1. Introduction
Cystic fibrosis (CF) management recognizes the critical role of nutrition. Historically, weight gain has been a primary objective due to the high prevalence of malnutrition and low body weight observed in this patient population [
1]. To address this, the CF Nutrition Guidelines have established recommendations for target nutritional indices based on Body Mass Index (BMI) [
2,
3].
There exists a well-established association between body mass index (BMI) and pulmonary function in CF management. Data consistently collected from various patient registries demonstrate a positive correlation between these two variables. This correlation is typically assessed through percent predicted forced expiratory volume in one second (ppFEV
1). Notably, patients with a BMI at the 50
th percentile exhibit a normal average ppFEV
1. Interestingly, this average ppFEV
1 continues to improve even as BMI increases into the overweight and obese categories [
4,
5]. Furthermore, evidence suggests that early life nutritional status has a long-term impact on pulmonary function within the paediatric CF population [
6]. Conversely, undernutrition remains a significant risk factor associated with poorer pulmonary outcomes, increased colonization with Pseudomonas aeruginosa (Pa), diminished quality of life, and heightened mortality rates [
7].
Clinicians involved in the care and management of patients with CF have made nutrition and weight gain a priority in their quality improvement initiatives. This focus has led to an increase in the median BMI, even surpassing the 50
th percentile in many CF Centres. This trend suggests a substantial proportion of patients with CF now fall into the overweight or obese categories [
8,
9]. This shift in emphasis aligns with the broader trend of rising obesity rates in the general population.
Multiple advancements have contributed to the improved outcomes observed in CF management. These advancements include earlier diagnosis, facilitated by newborn screening programs, the prompt initiation of pancreatic enzyme replacement therapy (PERT), more effective and timely nutritional and medical interventions, and, most notably, the recent introduction of CFTR modulators. The introduction of CFTR modulators, beginning with Ivacaftor in 2012 and culminating in the late 2019 approval of Elexacaftor-Tezacaftor-Ivacaftor (ETI), has revolutionized CF care. CFTR modulators have been incorporated into care programs across several countries and have demonstrated substantial benefits in both nutritional and respiratory outcomes [
10]. Several International initiatives are currently estimating the efficacy and the effects of these drugs through the increasing amount of data included into CF Registries, including the Italian CF Patient’s registry (ICFR).
This paper explores the evolving landscape of nutritional status in Italian people with CF (pwCF), assessing the prevalence of overweight and obese individuals within this population, and also verifying whether malnutrition still remains a problem to be addressed in pwCF.
2. Materials and Methods
We utilized data from the Italian CF Patient’s Registry (ICFR). ICFR collects data from 29 different Italian CF Centres; data are focussed on demography, diagnosis (including new diagnosis per year), genetics, lung function, nutrition, complication, microbiology, transplantation and mortality. Aspects relating to eligibility criteria, data collection, generalizability, and validity of the data have been comprehensively documented [
4].
Inclusion Criteria
To ensure the relevance and integrity of our analysis, the following criteria were applied to the study population:
Individuals with a confirmed diagnosis of CF;
Inclusion in the ICFR for at least one year within the period spanning from 2010 to 2021.
Patients aged two years or older at the time of inclusion.
At least one follow-up visit during the year, with a documented height and weight measurement.
Transplanted patients were included in the study population.
Study Components
Our research encompassed two key components:
Trends Analysis (2010–2021): ICFR collects quality data since 2010. For this study purposes, we conducted an in-depth examination of trends in the nutritional status of the entire CF population aged two years and older. Specifically, we assessed the proportion of individuals falling into underweight, target weight, overweight, and obese categories over the period spanning from 2010 to 2021.
Patient Level Comparison data of the year 2021: Focusing on the year 2021, we compared factors between adults aged 18 and older and children and adolescents aged 2 – <18 years, who were classified as overweight and obese and those who were not. This analysis aimed to identify potential determinants of overweight and obesity within the CF patient population.
Ethical Considerations
This study on anonymous patient data was approved by the Scientific and Steering Committees of the ICFR on June 26, 2023. Data collected within the ICFR and used for this study purposes, are in compliance with the indication and the approval of the Italian National Ethical Committee; informed consent was obtained from eligible individuals or their legal representatives as per the established protocols of the ICFR.
Variables and Data Analysis
Weight Group Classification: To categorize individuals based on their nutritional status, we utilized annualized BMI percentiles or values, depending on the age of the patient. For individuals younger than 18 years, we employed BMI percentiles, while those aged 18 and above were classified based on BMI values in kg/m
2;. The weight group designations were as follows: [
11]
- ο
BMI < 18.5 kg/m2; or < 5th percentile (underweight)
- ο
BMI 18.5–24.9 kg/m2; or 5th - < 85th percentile (target weight)
- ο
BMI 25–29.9 kg/m2; or 85th - < 95th percentile (overweight)
- ο
BMI > 30 kg/m2; or > 95th percentile (obese)
- 2.
Demographic and Clinical Factors: We considered a range of demographic and clinical patient-level factors to gain a comprehensive understanding of the factors associated with nutritional status in CF patients. These factors included:
- ο
Sex
- ο
Age
- ο
Genotype
- ο
Diagnosis by Newborn Screening (NBS)
- ο
Annualized percent predicted forced expiratory volume at one second (ppFEV1)
- ο
Pancreatic status, defined by proxy according to the utilization of pancreatic enzyme replacement therapy (PERT).
- ο
Diabetes status
- ο
Colonization by Pseudomonas aeruginosa (Pa)
- ο
Use of inhaled hypertonic saline (HS) and inhaled antibiotics
- ο
Use of CFTR modulators
Methodology for Weight Group Classification
The assignment of individuals to weight groups was carried out based on their annualized BMI percentile or value, depending on their age. To calculate annualized measures, we used the programs written in SAS language available on the site
https://www.cdc.gov/growthcharts/computer_programs.htm. For individuals aged less than 18 years, weight group classification relied on BMI percentile, while individuals aged 18 or older were categorized based on BMI values in kg/m
2;.
Statistical Analyses
Continuous variables presented as mean ± standard deviation (SD) and categorical variables showed as absolute frequency and percentage.
