Submitted:
25 March 2024
Posted:
26 March 2024
You are already at the latest version
Abstract
Keywords:
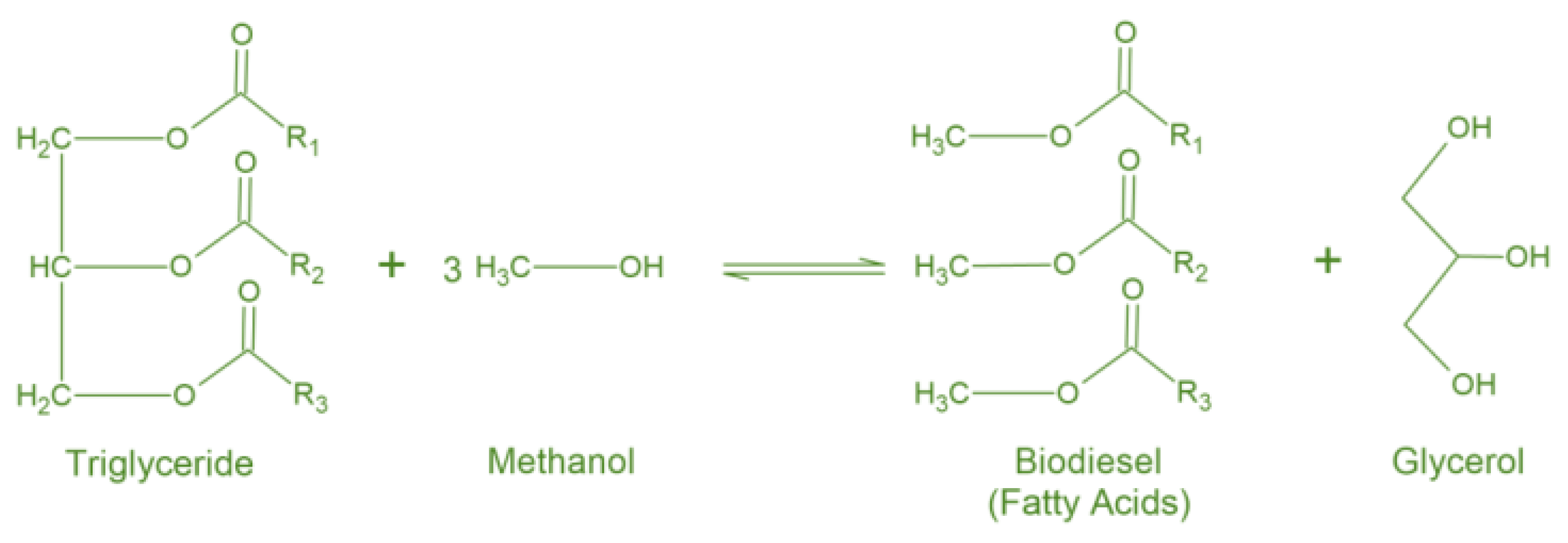
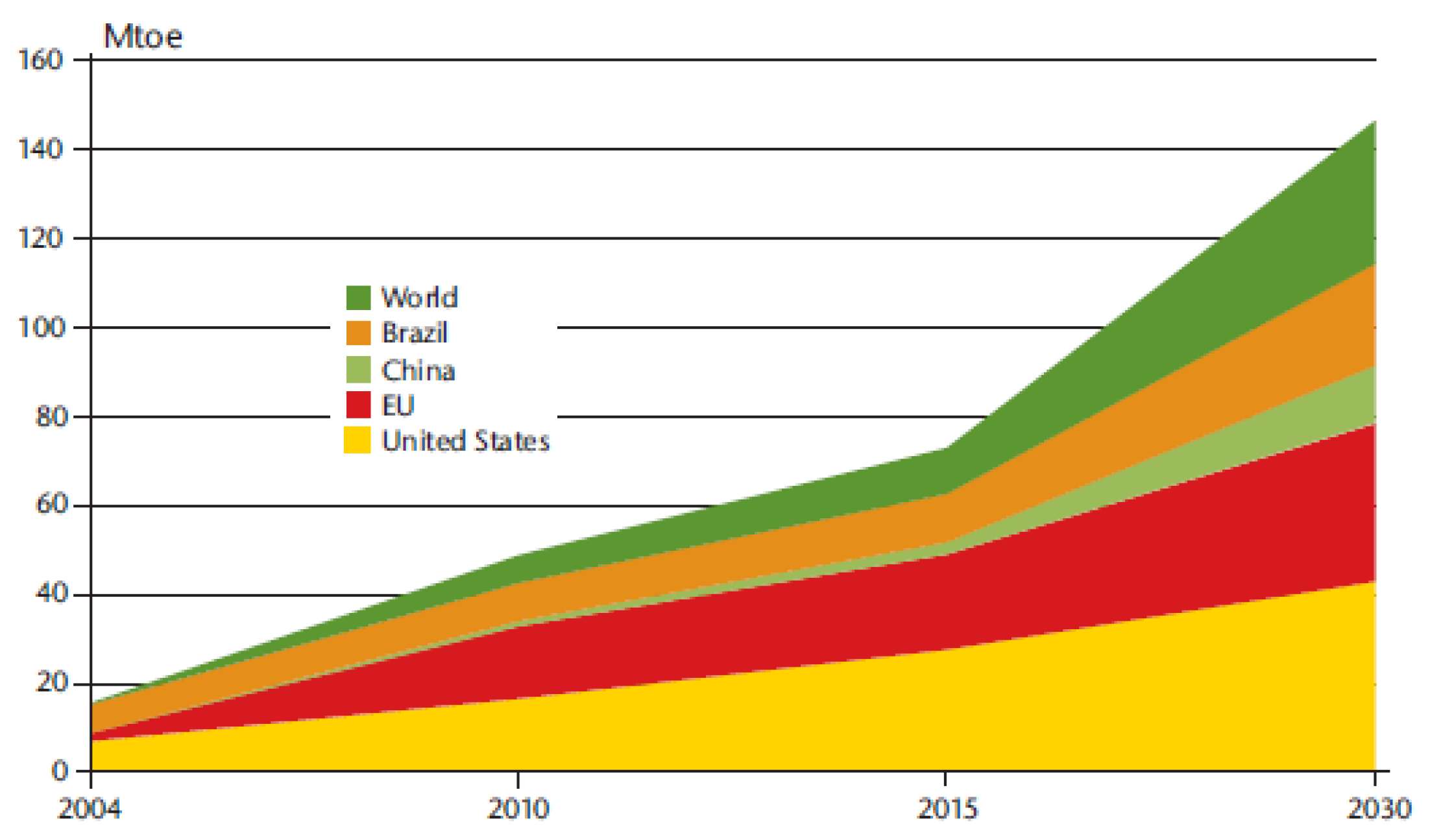
1. Introduction
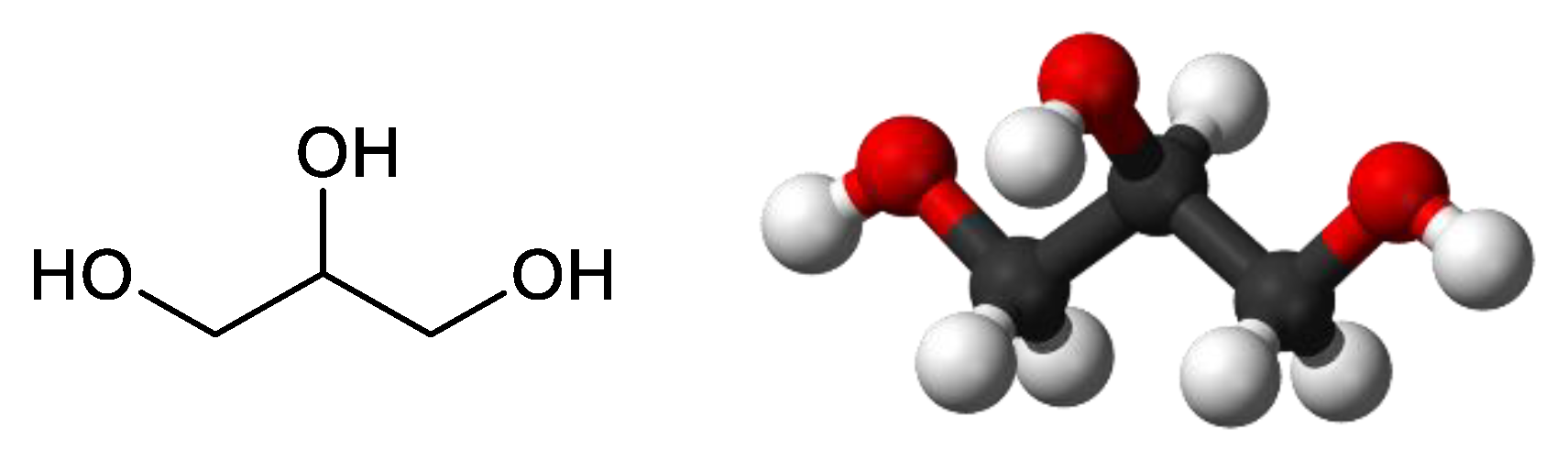
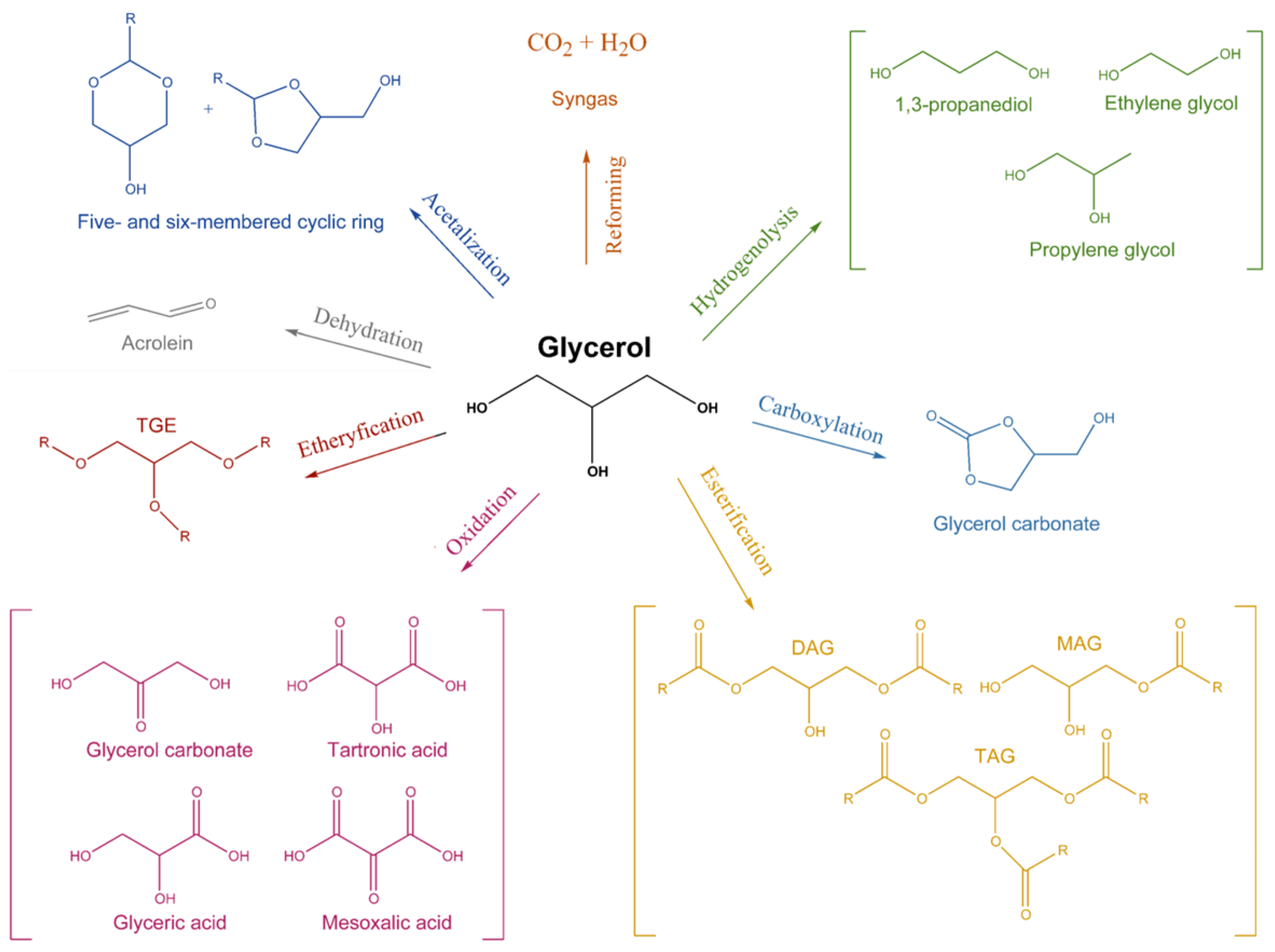
2. Glycerol Valorisation
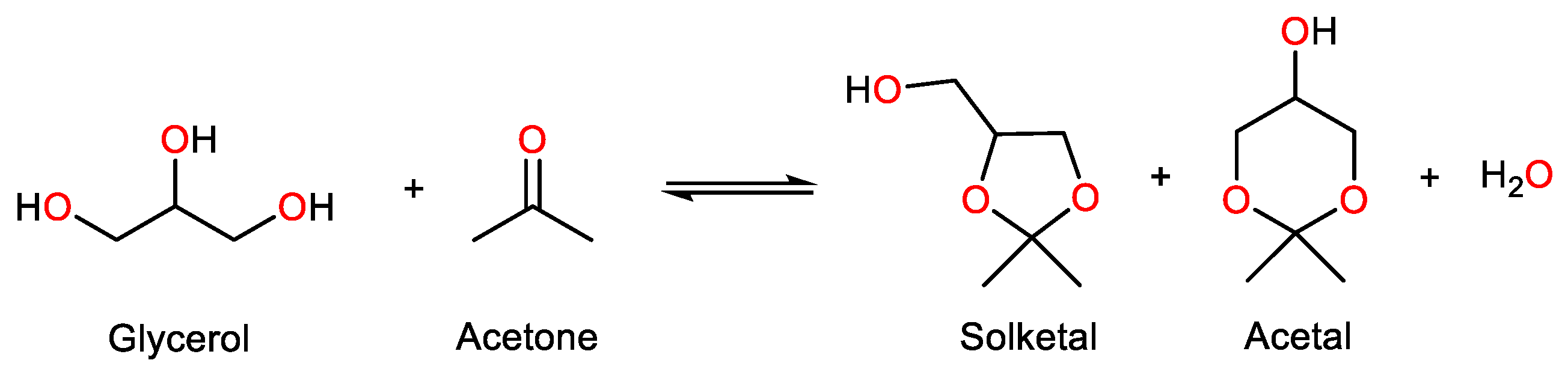
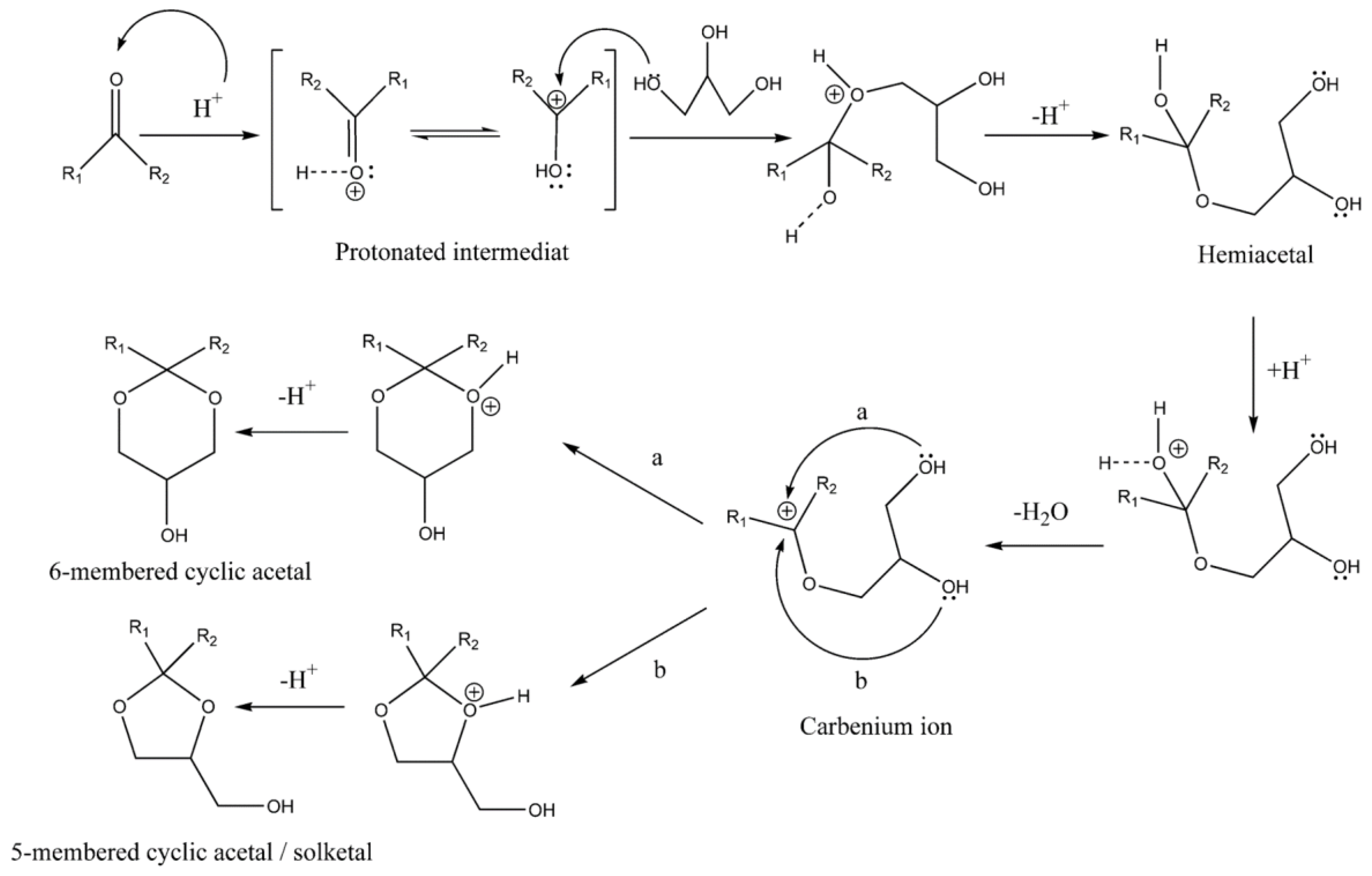
3. Acetalization Reaction
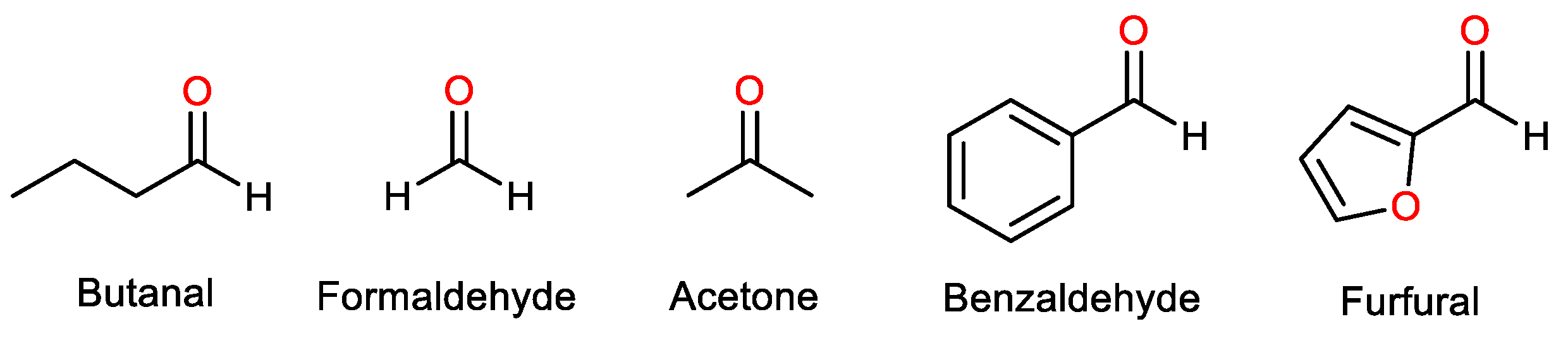
3.1. Substrate
3.2. Solvent
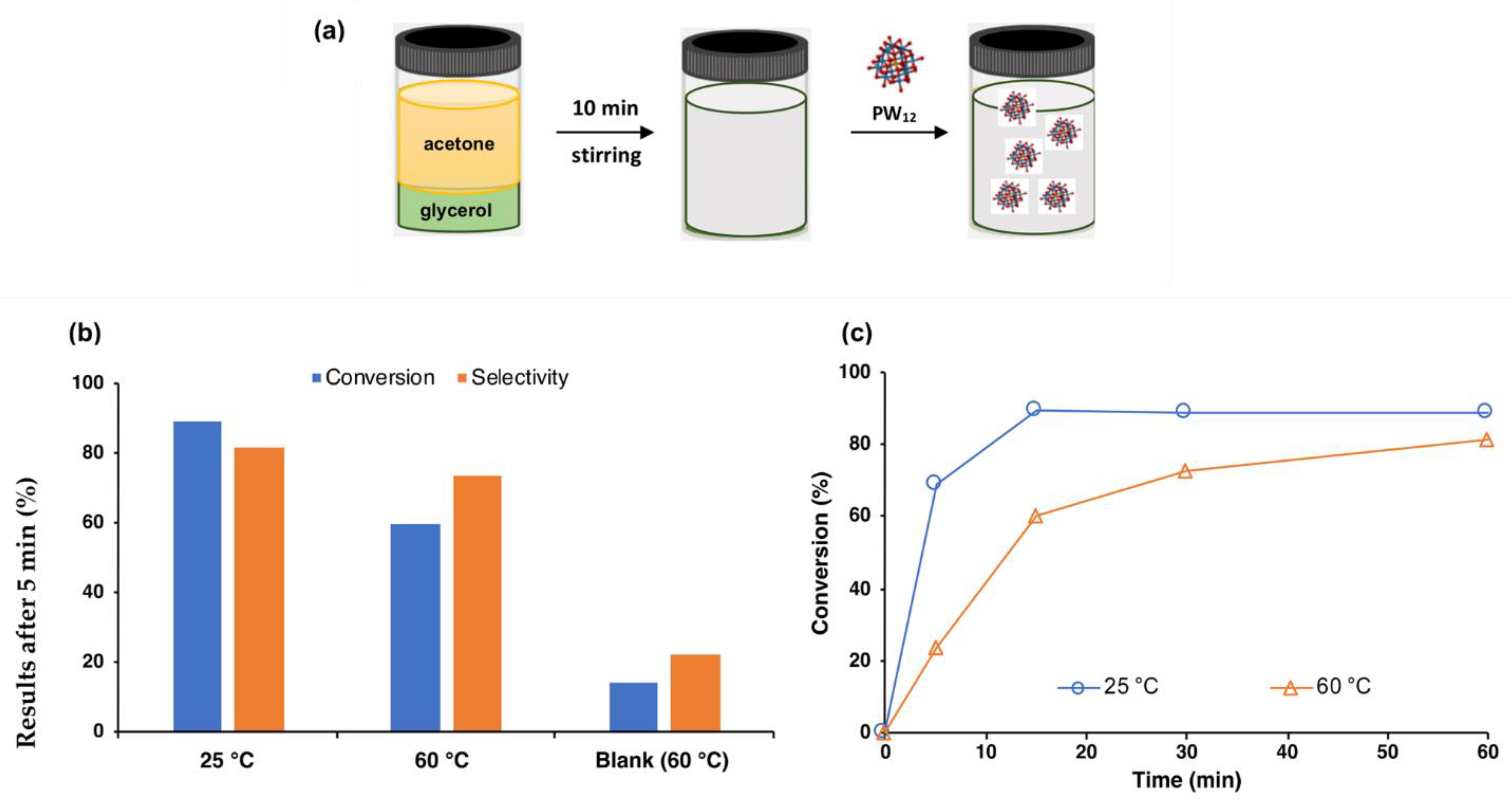
3.3. Catalyst
