1. Justification for Hypothesis
In the UK during winter/spring 2020, about 50% of SARS-2-infected subjects were non-symptomatic, the proportion rising to about 75% in the summer 2020. Of course some patients did have symptoms but usually comparable to patients with influenza, maybe needing home bed-rest but then resolving within about 7 days. Although there have been in vitro studies indicating a link between the virus and clot formation (eg activation of platelets), I give weight to the clinical data – patients do not need hospital care during the initial SARS-2 replication phase. This was well illustrated by our Prime Minister – he had mild symptoms for about 7 days. He was sent to hospital “as a precaution”. A day later, he was transferred to intensive care unit (ICU) “as a precaution” - within hours, he was on oxygen therapy. The sudden onset of life-threatening disease, late after infection, needs an explanation.
It is well known that influenza-IgG-antibody-complex consists of many virions bound together by the antibody. It seems to be accepted that corona viruses similarly form large complexes. To my knowledge, my innovation is to propose that SARS-2, being a large virus and having many spike proteins, can form such large clumps of virus-IgG-antibody complexes that these clumps can be sufficiently large to block micro-arteries (see
Table 1 and
Table 2). Micro-arteries are lined with epithelial cells which have the ACE2 receptor for coronaviruses. Although there is an apparent lack of free virus in these micro-arteries, that may be a result of the efficient binding of virus to the SARS-2 receptors on the epithelial cells, leading to infection of those cells. With rising levels of IgG antibodies, clumps would form, the complexed antibody would bind to complement which would assist the binding of platelets. The addition of platelets would form a long-lasting clot which would cause tissue death. I suggest that aspirin, through its inactivation of platelets, would prevent the formation of stable clots and enable the excess antibodies to disperse the virion-antibody clumps.
I conclude that there are more than enough SARS-2 virions and IgG anti-bodies to create many large clumps which could thereby block multiple micro-arteries.
The report on the first 20 children (see abstract and ref. [
1] focused my attention on the moment of sero-conversion. Early in the pandemic, it was clear that hospitalised patients had micro-clots but that anti-viral and anti-coagulant therapies gave essentially no benefit. This convinced me that the moment of sero-conversion was the critical period.
2. To Be Useful, a Hypothesis Should Be Testable, Provide Explanations and Give Predictions
Our hypothesis is easily testable, preparing stocks of virus in cell culture, titrating with a known stock of SARS-CoV-2 antibodies and measuring the sizes of the complexes.
The period of risk for micro-clot formation is short, being restricted to the period when there are equally high concentrations of both the SARS-2 virus and IgG antibody which, together, are needed to form the virion-antibody complex. After sero-conversion, our hypothesis predicts that the virion-IgG-antibody concentrations would be too low to create clumps large enough to cause micro-clots. Because serious disease follows sero-conversion and mini-clot formation, neither antiviral nor anti-coagulant drugs can be expected to be of much use in hospital patients.
The symptoms seen during the virus replication phase and those during sero-conversion, causing micro-clot formation, are likely to differ because the viral moieties differ. Although the former phase may have mild or no symptoms, the latter phase can cause such serious symptoms the patients need immediate hospital care, often intensive care. New variants illustrated this apparent mis-match in pathogenicity (see Page 4) [
3].
Hospital patients are well-known to have tissue damage due to micro-clots at multiple sites. It has been suggested that long-covid may be a different clinical disorder because no tissue damage is detectable. I have thought that long-covid, being so clinically variable, is a milder form of the same disease but caused by “nano-clots”, too small to cause detectable damage. The recent Oxford study proves that “no detectable tissue damage” does not equate to “no tissue damage” (see Page 5) [
4].
Long-covid, especially those cases without detectable tissue damage, has been widely likened to post-viral fatigue syndrome (PVFS), which is chronic fatigue syndrome (CFS) after a viral infection. I suggest that this similarity is due to an infection with an endemic coronavirus, perhaps OC43 because its antibodies cross-protect against SARS-1 (see Page 5) [
4].
My hypothesis suggests that anti-coagulant therapy starting before sero-conversion, may prevent long-covid and reduce hospitalisations. Being so widely available worldwide and in the home, aspirin therapy is a clear option to be considered (see Page 7) [
6].
Vaccinations have been hugely beneficial in restraining this pandemic. Unfortunately, the AstraZeneca vaccine (and other similar products) have been associated with deaths, albeit very rare (about 2/million vaccines). Although it is hard to establish a link, the rarity of the cause of death (micro-clots with low platelet counts) supports a link. How can micro-clots form when the platelet count is low? (See Page 6) [
5]
In a few patients recovering from a prolonged covid illness, a PCR test has been positive several months after symptom onset. Although such late positive tests have been assumed to represent a non-infectious fragment of a virion, there has been no explanation for such test results. If a large clump of virion-antibody complex initiates a blood clot, it is reasonable to think that this complex would be enclosed by the clot and protected by it. Months later during recovery, the clot may be dispersing just as a sample is being taken for a PCR test. Being such a sensitive test, that could give a positive result.
3. Apparent Mis-Match in Pathogenicity
Alpha, delta and omicron variants were shown to be more transmissible than previous strains (
Table 3). Because many of the most transmissible viruses spread silently, I expected each new variant should have more symptom-free days during virus replication, including an increased proportion of non-symptomatic infections. Thus the increase in transmissibility would not only be due to the changes to SARS-2 biochemistry (perhaps the spike protein) but also to the longer periods for silent transmission. Because the alpha and delta variants appeared before there was widespread immunity, the data can show which part of the virus replication cycle, virus replication or sero-conversion, has either increased or decreased pathogenicity.
