Submitted:
08 April 2024
Posted:
09 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Field Experiment
2.2. Greenhouse Experiment
3. Discussion
4. Materials and Methods
4.1. Field Experiment
4.1.1. Plant Material, Experimental Design, and Treatments
4.1.2. Candidatus Liberibacter asiaticus (CLas) Detection
4.1.4. CRM and ACP Samplings
4.2. Greenhouse Experiment
4.2.1. Plants and Insects
4.2.2. Diaphorina Citri Performance on HBr-Treated Plants
4.2.3. RNA Extraction and qRT PCR Analysis of Gene Expression
4.3. Statistical Analysis
| Genea | Gene name | Citrus ID | Primer Sequence (5’→3’) | |
|---|---|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase, | LOC102624117 | FW: GGAAGGTCAAGATCGGAATCAA | |
| RV: CGTCCCTCTGCAAGATGACTCT | ||||
| PAL | Phenylalanine ammonia-lyase-like | LOC102620464 | FW: CACATTCTTGGTAGCGCTTTG | |
| RV: AGCTACTTGGCTGACAGTATTC | ||||
| ICS | Isochorismate synthase 2, Chloroplastic | LOC102630235 | FW: GGAGGAGGAGAGAGTGAATTTG | |
| RV: GGGTTGCTTCCTTCTACTATCC | ||||
| NPR1 | BTB/POZ domain and ankyrin repeat-containing protein | LOC102617188 | FW: GTACCTTGAAAACAGAGTTGGACTGG | |
| RV: TGCTCCTCTTGCATTTTGAAAGGTG | ||||
| MYC2 | Transcription factor MYC2 | LOC102626457 | FW: TGCATCTACAGCCGACCC | |
| RV: TAGGTCCAGCCCTCACGA | ||||
| LOX2 | Linoleate 13S-lipoxygenase 2-1, chloroplastic-like | LOC102629656 | FW: GAACCATATTGCCACTTTCG | |
| RV: CGTCATCAATGACTTGACCA | ||||
| AOS | Allene oxide synthase | AY243478 | FW: AGATCTTATTCCCGAACATGGT | |
| RV: CGGACTTCATCAACGGCAT | ||||
| JAR1 | Jasmonate Resistant 1 | LOC102611440 | FW: AAGGCGATGCAGTCACAATG | |
| RV: TGGTGGAAATCAGGACCAAAG | ||||
| SAMP | response A/B barrel domain-containing protein HS1 | LOC102628374 | FW: AACAGGGGCAAGAATGTGAGCAT | |
| RV: ACACGTACTGTTGTCGGTTTGTAGTCA |
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urbaneja, A.; Grout, T.G.; Gravena, S.; Wu, F.; Cen, Y.; Stansly, P.A. Citrus Pests in a Global World. In The Genus Citrus; Elsevier, 2020; pp. 333–348.
- Ferrarezi, R.S.; Vincent, C.I.; Urbaneja, A.; Machado, M.A. Editorial: Unravelling Citrus Huanglongbing Disease. Front Plant Sci 2020, 11, 609655. [CrossRef]
- Bové, J.M. Huanglongbing: A Destructive Newly Emerging, Century-Old Disease of Citrus. Journal of Plant Pathology 2006, 88, 7–37.
- Gottwald, T.R. Current Epidemiological Understanding of Citrus Huanglongbing. Annu Rev Phytopathol 2010, 48, 119–139. [CrossRef]
- Hall, D.G.; Richardson, M.L.; Ammar, E.; Halbert, S.E. Asian Citrus Psyllid, <scp>D</Scp> Iaphorina Citri , Vector of Citrus Huanglongbing Disease. Entomol Exp Appl 2013, 146, 207–223. [CrossRef]
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) Outbreaks in Florida, California and Texas. Trop Plant Pathol 2020, 45, 265–278. [CrossRef]
- Graham, J.; Morgan, K. Why Bicarbonates Matter for HLB Management. Citrus Industry 2017, 98, 16–21, doi:2017_April_bicarbonates.pdf (ufl.edu).
