1. Introduction
Hemophilia is a recessive X-linked inherited coagulopathy that affects male subjects of all ethnicities. It is divided in Hemophilia A, caused by the absence or disfunction of clotting factor VIII (1:5000), and Hemophilia B, caused by the absence or disfunction of clotting factor IX (1:20000). The severity of the disease is assessed based on the quantity of the functional clotting factor in the blood circulation. Hemophilic arthropathy is the leading cause of morbidity in patients with severe hemophilia, with levels of clotting factor VIII or IX less than 0.01 UI ml-1[
1]. The mean age of the first episode of hemarthrosis ranges from 17 months to 2.2 years [
2]. In addition, 90% of those affected with severe hemophilia at the age of 25 years old show chronic degenerative changes that involve from 1 to 6 joints [
3]. The most affected joints are the knee (50.9%), the ankle (42.8%), the elbow (38.5%) and the shoulder (13.3%). Elbow arthropathy affects 13% to 87% of hemophilic patients [
4] and ranks third or second as the site affected depending on the studies [
5].
Hemarthrosis is often the onset manifestation of hemophilic pathology. At the anatomopathological level, there are essentially two characteristics: chronic synovitis and cartilaginous damage [
6]. At first, following acute intra-articular bleeding, the capsule is stretched and tensioned and a synovial reaction with infiltration of inflammatory cells occurs [
7]. With relapses of bleeding, the blood resorption capacity decreases, causing an accumulation of intra-articular hemosiderin [
6]. The storage of iron and the inflammatory blood cells in the articulation leads to local expression of proto-oncogene c-myc in the synovial cells. These cause an activation cascade of different kinds of proteins like VEGF, MDM2, MMP9, IL1 and with the mechanical damage because of the increased pressure in the articulation the final result is local tissue damage, with apoptosis of the chondrocytes and decreased synthesis of the proteoglycans with a degenerative and fibrotic joint [
8,
9,
10,
11,
12,
13]. Many factors contribute to the tissue damage, most experiments suggest that even a single episode of intra-articular bleeding would cause negative modifications in the chondrocytes and in the matrix causing damages in the long time. The direct effect of the blood on the cartilage precedes the indirect one of the synovial inflammation [
14,
15,
16].
These occurrences all result in persistent hemophilic synovitis, which prematurely triggers rebleeding and sets off a deadly cycle of hemarthrosis, synovitis, and hemarthrosis. If these cycles are not broken, the chondrocytes' premature death will cause the joint cartilage to be destroyed, leading in a few years to hemophilic arthropathy (HA), which negatively affects the quality of life for those affected, particularly in cases of severe hemophilia and in those who have inhibitor antibodies against the intravenously infused deficient factor [
17].
The result of repeated episodes of bleeding in the bone fracture site or as a result of sub-periosteal hemorrhage or bleeding into soft tissue due to the absence or lack of effective factor replacement could also be the reason for the onset of the hemophilic pseudotumor [
18].
About 1%–2% of hemophiliac patients develop hemophilic pseudotumors, which are gradually expanding cystic collections brought on by recurrent extra-articular or soft-tissue bleeding. Recurrent bleeding into these areas combined with insufficient reabsorption of blood products results in an expanding region of clotted blood surrounded by fibrous tissue that generally manifests as a mass effect. Usually, the patient refers chronic deep pain that improves with rest, the mass effect symptoms could be vascular or neurological, particularly in the limbs with paraesthesias. If the mass is superficial can irritate the muscles and the tendons around with pain during the movement of the articulation and joint impairment. [
19]. The patient also had typical symptoms of elbow arthropathy, including pain, discomfort, stiffness in active and passive range of motion, and local swelling.
The first line treatment option for the arthritic elbow should be the nonsurgical treatment with rest, nonsteroidal anti-inflammatory medications and long-term activity modification. However, activity modification is difficult for manual laborers and professional athletes. Physical therapy and dynamic hinged and static progressive splinting have also been recommended. Viscosupplementation has been used to treat arthritic conditions of the main body articulations. Surgical treatment is indicated for elbow arthritis following failure of nonsurgical treatment. It is useful to consider the patient’s age, functional demands, etiology, and severity of elbow arthritis before surgery. The surgical options for arthritis with moderate degenerative changes are arthroscopic or open synovectomy. Patients with severe elbow arthritis can be treated with distraction interposition arthroplasty and total elbow arthroplasty (TEA) [
20].
Our aim it’s to show a complex clinical case of hemophilic elbow arthropathy from the first presentation and to compare what was our diagnostic process and clinical path and what the medical scientific literature says about treatments and complications.
2. Case Presentation
We report a case of a 40-year-old smoker male, Caucasic, with severe hemophilia A under factor replacement prophylaxis with an elbow arthropathy. He’s an office worker with limitations, right dominant limb and in his free time usually he doesn’t play any contact sport.
2.1. History (Table 1)
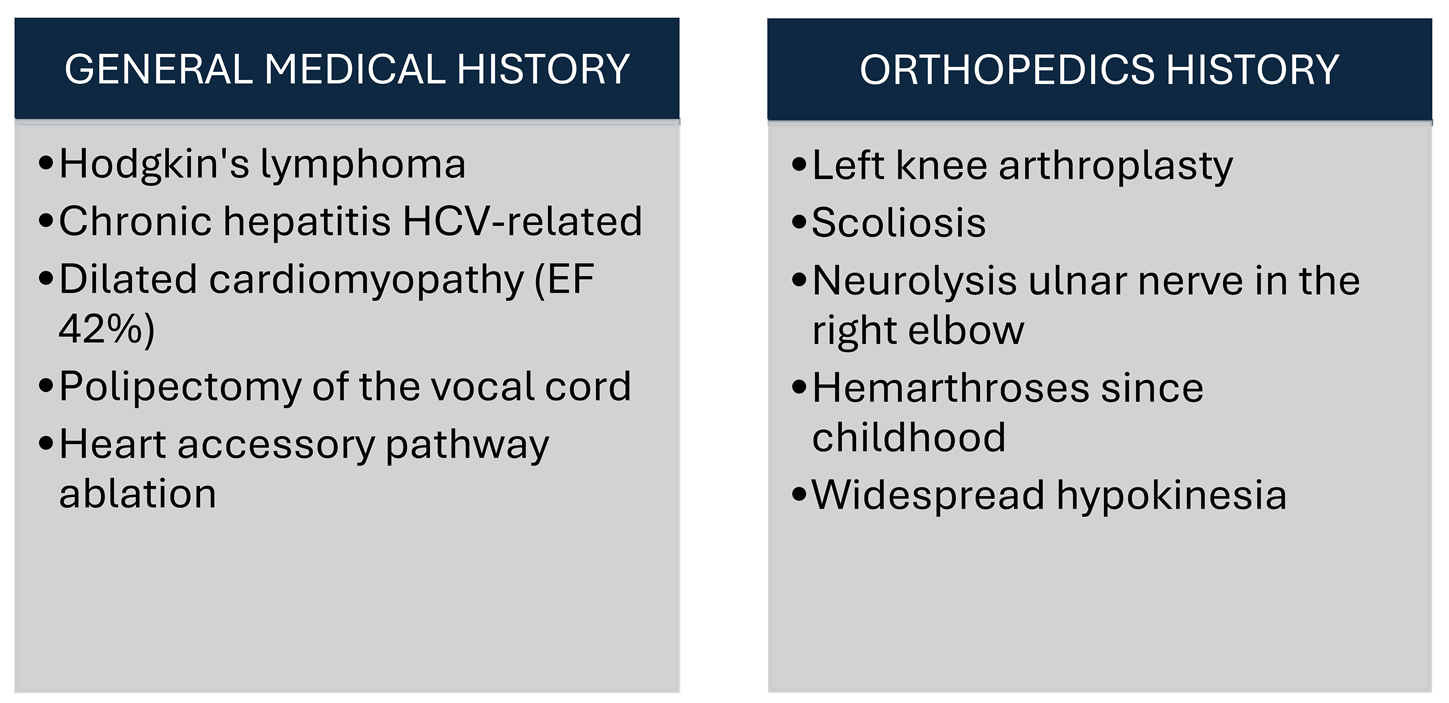
In addition to hemophilia, the patient has a medical history that includes Hodgkin's lymphoma treated with ABVD (doxorubicin/bleomycin/vinblastine/dacarbazine) protocol, chronic hepatitis HCV-related, dilated cardiomyopathy with an ejection fraction of 42% and widespread hypokinesia, moderate mitral insufficiency with left atrial dilatation, previous polypectomy of the vocal cord, occult right posterior-septal accessory pathway ablation with radio frequency (RF), scoliosis, left knee arthroplasty for a hemophilic arthropathy with varus deformity, history of conservative treatments and neurolysis of the ulnar nerve on the right elbow in other hospital, hemarthroses since childhood. Comorbidities influencing clinical presentation and surgical outcomes in hemophilic patients [
21] [
20].
2.2. Clinical Presentation
In March 2013, the patient presented to our Orthopedic and Trauma Surgery department with continuous pain [He referred Visual Analog Scale (VAS) 6], swelling and limited active range of motion in the right elbow, which was affecting his daily activities. Fortunately, at that moment the patient didn’t have any problems in the other joints, including in the left total knee arthroplasty (TKA). The range of motion (ROM) of the right shoulder was quite good without limitation or pain.
It is important to assess the involvement of other joints as this may affect the global functional outcome. A study by Malhotra showed that the majority of patients with elbow arthropathy also had involvement of other joints such as the knee joint. This is probably related to the repetitive load passing through the elbow, as these patients require walking aids for their mobility. In their study group, about 25% of the patients with severe arthropathy of the elbow had involvement of other joints. Aronstam concluded that patients presenting with pain and tenderness with a loss of more than 50% of the ROM are likely to have poor long-term prognosis. Gamble studied the loss of elbow and wrist motion in hemophilia and concluded that the loss of motion was significantly higher in older patients (more than 25 years of age) when compared with younger patients so the importance of early treatment [
21]. Around 80–90% of patients with hemophilia without primary prophylaxis treatment present elbow arthropathy. This is due, among other causes, to the role played by the upper limbs as ancillary appendages for ambulation, when patients suffer bleeding in the lower limbs. Aid from the upper limbs is manifested in the transition from sitting to standing and by their supporting functions (acting as support or partial support of body weight utilizing walking sticks, crutches, or walkers)[
22]. The elbow is not a weight-bearing joint, and early limitations of ROM (flexion and extension) rarely interfere with activity in daily living [
23,
24]. As the joint deterioration progresses the humerus-ulnar joint becomes affected, limiting flexion and extension movements. In this way, the normal development of the routine activities of daily life is affected. In some cases, bone deformity may lead to ulnar nerve neuropathy [
25].
