Submitted:
19 March 2024
Posted:
21 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Comparative Study of Different Fungi in the Production of L-Asparaginase
2.2.1. Solid-State Fermentation (SsF) of L-Asparaginase
2.2.2. Submerged Fermentation (SmF) of L-Asparaginase
2.3. Fungal Culture and Isolation
2.3.1. DNA Extraction
2.3.2. Primers and Polymerase Chain Reaction (PCR)
2.4. Purification of Crude L-Asparaginase Extracts
2.5. Protein Concentration
2.6. Determination of L-Asparaginase Activity
2.7. Biochemical Characterization of Purified L-Asparaginase from Fungus Extract
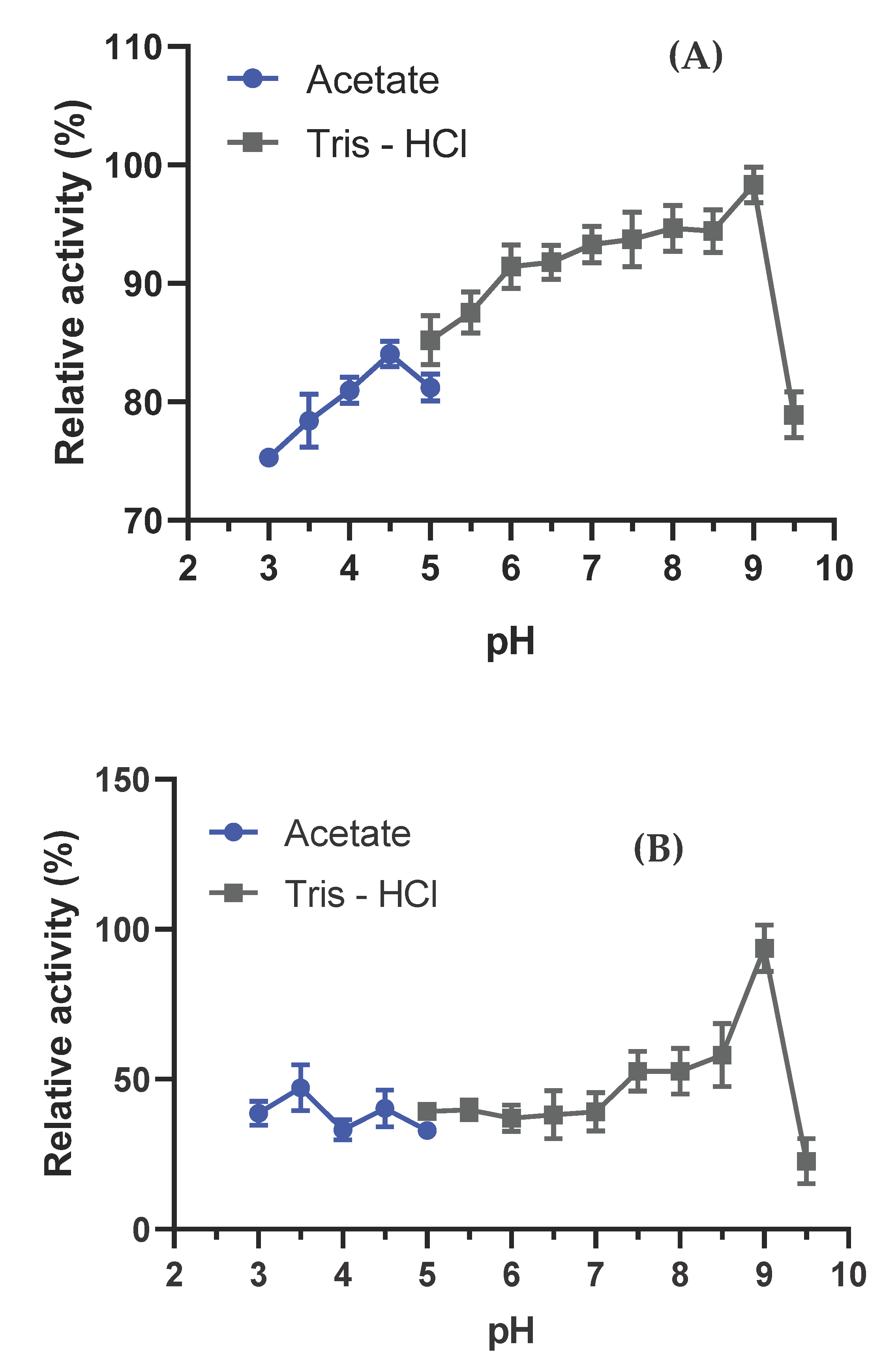
2.7.1. Fungo Optimal pH and Stability
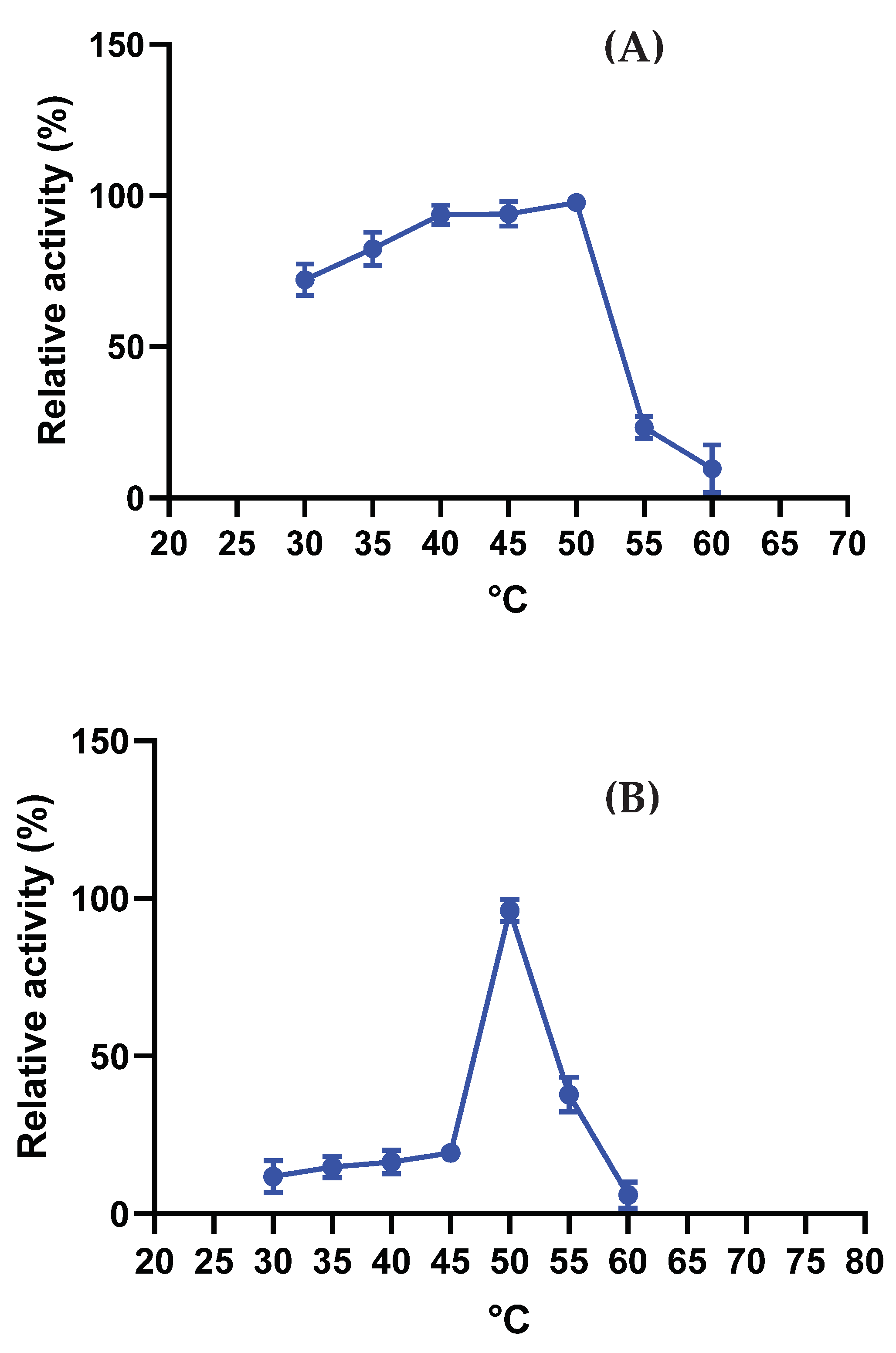
2.7.2. Optimal Temperature and Stability
2.7.3. Effect of Surfactants and Metal Ions in Salts
2.7.4. Stability in Organic Solvents
2.8. Cytotoxic Activity of L-Asparaginase
2.9. Morphological Viability in HeLa Cells by Inverted Light Microscopy
2.10. Statistical Analysis
3. Results
3.1. Tracking and Optimization of L-Asparaginase production by Fungi