Initially, the rapid spread of variant B.1.1.7 (later named alpha) indicated that this strain was more transmissible than previous strains. There was a wide expectation, perhaps hope, that new more transmissible strains would be steps towards a less pathogenic endemic coronavirus. The higher transmission rate was soon confirmed [
4]. Later, the same group reported on the increased mortality (about 50%) following infection with alpha [
5]. I quote the start of the first two paragraphs in the Discussion:
We previously found that B.1.1.7 is substantially more transmissible than pre-existing SARS-CoV-2 variants, but could not robustly identify any associated change in disease severity using population-level analysis of early data.
We do not identify any mechanism for the increased mortality here. Infections with the B.1.1.7 variant are associated with higher viral concentrations in nasopharyngeal swabs, as measured by Ct values using PCR testing (Extended Data Figure 6). Higher viral load could therefore be partly responsible for the observed increase in mortality.
My hypothesis explains the apparent mis-match in pathogenicity; the higher viral loads are not directly responsible for the increased mortality, but these higher viral loads will produce higher concentrations of the virus-IgG-antibody complexes, in turn producing larger clumps, more blocking of micro-arteries, more clots, leading to more deaths.
A cohort study was done among patients comparing alpha and delta infections, which were confirmed by whole-genome sequencing, with data being linked to hospital admission and mortality [
6]. Patients with delta had more than two times the risk of hospital admission compared with patients with alpha. This study did not assess the severity of the infection during the early, virus replication, phase of the infection, but, within the discussion, the authors said:
“Furthermore, the estimated HRs were similar for patients who were asymptomatic at the time the specimen was taken”. It is likely that some of these patients remained non-symptomatic yet those with delta had increased risk of hospitalisation and death. Such cases remind me of the children [
1] who had no symptoms before suddenly becoming seriously ill.
4. Long-Covid and Post-Viral Fatigue Syndrome (PVFS)
I start with the ideas (see page 3) [
2]:
The period of risk for micro-clot formation is short and associated with the time of sero-conversion.
During virus replication and during sero-conversion, symptoms will differ.
Long-covid is a milder form of the disease in hospital patients but caused by “nano-clots”, too small to cause detectable damage.
The short period of risk was illustrated by the report on the first 20 children needing urgent hospital care [
1].
The Kings/Zoe covid group reported that there are many covid symptoms which appear within 7 days of infection, but shortness of breath usually occurs a week after initial infection and is associated with having a more serious case of COVID-19 and needing hospital support. [
7]
A new technique can make “undetectable” damage visible.
In May 2021, a collaboration of Sheffield and Oxford Universities reported on the use of a new technique to examine patients continuing to suffer breathlessness at least 3 months after hospital discharge [
8]. At the same time that a CT scan was normal, MRI scans using Xenon showed marked reductions of lung function compared with normal controls. Recently (2nd February 2022) this group posted their findings on a small group of long-covid subjects who had never been hospitalised but had continuing breathlessness in spite of normal CT scans[
9]. The authors’ summary statement:
Hyperpolarized Xenon MRI and TLcoa demonstrate significantly impaired gas transfer in non-hospitalised long-COVID patients when all other investigations are normal. [a.
TLco refers to the Transfer capacity of the Lung, for the uptake of Carbon Monoxide, a frequently used test but CO distribution differs from that of O
2]
My conclusion, we should assume that long-covid is caused by nano-clots which lead to tissue damage – until proven otherwise.
Long-covid, especially those cases without detectable tissue damage, has been widely likened to post-viral fatigue syndrome (PVFS) (see page 3). I suggest that this similarity is due to an infection with an endemic coronavirus, perhaps OC43 because its antibodies cross-react in antibody tests and cross-protect in cell culture against SARS-1 infection (see page 3). Also, we know that the antibodies to at least one of the endemic coronaviruses cross-protects against SARS-2. The Francis Crick Institute reported full cross-protection by historic antibodies, dating from 2011 to 2018, in SARS-CoV-2-infected cell cultures [
10]. Because the historic antibodies can cross-protect against SARS-2, it is likely that those historic antibodies are able to bind SARS-2 into large clumps. If so, then my hypothesis suggests that an endemic-virion-antibody complex can cause nano-clots. Therefore, could PVFS be caused by an endemic coronavirus, perhaps OC43?
For those medics with long memories, PVFS is a “recent” medical problem. It may be a historical coincidence that CFS/PVFS was becoming widely recognised (late 1980s) soon after paracetamol replaced aspirin as the therapy of choice for respiratory infections in the 1980s. Alternatively, while aspirin was the drug of choice for treating the symptoms of virus infections, was PVFS being prevented? At that time, episodic low-dose (75 or 150mg) aspirin therapy was considered to have a good safety profile. Whereas aspirin inhibits platelets, paracetamol does not – inadvertently, the switch to paracetamol may have enabled a clump of coronavirus-anti-body-complex to start a nano-clot, thereby initiating PVFS.
In the USA, single assays, with multi-virus detection and bedside results within about one hour, are available [see a summary in the ICAR meeting report [
11]]. If the use of such assays were more widespread, expanding into other countries such as the UK, a connection between PVFS and a particular virus may have been discovered in a more timely fashion.