2.3. Further Investigations
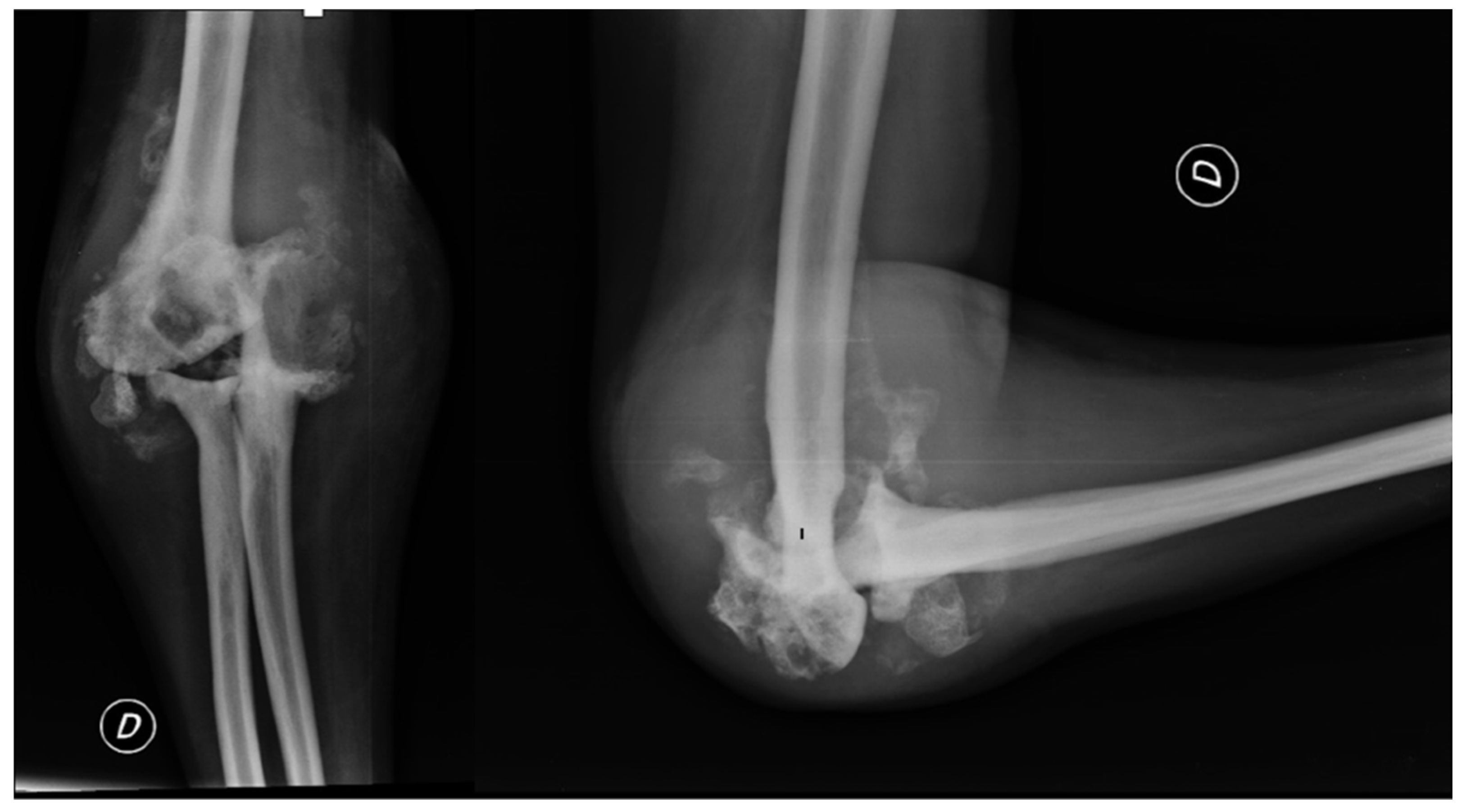
Due to the patient's clinical symptoms, a right elbow X-ray was performed (
Figure 1), which revealed significant morpho-structural changes in the bone structures of the joint, with osteolytic areas of bone. The X-ray also showed calcification of the periarticular soft tissue and dislocation of the radial head. Additionally, the patient had an enlarging ulnar elbow mass, which was attributed to recurrent intra-articular bleeding that involved the periarticular soft tissue.
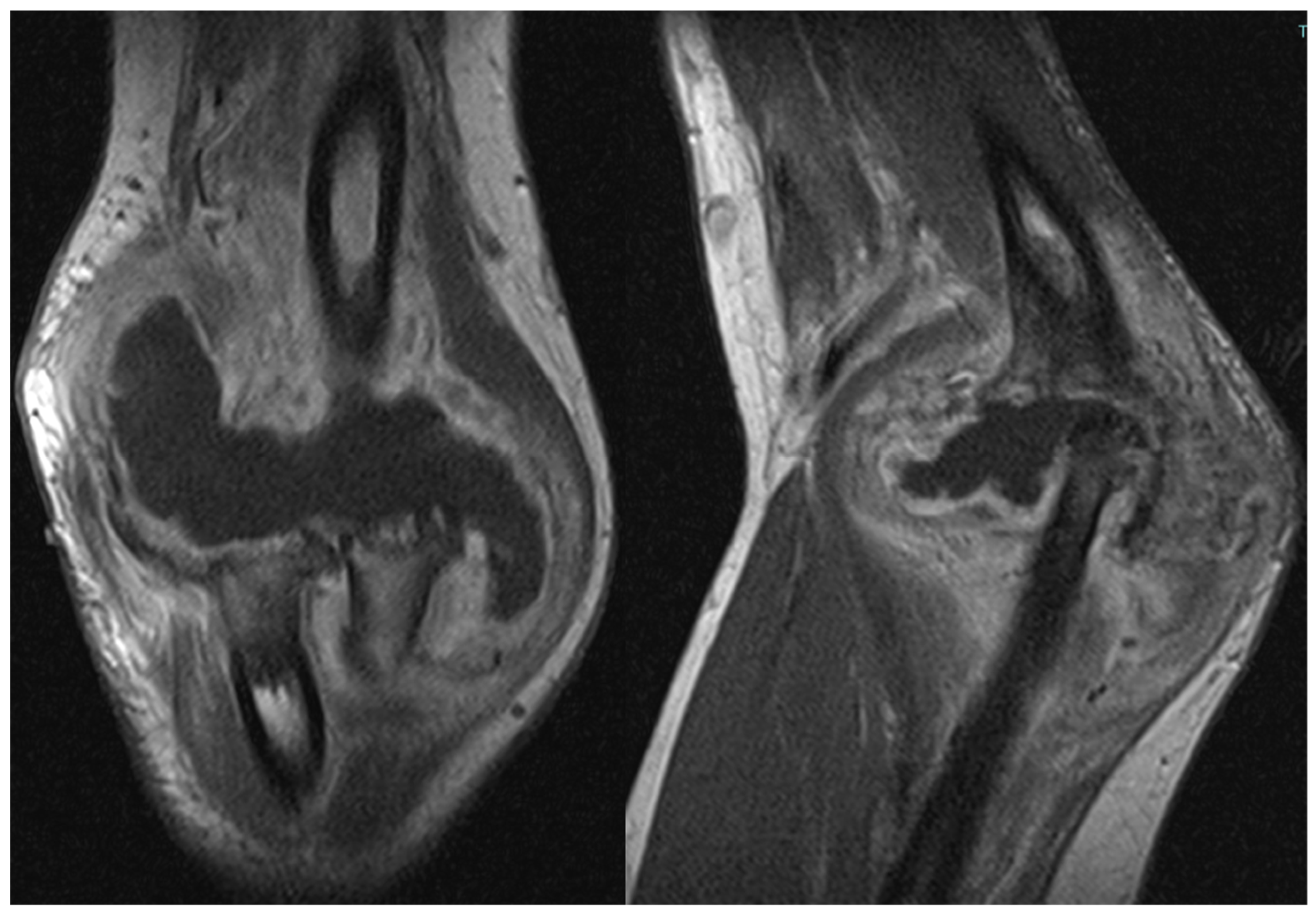
The patient also underwent magnetic resonance imaging (MRI) with and without contrast medium, which revealed a subluxation between the articular surfaces of the elbow joint and contrast enhancement and edema in the spongy bone of the distal third of the humerus and the proximal third of the radius. Additionally, there was a periosteal reaction in the humeral site without identifiable bone lesions on the imaging. An abundant layer of joint effusion with blood clots and loose bodies was evident at the joint level. The synovium appeared thickened and full of contrast enhancement, and periarticular soft tissues also appeared thickened with edema and soft contrasting infiltration in the proximal ulnar side. In the context of the known basic arthropathy and the clinical presentation, these findings based on radiological evidence suggested more for an arthritic arthro-synovitis picture with soft tissue involvement, without excluding the infectious nature, than osteomyelitis because despite the bone subversion there weren’t cavity of necrotic bone with fistula and bone marrow inflammation (
Figure 2).
Three are the most used kinds of imaging modalities for studying hemophilic arthropathy: conventional radiography, ultrasound and MRI. Two are the most used classifications to describe hemophilic arthropathy with conventional radiography: Pettersson’s score and Arnold Hilgartner’scale [
26,
27]. They are based on plain films that can demonstrate osteoporosis, bone cysts, osteonecrosis, bone fusion, joint space irregularity and narrowing, and epiphyseal overgrowth. They are, however, limited in soft tissue evaluation. Plain films are useful in the assessment of late arthropathy, but they may not detect early changes [
28].
However, the correlation between the clinical and the radiographic features is not yet well defined. The ultrasound can visualize with more precision soft tissues and guide any infiltrations [
29]. Synovitis of the elbow can appear as a hypoechogenic line that thickens and becomes irregular as the inflammation progresses with increased vascularity on color or power Doppler.
The synovial membrane is hardly perceptible on ultrasound in a normal joint, but it can be distinct in synovial inflammation. Variability among those performing and interpreting ultrasound images could be a problem for standardization and quantification, but ultrasound is nonetheless an effective and economical tool in the monitoring of hemophiliac joints, and its use is increasing.
The majority believe that MRI is the most sensitive test for arthropathy. Whereas MRI is less sensitive in the elbow than in other frequently affected joints, it is still more sensitive than other imaging modalities for arthropathy. When evaluating the elbow, a coronal, sagittal, and axial examination should be performed. Cross et al. recommend a T1 sequence for osteochondral lesions, whereas unenhanced gradient echo is best for viewing synovium, cartilage, and hemosiderin deposition. T2 gradient echo works well in looking for acute and chronic bleeding, and it can differentiate between simple effusion and hemorrhage [
28].
MRI allows to evaluation of even little changes in the affected joint and may have a significant impact on patient management [
30].
Various rating scales of the hemophilic arthropathy with MRI have been proposed [
31,
32,
33,
34].