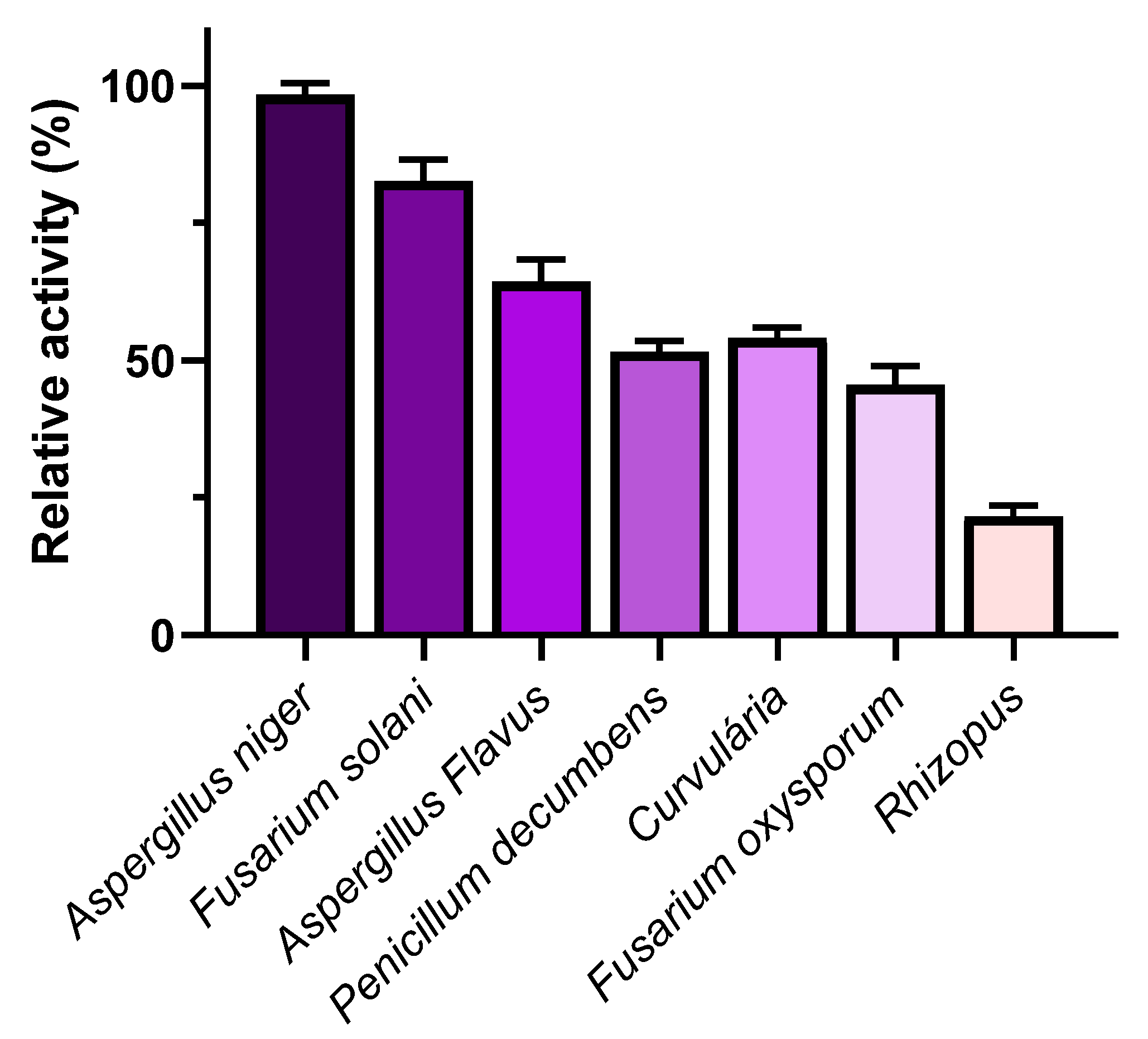
3.1.1. Screening for L-Asparaginase Producers
3.1.2. Fermentation Type for L-Asparaginase Production
3.2. Molecular Identification of A. niger
3.3. Precipitant Type for L-Asparaginase Purification
3.4. Biochemical Characterization of L-Asparaginase from A.niger
3.4.1. Optimal pH and Stability
3.4.2. Optimal Temperature and Stability
3.4.3. Effect of Surfactants and Metal Ions in Salts
3.4.4. Stability in Organic Solvents
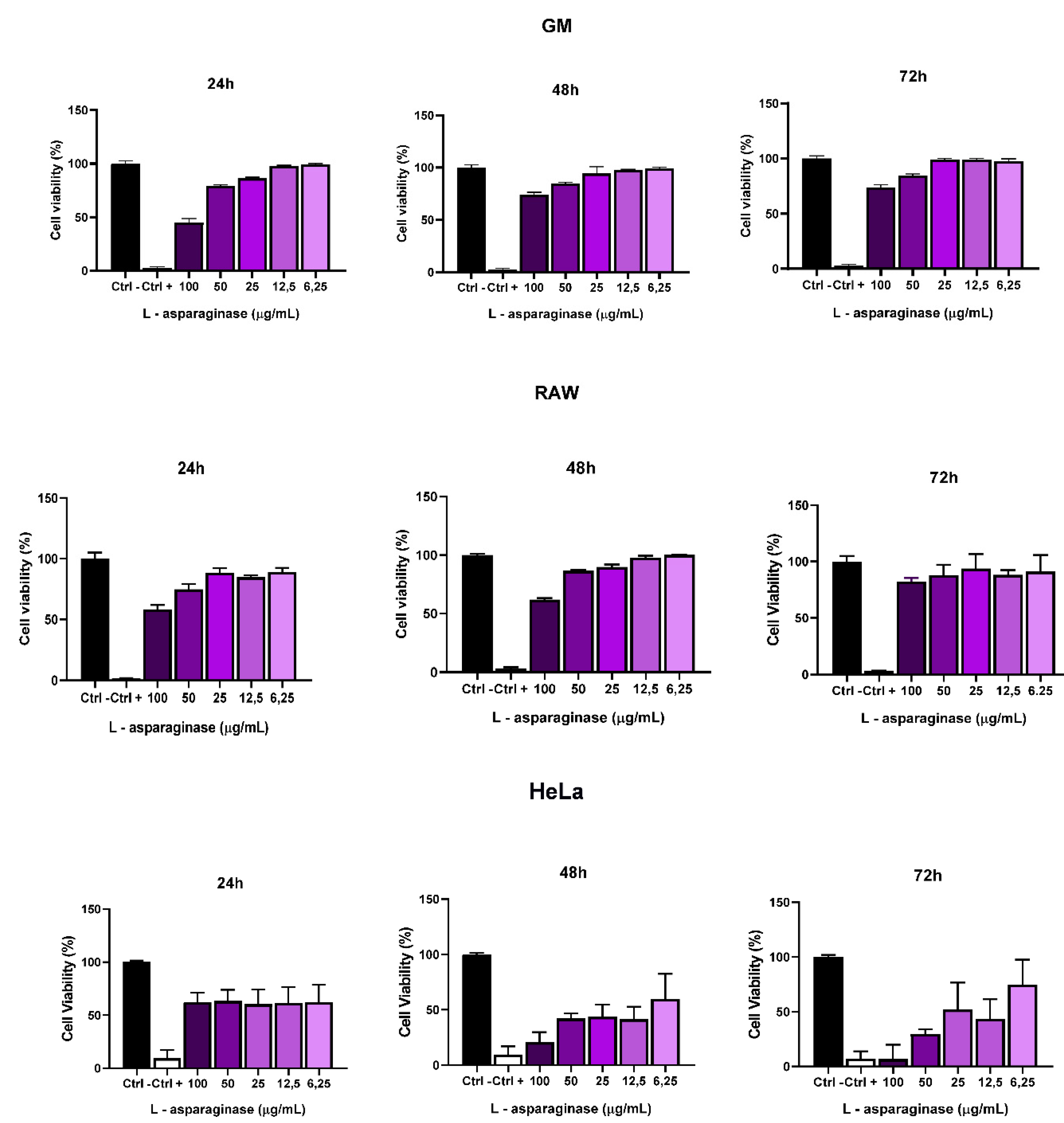
3.5. Cytotoxic Activity of L-Asparaginase in GM, RAW and HeLa Cells
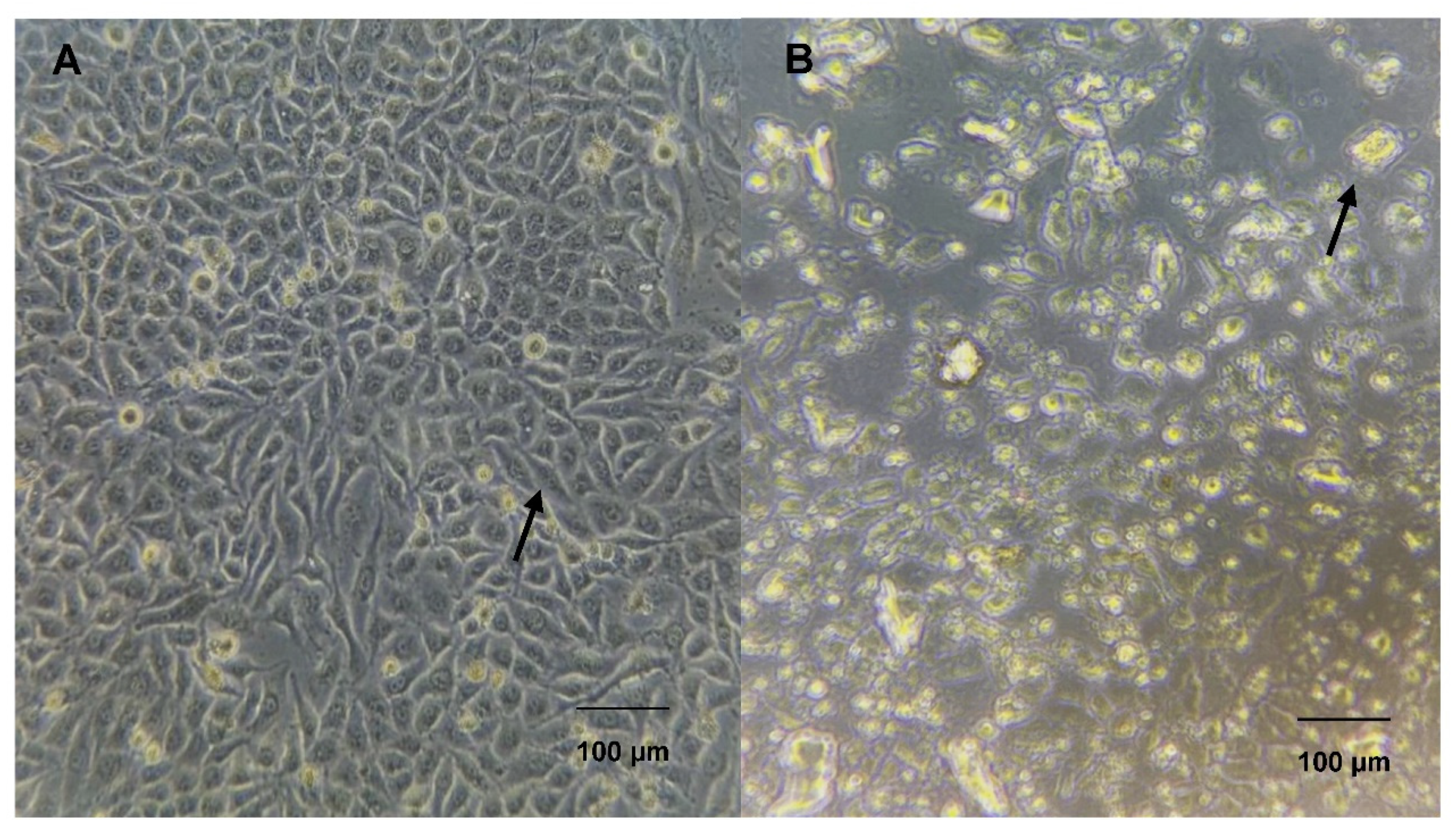
3.6. Morphological Viability of the HeLa Cell Line under L-Asparaginase Treatment
4. Discussion
5. Conclusion
Acknowledgments
References
- Okunade, K.S. (2020). Human papillomavirus and cervical cancer. Journal of Obstetrics and Gynaecology, 40(5), 602-608. [CrossRef]
