Submitted:
29 March 2024
Posted:
02 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
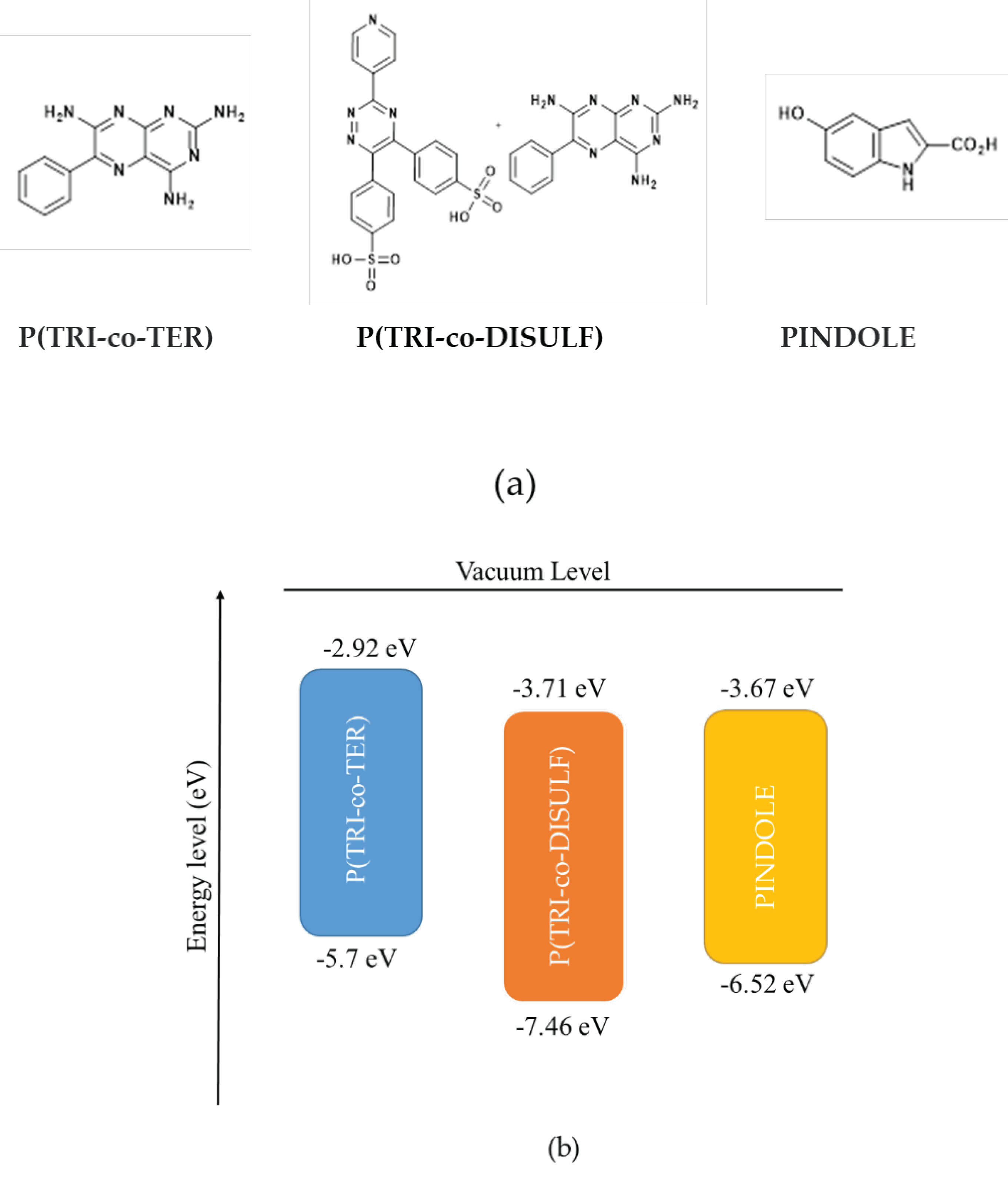
2.1. Materials