The International Prophylaxis Study Group’s (IPSG) 2005 scale has been revised in the IPSG MRI scale. It’s an additive scale with a single scoring system, and it includes soft tissue changes, such as effusion and hemarthrosis separate soft tissue and osteochondral changes that more closely reflect the progression from early disease to severe arthropathy [
28].
Computed tomography (CT) scan allows a very specific study of the bone’s primary structures and their structural modifications [
35].
Both CT scan and MRI help to detect the extent of the hemophilic pseudotumor it’s a chronic, encapsulated, hemorrhagic fluid collection that usually destroys bone and may become quite large [
30].
Then the role of the CT with three-dimensional (3D) it’s useful for surgical planning for total elbow arthroplasty (TEA) and helpful for the estimation of the implant size and accurate placement of implants [
36].
Another imaging technique is the angiography with arterial embolization. Even if it is an invasive procedure with some risk of complications the results of angiographic imaging revealed vascular blush, false aneurysm, true aneurysm, and arteriovenous shunt in combination with an aneurysm as cause of bleeding.
The incidence of hemarthrosis as a result of a spontaneous periarticular aneurysm is very low. In these circumstances, in patients with joint bleeds not responding to replacement coagulation factor therapy, angiographic embolization might be considered as a promising therapeutic and coagulation factor-saving option.
Rodriguez-Merchan at all. also suggest that therapeutic arterial embolization of hemophilic pseudotumors should be considered in lesions of large size because it may effectively reduce its size and decrease the risk of bleeding complications during surgery. Nevertheless, because of its temporary effect, embolization had better be performed, as a preparatory procedure, at best about 2 weeks before surgery. This time-lapse will allow for mass shrinkage but is insufficient for vessel restoration [
37].
2.4. Treatments
2.4.1. Available Treatment Options (Table 2)
Well-coordinated multidisciplinary teams in specialized centers: appropriate collaboration between all members of the multidisciplinary team is very important. It’s essential in the good management of this kind of patients the appropriate blood factor therapy.
Joint aspiration (arthrocentesis) of acute hemarthrosis: a study has demonstrated that arthrocentesis under hemostatic cover and in rigorously aseptic circumstances is a well-tolerated procedure that speeds the recovery of acute articular hemorrhages of patients with hemophilia.
Exercise: it is an effective way to manage hemophilia and has demonstrated a low frequency of related complications [
17].
In elbow arthropathy, the variables measured are ROM of the elbow, biceps strength, circumference of the arm, and elbow pain. The treatment with manual therapy improved the circumference of the arm, flexion and elbow pain [
22].
According to Sandford et al., 67% of patients with hemophilic elbow arthropathy who needed to wear splints all the time had poor or no adherence to their use, which made it challenging to get the expected benefit of orthotic treatment [
38].Conservative management of mild osteochondral damage (mild hemophilic arthropathy): hematological prophylaxis, pain killers, cyclooxygenase 2 (COX-2) inhibitors and intra-articular injections of corticosteroids, hyaluronic acid, platelet-rich plasma [PRP] and mesenchymal stem cells [MSCs] are good options [
17].
It has been observed functional improvement with better quality of life in the patients that received joint lavage with saline followed by injection of corticosteroids and hyaluronic acid [
39].
Synoviorthesis, that is the destruction of the hypertrophic synovium with the infiltration of a radiopharmaceutical, is indicated if a three-month medical and physiotherapy treatment failed in controlling the synovitis [
40].
Three synoviorthesis failed in six months justify the indication for an open surgery synovectomy [
41].
Synovectomy: When there is recurrent hemarthrosis in chronic hemophilic synovitis, it is recommended. Although synovitis is usually painless, it is important to suspect chronic synovitis in individuals who have experienced one or more hemarthroses in the past few months if they have hemophilia. It could be carried out by arthroscopy, chemical, or radiosynovectomy techniques [
17]. According to Van Vulpen, radiosynovectomy presents the best risk-benefit alternative to treat chronic hemophilic synovitis. Alternatively, chemical synoviorthesis can be used. Patients with single joint involvement having subacute or chronic synovitis not responding to conservative measures after 3-6 months are ideal for arthroscopic synovectomy [
42].
Surgery: it must be considered in the advanced stage of elbow hemophilic arthropathy after the conservative options. The main surgical procedures currently available in the literature are arthroscopic debridement, radial head resection, transposition of the ulnar nerve, removal of heterotopic ossifications and TEA. Compared with total hip and knee arthroplasties the TEA has suboptimal outcomes and a shorter prosthetic survival rate [
17].
2.4.2. Hematological Treatment
Preventing and treating bleeding in patients with low clotting factors is the main goal of therapy. When possible specific factor concentrations should be used. Antifibrinolytic drugs (e.g., tranexamic acid, epsilon aminocaproic acid) work very well as adjuvant therapies in surgical procedures like orthopedic surgery [
43].
The standard of care for patients with hemophilic arthropathy with a frequent bleeding phenotype (mostly severe hemophilia) is regular prophylactic intravenous infusions of factor VIII (FVIII), the goal being to maintain target through FVIII activity levels of ≥ 1 U/dL to prevent bleeds and relieve long-term secondary complications. Approximately 30% of these patients develop neutralizing alloantibodies (FVIII inhibitors), which render FVIII replacement therapy ineffective. Before the development of the bispecific monoclonal antibody, hemophilic patients were treated with prothrombotic coagulation factors that avoided FVIII. However, bypassing agents (BPAs) such as activated prothrombin complex concentrate (aPCC) and recombinant activated human factor VII (rFVIIa) have suboptimal hemostatic effects and unfavorable pharmacokinetics (short half-life, slow intravenous infusion rate).
