1. Introduction
The incidence of chronic diseases is high in today’s world, and this has raised concerns [
1,
2,
3]. Diabetes mellitus is one of the most common pathologies caused by either the failure of the pancreas to secrete insulin (type 1) or the body’s inadequate response to insulin (type 2). Diabetes is a group of metabolic diseases characterized by increased glucose production in the liver and decreased glucose elimination in muscles and fat, leading to abnormal glucose accumulation in the blood and disrupting insulin levels in the body [
4]. It shows promising potential to fulfill the need for patient-centric personalized treatment.
The impact of diabetes on sustainable development has been significant [
1], leading to the creation of a global goal to stop the increase in diabetes and obesity by 2025 [
5]. Diabetes is a severe chronic disease that affects around 422 million individuals worldwide [
6], with the highest prevalence observed in countries with low and middle-income populations. Colombia, with an estimated population of 50 million, has a diabetes prevalence rate of around 10.1% [
7]. The International Diabetes Federation estimates that the current prevalence of diabetes in Colombia is 9.9% [
8].
As of today, there is no cure for diabetes despite various technological advances. However, there are treatments available that allow patients to counteract or minimize the effects of the disease. The typical treatment for diabetes involves self-administering insulin injections subcutaneously several times a day at home. This procedure requires caution as it is susceptible to human error. The prescribed dose for each individual dictates the amount of insulin to inject, which may sometimes differ from the doctor’s recommendation. Unfortunately, to date, there is no cure for diabetes despite significant technological advances. However, available treatments allow patients to counteract or minimize its effects.
The effectiveness of the treatment depends on the patient’s adherence, the storage conditions of the drug at home, and the consistency of its application [