2.2. Device Fabrication
2.3. Active Layers and Devices Characterization
3. Results and Discussion
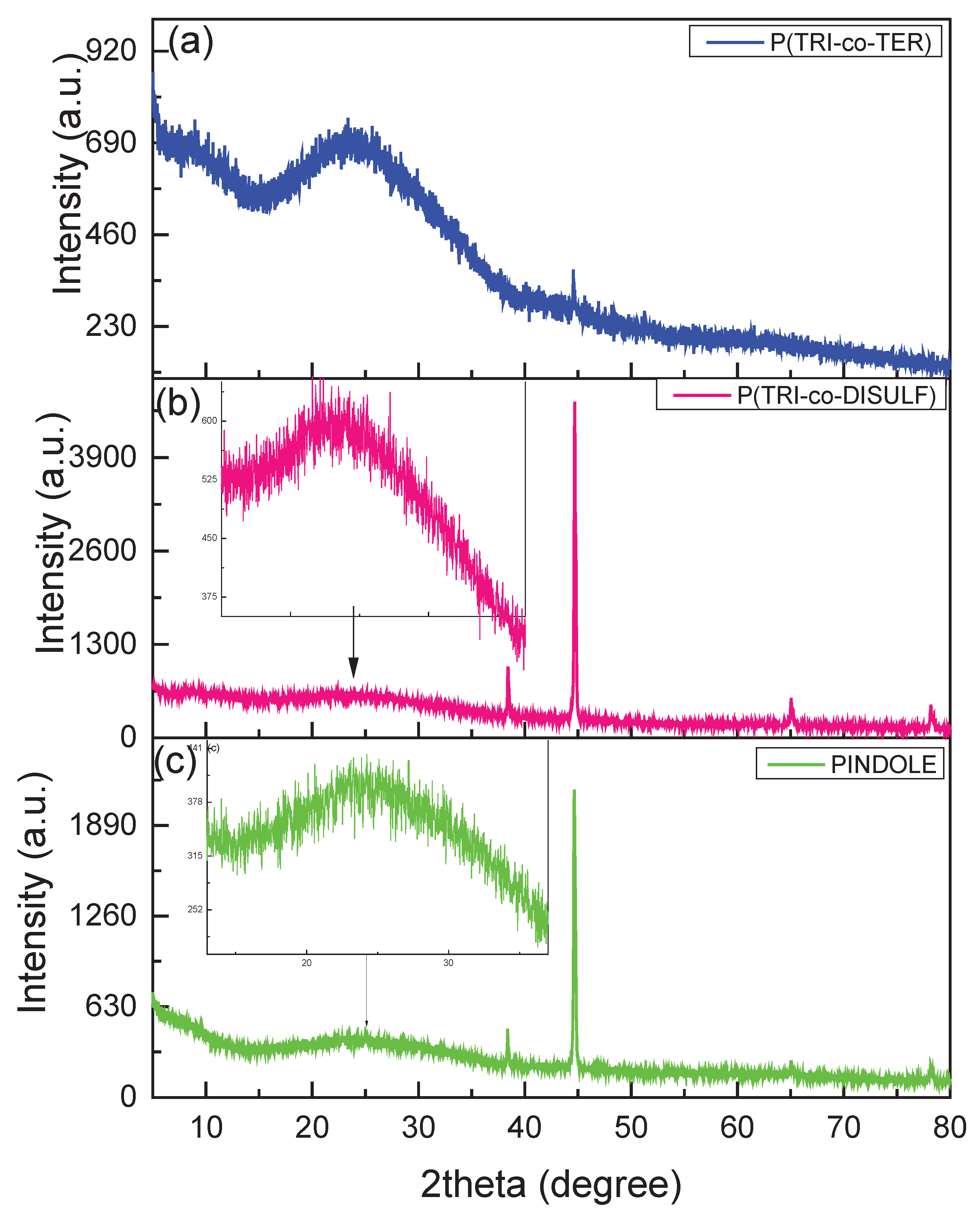
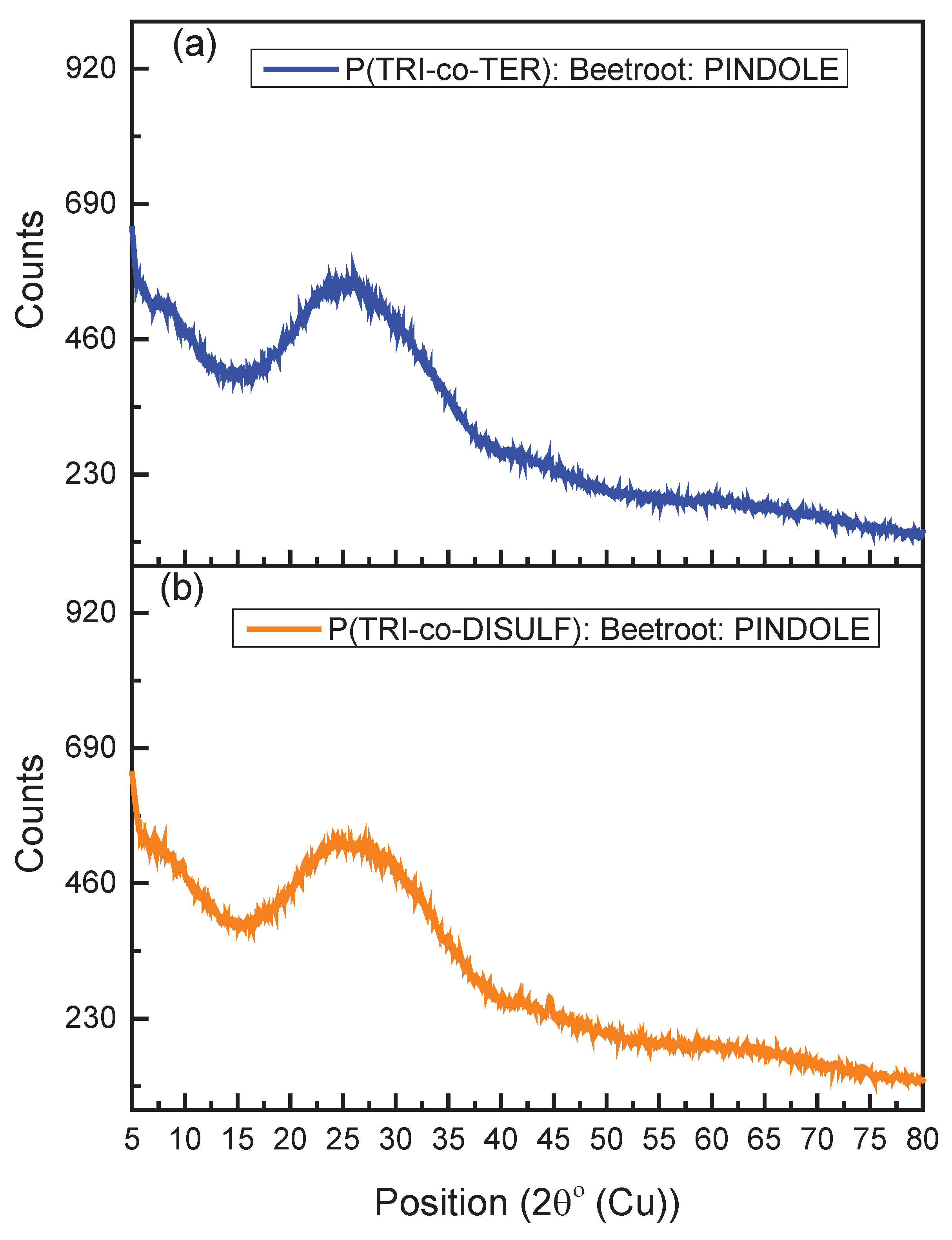
