Submitted:
20 March 2024
Posted:
20 March 2024
You are already at the latest version
Abstract
Keywords:
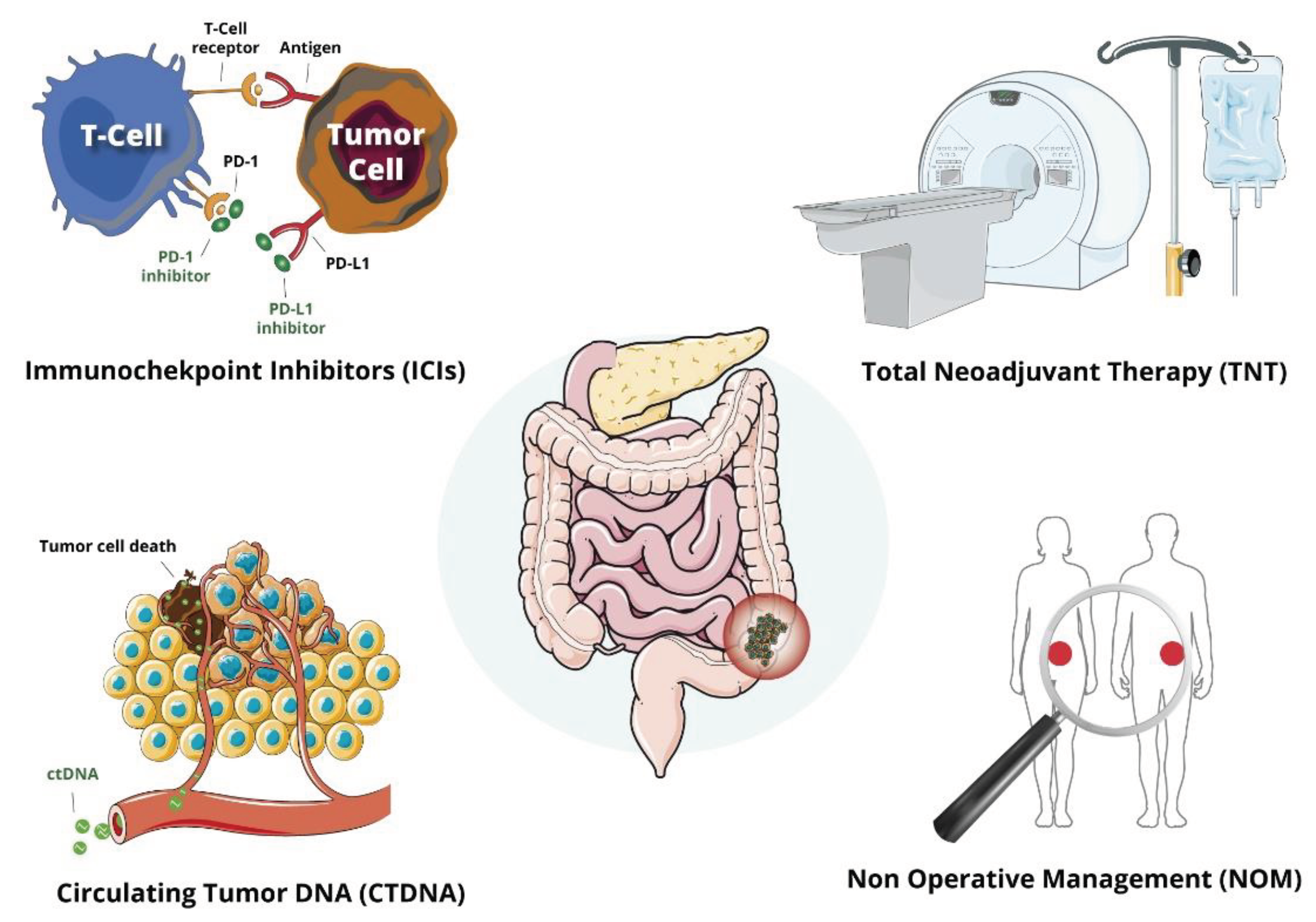
1. Introduction
2. Total Neoadjuvant Treatment (TNT)
2.1. Results of Phase III Trials
2.2. Omission of Radiotherapy
2.3. Induction vs Consolidation Chemotherapy
3. Immunocheckpoint Inhibitors (ICIs)
3.1. Mismatch Repair-Deficient (dMMR) or Microsatellite Instability-High (MSI-H) LARC
3.2. Mismatch Repair-Proficient (pMMR) or Microsatellite Stability (MSS) LARC
5. Non Operative Management (NOM)
| First Autor | Year | Study design | Control group | Number of patients | DFS (WW vs control) |
|||
|---|---|---|---|---|---|---|---|---|
| Control group | WW group | 3-years | 5-years | |||||
| Araujo [71] | 2015 | Retrospective cohort study | TME | 69 | 42 | 60.9% vs 82.8% (p 0.011) | ||
| Martens [73] | 2016 | Prospective cohort study | TEM | 15 | 85 | [LRFS] 85.8% vs 80% (p 0.57) |
||
| Smith [64] | 2019 | Retrospective case series analysis | TME | 136 | 113 | 75% vs 92% (p N.D.) |
||
| Beard [65] | 2020 | Retrospective cohort study | RS | 42 | 53 | [LRFS] 85 vs 92% (p 0.36) | ||
| Wang [70] | 2021 | Multicenter retrospective cohort study | RS | 94 | 94 | [LRFS] 98% vs 98% (p 0.506) |
||
| Najami [74] | 2021 | Observation descriptive cohort study | LE | 22 | 42 | 74.9% vs 66.2% (p N.D.) | ||
| Han [61] | 2022 | Prospective cohort study | TME | 26 | 58 | 81.1% vs 84.6% (p 0.819) | ||
| Wang [55] | 2023 | Retrospective cohort study | RS | 171 | 89 | 93.3% vs 92% (p 0.66) | ||
6. Circulating Tumor DNA (ctDNA) Evaluation in LARC Patients
7. Discussion
| NCT Number | Treatment strategy | Status | Phase |
|---|---|---|---|
| NCT03038256 | Ctr: CTRT→S→ADT Exp: CTRT → CAPOX x4→S Exp1: CTRT → CAPOX x6→S |
Recruiting | Phase II |
| NCT05054959 | Exp1: CTRT → CAPOX x6→S Exp 2: CAPOXx4→ CTRT → CAPOX x2→S |
Recruiting | Phase II |
| NCT05673772 | Exp1: SCRT → FOLFOX x4→S→ADT Ctr: CTRT→S→ADT |
Recruiting | Phase II |
| NCT05610163 | Exp1: CTRT → FOLFOX/CAPOX → S or NOM Exp2: CTRT → FOLFIRINOX → S or NOM Ctr: CTRT→S→ADT |
Recruiting | Phase II |
| NCT04246684 | Exp: CTRT (+ Oxa) → CT (FOLFOX x 6/ CAPOX x 4) Ctr: SCRT → CT (FOLFOX x 9/ CAPOX x 6) If cCR → NOM If not cCR → S |
Recruiting | Phase III |
| NCT04215731 | Exp1: FOLFOXIRI + bevacizumab x 4 → FOLFOXIRI x 2 → immediate S or CTRT→ S according to ycT Exp2: FOLFOX x 4 → CTRT → S |
Recruiting | Phase III |
| NCT05646511 | Exp 1: SCRT → CAPOX x 6 → S or NOM Exp 2: SCRT → CAPOXIRI x 6 → S or NOM |
Recruiting | Phase III |
| MSI-H Tumors | |||
|---|---|---|---|
| NCT Number | Study Title | Study Status | Phases |
| NCT05645094 | Neoadjuvant Envafolimab in Resectable and Locally Advanced MSI-H/dMMR Rectal Cancer | NOT YET RECRUITING | PHASE2 |
| NCT04411524 | The Combination of Immunotherapy and Neoadjuvant Chemoradiotherapy in MSI-H Locally Advanced Rectal Cancer | UNKNOWN STATUS |
PHASE2 |
| NCT04301557 | PD1 Antibody Toripalimab and Chemoradiotherapy for dMMR/MSI-H Locally Advanced Colorectal Cancer | RECRUITING | PHASE2 |
| NCT05723562 | A Study of Dostarlimab in Untreated dMMR/MSI-H Locally Advanced Rectal Cancer (AZUR-1) | RECRUITING | PHASE2 |
| NCT04357587 | Safety and Feasibility of PD-1 Blockade in the Treatment of dMMR or MSI-H Rectal Cancer | RECRUITING | PHASE2 |
| NCT04304209 | Pd1 Antibody Sintilimab ± Chemoradiotherapy for Locally Advanced Rectal Cancer | ACTIVE, NOT RECRUITING | PHASE2 |
| NCT04751370 | Testing Nivolumab and Ipilimumab With Short-Course Radiation in Locally Advanced Rectal Cancer | SUSPENDED | PHASE2 |