Symmetry/normality of BMI and BMI percentile was evaluated by Shapiro-Wilk test and checking Q–Q plot.
The associations between BMI and BMI percentile classes with respect to the categorical variables analysed in
Table 1 and 2 were evaluated by X
2 or Cochran-Armitage trend test.
Bubble plots were realised for weight categories rates of paediatric and adult patients from 2010–2021. A p value < 0.05 was considered statistically detectable.
Statistical analysis was performed using SAS version 9.4 TS Level 1 M8 and JMP PRO version 17 (SAS Institute, Cary, NC, USA).
3. Results
In 2021, ICFR patient’s cohort consisted of 3,332 individuals aged 18 years and above and 2,015 aged between 2 and 18 years. The analysis of the CF population over the review period from 2010 to 2021 revealed notable trends in nutritional status. The proportion of patients deemed eligible for this analysis consistently represented a substantial portion, ranging from 94% to 97% of individuals included in the ICFR.
3.1. Nutritional Trends Over Time
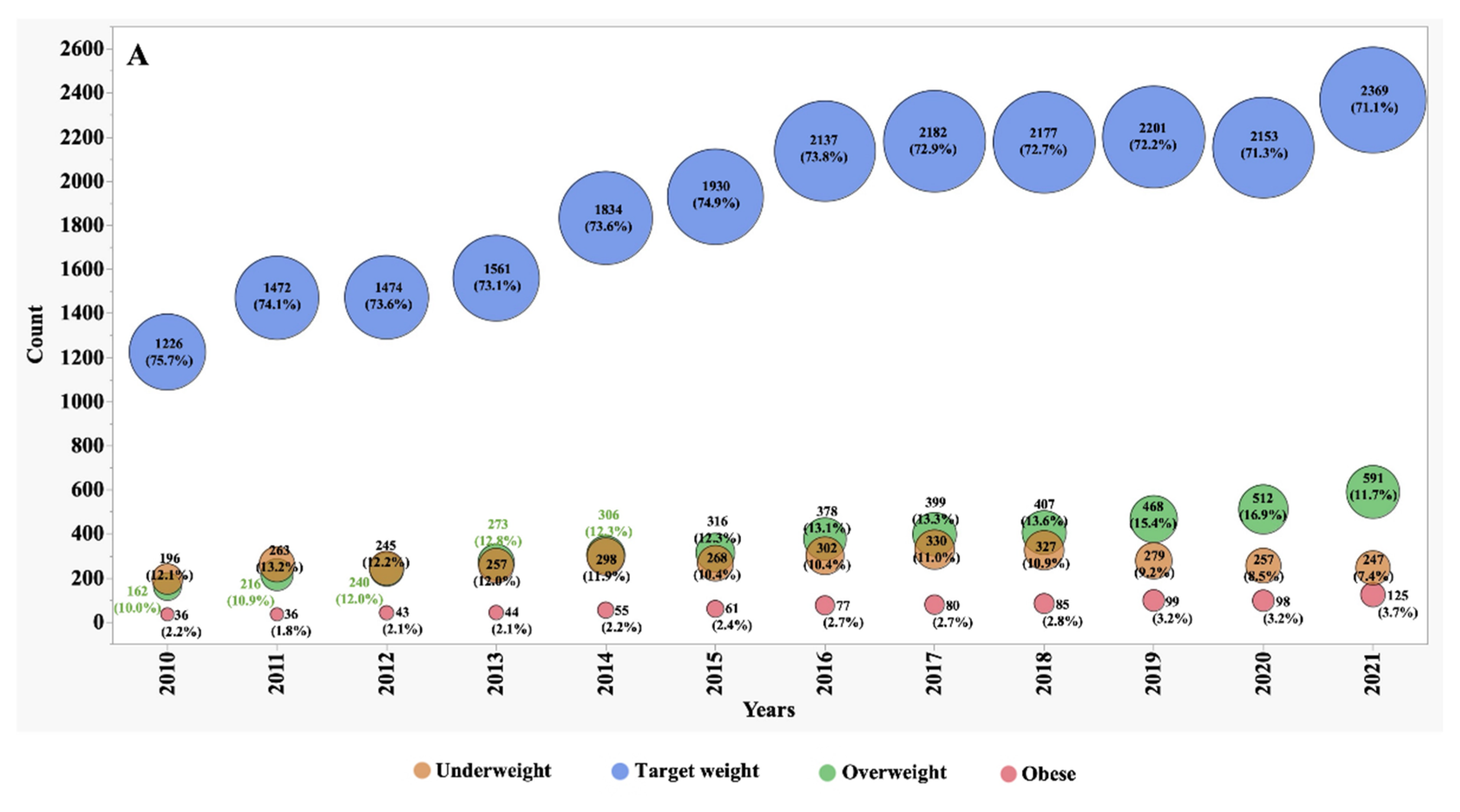
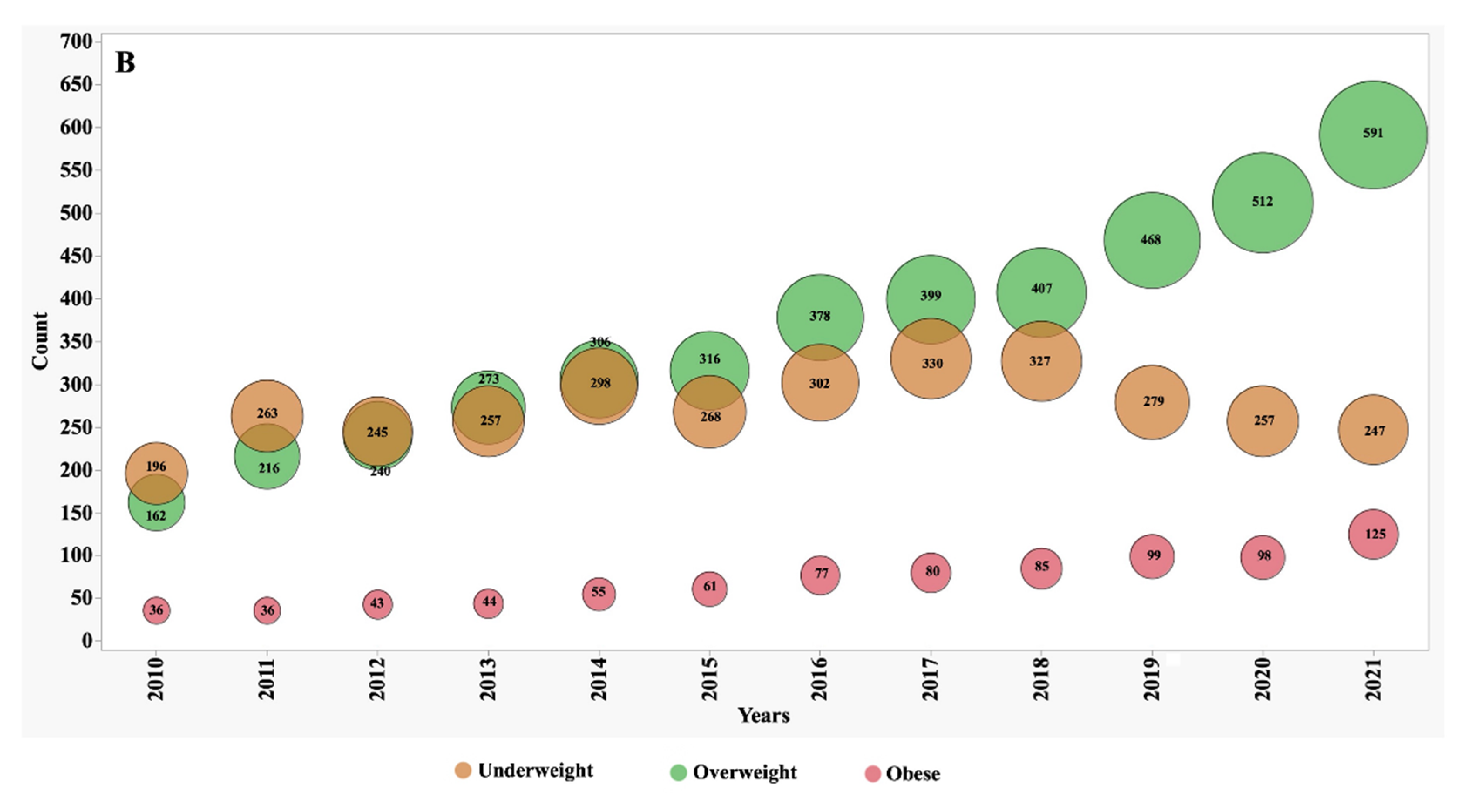
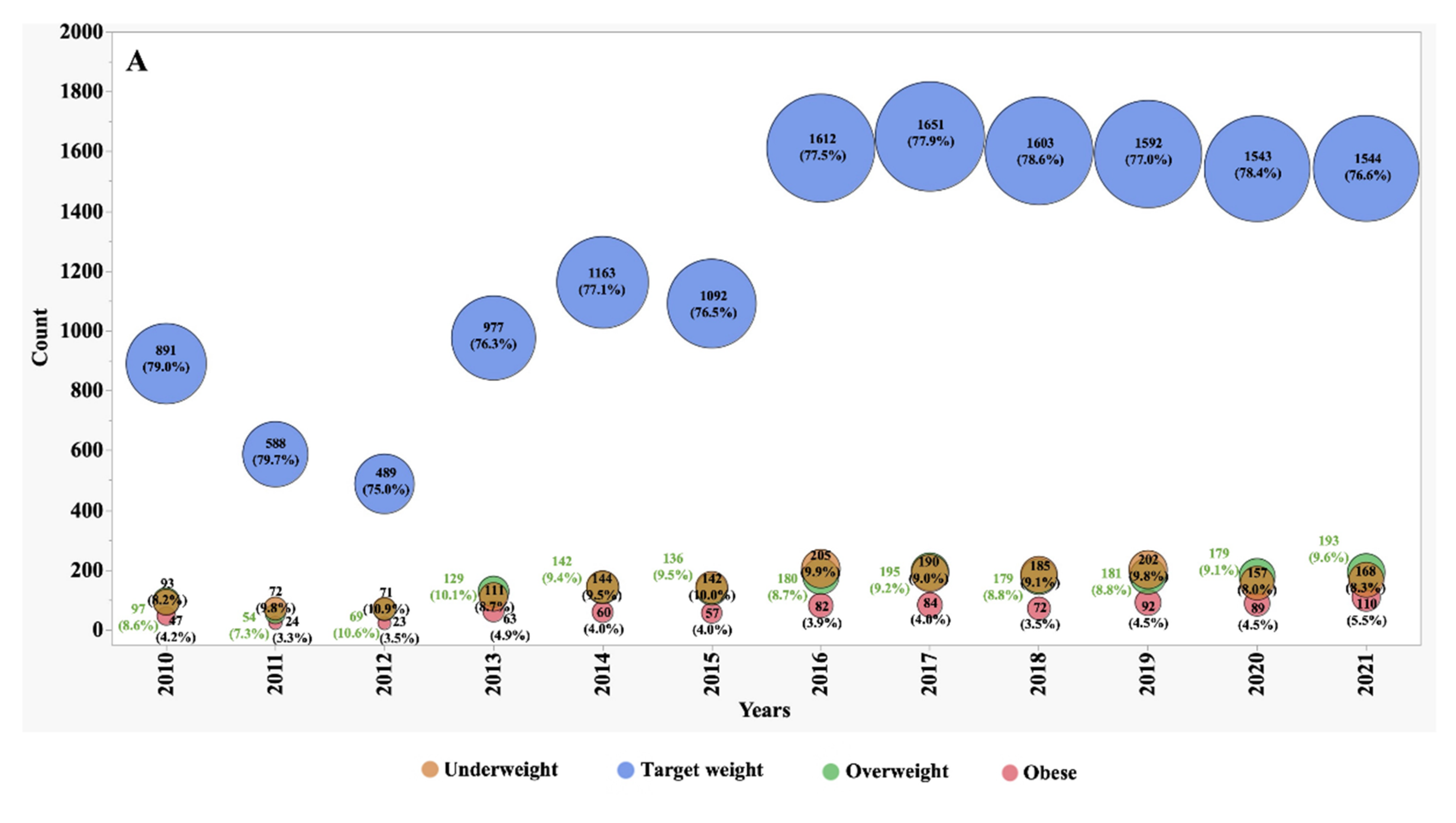
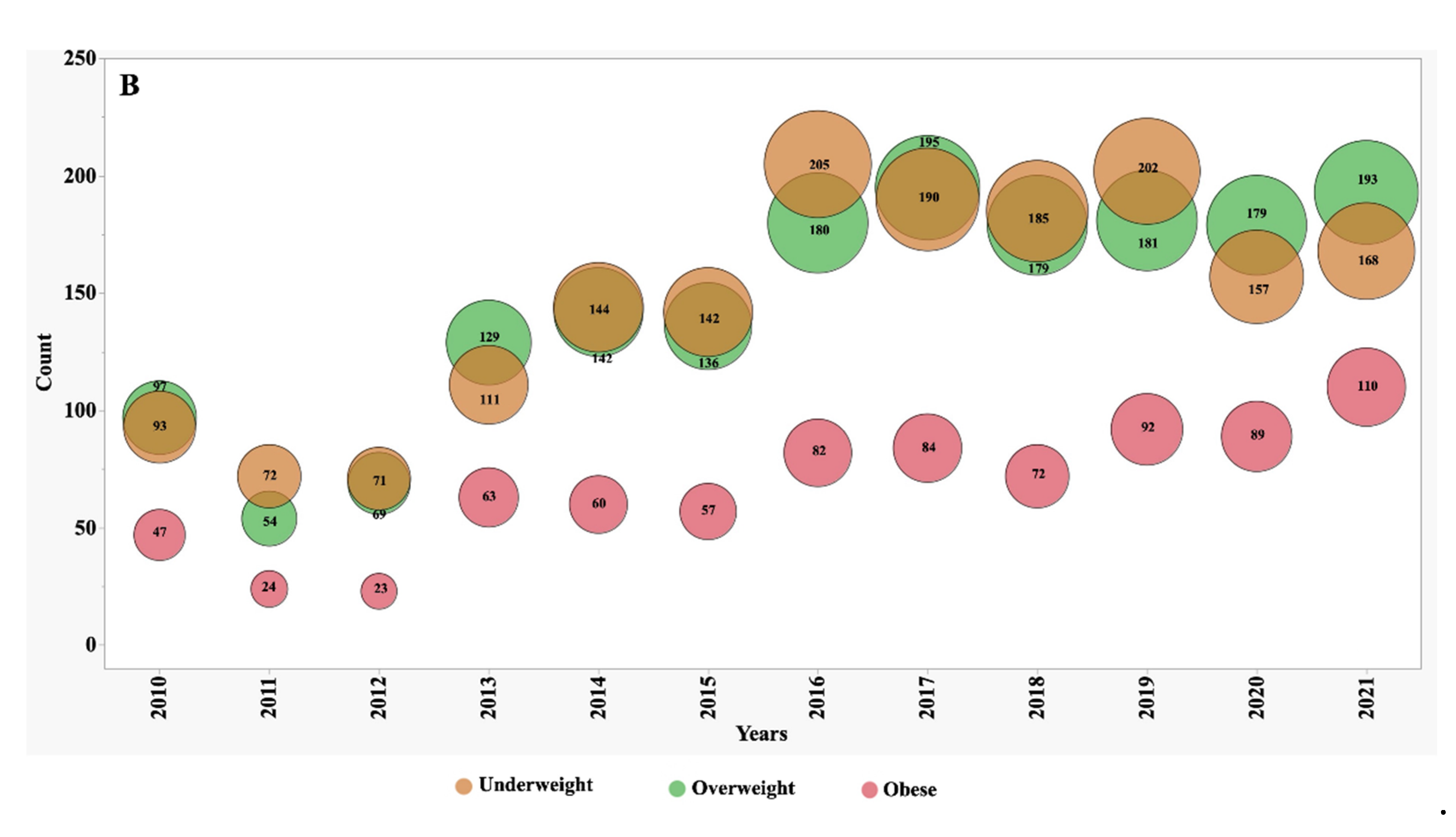
Between the years 2010 and 2021, we observed significant shifts in the numbers, proportions, and frequency of underweight, overweight, and obese patients (
Figure 1 and
Figure 2). In 2010, individuals classified as underweight accounted for 12.1% of the adult CF population and 8.2% of the paediatric subgroup. However, these percentages exhibited distinct patterns of change over the observation period. In the adult CF population, the percentage of underweight individuals, after remaining stable during the initial half of the observation period, markedly decreased to 7.4% by 2021. Conversely, in the paediatric group, the percentage of underweight individuals remained relatively stable, with minor variations, and stood at 8.3% in 2021.
During the first five years of observation, approximately 74% of adult patients fell within the target BMI range. However, this percentage experienced a slight decline to 71% during the subsequent five years. This decline was primarily driven by an increase of over 70% in the number of patients categorized as overweight, surging from 10% to 17%, and over 85% those classified as obese, which rose from 2% to 3.7%.
In contrast, the paediatric subgroup exhibited only minor variations in the proportion of subjects falling within the target BMI z-score, overweight, and obese categories. These nuanced fluctuations suggest that the nutritional trends in the paediatric CF population were relatively stable during the observation period.
3.2. Patient Characteristics Associated with Different Nutritional Status (Patient Level Comparison on data of the year 2021)
Table 1 and
Table 2 offers a comprehensive summary of patient-level factors, categorized by nutritional status. Our analysis revealed statistically significant differences in nutritional status rates across various patient-level factors.