5. Rare Clots after Vaccinations
To provide an independent view of the Oxford/AstraZeneca vaccine, I have chosen to quote from the EMA press conference, 7th April 2021 [12]. Although EMA confirmed overall benefit-risk remained positive, the statement continues: “EMA’s safety committee (PRAC) had concluded today that unusual blood clots with low blood platelets should be listed as very rare side effects of Vaxzevria (formerly COVID-19 Vaccine AstraZeneca). EMA is reminding healthcare professionals and people receiving the vaccine to remain aware of the possibility of very rare cases of blood clots combined with low levels of blood platelets occurring within 2 weeks of vaccination. So far, most of the cases reported have occurred in women under 60 years of age within 2 weeks of vaccination. Based on the currently available evidence, specific risk factors have not been confirmed.”
Of 86 reported cases (to 22nd March 2021) 18 were fatal, with about 25M people having had the vaccine.
Although it has been hard to prove that this vaccine (and similar vaccines) may cause death, the combination of blood clots with low blood platelets is so very rare, the association has been widely accepted.
In contrast, systemic side-effects are common. The Kings/Zoe Covid Group reported [13]: “people who have had a previous COVID-19 infection are almost twice as likely to experience one or more mild whole body (systemic) after effects compared to people who didn’t have COVID-19 (33% vs 19%) from a Pfizer/BioNTech vaccine dose.” Clearly, it should be noted that some vaccinees may have had covid unknowingly – there may be a greater difference. Most systemic symptoms appeared in the first two days after the vaccination. This result indicates to me that the sudden production of spike protein can combine with pre-existing antibodies to give systemic symptoms. That raises the question – is it possible that a person with exceptionally high antibody levels can bind to the burst of spike protein to form a clump, comparable to virus-antibody clumps?
Using the data in
Table 1 and repeating the calculation in
Table 2, 10
9 spike trimers could form a clump sufficiently large to block a micro-artery. Although I have not found any data on the number of spike trimers produced by vaccination, I note that 10
9 spike trimers would be present in 10
7 virions, not a large number of virions.
Therefore, my explanation is that exceptionally high pre-existing antibody concentrations can combine with the surge of spike trimer produced within a few days to give many large clumps, leading to so many micro-clots that the platelet levels are reduced. Because platelets take about 2 weeks to re-populate, the platelet levels do not have time to recover. This rare side effect seems to confirm that platelets are involved in micro-clot formation.
It must be shattering for families when a healthy person dies within about 2 weeks of vaccination. Naturally, the BBC gave wide coverage when an award-winning BBC radio presenter died in hospital after suffering blood clots having received the AstraZeneca vaccine. Her devastated family said that Lisa Shaw, who worked for BBC Newcastle, developed “severe” headaches a week after having the jab and fell seriously ill a few days later. The 44-year-old died at the Royal Victoria Infirmary, Newcastle, on Friday afternoon [21st May 2021] having been treated in intensive care for blood clots and bleeding.
Not only do I have huge sympathy for the families, I can well understand such news deterring people from accepting vaccination. If my explanation for the formation of clots is correct, then inactivation of platelets may prevent clots forming. Aspirin therapy seems to be the obvious choice, being readily available worldwide and in the home (see page 7). It seems important to give people a safety net following vaccination.
6. Aspirin Therapy
I have hinted that aspirin therapy may be useful to prevent long-covid and reduce hospitalisations (see page 3) and to avoid complications after vaccinations (see page 6). Also, while aspirin was the drug of choice for home-treatment of respiratory viruses, PVFS seemed not to occur. Is there any evidence to indicate that aspirin therapy may be useful for covid?
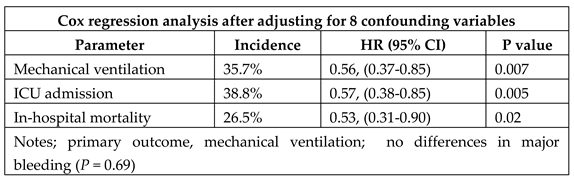
My hypothesis indicates that treatment must start before sero-conversion. A hospital PCR positive test may not indicate replicating virus. I know of only one report which included patients starting aspirin therapy before hospital admission – even in this retrospective study in Maryland, USA, only 74 of 98 patients had aspirin before admission [14]. Nevertheless, aspirin use was independently associated with decreased risk for all three parameters (
Table 4).
This retrospective study revealed nearly 50% lower mortality in hospitalised covid patients, many of whom had routinely been taking aspirin. I have not seen a corresponding prospective study which could assess the proportion of patients needing hospital care following aspirin therapy starting soon after infection and continuing through the period of sero-conversion.
Low-dose aspirin has long been used as a mild anti-coagulant and its safety profile is well known. Current virus-control strategies (vaccines or anti-viral drugs) exert evolutionary pressure on the virus, resulting in the selection of variants resistant to that therapy. Aspirin therapy, having no direct effect on viral replication, would not exert evolutionary pressure on the virus and therefore retain its efficacy with all variants. If the benefit of aspirin therapy was proven, being widely available throughout the world, it could be quickly adopted by the healthy working-age population. As long as the most vulnerable population is vaccinated, aspirin could protect the healthy younger population from long-covid, PVFS and reduce the need for hospitalisations, encouraging a return to a near-normal economy. Although vaccines have a central role to play, there will always be a need for aspirin to help those individuals not fully protected by vaccination.