| NCT05732389 | Immunotherapy in Patients With Early dMMR Rectal Cancer (RESET-R) | RECRUITING | PHASE2 |
| NCT04636008 | Neoadjuvant treatment of sintilimab plus hypofractionated radiotherapy for MSI-H/dMMR rectal cancer: A prospective, multicenter, phase Ib study | RECRUITING | PHASE2 |
| NCT05645094 | Neoadjuvant Envafolimab in Resectable and Locally Advanced MSI-H/dMMR Rectal Cancer | NOT YET RECRUITING | PHASE2 |
| NCT04411524 | The Combination of Immunotherapy and Neoadjuvant Chemoradiotherapy in MSI-H Locally Advanced Rectal Cancer | UNKNOWN STATUS |
PHASE2 |
| NCT04301557 | PD1 Antibody Toripalimab and Chemoradiotherapy for dMMR/MSI-H Locally Advanced Colorectal Cancer | RECRUITING | PHASE2 |
| NCT05723562 | A Study of Dostarlimab in Untreated dMMR/MSI-H Locally Advanced Rectal Cancer (AZUR-1) | RECRUITING | PHASE2 |
| NCT04357587 | Safety and Feasibility of PD-1 Blockade in the Treatment of dMMR or MSI-H Rectal Cancer | RECRUITING | PHASE2 |
| NCT04304209 | Pd1 Antibody Sintilimab ± Chemoradiotherapy for Locally Advanced Rectal Cancer | ACTIVE, NOT RECRUITING | PHASE2 |
| NCT04751370 | Testing Nivolumab and Ipilimumab With Short-Course Radiation in Locally Advanced Rectal Cancer | SUSPENDED | PHASE2 |
| NCT05732389 | Immunotherapy in Patients With Early dMMR Rectal Cancer (RESET-R) | RECRUITING | PHASE2 |
| NCT04636008 | Neoadjuvant treatment of sintilimab plus hypofractionated radiotherapy for MSI-H/dMMR rectal cancer: A prospective, multicenter, phase Ib study | RECRUITING | PHASE2 |
| MSS TUMORS | |||
| NCT05215379 | Neoadjuvant Chemoradiation Therapy Combined With Immunotherapy for MSS Ultra-low Rectal Cancer | RECRUITING | PHASE2|PHASE3 |
| NCT04109755 | Neo-adjuvant Pembrolizumab and Radiotherapy in Localized MSS Rectal Cancer | RECRUITING | PHASE2 |
| NCT04895137 | mFOLFOX6+Bevacizumab+PD-1 Monoclonal Antibody in Local Advanced MSS CRC | RECRUITING | PHASE2 |
| NCT04833387 | PD-1 Antibody Following Preoperative Chemoradiotherapy for Locally Advanced pMMR/MSS Rectal Cancer | ACTIVE, NOT RECRUITING | PHASE2 |
| NCT05731726 | Serplulimab Combined With CAPOX + Celecoxib as Neoadjuvant Treatment for Locally Advanced Rectal Cancer | RECRUITING | PHASE2 |
| NCT05972655 | Nodes-sparing Short-course Radiation Combined With CAPOX and Tislelizumab for MSS Middle and Low Rectal Cancer | RECRUITING | PHASE2 |
| NCT04940546 | Neoadjuvant Safety of Sintilimab + XELOX + Bevacizumab in pMMR/MSS CRLM Patients | ACTIVE, NOT RECRUITING | PHASE1|PHASE2 |
| NCT05858567 | Total Neoadjuvant Therapy With Short-course Radiation Followed by Envafolimab Plus CAPOX for MSS Locally Advanced Ultra Low Rectal Adenocarcinoma | RECRUITING | PHASE2 |
| NCT05216653 | Preoperative Short-course Radiation Followed by Envafolimab Plus CAPOX for MSS Locally Advanced Rectal Adenocarcinoma (PRECAM) | ACTIVE, NOT RECRUITING | PHASE2 |
| NCT05752136 | Preoperative Short-course Radiation Followed by Envafolimab Plus CAPOX for MSS Locally Advanced Rectal Adenocarcinoma | RECRUITING | PHASE3 |
| NCT05815303 | XELOX Combined With Cadonilimab Versus XELOX as Neoadjuvant Treatment for Locally Advanced, pMMR Rectal Cancer | RECRUITING | PHASE2 |
| NCT04304209 | Pd1 Antibody Sintilimab ± Chemoradiotherapy for Locally Advanced Rectal Cancer | ACTIVE, NOT RECRUITING | PHASE2 |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. Epub 2021 Feb 4. [CrossRef] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7-33. Epub 2022 Jan 12. [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. Version 3.2022. Published 2022. Accessed January 28, 2024. [https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf].