9]. Complications associated with this treatment include the risk of microbial contamination, local skin necrosis, and nerve damage—additionally, many users express dissatisfaction with this traditional injection method [
10]. Thus, researchers should seek treatment options that minimize discomfort, and one alternative is microdosing devices.
This research aims to contribute to these microdosing biodevices with two systems: the first is a microvalve for drug regulation, and the second is an array of microchannels for flow transport. To produce these systems, we will explore some additive manufacturing techniques. The intention is to obtain models that are easily manufacturable and producible using 3D printing.
2. Materials and Methods
2.1. Materials
The material used to manufacture the microvalve and the microchannel matrix was green translucent standard resin, which is a stable resin in various climatic conditions, which facilitates its storage, has a hardness of 79D, and achieves optimum mechanical properties with the post-curing recommendations, including the solid density of 1.184 g/cm3, a maximum tensile strength of 23.4 MPa and an elongation of 14.2%.
The post-curing process involved cleaning and removing resin residues using 99% isopropyl alcohol, following the manufacturer’s specifications.
2.2. Computer-Aided Design (CAD) of the Microvalve-Microchannel System
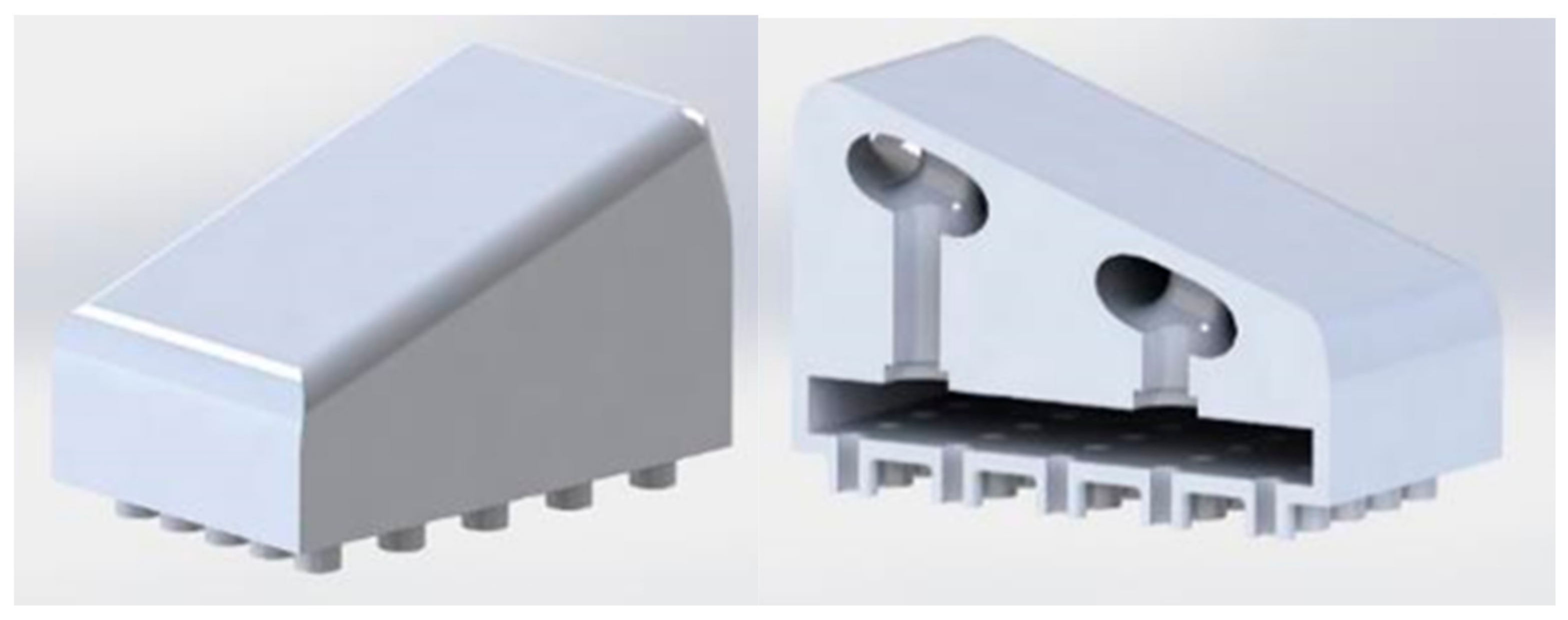
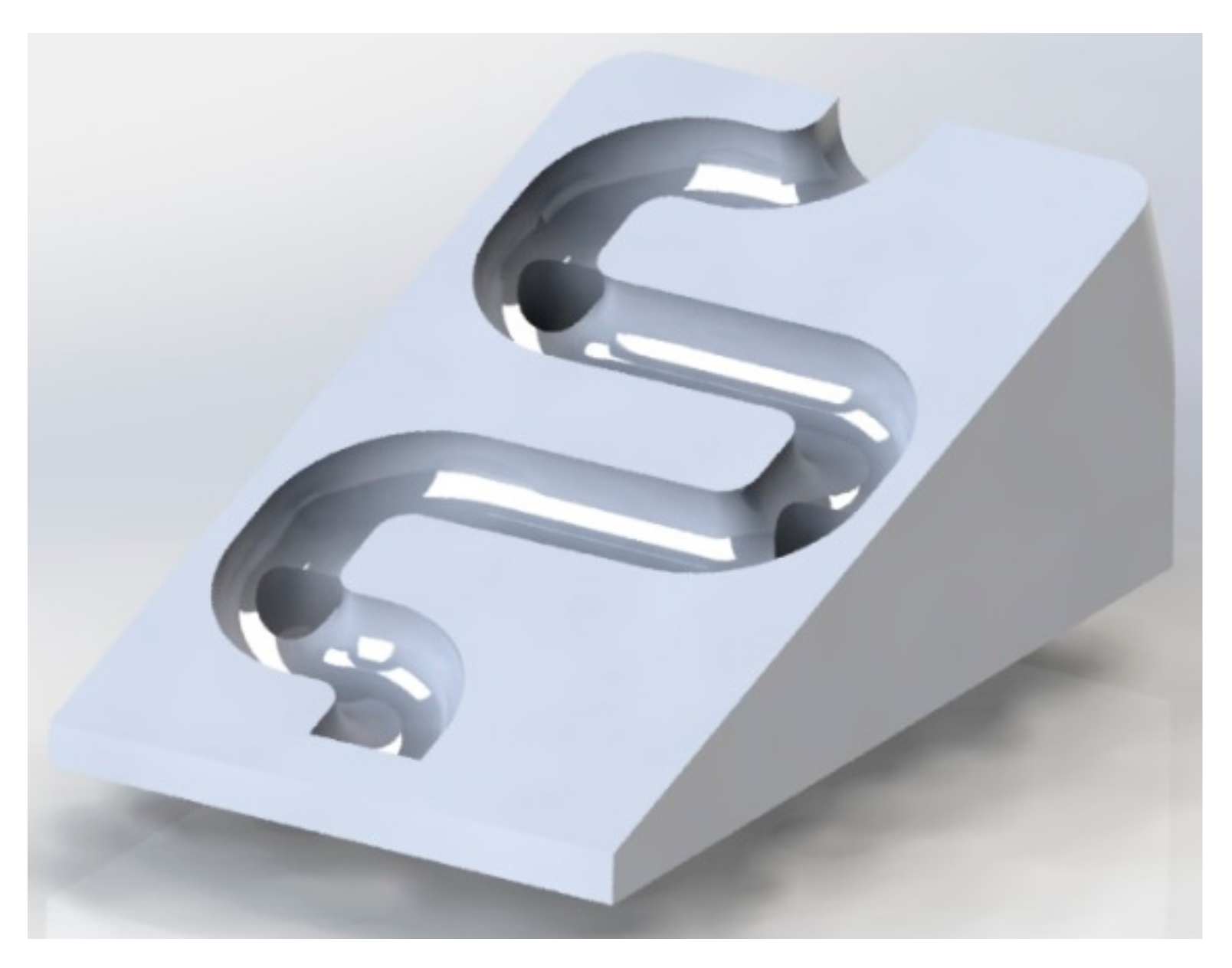
We used Fusion 360 software to create a structural model of the microvalve, which has an innovative mechanical design inspired by passive fluid mixing microvalves. The microvalve’s external dimensions are 9 mm x 15 mm, and fluid passes through a spiral-shaped path with an approximate diameter of 1 mm. To ensure a homogeneous fluid supply, we strategically placed four perforations with a radius of 1.5 mm at each path curve and the end of the run.
Figure 1 illustrates the average fluid speed through the microvalve, which is 7.13 x 10
−4 m/s. We followed this process to obtain the optimal design for the microvalve.