4. Heterogeneous Catalysts for Glycerol Conversion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akram, F.; Haq, I.u.; Raja, S.I.; Mir, A.S.; Qureshi, S.S.; Aqeel, A.; Shah, F.I. Current trends in biodiesel production technologies and future progressions: A possible displacement of the petro-diesel. Journal of Cleaner Production 2022, 370, 133479–133496. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumar Sharma, P.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262. [Google Scholar] [CrossRef]
- Zahid, I.; Ayoub, M.; Abdullah, B.B.; Nazir, M.H.; Ameen, M.; Zulqarnain; Mohd Yusoff, M. H.; Inayat, A.; Danish, M. Production of Fuel Additive Solketal via Catalytic Conversion of Biodiesel-Derived Glycerol. Industrial & Engineering Chemistry Research 2020, 59, 20961–20978. [Google Scholar]
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Progress in Energy and Combustion Science 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Ramos; Dias; Puna; Gomes; Bordado. Biodiesel Production Processes and Sustainable Raw Materials. Energies 2019, 12. [Google Scholar] [CrossRef]
- TEA. World Energy Outlook 2023, IEA, Paris https://www.iea.org/reports/world-energy-outlook-2023, Licence: CC BY 4.0 (report); CC BY NC SA 4.0 (Annex A) 2023.
- Statistical Review of World Energy. Energy Institute 2023, 7th Edition.
- Julião, D.; Gomes, A.C.; Pillinger, M.; Gonçalves, I.S.; Balula, S.S. Desulfurization and Denitrogenation Processes to Treat Diesel Using Mo(VI)-Bipyridine Catalysts. Chemical Engineering & Technology 2020, 43, 1774–1783. [Google Scholar] [CrossRef]
- Comission, E. A European Green Deal: Striving to be the first climate-neutral continent. Availabe online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 22/01/2023).
