1. Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the loss of both upper and lower motor neurons in the central nervous system, resulting in muscle weakness, paralysis, and eventually death within 2 to 5 years of diagnosis [
1]. While the majority of ALS cases (90% to 95%) are sporadic, the remaining cases are familial [
1]. So far, over 30 causative genes have been identified in familial ALS [
2], one of which is the gene encoding superoxide dismutase-1 (SOD1); mutations in this gene contribute to approximately 20% of familial cases [
3]. Wild-type SOD1 (WT SOD1) is an antioxidant that catalyzes the conversion of superoxide anion to hydrogen peroxide and molecular oxygen [
4]. However, the enzymatic activity of the ALS-linked SOD1 mutants varies depending on the variant, from 0% to 100% compared with that of WT SOD1 [
5,
6]. It is now accepted that the cytotoxicity of ALS-linked SOD1 mutants does not arise from a loss of their enzymatic activity [
7]. A common property of ALS-linked SOD1 mutations is that they compromise the stability of the protein’s quaternary structure, which promotes misfolding or disordered conformations [
8,
9]. Natively folded SOD1 is conformationally characterized by a homodimer containing one Cu and one Zn ion in each subunit, as well as by the formation of a disulfide bond between Cys57 and Cys146, which exhibits high thermostability (
Tm >90ºC) [
10]. However, once SOD1 loses these metal ions, undergoes dimer dissociation, or undergoes cleavage of the disulfide bond, its stability is remarkably decreased. For instance, metal-free, disulfide bond-cleaved WT SOD1 exhibits lower thermostability (
Tm = 42ºC) [
10,
11].
The concept that misfolded SOD1 participates in the pathogenesis of familial or even sporadic ALS has been reinforced by the development of antibodies specifically targeting misfolded SOD1 [
12,
13]. To our knowledge, more than 20 specific antibodies have been developed, a subset of which have the potential for use in therapeutic interventions [
13,
14,
15]. Most specific antibodies of this kind are designed to bind to regions that are inaccessible in the natively folded form. In other words, the specific antibodies recognize regions that are exposed at the surface only when SOD1 adopts a non-native conformation initiated by mutations or loss of cofactors involved in the conformational stability (
e.g., Zn ions). Unlike for natively folded SOD1, it is difficult to define the inherent conformation of misfolded SOD1 due to the huge number of influential factors acting on this in combination (
e.g., monomer/dimer, metal-free/bound, or disulfide bond cleavage/formation). Thus, misfolded SOD1 could consist of a heterogeneous population with distinct conformation. To better understand which misfolded SOD1 species participate in which disease process, it is critical to determine which misfolded SOD1 species are recognized by a specific antibody. However, the mechanism behind the selectivity of most developed antibodies that specifically recognize misfolded SOD1 has not been well characterized at the molecular level. In most cases, it has just been determined by a comparison of specificity between natively folded and unfolded forms treated with chaotropic reagents (e.g., guanidinium ions).
MS785 and MS27 are commercially available antibodies specifically targeting misfolded SOD1 [
16,
17]. Although each of these antibodies fails to recognize ALS-linked SOD1 mutants with a point mutation in their epitope region (amino acids 8-14 for MS785; amino acids 30-40 for MS27), the use of an MS785 and MS27 cocktail compensates for each of their shortcomings, with this mixture recognizing over 100 ALS-linked SOD1 mutants as determined by immunoprecipitation [
17]. Thus, the MS785-MS27 cocktail is the only antibody in which the affinity for a variety of ALS-linked SOD1 mutants has been experimentally confirmed. According to previous cell-based studies [
17,
18], this antibody cocktail recognizes SOD1 species that show an increase in level upon the treatment of cells with a Zn-chelating reagent, implying that the antibody cocktail recognizes “Zn-deficient SOD1 species.” However, despite substantial efforts, it is unclear which SOD1 species whose misfolding is induced by Zn deficiency can be recognized by this antibody cocktail at the molecular level.
Against the above background, the aim of the present study is to molecularly characterize the recognition by the MS785-MS27 antibody cocktail of various SOD1 species with altered conformational integrity.
3. Discussion
In this study, we molecularly characterized which misfolded SOD1 species are recognized by the MS785-MS27 antibody cocktail, which binds over 100 ALS-linked SOD1 mutants. Using indirect ELISA with recombinant SOD1, we found that the antibody cocktail recognized WT SOD1 as well as the ALS-linked mutants lacking incorporated Zn ion (
Figure 1 and
Figure 3). These results build on the earlier cell-based studies [
17,
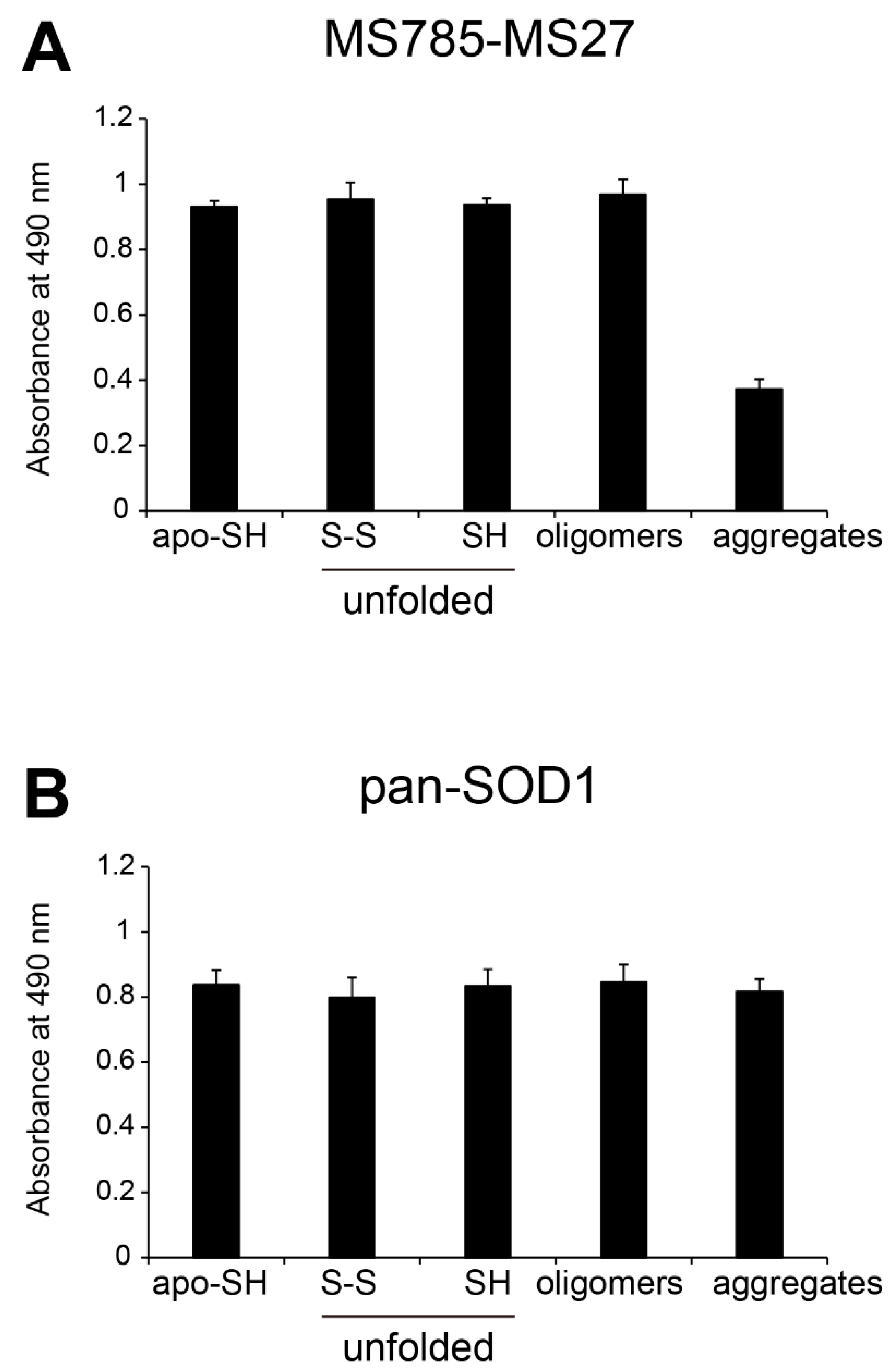
18], which demonstrated that treatment of cultured cells overexpressing WT SOD1 with a Zn-chelating reagent increased the range of SOD1 species that are recognized by the antibody cocktail. We also found that the antibody cocktail had strong reactivity to various conformation-disordered SOD1 species, including unfolded and oligomeric forms, but less for the aggregated one (
Figure 2 and
Figure 3). Thus, the present study deepens our understanding of the antibody cocktail recognition of misfolded SOD1 species at the molecular level.