- Pustika, A.B.; Subandiyah, S.; Holford, P.; Beattie, G.A.C.; Iwanami, T.; Masaoka, Y. Interactions between Plant Nutrition and Symptom Expression in Mandarin Trees Infected with the Disease Huanglongbing. Australas Plant Dis Notes 2008, 3, 112–115. [CrossRef]
- Etxeberria, E.; Gonzalez, P.; Achor, D.; Albrigo, G. Anatomical Distribution of Abnormally High Levels of Starch in HLB-Affected Valencia Orange Trees. Physiol Mol Plant Pathol 2009, 74, 76–83. [CrossRef]
- Tang, L.; Chhajed, S.; Vashisth, T. Preharvest Fruit Drop in Huanglongbing-Affected ‘Valencia’ Sweet Orange. Journal of the American Society for Horticultural Science 2019, 144, 107–117. [CrossRef]
- Rogers, M.E.; Stansly, P.A.; Stelinski, L.L. 2012 Florida Citrus Pest Management Guide: Asian Citrus Psyllid and Citrus Leafminer 1 Asian Citrus Psyllid Psyllid Management. Entomol. Nematol. Dept., Fla. Coop. 2012, doi:00003029.pdf (ufl.edu).
- Hall, D.G.; Albrecht, U.; Bowman, K.D. Transmission Rates of ‘ Ca. Liberibacter Asiaticus’ by Asian Citrus Psyllid Are Enhanced by the Presence and Developmental Stage of Citrus Flush. J Econ Entomol 2016, 109, 558–563. [CrossRef]
- Canales, E.; Coll, Y.; Hernández, I.; Portieles, R.; Rodríguez García, M.; López, Y.; Aranguren, M.; Alonso, E.; Delgado, R.; Luis, M.; et al. ‘Candidatus Liberibacter Asiaticus’, Causal Agent of Citrus Huanglongbing, Is Reduced by Treatment with Brassinosteroids. PLoS One 2016, 11, e0146223. [CrossRef]
- Sirhindi, G.; Kumar, S.; Bhardwaj, R.; Kumar, M. Effects of 24-Epibrassinolide and 28-Homobrassinolide on the Growth and Antioxidant Enzyme Activities in the Seedlings of Brassica Juncea L. Physiology and Molecular Biology of Plants 2009, 15, 335–341. [CrossRef]
- Choudhary, S.P.; Yu, J.-Q.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Benefits of Brassinosteroid Crosstalk. Trends Plant Sci 2012, 17, 594–605. [CrossRef]
- Yu, M.-H.; Zhao, Z.-Z.; He, J.-X. Brassinosteroid Signaling in Plant–Microbe Interactions. Int J Mol Sci 2018, 19, 4091. [CrossRef]
- Bürger, M.; Chory, J. Stressed Out About Hormones: How Plants Orchestrate Immunity. Cell Host Microbe 2019, 26, 163–172. [CrossRef]
- De Bruyne, L.; Höfte, M.; De Vleesschauwer, D. Connecting Growth and Defense: The Emerging Roles of Brassinosteroids and Gibberellins in Plant Innate Immunity. Mol Plant 2014, 7, 943–959. [CrossRef]
- Khripach, V. Twenty Years of Brassinosteroids: Steroidal Plant Hormones Warrant Better Crops for the XXI Century. Ann Bot 2000, 86, 441–447. [CrossRef]
- Wang, Z.-Y. Brassinosteroids Modulate Plant Immunity at Multiple Levels. Proceedings of the National Academy of Sciences 2012, 109, 7–8. [CrossRef]
- Ali, S.S.; Kumar, G.B.S.; Khan, M.; Doohan, F.M. Brassinosteroid Enhances Resistance to Fusarium Diseases of Barley. Phytopathology 2013, 103, 1260–1267. [CrossRef]
- Kim, Y.-W.; Youn, J.-H.; Roh, J.; Kim, J.-M.; Kim, S.-K.; Kim, T.-W. Brassinosteroids Enhance Salicylic Acid-Mediated Immune Responses by Inhibiting BIN2 Phosphorylation of Clade I TGA Transcription Factors in Arabidopsis. Mol Plant 2022, 15, 991–1007. [CrossRef]
- Pan, G.; Liu, Y.; Ji, L.; Zhang, X.; He, J.; Huang, J.; Qiu, Z.; Liu, D.; Sun, Z.; Xu, T.; et al. Brassinosteroids Mediate Susceptibility to Brown Planthopper by Integrating with the Salicylic Acid and Jasmonic Acid Pathways in Rice. J Exp Bot 2018, 69, 4433–4442. [CrossRef]
- Alférez, F.; Vincent, C.; Vashisth, T. Update on Brassinosteroids for HLB Management. Citrus Industry 2019, June, 16–18.