We designed the microchannels using Fusion 360 software, arranging them in a 10 mm x 15 mm array containing 25 microchannels, each with an internal diameter of 770 μm (see
Figure 1).
The systems were then coupled, and the result is presented in the following figure:
2.3. Fluid Simulation of Microvalve-Microchannel Systems
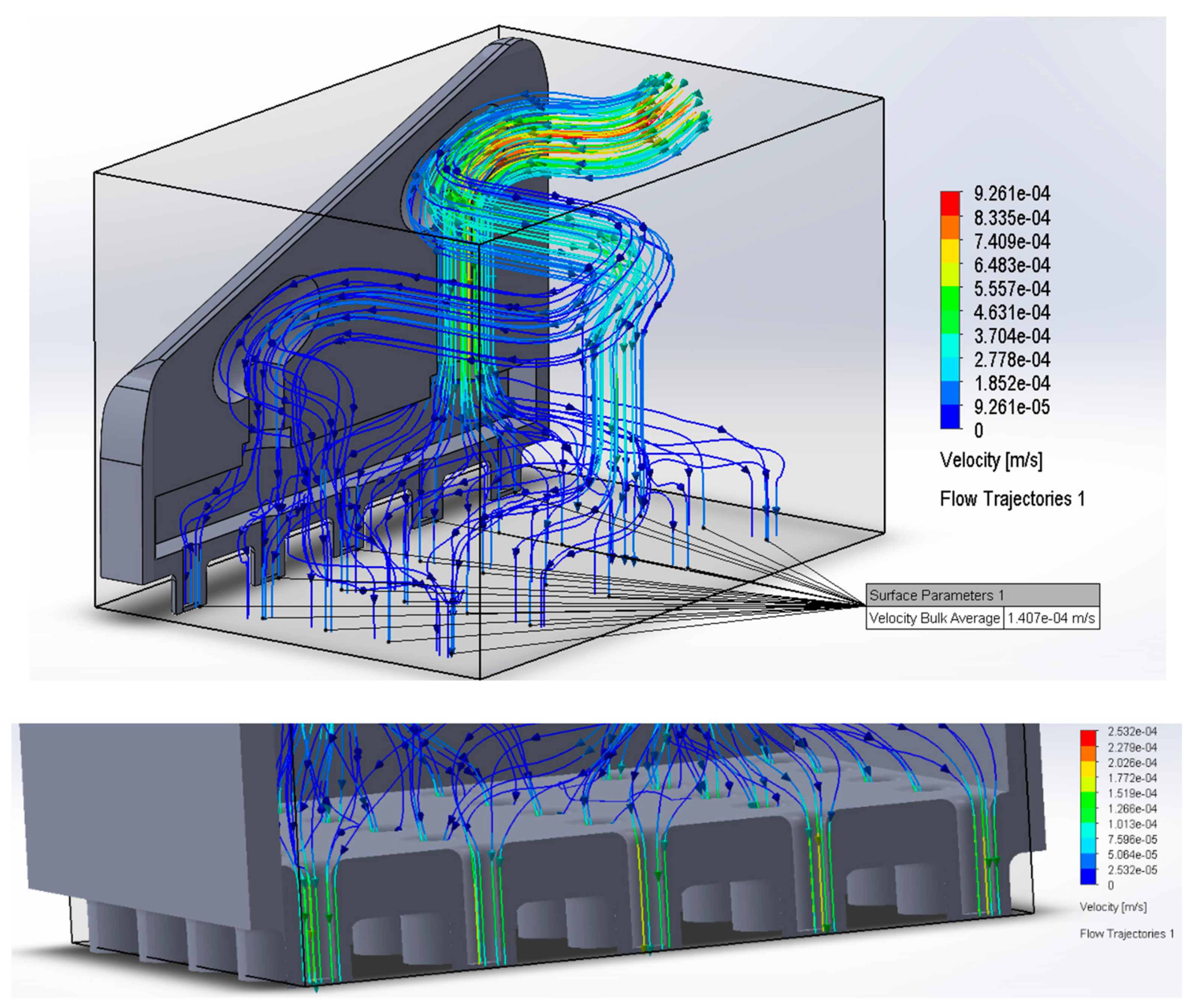
The two systems were coupled by the Solidworks 2020 software to perform fluid simulations to visualize fluid distribution in the system and obtain outlet velocity and thus the flow rate symbolized by Q of the form:
To conduct simulations with real-world values, we used reference microneedles from the study [
11], where the key parameters are established for fluid simulation, starting with the total inlet flow in the microneedles, which is 1.11 × 10
−3 cm3/
s so that the insulin does not lose effectiveness, that is, this must be the flow that passes through the microvalve. We also assigned an input parameter equivalent to that established by the ref. [
11] as mass flow, which is 1.38 × 10
−6 kg/s. See
Figure 2.
Figure 2 shows that the flow distribution is similar in each microchannel design because their velocity values are within the same range, and their area is constant (Q =A*V), allowing adequate flow to the microneedles.