The present study sheds light on the conformational features of the MS785-MS27-reactive SOD1 species of both WT SOD1 and the ALS-linked mutants. We prepared conformation-disordered SOD1 species including unfolded, oligomeric, and aggregated forms from apo-SOD1
S-S as a precursor. The antibody cocktail had similar affinity between apo-SOD1
S-S and the conformation-disordered SOD1 species, with the exception of the aggregated form (
Figure 2 and
Figure 3). This suggested that there could be common conformations among the MS785-MS27-reactive SOD1 species with various disordered conformations regardless of whether SOD1 is mutated or not. Here, we can speculate about the possible conformation that is shared among the MS785-MS27-reactive SOD1 species with regard to the epitope region of each antibody. The epitope region of MS785 is the peptide corresponding to amino acids 8 to 14 in human SOD1 [
16], which is located in the small loop between the β
1 and β
2 strands. Meanwhile, the epitope region of MS27 is the peptide corresponding to amino acids 30 to 40 in human SOD1 [
17], which covers the β
3 strand, followed by a small loop. It is possible that the apo-form, unfolded form, and oligomeric form share a common disordered conformation around the β
1 to β
3 strands. In support of this idea, the X-ray crystal structure of apo-SOD1 revealed that the regions of the β
1 to β
3 strands are exposed to the solvent, in contrast to the case for the natively folded SOD1 (PDB: 3ECU), which provokes the misfolding of SOD1 [
31].
Besides determining recognition by the MS785-MS27 antibody cocktail at the molecular level, we also characterized the toxicological features of MS785-MS27-reactive SOD1 species. We found that both apo-WT SOD1
SH and apo-SOD1
SH that lacks the Zn binding site exhibited toxic effects on NSC-34 cells (
Figure 4A,B). It would thus be beneficial to decrease the amounts of Zn-deficient SOD1 species in order to protect motor neurons. One approach to achieve this is to perform Zn coordination to SOD1 species. This approach is promising given the evidence that the disordered conformation and cytotoxicity observed in Zn-deficient SOD1 were reversed by exogenous treatment of these species with Zn ions (
Figure 1E,
Figure 3H,
Figure 4C,D). An earlier in vivo study demonstrated that treatment with Zn
II (atsm), a small compound that coordinates Zn ions, improved motor functions of different mouse lines of
SOD1-ALS [
32]. We have also shown that metallothionein-I, a major Zn binding protein in the cells, suppresses the loss of motor neurons in G93A SOD1 mice [
33,
34]. Thus, restoration of the Zn bioavailability in SOD1 itself or in the microenvironment surrounding SOD1 would be important for the correction of SOD1 misfolding and suppression of motor neuron death. We propose that the reactivity of the MS785-MS27 antibody cocktail could be used as an indicator of Zn bioavailability in SOD1, since its recognition negatively correlates with the incorporation of Zn ions in SOD1 (
Figure 1E and
Figure 3H).
We provide evidence of the cell types where the MS785-MS27-reactive SOD1 species were distributed in the spinal cord during the disease course of G93A SOD1 mice. Throughout the disease course, the MS785-MS27-reactive SOD1 species were mainly localized in the motor neurons (
Figure 5A–C), suggesting that misfolded SOD1 species present in motor neurons are deficient in Zn ions. These findings are consistent with a study [
35] showing that 42% of ALS cases exhibit Zn deficiency in WT or mutated SOD1 localized in the ventral horn. We also provide evidence for there being divergent populations of misfolded SOD1 species that dynamically change over the disease course of G93A SOD1 mice (
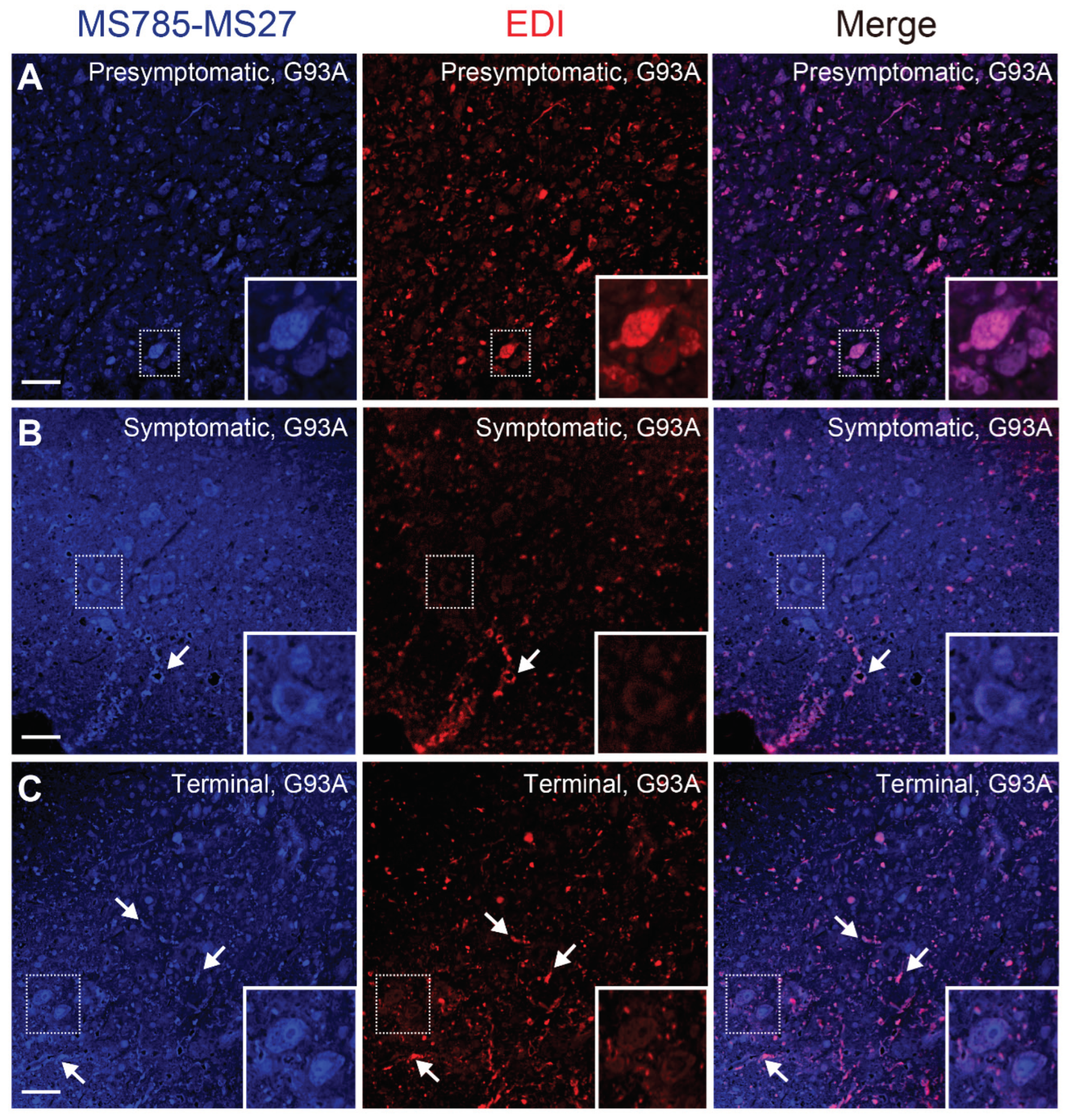
Figure 6). Co-immunostaining with the antibody cocktail and EDI revealed that the MS785-MS27-reactive SOD1 co-existed with EDI-reactive SOD1 species at the presymptomatic stage (
Figure 6A), suggesting that the conformation of misfolded SOD1 at the early stage is likely to be homogeneous. However, as the disease progressed, the EDI-reactive SOD1 species were mainly observed outside of the motor neurons, which decreased the overlap with the MS785-MS27-reactive SOD1 species (
Figure 6B,C). These results indicate that misfolded SOD1 species appear to adopt various conformations after the onset of symptoms of the disease. Our histological data are supported by a previous study reporting that there were distinct distribution patterns of misfolded SOD1 species when these species were immunolabeled with a panel of specific antibodies [
