Submitted:
19 March 2024
Posted:
20 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Nonmarine occurrences
2.1 Middle Jurassic
2.1.1 Thailand
2.2 Late Jurassic
2.2.1 China
2.2.2. North America
2.2.3. Africa
2.3. Early Cretaceous
2.3.1. Thailand
2.3.2. United Kingdom
2.3.3 Germany
2.3.4. Belgium
2.3.5. France
2.3.6. Spain
2.3.8. North America
2.3.9. South America
2.3.10. Africa
2.4. Late Cretaceous
2.4.1. China
2.4.2. India
2.4.3. Austria
2.4.4 Hungary
2.4.5. France
2.4.6. Spain
2.4.7. North America
2.4.8. South America
2.4.9. Africa
2.4.10. Madgascar
2.5. Palaeogene
2.5.1. India
2.5.2. North America
2.5.3. South America
2.5.4. Africa
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Taxonomy, affinities and palaeogeographic distribution of nonmarine pycnodontiforms in stratigraphic order from oldest to youngest.
| Taxa | Family | Country | Locality | Period | Stage | Ref. |
| cf. Gyrodus | Gyrodontidae | Thailand | Khlong Min Formation (Mab Ching) | Middle-Late Jurassic | Unknown | [21] |
| Tibetodus gyroides | Family incertae sedis | China | Tibet | Late Jurassic | Unknown | [25,26] |
| Pycnodontoidea gen et spp. indet | Family incertae sedis | USA | Morrison Formation, Dinosaur National Monument | Late Jurassic | Oxfordian-Tithonian | [27] |
| cf. Pycnodus | Pycnodontidae | Ethiopia | Abay Gorge, Mugher Mudstone | Late Jurassic | ?Kimmeridgian-Tithonian | [39] |
| Congopycnodus cornutus | Family incertae sedis | Democratic Republic of Congo | Stanleyville | Late Jurassic-Early Cretaceous | Kimmeridgian-Valanginian | [32] |
| ?Pycnodontiformes indet. (tooth morphotype 2) | Family incertae sedis | France | Champblanc Quarry, Cherves-de-Cognac | Early Cretaceous | Berriasian | [77] |
| aff. Gyrodus (tooth morphotype 6) | Gyrodontidae | France | Champblanc Quarry, Cherves-de-Cognac | Early Cretaceous | Berriasian | [77] |
| cf. Arcodonichthys (tooth morphotype 7) | Family incertae sedis | France | Champblanc Quarry, Cherves-de-Cognac | Early Cretaceous | Berriasian | [77,80] |
| Pycnodontiformes indet. (tooth morphotype 8) | Family incertae sedis | France | Champblanc Quarry, Cherves-de-Cognac | Early Cretaceous | Berriasian | [77] |
| Pycnodontidae gen. et spp. Indet (tooth morphotype 9) | Pycnodontidae | France | Champblanc Quarry, Cherves-de-Cognac | Early Cretaceous | Berriasian | [77] |
| Pycnodontidae gen. et spp. Indet (tooth morphotype 10) | Pycnodontidae | France | Champblanc Quarry, Cherves-de-Cognac | Early Cretaceous | Berriasian | [77] |
| Pycnodontidae gen. et spp. Indet (tooth morphotype 11) | Family incertae sedis | France | Champblanc Quarry, Cherves-de-Cognac | Early Cretaceous | Berriasian | [77] |
| Coelodus | Pycnodontidae | England | Lulworth Formation | Early Cretaceous | Berriasian | [26,49] |
| Coelodus** | Pycnodontidae | England | Durlston Formation | Early Cretaceous | Berriasian | [26,49] |
| “Pycnodus” mantelli | Pycnodontidae | Germany | Gronau, North Rhine-Westphalia | Early Cretaceous | Berriasian | [29,60] |
| “Pycnodus” mantelli | Pycnodontidae | Germany | Osterwald/Otternhagen, Lower Saxony | Early Cretaceous | Berriasian | [29,58] |
| Turbomesodon cf. arcuatus | Pycnodontidae | Germany | Bückeberg Group, Istenberg Formation | Early Cretaceous | Berriasian | [29,63] |
| Turbomesodon cf. arcuatus | Pycnodontidae | Germany | North Rhine-Westphalia | Early Cretaceous | Berriasian | [29,60,61,62] |
| Pycnodontiformes indet. | Family incertae sedis | Germany | Bückeberg Group, Fuhse Formation | Early Cretaceous | Berriasian | [29,55,56] |
| Pycnodontiformes indet. | Family incertae sedis | Germany | Bückeberg Group, Istenberg Formation | Early Cretaceous | Berriasian | [29,57] |
| Pycnodontiformes indet. | Family incertae sedis | Germany | Bückeberg Group, Oesede Formation or Istenberg Formation | Early Cretaceous | Berriasian | [29,58] |
| Pycnodontiformes indet. | Family incertae sedis | Germany | Lobber Ort, Island of Rügen | Early Cretaceous | Berriasian | [29,59] |
| Turbomesodon arcuatus | Pycnodontidae | England | Middle Purbeck Limestone | Early Cretaceous | Berriasian | [29] |
| Proscinetes sp. cf. P. bernardi | Pycnodontidae | Spain | Lérida | Early Cretaceous | Berriasian | [93,129,130,131,132] |
| Ocloedus subdiscus** | Pycnodontidae | Spain | El Montsec, Lérida | Early Cretaceous | Berriasian-Valanginian | [48,89,101] |
| Turbomesodon laevidens | Pycnodontidae | England | Middle Purbeck Limestone | Early Cretaceous | Berriasian-Valanginian | [29] |
| Ocloedus | Pycnodontidae | England | Pevensey Pit, Ashdown Brickworks quarry (Wealden Group) | Early Cretaceous | Valanginian | [50,51,53] |
| Turbomesodon microdon | Pycnodontidae | England | Grinstead Clay Formation | Early Cretaceous | Valanginian | [29] |
| cf. Anomoeodus | Pycnodontidae | Thailand | Sao Khua Formation (Phu Phan Thong) | Early Cretaceous | Hauterivian-Barremian | [21] |
| Turbomesodon multidens | Pycnodontidae | England | Weald Clay Group, Sevenoaks, Kent | Early Cretaceous | Hauterivian-Barremian | [29] |
| Arcodonichthys pasiegae | Family incertae sedis | Spain | Vega de Pas Formation, Basque-Cantabrian Basin | Early Cretaceous | Hauterivian-Barremian | [80] |
| Pycnodontiformes indet. | Family incertae sedis | Spain | El Castellamar Formation | Early Cretaceous | Berriasian-Barremian | [107] |
| Stenamara mia | Pycnodontidae | Spain | Las Hoyas | Early Cretaceous | Barremian | [118] |
| Turbomesodon praeclarus | Pycnodontidae | Spain | Las Hoyas | Early Cretaceous | Barremian | [76] |
| Coelodus* | Pycnodontidae | England | Wessex (Wealden Group) | Early Cretaceous | Barremian | [46] |
| Pycnodontiformes indet. | Family incertae sedis | England | Wessex (Wealden Group) | Early Cretaceous | Barremian | [46] |
| Pycnodontidae gen. et spp. indet | Pycnodontidae | Spain | Camarillas Formation | Early Cretaceous | Barremian | [109] |
| cf. Proscinetes | Pycnodontidae | Spain | Camarillas Formation | Early Cretaceous | Barremian | [109] |
| Coelodus | Pycnodontidae | Spain | Camarillas Formation | Early Cretaceous | Barremian | [109] |
| Anomoeodus | Pycnodontidae | Spain | Camarillas Formation | Early Cretaceous | Barremian | Kriwet, pers. obser, |
| Pycnodontiformes indet. | Family incertae sedis | Spain | Artoles Formation | Early Cretaceous | Barremian | Kriwet, pers. obser, |
| cf. Ocloedus sp. 1 | Pycnodontidae | Spain | Huérgiuna Formation | Early Cretaceous | Barremian | [44] |
| cf. Ocloedus sp. 2 | Pycnodontidae | Spain | Huérgiuna Formation | Early Cretaceous | Barremian | [44] |
| Anomoeodus nursalli | Pycnodontidae | Spain | Huérgiuna Formation | Early Cretaceous | Barremian | [44] |
| Coelodus | Pycnodontidae | Spain | Aguilón | Early Cretaceous | Barremian | [93,129,130,131,132] |
| Turbomesodon bernissartensis | Pycnodontidae | Belgium | Bernissart | Early Cretaceous | Barremian-Aptian | [75,76] |
| “Palaeobalistum” geiseri | Pycnodontidae | USA | Twin Mountains Formation, Paluxy Church | Early Cretaceous | Aptian | [141] |
| “Proscinetes” texanus | Pycnodontidae | USA | Twin Mountains Formation, Paluxy Church | Early Cretaceous | Aptian | [30,141] |
| Coelodus | Pycnodontidae | USA | Twin Mountains Formation, Paluxy Church | Early Cretaceous | Aptian | [141] |
| Thurmondella estesi | Family incertae sedis | USA | Twin Mountains Formation, Paluxy Church | Early Cretaceous | Aptian | [141,145] |
| Pycnodontidae indet. | Pycnodontidae | USA | Cloverly Formation, Little Sheep Mudstone Member | Early Cretaceous | Aptian | [133] |
| Anomoeodus complanatus | Pycnodontidae | Spain | Cintorres, Valencia | Early Cretaceous | Aptian | [93,129,130,131,132] |
| Anomoeodus complanatus | Pycnodontidae | Spain | Mirambel, Tereul | Early Cretaceous | Aptian | [93,129,130,131,132] |
| Coelodus sp. aff. C. soleri | Pycnodontidae | Spain | Morella, Valencia | Early Cretaceous | Aptian | [93,129,130,131,132] |
| aff. Gyrodus | Gyrodontidae | Tunisia | Jebel Boulouha North | Early Cretaceous | Albian | [152] |
| Thurmondella estesi | Family incertae sedis | USA | Paluxy Formation, Stephenville Printing | Early Cretaceous | Albian | [30,141] |
| Texasensis coronatus | Pycnodontidae | USA | Paluxy Formation, Pecan Valley Estates | Early Cretaceous | Albian | [30,31,141] |
| “Macromesodon” dumblei | Family incertae sedis | USA | Paluxy Formation, Pecan Valley Estates | Early Cretaceous | Albian | [30,141] |