- Glimelius B, Tiret E, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi81-8. [CrossRef]
- Molinari C, Passardi A. Why is neoadjuvant chemoradiation therapy underused for locally advanced rectal cancer? Expert Rev Gastroenterol Hepatol. 2016 Dec;10(12):1317-1319. Epub 2016 Oct 19. [CrossRef] [PubMed]
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004 Oct 21;351(17):1731-40. [CrossRef] [PubMed]
- Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, Bessell E, Griffiths G, Thompson LC, Parmar M. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009 Mar 7;373(9666):811-20. [CrossRef] [PubMed] [PubMed Central]
- Pettersson D, Cedermark B, Holm T, Radu C, Påhlman L, Glimelius B, Martling A. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg. 2010 Apr;97(4):580-7. [CrossRef] [PubMed]
- Marijnen CA, van de Velde CJ, Putter H, van den Brink M, Maas CP, Martijn H, Rutten HJ, Wiggers T, Kranenbarg EK, Leer JW, Stiggelbout AM. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005 Mar 20;23(9):1847-58. [CrossRef] [PubMed]
- Pettersson D, Holm T, Iversen H, Blomqvist L, Glimelius B, Martling A. Preoperative short-course radiotherapy with delayed surgery in primary rectal cancer. Br J Surg. 2012 Apr;99(4):577-83. Epub 2012 Jan 12. [CrossRef] [PubMed]
- Bujko K, Richter P, Smith FM, Polkowski W, Szczepkowski M, Rutkowski A, Dziki A, Pietrzak L, Kołodziejczyk M, Kuśnierz J, Gach T, Kulig J, Nawrocki G, Radziszewski J, Wierzbicki R, Kowalska T, Meissner W, Radkowski A, Paprota K, Polkowski M, Rychter A. Preoperative radiotherapy and local excision of rectal cancer with immediate radical re-operation for poor responders: a prospective multicentre study. Radiother Oncol. 2013 Feb;106(2):198-205. Epub 2013 Jan 17. [CrossRef] [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, Ackland SP, Schache D, McClure B, McLachlan SA, McKendrick J, Leong T, Hartopeanu C, Zalcberg J, Mackay J. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012 Nov 1;30(31):3827-33. Epub 2012 Sep 24. Erratum in: J Clin Oncol. 2013 Jan 20;31(3):399. [CrossRef] [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006 Oct;93(10):1215-23. [CrossRef] [PubMed]
- Breugom AJ, van Gijn W, Muller EW, Berglund Å, van den Broek CBM, Fokstuen T, Gelderblom H, Kapiteijn E, Leer JWH, Marijnen CAM, Martijn H, Meershoek-Klein Kranenbarg E, Nagtegaal ID, Påhlman L, Punt CJA, Putter H, Roodvoets AGH, Rutten HJT, Steup WH, Glimelius B, van de Velde CJH. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015 Apr;26(4):696-701. Epub 2014 Dec 5. [CrossRef] [PubMed]
- Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, Glynne-Jones R, Counsell N, Bastiaannet E, van den Broek CB, Liefers GJ, Putter H, van de Velde CJ. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015 Feb;16(2):200-7. Epub 2015 Jan 12. [CrossRef] [PubMed]
- Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, Radosevic-Jelic L, Piérart M, Calais G; European Organisation for Research and Treatment of Cancer Radiation Oncology Group. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007 Oct 1;25(28):4379-86. [CrossRef] [PubMed]
- Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, Guillem JG, Paty PB, Yaeger R, Stadler ZK, Seier K, Gonen M, Segal NH, Reidy DL, Varghese A, Shia J, Vakiani E, Wu AJ, Crane CH, Gollub MJ, Garcia-Aguilar J, Saltz LB, Weiser MR. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018 Jun 14;4(6):e180071. Epub 2018 Jun 14. [CrossRef] [PubMed] [PubMed Central]
- Passardi A, Canale M, Valgiusti M, Ulivi P. Immune Checkpoints as a Target for Colorectal Cancer Treatment. Int J Mol Sci. 2017 Jun 21;18(6):1324. [CrossRef] [PubMed] [PubMed Central]
- Tivey A, Church M, Rothwell D, Dive C, Cook N. Circulating tumour DNA - looking beyond the blood. Nat Rev Clin Oncol. 2022 Sep;19(9):600-612. Epub 2022 Aug 1. [CrossRef] [PubMed] [PubMed Central]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Etienne, P.-L.; Rio, E.; Evesque, L.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Boileve, A.; Delaye, M.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J. Clin. Oncol. 2023, 41, LBA3504. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, E.A.; Nilsson, P.J.; Hospers, G.A.; Bahadoer, R.R.; Kranenbarg, E.M.-K.; Roodvoets, A.G.; Putter, H.; Berglund, Å.; Cervantes, A.; Crolla, R.M.; et al. Locoregional Failure During and After Short-course Radiotherapy followed by Chemotherapy and Surgery Compared to Long-course Chemoradiotherapy and Surgery—A Five-year Follow-up of the RAPIDO Trial. Ann. Surg. 2023, 10–109. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.-M.; Jiang, J.; Li, N.; Liu, W.-Y.; Chen, S.-L.; Li, S.; Lu, N.-N.; et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J. Clin. Oncol. 2022, 40, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Wu X, Peng J, Ren D, Wang J. Neoadjuvant Modified FOLFOX6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial J Clin Oncol. 2019 Dec 1;37(34):3223-3233. Epub 2019 Sep 26. [CrossRef]