Prophylactic aspirin (75 or 150mg daily with food) would be a novel therapy. Each household should have aspirin ready for immediate use because the need may arise unexpectedly – a non-symptomatic SARS-2 infection can be followed by sero-conversion with sudden onset of nano-clots and serious illness. Clinical indications for aspirin therapy within the community:
The whole household with a confirmed index case.
Having typical covid symptoms.
Suddenly feeling frightenly ill (take aspirin immediately).
At the first signs of post-vaccine systemic symptoms.
It would be prudent to start early after infection and continue for about two weeks in order to ensure that the period of sero-conversion is covered. That would allow time for the antibodies to stop viral replication and enable the patient to recover normally. Early in the pandemic, the viral replication stage was often about 7 days. With more people having immunity, either through vaccination or prior infection, the time to sero-conversion would be much reduced. Also, the viral loads are likely to be low, but take aspirin if there are symptoms.
I am not advocating chronic aspirin use. I am aware that high-dose chronic aspirin therapy has safety issues but this generation of medics fear to use aspirin - that perception of its safely should be re-examined.
I had thought that the efficacy of aspirin would need to be tested clinically. Placebo-controlled trials, with vulnerable patients, raise difficult ethical issues. Placebo would be avoided if aspirin and remdesivir (RDV) were evaluated together, using viral load and blood parameters as endpoints and having individual and combination arms. All subjects have potentially life-saving therapy and each drug has a suitable control group.
I now think that a clinical trial is not necessary because the data has been prospectively collected by the Kings College/Zoe covid team (15). In spring 2020, this team invited people to download their app and report any symptoms – a year later, over 4.7 M people had joined. When registering for the app, one was asked to give current medication, included on the list, low and high dose aspirin. I have emailed several times asking the covid team to mine their data-base, but with no response. I ask that ISAR members send an email – if enough do so, we may get a result.
7. Conclusions
Hypothesis: after SARS-2 infection, micro-clots are formed only during sero-conversion.This concept implies that the viral-replication stage is relatively benign but the micro-clots cause tissue damage which is both serious and long-lasting.
New variants tend to support this concept, that virus and virus-antibody complexes are two different viral entities and therefore cause differing symptoms.
The virus-IgG-antibody complex forms clumps large enough to block micro-arteries and initiates micro-clot formation.
During the virus replication stage and after sero-conversion, the levels of virus-IgG-antibody complex are too low to initiate clot formation. The period for clot formation is very short, just a couple of days.
Because micro-clots cause illness serious enough for hospitalisation, anti-viral and anti-coagulant therapies are ineffective during hospital care
Serious illness, long-covid and PVFS represent parts of the same spectrum, caused by micro-clots and nano-clots respectively. Likewise, post-vaccination side effects, from lethal to mild, may be opposite ends of one spectrum in which symptoms may be due to spike-trimer-antibody complexes.
Platelet levels may be markedly depleted during multiple clot formation
Low dose aspirin, with well-known efficacy (to inactivate platelets) and safety profile, is a good candidate for preventing the serious consequences of SARS-2 infections. Its wide availability, around the world and in the home, may open the door to transform SARS-2 into a relatively benign endemic virus.
Please email covidteam@joinzoe.com to ask that team to examine their prospectively gathered information on aspirin – ask that they look at their data-base, choosing 3 groups, regular users of aspirin, regular users of another anti-coagulant, and all others. The outcomes are: uneventful recovery, long-covid (with 4, 8 and 12 week sub-groups), hospitalisation and death.
I like the way these many aspects and observations have come together to form one consistent picture
8. Questions for ISAR Members
What is the right question? For example: are viruses living organisms? What organisms are the most highly evolved? Answer the 1st question and one is looking in the wrong direction. The 2nd question leads one to respect the ability of Wuhan, Alpha, Delta and Omicron strains of SARS-2 to spread around the world within 2 years. In contrast, we humans still have less than 10 billion living copies of our genes.
A question for myself: Why am I apparently the first to propose the ideas in this poster? Mainly, being retired, I have time to ponder. Also, preparing the ICAR reports for a decade has given me a deeper understanding of many key ICAR lectures– that has given me a broad knowledge base.
Had these ideas been discussed two years ago, aspirin, once again, may have become the drug of choice for home use. How would the pandemic scene have been changed?
SARS-2 antibody tests: Is there an antibody test which has been evaluated using samples from PCR positive subjects with full range of symptoms, including non-symptomatic infections? In early 2020, vast number of tests were rejected due to “false positives” – surely “false positives” should have been tested for cross protection (see data from Francis Crick Institute).
Are we aware of the limitations of the Abbott and Roche tests? Both were evaluated using hospital samples, both had the test threshold set high to gain specificity but lack sensitivity. Abbott test measures only IgG, Roche, IgA, IgM and IgG, the latter being unsuitable to estimate duration of antibody response.
Raj Kalkeri and I could not have our manuscript published, even on a preprint site, because we were advocating an untested therapy for SARS-2. How can one have a more balanced approach?
Where do we go from here? Working members have to give priority to their “day” jobs. Please could ISAR consider setting up a forum for retired members to ponder on various SARS-2 issues?
9. Addendum 1 (Added 2022-10-05)
Background
By considering a single case of a couple (H & A), in which the time course seems to be typical, I am using it to illustrate my ideas about the underlying biology at each stage of this case. Following our (my wife and I) advice, the couple took low dose (75mg) aspirin once daily for 14 days from first positive test. I wish to emphasise that a single case does not prove that this aspirin therapy is effective although even a single case resulting in long-covid would cause me concern. This account is derived from notes taken the Saturday after the couple had recovered (2021-02-20).