- Corrêa, I.; Faria, R.P.V.; Rodrigues, A.E. Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustainable Chemistry 2021, 2, 286–324. [Google Scholar] [CrossRef]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renewable and Sustainable Energy Reviews 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Najaafi, N.; Tarighi, S. A Review on the Catalytic Acetalization of Bio-renewable Glycerol to Fuel Additives. Front Chem 2018, 6, 573. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renewable and Sustainable Energy Reviews 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Fangxia Yang, M.A.H. , Runcang Sun. Value-added uses for crude glycerol - a byproduct of biodiesel production. Biotechnology for Biofuels 2012, 5. [Google Scholar]
- OECD; Food; Nations, A.O.o.t.U. Biodiesel projections: Production and use; 2023;. https://doi.org/.
- Aydogan, H.; Hirz, M.; Brunner, H. The use and future of biofuels. International journal of social sciences ISSN 1804-980X 2014.
- da Silva, C.X.A.; Mota, C.J.A. The influence of impurities on the acid-catalyzed reaction of glycerol with acetone. Biomass and Bioenergy 2011, 35, 3547–3551. [Google Scholar] [CrossRef]
- Smirnov, A.; Selishcheva, S.; Yakovlev, V. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8, 595–620. [Google Scholar] [CrossRef]
- Fatimah, I.; Sahroni, I.; Fadillah, G.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Glycerol to Solketal for Fuel Additive: Recent Progress in Heterogeneous Catalysts. Energies 2019, 12, 2872–2886. [Google Scholar] [CrossRef]
- Smirnov, A.; Selishcheva, S.; Yakovlev, V. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Applied Catalysis B: Environmental 2016, 193, 75–92. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From glycerol to value-added products. Angew Chem Int Ed Engl 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Tomishige, K. Heterogeneous catalysis of the glycerol hydrogenolysis. Catalysis Science & Technology 2011, 1, 179–190. [Google Scholar] [CrossRef]
- Chaminand, J.; Djakovitch, L.a.; Gallezot, P.; Marion, P.; Pinel, C.; Rosier, C. Glycerol hydrogenolysis on heterogeneous catalysts. Green Chemistry 2004, 6, 359–361. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.-S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paul, S.; Dumeignil, F. Selective catalytic oxidation of glycerol: perspectives for high value chemicals. Green Chemistry 2011, 13. [Google Scholar] [CrossRef]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Kiely, C.J.; Hutchings, G.J. Oxidation of glycerol using supported Pt, Pd and Au catalysts. Physical Chemistry Chemical Physics 2003, 5, 1329–1336. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Canton, P.; Dimitratos, N.; Porta, F.; Prati, L. Selective oxidation of glycerol with oxygen using mono and bimetallic catalysts based on Au, Pd and Pt metals. Catalysis Today 2005, 102-103, 203–212. [Google Scholar] [CrossRef]
- Dodekatos, G.; Schünemann, S.; Tüysüz, H. Recent Advances in Thermo-, Photo-, and Electrocatalytic Glycerol Oxidation. ACS Catalysis 2018, 8, 6301–6333. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Bellière-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chemistry 2010, 12. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Hezaveh, H. Glycerol for renewable acrolein production by catalytic dehydration. Renewable and Sustainable Energy Reviews 2014, 40, 28–59. [Google Scholar] [CrossRef]
- Palanychamy, P.; Lim, S.; Yap, Y.H.; Leong, L.K. Critical Review of the Various Reaction Mechanisms for Glycerol Etherification. Catalysts 2022, 12, 1487–1517. [Google Scholar] [CrossRef]
- Frusteri, F.; Arena, F.; Bonura, G.; Cannilla, C.; Spadaro, L.; Di Blasi, O. Catalytic etherification of glycerol by tert-butyl alcohol to produce oxygenated additives for diesel fuel. Applied Catalysis A: General 2009, 367, 77–83. [Google Scholar] [CrossRef]
- Clacens, J.M.; Pouilloux, Y.; Barrault, J. Selective etherification of glycerol to polyglycerols over impregnated basic MCM-41 type mesoporous catalysts. Applied Catalysis A: General 2002, 227, 181–190. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chemical Society Reviews 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Mirante, F.; Leo, P.; Dias, C.N.; Cunha-Silva, L.; Balula, S.S. MOF-808 as an Efficient Catalyst for Valorization of Biodiesel Waste Production: Glycerol Acetalization. Materials (Basel) 2023, 16. [Google Scholar] [CrossRef]