3.1. XRD Analysis
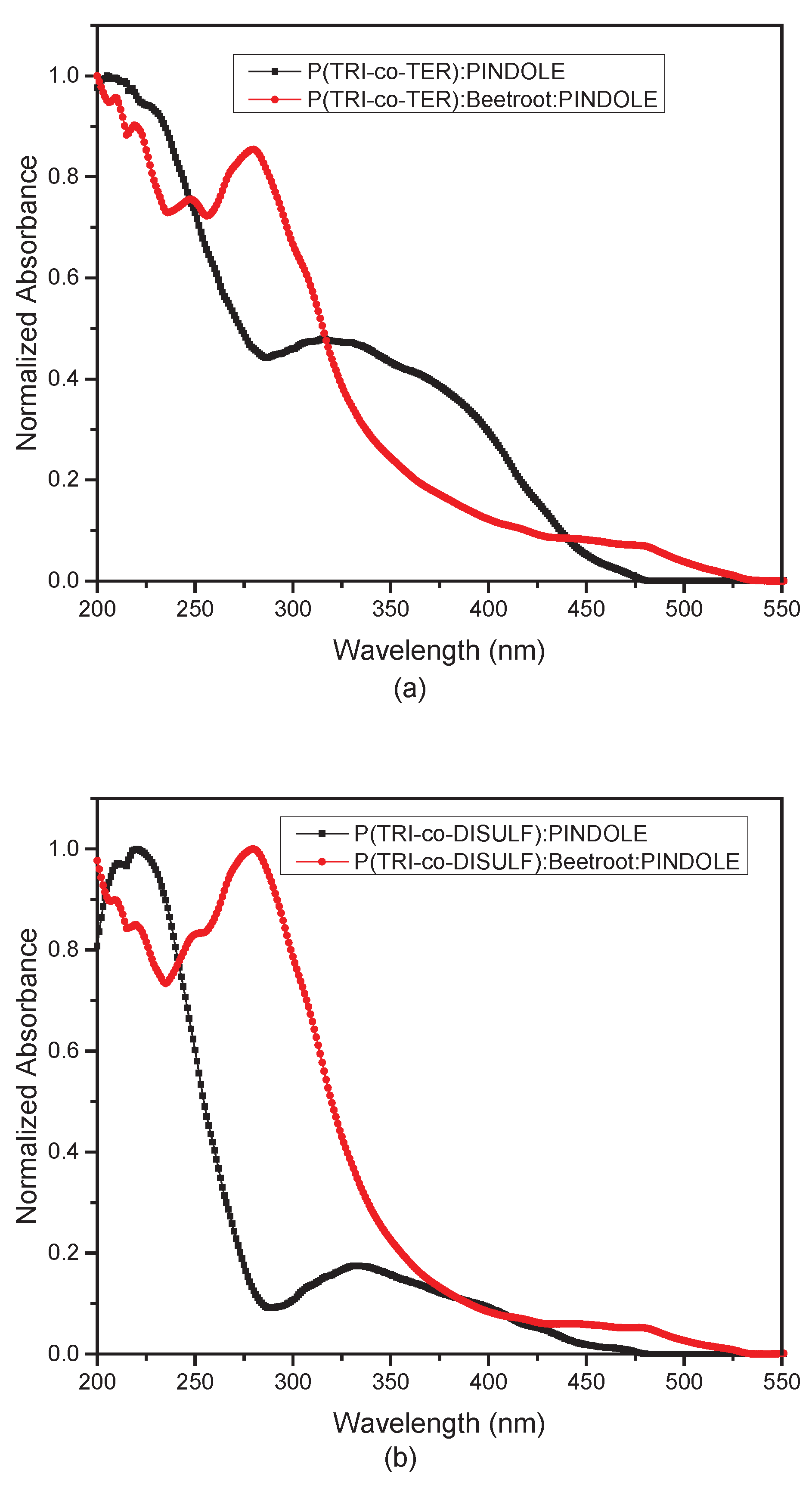
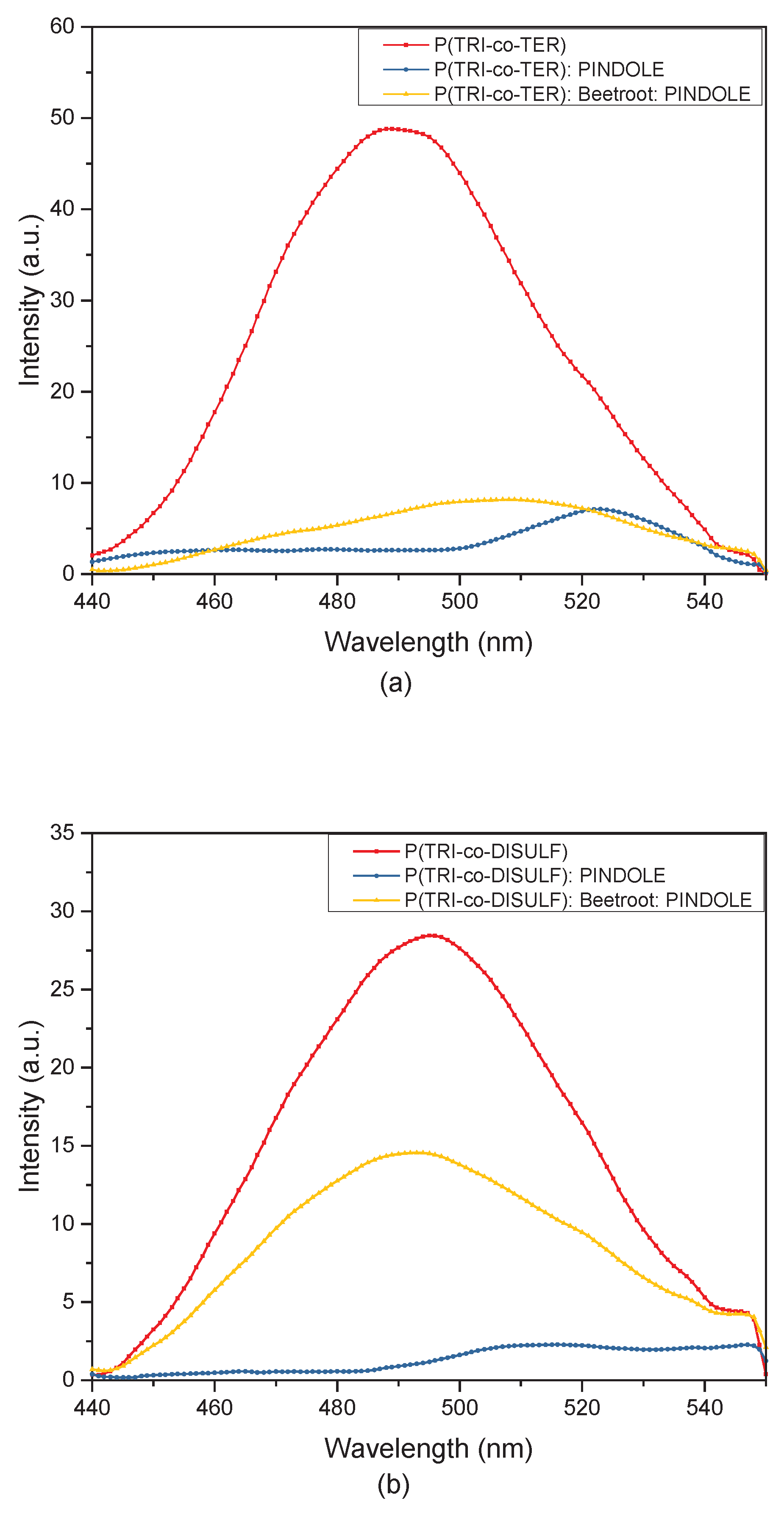
3.2. Optical Study
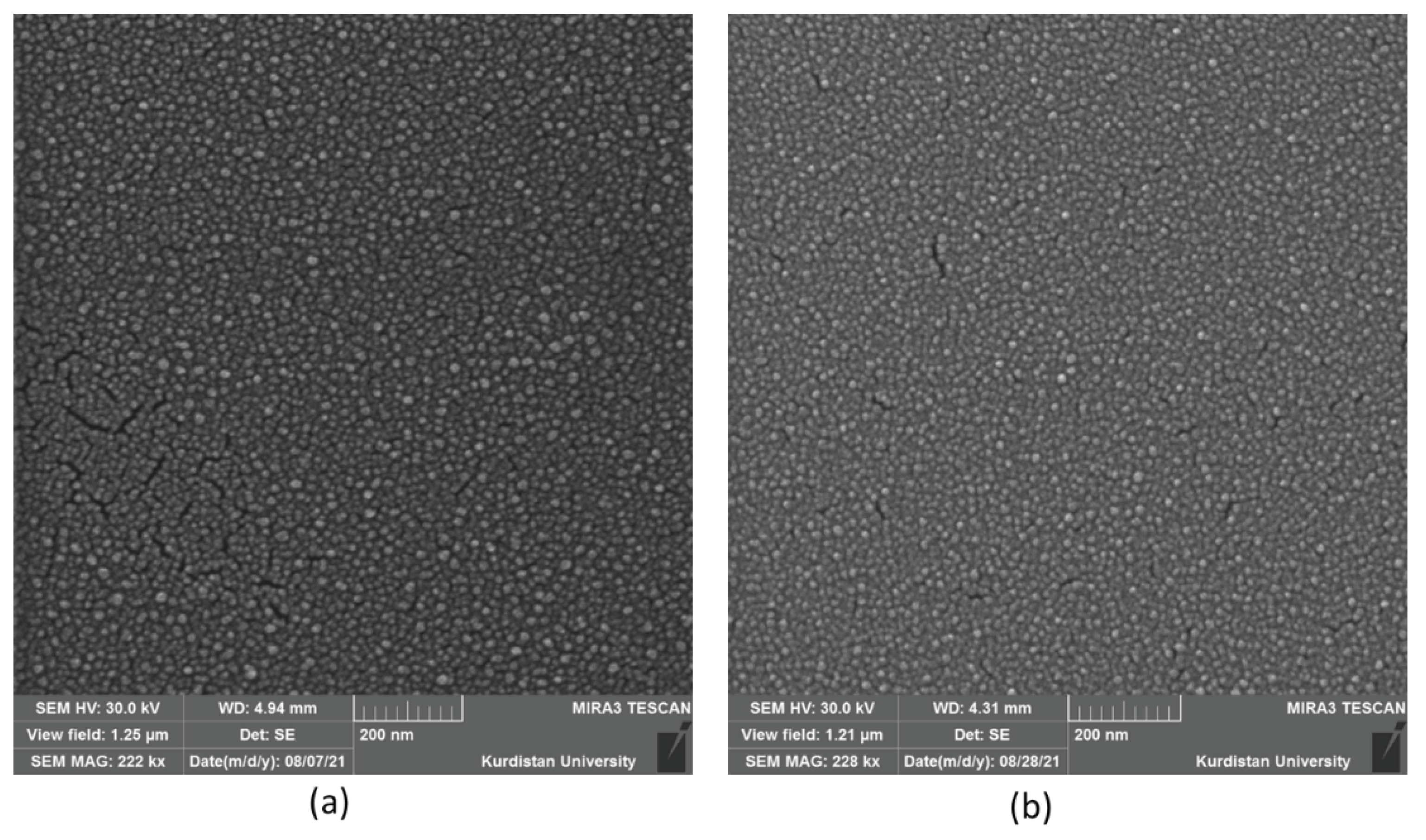