H & A regularly care for their young grandchildren. He is diabetic, she is on regular steroid therapy for her asthma, and both are slightly overweight. He had his 60th birthday during this infection. They were well aware that they were at high risk for long-covid. Our suggested course of aspirin therapy for 14 days is to ensure that the period of sero-conversion is covered.
Time Course
Day 0, Saturday 6th February, they were informed that the grandchildren and parents had tested positive for Covid. Infection was probably within the previous few days.
Day 1, Sunday, A feels ill.
Day 2, Monday, A tests positive. H feels ill but tests negative.
Day 4, Wednesday, H tests positive.
For 2/3 days, both have typical common-cold symptoms, including feeling dizzy, H also has a headache. They feel ill enough to need home bed-rest for 2/3 days.
Day 5, both start to feel better but ill enough to be restricted to light duties.
Day 6, both still on light duties.
Day 7 & 8, both suddenly feel very ill, described as “wiped out, in bed, hardly able to have a cup of tea”.
Day 9, both feeling much better, able to do light duties.
Day 10 & next few days, returning to normal, on grandchildren duties by the end of the week.
Under-Lying Biology
Days 0 to 4, SARS-2 virus replicating.
Days 5 & 6, IgA and IgM antibodies limit but do not control virus replication.
Day 7, IgG antibodies levels rising. When the levels of virus and IgG antibodies are similar, it is known that large clumps of virus-IgG-antibodies are formed. My hypothesis proposes that these clumps become large enough to block many micro-arteries (see my ICAR poster above). The sudden “wiped out” illness, which was much worse than the initial symptoms, can be explained by the blockage of many micro-arteries. Aspirin therapy would not prevent this step. The SARS-2 virus within a clump would probably not be infectious. In the absence of aspirin therapy, first complement and then platelets would bind to the clumps to form long-lasting clots (maybe months or years) (see ICAR Poster above). I expect this clot-formation process is inhibited by aspirin therapy.
Day 8, IgG antibody levels continue to rise. Because H & A had been taking aspirin throughout this period, excess IgG antibodies will disperse the virus-IgG-antibody clumps by coating each virus particle. This clinical case indicates that a 2-day period covers the whole process of virus-IgG-antibody clump formation, micro-arteries becoming blocked, clump dispersal by excess IgG antibody and restoration of blood flow through the micro-arteries. I expect that the 2-day period is short enough to avoid the death of tissue which, being so small, is likely to have some benefit by diffusion of oxygen from neighbouring tissues.
Day 9 onwards, full recovery.
With this couple, aspirin therapy was prophylatic, prior to sero-conversion, albeit after SARS-2 infection.
10. Conclusion
This account, of a single couple’s SARS-2 infection, indicates that the window of opportunity for aspirin therapy is strictly limited – it must start before sero-conversion. There have been many clinical trials of aspirin in hospital patients but even when therapy is started on day of entry, it is too late. Patients become ill enough to require hospital care only after sero-conversion when many micro-arteries are blocked by long-lasting clots. Please note that the PCR test may still give positive results after seroconversion but before the body had removed the virus-antibody complexes.
Prior to the 1980s when aspirin was the drug of choice for treating respiratory infections at home, aspirin was often taken for only a few days to treat the early respiratory symptoms. Because aspirin irreversibly inactivates platelets and because it takes about 2 weeks to repopulate platelets, even a short course of aspirin (commonly 300mg daily dose) may have been sufficient to inhibit long-lasting clot formation and PVFS.
Although one clinical case does not prove anything, I think that the evidence for the efficacy of aspirin, and perhaps other anticoagulants, remains hidden in the Kings/Zoe data base.
Please be aware that paracetamol does not inactivate platelets.
11. Addendum 2 (2023-03-15)
When preparing the ICAR 2022 poster, I was unaware that Pretorius was looking for micro-clots in long-covid patients. In my poster (page 3, section 4), I gave my justification for assuming micro-clots caused long-covid:
Hospital patients are well-known to have tissue damage due to micro-clots at multiple sites. It has been suggested that long-covid may be a different clinical disorder because no tissue damage is detectable. I have thought that long-covid, being so clinically variable, is a milder form of the same disease but caused by “nano-clots”, too small to cause detectable damage. The recent Oxford study proves that “no detectable tissue damage” does not equate to “no tissue damage.”