30]. The divergent populations of misfolded SOD1 species might explain why several passive immunotherapies targeting misfolded SOD1 fail to rescue disease phenotypes in mouse models of
SOD1-ALS [
36].
4. Materials and Methods
4.1. Expression, Purification, and Demetallation of Recombinant SOD1 Proteins
A bacterial expression plasmid, pET28a(+), encoding human SOD1 with I113T that was N-terminally tagged with hexahistidine, was purchased from Addgene (#117703; Watertown, MA, USA). Plasmids encoding WT, A4V, G37R, H46R, G85R, D90A, or G93A human SOD1 gene were prepared using site-directed mutagenesis, and correct sequences of each construct were confirmed by Azenta (Tokyo, Japan). Human SOD1 with mutations in the Zn binding sites was designed from the WT sequence by replacement of residues H63A, H71A, H80G, and D83A, and subcloned into the pET28a(+) vector. A bacterial expression plasmid, pET28a(+) encoding mouse WT Sod1 that was N-terminally tagged with hexahistidine, was also obtained from Addgene (#117701). Each expression vector was transformed into SHuffle E. coli (New England Biolabs, Ipswich, MA, USA). Colonies isolated from the LB-agar plate were inoculated onto LB medium with 100 μg/mL kanamycin sulfate at 200 rpm and 30ºC for 14 h. Bacterial overexpression was initiated by adding the preculture medium to 500 mL of the LB medium. When turbidity (OD600) of the E. coli reached between 0.60 and 0.80, the cells were cultured with 0.5 mM isopropyl-β-D-thiogalactopyranoside (Bio Medical Science, Tokyo, Japan) at 200 rpm and 20ºC for 20 h. The E. coli was lysed by sonication (Sonifier SFX250; Branson Emerson, Brookfield, CT, USA) in a buffer (pH 7.0) containing 2% (v/v) Triton X-100, 50 mM Tris, 500 mM NaCl, EDTA-free Complete Protease Inhibitor Cocktail (Merck, Darmstadt, Germany), 1 U DNase I (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan), and 5 mM MgSO4. The cell lysates were centrifuged at 20,000 × g and 4ºC for 30 min, and the supernatant was filtered with a 0.22 μm syringe-driven filter unit (Millipore, Burlington, MA, USA). The SOD1 with a hexahistidine tag was isolated using a Ni2+ affinity chromatograph (Complete His-Tag Purification Column; GE Healthcare, Chicago, IL, USA) by an ÄKTA Start (Cytiva, Tokyo, Japan). Pre-equilibration of the column was carried out using a buffer (pH 7.0) containing 50 mM Tris, 1 M NaCl, and 50 mM imidazole. Elution of the fusion protein was performed with a buffer (pH 7.0) containing 50 mM Tris, 100 mM NaCl, and 250 mM imidazole.
The SOD1 with a hexahistidine tag was demetallated by dialysis in a Spectra/Por® (molecular mass cut-off: 6-8 kDa; Repligen, Waltham, MA, USA) against a buffer (pH 4.0) containing 50 mM sodium acetate, 100 mM NaCl, and 5 mM EDTA at 4ºC for 20 h. The apo-SOD1 with a hexahistidine tag was neutralized by dialysis with a buffer (pH 7.0) containing 50 mM Tris, 100 mM NaCl, and 5 mM EDTA for 4 h at 4ºC. The hexahistidine tag was cleaved by treatment with 4 U thrombin, a serine protease (GE Healthcare), at 20ºC for 20 h. The non-cleaved fusion protein and thrombin were removed using a Complete His-Tag Purification Column and Benzamidine Column (Cytiva), respectively. The SOD1 was further purified by size exclusion chromatography (Superdex™ 200 Increase Column 10/300 GL; Cytiva) with an ÄKTA Go (Cytiva). The concentration of SOD1 protein was determined by an Eppendorf BioSpectrometer (Eppendorf, Hamburg, Germany) using a monomeric molar extinction coefficient at 280 nm of 5500 M−1 cm−1. The purity of SOD1 was confirmed by SDS-PAGE followed by InstantBlue® Coomassie Protein Stain (Abcam, Cambridge, UK).
4.2. Preparation of Metal Binding Forms and Conformation-Disordered SOD1
Metal binding forms of WT SOD1 were prepared as described elsewhere with slight modification [
22,
24]. A buffer (pH 7.0) containing 50 mM Tris and 100 mM NaCl was treated with a Chelex 100 Resin (100-200 mesh; Bio-Rad, Hercules, CA, USA) to remove divalent ions. Apo-WT SOD1
S-S was treated with 10 mM (±) dithiothreitol (DTT) at 37ºC for 1 h to obtain the disulfide bond-cleaved form. DTT was removed using a Zeta Spin Desalting Column (molecular mass cut-off: 7 kDa; Thermo Fisher Scientific, Waltham, MA, USA), in accordance with the manufacturer’s protocol. Apo-WT SOD1
SH was treated with 1 to 4 molar equivalents of Zn or Cu ions at 4ºC for 3 h. The Cu- and Zn-bound form of WT SOD1 was purchased from Sigma-Aldrich (S9636; St. Louis, MO, USA), which was isolated from human erythrocytes and used as a natively folded form (also known as an enzymatically active form).