2.4. Microvalve—Microchannel System Manufacturing Process
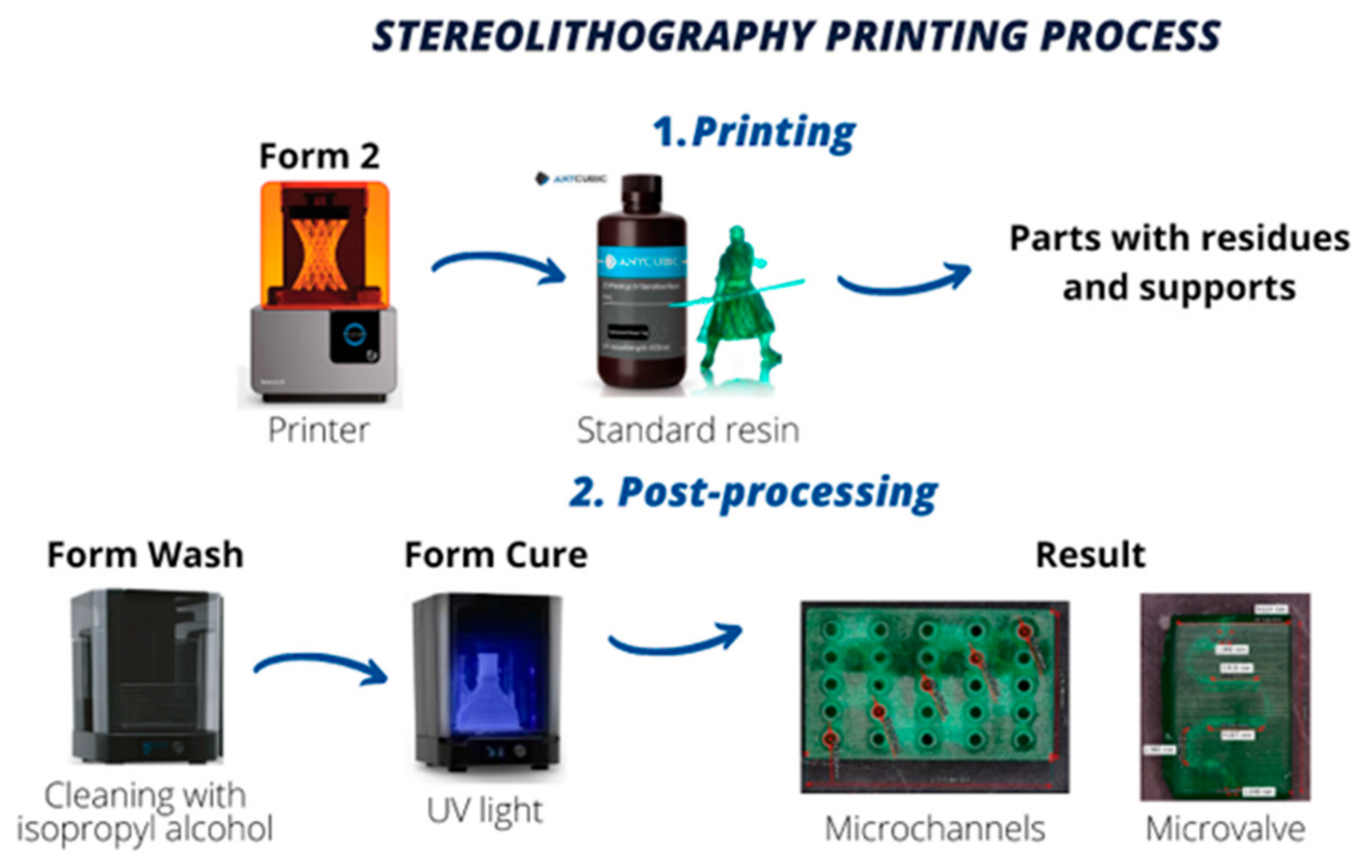
To model the microchannels and microvalve, we used Fusion 360 software. Subsequently, we exported the designs to PreForm, a 3D print preparation software, where we established the supports and configured vital parameters to achieve optimal results. Both parts were assigned a print angle of 0° relative to the printing platform to ensure the highest quality print output [
12,
13]. This methodological approach is vital to provide precise and reliable results in microfluidic devices that rely on the accurate manipulation of fluids at the microscale.
After preparing the file, we sent it to the Photon Mono X printer, setting a minimum resolution of 50 µm that matched the size of the laser focal spot. We initiated the printing process and waited approximately 3 h until the parts were ready for post-processing. This meticulous approach is crucial to ensure accurate and reliable results in microfluidic devices, as even minor deviations can lead to significant errors in fluid manipulation.
In the post-processing stage, the first step involves manually removing the supports from the parts using small tweezers. Subsequently, the parts undergo a 10-min centrifugal wash in Form Wash with 99% isopropyl alcohol to eliminate any remaining resin residues, specifically in the cavities. After the wash, we allow the parts to air-dry for 20 min, then curing in the FormCure for 30 min at 60 °C.
Figure 3 depicts the entire process.
3. Results and Discussions
3.1. Flow Simulation
This simulation was set up with the parameters extracted from the literature as mass flow to obtain the final flow value provided by the system.
The flow rate was calculated based on the mean velocity value obtained from the simulation presented in
Figure 2, considering that the internal radius of each microchannel is 754 μm.
Finally, the average flow value was obtained:
Since the program provides the average velocity value, each microchannel obtains a velocity close to that value. Consequently, the resulting parameter must be multiplied by 25 to calculate the total flow rate. The conversion is made to cm
3/s to compare the results with the reference value found in the literature.