- Oliveira, M A A, Mascarenhas, G M S, de Sousa Lima, A A, Carneiro, V M S, Silva, C D C M, Silva, M V C M (2022). Correlation of genetic factors of hpv 16/18 virus and cervical cancer. Advanced Studies on Health and Nature, 3. [CrossRef]
- El-Salem, F, Mansour, M, Gitman, M, Miles, B A, Posner, M R, Bakst,. L, Genden E M, Westra, W H. Real-time PCR HPV genotyping in fine needle aspirations of metastatic head and neck squamous cell carcinoma: Exposing the limitations of conventional p16 immunostaining. Oral Oncology, 2019; 90, 74–79.
- Pedreschi F, Mariotti S, Granby K, Risum, J. (2011). Acrylamide reduction in potato chips using commercial asparaginase in combination with conventional bleaching. LWT - Food Sci Technol 44(6):1473–1476. [CrossRef]
- Shakambari, G, Birendranarayan, A K, Lincy, M J A, Rai, S K, Ahamed, Q T, Ashokkumar, B, Varalakshmi, P (2016). Hemocompatible glutaminase free L-asparaginase from marine Bacillus tequilensis PV9W with anticancer potential modulating p53 expression. RSC advances, 6(31), 25943-25951. [CrossRef]
- Nunes, J.C.; Cristóvão, R.O. Santos-Ebinuma, V.C. Faria, J.L. Silva, C.G. Neves, M.C. Freire, M.G. Tavares, A.P. (2021). L-Asparaginase-based biosensors. Encyclopedia 2021, 1, 848–858. [Google Scholar] [CrossRef]
- Alam, S. Pranaw, K. Tiwari, R. Khare, S.K. (2019). Recent development in the uses of asparaginase as food enzyme. Green Bio-processes: Enzymes in Industrial Food Processing 2019, 55–81. [Google Scholar]
- Hijiya, N.Van Der Sluis, I.M. (2016). Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leukemia & lymphoma, 57(4), 748-757. [CrossRef]
- De Melo, D.W. Fernandez-Lafuente, R. Rodrigues, R.C. (2023). Enhancing biotechnological applications of l-asparaginase: Immobilization on amino-epoxy-agarose for improved catalytic efficiency and stability. Biocatalysis and Agricultural Biotechnology, 52, 102821. [CrossRef]
- Feenstra, L. R. Gehring, R van Geijlswijk, I. M., König, T. Prinsen, H. C. M. T. Vandemeulebroecke, et al. (2022). Evaluation of PEG-L-asparaginase in asparagine suppression and anti-drug antibody development in healthy Beagle dogs: A multi-phase preclinical study. The Veterinary Journal, 286, 105854. [CrossRef]
- Fonseca, M.H.G. da Silva Fiúza, T. de Morais, S.B. Trevizani, R. (2021). Circumventing the side effects of L-asparaginase. Biomedicine & Pharmacotherapy, 139 111616. [CrossRef]
- Khokhar, I. Haider, M.S. Mukhtar, I. Mushtaq, S. (2012). Biological control of Aspergillus niger, the cause of Black-rot disease of Allium cepa L.(onion), by Penicillium species. Journal of Agrobiology 2012, 29, 23. [Google Scholar] [CrossRef]
- Kim, T. Mullaney, E.J. Porres, J. M, Roneker, K.R. Crowe, S., Rice, S. Lei, X.G. (2006). Shifting the pH profile of Aspergillus niger PhyA phytase to match the stomach pH enhances its effectiveness as an animal feed additive. Applied and environmental microbiology 2006, 72, 4397–4403. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G. Arif, R. Bukhari, S.A. Ali, M., Sharif, S. Atta, A. (2018). Structural and functional annotation of citrate synthase from Aspergillus niger ANJ-120. Pakistan journal of pharmaceutical sciences, 31.