Conformation-disordered SOD1 species, including unfolded, oligomeric, and aggregated forms, were prepared as described previously with slight modification [
22,
24]. For preparation of the unfolded form, Apo-SOD1
S-S at 100 μM was treated with 10 mM DTT at 37ºC for 1 h. After removing the DTT using a Zeta Spin Desalting Column (molecular mass cut-off: 7 kDa; Thermo Fisher Scientific), apo-SOD1
SH was treated with a buffer (pH 7.0) containing 50 mM Tris, 100 mM NaCl, 5 mM EDTA, and 6 M guanidine hydrochloride for 37ºC for 2 h. For preparation of the oligomeric form, apo-SOD1
S-S at 100 μM was incubated at 37ºC for 5 days to induce non-native cross-linking between Cys residues in SOD1 [
37]. For preparation of the aggregated form, apo-SOD1
SH at 100 μM was added into a ProteoSave 96-well plate (Sumitomo Bakelite Co. Ltd., Tokyo, Japan) and agitated in the presence of a plastic POM ball (
φ 2.4 mm; Sanplatec Co. Ltd., Tokyo, Japan) at 1,200 rpm and 37ºC for 5 days. Suspension of the SOD1 aggregates was collected and subjected to centrifugation at 20,000 ×
g for 30 min at 4ºC. The supernatant was discarded and the pellet was resuspended in a buffer (pH 7.0) containing 50 mM Tris, 100 mM NaCl, and 5 mM EDTA. The SOD1 aggregates were sonicated using a Handy Sonic (UR-21P; Tony Seiko Co. Ltd., Tokyo, Japan). The concentration of SOD1 aggregates was determined by an Eppendorf BioSpectrometer using a monomeric molar extinction coefficient at 280 nm of 5,500 M
−1 cm
−1.
4.3. ELISA
To analyze the conformation-disordered SOD1 species, EDTA at a final concentration of 5 mM was added to Tris-buffered saline (TBS, pH 7.4) or TBS with 0.05% (v/v) Tween 20 (TBST, pH 7.4) to avoid the metal re-coordination to SOD1. For experiments on the metal binding forms of SOD1, TBS or TBST was treated with a Chelex 100 Resin (100-200 mesh; Bio-Rad) to remove divalent metal ions. SOD1 species at 5 μM were coated onto a Nunc Immuno 96-well Plate with a Maxi Soap (Thermo Fisher Scientific) at 4ºC for 18 h. The plates were blocked with 3% (w/v) bovine serum albumin in TBS (pH 7.4) at room temperature for 1 h. After three washes with TBST, primary antibodies including MS785-MS27 cocktail (0.025 μg/mL, FDV-0021A; Funakoshi, Tokyo, Japan), MS785 (0.025 μg/mL, FDV-0021B; Funakoshi), MS27 (0.025 μg/mL, FDV-0021C; Funakoshi), or pan-SOD1 (0.5 μg/mL, sc-11407; Santa Cruz Biotechnologies, Dallas, TX, USA) were added, and the plates were incubated at 4ºC for 18 h. After three washes with TBST, HRP-conjugated secondary antibody against rat IgG (1:1,000, AS028; AB Clonal, Woburn, MA, USA) or rabbit IgG (1:1,000, A9169; Sigma-Aldrich) was added, and the plates were incubated at room temperature for 1 h. As a chromogenic substrate, 100 mM citrate buffer (pH 5.0) containing 0.03% (v/v) hydrogen peroxide and 1 mg/mL O-phenylenediamine dihydrochloride (Fujifilm Wako Pure Chemical Corporation) was added, and the color reaction was terminated by treatment with 1 M hydrochloric acid. The absorbance at 490 nm was measured on a microplate reader (FLUOstar Omega; BMG LABTECH, Ortenberg, Germany).
For competitive inhibition of ELISA, the MS785-MS27 antibody cocktail at 1 μg/mL was mixed with apo-WT SOD1SH over a concentration range of 10−4 to 101 μΜ at 4ºC for 18 h. The complex of MS785-MS27 and apo-WT SOD1SH was used as a primary antibody for indirect ELISA, where apo-WT SOD1SH at 5 μM was coated onto the Nunc Immuno 96-well plate. The subsequent procedures of competitive ELISA were the same as mentioned above for the indirect ELISA.
4.4. Assessment of Cell Proliferation and Cytotoxicity
The culture and maintenance of NSC-34 cells were performed as described previously [
38,
39]. In brief, NSC-34 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 g/L
D-glucose (Nacalai Tesque, Kyoto, Japan) supplemented with 10% (v/v) fetal bovine serum at 37ºC in a humidified chamber with 5% (v/v) CO
2. For experiments on cell proliferation and cytotoxicity after treatment with Zn-deficient SOD1, NSC-34 cells (1.5×10
3 cells/well) were harvested in 96-well plates (Falcon, Corning, NY, USA). Zn-deficient SOD1 species, such as apo-WT SOD1
SH or apo-SOD1
SH that lacks the Zn binding site (H63A/H71A/H80G/D83A), were dissolved in 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, pH 7.2) pretreated with a Chelex 100 Resin (100-200 mesh; Bio-Rad) followed by filtered sterilization, and were diluted in DMEM/F-12 with GlutaMAX medium (Thermo Fisher Scientific) containing 1% (v/v) non-essential amino acids (Thermo Fisher Scientific) and 1% (v/v) fetal bovine serum. The cells were exposed to the Zn-deficient SOD1 species at a concentration range from 0.01 to 10 μM for 48 h. In parallel with the exposure of the Zn-deficient SOD1, the cells were also treated with 20 mM HEPES (pH 7.2), which was used as a vehicle control. For experiments on the coordination of Zn ions to the Zn-deficient SOD1 species, 1 μM apo-WT SOD1
SH or apo-SOD1
SH that lacks the Zn binding site was treated with 1 to 4 molar equivalents of Zn ions at 4ºC for 3 h. The cell proliferation and cytotoxicity were evaluated using the Cell Counting Kit-8 and the Cytotoxicity LDH Assay Kit-WST, in accordance with the manufacturer’s protocol (Dojindo Laboratories, Kumamoto, Japan), respectively.
4.5. Immunofluorescence
Transgenic mice carrying human mutated G93A SOD1 [B6SJL-Tg(SOD1-G93A)1Gur/J, 002726] or human WT SOD1 [B6SJL-Tg(SOD1)2Gur/J, 002297] were purchased from the Jackson Laboratory (Bar Harbor, MA, USA) [
40]. Both transgenic lines were maintained through hemizygotes crossing transgenic males with F
1 non-transgenic females on a B6SJL background. All animal protocols adhered to the Nihon University Animal Committee guidelines for the care and use of laboratory animals, and were approved by the Institutional Animal Care and Use Committee of Nihon University (#2026).