The flow rate value is converted to mL in 6 min to compare it with the minimum value of the reported parameter and verify that it allows compliance with the dose-volume relationship.
The value obtained states that it is higher than the required value and allows compliance with the dose-volume relationship [
14,
15], ensuring the correct dosing of the drug. The microvalve and microchannel system delivers a dose of 2.3 mL in 6 min, exceeding the reference value mentioned, which is 0.4 mL in 6 min [
11], and a uniform flow distribution is also evident, which guarantees a correct distribution of the drug to the patient’s skin.
3.2. Microvalve and Microchannel System Fabrication
We fabricated the 3D-printed microvalve and microchannel system using Photon Mono X stereolithography (SLA) technology. We employed a Class 1 resin that underwent evaluation according to ISO 10993-1 standards [
16]. After printing, all parts underwent washing with 99% isopropyl alcohol and subsequent curing under UV radiation in the FormCure at 60 °C for 30 min. The fabricator specified these parameters.
This technique for manufacturing microvalve and microchannel systems constitutes a valuable contribution to fabricating microdosing biodevices. Utilizing SLA printing in biodevice production provides notable advantages, such as cost-effective printers, printing resins, rapid manufacturing times, and the ability to scale at the micrometer level [
17,
18,
19,
20]. These advantages position the technique to contribute to transdermal drug delivery device production substantially.
This research utilized the capabilities of 3D printing technologies to fabricate small structures on the micrometer scale, providing an efficient, reproducible, and accurate method.
Furthermore, incorporating a spiral design, micro reservoirs, and evenly distributed channels enhances the system’s essential characteristics. Among these, the increased drug flow surpasses the minimum threshold required for precise dosing and optimal drug distribution. Consequently, the device achieves uniform insulin delivery, ensuring effective administration.
In the following figures, you can see the results obtained from the manufacturing process of the microchannels with two types of resins.
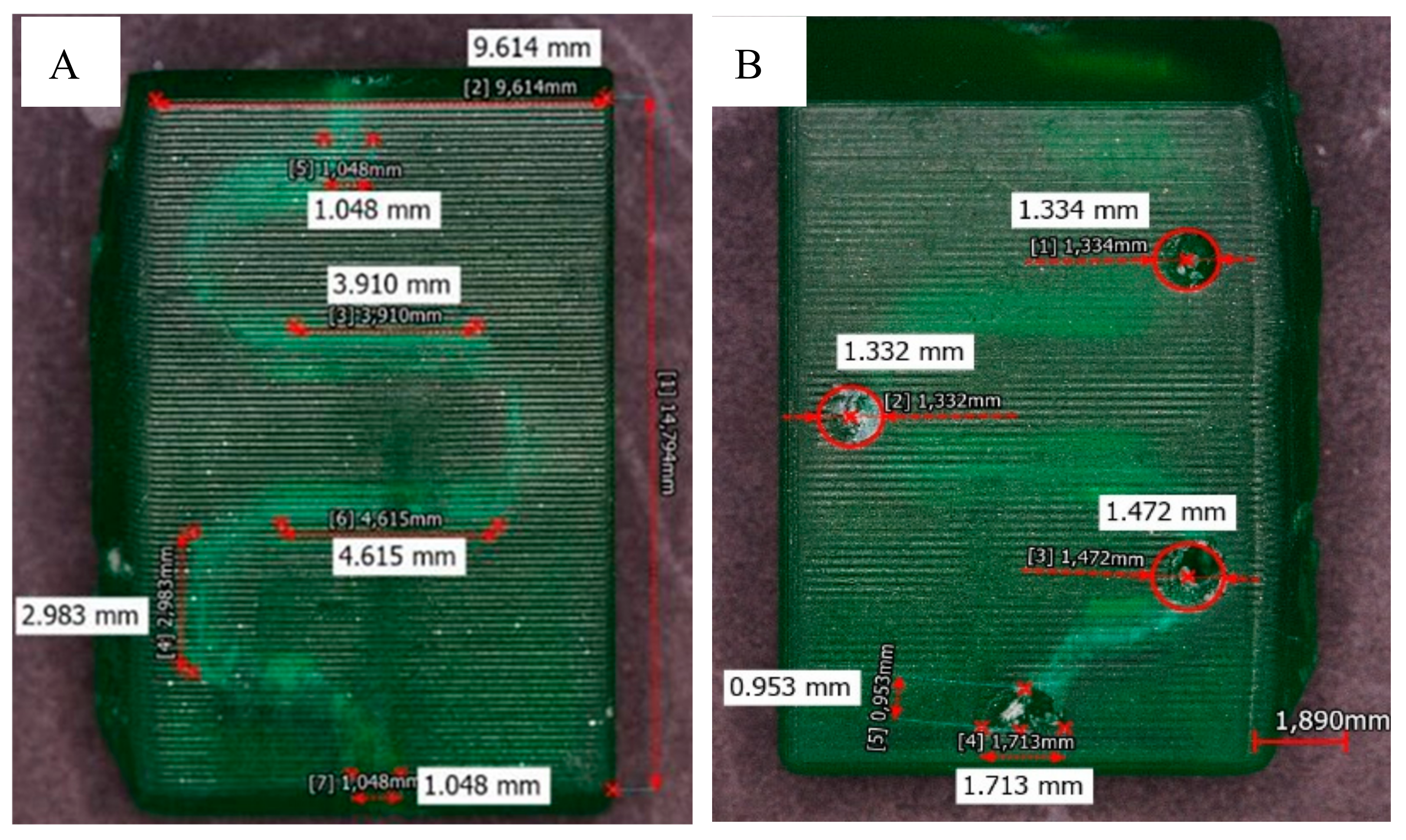
Figure 4A shows the fabricated valve spirals through the translucent resin. The diameters obtained from the channels fabricated by the established methodology are around 1 mm, as seen in
Figure 4B.