- Schrag, D.; Shi, Q.; Weiser, M.R.; Gollub, M.J.; Saltz, L.B.; Musher, B.L.; Goldberg, J.; Al Baghdadi, T.; Goodman, K.A.; McWilliams, R.R.; et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N. Engl. J. Med. 2023, 389, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Basch E, Dueck AC, Mitchell SA, Mamon H, Weiser M, Saltz L, Gollub M, Rogak L, Ginos B, Mazza GL, Colgrove B, Chang G, Minasian L, Denicoff A, Thanarajasingam G, Musher B, George T, Venook A, Farma J, O'Reilly E, Meyerhardt JA, Shi Q, Schrag D. Patient-Reported Outcomes During and After Treatment for Locally Advanced Rectal Cancer in the PROSPECT Trial (Alliance N1048). J Clin Oncol. 2023 Jul 20;41(21):3724-3734. Epub 2023 Jun 4. [CrossRef] [PubMed]
- Fokas, E.; Allgäuer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.-L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.-L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients with Locally Advanced Rectal Cancer. JAMA Oncol. 2022, 8, e215445. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022 Jun 23;386(25):2363-2376. Epub 2022 Jun 5. [CrossRef] [PubMed] [PubMed Central]
- Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, Liu M, Lin JZ, Wang FL, Li C, Quan TT, Xi SY, Zhang HZ, Pan ZZ, Wang F, Xu RH. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol. 2023 May;8(5):422-431. Epub 2023 Mar 1. [CrossRef] [PubMed]
- Zhang X, Yang R, Wu T, Cai X, Li G, Yu K, Li Y, Ding R, Dong C, Li J, Hu R, Feng Q and Li Y (2022) Efficacy and Safety of Neoadjuvant Monoimmunotherapy With PD-1 Inhibitor for dMMR/MSI⁃H Locally Advanced Colorectal Cancer: A Single-Center Real-World Study. Front. Immunol. 13:913483. [CrossRef]
- Yang R, Wu T, Yu J, Cai X, Li G, Li X, Huang W, Zhang Y, Wang Y, Yang X, Ren Y, Hu R, Feng Q, Ding P, Zhang X, Li Y. Locally advanced rectal cancer with dMMR/MSI-H may be excused from surgery after neoadjuvant anti-PD-1 monotherapy: a multiple-center, cohort study. Front Immunol. 2023 Jun 27;14:1182299. [CrossRef] [PubMed] [PubMed Central]
- Kothari A, White MG, Peacock O, Kaur H, Palmquist SM, You N, Taggart M, Salem U, Overman M, Kopetz S, Chang GJ. Pathological response following neoadjuvant immunotherapy in mismatch repair-deficient/microsatellite instability-high locally advanced, non-metastatic colorectal cancer. Br J Surg. 2022 May 16;109(6):489-492. [CrossRef] [PubMed]
- Demisse, R., Damle, N., Kim, E., Gong, J., Fakih, M., Eng, C., Oesterich, L., McKenny, M., Ji, J., Liu, J., Louie, R., Tam, K., Gholami, S., Halabi, W., Monjazeb, A., Dayyani, F., & Cho, M. (2020). Neoadjuvant Immunotherapy–Based Systemic Treatment in MMR-Deficient or MSI-High Rectal Cancer: Case Series. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw, 18(7), 798-804. [CrossRef]
- Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C, Beets GL, Snaebjornsson P, Maas M, Mertz M, Veninga V, Bounova G, Broeks A, Beets-Tan RG, de Wijkerslooth TR, van Lent AU, Marsman HA, Nuijten E, Kok NF, Kuiper M, Verbeek WH, Kok M, Van Leerdam ME, Schumacher TN, Voest EE, Haanen JB. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020 Apr;26(4):566-576. Epub 2020 Apr 6. [CrossRef] [PubMed]
- Phipps AI, Lindor NM, Jenkins MA, Baron JA, Win AK, Gallinger S, Gryfe R, Newcomb PA. Colon and rectal cancer survival by tumor location and microsatellite instability: the Colon Cancer Family Registry. Dis Colon Rectum. 2013 Aug;56(8):937-44. [CrossRef] [PubMed] [PubMed Central]
- Fabrizio DA, George TJ Jr, Dunne RF, Frampton G, Sun J, Gowen K, Kennedy M, Greenbowe J, Schrock AB, Hezel AF, Ross JS, Stephens PJ, Ali SM, Miller VA, Fakih M, Klempner SJ. Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J Gastrointest Oncol. 2018 Aug;9(4):610-617. [CrossRef] [PubMed] [PubMed Central]
- Ma X, Riaz N, Samstein RM, Lee M, Makarov V, Valero C, Chowell D, Kuo F, Hoen D, Fitzgerald CWR, Jiang H, Alektiar J, Alban TJ, Juric I, Parthasarathy PB, Zhao Y, Sabio EY, Verma R, Srivastava RM, Vuong L, Yang W, Zhang X, Wang J, Chu LK, Wang SL, Kelly DW, Pei X, Chen J, Yaeger R, Zamarin D, Zehir A, Gönen M, Morris LGT, Chan TA. Functional landscapes of POLE and POLD1 mutations in checkpoint blockade-dependent antitumor immunity. Nat Genet. 2022 Jul;54(7):996-1012. Epub 2022 Jul 11. [CrossRef] [PubMed] [PubMed Central]
- Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther. 2022 Jul 29;7(1):258. [CrossRef] [PubMed] [PubMed Central]
- Yuki S, Bando H, Tsukada Y, Inamori K. Short-term results of VOLTAGE-A: Nivolumab monotherapy and subsequent radical surgery following preoperative chemoradiotherapy in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. JCO. 2020;38(15_suppl):4100-4100. [CrossRef]