- Yao, T.; Xie, R.; Zhou, C.; Wu, X.; Li, D. Roles of Brossinosteroids Signaling in Biotic and Abiotic Stresses. J Agric Food Chem 2023, 71, 7947–7960. [CrossRef]
- Rashidi, H.; Amiri, J.; Shirzad, H. Effect of Postharvest Treatment with 24-Epibrassinolide and Fennel (Foeniculum Vulgare) Essential Oil on Quality Attributes and Storage Life of Orange (Citrus Sinensis Cv. ‘Valencia’). Erwerbs-Obstbau 2023, 65, 927–939. [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [CrossRef]
- Ibanez, F.; Suh, J.H.; Wang, Y.; Stelinski, L.L. Long-Term, Sustained Feeding by Asian Citrus Psyllid Disrupts Salicylic Acid Homeostasis in Sweet Orange. BMC Plant Biol 2019, 19, 493. [CrossRef]
- Wu, Q.; Moniruzzaman, M.; Yan, H.; Lv, Y.; Jiang, B.; Jiang, N.; Zhong, Y. The CsNPR1 Gene Expression Modulation in Citrus and Understanding the Defense Mechanism against Huanglongbing by Screening CsNPR1-Interacting Proteins. Sci Hortic 2021, 288, 110375. [CrossRef]
- Zhang, S.; Wang, X.; He, J.; Zhang, S.; Zhao, T.; Fu, S.; Zhou, C. A Sec-Dependent Effector, CLIBASIA_04425, Contributes to Virulence in ‘Candidatus Liberibater Asiaticus.’ Front Plant Sci 2023, 14. [CrossRef]