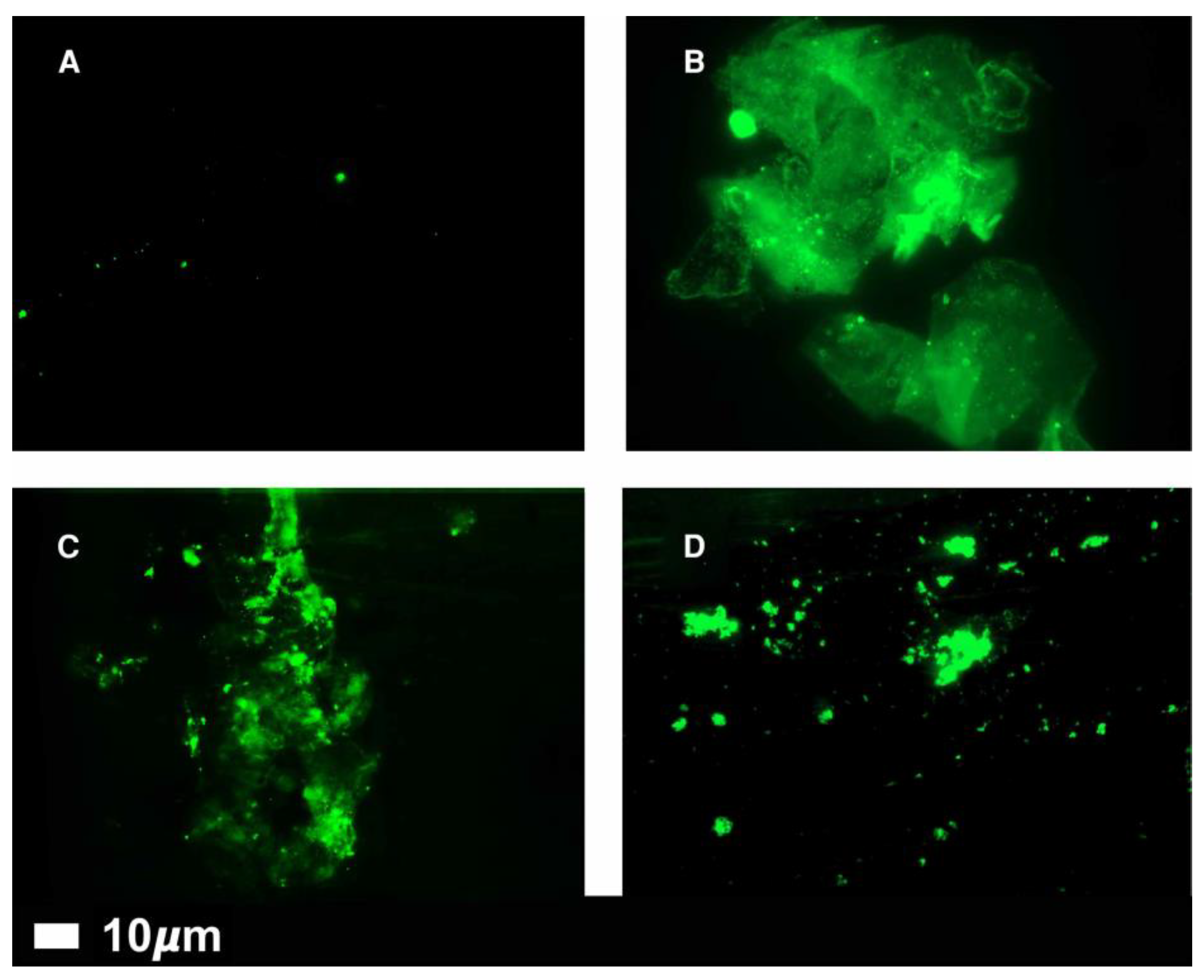
The 2022 review in Nature attracted my attention – for information, I have included a summary (see below). This review highlights the discovery, by Pretorius, of micro-clots circulating in all of 80 long-covid patients but essentially none in healthy controls. These micro-clots can be close to 10 μM in size (see Fig 4 below). There are comments from others who question the role of these micro-clots in causing clinical disease. In part, I agree but I consider these mico-clots to be an indication that, during their formation, slightly larger micro-clots would have been formed, these being large enough to block micro-arteries (capillaries) as proposed in my poster. These larger micro-clots would quickly disappear from circulation.
The review also mentions that long-covid and chronic fatigue syndrome are similar (see my poster, p3, 5) and that spike protein, when added to plasma from healthy volunteers, prompted the formation of abnormal clots. In my poster (page 6), I suggest that vaccination produces large amounts of spike protein which could bind to pre-existing antibody to form clumps. Pretorius does not mention the possibility of having antibody, very likely by 2021.
Also, I have copied
Figure 4 (see below) from a 2022 review by Pretorius: Fluorescence microscopy of representative micrographs showing micro-clots in the circulation of controls (A) and in patients with Long COVID (B–D).
From patients, some dense-looking clots are close to 10μM size. These micrographs support my hypothesis that clots >10μM diameter can be produced, that these larger clots would block micro-arteries and, hence, be removed from circulation.
The Mystery in Micro-Clots
Tiny blood clots might explain some of long COVID’s puzzling array of symptoms. But many researchers worry about people seeking unproven therapies.
By Cassandra Willyard
Nature, Vol 608, 25 August 2022, Pages 662-664
This well-written summary describes the work of Etheresia Pretorius (Stellenbosch University in South Africa) and Douglas Kell (University of Liverpool, UK), who discovered micro-clots in the blood of people with long COVID. They had developed an assay using a fluorescence dye to visualise micro-clots in patients with diabetes or various other inflammatory diseases. With the start of the Covid pandemic in 2020, it seemed natural for this team to study covid patients. They had plasma samples from healthy volunteers (n = 13), those with diabetes (n=10), those with covid (n=15) and those with long-covid (n=11). This study showed that both acute and long-covid patients had far higher levels of circulating micro-clots than had been seen previously in those with diabetes and essentially none in healthy volunteers. Then they tested 80 long-covid patients – all of them had micro-clots.