3.3. Morphology Investigation
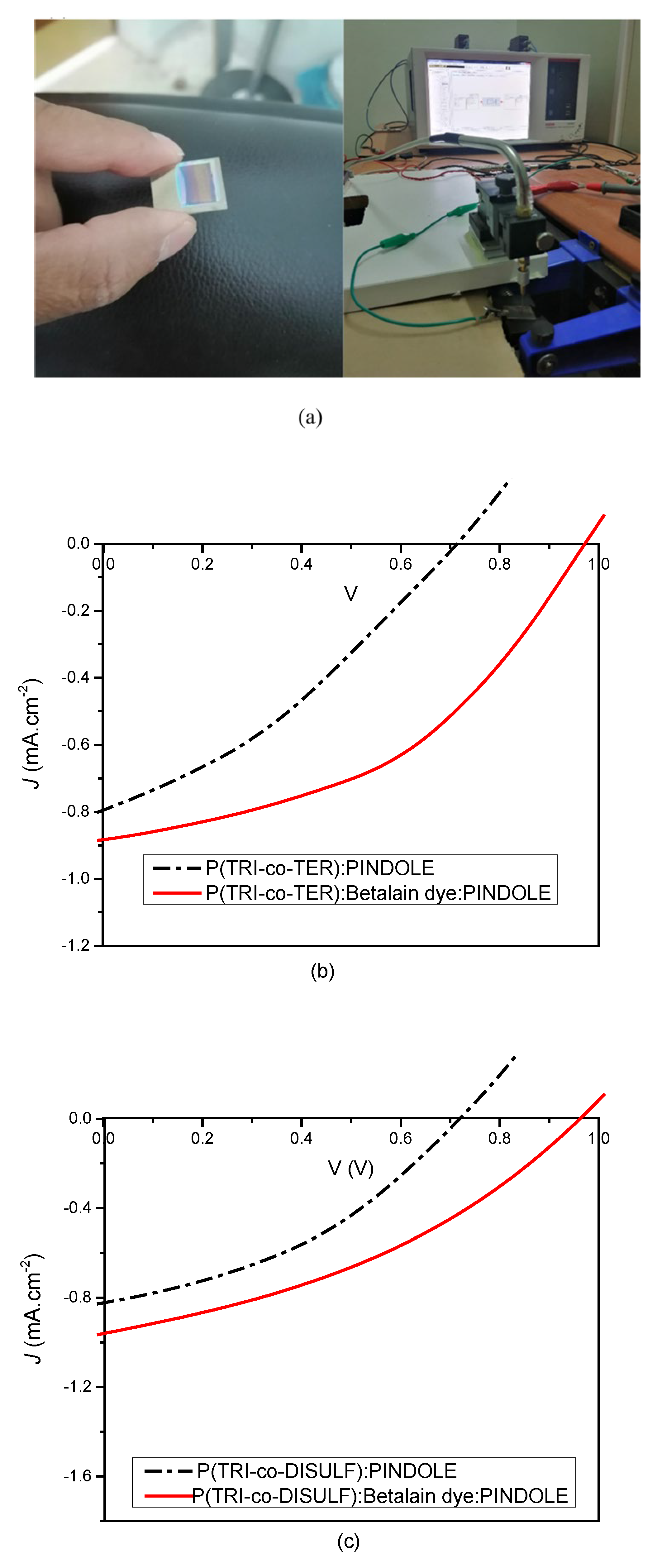
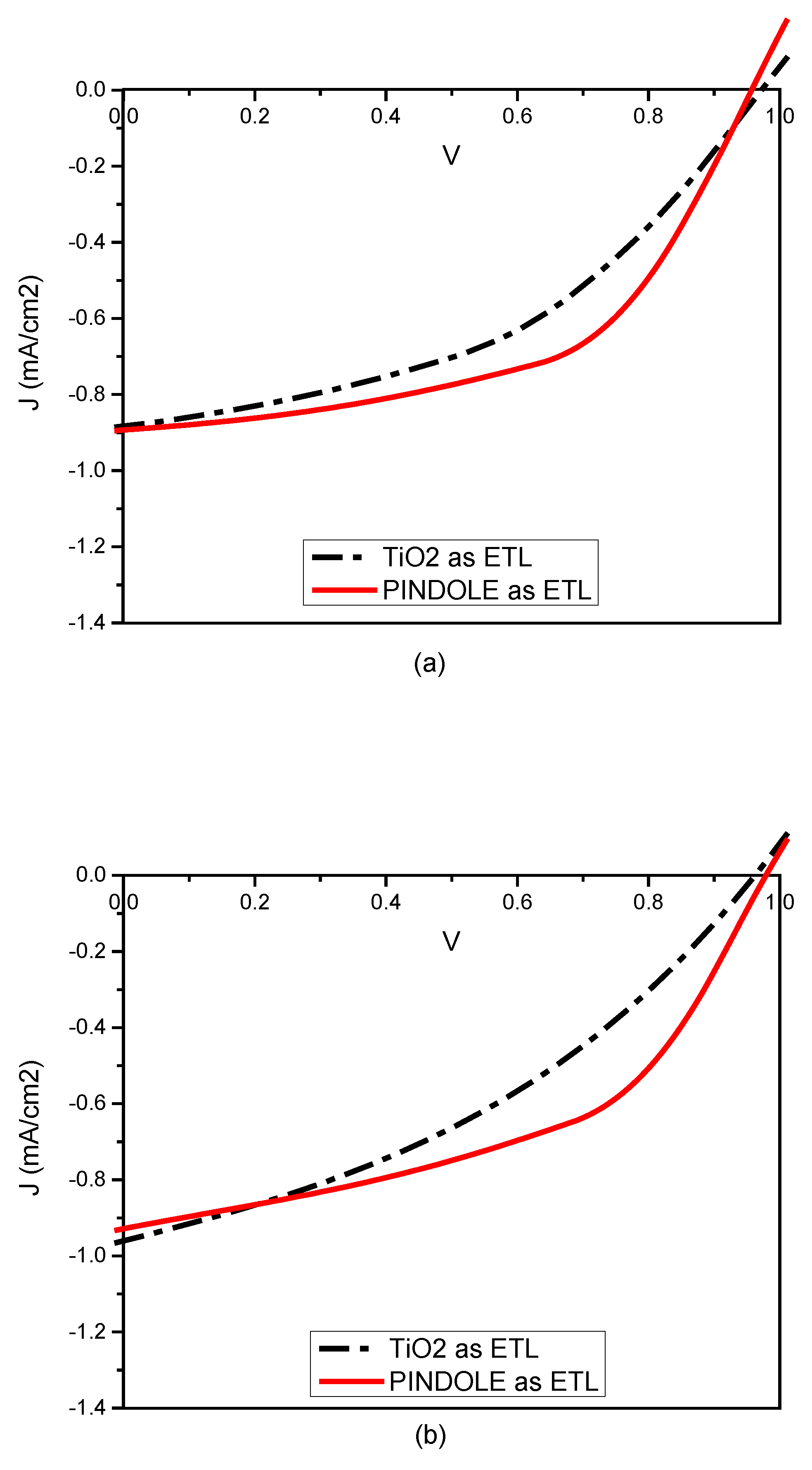
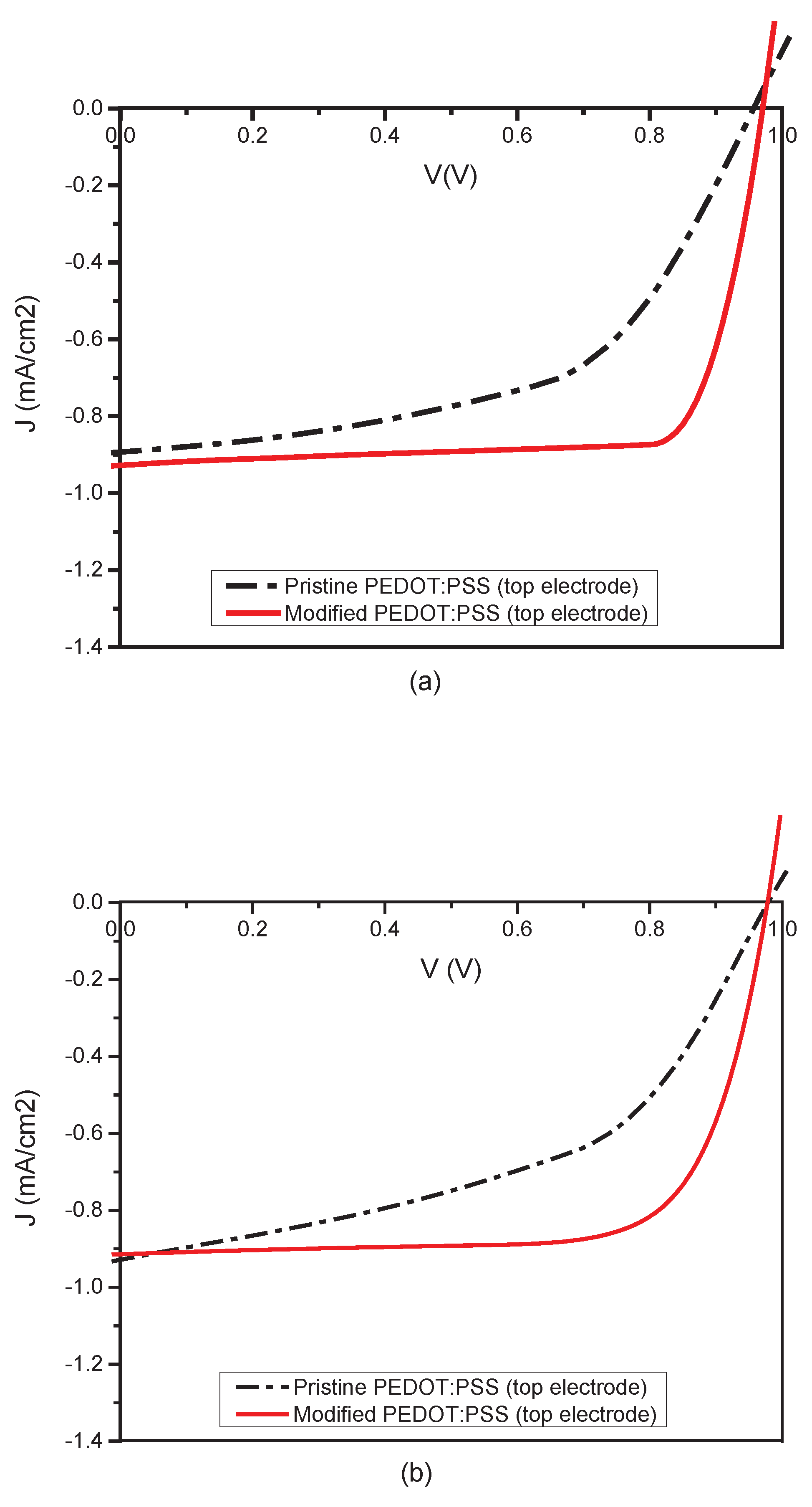
3.4. Device Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA (2021), Renewable Power, IEA, Paris https://www.iea.org/reports/renewable-power.
