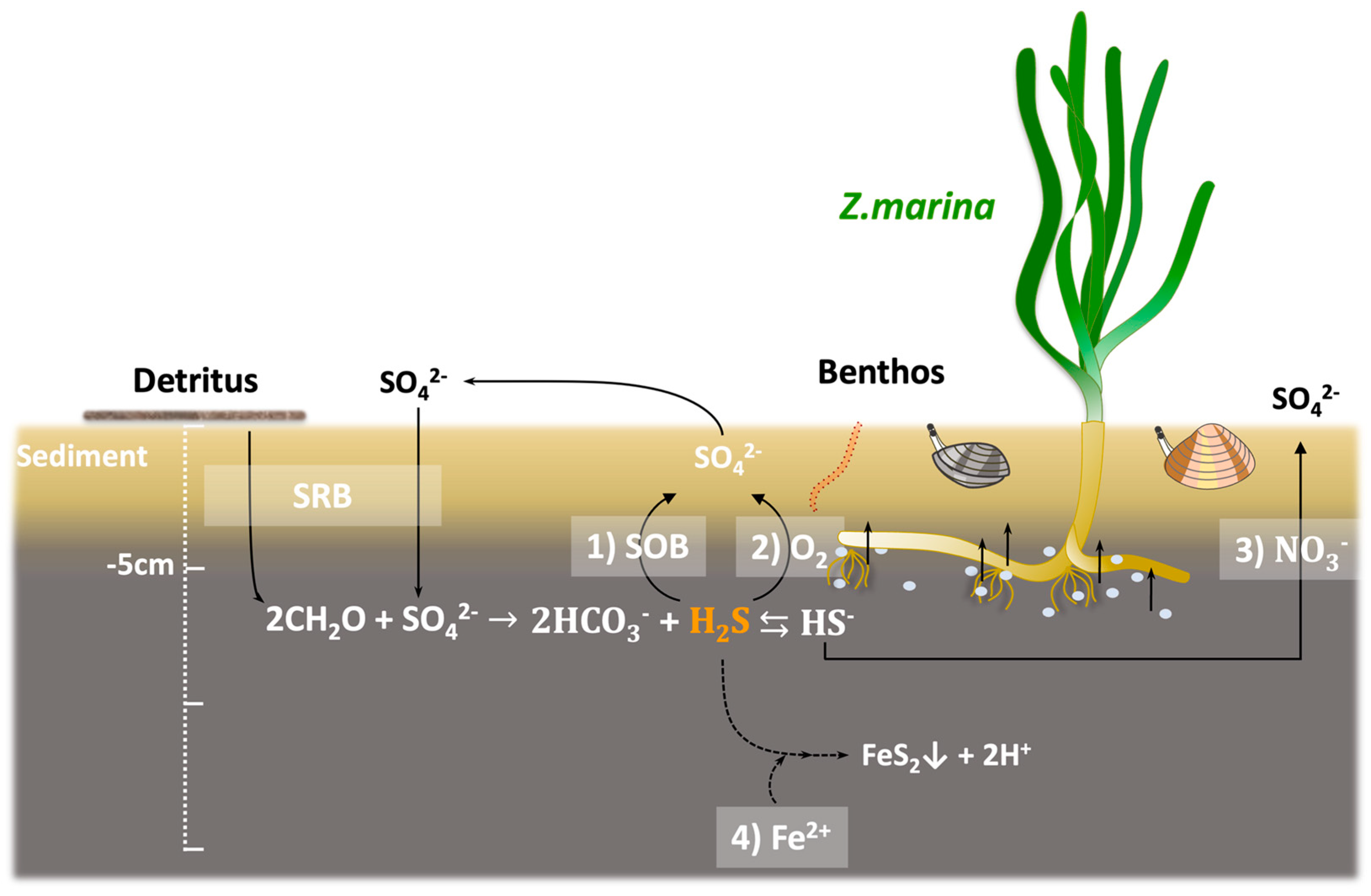
3.2. Detoxification System in Eelgrass Sediments
4. Materials and Methods
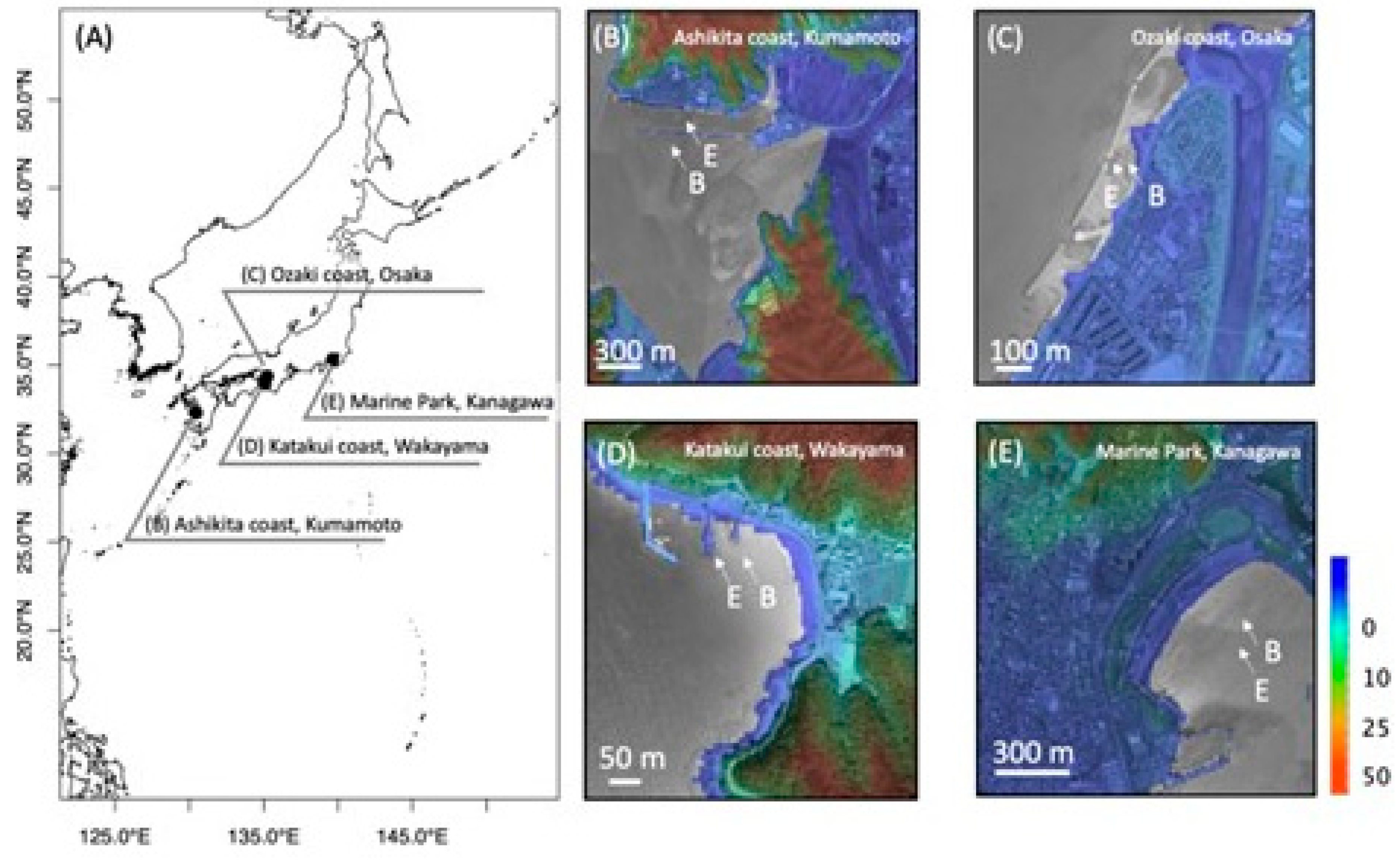
4.1. Sampling Sites and Sample Collection
4.2. 16S Metagenomic Sequencing
4.3. Microbial Community Composition and Diversity Analysis
4.4. qPCR Analysis for Total Bacteria and Sulfur-Oxidizing Bacteria Population in Sediments
4.5. Chemical Components Analysis
4.6. Total Organic Component Analysis
4.7. Statistical Analysis and Data Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, K.A.; Short, F.T. Zostera: Biology, Ecology, and Management. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: The Netherlands, USA, 2006; Volume 354, pp. 361–386. [Google Scholar]
- Kusube, M. Research on innovative marine environmental conservation with no environmental impact using marine bio-cement. Impact 2020, 3, 57–59. [Google Scholar] [CrossRef]
- Nishijima, W.; Nakano, Y.; Hizon-Fradejasc, B.A.; Nakai, S. Evaluation of substrates for constructing beds for the marine macrophyte Zostera marina L. Ecol. Eng. 2015, 83, 43–48. [Google Scholar] [CrossRef]
- Watanabe, K.; Kuwae, T. Radiocarbon isotopic evidence for assimilation of atmospheric CO2 by the seagrass Zostera marina. Biogeosciences. 2015, 12, 6251–6258. [Google Scholar] [CrossRef]
- Nishijima, W.; Nakano, Y.; Nakai, S.; Okuda, T.; Imai, T.; Okada, M. Macrobenthic succession and characteristics of a man-made intertidal sandflat constructed in the diversion channel of the Ohta River Estuary. Mar. Pollut. Bull. 2014, 82, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, B.B.; Findlay, J.A.; Pellerin, A. The Biogeochemical sulfur cycle of marine sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Dooley, D.F.; Sandy, W.E.; Roth, B.M.; Ward, D.P. ; Tolerance and response of Zostera marina seedlings to hydrogen sulfide. Aquat. Bot. 2013, 105, 7–10. [Google Scholar] [CrossRef]