- Peng, A.; Zou, X.; He, Y.; Chen, S.; Liu, X.; Zhang, J.; Zhang, Q.; Xie, Z.; Long, J.; Zhao, X. Overexpressing a NPR1-like Gene from Citrus Paradisi Enhanced Huanglongbing Resistance in C. Sinensis. Plant Cell Rep 2021, 40, 529–541. [CrossRef]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Achor, D.S.; Vasconcelos, F.N.C.; Hendrich, C.; Huang, Y.; et al. Citrus Huanglongbing Is a Pathogen-Triggered Immune Disease That Can Be Mitigated with Antioxidants and Gibberellin. Nat Commun 2022, 13, 529. [CrossRef]
- Robertson, C.J.; Zhang, X.; Gowda, S.; Orbović, V.; Dawson, W.O.; Mou, Z. Overexpression of the Arabidopsis NPR1 Protein in Citrus Confers Tolerance to Huanglongbing. J Citrus Pathol 2018, 5. [CrossRef]
- Sarkar, P.; El-Mohtar, C.; Turner, D.; Welker, S.; Robertson, C.J.; Orbovic, V.; Mou, Z.; Levy, A. NONEXPRESSOR OF PATHOGENESIS-RELATED GENES Control Huanglongbing Tolerance by Regulating Immune Balance in Citrus Plants. bioRxiv 2024.03.18.585579 2024, 03.18.585579. [CrossRef]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic Acid Signaling Pathway in Plants. Int J Mol Sci 2019, 20, 2479. [CrossRef]