- Qin, S. Zhou, M. Wang, Z. Li, P. Huang, S. Meng, J. (2023). Effect of pulsed electric field on spore germination rate and enzyme activity of Aspergillus niger. Innovative Food Science & Emerging Technologies, 89, 103473. [CrossRef]
- Cairns, T.C. Nai, C. Meyer, V. (2018). How a fungus shapes biotechnology: 100 years of Aspergillus niger research. Fungal biology and biotechnology, 5, 1-14. [CrossRef]
- Bellaouchi, R. Abouloifa, H. Rokni, Y. Hasnaoui, A. Ghabbour, N. Hakkou, A. Asehraou, A. (2021). Characterization and optimization of extracellular enzymes production by Aspergillus niger strains isolated from date by-products. Journal of Genetic Engineering and Biotechnology, 19(1), 1-8. [CrossRef]
- Valenzuela-Lopez, N. Sutton, D.A. Cano-Lira, J.F. Paredes, K., Wiederhold, N. Guarro, J. Stchigel, A.M. (2017). Coelomycetous fungi in the clinical setting: morphological convergence and cryptic diversity. Journal of clinical microbiology, 55 (2), 552-567. [CrossRef]
- White, T.J. Bruns, T. Lee, S.J.W.T. Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications 1990, 18, 315–322. [Google Scholar]
- labdalall, A H, ALanazi, N A, Aldakeel, S A, AbdulAzeez, S, Borgio, J F. 2020. Molecular, physiological, and biochemical characterization of extracellular lipase production by Aspergillus niger using submerged fermentation. PeerJ, 8, e9425. [CrossRef]
- Dias, F.F.G. Ruiz, A.L.T.G. Della Torre, A. Sato, H.H. (2016). Purification, characterization and antiproliferative activity of L-asparaginase from Aspergillus oryzae CCT 3940 with no glutaminase activity. Asian Pacific Journal of Tropical Biomedicine, 6(9), 785-794. [CrossRef]
- Mahajan, R.V. Saran, S. Saxena, R.K. Srivastava, A.K. (2013). A rapid, efficient and sensitive plate assay for detection and screening of l-asparaginase-producing microorganisms. FEMS microbiology letters, 341(2), 122-126. [CrossRef]
- Vala, A.K. Sachaniya, B. Dudhagara, D. Panseriya, H.Z. Gosai, H., Rawal, R., & Dave, B.P. (2018). Characterization of L-asparaginase from marine-derived Aspergillus niger AKV-MKBU, its antiproliferative activity and bench scale production using industrial waste. International journal of biological macromolecules, 108, 41-46. [CrossRef]
- Warburg, O and Christian, W. Isolation and Crystallization of Enolase. Biochemische Zeitschrift 1942, 310, 384–421.
- Drainas, C. Kinghorn, J.R. Pateman, J.A. Aspartic hydroxamate resistance and asparaginase regulation in the fungus Aspergillus nidulans. Microbiology 1977, 98, 493–501. [Google Scholar]
- Oliveira, B.H. Coradi, G.V. de Oliva-Neto, P. do Nascimento, V.M.G. (2020). Biocatalytic benefits of immobilized Fusarium sp. (GFC) lipase from solid state fermentation on free lipase from submerged fermentation. Industrial Crops and Products, 147, 112235. [CrossRef]
- El-Gendy, M.M.A.A. Yahya, S.M., Hamed, A.R. Soltan, M.M. El-Bondkly, A.M.A. (2018). Phylogenetic analysis and biological evaluation of marine endophytic fungi derived from Red Sea sponge Hyrtios erectus. Applied biochemistry and biotechnology 185, 755–777. [CrossRef]
- Babu, U.K., Ramagopal, N. Reddy, D.R. (2010). Optimization of L-Asparaginase production from Isolated Aspergillus niger by using Solid State Fermentation on sesame cake via application of Genetic Algorithm, and Artificial Neural Network-based design model. Journal of Biotechnology, (150), 538-539. [CrossRef]
- Karanam, S.K. Medicherla, N.R. Application of Doehlert experimental design for the optimization of medium constituents for the production of L-asparaginase from Palm Kernal cake (Elaeis guineensis). J Microbial Biochem Technol 2010, 2, 1–7. [Google Scholar] [CrossRef]