G93A SOD1 mice were anesthetized with pentobarbital when they reached 60 days (presymptomatic stage), 90 days (symptomatic stage), and 120 days of age (terminal stage), and the mice were transcardially perfused with ice-cold phosphate-buffered saline (PBS, pH 7.4) followed by 4% (v/v) paraformaldehyde in PBS. The spinal cord was postfixed with 4% (v/v) paraformaldehyde in PBS (pH 7.4) at 4 °C for 24 h and embedded in paraffin using a Tissue-Tek VIP5 Jr. (Sakura Finetek, Tokyo, Japan). The lumbar spinal cords of the mice (n=3 per genotype per disease stage) were sliced into 6 μm thick sections. These sections were deparaffinized in xylene, rehydrated in ethanol, and rinsed with deionized water. Epitope retrieval was performed using an autoclave for 20 min in the presence of 10 mM citrate buffer (pH 6.0). To inhibit endogenous mouse immunoglobulins, the lumbar spinal cord sections were treated with an M.O.M. blocking reagent (Vector Labs, Newark, CA, USA) at room temperature for 1 h. Non-specific binding of antibodies was blocked with PBS (pH 7.4) containing 10% (v/v) donkey normal serum (abcam), 3% (w/v) IgG- and protease-free bovine serum albumin (Jackson Immunoresearch, West Grove, PA, USA), and 0.1% (v/v) Trion X-100 at room temperature for 1 h. The following primary antibodies were used: rat monoclonal MS785-MS27 cocktail (2.5 μg/mL, FDV-0021A; Funakoshi, Tokyo, Japan), rabbit polyclonal anti-SOD1 EDI (2.5 μg/mL, SPC-206; StressMarq Bioscences, Victoria, Canada), mouse monoclonal anti-NeuN (2.5 μg/mL, MAB377; Merck Millipore), mouse monoclonal anti-Glial fibrillary acidic protein (GFAP) cocktail (1 μg/mL, 556330; BD Bioscience, Franklin Lakes, NJ, USA), or rabbit polyclonal anti-Ionized calcium-binding adapter molecule 1 (Iba1, 1 μg/mL, 013-27691; Fujifilm Wako Pure Chemical Corporation). As secondary antibodies, Alexa Fluor 405-conjugated donkey anti-rat IgG (5 μg/mL, ab175670; abcam), Alexa Fluor 488-conjugated donkey anti-mouse IgG (5 μg/mL, ab150109; abcam), or Alexa Fluor 594-conjugated donkey anti-rabbit IgG (5 μg/mL, ab150064, abcam) was used. The sections were mounted in ProLong Diamond antifade reagent (Thermo Fisher Scientific). Fluorescence images were acquired using a confocal microscope, Laser Scanning System Z710 (Carl Zeiss, Oberkochen, Germany).
4.6. Statistics
The results are given as the mean ± SD. All statistical tests were performed using Statcel 4 software (OMS Publishing Inc., Saitama, Japan). After determining the data normality, multiple group comparisons were performed using one-way ANOVA followed by Tukey–Kramer’s post hoc test. Statistical significance was defined as P<0.05.
Author Contributions
Conceptualization, E.T.; Methodology, E.T.; Software, E.T.; Validation, E.T., Y.S., and N.T.; Formal Analysis, E.T.; Investigation, E.T., Y.S., N.T., and A.D.; Resources, Y.K.; Data Curation, E.T.; Writing – Original Draft Preparation, E.T.; Writing – Review & Editing, E.T., Y.K., and T.M.; Visualization, E.T.; Supervision, E.T.; Project Administration, E.T.; Funding Acquisition, E.T. and T.M. All authors read and accepted the final version of the manuscript.
Figure 1.
MS785-MS27 antibody cocktail recognizes Zn-deficient WT SOD1 species. (A) A representative image showing Instant Blue Coomassie staining with human wild-type SOD1 (WT) and human SOD1 that lacks the Zn binding site by the replacement of amino acids, H63A/H71A/H80G/D83A (DZn binding site). Note that SOD1 that lacks the Zn binding site showed faster mobility than WT SOD1. For analysis of SOD1 with the disulfide bond, the protein at 10 mM was treated with 40 mM iodoacetamide at 37ºC for 1 h and was subjected to SDS-PAGE under non-reducing conditions. SH = disulfide bond-cleaved SOD1; S-S = disulfide bond-formed SOD1. (B) Specificity and reproducibility of indirect ELISA coupled with MS785-MS27 antibody cocktail. Apo-WT SOD1SH at 5 mM (black) or vehicle control (Tris-buffered saline with EDTA, gray) was coated onto the 96-well plates. n = 48 per group. (C) Competitive ELISA for MS785-MS27 antibody cocktail. The antibody cocktail at 1 mg/mL was preincubated with apo-WT SOD1SH at a concentration range from 10−4 to 101 mM. The complex of the antibody cocktail and apo-WT SOD1SH was used as a primary antibody in indirect ELISA. n = 6 per concentration. (D) Indirect ELISA with the antibody cocktail for WT SOD1SH with different incorporated metal ions. n = 6 per group. (E) A plot of the ELISA signals of apo-WT SOD1SH or apo-SOD1SH that lacks the Zn binding site treated with 1 to 4 molar equivalents of Zn ions. n = 4 per group. The significance of differences was analyzed using one-way ANOVA followed by Tukey–Kramer’s post hoc test. **P<0.01 (vs. Zn-untreated apo-WT SOD1SH). N.S. = not significant. (F) Indirect ELISA with the antibody cocktail for WT SOD1S-S with different incorporated metal ions. n = 6 per group. All data are given as the mean ± SD.
Figure 1.
MS785-MS27 antibody cocktail recognizes Zn-deficient WT SOD1 species. (A) A representative image showing Instant Blue Coomassie staining with human wild-type SOD1 (WT) and human SOD1 that lacks the Zn binding site by the replacement of amino acids, H63A/H71A/H80G/D83A (DZn binding site). Note that SOD1 that lacks the Zn binding site showed faster mobility than WT SOD1. For analysis of SOD1 with the disulfide bond, the protein at 10 mM was treated with 40 mM iodoacetamide at 37ºC for 1 h and was subjected to SDS-PAGE under non-reducing conditions. SH = disulfide bond-cleaved SOD1; S-S = disulfide bond-formed SOD1. (B) Specificity and reproducibility of indirect ELISA coupled with MS785-MS27 antibody cocktail. Apo-WT SOD1SH at 5 mM (black) or vehicle control (Tris-buffered saline with EDTA, gray) was coated onto the 96-well plates. n = 48 per group. (C) Competitive ELISA for MS785-MS27 antibody cocktail. The antibody cocktail at 1 mg/mL was preincubated with apo-WT SOD1SH at a concentration range from 10−4 to 101 mM. The complex of the antibody cocktail and apo-WT SOD1SH was used as a primary antibody in indirect ELISA. n = 6 per concentration. (D) Indirect ELISA with the antibody cocktail for WT SOD1SH with different incorporated metal ions. n = 6 per group. (E) A plot of the ELISA signals of apo-WT SOD1SH or apo-SOD1SH that lacks the Zn binding site treated with 1 to 4 molar equivalents of Zn ions. n = 4 per group. The significance of differences was analyzed using one-way ANOVA followed by Tukey–Kramer’s post hoc test. **P<0.01 (vs. Zn-untreated apo-WT SOD1SH). N.S. = not significant. (F) Indirect ELISA with the antibody cocktail for WT SOD1S-S with different incorporated metal ions. n = 6 per group. All data are given as the mean ± SD.