- Grassi E, Zingaretti C, Petracci E, Corbelli J, Papiani G, Banchelli I, Valli I, Frassineti GL, Passardi A, Di Bartolomeo M, Pietrantonio F, Gelsomino F, Carandina I, Banzi M, Martella L, Bonetti AV, Boccaccino A, Molinari C, Marisi G, Ugolini G, Nanni O, Tamberi S. Phase II study of capecitabine-based concomitant chemoradiation followed by durvalumab as a neoadjuvant strategy in locally advanced rectal cancer: the PANDORA trial. ESMO Open. 2023 Oct;8(5):101824. Epub 2023 Sep 27. [CrossRef] [PubMed] [PubMed Central]
- Lin Z, Cai M, Zhang P, Li G, Liu T, Li X, Cai K, Nie X, Wang J, Liu J, Liu H, Zhang W, Gao J, Wu C, Wang L, Fan J, Zhang L, Wang Z, Hou Z, Ma C, Yang K, Wu G, Tao K, Zhang T. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J Immunother Cancer. 2021 Nov;9(11):e003554. Erratum in: J Immunother Cancer. 2022 Feb;10(2):. [CrossRef] [PubMed] [PubMed Central]
- Shamseddine, Y. Zeidan, Y. Bouferraa, R. Turfa, J. Kattan, D. Mukherji, S. Temraz, K. Alqasem, R. Amarin, T. Al Awabdeh, S. Deeba, S. Doughan, I. Mohamad, M. Elkhaldi, F. Daoud, M. Al Masri, A. Dabous, A. Hushki, M. Charafeddine, M. Al Darazi, F. Geara. Efficacy and safety of neoadjuvant short-course radiation followed by mFOLFOX-6 plus avelumab for locally-advanced rectal adenocarcinoma: Averectal study. Annals of Oncology. 2021;32:S215. [CrossRef]
- Salvatore L, Bensi M, Corallo S, Bergamo F, Pellegrini I, Rasola C, Borelli B, Tamburini E. Randon G, Galuppo S, Boccaccino A, Viola M, Auriemma A, Fea E, Barbara C, Bustreo S, Smiroldo V, Barbaro B, Gambacorta M, Tortora G. (2021). Phase II study of preoperative (PREOP) chemoradiotherapy (CTRT) plus avelumab (AVE) in patients (PTS) with locally advanced rectal cancer (LARC): The AVANA study.. Journal of Clinical Oncology. 39. 3511-3511. [CrossRef]
- Kasi PM, Hidalgo M, Jafari MD, Yeo H, Lowenfeld L, Khan U, Nguyen ATH, Siolas D, Swed B, Hyun J, Khan S, Wood M, Samstein B, Rocca JP, Ocean AJ, Popa EC, Hunt DH, Uppal NP, Garrett KA, Pigazzi A, Zhou XK, Shah MA, Hissong E. Neoadjuvant botensilimab plus balstilimab response pattern in locally advanced mismatch repair proficient colorectal cancer. Oncogene. 2023 Oct;42(44):3252-3259. Epub 2023 Sep 21. [CrossRef] [PubMed] [PubMed Central]
- El-Khoueiry A, Fakih M, Gordon M, Tsimberidou A, Bullock A, Wilky B, Trent J, Margolin K, Mahadevan D, Balmanoukian A, Sanborn R, Schwartz G, Bockorny B, Moser J, Grossman J, Feliu W, Rosenthal K, O'Day S, Lenz HJ, Schlechter B (2023). Results from a phase 1a/1b study of botensilimab (BOT), a novel innate/adaptive immune activator, plus balstilimab (BAL; anti-PD-1 antibody) in metastatic heavily pretreated microsatellite stable colorectal cancer (MSS CRC).. Journal of Clinical Oncology. 41. LBA8-LBA8. [CrossRef]
- Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010 Sep;11(9):835-44. Epub 2010 Aug 6. [CrossRef] [PubMed]
- Zhang X, Ma S, Guo Y, Luo Y, Li L. Total neoadjuvant therapy versus standard therapy in locally advanced rectal cancer: A systematic review and meta-analysis of 15 trials. PLoS One. 2022 Nov 4;17(11):e0276599. [CrossRef] [PubMed] [PubMed Central]
- Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004 Oct;240(4):711-7; discussion 717-8. [CrossRef] [PubMed] [PubMed Central]
- Loria A, Tejani MA, Temple LK, Justiniano CF, Melucci AD, Becerra AZ, Monson JRT, Aquina CT, Fleming FJ. Practice Patterns for Organ Preservation in US Patients With Rectal Cancer, 2006-2020. JAMA Oncol. 2024 Jan 1;10(1):79-86. [CrossRef] [PubMed] [PubMed Central]
- Zhang X, Ding R, Li J, Wu T, Shen Z, Li S, Zhang Y, Dong C, Shang Z, Zhou H, Li T, Li G, Li Y. Efficacy and safety of the "watch-and-wait" approach for rectal cancer with clinical complete response after neoadjuvant chemoradiotherapy: a meta-analysis. Surg Endosc. 2022 Apr;36(4):2233-2244. Epub 2022 Jan 3. [CrossRef] [PubMed]
- van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, Habr-Gama A, Perez RO, Renehan AG, van de Velde CJH; IWWD Consortium. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018 Jun 23;391(10139):2537-2545. [CrossRef] [PubMed]
- Wang J, Zhang L, Wang M, Zhang J, Wang Y, Wan J, Li G, Zhang H, Wang Y, Wu R, Zhang Z, Li X, Xu Y, Zhu J, Shen L, Xia F, Zhang Z. Long-term outcomes in a retrospective cohort of patients with rectal cancer with complete response after total neoadjuvant therapy: a propensity-score weighted analysis. Ther Adv Med Oncol. 2023 Sep 8;15:17588359231197955. [CrossRef] [PubMed] [PubMed Central]
- Verheij FS, Omer DM, Williams H, Lin ST, Qin LX, Buckley JT, Thompson HM, Yuval JB, Kim JK, Dunne RF, Marcet J, Cataldo P, Polite B, Herzig DO, Liska D, Oommen S, Friel CM, Ternent C, Coveler AL, Hunt S, Gregory A, Varma MG, Bello BL, Carmichael JC, Krauss J, Gleisner A, Guillem JG, Temple L, Goodman KA, Segal NH, Cercek A, Yaeger R, Nash GM, Widmar M, Wei IH, Pappou EP, Weiser MR, Paty PB, Smith JJ, Wu AJ, Gollub MJ, Saltz LB, Garcia-Aguilar J. Long-Term Results of Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J Clin Oncol. 2024 Feb 10;42(5):500-506. Epub 2023 Oct 26. [CrossRef] [PubMed]