Within the adult population, certain subgroups exhibited significantly higher rates of overweight and obesity. Males showed higher rates, with 21.9% classified as overweight and 4.2% as obese, compared to females who exhibited 13.1% overweight and 3.3% obesity. Furthermore, individuals aged over 35, particularly those aged 45 or older, had overweight rates reaching 23.7% and obesity at 6∙9% (
Table 1). As regards the effect of genotypes, those carrying at least one allele with a CFTR mutation with
residual function (RF) displayed overweight at 24.8% and obesity at 7.5%. In terms of lung function, individuals with a ppFEV
1 exceeding 90% exhibited overweight at 24.1% and obesity at 5.4%. Patients prescribed CFTR modulators displayed a different distribution of weight categories compared to untreated subjects. Patients under treatment had higher rates of individuals within the target weight category (76.6% vs. 65.3% in adults,), along with fewer overweight and obese subjects (overall 16.0% in treated adults vs. 27.1% in untreated, respectively). Those not prescribed PERT exhibited overweight rates of 24.9%, with obesity at 9.4% whereas patients with pancreatic insufficiency exhibited 14.6% overweight and 1.2% obese, respectively. Lastly, rates of overweight and obesity were higher among subjects with lower prevalence of
Pa colonization and lower burden of inhaled treatments (hypertonic saline and antibiotics).
Among younger subjects, subgroups with higher rates of overweight and obesity were characterized by ppFEV
1 exceeding 90%, the absence of PERT, and the presence of
RF CFTR mutations in the genotype (
Table 2). Children also displayed a distinct distribution of weight categories concerning the use of CFTR modulators. Specifically, the target weight category included 80.5% of treated children compared to 75.3% of untreated children. On the other side, fewer overweight and obese subjects were observed in the treated group, with 11.4% of treated children falling into these categories as opposed to 16.3% in the untreated group.
In terms of underweight individuals, within the adult subgroup, associations were found with female sex, age younger than 35, ppFEV1 levels lower than 70%, especially those below 40%, and the presence of CF-related diabetes (CFRD). Conversely, in the younger group, notable factors associated with underweight were ppFEV1 levels lower than 70%, particularly below 40%, and the presence of CFRD.
Table 1.
Characteristics of weight categories in adult patients (age 18 and older).
Table 1.
Characteristics of weight categories in adult patients (age 18 and older).
| Parameter |
Overall
n=3332 |
Underweight
(n=247)
(%=7.41)
n (%) |
Target weight
(n=2369)
(%=71.10)
n (%) |
Overweight
(n=591)
(%=17.74)
n (%) |
Obese
(n=125)
(%=3.75)
n (%) |
p |
| Sex |
|
|
|
|
|
<0.0001 |
| Male
|
1742 |
90 (5.17) |
1197 (68.71) |
382 (21.93) |
73 (4.19) |
|
| Female
|
1590 |
157 (9.87) |
1172 (73.71) |
209 (13.14) |
52 (3.27) |
|
| Age |
|
|
|
|
|
<0.0001 |
| 18 to 35
|
1907 |
184 (9.65) |
1398 (73.31) |
278 (14.58) |
47 (2.46) |
|
| 35 to 45
|
715 |
43 (6.01) |
498 (69.65) |
145 (20.28) |
29 (4.06) |
|
| 45+
|
710 |
20 (2.82) |
473 (66.62) |
168 (23.66) |
49 (6.9) |
|
| ppFEV1 |
|
|
|
|
|
<0.0001 |
| <40
|
230 |
43 (18.7) |
159 (69.13) |
26 (11.3) |
2 (0.87) |
|
| 40 to 70
|
976 |
89 (9.12) |
735 (75.31) |
130 (13.32) |
22 (2.25) |
|
| 70 to 90
|
864 |
56 (6.48) |
632 (73.15) |
143 (16.55) |
33 (3.82) |
|
| 90+
|
1139 |
46 (4.04) |
758 (66.55) |
274 (24.06) |
61 (5.36) |
|
| Inhaled HS |
|
|
|
|
|
0.02 |
| No
|
1833 |
139 (7.58) |
1270 (69.28) |
343 (18.71) |
81 (4.42) |
|
| Yes
|
1496 |
108 (7.22) |
1098 (73.4) |
247 (16.51) |
43 (2.87) |
|
| Inhaled antibiotic |
|
|
|
|
|
<0.0001 |
| No
|
1745 |
113 (6.48) |
1166 (66.82) |
371 (21.26) |
95 (5.44) |
|
| Yes
|
1585 |
134 (8.45) |
1203 (75.9) |
219 (13.82) |
29 (1.83) |
|
| Diabetes |
|
|
|
|
|
<0.0001 |
| No
|
2135 |
137 (6.42) |
1473 (68.99) |
422 (19.77) |
103 (4.82) |
|
| Yes
|
802 |
70 (8.73) |
601 (74.94) |
119 (14.88) |
12 (1.5) |
|
|
Pacolonization
|
|
|
|
|
|
<0.0001 |
| No
|
1529 |
92 (6.02) |
1050 (68.67) |
306 (20.01) |
81 (5.3) |
|
| Yes
|
1299 |
109 (8.39) |
971 (74.75) |
195 (15.01) |
24 (1.85) |
|
| PERT |
|
|
|
|
|
<0.0001 |
| No
|
1012 |
40 (3.95) |
625 (61.76) |
252 (24.9) |
95 (9.39) |
|
| Yes
|
2319 |
207 (8.93) |
1744 (75.2) |
339 (14.62) |
29 (1.25) |
|
| CFTR Modulator |
|
|
|
|
|
<0.0001 |
| Treated
|
1446 |
108 (7.47) |
1109 (76.69) |
210 (14.52) |
19 (1.31) |
|
| Not treated
|
1886 |
139 (7.37) |
1260 (66.81) |
381 (20.20) |
106 (5.62) |
|
| Genotype |
|
|
|
|
|
|
| F/F
|
693 |
61 (8.8) |
536 (77.34) |
92 (13.28) |
4 (0.58) |
<0.0001 |
| F/G
|
62 |
3 (4.84) |
44 (70.97) |
11 (17.74) |
4 (6.45) |
0.62 |
| F/RF
|
312 |
13 (4.17) |
194 (62.18) |
80 (25.64) |
25 (8.01) |
<0.0001 |
| F/MF
|
875 |
70 (8) |
680 (77.71) |
119 (13.6) |
6 (0.69) |
<0.0001 |
| MF/MF
|
365 |
50 (13.7) |
270 (73.97) |
39 (10.68) |
6 (1.64) |
<0.0001 |
| MF/G
|
33 |
4 (12.12) |
20 (60.61) |
8 (24.24) |
1 (3.03) |
0.51 |
| MF/RF
|
221 |
8 (3.62) |
146 (66.06) |
52 (23.53) |
15 (6.79) |
0.001 |
Table 2.