| Nonaphalgodus trinitiensis | Pycnodontidae | USA | Antlers Formation, Butler Farm | Early Cretaceous | Albian | [30,141] |
| Anomoeodus caddoi** | Pycnodontidae | USA | Holly Creek Formation | Early Cretaceous | Albian | [137] |
| Pycnodontiformes indet.** | Family incertae sedis | USA | Holly Creek Formation | Early Cretaceous | Albian | [137] |
| ?Pycnodontiformes indet.** | Family incertae sedis | Tunisia | Oued el Khil | Early Cretaceous | Albian | [152] |
| Pycnodontiformes indet. | Family incertae sedis | Brazil | Açu Formation, Potiguar Basin | Early Cretaceous | Albian | [151] |
| Anomoeodus sp. aff. A. muensteri | Pycnodontidae | Spain | Condemios de Abajo, Castilla-La-Mancha | Early Cretaceous | Albian | [93,129,130,131,132] |
| Pycnodontiformes indet. | Family incertae sedis | Spain | Ceceda, Asturias | Early Cretaceous | Albian | [93,129,130,131,132] |
| Neoproscinetes africanus | Pycnodontidae | Morocco | Kem Kem | Early-Late Cretaceous | Albian-Cenomanian | [47] |
| cf. Macromesodon | Pycnodontidae | Morocco | Kem Kem | Early-Late Cretaceous | Albian-Cenomanian | [47] |
| Agassizilia erfoudina | ?Pycnodontidae | Morocco | Kem Kem | Early-Late Cretaceous | Albian-Cenomanian | [47] |
| cf. Coelodus | Pycnodontidae | Morocco | Kem Kem | Early-Late Cretaceous | Albian-Cenomanian | [47] |
| Coelodus soleri | Pycnodontidae | Spain | Girona, Catalonia | Early-Late Cretaceous | Albian-Cenomanian | [93,129,130,131,132] |
| Pycnodontiformes indet.** | Family incertae sedis | Brazil | Alcântara Formation, Laje do Coringa | Late Cretaceous | Cenomanian | [221,223] |
| Pycnodontiformes indet.** | Family incertae sedis | Algeria | Continental Intercalaire, Guir Basin | Late Cretaceous | Cenomanian | [238] |
| Pycnodontidae gen. et spp. Indet 1 | Pycnodontidae | USA | Cedar Mountain Formation | Late Cretaceous | Cenomanian | [214] |
| Pycnodontidae gen. et spp. Indet 2 | Pycnodontidae | USA | Cedar Mountain Formation | Late Cretaceous | Cenomanian | [214] |
| Coelodus | Pycnodontidae | USA | Dakota Formation | Late Cretaceous | Cenomanian | [217] |
| Coelodus | Pycnodontidae | USA | Straight Cliffs Formation, Smoky Hollow Member | Late Cretaceous | Turonian | [217] |
| Pycnodontiformes indet./Pycnodontidae indet.** | ?Pycnodontidae | Austria | Gams, Schönleiten Formation | Late Cretaceous | Turonian | [192,194] |
| Coelodus | Pycnodontidae | USA | Straight Cliffs Formation, John Henry Member | Late Cretaceous | Coniacian | [217] |
| Xinjiangodus gyrodoides | Pycnodontidae | China | Donggou Formation, Junggar Basin | Late Cretaceous | Coniacian-Campanian | [163] |
| cf. Coelodus | Pycnodontidae | Hungary | Csehbánya Formation, Iharkút | Late Cretaceous | Santonian | [198.201] |
| Micropycnodon** | Family incertae sedis | USA | Straight Cliffs Formation, John Henry Member | Late Cretaceous | Santonian | [217] |
| Pycnodontiformes indet. | Family incertae sedis | Hungary | Ajka Formation | Late Cretaceous | Santonian | [203] |
| cf. Phacodus | Family incertae sedis | France | Villeveyrac - L’Olivet | Late Cretaceous | Campanian | [45] |
| Micropycnodon** | Family incertae sedis | USA | Wahweap Formation | Late Cretaceous | Campanian | [217] |
| Pycnodontidae gen. et spp. indet | Pycnodontidae | Bolivia | Chaunaca Formation | Late Cretaceous | Campanian | [232] |
| Pycnodontoidea gen. et spp. indet | Family incertae sedis | Spain | Lo Hueco (Cuenca) | Late Cretaceous | Campanian-Maastrichtian | [208,209,210,211] |
| Pycnodus lametae | Pycnodontidae | India | Lameta Formation (Maharashtra) | Late Cretaceous | Maastrichtian | [168,169,174] |
| cf. Coelodus** | Family incertae sedis | Spain | Els Neret | Late Cretaceous | Maastrichtian | [212] |
| cf. Coelodus** | Family incertae sedis | Spain | L’Espinau | Late Cretaceous | Maastrichtian | [212] |
| Pycnodontiformes indet.** | Family incertae sedis | Spain | Serrat del Rostiar-1 | Late Cretaceous | Maastrichtian | [212] |
| Pycnodontiformes indet.** | Family incertae sedis | Spain | Cami del Soldat | Late Cretaceous | Maastrichtian | [212] |
| Pycnodontiformes indet.** | Family incertae sedis | Spain | Fontllonga-6 | Late Cretaceous | Maastrichtian | [212] |
| ?Pycnodontiformes indet.** | Family incertae sedis | Spain | Fontllonga-6 | Late Cretaceous | Maastrichtian | [212] |
| ?Pycnodontiformes indet.** | Family incertae sedis | Spain | L’Espinau | Late Cretaceous | Maastrichtian | [212] |
| Pycnodontidae gen. et spp. indet | Pycnodontidae | India | Madhya Pradesh (Kisalpuri) | Late Cretaceous | Maastrichtian | [180] |
| Pycnodontidae gen. et spp. indet | Pycnodontidae | India | Lameta Formation (Pisdura) | Late Cretaceous | Maastrichtian | [169,175,176] |
| Pycnodus bicresta | Pycnodontidae | India | Naskal, Andhra Pradesh | Late Cretaceous | Maastrichtian | [181,182] |
| Pycnodontidae gen. et spp. indet** | Pycnodontidae | India | Chhindwara District, Madhya Pradesh | Late Cretaceous | Maastrichtian | [186] |
| Pycnodus | Pycnodontidae | India | Papro Formation, Uttar Pradesh | Late Cretaceous | Maastrichtian | [177] |
| “Pycnodus” lametae*** | Pycnodontidae | India | Naskal, Andhra Pradesh | Late Cretaceous | Maastrichtian | [183] |
| Pycnodus | Pycnodontidae | India | Lameta Formation (Madhya Pradesh) | Late Cretaceous | Maastrichtian | [178,179] |
| Pycnodus bicresta | Pycnodontidae | India | Asifabad | Late Cretaceous | Maastrichtian | [169,176,185,186] |
| Pycnodus lametae | Pycnodontidae | India | Nagpur | Late Cretaceous | Maastrichtian | [184] |
| Pycnodus lametae | Pycnodontidae | India | Asifabad | Late Cretaceous | Maastrichtian | [184,185] |
| Pycnodus lametae | Pycnodontidae | India | Rangapur | Late Cretaceous | Maastrichtian | [184] |
| Pycnodus cf. P. praecursor | Pycnodontidae | India | Asifabad | Late Cretaceous | Maastrichtian | [184] |
| Coelodus toncoensis** | Pycnodontidae | Argentina | Yacoraite Formation | Late Cretaceous | Maastrichtian | [224,225,226,227,228] |
| Pycnodontiformes indet.** | Family incertae | Argentina | Yacoraite Formation | Late Cretaceous | Maastrichtian | [231] |
| Coelodus toncoensis** | Pycnodontidae | Bolivia | El Molino Formation, Paja Patcha | Late Cretaceous | Maastrichtian | [234,235,236] |
| Coelodus** | Pycnodontidae | Bolivia | El Molino Formation, Paja Patcha | Late Cretaceous | Maastrichtian | [235] |
| Pycnodontidae gen. et spp. indet** | Pycnodontidae | Bolivia | El Molino Formation, Paja Patcha | Late Cretaceous | Maastrichtian | [234,235] |
| Pycnodontidae gen. et spp. indet** | Pycnodontidae | Bolivia | El Molino Formation, Vila Vascarra | Late Cretaceous | Maastrichtian | [237] |
| Coelodus | Pycnodontidae | Madagascar | Maevarano Formation, Anembalemba Member | Late Cretaceous | Maastrichtian | [242] |
| Pycnodus jonesae** | Pycnodontidae | Mali | Ménaka Formation, Iullemmeden Basin | Late Cretaceous | Maastrichtian | [239,240] |
| Pycnodus** | Pycnodontidae | Mali | Ménaka Formation, Iullemmeden Basin | Late Cretaceous | Maastrichtian | [239,240] |
| Pycnodus | Pycnodontidae | India | Jhilmili, Chhindwara District | Late Cretaceous-Palaeocene | Maastrichtian-Danian | [191] |
| Pycnodus** | Pycnodontidae | India | Fatehgarh Formation, Rajasthan | Late Cretaceous-Palaeocene | Maastrichtian-Selandian | [189,190] |
| Coelodus toncoensis** | Pycnodontidae | Argentina | Yacoraite Formation | Palaeocene | Danian | [225] |
| Pycnodontidae gen. et spp. indet** | Pycnodontidae | India | Rajahmundry | Palaeocene | Danian | [169,247,248] |
| Pycnodus cf. P. praecursor** | Pycnodontidae | India | Rajahmundry | Palaeocene | Danian | [184] |
| Pycnodus** | Pycnodontidae | India | Rajahmundry | Palaeocene | Danian | [184] |
| Pycnodus jonesae** | Pycnodontidae | Mali | Teberemt Formation, Gao Trench Basin | Palaeocene | Selandian-Thanetian | [239,240] |
| Pycnodus jonesae** | Pycnodontidae | Mali | Teberemt Formation, Iullemmeden Basin | Palaeocene | Thanetian | [239,240] |
| Pycnodus** | Pycnodontidae | Mali | Teberemt Formation, Iullemmeden Basin | Palaeocene | Thanetian | [240] |
| Pycnodus maliensis** | Pycnodontidae | Mali | Tamaguélelt Formation, Taoudenit Basin | Eocene | Ypresian | [239,240] |
| Pycnodus zeaformis** | Pycnodontidae | Mali | Tamaguélelt Formation, Taoudenit Basin | Eocene | Ypresian | [239,240] |
| Pycnodus** | Pycnodontidae | Mali | Tamaguélelt Formation, Taoudenit Basin | Eocene | Ypresian | [240] |
| Pycnodus** | Pycnodontidae | USA | Tallahata Formation | Eocene | Ypresian-Lutetian | [250] |