- Fernandez LM, São Julião GP, Figueiredo NL, Beets GL, van der Valk MJM, Bahadoer RR, Hilling DE, Meershoek-Klein Kranenbarg E, Roodvoets AGH, Renehan AG, van de Velde CJH, Habr-Gama A, Perez RO; International Watch & Wait Database Consortium. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: a retrospective, international, multicentre registry study. Lancet Oncol. 2021 Jan;22(1):43-50. Epub 2020 Dec 11. [CrossRef] [PubMed]
- Lin W, Wee IJY, Seow-En I, Chok AY, Tan EK. Survival outcomes of salvage surgery in the watch-and-wait approach for rectal cancer with clinical complete response after neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Ann Coloproctol. 2023 Dec;39(6):447-456. Epub 2023 Dec 28. [CrossRef] [PubMed] [PubMed Central]
- Dattani M, Heald RJ, Goussous G, Broadhurst J, São Julião GP, Habr-Gama A, Perez RO, Moran BJ. Oncological and Survival Outcomes in Watch and Wait Patients With a Clinical Complete Response After Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Systematic Review and Pooled Analysis. Ann Surg. 2018 Dec;268(6):955-967. [CrossRef] [PubMed]
- Ferri V, Vicente E, Quijano Y, Duran H, Diaz E, Fabra I, Malave L, Ruiz P, Costantini G, Pizzuti G, Cubillo A, Rubio MC, Cañamaque LG, Alfonsel JN, Caruso R. Light and shadow of watch-and-wait strategy in rectal cancer: oncological result, clinical outcomes, and cost-effectiveness analysis. Int J Colorectal Dis. 2023 Dec 5;38(1):277. [CrossRef] [PubMed]
- Han Z, Li M, Chen J, Ji D, Zhan T, Peng Y, Xue W, Li Y, Cai Y, Sun Y, Wu Q, Du C, Gu J. Surgery may not benefit patients with locally advanced rectal cancer who achieved clinical complete response following neoadjuvant chemoradiotherapy. Asian J Surg. 2022 Jan;45(1):97-104. Epub 2021 Apr 20. [CrossRef] [PubMed]
- Yu G, Lu W, Jiao Z, Qiao J, Ma S, Liu X. A meta-analysis of the watch-and-wait strategy versus total mesorectal excision for rectal cancer exhibiting complete clinical response after neoadjuvant chemoradiotherapy. World J Surg Oncol. 2021 Oct 18;19(1):305. [CrossRef] [PubMed] [PubMed Central]
- Fernandez LM, São Julião GP, Renehan AG, Beets GL, Papoila AL, Vailati BB, Bahadoer RR, Kranenbarg EM, Roodvoets AGH, Figueiredo NL, Van De Velde CJH, Habr-Gama A, Perez RO; International Watch & Wait Database (IWWD) Consortium. The Risk of Distant Metastases in Patients With Clinical Complete Response Managed by Watch and Wait After Neoadjuvant Therapy for Rectal Cancer: The Influence of Local Regrowth in the International Watch and Wait Database. Dis Colon Rectum. 2023 Jan 1;66(1):41-49. Epub 2022 Oct 21. [CrossRef] [PubMed]
- Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A, Widmar M, Ganesh K, Yaeger R, Cercek A, Weiser MR, Nash GM, Guillem JG, Temple LKF, Chalasani SB, Fuqua JL, Petkovska I, Wu AJ, Reyngold M, Vakiani E, Shia J, Segal NH, Smith JD, Crane C, Gollub MJ, Gonen M, Saltz LB, Garcia-Aguilar J, Paty PB. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019 Apr 1;5(4):e185896. Epub 2019 Apr 11. [CrossRef] [PubMed] [PubMed Central]
- Beard BW, Rettig RL, Ryoo JJ, Parker RA, McLemore EC, Attaluri V. Watch-and-Wait Compared to Operation for Patients with Complete Response to Neoadjuvant Therapy for Rectal Cancer. J Am Coll Surg. 2020 Dec;231(6):681-692. Epub 2020 Oct 26. [CrossRef] [PubMed]
- Socha J, Kępka L, Michalski W, Paciorek K, Bujko K. The risk of distant metastases in rectal cancer managed by a watch-and-wait strategy - A systematic review and meta-analysis. Radiother Oncol. 2020 Mar;144:1-6. Epub 2019 Nov 8. [CrossRef] [PubMed]
- Meyer VM, Meuzelaar RR, Schoenaker IJH, de Groot JB, Reerink O, de Vos Tot Nederveen Cappel WH, Beets GL, van Westreenen HL. Delayed TME Surgery in a Watch-and-Wait Strategy After Neoadjuvant Chemoradiotherapy for Rectal Cancer: An Analysis of Hospital Costs and Surgical and Oncological Outcomes. Dis Colon Rectum. 2023 May 1;66(5):671-680. Epub 2021 Nov 24. [CrossRef] [PubMed]
- Tan S, Gao Q, Cui Y, Ou Y, Huang S, Feng W. Oncologic outcomes of watch-and-wait strategy or surgery for low to intermediate rectal cancer in clinical complete remission after adjuvant chemotherapy: a systematic review and meta-analysis. Int J Colorectal Dis. 2023 Oct 3;38(1):246. [CrossRef] [PubMed]
- Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, Verheij FS, Omer DM, Lee M, Dunne RF, Marcet J, Cataldo P, Polite B, Herzig DO, Liska D, Oommen S, Friel CM, Ternent C, Coveler AL, Hunt S, Gregory A, Varma MG, Bello BL, Carmichael JC, Krauss J, Gleisner A, Paty PB, Weiser MR, Nash GM, Pappou E, Guillem JG, Temple L, Wei IH, Widmar M, Lin S, Segal NH, Cercek A, Yaeger R, Smith JJ, Goodman KA, Wu AJ, Saltz LB. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol. 2022 Aug 10;40(23):2546-2556. Epub 2022 Apr 28. [CrossRef] [PubMed] [PubMed Central]
- Wang QX, Zhang R, Xiao WW, Zhang S, Wei MB, Li YH, Chang H, Xie WH, Li LR, Ding PR, Chen G, Zeng ZF, Wang WH, Wan XB, Gao YH. The watch-and-wait strategy versus surgical resection for rectal cancer patients with a clinical complete response after neoadjuvant chemoradiotherapy. Radiat Oncol. 2021 Jan 19;16(1):16. [CrossRef] [PubMed] [PubMed Central]