Characteristics of weight categories in paediatric patients (2 - < 18 years).
Table 2.
Characteristics of weight categories in paediatric patients (2 - < 18 years).
| Parameter |
Overall
n=2015 |
Underweight
(n=168)
(%=8.34)
n (%) |
Target weight
(n=1544)
(%=76.63)
n (%) |
Overweight
(n=193)
(%=9.58)
n (%) |
Obese
(n=110)
(%=5.46)
n (%) |
p |
| Sex |
|
|
|
|
|
0.03 |
| Male
|
1000 |
99 (9.90) |
745 (74.50) |
94 (9.40) |
62 (6.20) |
|
| Female
|
1015 |
69 (6.80) |
799 (78.72) |
99 (9.75) |
48 (4.73) |
|
| ppFEV1 |
|
|
|
|
|
<0.0001 |
| <40
|
14 |
7 (50.00) |
7 (50.00) |
0 (0.00) |
0 (0.00) |
|
| 40 to 70
|
104 |
25 (24.04) |
73 (70.19) |
4 (3.85) |
2 (1.92) |
|
| 70 to 90
|
335 |
28 (8.36) |
277 (82.69) |
22 (6.57) |
8 (2.39) |
|
| 90+
|
1082 |
53 (4.9) |
842 (77.82) |
123 (11.37) |
64 (5.91) |
|
| Inhaled HS |
|
|
|
|
|
0.32 |
| No
|
1002 |
81 (8.08) |
757 (75.55) |
108 (10.78) |
56 (5.59) |
|
| Yes
|
1013 |
87 (8.59) |
787 (77.69) |
85 (8.39) |
54 (5.33) |
|
| Inhaled antibiotic |
|
|
|
|
|
0.11 |
| No
|
1521 |
122 (8.02) |
1154 (75.87) |
157 (10.32) |
88 (5.79) |
|
| Yes
|
494 |
46 (9.31) |
390 (78.95) |
36 (7.29) |
22 (4.45) |
|
| Diabetes |
|
|
|
|
|
0.01 |
| No
|
1758 |
141 (8.02) |
1334 (75.88) |
177 (10.07) |
106 (6.03) |
|
| Yes
|
86 |
12 (13.95) |
70 (81.39) |
4 (4.65) |
0 (0.00) |
|
| Pa colonization |
|
|
|
|
|
0.06 |
| No
|
1473 |
112 (7.60) |
1122 (76.17) |
146 (9.91) |
93 (6.31) |
|
| Yes
|
159 |
15 (9.43) |
128 (80.50) |
14 (8.80) |
2 (1.26) |
|
| PERT |
|
|
|
|
|
<0.0001 |
| No
|
690 |
40 (5.80) |
474 (68.70) |
102 (14.78) |
74 (10.72) |
|
| Yes
|
1325 |
128 (9.66) |
1070 (80.75) |
91 (6.87) |
36 (2.72) |
|
| CFTR Modulator |
|
|
|
|
|
0.01 |
| Treated
|
459 |
37 (8.06) |
370 (80.61) |
40 (8.71) |
12 (2.61) |
|
| Not treated
|
1556 |
131 (8.42) |
1174 (75.45) |
153 (9.83) |
98 (6.30) |
|
| Genotype |
|
|
|
|
|
|
| F/F
|
432 |
49 (11.34) |
342 (79.17) |
32 (7.41) |
9 (2.08) |
0.0001 |
| F/G
|
40 |
3 (7.50) |
29 (72.50) |
5 (12.50) |
3 (7.50) |
0.85 |
| F/RF
|
134 |
12 (8.95) |
88 (65.67) |
17 (12.69) |
17 (12.69) |
0.0006 |
| F/MF
|
517 |
42 (8.12) |
421 (81.43) |
36 (6.96) |
18 (3.48) |
0.007 |
| MF/MF
|
228 |
23 (10.09) |
180 (78.95) |
16 (7.02) |
9 (3.95) |
0.27 |
| MF/G
|
19 |
1 (5.26) |
15 (78.95) |
2 (10.53) |
1 (5.26) |
0.97 |
| MF/RF
|
130 |
8 (6.15) |
98 (75.38) |
15 (11.54) |
9 (6.92) |
0.59 |
4. Discussion
Our study reveals a noteworthy and concerning trend: a consistent rise in the prevalence of overweight and obese individuals among the Italian CF adult population over the past decade. However, it is crucial to note that this trend is not extended to the paediatric age group. Despite significant efforts within CF programs to reduce the number and proportion of underweight patients, the most striking shift has been the substantial increase in the number of individuals entering the overweight or obese categories, a pattern not observed among paediatric patients. These divergent trajectories in nutritional status are complex and merit thorough exploration and consideration within the Italian (and international) CF community.
A key finding of our study pertains to patient factors associated with overweight and obesity among individuals with CF. Particularly notable are the so-called
RF CFTR mutations. This observation aligns with prior studies from the ICFR and the European CF Registry (EU CF Registry), which also demonstrated better nutritional status in individuals with
RF mutations compared to those homozygous
for F508del or carrying CFTR
minimal function mutations. The lower prevalence of pancreatic insufficiency, lower sweat chloride levels at diagnosis, and better lung function at all age groups all reflect a relatively milder disease, even though progression of lung disease is still evident [
12,
13].
Adult patients categorized as overweight or obese are more likely to be male, older than 45 years, exhibit pancreatic sufficiency, possess better lung function, and receive treatment with CFTR modulators. Additionally, they are less prone to CFRD and Pa colonization, and have, as a logic consequence, a minor burden of inhaled treatments. Similarly, overweight and obese children were characterized by normal lung function, pancreatic sufficiency, and RF CFTR mutations. These observations suggest a milder overall disease severity in overweight and obese CF patients, consistent with the higher prevalence of less severe CFTR mutations, particularly Class IV–V mutations (i.e., RF mutations).