- Sheetal, H.; Holmer, H. Sulfide intrusion and detoxification in the seagrass Zostera marina. PLoS ONE 2013, 10, 6. [Google Scholar] [CrossRef]
- Miyamoto, H.; Kawachi, N.; Kurotani, A.; Moriya, S.; Suda, W.; Suzuki, K.; Matsuura, M.; Tsuji, N.; Nakaguma, T.; Ishii, C.; et al. Computational estimation of sediment symbiotic bacterial structures of seagrass overgrowing downstream of onshore aquaculture. Environ Res. 2023, 219, 115130–115142. [Google Scholar] [CrossRef] [PubMed]
- Crump, B.C.; Wojahn, J.M.; Tomas, F.; Mueller, R.S. Metatranscriptomics and amplicon sequencing reveal mutualisms in seagrass microbiomes. Front. Microbiol. 2018, 9, 388. [Google Scholar] [CrossRef]
- Cúcio, C.; Overmars, L.; Engelen, A.; Muyzer, G. Metagenomic analysis shows the presence of bacteria related to free-living forms of sulfur-oxidizing chemolithoautotrophic symbionts in the rhizosphere of the seagrass Zostera marina. Front. Mar. Sci. 2018, 5, 171. [Google Scholar] [CrossRef]
- Chen, J.; Hanke, A.; Tegetmeyer, H.E.; Kattelmann, I.; Sharma, R.; Hamann, E.; Hargesheimer, T.; Kraft, B.; Lenk, S.; Geelhoed, J.S.; et al. Impacts of chemical gradients on microbial community structure. The ISME J. 2017, 11, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, Z.; Wang, P.; Zheng, X.; Fan, J. Comparative metagenomics reveals the microbial diversity and metabolic potentials in the sediments and surrounding seawaters of Qinhuangdao mariculture area. PLoS ONE 2020, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, R.J.; Dillon, R.M.; Bokulich, A.N.; Abnet, C.C.; Gabriel, A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Muwawa, M.E.; Obieze, C.C.; Makonde, M.H.; Jefwa, M.J.; Kahindi, H.P.; Khasa, P.D. 16S rRNA gene amplicon-based metagenomic analysis of bacterial