- Erb, M.; Reymond, P. Molecular Interactions Between Plants and Insect Herbivores. Annu Rev Plant Biol 2019, 70, 527–557. [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of Inducible Defense-Related Proteins in Infected Plants. Annu Rev Phytopathol 2006, 44, 135–162. [CrossRef]
- Thaler, J.S.; Farag, M.A.; Paré, P.W.; Dicke, M. Jasmonate-Deficient Plants Have Reduced Direct and Indirect Defences against Herbivores. Ecol Lett 2002, 5, 764–774. [CrossRef]
- Dicke, M.; Gols, R.; Ludeking, D.; Posthumus, M.A. Jasmonic Acid and Herbivory Differentially Induce Carnivore-Attracting Plant Volatiles in Lima Bean Plants. J Chem Ecol 1999, 25, 1907–1922. [CrossRef]
- Pérez-Hedo, M.; Arias-Sanguino, Á.M.; Urbaneja, A. Induced Tomato Plant Resistance against Tetranychus Urticae Triggered by the Phytophagy of Nesidiocoris Tenuis. Front Plant Sci 2018, 9, 1419. [CrossRef]
- Cruz-Miralles, J.; Cabedo-López, M.; Guzzo, M.; Pérez-Hedo, M.; Flors, V.; Jaques, J.A. Plant Defense Responses Triggered by Phytoseiid Predatory Mites (Mesostigmata: Phytoseiidae) Are Species-Specific, Depend on Plant Genotype and May Not Be Related to Direct Plant Feeding. BioControl 2021, 66, 381–394. [CrossRef]