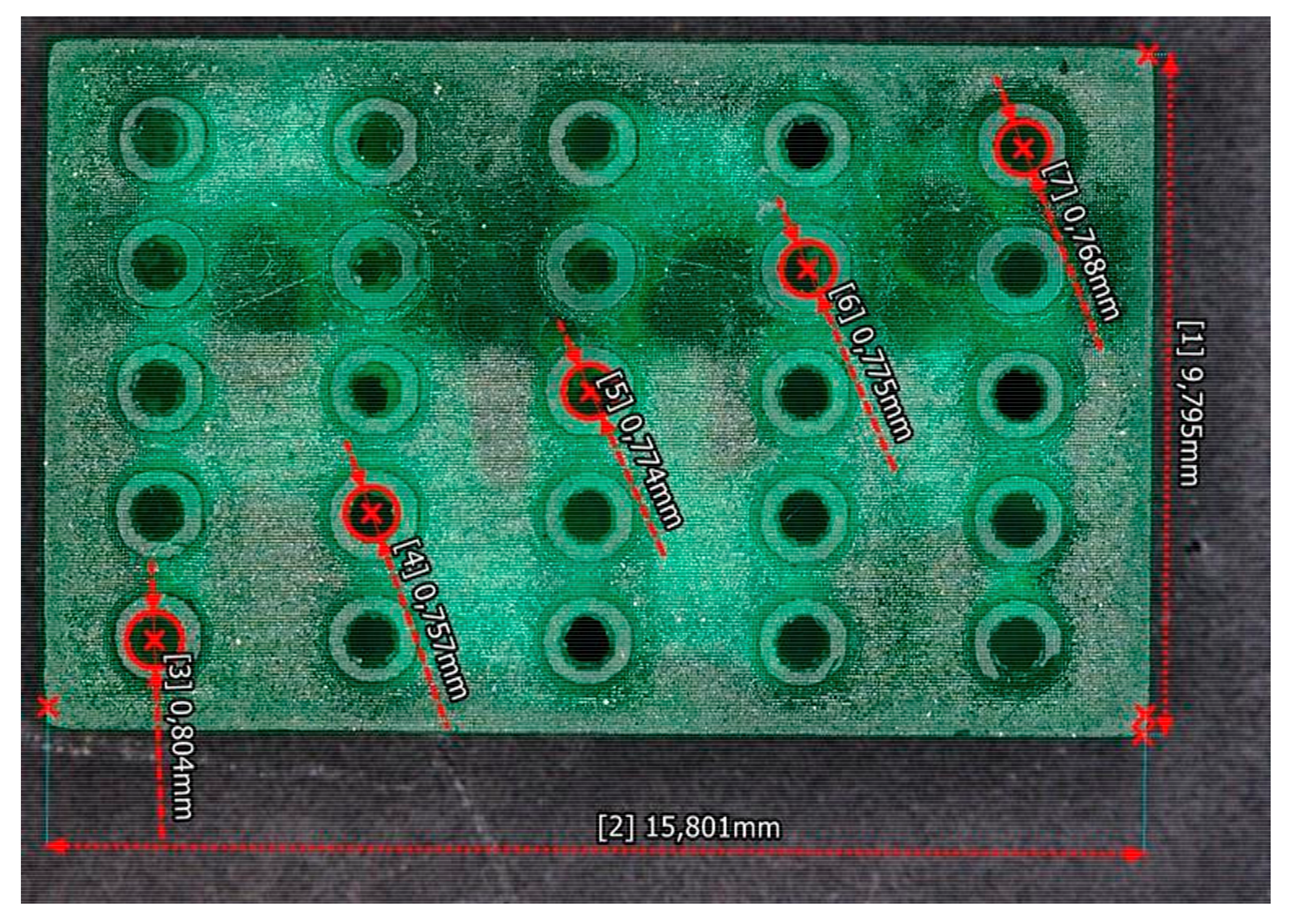
Figure 5 shows the microchannels fabricated through the translucent resin. The diameters obtained from the channels fabricated are around 775 µm.