- Elshafei, A.M. El-Ghonemy, D.H. (2015). Screening and media optimization for enhancing L-asparaginase production, an anticancer agent, from different filamentous fungi in solid state fermentation. British Biotechnology Journal, 9(3), 1-15. [CrossRef]
- Cardoso, S.L. de Freitas, M.M. de Souza, P.M. Homem-de-Mello, M. Silveira, D Fonseca-Bazzo, Y. M Magalhães, P.O. (2020). Optimization of aqueous two-phase micellar system for partial purification of L-asparaginase from Penicillium sp. grown in wheat bran as agro-industrial residue. Brazilian Journal of Microbiology, 51, 979-988. [CrossRef]
- Castro, A.M.D. Pereira Jr, N. (2010). Production, properties and application of cellulases in the hydrolysis of agro-industrial waste. Química Nova, 33, 181-188. [CrossRef]
- Tormo, M. A Gil-Exojo, I. de Tejada, A.R. Campillo, J.E. (2006). White bean amylase inhibitor administered orally reduces glycaemia in type 2 diabetic rats. British journal of nutrition, 96(3), 539-544. [CrossRef]
- Pereira, L.L.S. Santos, C.D.D. Pereira, C.A., Marques, T.R. Sátiro, L.C. Precipitation of α-amylase inhibitor from white beans: evaluation of methods. Food and Nutrition Araraquara 2010, 21, 15–20. [Google Scholar]
- El-Naggar, N.E.A. Deraz, S.F. Soliman, H.M. El-Deeb, N.M. El-Ewasy, S.M. (2016). Purification, characterization, cytotoxicity and anticancer activities of L-asparaginase, anti-colon cancer protein, from the newly isolated alkaliphilic Streptomyces fradiae NEAE-82. Scientific reports, 6(1), 32926. [CrossRef]
- Dunn, J.B., Mueller, S., Wang, M. Han, J. (2012). Energy consumption and greenhouse gas emissions from enzyme and yeast manufacture for corn and cellulosic ethanol production. Biotechnology letters, 34, 2259-2263. [CrossRef]
- Pourkhanali, K. Khayati, G. Mizani, F. Raouf, F. (2022). Characterization of free and immobilized lipase from Penicillium sp. onto three modified bentonites: A comparative study. Journal of Biotechnology, 344, 57-69. [CrossRef]
- El-Bessoumy, A.A. Sarhan, M. Mansour, J. Production, isolation, and purification of L-asparaginase from Pseudomonas aeruginosa 50071 using solid-state fermentation. BMB Reports 2004, 37, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R. Bera, D. (2022). Biochemical characterization and thermodynamic principles of purified L-asparaginase from novel Brevibacillus borstelensis ML12. Biocatalysis and Agricultural Biotechnology, 39, 102260. [CrossRef]
- Singh, Y. Gundampati, R.K. Jagannadham, M.V. Srivastava, S.K. (2013). Extracellular L-asparaginase from a protease-deficient Bacillus aryabhattai ITBHU02: purification, biochemical characterization, and evaluation of antineoplastic activity in vitro. Applied biochemistry and biotechnology, 171, 1759-1774. [CrossRef]
- Moreno-Enríquez, A. Evangelista-Martínez, Z. González-Mondragón, E.G. Calderón-Flores, A. Arreguín, R. Pérez-Rueda, E. Huerta-Saquero, A. (2012). Biochemical characterization of recombinant L-asparaginase (AnsA) from Rhizobium etli, a member of an increasing rhizobial-type family of L-asparaginases. [CrossRef]
- Krishnapura, P.R. Belur, P.D. (2016). Partial purification and characterization of L-asparaginase from an endophytic Talaromyces pinophilus isolated from the rhizomes of Curcuma amada. Journal of Molecular Catalysis B: Enzymatic, 124, 83-91. [CrossRef]
- Meghavarnam, A.K. Janakiraman, S. (2018). Evaluation of acrylamide reduction potential of l-asparaginase from Fusarium culmorum (ASP-87) in starchy products. Lwt, 89, 32-37. [CrossRef]
- Asthana, N, Azmi, W (2003). Microbial L-asparaginase: A potent antitumour enzyme.
- Rani, S A, Sundaram, L, Vasantha, P B. (). A study on in vitro antioxidant and anticancer activity of l-asparaginase. J Pharm Res 2012, 5, 1463–1466.