communities in the rhizospheres of selected mangrove species from Mida Creek and Gazi Bay, Kenya. PLoS ONE 2021, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Beals, W.E. Bray-Curtis ordination: An effective strategy for analysis of multivariate ecological data. Adv. Ecol. Res. 1984, 14, 1–55. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Nishimura, M.; Haider, N.M.; Yoshizawa, S. Microbial communities on eelgrass (Zostera marina) thriving in Tokyo Bay and the possible source of leaf-attached microbes. Front. Microbiol. 2023, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Martin-Orue, M.S.; Manzanilla, G.E.; Badiola, I.; Martin, M.; Gasa, J. Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR. Vet. Microbiol. 2006, 114, 165–170. [Google Scholar] [CrossRef]
- Larionov, A.; Krause, A.; Miller, W. A standard curve based method for relative real time PCR data processing. BMC Bioinform. 2005, 6, 62. [Google Scholar] [CrossRef]
- Millero, F.; Hublnger, S.; Fernandez, M.; Garnett, S. Oxidation of H2S in seawater as a function of temperature, pH, and ionic strength. Environ. Sci. Technol. 1987, 21, 439–443. [Google Scholar] [CrossRef]
- Schnell, S.; Ratering, S.; Jansen, H.K. Simultaneous determination of Iron(III), Iron(II), and Manganese(II) in environmental samples by Ion chromatography. Environ. Sci. Technol. 1998, 32, 1530–1537. [Google Scholar] [CrossRef]
- Jaffer, Y.D.; Kumar, H.S.; Vinothkumar, R.; Irfan, A.B.; Ishfaq, N.M.; Ganie, P.A.; Bhat, R.A.H.; Vennila, A. Isolation and characterization of heterotrophic nitrification-aerobic denitrification and sulphur-oxidizing bactcterium Paracoccus saliphilus strain SPUM from coastal shrimp ponds. Aquac. Int. 2019; 27, 1513–1524. [Google Scholar] [CrossRef]