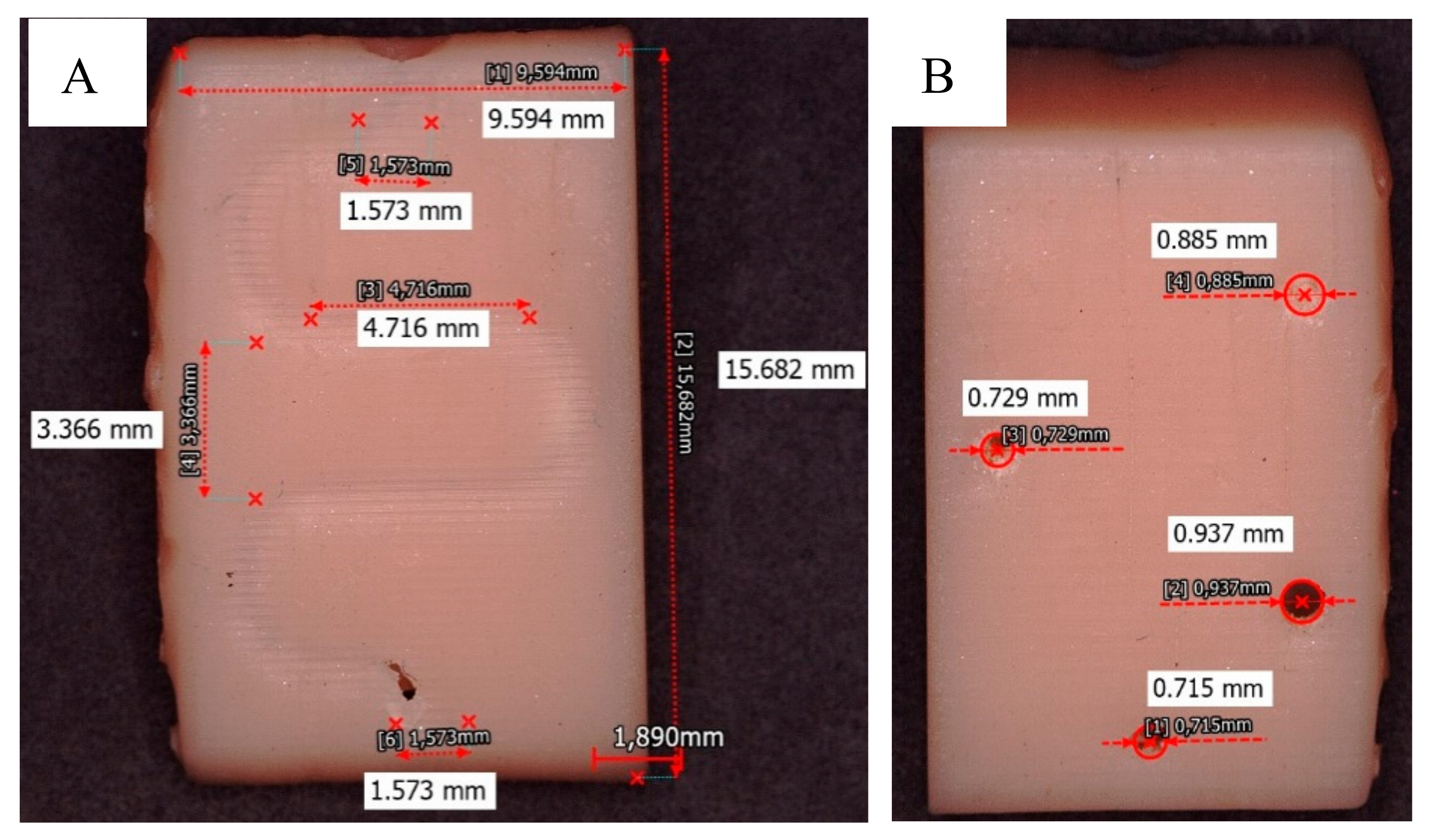
Figure 6 shows the results obtained with the Dental Non-Castable UV resin. The spiral-shaped microvalve was fabricated (see
Figure 6A), but smaller diameters were obtained in the microchannels, ~ 817 µm (see
Figure 6B).
4. Conclusions
The research concludes that the bio device manufactured by additive manufacturing techniques allows an efficient and rapid micro-dosing of insulin, complying with the dosing limit established in the dose-volume ratio (0.4 mL / 6 min), with a registered value of 2.3 mL in 6 min.
The designs of both the microchannels and the microvalve optimize fluid dynamics, thereby guaranteeing uniform distribution. These characteristics render the device generic and potentially suitable for other drug microdosing research endeavors. Nevertheless, it is crucial to investigate the chemical compatibility between the administered substance and the resin employed during manufacturing.
It is worth noting that standard translucent resin is utilized for printing the final components due to the absence of optimal printing conditions for the printer and resin selection. Nevertheless, the Dental Non-Castable UV resin offers excellent properties and does not exhibit toxicity indices when in contact with the human body. Given the right printing conditions, this material can be considered appropriate for the design dimensions of the valve and microchannels.