Emicizumab (HEMLIBRA ®; F. Hoffmann-La Roche Ltd, Basel, Switzerland) is a humanized, bispecific monoclonal antibody that mimics the cofactor function of absent activated FVIII (FVIIIa), bridging the activated factor IX (FIXa) and factor X (FX) for restoring the effective hemostasis. It has no sequence homology with FVIII and is therefore unlikely to induce FVIII inhibitors and is unaffected by their presence. Emicizumab has high subcutaneous bioavailability and a half-life of approximately 30 days, enabling treatment once weekly (1.5 mg/kg), every 2 weeks, or every 4 weeks subcutaneous dosing regimens, thus avoiding the need for frequent intravenous administration [
44].
2.4.3. Open Debridement
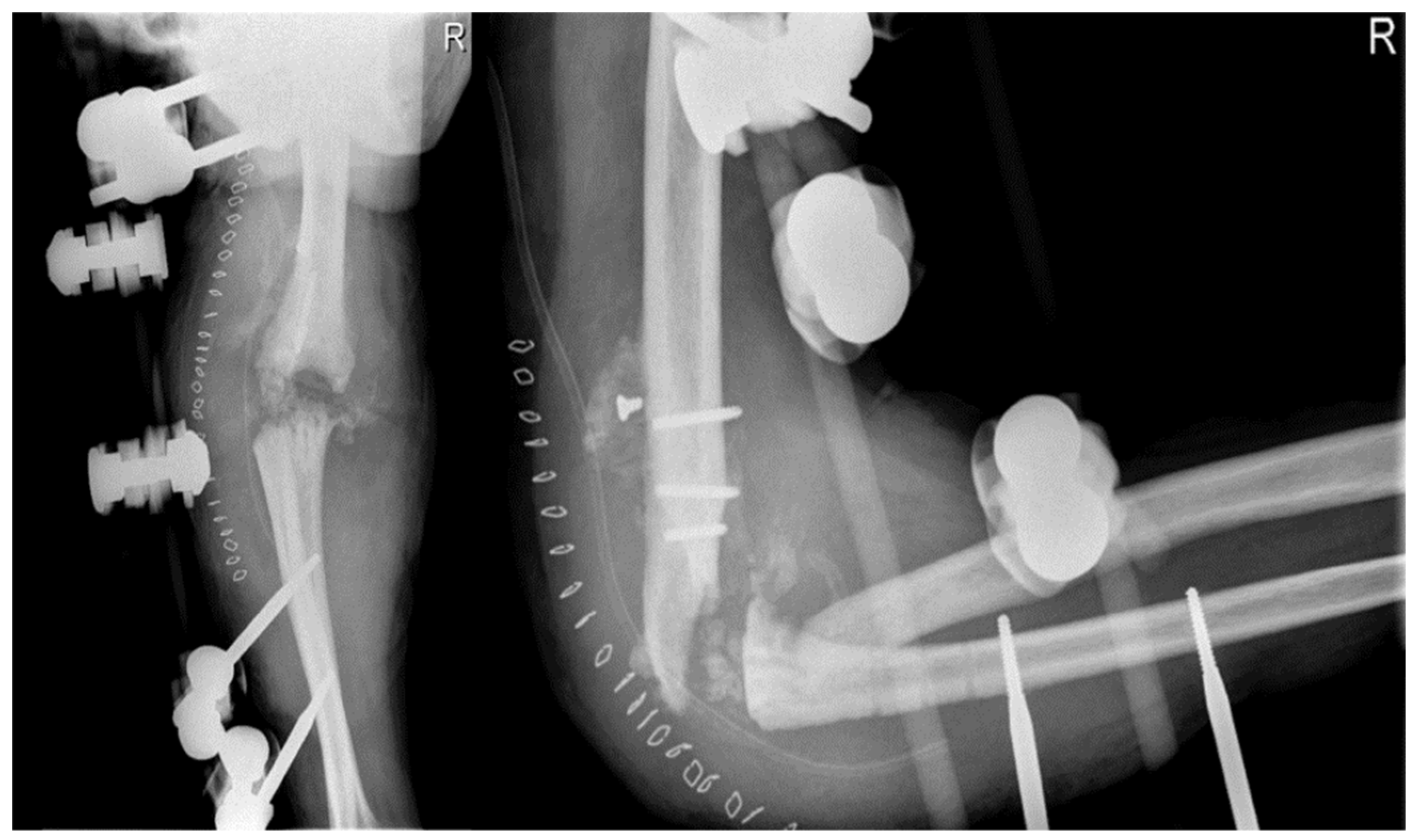
In April 2012, the patient underwent open debridement with synovectomy and removal of bone fragments in arthroscopy due to the advanced stage of hemophilic elbow arthropathy. During the procedure, bone resorption phenomena with free fragments were observed in the joint, mainly at the radial head, and the capsule was thickened with evidence of synovitis. The dislocation was structured and non-reducible. Samples of bone and synovium were taken and cultured for pathogen microorganisms, which turned out positive for different types of coagulase-negative
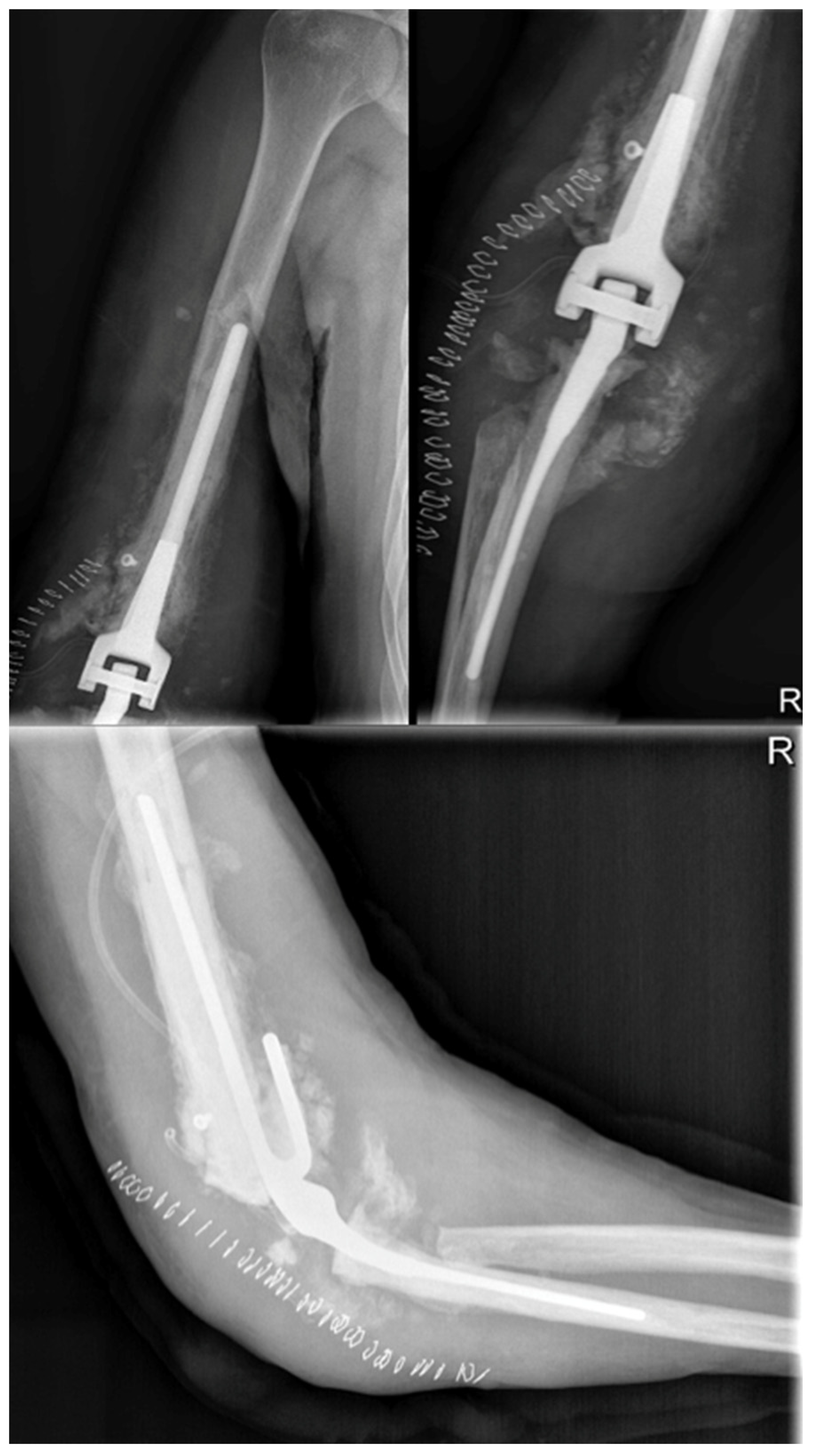
Staphylococcus. Postoperative X-ray showed the removal of some fragments at the joint level, but the elbow joint dislocation persisted (
Figure 3). Synovium fragments showed fibrosis, calcifications, scattered foci of chronic inflammation, synovial hyperplasia, and abundant superficial fibrin stores. Bone samples showed trabecular remodeling and peritrabecular fibrosis with spared perivascular reactive lymphoid aggregates.
Hemophilic arthropathy manifests itself with histological findings of severe arthropathy, intra-articular iron deposition, synovial proliferation, neo-angiogenesis, destruction and loss of cartilage and subchondral bone with osteoporosis [
45].
In hemophilic patients with major subversions of bone and soft tissue anatomy, it is important to perform tissue samples and cancer biopsy during surgery to rule out other diseases such as true tumors (chondrosarcoma, liposarcoma, synovial sarcoma) which sometimes mimic hemophilia pseudotumors [
46].
2.4.3.1. Joint Debridement and Complications
After being discharged, the patient was prescribed tranexamic acid every 12 hours for 10 days, along with physiotherapy for active and passive mobilization in flexion, extension, and supination. The patient was advised to monitor their inflammation index every seven days until normal values were reached and began an antibiotic therapy based on the antibiogram. However, the patient was readmitted to the hospital a week later due to a hematoma in the right elbow. A sterile aspiration drainage was performed and the patient was discharged with an articulated elbow brace set between 10 and 80°, allowing active mobilization within that ROM. During subsequent outpatient clinic visits, the wound showed proximal dehiscence with serum-corpuscular material secretion, and the elbow was flush and swollen, leading to arthrocentesis and continuation of antibiotic therapy with amoxicillin-clavulanate 1 g per 3/day.
The patient showed signs of improvement after 15 days, with no signs of inflammation but a permanent wound dehiscence of 0.5 cm and discharge of serum corpuscle material. Antibiotic therapy was continued, and the elbow was locked in an articular brace at 90 degrees. After 40 days, the wound dehiscence persisted, and the decision was made to discontinue antibiotic therapy and proceed with elbow arthrodesis, as advised by the infectious disease specialist. The patient was also scheduled for a week-long follow-up to monitor the inflammation index and a CT scan of the elbow. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values were 7 in the first hour and 0.35 mg/d.
The CT scan conducted with a 64 md spiral method revealed a significant and widespread osteo-structural abnormality of a stony nature. This led to an articulated deformity that affected all joint bone segments, leading to the loss of joint relationship and joint deformity. There were multiple peri-skeletal bone fragments and the presence of significant swelling due to soft tissue alterations. This clinical presentation was consistent with advanced hemophilic arthropathy, but an arthrosinovitis process of infectious origin was ruled out. In late June, due to the presence of a fluctuating swelling at the back of the elbow, 50 cc of blood fluid was aspirated, followed by an arthrocentesis in early July which evacuated 100 cc of serum with fibrin deposition. However, there were no signs of infection in the elbow. The ESR and CRP values were 12 and 0.56 mg/dL, respectively.
Wound infection, shoulder-hand syndrome, deep wound infection, ulnar nerve symptoms, radial nerve palsy, residual loose bodies, hematoma, and recurrent effusions could be complications of open debridement [
47].
The overall incidence of complications is 6.1% (range 0-25%) in the open joint debridement literature. The most common complication type is neurological, with an incidence of 1.9% (range 0-12%). The deep infection rate is 0.7% (range 0-10%). Most of the neurological complications can be treated with neurotrophic drugs or spontaneously, the others remained with surgical intervention despite no good results. For superficial and deep infections, the most important things are prevention and specific antibiotic therapy on the antibiograms [
48].
2.4.4. Arthrodesis
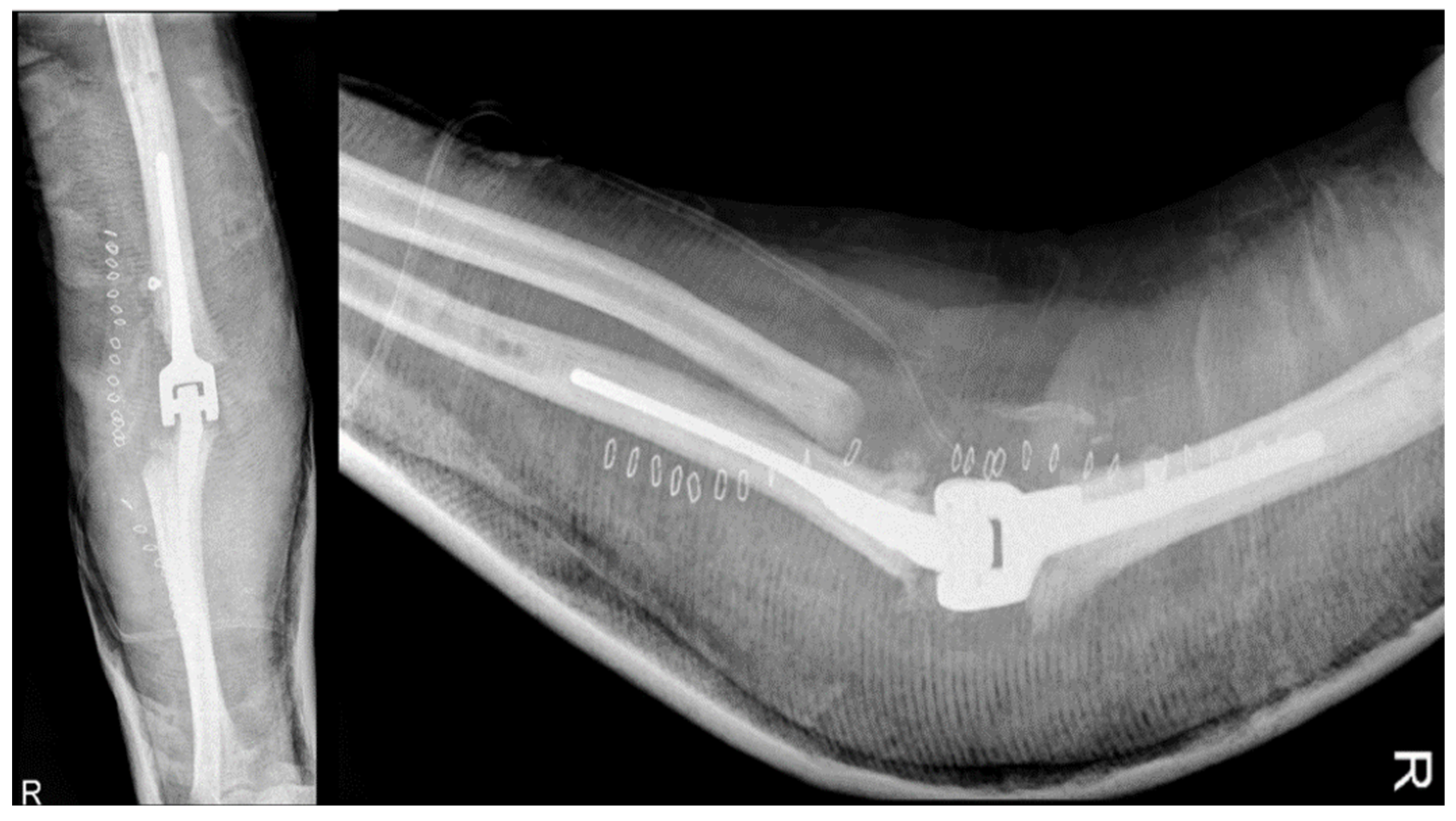
In early August 2013, a surgical procedure was performed to fuse the elbow joint using a plate with 12 holes and a 90-degree flexion angle, using the same surgical access point as the previous surgery (
Figure 4). During the procedure, it was observed that the anatomical structures of the ulna, radial head, and distal humerus were severely distorted and not recognizable. A new synovectomy and osteotomy were performed to apply the plate. After discharge from the hospital, the patient was instructed to wear a brace at 90 degrees and an arm sling, undergo physiotherapy to mobilize the fingers and wrist, and have an X-ray examination 30 days after the surgery.