- Hirano, H.; Tomoya, T.; Nishimiya, N.; Setiamarga, D.H.E.; Morita, S.; Uragaki, Y.; Okamoto, K. Artificial sludge based on compositional information of a natural sea sludge. Int. J. GEOMATE. 2017, 12, 95–99. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Nishimura, M.; Haider, M.N.; Sano, M.; Ijichi, M.; Kogure, K.; Yoshizawa, S. Diversity and composition of microbial communities in an eelgrass (Zostera marina) bed in Tokyo bay, Japan. Microbes Environ. 2021, 36, 4. [Google Scholar] [CrossRef]
- Wojahn, J.M.A. Metagenomics and metatranscriptomics of the leaf- and root-associated microbiomes of Zostera marina and Zostera japonica. dissertation/bachelor’s thesis, Oregon State University, USA, 2016. [Google Scholar]
- Crump, B.C.; Wojahn, J.M.; Tomas, F.; Mueller, R.S. Metatranscriptomics and Amplicon Sequencing Reveal Mutualisms in Seagrass Microbiomes. Front. Microbiol. 2018, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Fahimipour, K.A.; Kardish, M.R.; Lang, M.J.; Green, L.J.; Eisen, A.J.; Stachowicz, J.J. Global-Scale Structure of the Eelgrass Microbiome. Appl. Environ. Microbiol. 2017, 83, e03391–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Song, Y.; Zhang, H.; Liu, P.; Hu, X. Seagrass vegetation affect the vertical organization of microbial communities in sediment. Mar. Environ. Res. 2020, 162, 105174. [Google Scholar] [CrossRef] [PubMed]
- Krause-Jensen, D.; Carstensen, J.; Nielsen, S.L.; Dalsgaard, T.; Christensen, P.B.; Fossing, H.; Rasmussen, M.B. Sea bottom characteristics affect depth limits of eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 2011, 425, 91–102. [Google Scholar] [CrossRef]
- Calderwood, A.; Kopriva, S. Hydrogen sulfide in plants: From dissipation of excess sulfur to signaling molecule. Nit. Oxide. 2014, 41, 72–78. [Google Scholar] [CrossRef]
- Tanaka, A.; Mulleriyawa, R.P.; Yasu, T. Possibility of hydrogen sulfide induced iron toxicity of the rice plant. Soil Sci. Plant Nutr. 1968, 14, 1–6. [Google Scholar] [CrossRef]