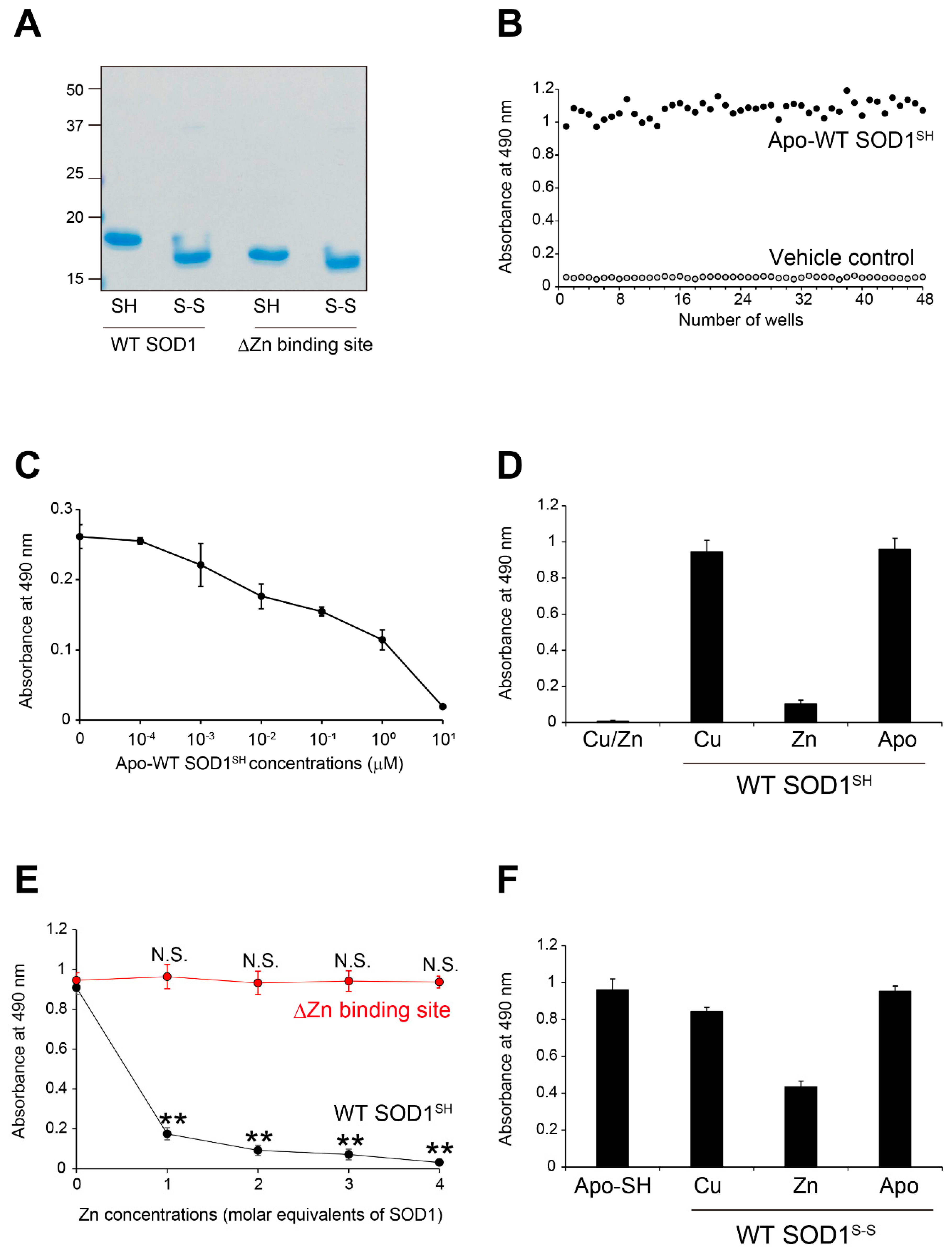
Figure 2.
Recognition by MS785-MS27 antibody cocktail of conformation-disordered WT SOD1 species. Conformation-disordered WT SOD1 species, including unfolded, oligomeric, and aggregated forms, were prepared from apo-WT SOD1S-S as a precursor. (A, B) Indirect ELISA using the conformation-disordered WT SOD1 species analyzed with (A) MS785-MS27 antibody cocktail and (B) anti-pan SOD1 antibody that reacts with various SOD1 conformers. Apo-WT SOD1SH was used as an internal control for the comparisons among different 96-well plates. All data are given as the mean ± SD (n = 6 per group).
Figure 2.
Recognition by MS785-MS27 antibody cocktail of conformation-disordered WT SOD1 species. Conformation-disordered WT SOD1 species, including unfolded, oligomeric, and aggregated forms, were prepared from apo-WT SOD1S-S as a precursor. (A, B) Indirect ELISA using the conformation-disordered WT SOD1 species analyzed with (A) MS785-MS27 antibody cocktail and (B) anti-pan SOD1 antibody that reacts with various SOD1 conformers. Apo-WT SOD1SH was used as an internal control for the comparisons among different 96-well plates. All data are given as the mean ± SD (n = 6 per group).
Figure 3.
Recognition by MS785-MS27 antibody cocktail of ALS-linked SOD1 mutants with distinct biophysical properties. (A) The location of the ALS-linked SOD1 mutations, including A4V, G37R, H46R, G85R, D90A, G93A, and I113T. They are categorized into three groups: (i) wild-type-like mutants (blue; G37R, D90A, and G93A), (ii) dimer interface mutants (green; A4V and I113T), and (iii) metal binding region mutants (red; H46R and G85R). Each mutation is highlighted in the X-ray crystal structure of human WT SOD1 (PDB: 2C9V). Cu and Zn ions are represented by cyan and pink colors, respectively. (B) A representative image showing Instant Blue Coomassie staining with WT SOD1 and the ALS-linked SOD1 mutants. For the analysis of SOD1 with a disulfide bond, the protein at 10 mM was treated with 40 mM iodoacetamide at 37ºC for 1 h and subjected to SDS-PAGE under non-reducing conditions. SH = disulfide bond-cleaved SOD1; S-S = disulfide bond-formed SOD1. (C–G) Indirect ELISA of the recognition by MS785-MS27 antibody cocktail of (C) apo-SOD1SH, (D) apo-SOD1S-S, (E) unfolded SOD1SH, (F) SOD1 oligomers, and (G) SOD1 aggregates with the ALS-linked mutantions. Apo-WT SOD1SH (black, WT-SH) was used as an internal control. Data are given as the mean ± SD (n = 6 per mutant). (H) A plot of the ELISA signals of apo-SOD1SH with the ALS-linked mutations treated with 1 to 4 molar equivalents of Zn ions. Data are given as the mean ± SD (n = 3 per mutant). The significance of differences was analyzed using one-way ANOVA followed by Tukey–Kramer’s post hoc test. *P<0.05, **P<0.01 (vs. the same Zn-untreated mutant). N.S. = not significant.
Figure 3.
Recognition by MS785-MS27 antibody cocktail of ALS-linked SOD1 mutants with distinct biophysical properties. (A) The location of the ALS-linked SOD1 mutations, including A4V, G37R, H46R, G85R, D90A, G93A, and I113T. They are categorized into three groups: (i) wild-type-like mutants (blue; G37R, D90A, and G93A), (ii) dimer interface mutants (green; A4V and I113T), and (iii) metal binding region mutants (red; H46R and G85R). Each mutation is highlighted in the X-ray crystal structure of human WT SOD1 (PDB: 2C9V). Cu and Zn ions are represented by cyan and pink colors, respectively. (B) A representative image showing Instant Blue Coomassie staining with WT SOD1 and the ALS-linked SOD1 mutants. For the analysis of SOD1 with a disulfide bond, the protein at 10 mM was treated with 40 mM iodoacetamide at 37ºC for 1 h and subjected to SDS-PAGE under non-reducing conditions. SH = disulfide bond-cleaved SOD1; S-S = disulfide bond-formed SOD1. (C–G) Indirect ELISA of the recognition by MS785-MS27 antibody cocktail of (C) apo-SOD1SH, (D) apo-SOD1S-S, (E) unfolded SOD1SH, (F) SOD1 oligomers, and (G) SOD1 aggregates with the ALS-linked mutantions. Apo-WT SOD1SH (black, WT-SH) was used as an internal control. Data are given as the mean ± SD (n = 6 per mutant). (H) A plot of the ELISA signals of apo-SOD1SH with the ALS-linked mutations treated with 1 to 4 molar equivalents of Zn ions. Data are given as the mean ± SD (n = 3 per mutant). The significance of differences was analyzed using one-way ANOVA followed by Tukey–Kramer’s post hoc test. *P<0.05, **P<0.01 (vs. the same Zn-untreated mutant). N.S. = not significant.