Elbow function loss can have a serious impact on everyday life activities. Rarely is elbow arthrodesis necessary because of persistent functional impairment. With current techniques, fusion rates vary from 50% to 100%. More compensatory motion is observed in the wrist and spinal column. There is disagreement over the ideal location for arthrodesis. Post-traumatic arthritis, instability, or infection with extensive joint destruction are the most common causes of arthrodesis. There is no ideal angle or position to fuse the elbow. The literature suggests that the fusion angle lies somewhere between 45° and 110°. Historically, 90° has been accepted as the best position. Factors to consider when choosing the position include sex, occupation, dominant limb, functional ability of the opposite upper extremity and functional requirements. In addition, ipsilateral shoulder and wrist pathology and patient preference should be considered. It is advisable to brace or cast the patient's elbow at several angles in order to better establish the optimal position for fusion. After that, the patient can provide feedback on the best choice of arthrodesis angle [
49].
2.4.4.1. Arthrodesis and Complications
At the 30-day postoperative follow-up, X-rays revealed three broken screws in the proximal humeral shaft and a subtle periosteal reaction around the plate. The patient was provided with a new brace and referred for a fixation revision. The screws and plates were removed using the previous lateral elbow access from the surgery. The broken screw bodies and heads were left in place since they were embedded in the bone and soft tissue, respectively. However, due to the last 7 to 8 cm of the distal humerus appearing like a cortical lamina, internal fixation was not feasible. Consequently, an external fixator bar-to-bar was applied after the arthrodesis surfaces were prepared. A follow-up X-ray confirmed the device's appropriate positioning (
Figure 5). At hospital discharge, the orthopedic recommendation was to wear the external fixator for 60 days, rest, and undergo weekly ambulatory assessments of the local clinical findings.
The elbow external fixator can be used statically as temporary stabilization or as a hinged motion fixator.
The most common surgical indications are temporary stabilization of the elbow as damage control surgery, e.g. for fractures with extensive soft tissue damage or in multiple trauma patients, additional protection/motion control (motion fixator) after complex osteoligamentous surgery or isolated in case of concomitant injuries or comorbidities, in persistent intraoperative dislocation tendency after osteoligamentous stabilization or in distraction treatment/distraction arthrolysis (motion fixator).
The center of rotation must be precise because it could change the range of motion. This increases challenges to the surgical procedure. Soft tissue irritation, loosening, malposition or fracture/pull, infection, and hypertrophic scarring are among the problems linked to pins. Damage to arteries and nerves, especially the radial nerve, is a common surgical consequence. Understanding the anatomy of the neurovascular structures is essential. Soft tissue preparation with safe exposure of the humerus and ulna is preferable to risky stab incisions.
Postoperative complications could be periarticular calcifications/heterotopic ossifications, secondary loss of reduction, persistent instability, osteitis/osteomyelitis, residual pain and limitation of movement, and specific complications of the underlying pathology [
50].
2.4.5. Total Elbow Arthroplasty
The patient kept the external fixator for two months for the healing of the soft tissue and was deemed fit for TEA surgery, which was performed in November 2013. The broken screws were removed, and the distal epiphysis of the humerus and ulna were prepared for Coonrad-Morrey prosthesis implantation with humeral stem extra small/L 100 mm cemented. The extensor apparatus of the ulna was stabilized with non-resorbable fiber wire, and the postoperative X-rays showed the correct position of the prosthesis (
Figure 6). The patient was advised to maintain a 90-degree cast on the elbow until wound healing was complete, followed by careful mobilization. Tranexamic acid was prescribed for a week, and wound healing was good with a range of motion in flexion of about 100 degrees after 15 days from the TEA.
Seen the failure of the arthrodesis it was re-evaluated the surgical option of prosthetics. The passage from an arthrodesis, generally the extreme solution, to the prosthesis has been described with success by Burkhart et al [
51].
The prosthetic replacement of the elbow was born with R. Dee in 1972, subsequently, Coonrad introduced a prosthesis with greater bone sparing.
In 1978 Morrey modified the prosthesis. Ultimately the prosthesis was modified using a central pivot joint instead of the native C-lock [
52,
53].
The principal indication for TEA is elbow arthritis (usually because of rheumatoid arthritis) in a patient possibly over 65 years and with poor functional demands with excessive pain, significant loss of range of motion (ROM), impaired elbow function and failed nonoperative treatment. Relative indications are arthrosis, post-traumatic arthrosis, and comminuted fractures in old patients where it is not possible the internal fixation [
54].
Other indications of elbow prosthesis are large bone lack and hemophilic arthropathy [
56]. In the end there is indication of prosthesis even in the reconstruction after tumoral resections.
The contraindications include active infections, neuromuscular paralysis and open cutaneous wounds. Relative contraindications are non-compliant patients, people who do heavy work, massive bone loss, the presence of a functional not painful arthrodesis.
The complications include infections (2-4%), ulnar neuropathy (5%), so much that many surgeons always recommend the transposition in these patients, the insufficiency of the extensor apparatus that can be treated with a flap of the anconeus or an allograft from Achilles tendon. Instability with a 15% between dislocation and subluxation are treated with a splint (if they happen in the first weeks after the intervention) or with a revision with a linked prosthesis if they happen after. There are 2% of mechanic failures due to the aseptic mobilization of the prosthesis of Coonrad Morrey, 8% for the Souter and 18% for the Kudo.
The elbow prosthesis can be linked (with a joint system with a central pin) or non-linked. The linked prosthesis allows for a wider release of soft tissues, increasing range of motion, and guarantees greater joint stability even in the presence of significant bone loss and ligament insufficiency. Even though they increase the tension on both joint surfaces, this may cause the prosthesis loosening. In the first few days following the initial immobilization in extension with a linked prosthesis, mobilization without protection is permitted. The extent of the wound and the reconstruction of the extensor apparatus will determine this. If there are signs of a significant flexion contracture before the surgery, it is necessary to wear an extension splint at night for the first few weeks.
In a recent review of the Mayo Clinic on the prosthesis used for rheumatoid arthritis, on 461 implants in 2-25 years, 43 implants underwent a revision. Ten of them for infection, twenty-five for mobilization, eight for degeneration of polyethylene and three for periprosthetic fracture. In the same review, in case of post-traumatic prosthesis, 16 on 85 failed. In case of pseudoarthrosis of distal humerus, the prosthesis is a good indication, mostly in old patients with little bone stock. The results of the Mayo Clinic on 92 implants for pseudoarthrosis of distal humerus with a range of 2-20 years of follow-up showed that 79% did not have pain with a ROM between 22 and 135 °. Sixteen had an aseptic mobilization, five prosthesis ruptures, five deep infections [
57].
In a study of elbow arthroplasty on hemophilic arthropathy involving 7 patients with an average age of about 34 years, 3 of them were treated with prosthesis of Coonrad Morrey, and only one underwent revision, due to the pain and to the clicking, subsequently lasting for 12 years. The retrospective study has an average of 118 months. The others had benefit in terms of pain and ROM [
58].
TEA in patients with advanced hemophilic arthropathy is associated with a higher complication and revision rate than other patients without bleeding disorders. However, even after revision without implant removal, it provides good functional and subjective long-term results.
Indications for TEA have spread with increasing knowledge, and it has also become a favorable surgery in patients with advanced hemophilic arthropathy. Concerning meaningful long-term outcomes, however, there is still a paucity of reports of TEA in patients with hemophilic arthropathy [
59].
2.4.5.1. Total Elbow Arthroplasty and Complications
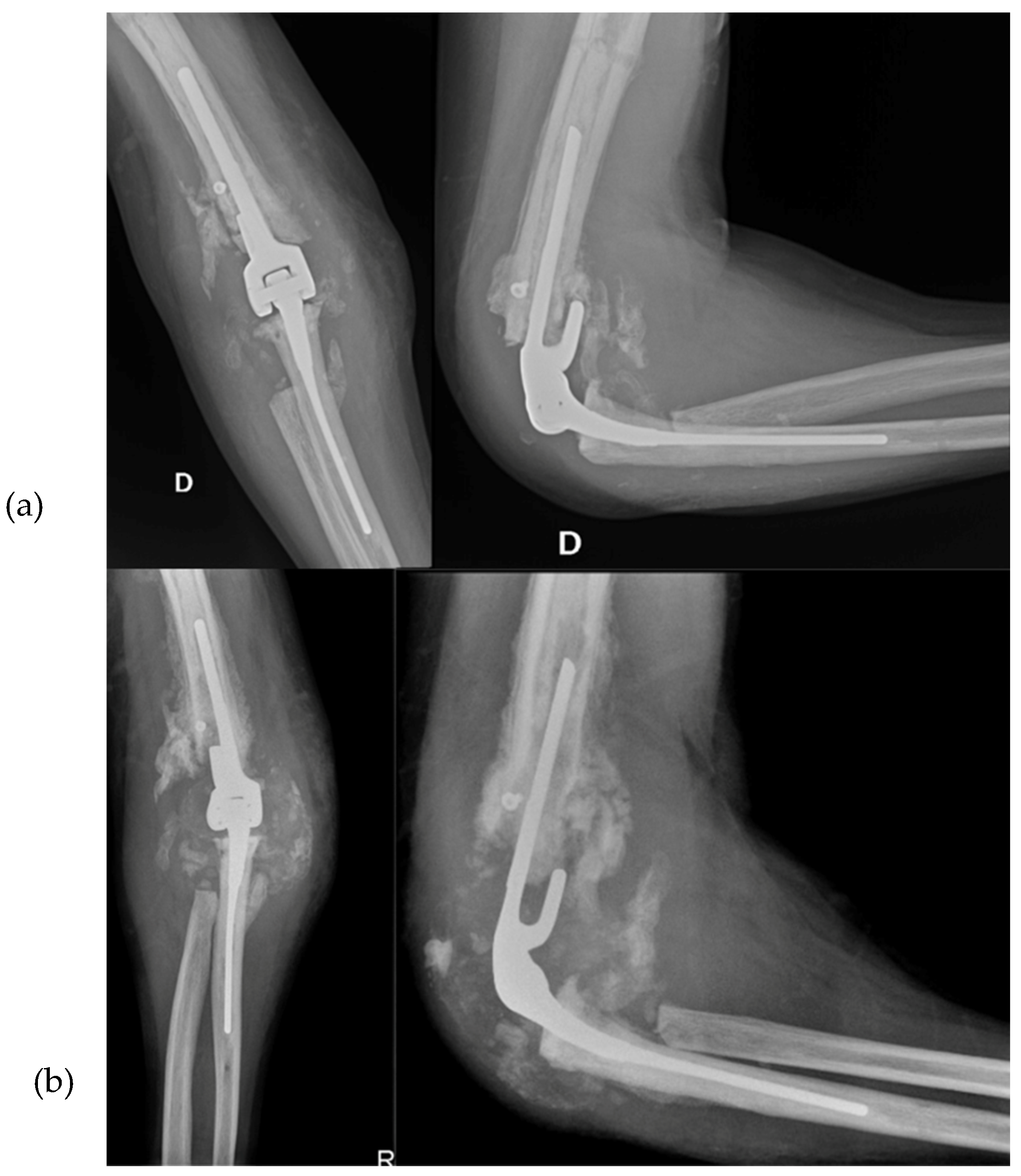
The patient experienced problems two months after the TEA surgery in February 2014, despite having good elbow flexion (about 120 degrees). Calcific stores had increased and a faint radio-transparent line was observed around the prosthetic humeral stem, while findings for the ulnar stem remained unchanged. A subsequent X-ray taken 15 days later revealed loosening of the humeral portion of the TEA, increased radio-transparency both at the apex and along the stem, and caudal dislocation relative to the bone, with many calcific stores being resorbed (
Figure 7a). In addition, the patient was hospitalized for a recurrence of Hodgkin's lymphoma and presented with local swelling, along with increased inflammation markers including ESR at 41, CRP at 7.68 mg/dl, and white blood cells (WBC) at 10.06 thousand/mm
3.