- Tan, C.A.-W.; Wong, B.T. Unraveling the Mystery of Ternary Organic Solar Cells: A Review on the Influence of Third Component on Structure–Morphology–Performance Relationships. Solar RRL 2021, 5, 2100503. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Roy, V.A.L.; Low, K.-H.; Che, C.-M. Bulk heterojunction photovoltaic cells based on tetra-methyl substituted copper(ii) phthalocyanine : P3HT : PCBM composite. Chemical Communications 2011, 47, 9654–9656. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, N.; Jiao, X.; Heumueller, T.; Baran, D.; Matt, G.J.; Fladischer, S.; Spiecker, E.; Ade, H.; Brabec, C.J.; Ameri, T. Designing ternary blend bulk heterojunction solar cells with reduced carrier recombination and a fill factor of 77%. Nature Energy 2016, 1, 16118. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Cheng, P.; Li, G.; Yang, Y. Transparent polymer photovoltaics for solar energy harvesting and beyond. Joule 2018, 2, 1039–1054. [Google Scholar] [CrossRef]
- Anctil, A.; Lee, E.; Lunt, R.R. Net energy and cost benefit of transparent organic solar cells in building-integrated applications. Applied Energy 2020, 261, 114429. [Google Scholar] [CrossRef]
- Brus, V.V.; Lee, J.; Luginbuhl, B.R.; Ko, S.J.; Bazan, G.C.; Nguyen, T.Q. Solution-Processed Semitransparent Organic Photovoltaics: From Molecular Design to Device Performance. Advanced Materials 2019, 31, 1900904. [Google Scholar] [CrossRef] [PubMed]
- Kouijzer, S.; Li, W.; Wienk, M.; Janssen, R. Charge transfer state energy in ternary bulk-heterojunction polymer–fullerene solar cells. Journal of Photonics for Energy 2014, 5, 057203–057203. [Google Scholar] [CrossRef]
- Torimtubun, A.A.A.; Follana-Berná, J.; Sánchez, J.G.; Pallarès, J.; Sastre-Santos, Á.; Marsal, L.F. Fluorinated Zinc and Copper Phthalocyanines as Efficient Third Components in Ternary Bulk Heterojunction Solar Cells. ACS Applied Energy Materials 2021, 4, 5201–5211. [Google Scholar] [CrossRef]
- Savoie, B.M.; Dunaisky, S.; Marks, T.J.; Ratner, M.A. The Scope and Limitations of Ternary Blend Organic Photovoltaics. Advanced Energy Materials 2015, 5, 1400891. [Google Scholar] [CrossRef]
- Khlyabich, P.P.; Rudenko, A.E.; Street, R.A.; Thompson, B.C. Influence of Polymer Compatibility on the Open-Circuit Voltage in Ternary Blend Bulk Heterojunction Solar Cells. ACS Applied Materials & Interfaces 2014, 6, 9913–9919. [Google Scholar]
- Goh, T.; Huang, J.-S.; Bartolome, B.; Sfeir, M.Y.; Vaisman, M.; Lee, M.L.; Taylor, A.D. Panchromatic polymer–polymer ternary solar cells enhanced by Förster resonance energy transfer and solvent vapor annealing. Journal of Materials Chemistry A 2015, 3, 18611–18621. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, H.; Price, S.C.; You, W. Parallel-like bulk heterojunction polymer solar cells. Journal of the American Chemical Society 2012, 134, 5432–5435. [Google Scholar] [CrossRef]
- Amin, P.O.; Muhammadsharif, F.F.; Saeed, S.R.; Ketuly, K.A. A Review of the Improvements in the Performance and Stability of Ternary Semi-Transparent Organic Solar Cells: Material and Architectural Approaches. Sustainability 2023, 15, 12442. [Google Scholar] [CrossRef]
- Amin, P.O.; Ketuly, K.A.; Saeed, S.R.; Muhammadsharif, F.F.; Symes, M.D.; Paul, A.; Sulaiman, K. Synthesis, spectroscopic, electrochemical and photophysical properties of high band gap polymers for potential applications in semi-transparent solar cells. BMC Chemistry 2021, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Amin, P.O.; Muhammadsharif, F.F.; Saeed, S.R.; Ketuly, K.A.; Sulaiman, K. The effect of donor-π-acceptor unit on the optoelectronic parameters of poly(triamterene-co-terephthalate):betalain dye composite system. AIP Conference Proceedings 2022, 2398, 020068. [Google Scholar]
- Amin, P.O.; Muhammadsharif, F.F.; Saeed, S.R.; Sulaiman, K. A Study On the Optoelectronic Parameters of Natural Dyes Extracted from Beetroot, Cabbage, Walnut, and Henna for Potential Applications in Organic Electronics. Journal of Fluorescence 2022, 32, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kazemifard, S.; Naji, L.; Afshar Taromi, F. Enhancing the photovoltaic performance of bulk heterojunction polymer solar cells by adding Rhodamine B laser dye as co-sensitizer. Journal of Colloid and Interface Science 2018, 515, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Bliznyuk, V.N.; Gasiorowski, J.; Ishchenko, A.A.; Bulavko, G.V.; Rahaman, M.; Hingerl, K.; Zahn, D.R.T.; Sariciftci, N.S. Photovoltaic cells based on ternary P3HT:PCBM:polymethine dye active layer transparent in the visible range of light. Applied Surface Science 2016, 389, 419–427. [Google Scholar] [CrossRef]
- Hwang, H.; Sin, D.H.; Park, C.; Cho, K. Ternary Organic Solar Cells Based on a Wide-Bandgap Polymer with Enhanced Power Conversion Efficiencies. Scientific Reports 2019, 9, 12081. [Google Scholar] [CrossRef]
- Amin, P.O.; Muhammadsharif, F.F.; Saeed, S.R.; Ketuly, K.A. A new approach to optimize the active layers of photovoltaic devices using area under the curve of absorption profile. Optical and Quantum Electronics 2022, 54, 510. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Ye, L.; Cui, Y.; Fan, H.; Hou, J. Investigations of the Conjugated Polymers Based on Dithienogermole (DTG) Units for Photovoltaic Applications. Macromolecules 2014, 47, 5558–5565. [Google Scholar] [CrossRef]
- Mir, F.A. Structural, morphological and ac conductivity study of PrFe0.5Ni0.5O3 thin film. Microelectronic Engineering 2014, 122, 59–63. [Google Scholar] [CrossRef]
- Ahmed, B.; Raghuvanshi, S.; Srivastava, A.; Krishna, J.; Wahab, M. Optical and structural study of aromatic polymers irradiated by gamma radiation. 2012.