| Pycnodus** | Pycnodontidae | USA | Lisbon Formation | Eocene | Lutetian | [250] |
References
- Reid, G.M. Introduction to Freshwater Fishes and Their Conservation. Int. Zoo Yearb. 2013, 47, 1–5. [Google Scholar] [CrossRef]
- Oberdoff, T.; Guégan, J.; Hugueny, B. Global scale patterns of fish species richness in rivers. Ecography 1995, 18, 345–352. [Google Scholar] [CrossRef]
- Amarasinghe, U.S.; Welcomme, R.L. An Analysis of Fish Species Richness in Natural Lakes. Environ. Biol. Fishes 2002, 65, 327–339. [Google Scholar] [CrossRef]
- Horne, A.J.; Goldman, C. Limnology; McGraw-Hill: University of Minnesota, USA, 1994. [Google Scholar]
- Boyd, C.E. Water Quality: An Introduction, 2nd ed.; Springer: Auburn, USA, 2015. [Google Scholar]
- Dodds, W.K.; Whiles, M.R. Freshwater ecology: concepts and environmental applications of limnology; 2nd ed.; Academic Press: Cambridge, USA, 2010. [Google Scholar]
- Lundberg, J.G. The temporal context for the diversification of neotropical fishes. In Phylogeny and Classification of Neotropical Fishes; Malabarba, L.R., Reis, R.E., Vari, R.P., Lucena, C.A.S., Lucena, Z.M.S., Eds.; EDIPUCRS: University of California, USA, 1998; pp. 49–68. [Google Scholar]
- Berra, T.M. An atlas of distribution of the freshwater fish families of the world; University of Nebraska Press: Lincoln, USA, 1981. [Google Scholar]
- McDowall, R.M. The occurrence and distribution of diadromy among fishes. Am Fish Soc Symp 1987, 1, 1–13. [Google Scholar]
- Cavin, L. Freshwater fishes: 250 million years of evolutionary history; ISTE Press Ltd.: London, UK, 2017. [Google Scholar]
- Patterson, C. The distribution of mesozoic freshwater fishes, Biogéographie et liaisons intercontinentales au cours du Mésozoïque. Mém Mus Natl Hist 1975, 11, 156–174. [Google Scholar]
- Bogan, S.; Taverne, L.; Agnolin, F. First triassic and oldest record of a South American amiiform fish: Caturus sp. from the Los Menucos Group (lower Upper Triassic), Rio Negro province, Argentina. Geol Belg 2013, 16, 191–195. [Google Scholar]
- Cavin, L. Diversity of Mesozoic semionotiform fishes and the origin of gars (Lepisosteidae). Sci. Nat. 2010, 97, 1035–1040. [Google Scholar] [CrossRef]
- Egerton, P.d.M.G. XXXV. On chondrosteus, an extinct genus of the sturionidæ, found in the lias formation at Lyme Regis. Philos. Trans. R. Soc. Lond. 1858, 148, 871–885. [Google Scholar] [CrossRef]
- Hilton E., J.; Grande, L.; Bemis, W.E. Morphology of †Coccolepis bucklandi Agassiz, 1843 (Actinopterygii, †Coccolepidae) from the Solnhofen Lithographic Limestone deposits (Upper Jurassic, Germany). In Mesozoic Fishes 3 – Systematics, Paleoenvironments and Biodiversity; Arratia, G., Tintori, A., Eds.; Verlag Dr. Friedrich Pfeil: Munich, Germany, 2004; pp. 209–238. [Google Scholar]
- Cawley, J.J.; Marramà, G.; Carnevale, G.; Villafaña, J.A.; López-Romero, F.A.; Kriwet, J. Rise and fall of †Pycnodontiformes: Diversity, competition and extinction of a successful fish clade. Ecol Evol 2021, 11, 1769–1796. [Google Scholar] [CrossRef]
- Nursall, J.R. The case of pycnodont fishes as the fossil sister-group of teleosts. In Origin and phylogenetic interrelationships of teleosts; Nelson, J.S., Schultze, H.P., Wilson, M.V.H., Eds.; Verlag Dr. Friedrich Pfeil: Munich, Germany, 2010; Volume 121, pp. 329–343. [Google Scholar]
- Poyato-Ariza, F.J. Studies on pycnodont fishes (I): evaluation of their phylogenetic position among actinopterygians. 2015; 37–60. [Google Scholar] [CrossRef]
- Kriwet, J. A comprehensive study of pycnodont fishes (Neopterygii, Pycnodontiformes): morphology, taxonomy, functional morphology, phylogeny, and palaeobiogeography. Unpublished PhD thesis, Humboldt University, Berlin, 2001.
- Poyato-Ariza, F.J. Pycnodont fishes: morphologic variation, ecomorphologic plasticity, and a new interpretation of their evolutionary history. Bull Kitakyushu Mus Nat Hist Hum Hist, Ser A 2005, 3, 169–184. [Google Scholar]
- Cavin, L.; Deesri, U.; Suteethorn, V. The Jurassic and Cretaceous bony fish record (Actinopterygii, Dipnoi) from Thailand. In Late Palaeozoic and Mesozoic Ecosystems in SE Asia; Buffetaut, E., Cuny, G., Le Loeuff, J., Suteethorn, V., Eds.; Geological Society: London, UK, 2009; pp. 125–139. [Google Scholar]
- Buffetaut, E.; Raksaskulwong, L.; Suteethorn, V.; Tong, H. First post-Triassic temnospondyl amphibians from the Shan-Thai block: intercentra from the Jurassic of peninsular Thailand. Geol. Mag. 1994, 131, 837–839. [Google Scholar] [CrossRef]
- Buffetaut, E.; Tong, H.; Suteethorn, V.; Raksaskulwong, L. Jurassic vertebrates from the southern peninsula of Thailand and their implications. In A preliminary report. In Proceedings of the International Symposium on Stratigraphic Correlation of Southeast Asia, Bangkok, Bangkok, Thailand, 15-20 November 1994; Angsuwathana, P., Wongwanich, T., Tansathien, W., Wongsomak, S., Tulyatid, S., Tulyatid, J., Eds.; Department of Mineral Resources: Bangkok, Thailand, 1994; pp. 253–256. [Google Scholar]
- Tong, H.; Buffetaut, E.; Suteethorn, V. Middle Jurassic turtles from southern Thailand. Geol. Mag. 2002, 139, 687–697. [Google Scholar] [CrossRef]
- Young, C.C.; Liu, H. Tibetodus, a new pycnodont fish from Changtu (in Chinese with English summary). Acta Palaeo Sin 1954, 2, 95–102. [Google Scholar]
- Schaeffer, B.; Patterson, C. Jurassic fishes from the western United States, with comments on Jurassic fish distribution. Am Mus Novit 1984, 2796, 1–86. [Google Scholar]
- Kirkland, J.I. Morrison fishes. Mod Geol 1998 22, 503–533.
- Woodward, A.S. The Fossil Fishes of the English Wealden and Purbeck Formations; Cambridge University Press (CUP): Cambridge, United Kingdom, 2014; ISBN 9781108076944. [Google Scholar]
- Hornung, J. Pycnodonte Fische (Actinopterygii: Pycnodontiformes) in der Unterkreide von Norddeutschland - Diversität und palökologische Beziehungen -- Pycnodont fishes (Actinopterygii: Pycnodontiformes) from the Lower Cretaceous of northern Germany - diversity and palecological relationships. Ber Naturwiss Ver Bielef Umgeg 2021, 58, 4–77. [Google Scholar]
- Thurmond, J.T. Lower vertebrate faunas of the Trinity Division in north-central Texas. Geosci Man 1974, 8, 103–129. [Google Scholar]
- zdikmen, H. Texasensis nom. nov., a new name for the preoccupied fossil fish genus Callodus Thurmond, 1974 (Osteichthyes: Pycnodontiformes). Mun Ent Zool 2009, 4, 616. [Google Scholar]
- Taverne, L. A horny pycnodont fish (Pycnodontiformes) in the continental Middle Jurassic (Stanleyville Formation) of the Democratic Republic of Congo. Geo-Eco-Trop 2019, 43, 25–34. [Google Scholar]
- Myers, T.S.; Tabor, N.J.; Jacobs, L.L. Late Jurassic paleoclimate of Central Africa. Palaeogeogr. Palaeoclim. Palaeoecol. 2011, 311, 111–125. [Google Scholar] [CrossRef]
- Caillaud, A.; Blanpied, C.; Delvaux, D. The Upper Jurassic Stanleyville Group of the eastern Congo Basin: An example of perennial lacustrine system. J. Afr. Earth Sci. 2017, 132, 80–98. [Google Scholar] [CrossRef]
- Taverne, L.; Capasso, L. Gladiopycnodontidae, a new family of pycnodontiform fishes from the Late Cretaceous of Lebanon, with the description of three genera. Eur. J. Taxon. 2013. [Google Scholar] [CrossRef]
- Gayet, M. Ichthyoceros spinosus nov. gen., nov. sp., du Cénomanien inférieur de Hakel (Liban) et ses affinités avec le genre Trewavasia (Pisces, Pycnodontiformes, Coccodontidae). Bull Mus Natl Hist Nat 4C 1984, 3, 287–307. [Google Scholar]
- Taverne, L.; Capasso, L. Ostéologie et phylogénie des Coccodontidae, une famille remarquable de poissons Pycnodontiformes du Crétacé supérieur marin du Liban, avec la description de deux nouveaux genres. Palaeontos 2014, 25, 3–43. [Google Scholar]
- Nursall, J.R.; Capasso, L. Gebrayelichthys (novum), an extraordinary genus of neopterygian fishes from the Cenomanian of Lebanon. In Mesozoic Fishes 3: Systematics, Paleoenvironments and Biodiversity; Arratia, G., Tintori, A., Eds.; Verlag Dr. Friedrich Pfeil: Munich, Germany, 2004; pp. 317–340. [Google Scholar]
- Goodwin, M.B.; Clemens, W.A.; Hutchison, J.H.; Wood, C.B.; Zavada, M.S.; Kemp, A.; Duffin, C.J.; Schaff, C.R. Mesozoic continental vertebrates with associated palynostratigraphic dates from the northwestern Ethiopian plateau. J. Vertebr. Paléontol. 1999, 19, 728–741. [Google Scholar] [CrossRef]