A subsequent CT scan confirmed that the humeral stem of the prosthesis had loosened and was displaced caudally and tilted in the mediolateral direction. There was significant bone loss around the prosthesis, with lithic bone remodeling and periosteal reaction. The ulnar portion appeared to be well-fixed but with periosteal reaction. The presence of intra-articular loss bodies, capsular calcifications, and soft tissue swelling was also noted. The radiologist suspected advanced hemophilic arthropathy but could not rule out the possibility of arthritic-synovitis changes of infectious origin or related to lymphoproliferative disease.
After the patient's elbow became swollen and hot with limited ROM and an audible click, an open elbow cast was applied, a blood culture was taken, and empiric antibiotic therapy with Trimethoprim/sulfamethoxazole (160mg/800mg) and Minocycline (100mg) was initiated. However, a follow-up X-ray after 15 days showed an increase in joint calcifications and no improvement in the loosening of the implants (
Figure 7b).
Infection, ulnar nerve neuropathy, compartment syndrome, polyethylene wear, periprosthetic infection, and loosening of the humeral component are potential post-operative complications of the TEA. The functional long-term benefits, including increased range of motion and patient-reported outcome measures, suggest that semi-constrained linked TEA is a reasonable and effective treatment option for hemophilic arthropathy of the elbow in this highly selected population. The increased risk of complications should be anticipated to the patient (complications rate of 62% and revision rate of 38%) [
59].
Despite the increasing use, long-term complications, such as infection, aseptic loosening, instability and periprosthetic fracture, remain a challenge in the linked TEA. It allows some varus-valgus motion, reducing stress concentration on the bone-cement interface. This design has been used to treat conditions including rheumatoid arthritis, degenerative arthritis, trauma reconstruction, and satisfactory outcomes have been reported with long-term follow-up. However, aseptic loosening together with bushing wear is a leading complication of implant failure and reducing the complication rate remains a challenge. The rise in revision-related problems TEA increases in tandem with primary elbow arthroplasty. It is commonly established that patients with inflammatory arthritis had a significantly longer life rate in TEA compared to those with trauma-related causes. Patients with significant comorbidity, smoking, obesity, and young age also have an elevated risk of complications. In constrained arthroprostheses, multiple revisions are performed due to wear of the polyethylene, while in cases with unconstrained prostheses, multiple revisions are performed due to instability and dislocations. Infection, aseptic loosening, and periprosthetic fracture are the most concerning common complications primarily requiring revision surgery. Aseptic loosening is one of the most common causes of revision surgery. Stress shielding is frequently the cause of osteolysis surrounding the implant and loosening at the elbow prosthesis. In TEA, as Wolff’s law state, the transmission of nonanatomic force results in stress shielding at the humeral condyles and olecranon, leading to progressive bone resorption. This bone resorption increases the moment of force on the arm between the hinge and the site where the stem transfers most of its load, which not only predisposes to loosening of the stem but also increases the likelihood of arthroplasty failure due to polyethylene wear, mechanical failure, or periprosthetic fracture. Revision TEA is often possible for loose stems using a larger stem, and bone grafting can be done if necessary. King reported a series of 31 patients who underwent revision TEA with semi-constrained prosthesis due to aseptic loosening with a mean 6-year follow-up. The mean MEPS (Mayo Elbow Performance Score) was 87, and the mean flexion-extension arc was > 100° [
60].
A preliminary working diagnosis is based on clinical examination, basic imaging (orthogonal radiographs), and preliminary serological tests. The presentation can be broadly categorized into three: the TEA may be stiff (reduced active range of motion), weak (reduced active power of motion) or unstable (prosthetic loosening or dissociation, or both). A combination of any or all of these conditions may be present, with or without infection.
One or more of the three functional diagnoses determines the course of future study if it can be positively determined that the implant and/or implant-bone interface are the source of the pain and dysfunction. To diagnose stiffness caused by infection, implant impingement, implant malrotation, or heterotopic ossification, anesthesia is required for the examination. Fluoroscopy and joint aspiration are useful diagnostic tools. The causes of intrinsic neuropathy and extrinsic nerve compression can be identified by nerve conduction tests and ulnar nerve ultrasonography (US, with Doppler augmentation). Assessing bone length loss and subduction of a loose implant, along with imaging of the triceps mechanism, can help determine whether a TEA is weak and deteriorating. A painful TEA that presents with instability from a failing linking mechanism, or insufficient collateral ligaments in an unlinked implant, can be assessed with stress radiographs. CT is essential to assess bone loss and implant alignment (and prepare for a bespoke implant solution if required). In all scenarios, infection needs to be considered and investigated.
Patients with fever, erythema, wound dehiscence/blister with sinus or fistula formation, persistent or worsening local discomfort, swelling, or radiolucent lines on radiographs to the bone-cement-implant interface are suspected of having a periprosthetic elbow joint infection. Several attempts to identify the infecting microorganism may be necessary to guide antimicrobial treatment and management of the implants. Finding the infection in the extracapsular compartment (skin and subcutaneous tissues, including the triceps), intracapsular compartment, or intraosseous compartment (osteomyelitis) can be supported by US investigation combined with guided aspiration and CT imaging. When planning revision surgery, the most important factors to consider are the condition of the neurovascular system, the soft tissues (skin and muscle-tendon envelope), the quality of the bone after the implants are removed, the safety of the implant in the bone, and the presence or absence of infection [
61].
2.4.6. Revision Total Elbow Arthroplasty
In January 2014, a surgical intervention via the usual lateral access was performed to replace the prosthesis with a Coonrad-Morrey humeral stem small/L 150mm/Flangia through deep washing and cementation in absent signs of infections in the operating room. The patient was advised to keep the arm in an arm sling pocket for a month. Considering the failure of the arthrodesis and the good seal of the ulnar component, the prosthetic revision was deemed the most reasonable choice. Postoperative X-rays indicated good implant positioning (
Figure 8).
The patient had a good postoperative course, except for a small wound dehiscence with fibrinous serum secretions, which resolved in May 2014 with outpatient clinic treatments. In July 2014, after four months, the patient achieved complete extension and flexion of 100 degrees, with no significant changes in the X-ray findings. Two months later, in September 2014, the X-rays remained unchanged (
Figure 9), and the patient had a flexion of about 105 degrees, complete extension and pronation, and limited supination of 5 degrees.
Postoperative follow-up in complex revision depends on the status of the reconstructed extensor mechanism and the initial stability of the bone-implant structure. Healing of the skin wound, reduction of distal limb edema, hand mobility and protection of the ulnar nerve determine the patient's postoperative management and recovery of flexion-extension and pronation-supination. The circumferential bandage must be used very carefully to avoid the appearance of edema with distal pain. Isometric activation of the shunt muscles (including biceps and triceps) will help increase joint stability and promote restoration of neural feedback mechanisms of motor control. From 3 weeks to 3 months, from wound healing to healing of the extensor system, functional rehabilitation is practiced. Here, the concept that the shoulder and elbow are the “servants of the hand” may play a role: rehabilitation is guided by the skills of the hand, and the elbow and shoulder “simply” follow the hand in its tasks. Weight-bearing and load-sharing in long lever arm activities can be introduced once implant-bone stability has been achieved (the absence of symptoms of failure of osteo-integration, and often no earlier than 3 months after surgery), depending on the patient's needs and goals.
In general, high forces and impacts should be avoided. Nevertheless, biomechanical investigations have not provided evidence for recommendations to avoid compression or distraction loads greater than 2 kg.
It’s documented in the literature that up to 40% of patients with elbow replacement perform activities that are “excessively demanding” for TEA despite receiving relevant advice.
Due to the multifactorial nature of TEA failure, revision total elbow arthroplasty (R-TEA) still represents a significant challenge requiring careful evaluation of the patient, their environment, functional goals, and optimal knowledge of the biomechanics of the elbow [
61].
4. Discussion
Elbow arthropathy is characteristic in patients with hemophilia. Arthropathy is manifested by decreased ROM, pain, loss of strength and muscular atrophy and axial changes [
22].