The association between CFTR modulators use and weight gain merits substantial attention and further investigation. It is worth noting that the impact of the highly efficient recent modulator ETI, commercially available in Italy since July 2021, is not yet fully evaluable in our study. Several proposed mechanisms underlie this association, encompassing changes in exocrine pancreas function, alterations in energy expenditure, shifts in body composition, and variations in intestinal microbiota [
14]. To comprehend these intricate mechanisms and their collective impact on nutritional status, vigilant monitoring of nutritional parameters is essential. Such monitoring will be pivotal in deciphering the precise mechanisms of action and facilitating the provision of tailored guidance and care to patients with CF.
A notable observation in our study is the presence of a sex disparity in the prevalence of overweight and obesity, a phenomenon documented in previous years within the general population in Italy [
15]. Our analysis of Italian pwCF reveals a somewhat analogous pattern. Specifically, we observe that the proportion of obese men exceeds that of obese women within the CF population. This sex-specific variation underscores the importance of considering sex as a potential modifier of nutritional status in CF. Our findings align with recent data from the US, Canadian, and German CF Registries, where comparable trends of increasing overweight and obese status have been documented [
5,
16,
17]. Simultaneously, there has been a noticeable decline in the prevalence of individuals classified as having normal weight or being underweight. However, it is crucial to acknowledge that European CF Registry data indicate divergent outcomes across various European nations [
18]. These regional disparities underscore the complex interplay of factors influencing nutritional status in CF patients, including genetics, healthcare practices, access to therapies, and cultural or environmental influences.
Additionally, we emphasize that the observed nutritional improvements seem largely limited to the adult patient population. Data from the paediatric subgroup of our CF population indicate that in the last decade, the relative percentages of underweight, target, overweight, and obese children remained relatively stable. However, it is important to note that the eligibility for ETI for children aged 12 and above, and subsequently for children aged 6–11, was obtained in Italy in July 2021 and September 2022, respectively. Therefore, only 43.4% of the adult subgroup and 22.8% of the paediatric subgroup were treated by CFTR modulators at the time of our analysis; so we probably cannot yet observe the effects of the highly efficient modulators. In Germany, one year of treatment with ETI reduced the percentage of underweight children from 28.9% to 17.9%, suggesting a significant impact in the near future [
17]. Nonetheless, ensuring adequate nutrition for children with CF remains a priority.
In this complex landscape characterized by a wide and dynamic spectrum of nutritional conditions, new challenges are emerging for CF care teams. Considering that obesity, dyslipidaemia, aging, and hyper caloric diets, enriched in fat, sugar, and salt, are well-established risk factors for cardiovascular disease (CVD), it is prudent to anticipate a potential increase in CVD cases among persons with CF in the coming years, particularly in those who are pancreatic sufficient [
19]. Consequently, it is imperative that future investigations, including case studies, observational research, and longitudinal studies, are conducted within this specific patient cohort to substantiate this hypothesis. There is also the need to integrate the data collection of the CF registers with information on the appearance of further adult complications, such as hypertension, or coronary artery disease [
20]. On the other hand, it is mandatory to maintain measures to improve nutrient intake and digestion and to adopt the appropriate nutritional intervention to paediatric pwCF where undernutrition is even now evident. The push towards improvement of nutrition from CFTR modulators in younger patients will be investigated in the next years.
5. Conclusions
In conclusion, our study underscores and confirms the evolving scenery of nutritional status in the Italian adult CF population, with a pronounced shift towards overweight and obesity over the past decade. These trends, which are likely to extend to younger CF patients in the near future, necessitate proactive measures within CF standards of care to adapt and address the changing needs of patients [
21,
22]. The association between obesity and milder CFTR mutations, along with the intriguing link between CFTR modulator use and weight gain, enriches our understanding of CF pathophysiology. Future research endeavours should focus on elucidating the mechanisms underlying these observations to enhance the management and care of individuals living with CF.
Author Contributions
Conceptualization, D.S., R.P. and M.S.; methodology, D.S. and G.C..; validation, A.A and G.C.; formal analysis, D.S., R.P. and G.C.; investigation, D.S. and R.P..; resources, M.S..; data curation, A.A. and G.C; writing—original draft preparation, D.S.; writing—review and editing, D.S., R.P., A.A., M.S. AND G.C..; visualization, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, on anonymous patient data and was approved by the Scientific and Steering Committees of the ICFR on June 26, 2023. Data collected within the ICFR and used for this study purposes, are in compliance with the indication and the approval of the Italian National Ethical Committee.
Informed Consent Statement
Informed consent was obtained from all subjects or their legal representatives involved in the study.
Acknowledgments
We would like to thank Lega Italiana Fibrosi Cistica – ONLUS (Rome, Italy) for supporting the activity of the Italian CF Registry. We would like to thank all of the other members of the Italian CF Registry Working group: M. Ambroni (CF Centre Cesena), R. Badolato (CF Centre Brescia), E. Bignamini (CF Centre Turin), F.A. Blasi (CF Centre Milan), M. Caloiero (CF Centre Lamezia Terme), V. Carnovale (Adult CF Centre Naples), C. Castellani (CF Centre Genoa), G. Cimino (CF Centre Rome), M. Cipolli (CF Centre (Verona), R. Cutrera ((CF Centre Rome-Hospital Bambino Gesù), B. Fabrizzi (CF Centre Ancona), S. Falorni (CF Centre Grosseto), F. Ficili (CF Centre Palermo), P.Giordano (CF Centre Bari), S. Leonardi (CF Centre Catania), M.C. Lucanto (CF Centre Messina), M. Maschio (CF Centre Trieste), B. Messore (Adult CF Centre Orbassano), N. Palladino (CF Centre Gubbio), G. Pisi (CF Centre Parma), S. Quinti (CF Centre Livorno), V. Raia (CF Centre Naples), P. Ripani (CF Centre Atri), G. Taccetti (CF Centre Florence), P. Vitullo (CF Centre Cerignola).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Culhane, S.; George, C.; Pearo, B.; Spoede, E. Malnutrition in cystic fibrosis: a review. Nutr Clin Pract 2013, 28, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Wilschanski, M.; Munck, A.; Carrion, E.; Cipolli, M.; Collins, S.; Colombo, C.; Declercq, D.; Hatziagorou, E.; Hulst, J.; Kalnins, D.; Katsagoni, C.N.; Mainz, J.G.; Ribes-Koninckx, C.; Smith, C.; Smith, T.; Van Biervliet, S.; Chourdakis, M. ESPEN-ESPGHAN-ECFS guideline on nutrition care for cystic fibrosis. Clin Nutr 2024, 43, 413–445. [Google Scholar] [CrossRef] [PubMed]
- Stallings, V.A.; Stark, L.J.; Robinson, K.A.; Feranchak, A.P.; Quinton, H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc 2008, 108, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Campagna, G.; Amato, A.; Majo, F.; Ferrari, G.; Quattrucci, S.; Padoan, R.; Floridia, G.; Salvatore, D.; Carnovale, V. , Puppo Fornaro, G.; Taruscio, D.; Salvatore, M. Rapporto 2019-2020 [Italian Cystic Fibrosis Registry (ICFR). Report 2019-2020]. Epidemiol Prev 2022, 46, 1–38. [Google Scholar] [PubMed]
- Cystic Fibrosis Foundation Patient Registry 2021 Annual Data Report Bethesda, Maryland ©2022 Cystic Fibrosis Foundation.