- Araujo RO, Valadão M, Borges D, Linhares E, de Jesus JP, Ferreira CG, Victorino AP, Vieira FM, Albagli R. Nonoperative management of rectal cancer after chemoradiation opposed to resection after complete clinical response. A comparative study. Eur J Surg Oncol. 2015 Nov;41(11):1456-63. Epub 2015 Aug 29. [CrossRef] [PubMed]
- Benson AB 3rd, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, Engstrom PF, Enzinger PC, Fenton MJ, Fuchs CS, Grem JL, Grothey A, Hochster HS, Hunt S, Kamel A, Kirilcuk N, Leong LA, Lin E, Messersmith WA, Mulcahy MF, Murphy JD, Nurkin S, Rohren E, Ryan DP, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Freedman-Cass D. Rectal Cancer, Version 2.2015. J Natl Compr Canc Netw. 2015 Jun;13(6):719-28; quiz 728. [CrossRef] [PubMed]
- Martens MH, Maas M, Heijnen LA, Lambregts DM, Leijtens JW, Stassen LP, Breukink SO, Hoff C, Belgers EJ, Melenhorst J, Jansen R, Buijsen J, Hoofwijk TG, Beets-Tan RG, Beets GL. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J Natl Cancer Inst. 2016 Aug 10;108(12):djw171. [CrossRef] [PubMed]
- Al-Najami I, Jones HJ, Dickson EA, Muirhead R, Deding U, James DR, Cunningham C. Rectal cancer: Watch-and-wait and continuing the rectal-preserving strategy with local excision for incomplete response or limited regrowth. Surg Oncol. 2021 Jun;37:101574. Epub 2021 Apr 3. [CrossRef] [PubMed]
- Chan HT, Nagayama S, Otaki M, Chin YM, Fukunaga Y, Ueno M, Nakamura Y, Low SK. Tumor-informed or tumor-agnostic circulating tumor DNA as a biomarker for risk of recurrence in resected colorectal cancer patients. Front Oncol. 2023 Jan 26;12:1055968. [CrossRef] [PubMed] [PubMed Central]
- Vidal J, Casadevall D, Bellosillo B, Pericay C, Garcia-Carbonero R, Losa F, Layos L, Alonso V, Capdevila J, Gallego J, Vera R, Salud A, Martin-Richard M, Nogué M, Cillán E, Maurel J, Faull I, Raymond V, Fernández-Martos C, Montagut C. Clinical Impact of Presurgery Circulating Tumor DNA after Total Neoadjuvant Treatment in Locally Advanced Rectal Cancer: A Biomarker Study from the GEMCAD 1402 Trial. Clin Cancer Res. 2021 May 15;27(10):2890-2898. Epub 2021 Mar 16. [CrossRef] [PubMed]
- Zhou J, Wang C, Lin G, Xiao Y, Jia W, Xiao G, Liu Q, Wu B, Wu A, Qiu H, Zhang F, Hu K, Xue H, Shen Z, Wang Z, Han J, Niu B, Xu Y, Yu Z, Yang L. Serial Circulating Tumor DNA in Predicting and Monitoring the Effect of Neoadjuvant Chemoradiotherapy in Patients with Rectal Cancer: A Prospective Multicenter Study. Clin Cancer Res. 2021 Jan 1;27(1):301-310. Epub 2020 Oct 12. [CrossRef] [PubMed]
- Wang Y, Yang L, Bao H, Fan X, Xia F, Wan J, Shen L, Guan Y, Bao H, Wu X, Xu Y, Shao Y, Sun Y, Tong T, Li X, Xu Y, Cai S, Zhu J, Zhang Z. Utility of ctDNA in predicting response to neoadjuvant chemoradiotherapy and prognosis assessment in locally advanced rectal cancer: A prospective cohort study. PLoS Med. 2021 Aug 31;18(8):e1003741. [CrossRef] [PubMed] [PubMed Central]
- Morais M, Pinto DM, Machado JC, Carneiro S. ctDNA on liquid biopsy for predicting response and prognosis in locally advanced rectal cancer: A systematic review. Eur J Surg Oncol. 2022 Jan;48(1):218-227. Epub 2021 Sep 3. [CrossRef] [PubMed]
- Chang L, Zhang X, He L, Ma Q, Fang T, Jiang C, Ma Z, Li Q, Wu C, Tao J. Prognostic Value of ctDNA Detection in Patients With Locally Advanced Rectal Cancer Undergoing Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis. Oncologist. 2023 Dec 11;28(12):e1198-e1208. [CrossRef] [PubMed] [PubMed Central]
- Piercey O, Tie J. Circulating tumour DNA in the evolving treatment landscape of locally advanced rectal cancer: where does it fit in? Ther Adv Med Oncol. 2023 Mar 14;15:17588359231160138. [CrossRef] [PubMed] [PubMed Central]
- Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, Knudsen M, Nordentoft I, Wu HT, Tin AS, Heilskov Rasmussen M, Vang S, Shchegrova S, Frydendahl Boll Johansen A, Srinivasan R, Assaf Z, Balcioglu M, Olson A, Dashner S, Hafez D, Navarro S, Goel S, Rabinowitz M, Billings P, Sigurjonsson S, Dyrskjøt L, Swenerton R, Aleshin A, Laurberg S, Husted Madsen A, Kannerup AS, Stribolt K, Palmelund Krag S, Iversen LH, Gotschalck Sunesen K, Lin CJ, Zimmermann BG, Lindbjerg Andersen C. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019 Aug 1;5(8):1124-1131. Erratum in: JAMA Oncol. 2019 Jun 13. [CrossRef] [PubMed] [PubMed Central]
- Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, Wong R, Kosmider S, Ananda S, McKendrick J, Lee B, Cho JH, Faragher I, Jones IT, Ptak J, Schaeffer MJ, Silliman N, Dobbyn L, Li L, Tomasetti C, Papadopoulos N, Kinzler KW, Vogelstein B, Gibbs P. Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. JAMA Oncol. 2019 Dec 1;5(12):1710-1717. Erratum in: JAMA Oncol. 2019 Dec 1;5(12):1811. [CrossRef] [PubMed] [PubMed Central]