- Connell, W.E.; Patrick Jr., W. H. Reduction of sulfate to sulfide in waterlogged soil. Soil Sci. Soc. Am. J. 1969, 33, 711–715. [Google Scholar] [CrossRef]
- Joshi, M.M.; Ibrahim, I.K.A.; Hollis, J.P. Hydrogen sulfide: Effect on the physiology of rice plants and relation to straighthead disease. Phytopathology. 1975, 65, 1165–1170. [Google Scholar] [CrossRef]
| ORP/mV | DO/ppm | H2S/ppm | TOC/% | Fe/ppm | K+/ppm | Ca2+/ppm | NO3-/ppm | ||
|---|---|---|---|---|---|---|---|---|---|
| Eelgrass | Kanagawa | -154.5(201.39) | 3.0(2.3) | 2.70(1.37) | 1.28(0.13) | 393.0(19.7) | 36.7(2.7) | 65.0(5.3) | 0.22(0.023) |
| Osaka | -175.0(63.25) | 1.9(0.9) | 12.32(6.68) | 2.76(0.42) | 671.4(57.5) | 58.6(0.9) | 108.4(14.7) | 95.10(23.92) | |
| Wakayama | -163.6(54.50) | 2.1(0.9) | 23.29(4.34) | 2.30(0.14) | 214.7(25.4) | 38.6(0.7) | 138.9(6.3) | 19.67(9.40) | |
| Kumamoto | -282.3(6.41) | 0.1(0) | 16.14(0.20) | 4.52(0.18) | 798.8(20.1) | 57.8(0.7) | 115.1(1.4) | 0.031(0.025) | |
| Bare | Kanagawa | 131.6(68.69) | 5.3(1.0) | 2.24(1.57) | 1.20(0.09) | 353.7(12.6) | 25.8(4.0) | 46.1(9.3) | 0.14(0.022) |
| Osaka | 166.3(1.00) | 5.9(0.7) | 0.42(0.21) | 1.36(0.33) | 508.6(28.8) | 36.7(2.7) | 84.0(4.4) | 40.49(12.49) | |
| Wakayama | 46.2(62.65) | 9.1(2.0) | 1.47(1.45) | 1.23(0.57) | 144.0(68.5) | 31.8(2.8) | 116.4(11.9) | 24.19(13.58) | |
| Kumamoto | -261.8(8.49) | 0.4(0.2) | 18.58(9.96) | 3.98(0.25) | 777.4(25.8) | 52.5(1.1) | 130.3(4.3) | 0.28(0.40) | |
| Primer Name | Target Organisms | Denaturation | Annealing | Extension |
|---|---|---|---|---|
| Eub519F/ U785R | Total bacteria | 5 sec at 95 ℃ | 30 sec at 60 ℃ | 30 sec at 60 ℃ |
| CHR986F/CHR1392R | Chromatiales | 30 sec at 95 ℃ | 60 sec at 55 ℃ | 120 sec at 72 ℃ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