- Cabedo-López, M.; Cruz-Miralles, J.; Vacas, S.; Navarro-Llopis, V.; Pérez-Hedo, M.; Flors, V.; Jaques, J.A. The Olfactive Responses of Tetranychus Urticae Natural Enemies in Citrus Depend on Plant Genotype, Prey Presence, and Their Diet Specialization. J Pest Sci (2004) 2019, 92, 1165–1177. [CrossRef]
- Dahmane, M.; Urbaneja, A.; Ruíz-Rivero, O.; Alonso-Valiente, M.; Pérez-Hedo, M. The Zoophytophagous Predator Pilophorus Clavatus (Hemiptera: Miridae) Induces Plant Defences in Citrus. J Pest Sci (2004) 2022, 95, 1519–1530. [CrossRef]
- Pappas, M.L.; Steppuhn, A.; Geuss, D.; Topalidou, N.; Zografou, A.; Sabelis, M.W.; Broufas, G.D. Beyond Predation: The Zoophytophagous Predator Macrolophus Pygmaeus Induces Tomato Resistance against Spider Mites. PLoS One 2015, 10, e0127251. [CrossRef]
- Santamaria, M.E.; Cambra, I.; Martinez, M.; Pozancos, C.; González-Melendi, P.; Grbic, V.; Castañera, P.; Ortego, F.; Diaz, I. Gene Pyramiding of Peptidase Inhibitors Enhances Plant Resistance to the Spider Mite Tetranychus Urticae. PLoS One 2012, 7, e43011. [CrossRef]