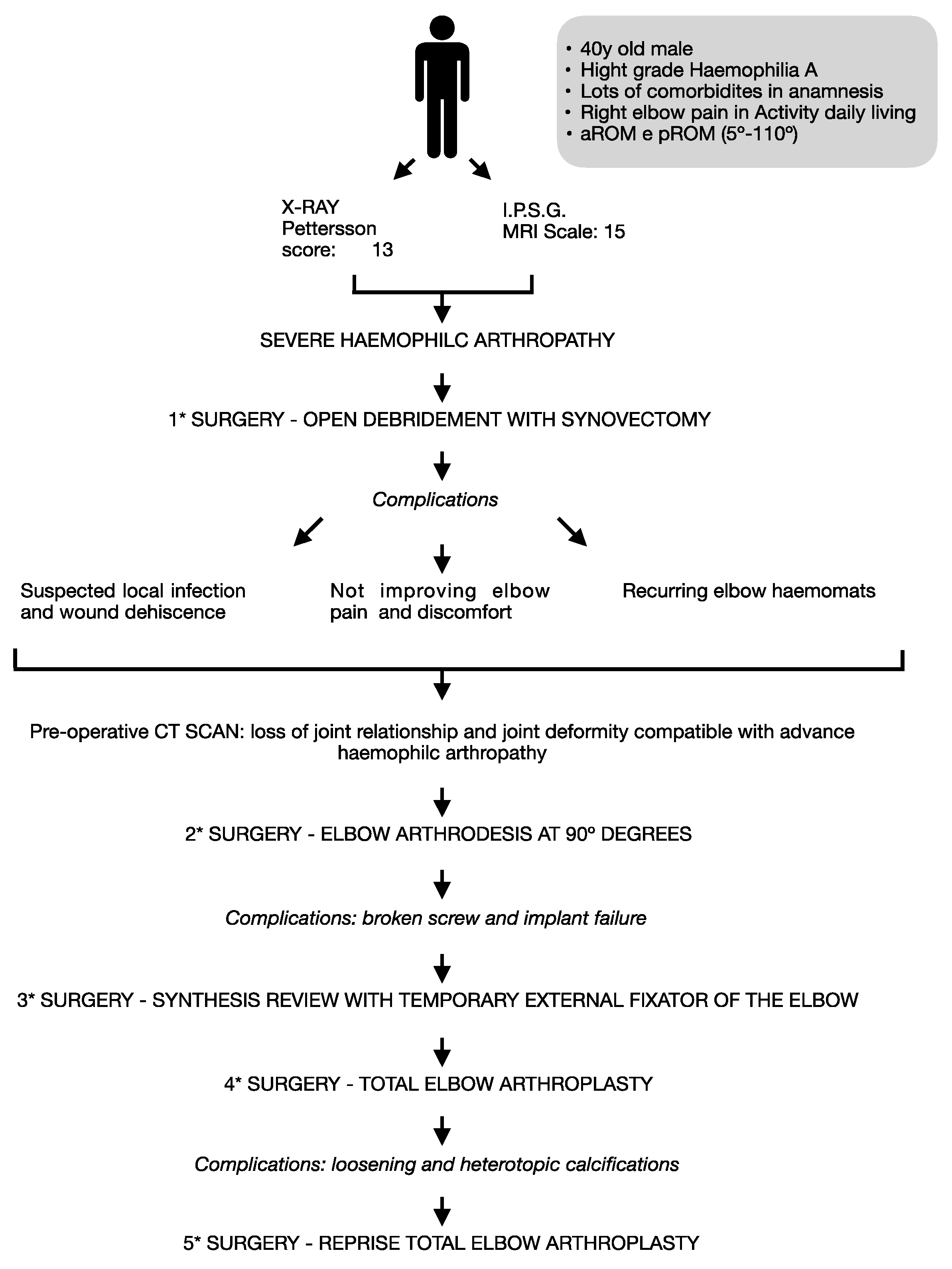
This case report provides a valuable example of clinical practice, as it involves multiple surgical and treatment interventions for a single patient along with associated complications (
Figure 10). The patient presented with elbow arthritis from the first medical visit, and the etiology was uncertain - whether it was due to primary arthritis caused by Hemophilia due to infection, or both. The decision to perform surgical synovectomy was made due to the patient's poor clinical and radiological condition, with suspected pseudotumor, and a conservative approach was unlikely to yield favorable outcomes.
It was challenging to perform an arthroscopic synovectomy in this patient due to the anatomic joint alterations caused by hemophilic arthropathy, even though it has been described and used in the treatment of this condition.
In patients with high-grade arthritis where anatomy is compromised, open synovectomy along with debridement of the calcifications can make sense [
62].
In this case, the severe hemophilic arthropathy with complete distortion of the joint anatomy may have been caused by an undetected hemophilic pseudotumor near the elbow joint. Hemophilic pseudotumors are often asymptomatic and painless until they become large enough to compress adjacent structures, leading to fractures, necrosis, fistulas, or infections. Arterial embolization is a treatment option for large tumors, particularly in the pelvic area, but surgery is typically considered the most effective cure.
However, the effect is temporary and it better serves as an adjunctive procedure, shrinking the lesion to make it more amenable to surgical resection [
63].
The surgical removal of hemophilic pseudotumor is considered the best option, and in this case, open synovectomy was performed to remove part of the atypical soft tissue around the joint. Preoperative artery embolization, ideally two weeks before surgery, would have been useful to control intraoperative bleeding during surgery and to prevent postoperative complications [
46].
The decision to perform arthrodesis was made due to the patient's young age and high functional demand, along with the instability of the joint caused by poor soft tissue quality. The aim was to reduce pain and maintain some degree of functionality. The failure of the arthrodesis was likely due to several factors including the use of a too-short plate on the humerus, lack of bone grafts, insufficient length of locking screws, and early mobilization of the arm. An external fixator was used as a salvage option due to surgical wound dehiscence, and the possibility of total elbow arthroplasty was reconsidered after arthrodesis failure.
The step from an arthrodesis, generally the extreme solution, to the prosthesis has been described with success by Burkhart et al [
51].
In this study, a Coonrad-Morrey model of linked TEA was used due to its ability to provide greater joint stability, even in cases of significant bone loss and ligament insufficiency, while still maintaining a good range of motion. However, it should be noted that the increased tension on both joint surfaces may lead to early loosening of the prosthesis. The patient was advised to maintain a pinstripe shower until the wound healed, followed by cautious mobilization of the elbow and taking 2 oral vials of Tranexamic acid every 12 hours for a week after the TEA surgery. Aftercare recommendations, including initial immobilization in extension for the first few days, followed by cautious mobilization, were followed.
Despite protective measures, prosthesis mobilization still occurred, and the cause of loosening is more likely attributed to under-sizing of the humeral stem component and bad osteointegration due to the inflammatory local environment from the basic pathology or the recurrence of the lymphoma. Based on the failure of the arthrodesis and the good positioning of the ulnar component, a prosthetic revision due to aseptic loosening seems to be the most reasonable choice.
5. Conclusion
This case highlights the complexity of managing hemophilic patients with elbow arthropathy, where the risk of failure is higher than the general population. Therefore, treatment decisions should be made with caution and consideration of the potential challenges. Close follow-up in a specialized hospital setting is crucial to minimize treatment delays and prevent end-stage damage. The path from diagnosis to surgical treatment of hemophilic elbow arthropathy can be a risky one, as it happened in this case.
Author Contributions
“Conceptualization, G.P. and F.B.; methodology, D.A., S.A. R.R.; formal analysis, G.P., EC.RM.; investigation, G.P., D.A., S.A., R.R., P.A., E.J., F.B., E.V., EC.RM., M.M.; writing—original draft preparation, D.A., R.R.; writing—review and editing, D.A., S.A.; visualization, E.V., P.A.,E.J,M.M.; supervision, G.P.,EC.RM. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study because it is a retrospective study.
Disclaimer: The authors: their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subjects of this article.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Madhok: R.: York: J.; Sturrock, R. Haemophilic Arthritis. Ann Rheum Dis 1991, 50, 588–591. [CrossRef]
- Manco-Johnson, M.; Abshire, T.; Shapiro, A.; Riske, B.; Hacker, M.; Kilcoyne, R.; Ingram, J.; Manco Johnson, M.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus Episodic Treatment to Prevent Joint Disease in Boys with Severe Hemophilia. N Engl J Med 2007, 357, 535–544. [CrossRef]
- Aledort, L.; Haschmeyer, R.; Petterson, H. A Longitudinal Study of Orthopaedic Outcomes for Severe Factor-VIII- Deficient Haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994, 236, 391–399. [CrossRef]
- Högh, J.; Ludlam, C.; Macnicol, M. Hemophilic Arthropaty of the Upper Limb. Clin Orthop Relat Res. 1987, 218, 225–231.
- Gilbert, M.; Glass, K. Hemophilic Arthropaty in the Elbow. Mt Sinai J Med N Y. 1977, 44, 389–396. [CrossRef]
- Raffini, L.; Manno, C. Modern Management of Haemophilic Arthropathy. Br J Haematol. 2007, 136, 777–787. [CrossRef]
- Letizia, G.; Piccione, F.; Ridola, C.; Zummo, G. Ultrastructural Appearance of Human Synovial Membrane in the Reabsorption Phase of Acute Haemarthrosis. Ital J Orthop Traumatol. 1980, 6, 275–277.
- Hooveld, M.; Rosendall, G.; Vianen, M.; Van den Berg, M.; Bijlsma, J.; Lafeber, F. Blood Induced Joint Damage: Longterm Effects in Vitro and in Vivo. J Rheumatol. 2003, 30, 339–344.
- AICE Online. La Gestione Dell’angiogenesi Nella Sinovite in Corso Di Artropatia Emofilica: Nuove Prospettive Patogenetiche e Terapeutiche, La Diagnostica per Immagini.
- Hakobyan, N.; Kazarian, T.; Valentino, L. Synovitis in a Murine Model of Human Factor VIII Deficiency. Haemoph Off J World Fed Hemoph. 2005, 11, 227–232. [CrossRef]
- Acharya, S.; Rule, B.; McMillan, O.; Humphries, T. Point of Care Ultrasonograpy (POCUS) in haemophiliaA: A Commentary on Current Status and Its Potential Role for Improving Prophylaxis Management in Severe Haemophilia A. Ther Adv Hematol. 2017, 8, 153–156. [CrossRef]
- Wen, F.; Jabbar, A.; Chen, Y.; Kazarian, T.; Patel, D.; Valentino, L. C-Myc Proto-Oncogene Expression in Hemophilic Synovitis:In Vitro Studies of Effects of Iron and Ceramide. Blood 2002, 100, 912–916. [CrossRef]
- Rosendall, G.; Lafeber, F. Pathogenesis of Haemophilic Arthropathy. . Haemoph Off J World Fed Hemoph 12, 177–121. [CrossRef]
- Rosendall, G.; Tekoppelle, J.; Vianen, M.; Van den Berg, H.; Lafeber, F.; Bijlsma, J. Articular Cartilage Is More Susceptible to Blood Induced Damage at Young than at Old Age. J Rheumatol. 2000, 27, 1740–1744, PMID: 12563692.