- Wolela, A. Sedimentation and depositional environments of the Barremian-Cenomanian Debre Libanose Sandstone, Blue Nile (Abay) Basin, Ethiopia. Cretac. Res. 2009, 30, 1133–1145. [Google Scholar] [CrossRef]
- Getaneh, W.; Atnafu, B. Geochemistry and lithostratigraphy of the mugher mudstone: Insights into the late jurassic-early cretaceous clastic sedimentation in Ethiopia and its surroundings. J. Afr. Earth Sci. 2020, 164, 103770. [Google Scholar] [CrossRef]
- Cawley, J.J.; Marrama, G.; Carnevale, G.; Kriwet, J. A quantitative approach to determine the taxonomic identity and ontogeny of the pycnodontiform fish Pycnodus (Neopterygii, Actinopterygii) from the Eocene of Bolca Lagerstätte, Italy. PeerJ 2018, 6, e4809. [Google Scholar] [CrossRef] [PubMed]
- Cuny, G. 2002. Requins d'eau douce, actuels et fossiles. PSci 2002, 295, 30–36. [Google Scholar]
- Kriwet, J. Pycnodont fishes. In Mesozoic Fishes 2: Systematics and Fossil Record; Arratia, G., Schultze, H.P., Eds.; Verlag Dr. Friedrich Pfiel: Munich, Germany, 1999; pp. 215–238. [Google Scholar]
- Cavin, L.; Garcia, G.; Valentin, X. A minute freshwater pycnodont fish from the Late Cretaceous of southern France: Palaeoecological implications. Cretac. Res. 2020, 106. [Google Scholar] [CrossRef]
- Sweetman, S.C.; Goedert, J.; Martill, D.M. A preliminary account of the fishes of the Lower Cretaceous Wessex Formation (Wealden Group, Barremian) of the Isle of Wight, southern England. Biol. J. Linn. Soc. 2014, 113, 872–896. [Google Scholar] [CrossRef]
- Cooper, S.L.; Martill, D.M. A diverse assemblage of pycnodont fishes (Actinopterygii, Pycnodontiformes) from the mid-Cretaceous, continental Kem Kem Group of south-east Morocco. Cretac. Res. 2020, 112, 104456. [Google Scholar] [CrossRef]
- Poyato-Ariza, F.J.; Wenz, S. A new insight into pycnodontiform fishes. Geodiversitas 2002, 24, 139–248. [Google Scholar]
- Coram, R.A.; Radley, J.D. Revisiting climate change and palaeoenvironments in the Purbeck Limestone Group (Tithonian – Berriasian) of Durlston Bay, southern UK. Proc. Geol. Assoc. 2021, 132, 392–404. [Google Scholar] [CrossRef]
- Austen, P.; Brockhurst, D.; Honeysett, K. Vertebrate fauna from Ashdown brickworks, Bexhill, east Sussex. Wealden News 2010, 8, 13–23. [Google Scholar]
- Naish, D.; Sweetman, S.C. A tiny maniraptoran dinosaur in the Lower Cretaceous Hastings Group: Evidence from a new vertebrate-bearing locality in south-east England. Cretac. Res. 2011, 32, 464–471. [Google Scholar] [CrossRef]
- Allen, P. Wealden of the Weald: a new model. Proc. Geol. Assoc. 1975, 86, 389–437. [Google Scholar] [CrossRef]
- Turmine-Juhel, P.; Wilks, R.; Brockhurst, D.; Austen, P.A.; Duffin, C.J.; Benton, M.J. Microvertebrates from the Wadhurst Clay Formation (Lower Cretaceous) of Ashdown Brickworks, East Sussex, UK. Proc. Geol. Assoc. 2019, 130, 752–769. [Google Scholar] [CrossRef]
- Hayward, R.J. The Geology of part of the Wadhurst Clay Formation and part of the Tunbridge Wells Formation at the Ashdown Brickworks, Bexhill, Sussex. Unpublished B.Sc. dissertation, University of Greenwich, London, 1996.
- Struckmann, C. Die Wealdenbildungen von Sehnde bei Lehrte. Neues Jahrb Mineral Geol Palaeontol 1891, 1891, 117–131. [Google Scholar]
- Jordan, R. Die Ziegeleitongrube westlich Wätzum, Blatt Sarstedt, an der Ostflanke des Salzstockes von Sarstedt-Lehrte (Dogger, Wealden, marine Unterkreide). Ber Naturhist Ges Hann 1959, 104, 5–24. [Google Scholar]
- Struckmann, C. Geognostische Studien am Deister. Jahresber Naturhist Ges Hann 1880, 29/30, 60–75.
- Dunker, W. Monographie der Norddeutschen Wealdenbildung. Ein Beitrag zur Geognosie und Naturgeschichte der Vorwelt. Oehme und Müller: Rome, Italy, 1846; 83 pp.
- Ansorge, J. Fischreste (Selachii, Actinopterygii) aus der Wealdentonscholle von Lobber Ort (Mönchgut/Rügen/DDR). PalZ 1990, 64, 133–144. [Google Scholar] [CrossRef]
- Nyhuis, C.; Herbig, H.G. Ichthyolithe aus dem Berriasium von Gronau/Westfalen (westliches niedersächsisches Kreidebecken) – Rekonstruktion einer trophischen Kette aus sturmkondensierten Bonebeds. Terra Nostra 2009, 2009, 85. [Google Scholar]
- Kemper, E. Geologischer Führer durch die Grafschaft Bentheim und die angrenzenden Gebiete. 3rd edition. Verlag Heimatverein der Grafschaft Bentheim: Nordhorn, Germany, 1968; 172 pp.
- Kemper, E. Geologischer Führer durch die Grafschaft Bentheim und die angrenzenden Gebiete mit Abriss einem der emsländischen Unterkreide. Verlag Heimatverein der Grafschaft Bentheim: Nordhorn, Germany, 1976; 206 pp.
- Kriwet, J. A comprehensive study of the skull and dentition of pycnodont fishes. Zitteliana 2005, 45, 135–188. [Google Scholar]
- Olive, S.; Taverne, L.; López-Arbarello, A. A new genus of coccolepidid actinopterygian from the Cretaceous Iguanodon-bearing locality of Bernissart, Belgium. Cretac. Res. 2018, 95, 318–335. [Google Scholar] [CrossRef]
- Cavin, L.; Deesri, U.; Olive, S. Scheenstia bernissartensis (Actinopterygii: Ginglymodi) from the Early Cretaceous of Bernissart, Belgium, with an appraisal of ginglymodian evolutionary history. J Syst Palaeontol 2020, 18, 513–527. [Google Scholar] [CrossRef]
- Cornet, J.; Schmitz, G. Note sur les puits naturels du terrain houiller du Hainaut et le gisement des iguanodons de Bernissart-Bull. Bull Soc belge Géol Paléontol Hydrol 1898, 12, 301–318. [Google Scholar]
- Cornet, J. L’époque wealdienne dans le Hainaut. Bull Soc belge Géol Paléontol Hydrol 1927, 50, 89–104. [Google Scholar]
- Yans, J. Lithostratigraphie, minéralogie et diagenèse des sédiments à faciès wealdien du Bassin de Mons (Belgique). Académie royale de Belgique: Brussels, Belgium, 2007; 179 pp.
- Schnyder, J.; Dejax, J.; Keppens, E.; Tu, T.T.N.; Spagna, P.; Boulila, S.; Galbrun, B.; Riboulleau, A.; Tshibangu, J.-P.; Yans, J. An Early Cretaceous lacustrine record: Organic matter and organic carbon isotopes at Bernissart (Mons Basin, Belgium). Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 281, 79–91. [Google Scholar] [CrossRef]
- Spagna, P. Les faciès wealdiens du Bassin de Mons (Belgique): paléoenvironnements, géodynamique et valorisation industrielle. Unpublished PhD thesis, Faculté Polytechnique de l'Umons, Mons, 2010.
- Spagna, P.; Yans, J.; Schnyder, J.; Dupuis, C. The Paleoenvironment of the Bernissart Iguanodons: Sedimentological Analysis of the Lower Cretaceous Wealden Facies in the Bernissart Area. In Bernissart Dinosaurs and Early Terrestrial Ecosystems; Godefroit, P., Ed.; Indiana University Press: Bloomington, USA, 2012; pp. 87–96. [Google Scholar]
- Dejax, J.; Pons, D.; Yans, J. Palynology of the dinosaur-bearing Wealden facies in the natural pit of Bernissart (Belgium). Rev. Palaeobot. Palynol. 2007, 144, 25–38. [Google Scholar] [CrossRef]
- Yans, J.; Dejax, J.; Pons, D.; Taverne, L.; Bultynck, P. The iguanodons of Bernissart (Belgium) are middle Barremian to earliest Aptian in age. Bull Inst R Sci Nat Belg Sci Terre 2006, 76, 91–95. [Google Scholar]
- Yans, J.; Dejax, J.; Schnyder, J. On the age of the Bernissart Iguanodons. In Bernissart Dinosaurs and Early Terrestrial Ecosystems; Godefroit, P., Ed.; Indiana University Press: Bloomington, USA, 2012; pp. 79–86. [Google Scholar]
- Traquair, R.H. Les poissons wealdiens de Bernissart. Mém Mus R Hist Nat Belg 1911, 6, 1–65. [Google Scholar]
- Poyato-Ariza, F.J.; Wenz, S. The new pycnodontid fish genus Turbomesodon, and a revision of Macromesodon based on new material from the Lower Cretaceous of Las Hoyas, Cuenca, Spain. In Mesozoic Fishes 3: Systematics, Paleoenvironments and Biodiversity; Arratia, G., Tintori, A., Eds.; Verlag Dr. Friedrich Pfeil: Munich, Germany, 2004; pp. 341–378. [Google Scholar]
- Pouech, J.; Mazin, J.-M.; Cavin, L.; Poyato-Ariza, F.J. A Berriasian actinopterygian fauna from Cherves-de-Cognac, France: Biodiversity and palaeoenvironmental implications. Cretac. Res. 2015, 55, 32–43. [Google Scholar] [CrossRef]
- Mazin, J.M.; Pouech, J.; Hantzpergue, P.; Lenglet, T. The Purbeckian site of Cherves-de-Cognac (Berriasian, Early Cretaceous, SW France): a first synthesis. In Mid-Mesozoic Life and Environments Symposium, Cognac, France, 24-28 June 2008; Mazin, J.M., Pouech, J., Hantzpergue, P., Lacombe, V.., Eds.; Documents des Laboratoires de Géologie de Lyon: Cognac, France, 2008; pp. 68–71. [Google Scholar]
- Pouech, J. Position des mammifères dans les écosystèmes mésozoïques d'Europe Occidentale: le gisement de Cherves-de-Cognac (Berriasien, Charente, France). Unpublished PhD thesis, Claude Bernard University Lyon 1, Lyon, 2008.