- Wan, J.; Chen, Z.; Zeng, L.; Liao, X.; He, Q.; Liu, S.; Zhu, P.; Zhu, H.; Chen, Y. Realizing high-performance organic solar cells through precise control of HOMO driving force based on ternary alloy strategy. Journal of Energy Chemistry 2022, 65, 133–140. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Z.-G.; Bin, H.; Xue, L.; Yang, Y.; Wang, C.; Liu, F.; Russell, T.P.; Li, Y. High-Efficiency Nonfullerene Polymer Solar Cells with Medium Bandgap Polymer Donor and Narrow Bandgap Organic Semiconductor Acceptor. Advanced Materials 2016, 28, 8288–8295. [Google Scholar] [PubMed]
- Yuanshuai Huang, F.W. , Min Zhang, Suli Mei, Ping Shen, Songting Tan. Synthesis and photovoltaic properties of conjugated polymers with an asymmetric 4-(2-ethylhexyloxy)-8-(2-ethylhexylthio)benzo[1,2-b:4,5-b] dithiophene unit. Dyes and Pigments 2015, 115, 58–66. [Google Scholar] [CrossRef]
- Jeong I, C.S. , Yi A, Kim J, Cho JH, Kim HJ, Suh H. Syntheses and photovoltaic properties of 6-(2-thienyl)-4H-thieno[3,2-b]indole based conjugated polymers containing fluorinated benzothiadiazole. Polymer 2017.
- Wang J, Y.P. , Wu Y, Liu G, Cui C, Shen P. Synthesis and optoelectronic property manipulation of conjugated polymer photovoltaic materials based on benzo[d]-dithieno[3,2-b;2′,3′-f]azepine. Polymer 2018.
- Joo Young Shim, T.K. , Juae Kim,, Jinwoo Kim, Il Kim,, Jin Young Kim, Hongsuk Suh. Trifluoromethyl benzimidazole-based conjugated polymers with deep HOMO levels for organic photovoltaics. Synthetic Metals 2015, 205, 112–120. [Google Scholar] [CrossRef]
- Juae Kima, Sangmin Chae, Ahra Yi, Seungyeon Hong, Hyo Jung Kim, Hongsuk Suh. Characterization of push-pull type of conjugated polymers containing 8H-thieno[2,3-b]indole for organic photovoltaics. Synthetic Metals 2018, 245, 267–275. [Google Scholar] [CrossRef]
- An, Q.; Zhang, F.; Zhang, J.; Tang, W.; Deng, Z.; Hu, B. Versatile ternary organic solar cells: A critical review. Energy Environ. Sci. 2015, 9. [Google Scholar]
- Ameri, T.; Khoram, P.; Min, J.; Brabec, C.J. Organic ternary solar cells: a review. Advanced Materials 2013, 25, 4245–4266. [Google Scholar] [CrossRef]
- Zhong, L.; Gao, L.; Bin, H.; Hu, Q.; Zhang, Z.-G.; Liu, F.; Russell, T.P.; Zhang, Z.; Li, Y. High Efficiency Ternary Nonfullerene Polymer Solar Cells with Two Polymer Donors and an Organic Semiconductor Acceptor. Advanced Energy Materials 2017, 7, 1602215. [Google Scholar] [CrossRef]
- Akel, S.; Sharif, M.A.; Al-Esseili, R.; Al-Wahish, M.A.; Hodali, H.A.; Müller-Buschbaum, P.; Schmidt-Mende, L.; Al-Hussein, M. Photovoltaic cells based on ternary P3HT:PCBM: Ruthenium(II) complex bearing 8-(diphenylphosphino)quinoline active layer. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 622, 126685. [Google Scholar] [CrossRef]
- Çaldıran, Z.; Erkem, Ü.; Baltakesmez, A.; Biber, M. Effects of the PENTACENE as doping material on the power conversion efficiency of P3HT:PCBM based ternary organic solar cells. Physica B: Condensed Matter 2021, 607, 412859. [Google Scholar] [CrossRef]
- Lewińska, G.; Jeleń, P.; Kanak, J.; Walczak, Ł.; Socha, R.; Sitarz, M.; Sanetra, J.; Marszałek, K.W. Investigation of Dye Dopant Influence on Electrooptical and Morphology Properties of Polymeric Acceptor Matrix Dedicated for Ternary Organic Solar Cells. Polymers 2021, 13, 4099. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Heo, D.K.; Yun, C.; Kim, Y.H.; Kang, M.H. Solution-Processed Semitransparent Inverted Organic Solar Cells from a Transparent Conductive Polymer Electrode. ECS Journal of Solid State Science and Technology 2019, 8, Q32–Q37. [Google Scholar] [CrossRef]
- Khlyabich, P.P.; Rudenko, A.E.; Thompson, B.C.; Loo, Y.-L. Structural Origins for Tunable Open-Circuit Voltage in Ternary-Blend Organic Solar Cells. Advanced Functional Materials 2015, 25, 5557–5563. [Google Scholar] [CrossRef]
- Gasparini, N.; Salvador, M.; Fladischer, S.; Katsouras, A.; Avgeropoulos, A.; Spiecker, E.; Chochos, C.L.; Brabec, C.J.; Ameri, T. An Alternative Strategy to Adjust the Recombination Mechanism of Organic Photovoltaics by Implementing Ternary Compounds. Advanced Energy Materials 2015, 5, 1501527. [Google Scholar] [CrossRef]
- Xiao, B.; Wu, H.; Cao, Y. Solution-processed cathode interfacial layer materials for high-efficiency polymer solar cells. Materials Today 2015, 18, 385–394. [Google Scholar] [CrossRef]