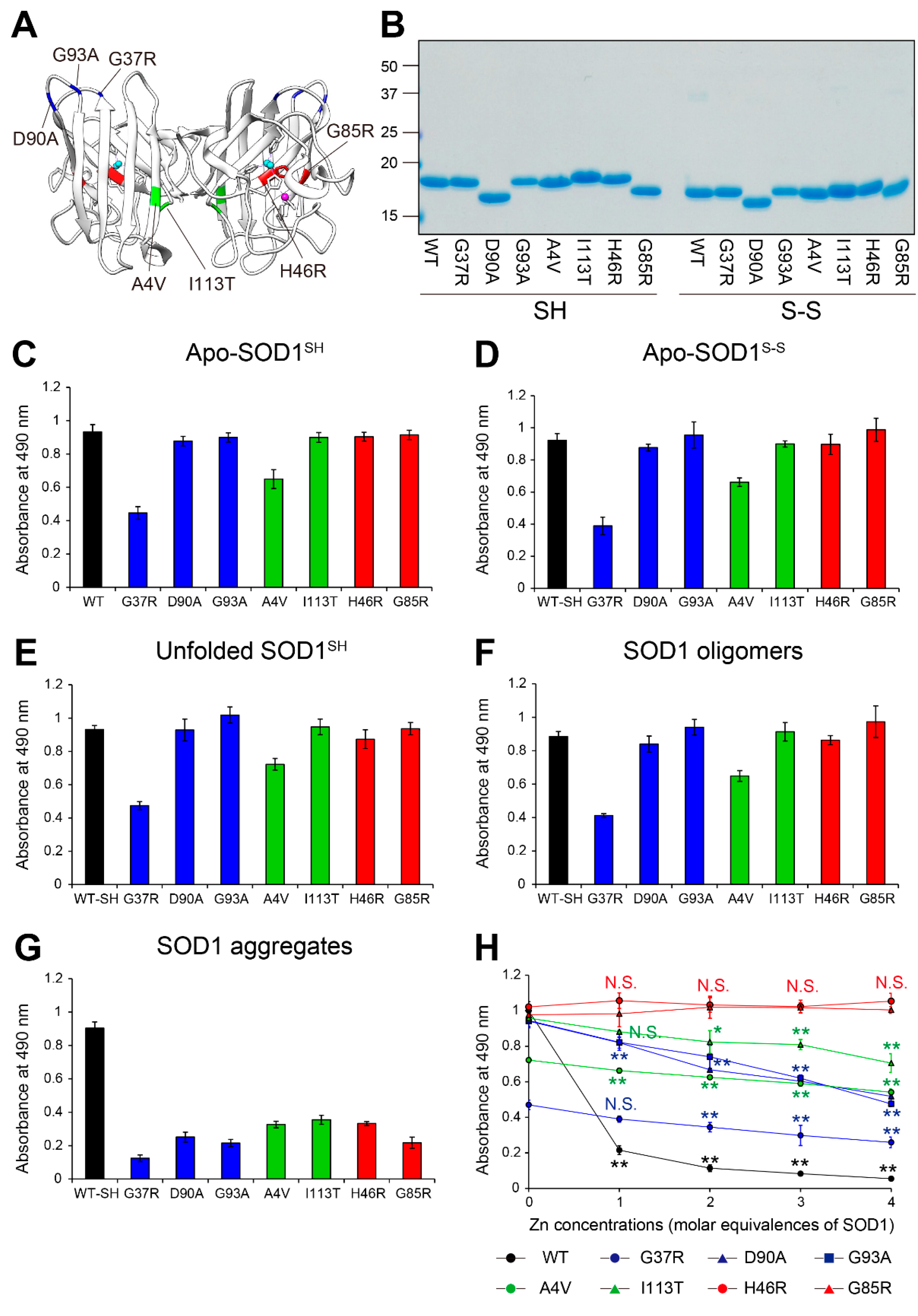
Figure 4.
Toxicological features of MS785-MS27-reactive SOD1 species against NSC-34 cells. (A, B) NSC-34, a cell model of motor neurons, was treated with apo-WT SOD1SH (black) or apo-SOD1SH that lacks the Zn binding site (red) at the indicated concentrations (0.01 mM to 10 mM) for 48 h. HEPES buffer was used as a vehicle control. (A) Cell proliferation and (B) cytotoxicity were assessed by CCK-8 and LDH assays, respectively. The right panels in A and B represent enlargements of the dotted area in the left panels. The significance of differences was analyzed using one-way ANOVA followed by Tukey–Kramer’s post hoc test. *P<0.05, **P<0.01 vs. HEPES-treated NSC-34. N.S. = not significant. (C, D) Apo-WT SOD1SH (black) or apo-SOD1SH that lacks the Zn binding site at 1 mM (red) was treated with 1 to 4 molar equivalents of Zn ions. NSC-34 cells were exposed to the Zn-pretreated SOD1 species. NSC-34 cells were also exposed to ZnSO4 resolved in HEPES buffer. (C) Cell proliferation and (B) cytotoxicity were assessed by CCK-8 and LDH assays, respectively. The significance of differences was analyzed using one-way ANOVA followed by Tukey–Kramer’s post hoc test. **P<0.01 vs. HEPES-treated NSC-34. N.S. = not significant. All data are given as the mean ± SD (n = 6 per group).
Figure 4.
Toxicological features of MS785-MS27-reactive SOD1 species against NSC-34 cells. (A, B) NSC-34, a cell model of motor neurons, was treated with apo-WT SOD1SH (black) or apo-SOD1SH that lacks the Zn binding site (red) at the indicated concentrations (0.01 mM to 10 mM) for 48 h. HEPES buffer was used as a vehicle control. (A) Cell proliferation and (B) cytotoxicity were assessed by CCK-8 and LDH assays, respectively. The right panels in A and B represent enlargements of the dotted area in the left panels. The significance of differences was analyzed using one-way ANOVA followed by Tukey–Kramer’s post hoc test. *P<0.05, **P<0.01 vs. HEPES-treated NSC-34. N.S. = not significant. (C, D) Apo-WT SOD1SH (black) or apo-SOD1SH that lacks the Zn binding site at 1 mM (red) was treated with 1 to 4 molar equivalents of Zn ions. NSC-34 cells were exposed to the Zn-pretreated SOD1 species. NSC-34 cells were also exposed to ZnSO4 resolved in HEPES buffer. (C) Cell proliferation and (B) cytotoxicity were assessed by CCK-8 and LDH assays, respectively. The significance of differences was analyzed using one-way ANOVA followed by Tukey–Kramer’s post hoc test. **P<0.01 vs. HEPES-treated NSC-34. N.S. = not significant. All data are given as the mean ± SD (n = 6 per group).

Figure 5.