- Poyato-Ariza, F.J.; Bermúdez-Rochas, D.D. New pycnodont fish (Arcodonichthys pasiegaegen. et sp. nov.) from the Early Cretaceous of the Basque-Cantabrian Basin, northern Spain. J. Vertebr. Paléontol. 2009, 29, 271–275. [Google Scholar] [CrossRef]
- Cooper, S.L.; Martill, D.M. Pycnodont fishes (Actinopterygii, Pycnodontiformes) from the Upper Cretaceous (lower Turonian) Akrabou Formation of Asfla, Morocco. Cretac. Res. 2020, 116, 104607–104607. [Google Scholar] [CrossRef] [PubMed]
- Pouech, J.; Amiot, R.; Lécuyer, C.; Mazin, J.-M.; Martineau, F.; Fourel, F. Oxygen isotope composition of vertebrate phosphates from Cherves-de-Cognac (Berriasian, France): Environmental and ecological significance. Palaeogeogr. Palaeoclim. Palaeoecol. 2014, 410, 290–299. [Google Scholar] [CrossRef]
- Kriwet, J. Dental morphology of the pycnodontid fish †Stemmatodus rhombus (Agassiz 1844) (Neopterygii, {Pycnodontiformes) f rom the Early Cretaceous, with comments on its systematic position. Trans R Soc Edinb Earth Sci 2004, 94, 145–155. [Google Scholar] [CrossRef]
- Bermúdez-Rochas, D.D.; Delvene, G.; Moratalla, J.; Hernán, J.; De La Fuente, M. Primeros datos paleontológicos del yacimiento del Cretácico Inferior Vega de Pas 1 (Cuenca Vasco-Cantábrica, Cantabria, España). In II Semana de Jóvenes Investigadores del IGME; Bermúdez, D.D., Najarro, M., Quesada, C., Eds.; Publicaciones del Instituto Geológico y Minero de España: Madrid, Spain, 2007; pp. 23–28. [Google Scholar]
- Darras, L.P.G. The evolution of macroecological consequences of grazing and shell-crushing in fishes. Unpublished PhD thesis, University of Leicester, Leicester, 2012.
- Kriwet, J. Feeding mechanisms and ecology of pycnodont fishes (Neopterygii, Pycnodontiformes). Foss. Rec. 2001, 4, 139–165. [Google Scholar] [CrossRef]
- Kriwet, J. Late Jurassic elasmobranch and actinopterygian fishes from Portugal and Spain. Cuad Geol Ibérica 1998, 24, 241–260. [Google Scholar]
- Gómez-Alba, J. Catálogo razonado de los vertebrados fósiles de España del Museo de Geología de Barcelona (1882-1982). Treb Mus Geol Barc 1997, 6, 1–296. [Google Scholar]
- Kriwet, J.; Poyato-Ariza, F.J.; Wenz, S. A revision of the pycnodontid fish Coelodus subdiscus Wenz 1989, from the Early Cretaceous of Montsec (Lleida, Spain). Treb Mus Geol Barc 1999, 8, 33–65. [Google Scholar]
- Vidal, L.M. Nota sobre la presencia del tramo Kimeridgense en el Montsech (Lérida) y hallazgo de un batracio en sus hiladas. Mems R Acad Cienc Artes Barcelona 1902, 4, 1–12. [Google Scholar]
- Peybernés, B.; Oertli, H. La série de passage du Jurassique au Crétacé dans le Bassin sud-pyrénéen (Espagne). C R Acad Sci 1972, 274, 3348–3351. [Google Scholar]
- Brenner, P. Ostracoden und Charophyten des spanischen Wealden (Systematik, Gkologie, Stratigraphie, Palaogeographie). Palaeontogr A 1976, 152, 113–201. [Google Scholar]
- Gomez Pallerola, J.E. Nuevas aportaciones a la ictiofauna y a la flora del Neocomiense del Montsech de Rubies (Lérida). Bol Geol Min 1982, 93, 199–213. [Google Scholar]
- Gomez Pallerola, J.E. Nuevos Hybodóntidos del Cretácio Inferior de Santa Maria de Meyá (Lérida). Bol Geol Min 1985, 96, 372–380. [Google Scholar]
- Gomez Pallerola, J.E. Nota sobre los tiburones hybodontos de las calizas litográficas del Cretácio Inferior del Motsech (Lérida). Bol Geol Min 1992, 103, 783–813. [Google Scholar]
- Wenz, S. Note preliminaire sur la faune ichthyologique du Jurassique superieur du Montsech (Espagne). BSGF - Earth Sci. Bull. [CrossRef]
- Wenz, S. Les Amiidés (Pisces, Halecomorphi) du Crétacé inférieur du Montsec (province de Lérida, Espagne): Amiopsis woodwardi (Sauvage, 1903). Quaderns Institut d’Estudis Ilerdencs 1988, 1, 1–50. [Google Scholar]
- Wenz, S. Peixos del Cretaci Inferior de la Serra del Montsec (Espanya). In Les calcàries litogràfiques del Cretaci inferior del Montsec. Deu anys de campanyes paleontològiques; Martínez-Delclòs, X., Ed.; Institut d'Estudis Ilerdencs: Lleida, Spain, 1991; pp. 111–132. [Google Scholar]
- Poyato-Ariza, F.J. Teleósteos primitivos del Cretácico inferior español: órdenes Elopiformes y Gonorhynchiformes. Unpublished. PhD Thesis, Universidad Autónoma, Madrid, 1991. [Google Scholar]
- Wenz, S.; Poyato-Ariza, F.J. Les actinoptérygiens juvéniles du Crétacé inférieur du Montsec et de Las Hoyas (Espagne). Geobios 1994, 27, 203–212. [Google Scholar] [CrossRef]
- Wenz, S. Une nouvelle espèce de Coelodus (Pisces, Pycnodontiformes) du Crétacé inférieur du Montsech (Province de Lérida, Espagne): Coelodus subdiscus n. sp. Geobios 1989, 22, 515–520. [Google Scholar] [CrossRef]
- Martínez-Delclos, X.; Nel, A. Arthropods. In Motsec and Montral-Alcover, Two Konservat-Lagerstätten, Catalonia, Spain; Martínez-Delclos, X., Ed.; Institut d'Estudis Ilerdencs: Lleida, Spain, 1995; pp. 39–46. [Google Scholar]
- de Gibert, J.; Fregenal-Martı́nez, M.; Buatois, L.; Mángano, M. Trace fossils and their palaeoecological significance in Lower Cretaceous lacustrine conservation deposits, El Montsec, Spain. Palaeogeogr. Palaeoclim. Palaeoecol. 2000, 156, 89–101. [Google Scholar] [CrossRef]
- Schairer, G.; Janicke, V. Sedimentologisch-paläontologische Untersuchungen an den Plattenkalken der Sierra de Montsech (Prov. Lérida. NE-Spanien). Neues Jahrb Geol Paläont Abh 1970, 135, 171–189. [Google Scholar]
- Schultze, H.P. The fossil record of the intertidal zone. In Intertidal fishes: Life in two worlds; Horn, M.H., Martin, K.L.M., Chotkowski, M.A., Eds.; Academic Press: San Diego, 1999; pp. 373–392. [Google Scholar]
- Cuenca-Bescós, G.; Amo, O.; Aurell, M.; Buscalioni, A.D.; Canudo, J.I.; Laplana, C.; Pérez Oñate, J.; Ruiz Omeñaca, J.I.; Sanz, J.L.; Soria, A.R. Los vertebrados del tránsito Jurásico-Cretácico de Galve (Teruel). In Comunicaciones de las X Jornadas Paleontología, Madrid, Spain, 3-; Sociedad Española de Paleontología, Madrid, Spain, 1994; pp. 50–53. 5 November.
- Sánchez-Hernández, B.; Benton, M.J.; Naish, D. Dinosaurs and other fossil vertebrates from the Late Jurassic and Early Cretaceous of the Galve area, NE Spain. Palaeogeogr Palaeoclimatol Palaeoecol 2007, 249, 180–215. [Google Scholar] [CrossRef]
- Díaz, M.; Yébenes, A. La sedimentación litoral y continental durante el Cretácico Inferior. Sinclinal de Galve, Teruel. Estud Geol 1987, 43, 3–21. [Google Scholar]
- Soria, A.R.; Meléndez, A.; Cuenca-Bescós, G.; Canudo, J.I.; Liesa, C.L. Los sistemas lacustres del Cretácico Inferior de la Cordillera Ibérica Central: la Cubeta de Aliaga. In XIII Congreso Español de Sedimentología. Guía de Excursiones, Zaragoza, Spain, 28-30 June 1995; Meléndez, A., Aurell, M., Eds.; Universidad de Zaragoza: Zaragoza, Spain, 1995; pp. 91–141. [Google Scholar]
- Kriwet, J. Neoselachier (Pisces, Elasmobranchii) aus der Unterkreide (unteres Barremium) von Galve und Alcaine (Spanien, Provinz Teruel). Palaeo Ichthyol 1999, 9, 113–142. [Google Scholar]
- Henkel, S.; Krebs, B. Zwei Säugetier-Unterkiefer aus der Unteren Kreide von Uña (Prov. Cuenca, Spanien). Neues Jahrb Geol Paläont Mh 1969, 1969, 449–463. [Google Scholar]
- Krebs, B. The search for Mesozoic Mammals in Spain and Portugal. Mesozoic Vert Life 1980, 1, 23–25. [Google Scholar]
- Krebs, B. The Barremian vertebrate locality Uña (Province of Cuenca) materials for a comparison with Las Hoyas. In 2nd International Symposium on Lithographic Limestones, Lleida - Cuenca (Spain), Extended Abstracts, Madrid, Spain, 9-; Melendez, N., Ed.; Ediciones de la Universidad Autónoma de Madrid; Madrid, Spain, 1995, pp. 95–97. 16 July.