To the date of this review, the group, led by Pretorius and Kell, had published the only studies on micro-clots in patients with long-covid. However, Caroline Dalton (Sheffield Hallam University’s Biomolecular Sciences Research Centre, UK) has used a quantitative version of the assay to study three groups (about 25/group), people who had never knowingly had covid, those who had covid and recovered, and people with long-covid. Dalton confirmed that all three groups had micro-clots, being larger and more numerous in long-covid patients. “This team’s hypothesis is that SARS-CoV-2 infection creates a burst of micro-clots that go away over time. In individuals with long COVID, however, they seem to persist.” My hypothesis not only reflects theirs, but also explains how and when there is a burst of micro-clot formation.
This review notes the similarity between long-covid and chronic fatigue syndrome (CFS) and reports the comments of Maureen Hanson (Cornell University, USA). A 1980s hypothesis about abnormal clots causing long-term symptoms is similar to the ideas arising from Pretorius and Kell’s work.
The review ends with a short paragraph, titled “Micro-clot mysteries”, starting with the sentence “Where these micro-clots come from isn’t entirely clear”. But Pretorius and Kell have found that clots are formed when SARS-2 spike protein is added to plasma from healthy subjects.
Review Article
A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications
Douglas B. Kell, Gert Jacobus Laubscher and Etheresia Pretorius
Biochemical Journal (2022) 479 537–559 ttps://doi.org/10.1042/BCJ20220016; open access.
Figure 4.
Fluorescence microscopy of representative micrographs showing microclots in the circulation of controls (A) and in patients with Long COVID (B–D).
Figure 4.
Fluorescence microscopy of representative micrographs showing microclots in the circulation of controls (A) and in patients with Long COVID (B–D).
Figure 4.
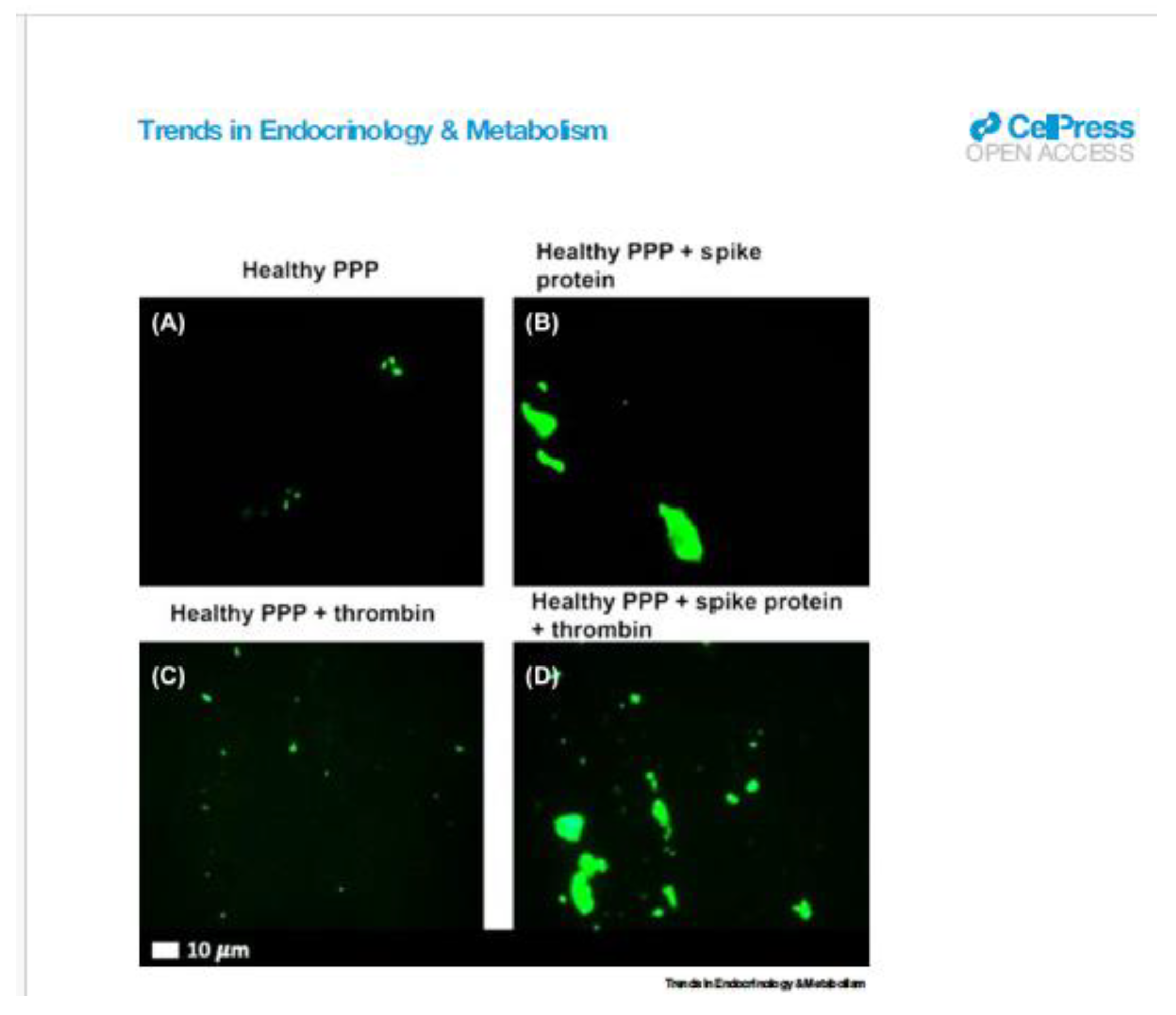
Representative fluorescence micrographs of platelet-poor plasma (PPP) from healthy individuals after addition of Thioflavin T (green fluorescent signal). (A) PPP smear. (B) PPP with spike protein. (C) PPP with thrombin to create extensive fibrin clot. (D) PPP exposed to spike protein followed by addition of thrombin. Reproduced from [
10]. Reference within this article:10. Grobbelaar, L.M. et al. (2021) SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci. Rep. 41, BSR20210611 11.