- Khakoo S, Carter PD, Brown G, Valeri N, Picchia S, Bali MA, Shaikh R, Jones T, Begum R, Rana I, Wotherspoon A, Terlizzo M, von Loga K, Kalaitzaki E, Saffery C, Watkins D, Tait D, Chau I, Starling N, Hubank M, Cunningham D. MRI Tumor Regression Grade and Circulating Tumor DNA as Complementary Tools to Assess Response and Guide Therapy Adaptation in Rectal Cancer. Clin Cancer Res. 2020 Jan 1;26(1):183-192. Epub 2019 Dec 18. [CrossRef] [PubMed]
- Murahashi S, Akiyoshi T, Sano T, Fukunaga Y, Noda T, Ueno M, Zembutsu H. Serial circulating tumour DNA analysis for locally advanced rectal cancer treated with preoperative therapy: prediction of pathological response and postoperative recurrence. Br J Cancer. 2020 Sep;123(5):803-810. Epub 2020 Jun 22. [CrossRef] [PubMed] [PubMed Central]
- McDuff SGR, Hardiman KM, Ulintz PJ, Parikh AR, Zheng H, Kim DW, Lennerz JK, Hazar-Rethinam M, Van Seventer EE, Fetter IJ, Nadres B, Eyler CE, Ryan DP, Weekes CD, Clark JW, Cusack JC, Goyal L, Zhu AX, Wo JY, Blaszkowsky LS, Allen J, Corcoran RB, Hong TS. Circulating Tumor DNA Predicts Pathologic and Clinical Outcomes Following Neoadjuvant Chemoradiation and Surgery for Patients With Locally Advanced Rectal Cancer. JCO Precis Oncol. 2021 Jan 12;5:PO.20.00220. [CrossRef] [PubMed] [PubMed Central]
- Hofste LSM, Geerlings MJ, von Rhein D, Rütten H, Westenberg AH, Weiss MM, Gilissen C, Hofste T, van der Post RS, Klarenbeek BR, de Wilt JHW, Ligtenberg MJL; Libic2 collaborators group. Circulating tumor DNA detection after neoadjuvant treatment and surgery predicts recurrence in patients with early-stage and locally advanced rectal cancer. Eur J Surg Oncol. 2023 Jul;49(7):1283-1290. Epub 2023 Jan 31. [CrossRef] [PubMed]
- Tie J, Cohen JD, Wang Y, Li L, Christie M, Simons K, Elsaleh H, Kosmider S, Wong R, Yip D, Lee M, Tran B, Rangiah D, Burge M, Goldstein D, Singh M, Skinner I, Faragher I, Croxford M, Bampton C, Haydon A, Jones IT, S Karapetis C, Price T, Schaefer MJ, Ptak J, Dobbyn L, Silliman N, Kinde I, Tomasetti C, Papadopoulos N, Kinzler K, Volgestein B, Gibbs P. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut. 2019 Apr;68(4):663-671. Epub 2018 Feb 2. [CrossRef] [PubMed] [PubMed Central]
- C. Molinari, G. C. Molinari, G. Marisi, G. Laliotis, E. Spickard, I.G. Rapposelli, E. Petracci, G.V. George, S. Sharma, A. Jurdi, H. Sethi, M.C. Liu, P. Ulivi, M. Canale, L. Saragoni, R. Panzacchi, G.L. Frassineti, M. Muratore, A. Romeo, G. Martinelli, A. Passardi. Assessment of circulating tumor (ct)DNA in patients (pts) with locally advanced rectal cancer (LARC) pts treated with neoadjuvant therapy (NAT). Ann Oncol 2023 Vol 34 Issue 52. [CrossRef]
| Phase III clinical trials evaluating TNT | |||||||||
| Ref | Study title | Study | N | Stage | Induction | Radiotherapy | Consolidation | pCR | Rec |
| Conroy T. | PRODIGE-23 | Prospective phase III | 461 | cT3-4 or N+ | FOLFIRINOX | LCRT | None | 27.8% | DFS 76% DM 17% |
| Bahadoer, R.R. | RAPIDO | Prospective phase III | 912 | II – III* | None | SCRT | CAPOX/FOLFOX | 28.4% | DrTF 23.7% LLR 8.3% DM 20% |
| Jin J. | STELLAR | Prospective phase III | 599 | cT3-4 or N+ | None | SCRT | CAPOX | cCR 11.1% | DFS 64.5% LLR 8.4% DM 22.8% |
| Omission of radiotherapy | |||||||||
| Ref | Study title | Study | Stage | Chemiotherapy | Radiotherapy | - | pCR | Rec | |
| Deng Y. | FOWARC | Prospective phase III | 495 | cT3-4 or N+ | mFOLFOX6 | None | - | 6.5% | DFS 73.5% LR 1.8% |
| Schrag D. | PROSPECT | Prospective phase III | 1128 | cT2-3 or N+ | FOLFOX | CTRT in selected cases ** | - | 21.9% | DFS 80.8% LR 1.8% |
| Induction vs consolidation chemoterapy | |||||||||
| Ref | Study title | Study | Stage | Induction | Radiotherapy | Consolidation | pCR | Rec | |
| Fokas E. | CAO/ARO/AIO-12 | Prospective phase II | 306 | II - III | FOLFOX (A) | LCRT | FOLFOX (B) | 17% (A) 25% (B) |
LLR 6%vs5% DM 18%vs16% |
| Garcia-Aguilar J. | OPRA | Prospective phase II | 324 | II - III | FOLFOX (A) | LCRT | FOLFOX (B) | 10% (A) 8% (B) | 5y DFS 71% vs 69% |
| ICI | Study title/ Reference |
Patients (N°) |
Induction | Radio therapy |
Consolidation | pCR |
|---|---|---|---|---|---|---|
| dMMR/MSI | ||||||
| Dostarlimab | NCT04165772 | 12 | Dostarlimab | LCRT | None | 100% |
| Sintilimab | NCT04304209 | 17 | Sintilimab | None | None | 75% |
| Tislelizumab, Sintilimab, Pembrolizumab | Zhang X. | 32 | None | Not Specified* | None | 100% |
| Tislelizumab, Sintilimab, Pembrolizumab | Yang R. | 20 | None | None | None | 90% |
| Pembrolizumab, Nivolumab +/- CT | Kothari A. | 9 | None | None | None | 89% |
| Pembrolizumab, Nivolumab +/- CT** | Demisse R. | 3 | None | Not Specified*** | None | 100% |
| MSS | ||||||
| Nivolumab | VOLTAGE-A | 37 | Nivolumab | LCRT | None | 30% 7 |
| Camrelizumab | NCT04231552 | 26 | None | SCRT | CAPOX + Camrelizumab (2 x 21 days cycles) |
46.2%9 |
| Durvalumab | PANDORA | 46 | None | LCRT | Durvalumab | 34.5%,8 |
| Avelumab | AVERECTAL | 26 | None | SCRT | FOLFOX + Avelumab | 37%10 |
| Avelumab | AVANA | 38 | None | LCRT | Avelumab | 23%11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