- Geyer, O.F. , Krautter, M. Die Unterkreide im Grenzgebiet von Aragonien, Neukastilien und Valencia (südwestliches Keltiberikum, Spanien). Profil 1998, 15, 163–239. [Google Scholar]
- Sanz, J.L.; Wenz, S.; Yebenes, A.; Estes, R.; Martinez-Delclos, X.; Jimenez-Fuentes, E.; Diéguez, C.; Buscalioni, A.D.; Barbadillo, L.J.; Via, L. An Early Cretaceous faunal and floral continental assemblage:Las Hoyas fossil site (Cuenca, Spain). Geobios 1988, 21, 611–635. [Google Scholar] [CrossRef]
- Poyato-Ariza, F.J.; Wenz, S. Ichthyofauna. In Las Hoyas. A Lacustrine Konservat-Lagerstätte, Cuenca, Spain. Field trip guide book of the II International Symposium on Lithographic Limestones; Meléndez, N., Ed.; Universidad Complutense de Madrid: Madrid, Spain, 1995; pp. 43–49. [Google Scholar]
- Poyato-Ariza, F.J. Palaeoecology of the fishes from the Early Cretaceous lake of Las Hoyas Cuenca, Spain, with a hypothesis of sexual dimorphism for the Chanidae Rubiesichthys. Bull Kitakyushu Mus Nat Hist Hum Hist, Ser A 2005, 3, 153–168. [Google Scholar]
- Poyato-Ariza, F.J.; Wenz, S. A new pycnodontiform fish from the Early Cretaceous of Las Hoyas (Cuenca, Spain). BSGF - Earth Sci. Bull. 2000, 171, 251–258. [Google Scholar] [CrossRef]
- Poyato-Ariza, F.J.; Talbot, M.R.; Fregenal-Martı́nez, M.A.; Meléndez, N.; Wenz, S. First isotopic and multidisciplinary evidence for nonmarine coelacanths and pycnodontiform fishes: palaeoenvironmental implications. Palaeogeogr. Palaeoclim. Palaeoecol. 1998, 144, 65–84. [Google Scholar] [CrossRef]
- Wenz, S.; Poyato-Ariza, F.J. Les Actinoptérygiens juvéniles du Crétacé inférieur du Montsec et de Las Hoyas. Geobios Mem Spec 1994, 16, 203–212. [Google Scholar] [CrossRef]
- Wenz, S.; Poyato-Ariza, F.J. Pycnodontiform fishes from the early Cretaceous of Las Hoyas (Spain). In Extended abstracts: II International Symposium on Lithographic Limestones, Cuenca, Spain, July 1995, Cuenca, Spain, 9-; Ediciones de la Universidad Autonoma de Madrid, Madrid, Spain, 1995; pp. 157–161. 16 July.
- Martín-Closas, C.; Diéguez, C. Charophytes from the Lower Cretaceous of the Iberian ranges (Spain). Palaeontology 1998, 41, 1133–1152. [Google Scholar]
- Tintori, A.; Renesto, S. THE MACROSEMIIDAE (PISCES, ACTINOPTERYGII) FROM THE UPPER TRIASSIC OF LOMBARDY (N. ITALY). Riv. Ital. DI Paléontol. E Strat. 2020, 89. [Google Scholar] [CrossRef] [PubMed]
- Capasso, L.L.; Abi Saad, P.; Taverne, L. Nursallia tethysensis sp. nov., a new pycnodont fish (Neopterygii:† Halecostomi) from the Cenomanian of Lebanon. Bull Inst R Sci Nat Belg Sci Terre 2009, 79, 117–136. [Google Scholar]
- Ebert, M. A new genus of Pycnodontidae (Actinopterygii) from the Upper Jurassic of France and Germany, included in a phylogeny of Pycnodontiformes. Zoo, 2019. [Google Scholar] [CrossRef]
- Vullo, R.; Bardet, N.; Gheerbrant, E.; Jalil, N.-E. Multicuspid tooth morphology in a gigantic Palaeocene pycnodont fish: evolutionary and palaeoecological significance. Geol. Mag. 2019, 156, 1618–1622. [Google Scholar] [CrossRef]
- Baines, D.C. Tooth Microwear in Fishes. Unpublished PhD thesis, University of Leicester, Leicester, 2010.
- De Gibert, J.M.; Buatois, L.A.; Fregenal-Martínez, M.A.; Mángano, M.G.; Ortega, F.; Poyato-Ariza, F.J.; Wenz, S. The fish trace fossil Undichna from the Cretaceous of Spain. Palaeontology 1999, 42, 409–427. [Google Scholar] [CrossRef]
- Bauzá Rullán, J. Contribución al conocimiento de la fauna ictiológica de Cataluña. Estud Geol 1948, 8, 241–242. [Google Scholar]
- Bauzá Rullán, J. Contribución a la fauna ictiológica de España. Brev Geol Asturica 1957, 1, 7–19. [Google Scholar]
- Bataller, J.R. Los vertebrados del Cretácico español. Not Comunic Inst Geol Miner España 1960, 60, 141–164. [Google Scholar]
- Soría, A.R. La sedimentación en las cuencas marginales del surco Ibérico durante el Cretácico Inferior y su control estructural. Unpublished PhD thesis, Universidad de Zaragoza, Zaragoza, 1996.
- Oreska, M.P.; Carrano, M.T.; Dzikiewicz, K.M. Vertebrate paleontology of the Cloverly Formation (Lower Cretaceous), I: faunal composition, biogeographic relationships, and sampling. J Vertebr Paleontol 2013, 33, 264–292. [Google Scholar] [CrossRef]
- D'Emic, M.D.; Foreman, B.Z.; Jud, N.A.; Britt, B.B.; Schmitz, M.; Crowley, J.L. Chronostratigraphic Revision of the Cloverly Formation (Lower Cretaceous, Western Interior, USA). Bull. Peabody Mus. Nat. Hist. 2019, 60, 3–40. [Google Scholar] [CrossRef]
- Rogers, R.R.; Brady, M.E. Origins of microfossil bonebeds: insights from the Upper Cretaceous Judith River Formation of north-central Montana. Paleobiology 2010, 36, 80–112. [Google Scholar] [CrossRef]
- Moberly, R. Morrison, Cloverly, and Sykes Mountain formations, northern Bighorn Basin, Wyoming and Montana. GSA Bull. 1960, 71, 1137–1176. [Google Scholar] [CrossRef]
- Suarez, C.A.; Frederickson, J.; Cifelli, R.L.; Pittman, J.G.; Nydam, R.L.; Hunt-Foster, R.K.; Morgan, K. A new vertebrate fauna from the Lower Cretaceous Holly Creek Formation of the Trinity Group, southwest Arkansas, USA. PeerJ 2021, 9, e12242. [Google Scholar] [CrossRef]
- Frucci, M.N. 2018, Oxygen isotopic composition of aquatic reptile phosphate from the Early Cretaceous Holly Creek Formation, southern Arkansas. Unpublished B.S. Honors Thesis, University of Arkansas, Fayetteville, 2018. [Google Scholar]
- Thurmond, J.T. Lower vertebrates and paleocology of the Trinity Group (Lower Cretaceous) in North Central Texas; Southern Methodist University: Southern Methodist University, USA, 1969. [Google Scholar]
- Winkler, D.A.; Murry, P.A.; Jacobs, L.L. Vertebrate paleontology of the Trinity Group, Lower Cretaceous of central Texas. In Field guide to the vertebrate paleontology of the Trinity Group, Lower Cretaceous of central Texas; Winkler, D.A., Murry, P.A., Jacobs, L.L., Eds.; Institute for the Study of the Earth and Man: Dallas, USA, 1989; pp. 1–22. [Google Scholar]
- Winkler, D.A.; Murry, P.A.; Jacobs, L.L. Early Cretaceous (Comanchean) vertebrates of central Texas. J. Vertebr. Paléontol. 1990, 10, 95–116. [Google Scholar] [CrossRef]
- Cifelli, R.L.; Gardner, J.D.; Nydam, R.L.; Brinkman, D.L. Additions to the vertebrate fauna of the Antlers Formation (Lower Cretaceous), Southwestern Oklahoma. Oklahoma Geol Notes 1997, 57, 124–131. [Google Scholar]
- Caughey, C.A. Depositional systems in the Paluxy Formation (Lower Cretaceous), Northeast Texas—oil, gas, and groundwater resources; Bureau of Economic Geology: Austin, USA, 1977. [Google Scholar]
- Thurmond, J.T. Cartilaginous fishes of the Trinity Group and related rocks (Lower Cretaceous) of north central Texas. Southeast Geol 1971, 13, 207–227. [Google Scholar]
- zdikmen, H.; Akbulut, A. Thurmondella nom. nov., A new name for the preoccupied genus Paramicrodon Thurmond, 1974 (Osteichtyes: Actinopterygii: Pycnodontiformes). Mun Ent Zool 2012, 7, 1280–1281. [Google Scholar]
- Costa, A.B.C.; Córdoba, V.C.; Netto, R.G.; Lima Filho, F.P. Registro faciológico e paleoambiental da transgressão que marca a passagem do Cenomaniano para o Turoniano na Bacia Potiguar, NE do Brasil. Comun Geol 2014, 101, 415–420. [Google Scholar]
- Araripe, P.T.; Feijó, F.J. Potiguar Basin; Bacia Potiguar. Bol geociênc Petrobras 1994, 8, 127–141. [Google Scholar]
- Maraschin, A.; Mizusaki, A.M.; Vasconcelos, P.M.; Hinrichs, R.; Luiz, D.E.; Dos Anjos, S. Depositional age definition of the Açu Formation (Potiguar Basin, northeastern Brazil) through 40 Ar- 39 Ar dating of early-authigenic K-feldspar overgrowths. Pesqui em Geocienc 2010, 37, 85–96. [Google Scholar] [CrossRef]
- Arai, M. Paleogeografia do Atlântico Sul no Aptiano: um novo modelo a partir de dados micropaleontológicos recentes. Bol geociênc Petrobras 2009, 17, e351. [Google Scholar]
- Arai, M. Aptian/Albian (Early Cretaceous) paleogeography of the South Atlantic: a paleontological perspective. Braz. J. Geol. 2014, 44, 339–350. [Google Scholar] [CrossRef]
- Veiga, I.M.M.G.; Bergqvist, L.P.; Brito, P.M. The fish assemblage of the Cretaceous (? Albian-Cenomanian) Açu Formation, Potiguar Basin, Northeastern Brazil. J South Am Earth Sci 2019, 93, 162–173. [Google Scholar]
- Cuny, G.; Cobbett, A.M.; Meunier, F.J.; Benton, M.J. Vertebrate microremains from the Early Cretaceous of southern Tunisia. Geobios 2010, 43, 615–628. [Google Scholar] [CrossRef]
- Anderson, P.E.; Benton, M.J.; Trueman, C.N.; Paterson, B.A.; Cuny, G. Palaeoenvironments of vertebrates on the southern shore of Tethys: The nonmarine Early Cretaceous of Tunisia. Palaeogeogr. Palaeoclim. Palaeoecol. 2007, 243, 118–131. [Google Scholar] [CrossRef]
- Kriwet, J.; Schmitz, L. New insight into the distribution and palaeobiology of the pycnodont fish Gyrodus. Acta Palaeontol Pol 2005, 50, 49–56. [Google Scholar]
- Srarfi, D. Biostratigraphie, biodiversité, taphonomie and paléoenvironnement des niveaux à vertébrés du Jurassique–Crétacé du Sud-Est de la Tunisie. Implications paléobiogéographiques. Unpublished PhD thesis, Claude Bernard University Lyon 1, Lyon, 2006.