- Schmidt, H.; Zilberberg, K.; Schmale, S.; Flügge, H.; Riedl, T.; Kowalsky, W. Transient characteristics of inverted polymer solar cells using titaniumoxide interlayers. Applied Physics Letters 2010, 96, 243305–224908. [Google Scholar] [CrossRef]
- Sun, F.-Z.; Shi, A.-L.; Xu, Z.-Q.; Wei, H.-X.; Li, Y.-Q.; Lee, S.-T.; Tang, J.-X. Efficient inverted polymer solar cells with thermal-evaporated and solution-processed small molecular electron extraction layer. Applied Physics Letters 2013, 102, 133303. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, X.; Zhang, L.; Chen, J.; Cao, Y. High efficiency inverted polymeric bulk-heterojunction solar cells with hydrophilic conjugated polymers as cathode interlayer on ITO. Solar Energy Materials and Solar Cells 2012, 97, 83–88. [Google Scholar] [CrossRef]
- Chou, T.-R.; Chen, S.-H.; Chiang, Y.-T.; Chang, T.-T.; Lin, C.-W.; Chao, C.-Y. Highly conductive PEDOT:PSS film by incorporating secondary doping and post-treatment for ITO-free polymer dispersed liquid crystal display. Molecular Crystals and Liquid Crystals 2017, 647, 28–36. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, Y.; Du, D.; Ouyang, J. PEDOT:PSS Films with Metallic Conductivity through a Treatment with Common Organic Solutions of Organic Salts and Their Application as a Transparent Electrode of Polymer Solar Cells. ACS Applied Materials & Interfaces 2016, 8, 11629–11638. [Google Scholar]
- Makha, M.; Testa, P.; Anantharaman, S.B.; Heier, J.; Jenatsch, S.; Leclaire, N.; Tisserant, J.-N.; Véron, A.C.; Wang, L.; Nüesch, F. , et al. Ternary semitransparent organic solar cells with a laminated top electrode. Science and Technology of Advanced Materials 2017, 18, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, D.; Shan, C.; Liu, Q.; Gu, X.; Li, W.; Choy, W.; Kyaw, A. High-Performance Semitransparent Organic Solar Cells Enabled by Improved Charge Transport and Optical Engineering of Ternary Blend Active Layer. Solar RRL 2021, 6. [Google Scholar] [CrossRef]
- An, N.G.; Lee, T.; Heo, J.; Kim, J.W.; Song, S.; Lee, W.; Walker, B.; Lim, E.; Kim, J.Y. Exploiting Ternary Blends to Accurately Control the Coloration of Semitransparent, Non-Fullerene, Organic Solar Cells. Solar RRL 2021, 5, 2000742. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Babu, D.; Li, W.; Gu, X.; Shan, C.; Kyaw, A.; Choy, W. Efficient Semi-Transparent Organic Solar Cells with High Color Rendering Index Enabled by Self-Assembled and Knitted AgNPs/MWCNTs Transparent Top Electrode via Solution Process. Advanced Optical Materials 2021, 9, 2002108. [Google Scholar] [CrossRef]
| Active Layer | ||||
|---|---|---|---|---|
| P(TRI-co-TER): PINDOLE | 0.71 | 0.79 | 12.83 | 0.072 |
| P(TRI-co-TER): Beetroot: PINDOLE | 0.97 | 0.88 | 27.6 | 0.23 |
| P(TRI-co-DISULF): PINDOLE. | 0.72 | 0.82 | 17.14 | 0.1 |
| P(TRI-co-DISULF): Beetroot: PINDOLE. | 0.96 | 0.96 | 21.39 | 0.19 |
| Active Layer | ETL | ||||
|---|---|---|---|---|---|
| P(TRI-co-TER): Beetroot: PINDOLE | TiO2 | 0.97 | 0.88 | 27.6 | 0.23 |
| P(TRI-co-TER): Beetroot: PINDOLE | PINDOLE | 0.96 | 0.89 | 37.14 | 0.31 |
| P(TRI-co-DISULF): Beetroot: PINDOLE. | TiO2 | 0.96 | 0.96 | 21.39 | 0.19 |
| P(TRI-co-DISULF): Beetroot: PINDOLE. | PINDOLE | 0.98 | 0.92 | 35.13 | 0.31 |
| Active Layer | ETL | Top Electrode | Year [Ref.] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P(TRI-co-TER): Beetroot: PINDOLE | PINDOLE | Pristine PEDOTE:PSS | 0.96 | 0.89 | 37.14 | 0.31 | 100.8 | 2.8 | This work |
| P(TRI-co-TER): Beetroot: PINDOLE | PINDOLE | Modified PEDOTE:PSS | 0.97 | 0.92 | 65.23 | 0.58 | 28.9 | 3.3 | This work |
| P(TRI-co-DISULF): Beetroot: PINDOLE. | PINDOLE | Pristine PEDOTE:PSS | 0.98 | 0.92 | 35.13 | 0.31 | 118.9 | 1.07 | This work |
| P(TRI-co-DISULF): Beetroot: PINDOLE. | PINDOLE | Modified PEDOTE:PSS | 0.98 | 0.91 | 58 | 0.52 | 34.2 | 6.1 | This work |
| P3HT:PCBM | ZnO | Modified PEDOTE:PSS | 0.57 | 6.9 | 51.2 | 2 | - | - | 2019[38] |
| PBDTTT-C : Cy7-T : PC70BM | TiO2 | Transparent laminated electrode | 0.71 | 10.4 | 68 | 5.5 | - | - | 2017[47] |
| PBDB-TF:Y6:BDC-4F-C8 | PDINN | 15 nm Ag | 0.82 | 20.32 | 66 | 11.5 | - | - | 2021[48] |
| PTB7-Th :IEICO-4F :T2-OEHRH | ZnO NPs | SAS | 0.72 | 15.01 | 63 | 6.73 | - | - | 2021[49] |
| J71: Y6: PC71BM | ZnO | Au 0.8nm:Ag 15nm | 0.802 | 14.68 | 60.15 | 7.08 | - | - | 2021[50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