- Fischer, K.; Van der Bom, J.; Mauser Bunschoten, E.; Roosendal, G.; Prejs, R.; de Kleijn, P.; Grobbee, D.; Van den Berg, M. The Effects of Postponing Prophylactic Treatment on Long-Term Outcome in Patients with Severe Haemophilia. Blood 2002, 99, 2337–2341. [CrossRef]
- Rosendall, G.; Lafeber, F. Blood Induced Joint Demage in Haemophilia. Semin Thromb Hemost. 2003, 29, 37–42. [CrossRef]
- Rodriguez-Merchan, E. Haemophilic Arthropathy: Contemporary Management Challenges and a Future Scenario. Haemophilia 2021, 27, e765–e767. [CrossRef]
- Duarte, M.; Duarte, E.; Solorzano, E. Hemophilic Pseudotumor - a Rare Complication. 2017;39:84–5. Rev Bras Hematol Hemoter 2017, 39, 5. [CrossRef]
- Yeung, C.; Blazar, P. Unusual Olecranon Mass with Ulnar Nerve Compressive Neuropathy Caused by a Haemophilic Pseudotumour. BMJ Case Rep 2019, 12. [CrossRef]
- Papatheodorou, L.; Baratz, M.; Sotereanos, D. Elbow Arthritis: Current Concepts. J Hand Surg Am 2013, 38, 605–613. [CrossRef]
- Utukuri, M.; Goddard, N. Haemophilic Arthropathy of the Elbow. Haemophilia 2005, 11, 565–570. [CrossRef]
- Cuesta-Burriuso, R.; Gomez Conesa, A.; Lopez Pina, J. Manual and Educational Therapy in the Treatment of Hemophilic Arthropaty of the Elbow: A Randomized Pilot Study. Orphanet J Rare 2018, 13, 151. [CrossRef]
- Adams, J.; Reding, M. Hemophilic Arthropathy of the Elbow. Hand Clin. 2011, 27, 151–163. [CrossRef]
- Ishiguro, N.; Yasuo, S.; Takamatu, S.; Iwata, H. Hemophilic Arthropathy of the Elbow. 1995, 15, 821–825. [CrossRef]
- Heim, M.; Beeton, K.; Blamey, G.; Goddard, N. Management of the Elbow Joint. Haemophilia 2012, 18, 101–104. [CrossRef]
- Arnold, W.; Hilgartner, M. Hemophilic Artrhopaty. Current Concepts of Pathogenesis and Management. J bone Joint Surg Am. 1977, 59, 287–305.
- Petterson, H.; Ahlberg, A.; Nilsson, I. A Radiologic Classification of Hemophilic Arthropathy. Clin Orthop Relat Res. 1980, 153–159.
- Dale, T.; Saucedo, J.; Rodriguez-Merchan, E. Hemophilic Arthropathy of the Elbow: Prophylaxis, Imaging, and the Role of Invasive Management. J Shoulder Elbow Surg 2015, 24, 1669–1678. [CrossRef]
- Bossard, D.; Carrillon, Y.; Stieltjes, N.; Larbre, J.; Laurian, Y.; Molina; Dirat, G. Management of Haemophilis Arthropaty. Haemoph Off J World Fed Hemoph 2008, 14, 11–19.
- Kerr, R. Imaging of Musculoskeletal Complications of Hemophilia. Semin Musculoskelet Radiol. 2003, 7, 127–136. [CrossRef]
- Acharya, S. Exploration of the Pathogenesis of Haemolitic Joint Arthropathy: Understanding Implications for Optimal Clinical Management. ritish Journal of Haematology – Wiley Online Library. 2012, 156, 13–23. [CrossRef]
- Lundin A New Magnetic Resonance Imaging Scoring Method for Assessment of Haemophilic Arthropaty, -. Haemophilia- Wiley Online Library. 2004, 10, 383–389. [CrossRef]
- Lundin, B.; Manco Johnson, M.; Ignas, D.; Moineddin, R.; Blanchette, V.; Dunn, A.; Gibikote, S.; Keshava, S.; Ljung, R.; Manco-Johnson, M.; et al. An MRI Scale for Assessment of Haemophilic Arthropaty from the International Prophylaxis Study Group. Haemophilia 2012, 18, 962–970. [CrossRef]
- Soler, R.; Loper Fernandez, F.; Rodriguez-Merchan, E.; Marini, M. Hemophilic Arthropathy. A Scoring System for Magnetic Resonance Imaging. Eur Radiol. 2002, 12, 836–843. [CrossRef]
- Cittadini, G.; Cittadini, G.; Sardanelli, F. Diagnostica per Immagini. Pg 144-145; Edra S.p.a., 2016;
- Iwamoto, T.; Suzuki, T.; Oki, S.; Matsumura, N.; Nakamura, M.; Matsumoto, M.; Sato, K. Computed Tomography-Based 3-Dimensional Preoperative Planning for Unlinked Total Elbow Arthroplasty. J Shoulder Elbow Surg. 2018, 27, 1792–1799. [CrossRef]
- Rodriguez-Merchan, E.; Jimenez-Yuste, V. The Role of Selective Angiographic Embolization of the Musculo-Skeletal System in Haemophilia. Haemophilia 2009, 15, 864–868. [CrossRef]
- Sandford, F.; Barlow, N.; Lewis, J. A Study to Examine Patient Adherence to Wearing 24-Hour Forearm Thermoplastic Splints after Tendon Repairs. J Hand Ther Off J Am Soc Hand Ther. 2008, 21, 44–52. [CrossRef]
- Zelada, F.; de Almeida, A.; Pailo, A.; Neto, R.; Okazaki, E.; de Rezende, M. Viscosupplementation in Patients with Hemophilic Arthropathy.I. Acta Ortop Bras 2013, 21, 12–17. [CrossRef]
- Rodríguez-Merchán, E.C.; Magallón, M.; Galindo, E.; López-Cabarcos, C. Hemophilic Synovitis of the Knee and the Elbow. Clin. Orthop. 1997, 47–53.
- Luck, J. Traumatic Arthrofibrosis; the Fibroplastic Response of Joints to Trauma. Bull Hosp Joint Dis. 1951, 12, 394–403.
- van Vulpen, L.; Thomas, S.; Keny, S.; Mohanty, S. Synovitis and Synovectomy in Haemophilia. Haemophilia. 2021, 27, 96–102. [CrossRef]
- Makris, M.; Kasper, C. The World Federation of Hemophilia Guideline on Management of Haemophilia. Haemophilia 2013, 19, 1. [CrossRef]
- Schmitt, C.; Adamkewicz, J.; Xu, J.; Petry, C.; Catalani, O.; Young, G.; Negrier, C.; Callaghan, M.; Levy, G. Pharmacokinetics and Pharmacodynamics of Emicizumab in Persons with Hemophilia A with Factor VIII Inhibitors: HAVEN 1 Study. Thromb Haemost 2021, 121, 351–360. [CrossRef]
- Melchiorre, D.; Manetti, M.; Matucci-Cerinic, M. Pathophysiology of Hemophilic Arthropathy. J Clin Med 2017, 6, 63. [CrossRef]
- Rodriguez-Merchan, E. Hemophilic Pseudotumors: Diagnosis and Management. Arch Bone Jt Surg. 2020, 8, 121–130. [CrossRef]
- Poonit, K.; Zhou, X.; Zhao, B.; Yao, C.; Zhang, F.; Zheng, J.; Yan, H. Treatment of Osteoarthritis of the Elbow with Open or Arthroscopic Debridement: A Narrative Review. BMC Musculoskeletal Disorders 2018, 19, 394. [CrossRef]
- White, C.; Ravi, V.; Watson, J.; Badhrinarayanan, S.; Phadnis, J. A Systematic Review of Arthroscopic Versus Open Debridement of the Arthritic Elbow. Arthroscopy 2021, 37, 747–758. [CrossRef]
- Kovack, T.; Jacob, P.; Mighell, M. Elbow Arthrodesis: A Novel Technique and Review of the Literature. 2014, 37, 313–319. [CrossRef]
- Leschinger, T.; Ott, N.; Hackl, M.; Müller, L.; Wegmann, K. Fixateur Externe Am Ellenbogen. Oper Orthop Traumatol 2020, 32, 387–395. [CrossRef]
- Burkhart, K.; Dargel, J.; Wegmann, K. Konvertierung Einer Ellenbogen Arthrodese Zur Ellenbogenprothese. Unfallchirurg 2013, 116, 371–375. [CrossRef]
- Aldridge, J.; Lightdale, N.; Mallon, W.; Coonrad, R. Total Elbow Arthroplasty with the Coonrad/Coonrad-Morrey Prosthesis: A 10 to 31 Years Survival Analysis. J Bone Joint Surg Br. 2006, 88, 509–514. [CrossRef]
- Dee, R. Total Replacement Arthroplasty of the Elbow for Rheumatoid Arthritis. J Bone Joint Surg Br. 1972, 54, 88–95.
- Kamineni, S.; Morrey, B. Distal Humeral Fractures Treated with Noncustom Total Elbow Replacement. Surgical Technique. J Bone Joint Surg Am. 2005, 87, 41–50. [CrossRef]
- Ramsey, M.L.; Adams, R.A.; Morrey, B.F. Instability of the Elbow Treated with Semiconstrained Total Elbow Arthroplasty. J. Bone Joint Surg. Am. 1999, 81, 38–47. [CrossRef]
- Kaminemi, S.; Adams, R.; O’Driscoll, S.; Morrey, B. Hemophilic Arthropathy of the Elbow Treated by Total Elbow Replacement. A Case Series. J Bone Joint Surg Am. 2004, 86, 584–589. [CrossRef]
- Sanchez-Sotelo, J. Total Elbow Arthroplasty. Open Orthop J. 2011, 5, 115–123. [CrossRef]
- Marshall Brooks, M.; Tobase, P.; Karp, S.; Francis, D.; Fogarty, P. Outcomes in Total Elbow Arthroplasty in Patients with Haemophilia at the University of California, San Francisco: A Retrospective Review. Haemoph Off J World Fed Hemoph. 2011, 17, 118–123. [CrossRef]
- Ernstbrunner, L.; Hingsammer, A.; Imam, M.; Sutter, R.; Brand, B.; Meyer, D.; Wieser, K. Long-Term Results of Total Elbow Arthroplasty in Patients with Hemophilia. J Shoulder Elbow Surg 2018, 27, 126–132. [CrossRef]
- Kwak, J.; Koh, K.; Jeon, I. Total Elbow Arthroplasty: Clinical Outcomes, Complications, and Revision Surgery, ReviewArticle. Clinics Orthopedic Surgery 2019, 11, 369–379. [CrossRef]
- Chin, K.; Lambert, S. Revision Total Elbow Replacement. Journal of Clinical Orthopaedics and Trauma 2021, 20. [CrossRef]
- Pasta, G.; Annunziata, S.; Forini, G.; Jannelli, E.; Minen, A.; Preti, P.; Mosconi, M.; Benazzo, F. A Rare Case of a Patient with Hemophilia Presenting Elbow Ankylosing Heterotopic Ossification:Surgery and Functional Outcomes. 2020, 4, 1021–1025. [CrossRef]
- Pasta, G.; Ruggieri, R.; Annunziata, S.; Gagliardi, V.; Cuzzocrea, F.; Ghiara, M.; Russo, M.; Preti, P.; Santi, R.; Mosconi, M.; et al. Haemophilic Pelvic Pseudotumour: A New Surgical Option. Healthcare (Basel). 2021, 9, 1269. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).