- Russell, D.A. Isolated dinosaur bones and teeth from the Middle Cretaceous of the Tafilalt, Morocco. Bull Mus Natl Hist Nat 4C 1996, 18, 349–402. [Google Scholar]
- Dal Sasso, C.; Maganuco, S.; Buffetaut, E.; Mendez, M.A. New information of the skull of the enigmatic theropod Spinosaurus, with remarks on its size and affinities. J Vertebr Paleontol 2005, 25, 888–896. [Google Scholar] [CrossRef]
- Ibrahim, N.; Sereno, P.C.; Dal Sasso, C.; Maganuco, S.; Fabri, M.; Martill, D.M.; Zouhri, S.; Myhrvold, N.; Lurino, D.A. S emiaquatic adaptations in a giant predatory dinosaur. Science 2014, 345, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Sereno, P.C.; Dutheil, D.B.; Iarochene, M.; Larsson, H.C.E.; Lyon, G.H.; Magwene, P.M.; Sidor, C.A.; Varricchio, D.J.; Wilson, J.A. Predatory Dinosaurs from the Sahara and Late Cretaceous Faunal Differentiation. Science 1996, 272, 986–991. [Google Scholar] [CrossRef]
- Martill, D.M.; Ibrahim, N. Aberrant rostral teeth of the sawfish Onchopristis numidus from the Kem Kem beds (?early Late Cretaceous) of Morocco and a reappraisal of Onchopristis in New Zealand. J. Afr. Earth Sci. 2012, 64, 71–76. [Google Scholar] [CrossRef]
- Ibrahim, N.; Sereno, P.C.; Varricchio, D.J.; Martill, D.M.; Dutheil, D.B.; Unwin, D.M.; Baidder, L.; Larsson, H.C.; Zouhri, S.; Kaoukaya, A. Geology and paleontology of the Upper Cretaceous Kem Kem group of eastern Morocco. ZooKeys 2020, 928, 1–216. [Google Scholar] [CrossRef] [PubMed]
- Yabumoto, Y.; Uyeno, T. New material of a Cretaceous coelacanth, Mawsonia lavocati Tabaste from Morocco. Bull Natn Sci Mus Tokyo 2005, 31, 39–49. [Google Scholar]
- Zhou, X.; You, J.; Jin, J.; Feng, Q.; Luo, Z.; Hu, J.; Li, Y. A minute freshwater pycnodont fish from the Late Cretaceous on the southern margin of the Junggar Basin: Palaeoecological implications. Hist. Biol. 2022, 35, 1528–1535. [Google Scholar] [CrossRef]
- Hao, Y.C.; Su, D.Y.; Yu, J. X.; Li, P.X.; Li, Y.G.; Wang, N.W.; Qi, H.; Guan, S.Z.; Hu, H.G.; Liu, X.; Yang, W.D.; Ye, L.S.; Shou, Z.X.; Zhang, Q.B. Strata of China 12: Cretaceous; Geological Publishing House: Beijing, China, 1986. [Google Scholar]
- Chen, P. A survey of the non-marine Cretaceous of China. Cretac Res 1983, 4, 123–143. [Google Scholar]
- Zheng, X.L.; Zheng, X.M.; Zheng, X.S.; Li, J.G.; Yang, Y.M.; Weng, Y.X.; Wu, C.X. The late Cretaceous ostracod fossils of the Junggar Basin (in Chinese). J Stratigr 2013, 37, 206–209. [Google Scholar]
- Xi, D.; Wan, X.; Li, G.; Li, G. Cretaceous integrative stratigraphy and timescale of China. Sci China Earth Sci 2019, 62, 256–286. [Google Scholar] [CrossRef]
- Woodward, A.S. On some fish-remains from the Lameta Beds at Dongargaon, Central Provinces. Palaeont Ind 1908, 3, 1–6. [Google Scholar]
- Prasad, G.V. Vertebrate biodiversity of the Deccan volcanic province of India: A review. BSGF - Earth Sci. Bull. 2012, 183, 597–610. [Google Scholar] [CrossRef]
- Sarkar, A.; Bhattacharya, S.K.; Mohabey, D.M. Stable isotope analyses of dinosaur eggshells: Paleoenvironmental implications: Geology 1991, 19, 1068–1107.
- Ghosh, P.; Bhattacharya, S.K.; Jani, R.A. Paleoclimate and paleovegetation in Central India during the Upper Cretaceous based on the stable isotope composition of the paleosol carbonates. Palaeogeogr Palaeoclimatol Palaeoecol 1995, 114, 285–296. [Google Scholar] [CrossRef]
- Tandon, S.K.; Sood, A.; Andrews, J.E.; Dennis, P.F. Paleoenvironments of dinosaur-bearing Lameta Beds (Maastrichtian), Narmada Valley, Central India. Palaeogeogr Palaeoclimatol Palaeoecol 1995, 117, 153–184. [Google Scholar] [CrossRef]
- Mohabey, D.M. Pycnodus lametae (Pycnodontidae), a holostean fish from freshwater Upper Cretaceous Lameta Formation of Maharashtra. J Geol Soc India 1996, 47, 593–598. [Google Scholar]
- Mohabey, D.M.; Udhoji, S.G. Fauna and flora from Late Cretaceous (Maastrichtian) non-marine Lameta sediments associated with Deccan volcanic episode, Maharashtra: its relevance to the K-T boundary problem, palaeoenvironment and palaeogeography. Gondwana Geol Mag 1996, 2, 349–364. [Google Scholar]
- Jain, S.L.; Sahni, A. Some Upper Cretaceous vertebrates from central India and their palaeogeographical implications. In Symposium on Cretaceous of India: palaeoecology, palaeogeography and time boundaries, Lucknow, India, 24–26 November; Maheshwari, H.K., Ed.; Indian Association Palynostratigraphers: Lucknow, India, 1983. [Google Scholar]
- Sahni, A. Cretaceous-Paleocene Terrestrial Faunas of India: Lack of Endemism During Drifting of the Indian Plate. Science 1984, 226, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Shome, S.; Chandel, R.S. Palaeontological studies of Papro Formation (Infratrappean) of Lalitpur District, Uttar Pradesh-Its age, correlation and palaeoecology. Indian J Geosci 2013, 65, 49–62. [Google Scholar]
- Khosla, A. Dinosaur eggshells from the Late Cretaceous Lameta Formation along the east-central Narbada River region: Biomineralization and morphotaxonomical studies. Unpublished. PhD Thesis, Panjab University, Chandigarh, 1996. [Google Scholar]
- Khosla, A.; Sahni, A. Late cretaceous (Maastrichtian) ostracodes from the Lameta formation, Jabalpur cantonment area, Madhya Pradesh, India. J Palaeontol Soc India 2000, 45, 57–78. [Google Scholar]
- Khosla, A.; Prasad, G.V.R.; Verma, O.; Jain, A.K.; Sahni, A. Discovery of a micromammal-yielding Deccan intertrappean site near Kisalpuri, Dindori District, Madhya Pradesh. Curr Sci 2004, 87, 380–383. [Google Scholar]
- Prasad, G.V.R.; Khajuria, C.K. Palaeoenvironments of the Late Cretaceous Mammal-Bearing Intertrappean Beds of Naskal, Andhra Pradesh, India. Mem Geol Soc India 1996, 37, 337–362. [Google Scholar]
- Khajuria, C.; Prasad, G. Taphonomy of a late Cretaceous mammal-bearing microvertebrate assemblage from the Deccan inter-trappean beds of Naskal, peninsular India. Palaeogeogr. Palaeoclim. Palaeoecol. 1998, 137, 153–172. [Google Scholar] [CrossRef]
- Prasad, G.V.R.; Khajuria, C.K. A record of microvertebrate fauna from the intertrappean beds of Naskal, Andhra Pradesh. J Palaeontol Soc India 1990, 35, 151–161. [Google Scholar]
- Rana, R.S. Palaeontology and Palaeoecology of the Intertrappean (Cretaceous–Tertiary transition) beds of the Peninsular India. J Palaeontol Soc India 1990, 35, 105–120. [Google Scholar]
- Prasad, G.V.R.; Sahni, A. Coastal-plain microvertebrate assemblage from the terminal Cretaceous of Asifabad, Peninsular India. J Palaeontol Soc India 1987, 32, 5–19. [Google Scholar]
- Lourembam, R.S.; Prasad, G.V.R.; Grover, P. Ichthyofauna (Chondrichthyes, Osteichthyes) from the Upper Cretaceous intertrappean beds of Piplanarayanwar, Chhindwara District, Madhya Pradesh, India. Isl. Arc 2017, 26. [Google Scholar] [CrossRef]
- Sahni, A. Upper Cretaceous palaeobiogeography of peninsular India and e Cretaceous-Paleocene transition: the vertebrate evidence. In Symposium on Cretaceous of India: palaeoecology, palaeogeography and time boundaries, Lucknow, India, 24–26 November; Maheshwari, H.K., Ed.; Indian Association Palynostratigraphers: Lucknow, India, 1983. [Google Scholar]
- Keller, G.; Adatte, T.; Bajpai, S.; Mohabey, D.; Widdowson, M.; Khosla, A.; Sharma, R.; Khosla, S.; Gertsch, B.; Fleitmann, D.; et al. K–T transition in Deccan Traps of central India marks major marine Seaway across India. Earth Planet. Sci. Lett. 2009, 282, 10–23. [Google Scholar] [CrossRef]
- Rana, R.S.; Kumar, K.; Singh, H. Palaeocene vertebrate fauna from the Fatehgarh Formation of Barmer district, Rajasthan, western India. In Micropalaeontology: Application in Stratigraphy and Paleoceanography; Sinha, D.K., Ed.; Narosa Publishing House: New Delhi, India, 2006; pp. 113–130. [Google Scholar]
- Dolson, J.; Burley, S.D.; Sunder, V.; Kothari, V.; Naidu, B.; Whiteley, N.P.; Farrimond, P.; Taylor, A.; Direen, N.; Ananthakrishnan, B. The discovery of the Barmer Basin, Rajasthan, India, and its petroleum geology. AAPG Bull. 2015, 99, 433–465. [Google Scholar] [CrossRef]
- Khosla, A. Palaeoenvironmental, palaeoecological and palaeobiogeographical implications of mixed fresh water and brackish marine assemblages from the Cretaceous-Palaeogene Deccan intertrappean beds at Jhilmili, Chhindwara District, central India. Rev Mex de Cienc Geol 2015, 32, 344–357. [Google Scholar]
- Schultz, O.; Paunović, M. Der Nachweis von Coelodus (Osteichthyes, Pycnodontidae) im Turonien (Oberkreide) von Gams bei Hieflau, Steiermark, Österreich, und aus der Oberkreide von Kroatien und Italien. Ann Naturh Mus Wien, Ser A 1997, 98A, 73–141.