- Serafim, H.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorization of glycerol into fuel additives over zeolites as catalysts. Chemical Engineering Journal 2011, 178, 291–296. [Google Scholar] [CrossRef]
- Wegenhart, B.L.; Liu, S.; Thom, M.; Stanley, D.; Abu-Omar, M.M. Solvent-Free Methods for Making Acetals Derived from Glycerol and Furfural and Their Use as a Biodiesel Fuel Component. ACS Catalysis 2012, 2, 2524–2530. [Google Scholar] [CrossRef]
- Castanheiro, J. Acetalization of Glycerol with Citral over Heteropolyacids Immobilized on KIT-6. Catalysts 2022, 12. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Arvind, N. Acetalization of glycerol and benzaldehyde to synthesize biofuel additives using SO(4) (2-)/CeO(2)-ZrO(2) catalyst. Heliyon 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Glycerol acetalization with formaldehyde using heteropolyacid salts supported on mesostructured silica. Applied Catalysis A: General 2018, 549, 207–215. [Google Scholar] [CrossRef]
- Juliao, D.; Mirante, F.; Balula, S.S. Easy and Fast Production of Solketal from Glycerol Acetalization via Heteropolyacids. Molecules 2022, 27, 6573–6583. [Google Scholar] [CrossRef] [PubMed]
- Mallesham, B.; Sudarsanam, P.; Raju, G.; Reddy, B.M. Design of highly efficient Mo and W-promoted SnO2solid acids for heterogeneous catalysis: acetalization of bio-glycerol. Green Chem. 2013, 15, 478–489. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, R.; Ye, B.; Hou, Z. Acetalization of glycerol over sulfated UiO-66 under mild condition. Journal of Industrial and Engineering Chemistry 2022, 110, 357–366. [Google Scholar] [CrossRef]
- Qing, W.; Chen, J.; Shi, X.; Wu, J.; Hu, J.; Zhang, W. Conversion enhancement for acetalization using a catalytically active membrane in a pervaporation membrane reactor. Chemical Engineering Journal 2017, 313, 1396–1405. [Google Scholar] [CrossRef]
- Umbarkar, S.B.; Kotbagi, T.V.; Biradar, A.V.; Pasricha, R.; Chanale, J.; Dongare, M.K.; Mamede, A.-S.; Lancelot, C.; Payen, E. Acetalization of glycerol using mesoporous MoO3/SiO2 solid acid catalyst. Journal of Molecular Catalysis A: Chemical 2009, 310, 150–158. [Google Scholar] [CrossRef]
- Shirani, M.; Ghaziaskar, H.S.; Xu, C. Optimization of glycerol ketalization to produce solketal as biodiesel additive in a continuous reactor with subcritical acetone using Purolite® PD206 as catalyst. Fuel Processing Technology 2014, 124, 206–211. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Khan, N.A.; Hasan, Z.; Prosvirin, I.P.; Tsybulya, S.V.; Jhung, S.H. Isostructural metal-carboxylates MIL-100(M) and MIL-53(M) (M: V, Al, Fe and Cr) as catalysts for condensation of glycerol with acetone. Applied Catalysis A: General 2017, 529, 167–174. [Google Scholar] [CrossRef]
- da Silva, M.J.; Julio, A.A.; Dorigetto, F.C.S. Solvent-free heteropolyacid-catalyzed glycerol ketalization at room temperature. RSC Advances 2015, 5, 44499–44506. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Hameed, B.H. Solventless acetalization of glycerol with acetone to fuel oxygenates over Ni–Zr supported on mesoporous activated carbon catalyst. Applied Catalysis A: General 2013, 464-465, 191–199. [Google Scholar] [CrossRef]
- Gadamsetti, S.; Rajan, N.P.; Rao, G.S.; V. R. Chary, K. Acetalization of glycerol with acetone to bio fuel additives over supported molybdenum phosphate catalysts. Journal of Molecular Catalysis A: Chemical 2015, 410, 49–57. [Google Scholar] [CrossRef]
- da Silva, M.J.; Teixeira, M.G.; Chaves, D.M.; Siqueira, L. An efficient process to synthesize solketal from glycerol over tin (II) silicotungstate catalyst. Fuel 2020, 281, 118724–118732. [Google Scholar] [CrossRef]
- Ammaji, S.; Rao, G.S.; Chary, K.V.R. Acetalization of glycerol with acetone over various metal-modified SBA-15 catalysts. Applied Petrochemical Research 2018, 8, 107–118. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Highly Efficient Glycerol Acetalization over Supported Heteropoly Acid Catalysts. ChemCatChem 2018, 10, 1918–1925. [Google Scholar] [CrossRef]
- Manjunathan, P.; Maradur, S.P.; Halgeri, A.B.; Shanbhag, G.V. Room temperature synthesis of solketal from acetalization of glycerol with acetone: Effect of crystallite size and the role of acidity of beta zeolite. Journal of Molecular Catalysis A: Chemical 2015, 396, 47–54. [Google Scholar] [CrossRef]
- Santos-Vieira, I.C.M.S.; Mendes, R.F.; Almeida Paz, F.A.; Rocha, J.; Simões, M.M.Q. Acetalization of glycerol with acetone over UAV-59 catalyst: Mild reaction conditions and enhanced selectivity. Catalysis Today 2023, 424. [Google Scholar] [CrossRef]