- Sudarkodi, C.; Sundar, S. Anticancer activity of L-asparaginase from Aspergillus oryzae against HEP-G2 and Hela cell lines. Int J Recent Sci Res 2018, 9, 25328–25330. [Google Scholar]
- Fatima, N, Khan, M M, Khan, I A. (2019). L-asparaginase produced from soil isolates of Pseudomonas aeruginosa shows potent anti-cancer activity on HeLa cells. Saudi Journal of Biological Sciences, 26(6), 1146-1153. [CrossRef]
| Type of Fermentation | Purification Stage | Total Activity (U/mL) | Total Protein (mg) | Specific Activity (U/mg) | Yield (%) | purification |
| SmF | Crude Extract | 3711,50 | 480,00 | 7,73 | 100 | 1 |
| Fraction 1 (0 - 40%) | 363,96 | 18,95 | 19,21 | 9,81 | 2,48 | |
| Fraction 2 (40 - 80%) | 767,20 | 44,93 | 17,07 | 20,67 | 2,21 | |
| SsF | Crude Extract | 5261 | 480 | 10,96 | 100,00 | 1,00 |
| Fraction 1 (0 - 40%) | 888,58 | 84,63 | 10,50 | 16,89 | 0,96 | |
| Fraction 2 (40 - 80%) | 2748,9 | 14,685 | 187,19 | 52,25 | 17,08 |
| Type of precipitation | Purification Stage | Total Activity (U/mL) | Total Protein (mg) | Specific Activity (U/mg) | Yield | purification (%) |
| Crude Extract | 5520,50 | 3367,5 | 1,64 | 100,00 | 1,00 | |
| Ethanol | Fraction 1 (0 - 40%) | 1205,68 | 84,63 | 14,25 | 21,84 | 8,69 |
| Fraction 2 (40 - 80%) | 2671,90 | 114,18 | 23,40 | 48,40 | 14,27 | |
| DEAE Cellulose Column | 1092,30 | 31,8 | 34,35 | 19,79 | 20,95 | |
| Ammonium Sulfate | Fraction 1 (0 - 40%) | 182,16 | 57,09 | 3,19 | 3,30 | 1,95 |
| Fraction 2 (40 - 80%) | 826,20 | 152,01 | 5,44 | 14,97 | 3,32 | |
| DEAE Cellulose Column | 265,00 | 21,5 | 12,33 | 4,80 | 7,52 | |
| Isopropanol | Fraction 1 (0 - 40%) | 113,40 | 64,35 | 1,76 | 2,05 | 1,07 |
| Fraction 2 (40 - 80%) | 306,60 | 143,955 | 2,13 | 5,55 | 1,30 | |
| DEAE Cellulose Column | 104,10 | 16,3 | 6,39 | 1,89 | 3,90 |
| Type | Concentrations | U/mL | Relative Activity (%) ± SD |
| Control | 47,48 | 100 ± 0,014 | |
| Tween 20 | |||
| 0,01% | 44,98 | 94,73 ± 0,035 | |
| 0,10% | 55,27 | 116,41± 0,084 | |
| 0,50% | 51,35 | 108,15 ± 0,028 | |
| Triton X – 100 | |||
| 0,01% | 45,91 | 96,69 ± 0,013 | |
| 0,10% | 36,56 | 77,00 ± 0,018 | |
| 0,50% | 33,92 | 71,44 ± 0,020 | |
| Tween 80 | |||
| 0,01% | 41,61 | 87,64 ± 0,011 | |
| 0,10% | 42,45 | 89,41± 0,006 | |
| 0,50% | 38,01 | 80,05 ± 0,027 | |
| SDS | |||
| 0,01% | 35,02 | 73,76 ± 0,012 | |
| 0,10% | 24,13 | 50,82 ± 0,009 | |
| 0,50% | 24,43 | 51,45 ± 0,025 | |
| E.D.T. A | |||
| 0,01% | 31,1 | 65,50 ± 0,005 | |
| 0,10% | 22,12 | 46,59 ± 0,004 | |
| 0,50% | 19,13 | 40,29 ± 0,003 | |
| Type | U/mL | Relative Activity (%) ± SD |
| Control | 31,31 | 100 ± 0,04 |
| Mg+2 | 46,4 | 148,20 ± 0,02 |
| Co2+ | 16,89 | 153,94 ± 0,01 |
| SO42- | 15,05 | 48,07 ± 0,03 |
| Zn+2 | 16,21 | 51,77 ± 0,01 |
| Ca2+ | 46,13 | 47,33 ± 0,01 |
| Fe3+ | 27,63 | 88,25 ± 0,01 |
| Na+ | 28,47 | 90,93 ± 0,02 |
| Type | Concentrations | U/mL | Relative Activity (%) ± SD |
| Control | 32,48 | 100 ± 0,014 | |
| Acetone | |||
| 25 | 16,79 | 51,72 ± 0,05 | |
| 50 | 14,53 | 44,72 ± 0,02 | |
| 80 | 5,76 | 17,73 ± 0,03 | |
| 100 | 2,49 | 7,67 ± 0,04 | |
| Ethanol | |||
| 25 | 24,15 | 74,38 ± 0,04 | |
| 50 | 15,02 | 46,18 ± 0,01 | |
| 80 | 9,54 | 29,34 ± 0,02 | |
| 100 | 6,28 | 19,33 ± 0,02 | |
| Isopropanol | |||
| 25 | 13,45 | 41,37 ± 0,03 | |
| 50 | 10,26 | 31,59 ± 0,01 | |
| 80 | 6,75 | 20,78 ± 0,03 | |
| 100 | 4,14 | 12,75 ± 0,01 | |
| Methanol | |||
| 25 | 8,55 | 26,32 ± 0,09 | |
| 50 | 7,82 | 24,05 ± 0,03 | |
| 80 | 5,19 | 16,00 ± 0,02 | |
| 100 | 2,34 | 7,20 ± 0,07 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