Author Contributions
Conceptualization, A.C.R.-C., C.A.R.-D. and F.F.; methodology, A.C.R.-C., C.A.R.-D., P.C.C. and F.F.; software, A.C.R.-C. and C.A.R.-D.; validation, A.C.R.-C., C.A.R.-D. and F.F.; formal analysis, A.C.R.-C. and C.A.R.-D.; investigation, A.C.R.-C., C.A.R.-D., L.J.V.-G. and F.F.; data curation, L.J.V.-G. and P.C.C.; writing—original draft preparation, A.C.R.-C. and C.A.R.-D.; writing—review and editing, A.C.R.-C., C.A.R.-D., P.C.C., L.J.V.-G. and F.F.; supervision, F.F.; Project administration, F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to the Universidad Autónoma de Occidente, the institution that supported this research project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nikpour, S.; Mehrdad, N.; Sanjari, M.; Aalaa, M.; Heshmat, R.; Mafinejad, M.K.; Larijani, B.; Nomali, M.; Ghezeljeh, T.N. Challenges of type 2 diabetes mellitus management from the perspective of patients: Conventional content analysis. Interact. J. Med. Res. 2022, 11, e41933. [Google Scholar] [CrossRef] [PubMed]
- Ghazanchaei, E.; Allahbakhshi, K.; Khorasani-Zavareh, D.; Aghazadeh-Attari, J.; Mohebbi, I. Challenges in providing care for patients with chronic diseases during disasters: A qualitative study with focus on diabetes and chronic respiratory diseases in Iran. Tanaffos 2022, 21, 83–101. [Google Scholar]
- Iglesias, M.N. Dificultades en los cuidados de las personas con enfermedades crónicas: Diabetes mellitus tipo 2: Estado de la cuestión. revistaprismasocial.es 2021, 32, 446–475. Available online: https://revistaprismasocial.es/article/view/4081.
- Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Kondapally Seshasai, Gobin, S.R.; Kaptoge, R.S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; A Stehouwer, C.D.; Lewington, S.; Pennells, L.; Thompson, A.; Sattar, N.; White, I.R.; Ray, K.K.; Danesh, J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [CrossRef]
- OMS, Diabetes—OPS/OMS | Organización Panamericana de la Salud. Available online: https://www.paho.org/es/temas/diabetes.
- Ruiz-Ramos, M.; Escolar-Pujolar, A.; Mayoral-Sánchez, E.; Corral-San Laureano, F.; Fernández-Fernández, I. Mellitus diabetes in Spain: Death rates, prevalence, impact, costs and inequalities. Gaceta Sanitaria 2006, 20, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Mendivil, C.O.; Gutiérrez, S.A.; Peláez-Jaramillo, M.J.; NievesBarreto, L.D.; Montaño-Rodríguez, A.; Betancourt-Villamizar, E. Diabetes and associated dietary intake among urban adults: COPEN (Colombian Nutritional Profiles)-a cross-sectional study. BMJ Open 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas Reports| Tenth Edition. Available online: https://diabetesatlas.org/.
- Misso, M.L.; Egberts, K.J.; Page, M.; O’Connor, D.; Shaw, J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst. Rev. 2010, 1, CD005103. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Advances in transdermal insulin delivery. Adv. Drug Deliv. Rev. 2019, 139, 51–70. [Google Scholar] [CrossRef] [PubMed]
- García, J.; Ríos, I.; Fonthal, F. Design and analyses of a transdermal drug delivery device (TD3). Sensors 2019, 19, 5090. [Google Scholar] [CrossRef] [PubMed]
- Villota, I.; Calvo, P.C.; Campo, O.I.; Fonthal, F. Optimization of design and manufacturing parameters of one-plane bevel tipped 3d printed microneedles. In: Marques, J.L.B.; Rodrigues, C.R.; Suzuki, D.O.H.; Marino Neto, J.; García Ojeda, R. (eds) IX Latin American Congress on Biomedical Engineering and XXVIII Brazilian Congress on Biomedical Engineering. CLAIB CBEB 2022 2022. IFMBE Proceedings 2024, 99. [Google Scholar] [CrossRef]
- Economidou, S.N.; Pissinato Pere, C.P.; Okereke, M.; Douroumis, D. Optimisation of design and manufacturing parameters of 3d printed solid microneedles for improved strength, sharpness, and drug delivery. Micromachines 2021, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Roxhed, N.T.; Gasser, C.; Griss, P.; Holzapfel, G.A.; Stemme, G. Penetration-enhanced ultrasharp microneedles and prediction on skin interaction for efficient transdermal drug delivery. J. Microelectromech. Syst. 2007, 16, 1429–1440. [Google Scholar] [CrossRef]
- Sanjay, S.T.; Zhou, W.; Dou, M.; Tavakoli, H.; Ma, L.; Xu, F.; Li, X. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev 2018, 128, 3–28. [Google Scholar] [CrossRef]
- Cambiaghi, A. Biological evaluation of medical devices as an essential part of the risk management process: Updates and challenges of ISO 10993-1: 2018. Eurofins Medical Device Testing. Lancaster, PA, USA (2018).
- Elumalai, A.; Nayak, Y.; Ganapathy, A.K.; Chen, D.; Tappa, K.; Jammalamadaka, U.; Bishop, G.; Ballard, D.H. Reverse engineering and 3d printing of medical devices for drug delivery and drug-embedded anatomic implants. Polymers 2023, 15, 4306. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Q.; Zourob, M. 3D Bioprinting at the frontier of regenerative medicine, pharmaceutical, and food industries. Front Med Technol. 2021, 2, 607648. [Google Scholar] [CrossRef] [PubMed]
- Economidou, S.N.; Pere, C.; Reid, A.; Uddin, M.J.; Windmill, J.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Lantada, A.D.; Morgado, P.L.; Stampfl, J. Additive Manufacturing Technologies for Enhancing the Development Process of Biodevices. In: Lantada, A. (eds) Handbook on Advanced Design and Manufacturing Technologies for Biomedical Devices. 2013. Springer. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).