Figure 4.
Representative fluorescence micrographs of platelet-poor plasma (PPP) from healthy individuals after addition of Thioflavin T (green fluorescent signal). (A) PPP smear. (B) PPP with spike protein. (C) PPP with thrombin to create extensive fibrin clot. (D) PPP exposed to spike protein followed by addition of thrombin. Reproduced from [
10]. Reference within this article:10. Grobbelaar, L.M. et al. (2021) SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci. Rep. 41, BSR20210611 11.
12. Addendum 3 (2024-04-02)
In addendum 2 [
11], I described the work of Pretorius & Kell who discovered micro-clots circulating in all of 80 long-covid patients but essentially none in healthy controls. In the micrographs, the solid-looking micro-clots are generally well below 10 µm and so are too small to block micro-arteries (capillaries). Whereas this discovery does not prove that larger micro-clots are formed at the point of seroconversion and therefore removed from circulation by being trapped by micro-arteries, it gives strong support for my hypothesis.
At the time I was preparing my ICAR Poster, I was able to quote “EMA’s safety committee (PRAC) had concluded today that unusual blood clots with low blood platelets should be listed as very rare side effects of Vaxzevria (formerly COVID-19 Vaccine AstraZeneca).”I assumed that, similarly to virions, SARS-2 spike protein and IgG-antibody could form clumps large enough (≥10µm) to block micro-arteries.
Here, I include a micrograph (B) showing micro-clots, larger than 10 µm, are formed when SARS-2 spike protein is added to platelet-poor plasma (PPP) from healthy subjects. By 2021, I expect that most samples of plasma from healthy subjects would contain IgG-antibodies to the SARS-2 spike protein, either from natural infection or after vaccination.
I conclude that this data supports my explanation for rare deaths and common systemic symptoms after vaccination. Also, this work strongly supports my idea that virion-IgG-antibodies form clumps with size at least as large (over 10 µm) as those formed with spike protein.
In vitro, I suggest that SARS-2-IgG-antibodies and spike protein will form clumps which self-disperse on adding excess antibody.
A Review Article
Feature Review Long COVID: pathophysiological factors and abnormalities of coagulation
Simone Turner,1 M. Asad Khan,2 David Putrino,3 Ashley Woodcock,4,5 Douglas B. Kell , 1,6,7,8,* and Etheresia Pretorius 1,6,9,*(see reference below).
Acknowledgments
I thank my wife, Helen, (a retired GP) for being the first to suggest to me that aspirin therapy may be useful. Also my thanks to Raj Kalkeri (formerly at Southern Research, Frederick, MD, USA) whose support was essential for preparation of the original (unpublished) manuscript of which he was a co-author. Also, I thank those ISAR colleagues, notably Lisa Ryan, who gave me useful comments during the 2022 ICAR at Seattle.
References
- Shelley Riphagen, Xabier Gomez, Carmen Gonzalez-Martinez, Nick Wilkinson, Paraskevi Theocharis. 2020. Hyperinflammatory shock in children during COVID-19 pandemic, The Lancet, 395, ISSN 0140-6736. [CrossRef]
- SARS-CoV-2 (COVID-19) by the numbers. Yinon M. Bar-On, Avi Flamholz, Rob Phillips, and Ron Milo. Published in eLife, 31st March 2020. https://elifesciences.org/articles/57309.
- Diameter of arterial microvessels trapping 8--10 micron, 15 micron and 25 micron microspheres as determined by vital microscopy of the hamster cheek pouch. W H Dickhoner, B R Bradley, G S Harell. Invest Radiol.. Jul-Aug 1978;13(4):313-7. [CrossRef]
- 4. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Davies NG, Abbott S, Barnard RC, et al. Science, 2021; 372. [CrossRef]
- Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nicholas G. Davies1 ✉, Christopher I. Jarvis1, CMMID COVID-19 Working Group, Published online 15th March 2021. Nature 593 270. [CrossRef]
- Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Katherine A Twohig*, Tommy Nyberg*, Asad Zaidi, et. al. Lancet Infect Dis 2022; 22: 35–42 Published Online 27th August, 2021. [CrossRef]
- Kings/Zoe Covid Group News reports, 18th March 2021 and 1st April 2021. Symptoms of covid.
- Hyperpolarized 129Xe MRI Abnormalities in Dyspneic Patients 3 Months after COVID-19 Pneumonia: Preliminary Results. James T. Grist, Mitchell Chen, Guilhem J. Collier, et. al. Radiology 2021; 301:E353–E360. [CrossRef]
- medRxiv preprint doi: https://doi.org/10.1101/2022.02.01.22269999; this version posted February 2, 2022. The Investigation of Pulmonary Abnormalities using Hyperpolarised Xenon Magnetic Resonance Imaging in Patients with Long-COVID. James T. Grist, Guilhem J. Collier. Huw Walters, et al.
- Inside Health, BBC Radio 4, 29th September 2020.
- Meeting report: 29th International Conference on Antiviral Research in La Jolla, CA, USA. Antiviral Research, R. Anthony Vere Hodge (2017). 137, 23-40.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).