MS785-MS27-reactive SOD1 species are distributed in motor neurons throughout the disease course of G93A SOD1 mice. (A–E) Confocal imaging of the lumbar spinal cord sections dually immunostained with MS785-MS27 (blue) and NeuN (green), a marker of neurons. G93A SOD1 mice at different disease stages, namely, (A) presymptomatic (60 days), (B) symptomatic (90 days), and (C) terminal (120 days) stages, were used. (D) Mice expressing human WT SOD1 and (E) non-transgenic (Non-Tg) mice at 120 days of age were also analyzed. n = 3 per genotype. Insets represent enlargements of the dotted area. (F–H) Confocal imaging of the lumbar spinal cord from G93A SOD1 mice at (F) presymptomatic, (G) symptomatic, and (H) terminal stages. The sections were immunostained with the MS785-MS27 cocktail (blue) and GFAP (green), a marker of astrocytes. (I–K) Confocal imaging of the lumbar spinal cord sections immunostained with the MS785-MS27 cocktail (blue) and Iba1 (red), a marker of microglia. G93A SOD1 mice at different disease stages, namely, (I) presymptomatic, (J) symptomatic, and (K) terminal stages, were used. n = 3 per disease stage. Scale bars = 50 mm.
Figure 5.
MS785-MS27-reactive SOD1 species are distributed in motor neurons throughout the disease course of G93A SOD1 mice. (A–E) Confocal imaging of the lumbar spinal cord sections dually immunostained with MS785-MS27 (blue) and NeuN (green), a marker of neurons. G93A SOD1 mice at different disease stages, namely, (A) presymptomatic (60 days), (B) symptomatic (90 days), and (C) terminal (120 days) stages, were used. (D) Mice expressing human WT SOD1 and (E) non-transgenic (Non-Tg) mice at 120 days of age were also analyzed. n = 3 per genotype. Insets represent enlargements of the dotted area. (F–H) Confocal imaging of the lumbar spinal cord from G93A SOD1 mice at (F) presymptomatic, (G) symptomatic, and (H) terminal stages. The sections were immunostained with the MS785-MS27 cocktail (blue) and GFAP (green), a marker of astrocytes. (I–K) Confocal imaging of the lumbar spinal cord sections immunostained with the MS785-MS27 cocktail (blue) and Iba1 (red), a marker of microglia. G93A SOD1 mice at different disease stages, namely, (I) presymptomatic, (J) symptomatic, and (K) terminal stages, were used. n = 3 per disease stage. Scale bars = 50 mm.
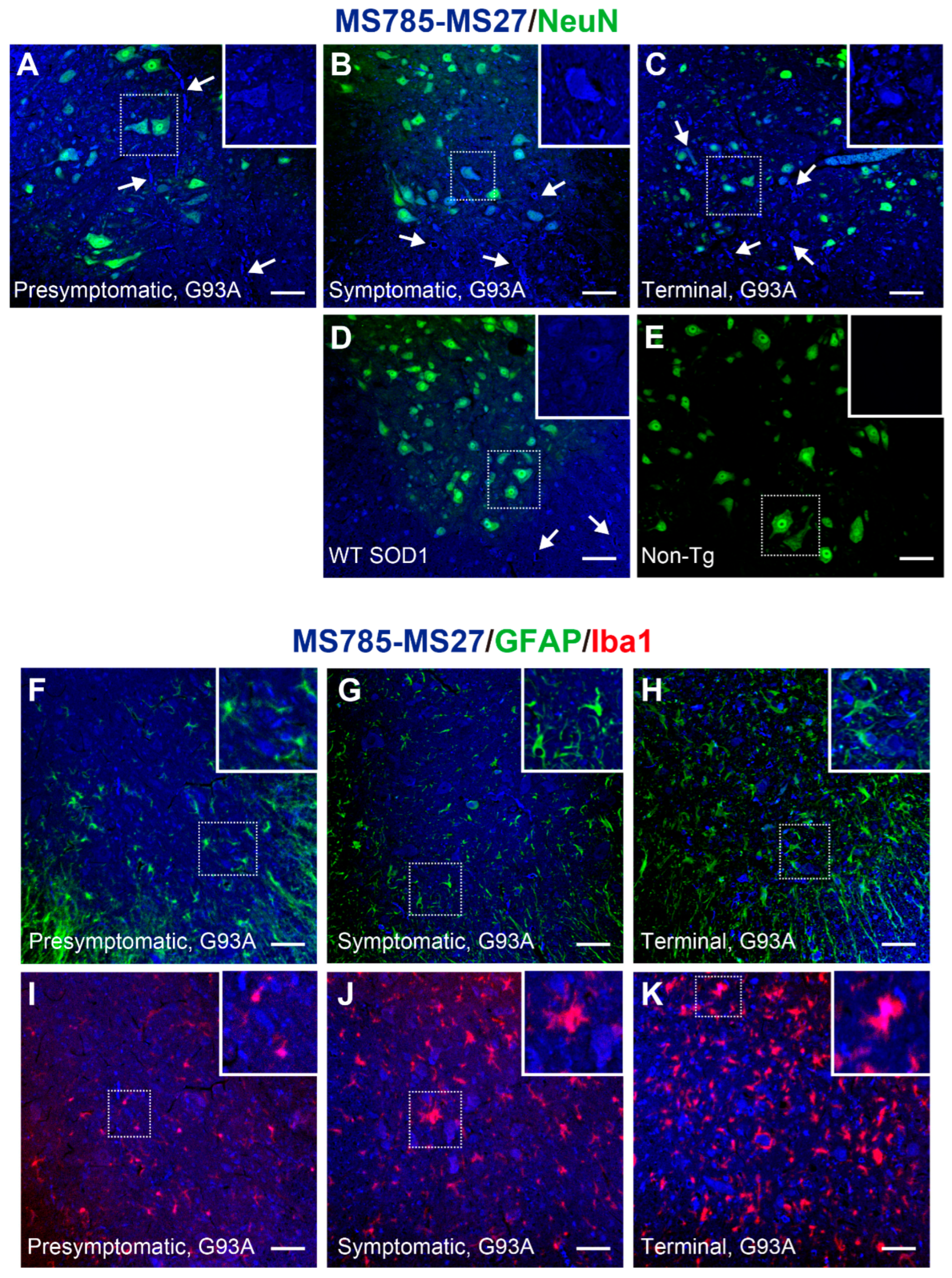
Figure 6.
Conformational heterogeneity of misfolded SOD1 species in G93A SOD1 mice revealed by co-immunostaining with MS785-MS27 cocktail and EDI (A–C) Confocal imaging of lumbar spinal cord sections from G93A SOD1 mice at (A) presymptomatic (60 days), (B) symptomatic (90 days), and (C) terminal (120 days) stages. The sections were dually immunostained with the MS785-MS27 cocktail (blue) and EDI (red), an antibody specific to misfolded SOD1 species for which the epitope is exposed at the dimer interface. n = 3 per disease stage. Scale bars = 50 mm.
Figure 6.
Conformational heterogeneity of misfolded SOD1 species in G93A SOD1 mice revealed by co-immunostaining with MS785-MS27 cocktail and EDI (A–C) Confocal imaging of lumbar spinal cord sections from G93A SOD1 mice at (A) presymptomatic (60 days), (B) symptomatic (90 days), and (C) terminal (120 days) stages. The sections were dually immunostained with the MS785-MS27 cocktail (blue) and EDI (red), an antibody specific to misfolded SOD1 species for which the epitope is exposed at the dimer interface. n = 3 per disease stage. Scale bars = 50 mm.