- Bakuru, V.R.; Churipard, S.R.; Maradur, S.P.; Kalidindi, S.B. Exploring the Bronsted acidity of UiO-66 (Zr, Ce, Hf) metal-organic frameworks for efficient solketal synthesis from glycerol acetalization. Dalton Trans 2019, 48, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Castanheiro, J.E.; Vital, J.; Fonseca, I.M.; Ramos, A.M. Glycerol conversion into biofuel additives by acetalization with pentanal over heteropolyacids immobilized on zeolites. Catalysis Today 2020, 346, 76–80. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: principles and practice. Chem Soc Rev 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikov, I.V. Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions. Chem Rev 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Drago, R.S.; Dias, J.A.; O. Maier, T. An Acidity Scale for Brönsted Acids Including H3PW12O40. J. AM. CHEM. SOC 1997, 119, 7702–7710. [Google Scholar] [CrossRef]
- Wang, S.S.; Yang, G.Y. Recent advances in polyoxometalate-catalyzed reactions. Chem Rev 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhao, X.; Li, D.; Chen, M.; Wei, X.; Fang, J.; Cui, K.; Ma, Y.; Hou, Z. Synthesis of bio-additive fuels from glycerol acetalization over a heterogeneous Ta/W mixed addenda heteropolyacid catalyst. Fuel Processing Technology 2021, 214, 106705–106717. [Google Scholar] [CrossRef]
- Hong, G.H.; Li, Z.; Park, J.S.; Li, Z.; Kim, K.Y.; Li, C.; Lee, J.; Jin, M.; Stucky, G.D.; Kim, J.M. Glycerol acetalization over highly ordered mesoporous molybdenum dioxide: Excellent catalytic performance, recyclability and water-tolerance. Journal of Industrial and Engineering Chemistry 2022, 107, 354–364. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.M. Eco-friendly synthesis of bio-additive fuels from renewable glycerol using nanocrystalline SnO2-based solid acids. Catalysis Science & Technology 2014, 4. [Google Scholar] [CrossRef]
- Huang, H.; Mu, J.; Liang, M.; Qi, R.; Wu, M.; Xu, L.; Xu, H.; Zhao, J.; Zhou, J.; Miao, Z. One-pot synthesis of MoO3-ZrO2 solid acid catalyst for solvent-free solketal production from glycerol. Molecular Catalysis 2024, 552. [Google Scholar] [CrossRef]
- Vicente, G.; Melero, J.A.; Morales, G.; Paniagua, M.; Martín, E. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chemistry 2010, 12. [Google Scholar] [CrossRef]
- Zhou, R.; Jiang, Y.; Zhao, H.; Ye, B.; Wang, L.; Hou, Z. Synthesis of solketal from glycerol over modified SiO2 supported p-phenolsulfonic acid catalyst. Fuel 2021, 291, 120207–120216. [Google Scholar] [CrossRef]
- Matkala, B.; Boggala, S.; Basavaraju, S.; Sarma Akella, V.S.; Aytam, H.P. Influence of sulphonation on Al-MCM-41 catalyst for effective bio-glycerol conversion to Solketal. Microporous and Mesoporous Materials 2024, 363. [Google Scholar] [CrossRef]
- Hu, Z.; Nalaparaju, A.; Peng, Y.; Jiang, J.; Zhao, D. Modulated Hydrothermal Synthesis of UiO-66(Hf)-Type Metal-Organic Frameworks for Optimal Carbon Dioxide Separation. Inorg Chem 2016, 55, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.Q.; Siddiqui, Z.N. Heteropoly Ionic Liquid Functionalized MOF-Fe: Synthesis, Characterization, and Catalytic Application in Selective Acetalization of Glycerol to Solketal as a Fuel Additive at Room Temperature, Solvent-Free Conditions. Precision Chemistry 2023, 1, 485–496. [Google Scholar] [CrossRef]
- Dashtipour, B.; Dehghanpour, S.; Sharbatdaran, M. Improvement of the acidic properties of MOF by doped SnO2 quantum dots for the production of solketal. Journal of Chemical Sciences 2022, 134. [Google Scholar] [CrossRef]
- Saini, B.; Tathod, A.P.; Saxena, S.K.; Arumugam, S.; Viswanadham, N. Sustainable Upgrade of Bioderived Glycerol to Solketal through Acetalization over Metal-Free Mordenite Catalysts. ACS Sustainable Chemistry & Engineering 2022, 10, 1172–1181. [Google Scholar] [CrossRef]
- Li, Z.; Miao, Z.; Wang, X.; Zhao, J.; Zhou, J.; Si, W.; Zhuo, S. One-pot synthesis of ZrMo-KIT-6 solid acid catalyst for solvent-free conversion of glycerol to solketal. Fuel 2018, 233, 377–387. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Phung, T.K.; Hossain, M.A.; Chowdhury, E.; Tulaphol, S.; Lalvani, S.B.; O’Toole, M.; Willing, G.A.; Jasinski, J.B.; Crocker, M. , et al. Hydrophobic functionalization of HY zeolites for efficient conversion of glycerol to solketal. Applied Catalysis A: General 2020, 592. [Google Scholar] [CrossRef]