- Huang, C.-Y.; Araujo, K.; Sánchez, J.N.; Kund, G.; Trumble, J.; Roper, C.; Godfrey, K.E.; Jin, H. A Stable Antimicrobial Peptide with Dual Functions of Treating and Preventing Citrus Huanglongbing. Proceedings of the National Academy of Sciences 2021, 118, e2019628118. [CrossRef]
- Alferez, F.; Albrecht, U.; Gaire, S.; Batuman, O.; Qureshi, J.; Zekri, M. Individual Protective Covers (IPCs) for Young Tree Protection from the HLB Vector, the Asian Citrus Psyllid. EDIS 2021, 2021. [CrossRef]
- Gaire, S.; Albrecht, U.; Batuman, O.; Qureshi, J.; Zekri, M.; Alferez, F. Individual Protective Covers (IPCs) to Prevent Asian Citrus Psyllid and Candidatus Liberibacter Asiaticus from Establishing in Newly Planted Citrus Trees. Crop Protection 2022, 152, 105862. [CrossRef]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative Real-Time PCR for Detection and Identification of Candidatus Liberibacter Species Associated with Citrus Huanglongbing. J Microbiol Methods 2006, 66, 104–115. [CrossRef]
- McCoy, C.W.; Albrigo, L.G. Feeding Injury to the Orange Caused by the Citrus Rust Mite, Phyllocoptruta Oleivora (Prostigmata: Eriophyoidea)1. Ann Entomol Soc Am 1975, 68, 289–297. [CrossRef]
- Monzo, C.; Arevalo, H.A.; Jones, M.M.; Vanaclocha, P.; Croxton, S.D.; Qureshi, J.A.; Stansly, P.A. Sampling Methods for Detection and Monitoring of the Asian Citrus Psyllid (Hemiptera: Psyllidae). Environ Entomol 2015, 44, 780–788. [CrossRef]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and Management of Asian Citrus Psyllid, Vector of the Huanglongbing Pathogens. Annu Rev Entomol 2013, 58, 413–432. [CrossRef]
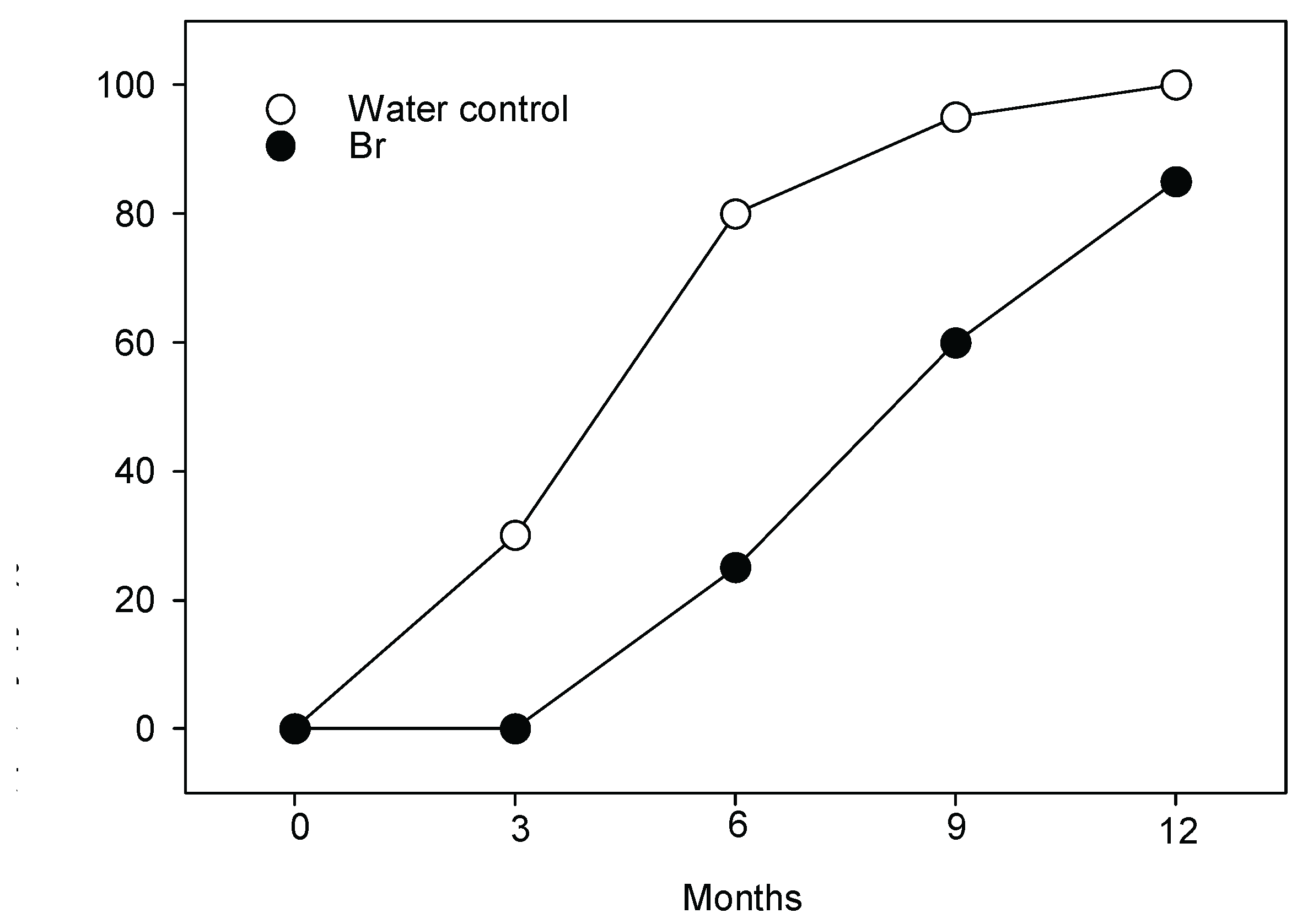
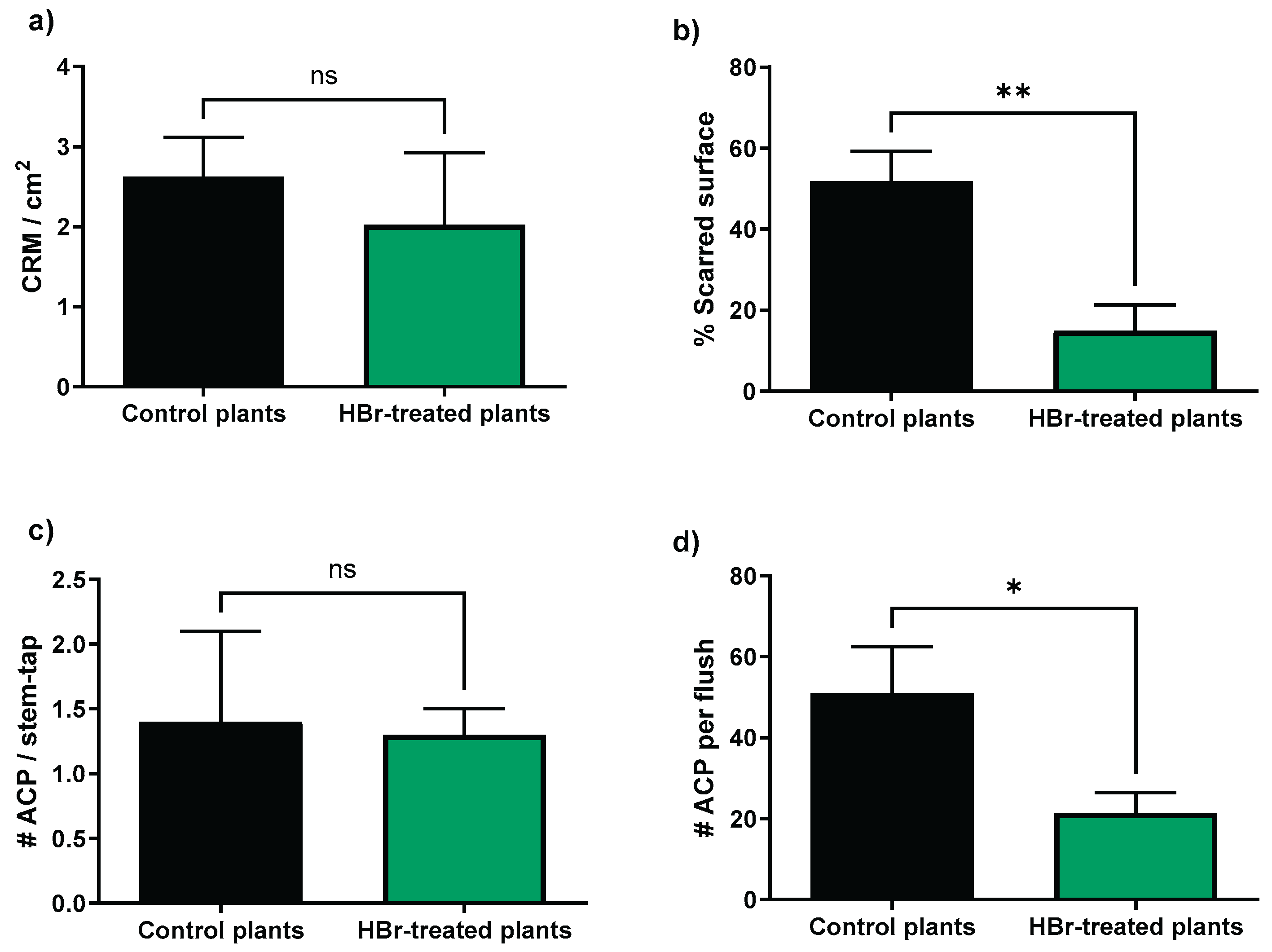
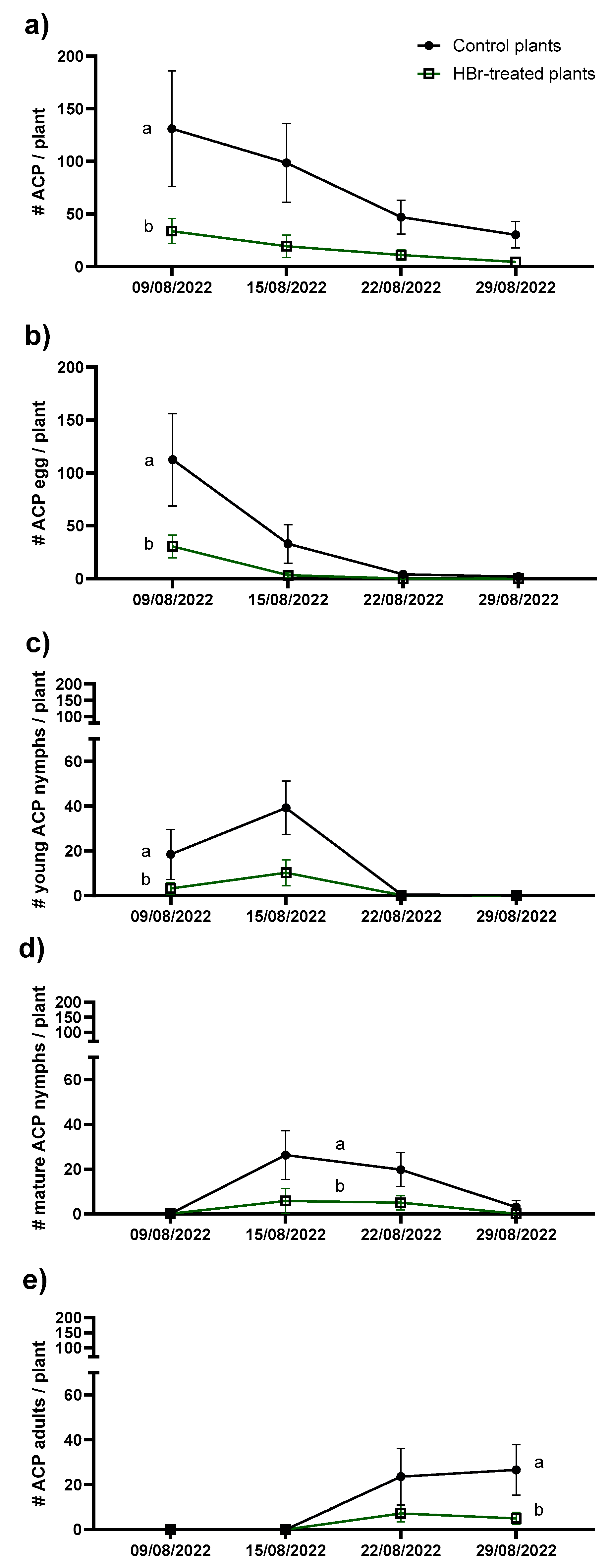
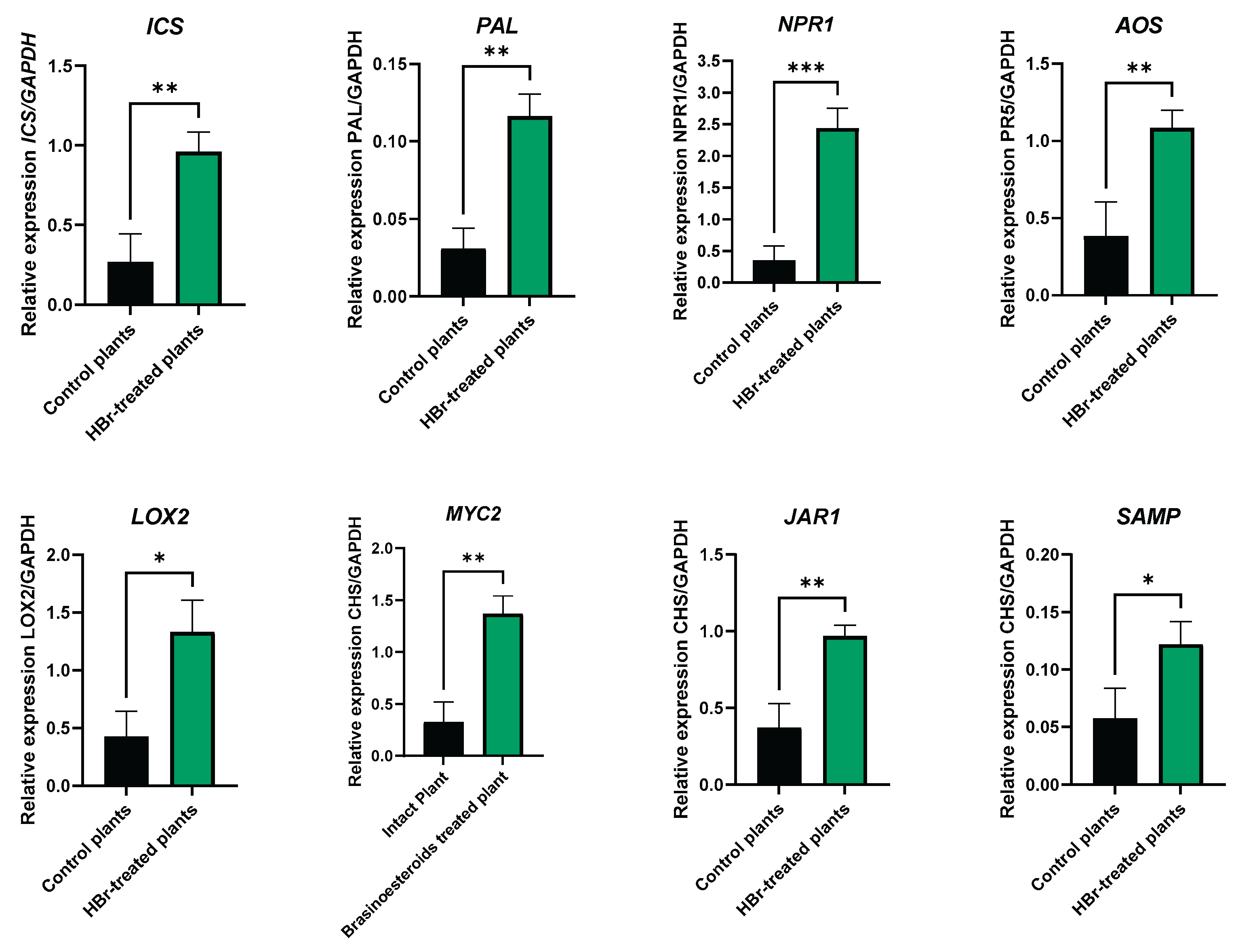
| Mortality | Control plants | HBr-treated plants | Statistics |
|---|---|---|---|
| Egg | 49.9 ± 16.3 | 65.0 ± 10.2 | t1, 7 = 0.817; P = 0.441 |
| Nymphal | 35.9 ± 18.2 | 68.6 ± 11.2 | t1, 7 = 1.600; P = 0.158 |
| Egg-adult | 76.6 ± 5.33 | 85.7 ± 6.8 | t1, 7 = 1.008; P = 0.347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