- Macdougall, A.; Jarvis, D.; Keogh, R.H.; Bowerman, C.; Bilton, D.; Davies, G.; Carr, S.B.; Stanojevic, S. Trajectories of early growth and subsequent lung function in cystic fibrosis: An observational study using UK and Canadian registry data. J Cyst Fibros 2023, 22, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, G.; Wiedemann, B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax 2002, 57, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Szentpetery, S.; Fernandez, G.S.; Schechter, M.S.; Jain, R.; Flume, P.A.; Fink, A.K. Obesity in cystic fibrosis: prevalence, trends and associated factors data from the US cystic fibrosis foundation patient registry. J Cyst Fibros 2022, 21, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, A.; Aliberti, S.; Contarini, M.; Savi, D.; Sotgiu, G.; Majo, F.; Saderi, L.; Lucidi, V.; Amati, F.; Pappalettera, M.; Palange, P.; Blasi, F. Overweight and obesity in adults with cystic fibrosis: An Italian multicenter cohort study. J Cyst Fibros 2022, 21, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Cousar, J.L.; Robinson, P.D.; Shteinberg, M.; Downey, D.G. CFTR modulator therapy: transforming the landscape of clinical care in cystic fibrosis. Lancet, 2023; 402, 1171–1184. [Google Scholar]
- Body Mass Index (BMI). Centers for Disease Control and Prevention. CDC 2022. Available on line: https://www.cdc.gov/ healthyweight/assessing/bmi/ index.html(accessed on 11 October 2023).
- Salvatore, D.; Padoan, R.; Buzzetti, R.; Amato, A.; Giordani, B.; Ferrari, G.; Majo, F. Patients with cystic fibrosis having a residual function mutation: Data from the Italian registry. Pediatr Pulmonol 2019, 54, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Mei Zahav, M.; Orenti, A.; Jung, A.; Hatziagorou, E.; Olesen, H.V.; Kerem, E. Disease severity of people with cystic fibrosis carrying residual function mutations: Data from the ECFS Patient Registry. J Cyst Fibros 2023, 22, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Bass, R.; Brownell, J. N, Stallings V.A. The impact of highly effective CFTR modulators on growth and nutrition status. Nutrients 2021, 13, 2907. [Google Scholar] [CrossRef] [PubMed]
- Masocco, M.; Minardia, V.; Contoli, B.; Minelli, G.; Manno, V.; Cobellis, L.; Greco, D. Inequality in overweight and obesity among adults in Italy: trends and geographic disparities with a focus on Campania Region. Boll Epidemiol Naz 2023, 4, 1–8. [Google Scholar]
- Cystic Fibrosis Canada. The Canadian Cystic Fibrosis Registry 2021 Annual Data Report. Toronto, Canada, 2023.
- Sutharsan, S.; Dillenhoefer, S.; Welsner, M.; Stehling, F.; Brinkmann, F.; Burkhart, M.; Ellemunter, H.; Dittrich, AM.; Smaczny, C.; Eickmeier, O.; Kappler, M.; Schwarz, C.; Sieber, S.; Naehrig, S.; Naehrlich, L. Impact of elexacaftor/tezacaftor/ivacaftor on lung function, nutritional status, pulmonary exacerbation frequency and sweat chloride in people with cystic fibrosis: real-world evidence from the German CF Registry. Lancet Reg Health Eur 2023, 32, 100690. [Google Scholar] [CrossRef] [PubMed]
- Zolin,A.; Orenti, A.; Jung, A.; van Rens, J. ECFSPR Annual Report 2021; 2023.
- Duckers, J.; Fitzgerald, R.; Proud, D.; Addy, C.; Datta, D. Forewarned is forearmed: The cardiovascular time bomb in Cystic Fibrosis. J Cyst Fibros 2022, 21, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Saunders, T.; Burgner, D.; Ranganathan, S. Identifying and preventing cardiovascular disease in patients with cystic fibrosis. Nat Cardiovasc Res 2022, 1, 187–188. [Google Scholar] [CrossRef]
- McDonald, C.M.; Alvarez, J.A.; Bailey, J.; Bowser, E.K.; Farnham, K.; Mangus, M.; Padula, L.; Porco, K.; Rozga, M. 2020 Cystic Fibrosis Evidence Analysis Center Evidence-Based Nutrition Practice Guideline. J Acad Nutr Diet 2021, 121, 1591–1636. [Google Scholar] [CrossRef] [PubMed]
- Southern, K.W.; Addy, C.; Bell, S.; Bevan, A.; Borawska, U.; Brown, C.; Burgel, PR.; Button, B.; Castellani, C.; Chansard, A.; et al. Standards for the care of people with cystic fibrosis; establishing and maintaining health. J Cyst Fibros, 2024; 23, 12–28. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).