- Santos-Vieira, I.C.M.S.; Mendes, R.F.; Almeida Paz, F.A.; Rocha, J.; Simões, M.M.Q. Solketal Production via Solvent-Free Acetalization of Glycerol over Triphosphonic-Lanthanide Coordination Polymers. Catalysts 2021, 11, 598–612. [Google Scholar] [CrossRef]
- Lopes, N.F.; Caiado, M.; Canhão, P.; Castanheiro, J.E. Synthesis of Bio-fuel Additives From Glycerol Over Poly(Vinyl Alcohol) With Sulfonic Acid Groups. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 2015, 37, 1928–1936. [Google Scholar] [CrossRef]
- Nandan, D.; Sreenivasulu, P.; Sivakumar Konathala, L.N.; Kumar, M.; Viswanadham, N. Acid functionalized carbon–silica composite and its application for solketal production. Microporous and Mesoporous Materials 2013, 179, 182–190. [Google Scholar] [CrossRef]
- Domínguez-Barroso, V.; Herrera, C.; Larrubia, M.Á.; González-Gil, R.; Cortés-Reyes, M.; Alemany, L.J. Continuous-Flow Process for Glycerol Conversion to Solketal Using a Brönsted Acid Functionalized Carbon-Based Catalyst. Catalysts 2019, 9. [Google Scholar] [CrossRef]
| Catalyst | Ratio of Glycerol/acetone |
Temperature (°C) | Time (h) |
Conversion (%) |
Selectivity to solketal (%) | Ref. |
|---|---|---|---|---|---|---|
| H3PW12040 | 1:15 | RT | 0.08 | 99.2 | 97 | [41] |
| H3PMo12040 | 1:15 | RT | 0.08 | 91.4 | 94 | [41] |
| H4SiW12O40 | 1:15 | RT | 0.08 | 90.7 | 85.7 | [41] |
| Sn2SiW12O40 | 1:16 | RT | 1 | 99 | 97 | [51] |
| Cs2.5H0.5PW12O40 | 1:6 | RT | 1 | 94 | 98 | [53] |
| Cs2.5H0.5PW12O40@KIT-6 | 1:6 | RT | 0.25 | 95 | 98 | [53] |
| meso-MoO2 | 1:10 | RT | 1 | 95.8 | 97.8 | [63] |
| meso-WO3 | 1:10 | RT | 1 | 34.7 | 71.2 | [63] |
| meso-SnO2 | 1:10 | RT | 1 | 28.9 | 68.9 | [63] |
| SnO2 | 1:1 | RT | 1.5 | 15 | 96 | [42] |
| WO3/SnO2 | 1:1 | RT | 1.5 | 55 | 90 | [42] |
| MoO3/SnO2 | 1:1 | RT | 1.5 | 61 | 96 | [42] |
| SO42-/SnO2 | 1:1.5 | RT | 4 | 98 | 96 | [64] |
| MoO3-ZrO2 | 1:8 | 50 | 0.2 | 89 | 97 | [65] |
| [HMIm]3[PW12O40] | 1:2 | RT | 1 | 85 | 87.06 | [70] |
| [HMIm]3[PMo12O40] | 1:2 | RT | 1 | 80 | 82.5 | [70] |
| [HMIm]4[SiW12O40] | 1:2 | RT | 1 | 76 | 78.94 | [70] |
| Catalyst | Ratio of Glycerol/acetone |
Temperature (°C) | Time (h) |
Conversion (%) |
Selectivity to solketal (%) | Ref. |
|---|---|---|---|---|---|---|
| MoPo@SBA-15 | 1:3 | RT | 1 | 100 | 98 | [50] |
| Nb-SBA-15 | 1:3 | RT | 1 | 95 | 100 | [52] |
| Zr-SBA-15 | 1:3 | RT | 1 | 92 | 98 | [52] |
| Ti-SBA-15 | 1:3 | RT | 1 | 65 | 98 | [52] |
| Al-SBA-15 | 1:3 | RT | 1 | 60 | 98 | [52] |
| Ar-SBA-15 | 1:6 | 70 | 0.5 | 82.5 | wi | [66] |
| Pr-SBA-15 | 1:6 | 70 | 0.5 | 79.0 | wi | [66] |
| PSF | 1:10 | RT | 1.5 | 75 | 98 | [67] |
| PSF/SiO2 | 1:10 | RT | 1.5 | 86.6 | 98 | [67] |
| PSF/K-SiO2 | 1:10 | RT | 1.5 | 86.3 | 98 | [67] |
| MoO3/SiO2 | 1:2 | RT | 1 | 46.8 | 90 | [54] |
| SO4-Al-MCM-41 | 1:10 | RT | 2 | 94.8 | 99 | [68] |
| Catalyst | Ratio of Glycerol/acetone |
Temperature (°C) | Time (h) |
Conversion (%) |
Selectivity to solketal (%) | Ref. |
|---|---|---|---|---|---|---|
| UiO-66 (Hf) | 1:4 | RT | 1 | 94.5 | 97.2 | [56] |
| UiO-66 (Ce) | 1:4 | RT | 1 | 70.9 | 90.1 | [56] |
| UiO-66 (Zr) | 1:4 | RT | 1 | 1.5 | 73.2 | [56] |
| UiO-SO3H-0.5 | 1:10 | 60 | 1 | 60.2 | 99.7 | [43] |
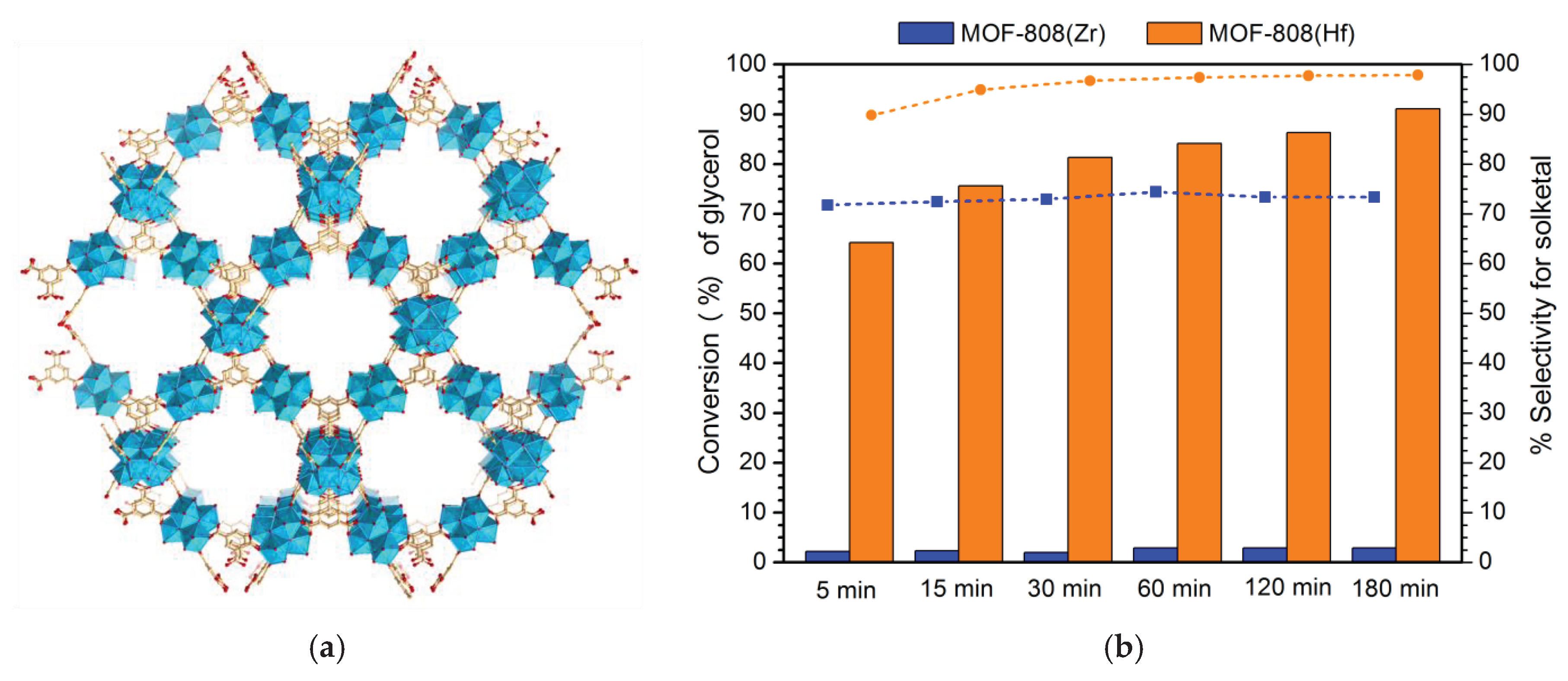
| MOF-808 (Hf) | 1:6 | 60 | 3 | 91 | 98 | [35] |
| MOF-808 (Zr) | 1:6 | 60 | 3 | 6 | 100 | [35] |
| MOF-Fe | 1:2 | RT | 1 | 72 | 72.22 | [70] |
| MIL-118 (Al) | 1:10 | wi | 4 | 43 | 58 | [71] |
| MIL-118-SnO2 | 1:10 | wi | 4 | 76 | 97 | [71] |
| UAV-59 | 1:10 | 55 | 2 | 94 | 97 | [55] |
| UAV-63 | 1:10 | 55 | 6 | 84 | 96 | [75] |
| UAV-20 | 1:10 | 55 | 6 | 56 | 90 | [75] |
| [HMIm]3[PW12O40]@MOF-Fe | 1:2 | RT | 1 | 100 | 100 | [70] |
| [HMIm]3[PMo12O40]@MOF-Fe | 1:2 | RT | 1 | 95 | 96.84 | [70] |
| [HMIm]4[SiW12O40]@MOF-Fe | 1:2 | RT | 1 | 90 | 93.33 | [70] |
| PVA40 | 1:6 | 70 | 3 | 94 | wi | [76] |
| SCS1/2 | 1:6 | 70 | 0.5 | 75 | 90 | [77] |
| HSCS1/2 | 1:6 | 70 | 0.5 | 82 | 99 | [77] |
| SO3H-C | 1:8 | 57 | 1 | 80 | wi | [78] |
| Catalyst | Ratio of Glycerol/acetone |
Temperature (°C) | Time (h) |
Conversion (%) |
Selectivity to solketal (%) | Ref. |
|---|---|---|---|---|---|---|
| ZrMo-KIT-6 | 1:8 | 50 | 4 | 85.8 | 97.8 | [73] |
| Zeolite HY | 1:2 | RT | 1 | 74.2 | 98.2 | [54] |
| Zeolite OTS-HY | 1:12 | 30 | 1 | 89 | 95 | [74] |
| Zeolite H-Beta-1 | 1:2 | RT | 1 | 86 | 98.5 | [54] |
| Zeolite HBEA | 1:10 | RT | 1.5 | 70.9 | 97.5 | [67] |
| Zeolite Mordenite | 1:10 | 60 | 4 | 99 | 99 | [72] |
| Amberlyst-15 | 1:2 | RT | 1 | 73.1 | 91 | [54] |
| Amberlyst-45 | 1:10 | RT | 1.5 | 80.6 | 97.4 | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