- Arambourg, C.; Joleaud, L. Vertébrés fossiles du Bassin du Niger. Bull Dir Mines Af Occid 1943, 7, 27–74. [Google Scholar]
- Ősi, A.; Szabó, M.; Kollmann, H.; Wagreich, M.; Kalmár, R.; Makádi, L.; Szentesi, Z.; Summesberger, H. Vertebrate remains from the Turonian (Upper Cretaceous) Gosau Group of Gams, Austria. Cretac. Res. 2019, 99, 190–208. [Google Scholar] [CrossRef]
- Kollmann, H.A. Megalonoda n. gen.(Melanopsidae, Gastropoda) aus der Oberkreide der Nördlichen Kalkalpen (Österreich). Ann Naturh Mus Wien, Ser A 1984, 86, 55–62. [Google Scholar]
- Kollmann, H.A.; Sachsenhofer, R.F. Zur Genese des Gagats von Gams bei Hieflau (Oberkreide, Steiermark). Mitt Ref Geol Paläont Landesmuseum Joanneum 1998, 2, 223–228. [Google Scholar]
- Neubauer, T.A.; Harzhauser, M.; Mandic, O.; Georgopoulou, E.; Kroh, A. Paleobiogeography and historical biogeography of the non-marine caenogastropod family Melanopsidae. Palaeogeogr. Palaeoclim. Palaeoecol. 2016, 444, 124–143. [Google Scholar] [CrossRef]
- Kocsis, L.; Ősi, A.; Vennemann, T.; Trueman, C.N.; Palmer, M.R. Geochemical study of vertebrate fossils from the Upper Cretaceous (Santonian) Csehbánya Formation (Hungary): Evidence for a freshwater habitat of mosasaurs and pycnodont fish. Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 280, 532–542. [Google Scholar] [CrossRef]
- Botfalvai, G.; Ősi, A.; Mindszenty, A. Taphonomic and paleoecologic investigations of the Late Cretaceous (Santonian) Iharkút vertebrate assemblage (Bakony Mts, northwestern Hungary). Palaeogeogr Palaeoclimatol Palaeoecol 2015, 417, 79–405. [Google Scholar] [CrossRef]
- Szabó, M.; Gulyás, P.; Ősi, A. Late Cretaceous (Santonian) pycnodontid (Actinopterygii, Pycnodontidae) remains from the freshwater deposits of the Csehbánya Formation, (Iharkút, Bakony Mountains, Hungary). Ann Paleontol 2016, 102, 123–134. [Google Scholar] [CrossRef]
- si, A.; Rabi, M.; Makádi, L.; Szentesi, Z.; Botfalvai, G.; Gulyás, P. The Late Cretaceous continental vertebrate fauna from Iharkút (western Hungary, Central Europe): a review. In Bernissart Dinosaurs and Early Terrestrial Ecosystems; Godefroit, P., Ed.; Indiana University Press: Bloomington, USA, 2012; pp. 532–569. [Google Scholar]
- Jocha-Edelényi, E. History of evolution of the Upper Cretaceous Basin in the Bakony Mts at the time of the terrestrial Csehbánya Formation. Acta Geol Hung 1988, 31, 19–31. [Google Scholar]
- Ősi, A.; Bodor, E.R.; Makádi, L.; Rabi, M. Vertebrate remains from the Upper Cretaceous (Santonian) Ajka Coal Formation, western Hungary. Cretac. Res. 2016, 57, 228–238. [Google Scholar] [CrossRef]
- Császár, G.H.; Góczán, F.A. Bakony felső-kréta kőszénkutatás és kőszén lápvizsgálatat (Upper Cretaceous coal prospecting and peat bog studies in the Bakony Mts). Magyar Állami Földtani Intézet Évi Jelentése 1988, 1986, 155–178. [Google Scholar]
- Siegl-Farkas, Á. Palynostratigraphy and evolution history of the Ajka Coal Formation, W. Hungary. (Hungarian with English summary). Magyar Állami Öldtani lntéźet Évi Jelentése 1988, 1986, 179–209. [Google Scholar]
- Bodrogi, I.; Fogarasi, A.; Yazikova, E.A.; Sztanó, O.; Báldi-Beke, M. Upper Cretaceous of the Bakony Mts. (Hungary): sedimentology, biostratigraphy, correlation. Zentbl Geol Paläontologie 1998, 1, 1179–1194. [Google Scholar]
- Garcia, G.; Bardet, N.; Houssaye, A.; Pereda-Suberbiola, X.; Valentin, X. Mosasauroid (Squamata) discovery in the Late Cretaceous (Early Campanian) continental deposits of Villeveyrac–L’Olivet, southern France. C R Palevol 2015, 14, 495–505. [Google Scholar] [CrossRef]
- Torices, A.; Diaz Berenguer, E.; Narvaéz, I.; Ortega, F.; Perez, S.; Serrano, H. Preliminary analysis of the microvertebrate fossils of“Lo Hueco” (Upper Cretaceous, Cuenca, Spain). In Mésogée-Bulletin du Muséum d’histoire Naturelle de Marseille 66, Proceedings of the 8ème Congrès de l’European Association of Vertebrate Palaeontology (EAVP), Aix-en-Provence, France, 7–12 June 2010; Muséum d’histoire naturelle: Marseille, France, 2010; p. 80. [Google Scholar]
- Torices, A.; Barroso-Barcenilla, F.; Cambra-Moo, O.; Perez, S.; Serrano, H. Vertebrate microfossil analysis in the Palaeontological site of “Lo Hueco” (Upper Cretaceous, Cuenca, Spain). In Abstracts 71st Meeting of the Society of Vertebrate Paleontology, Las Vegas, USA, 2–5 November 2011; Supplement to the Journal of Vertebrate Paleontology: Las Vegas, USA, 2011. [Google Scholar]
- Ortega, F.; Bardet, N.; Barroso-Barcenilla, F.; Callapez, P.M.; Cambra-Moo, O.; Daviero-Gómez, V.; Díez Díaz, V.; Domingo, L.; Elvira, A.; Escaso, F.; García-Oliva, M.; Gómez, B.; Houssaye, A.; Knoll, F.; Marcos-Fernández, F.; Martín, M.; Mocho, P.; Narváez, I.; Pérez-García, A.; Peyrot, D.; Segura, M.; Serrano, H.; Torices, A.; Vidal, D.; Sanz, J.L. The biota of the upper Cretaceous site of Lo Hueco (Cuenca, Spain). J Iber Geol 2015, 41, 83–99. [Google Scholar] [CrossRef]
- Marmi, J.; Blanco, A.; Fondevilla, V.; Vecchia, F.M.D.; Sellés, A.G.; Vicente, A.; Martín-Closas, C.; Oms, O.; Galobart. The Molí del Baró-1 site, a diverse fossil assemblage from the uppermost Maastrichtian of the southern Pyrenees (north-eastern Iberia). Cretac. Res. 2016, 57, 519–539. [Google Scholar] [CrossRef]
- Blanco, A.; Szabó, M.; Blanco-Lapaz. ; Marmi, J. Late Cretaceous (Maastrichtian) Chondrichthyes and Osteichthyes from northeastern Iberia. Palaeogeogr. Palaeoclim. Palaeoecol. 2017, 465, 278–294. [Google Scholar] [CrossRef]
- Díez-Canseco, D.; Arz, J.; Benito, M.; Díaz-Molina, M.; Arenillas, I. Tidal influence in redbeds: A palaeoenvironmental and biochronostratigraphic reconstruction of the Lower Tremp Formation (South-Central Pyrenees, Spain) around the Cretaceous/Paleogene boundary. Sediment. Geol. 2014, 312, 31–49. [Google Scholar] [CrossRef]
- Cifelli, R.L.; Nydam, R.L.; Gardner, J.D.; Weil, A.; Eaton, J.G.; Kirkland, J.I.; Madsen, S.K.; Gillette, D.D. Medial Cretaceous vertebrates from the Cedar Mountain Formation, Emery County, Utah: the Mussentuchit local fauna. In Vertebrate paleontology in Utah (MP 99–1); Gillette, D.D., Ed.; Utah Geological Survey: Salt Lake City, USA, 1999; pp. 219–242. [Google Scholar]
- Fiorillo, A.R.; Gillette, D.D. Non-mammalian microvertebrate remains from the Robison Eggshell site, Cedar Mountain Formation (Lower Cretaceous), Emery County. Utah. In Vertebrate paleontology in Utah (MP 99–1); Gillette, D.D., Ed.; Utah Geological Survey: Salt Lake City, USA, 1999; pp. 259–268. [Google Scholar]
- Eaton, J.G.; Kirkland, J.I.; Kauffman, E.G. Evidence and dating of mid-Cretaceous tectonic activity in the San Rafael Swell, Emery County, Utah. Mt Geol 1990, 27, 39–45. [Google Scholar]
- Brinkman, D.B.; Newbrey, M.G.; Neuman, A.G.; Eaton, J.G.; Titus, A.L. Freshwater Osteichthyes from the Cenomanian to late Campanian of Grand Staircase-Escalante National Monument, Utah. In At the Top of the Grand Staircase: The Late Cretaceous of Southern Utah; Titus, A.L., Loewen, M.A., Eds.; Indiana University Press: Bloomington, USA, 2013; pp. 195–236. [Google Scholar]
- Kirkland, J.I.; Eaton, J.G.; Brinkman, D.B. Elasmobranchs from Upper Cretaceous freshwater facies in southern Utah. In At the Top of the Grand Staircase: The Late Cretaceous of Southern Utah; Titus, A.L., Loewen, M.A., Eds.; Indiana University Press: Bloomington, USA, 2013; pp. 153–194. [Google Scholar]
- Titus, A.; Eaton, J.; Sertich, J. Late Cretaceous stratigraphy and vertebrate faunas of the Markagunt, Paunsaugunt, and Kaiparowits plateaus, southern Utah. Geol Intermt West 2016, 3, 229–291. [Google Scholar] [CrossRef]
- Pedrão, E.; Arai, M.; Barrilari, I.M.R.; Carvalho, I.S. Análise palinológica de uma amostra de superfície de Querru (Formação Itapecuru), Município de Itapecuru-Mirim – MA. Relatório Técnico Petrobrás, Rio de Janeiro, Brazil, 1993; 11 pp.
- Candeiro, C.R.A.; Fanti, F.; Therrien, F.; Lamanna, M.C. Continental fossil vertebrates from the mid-Cretaceous (Albian–Cenomanian) Alcântara Formation, Brazil, and their relationship with contemporaneous faunas from North Africa. J Afr Earth Sci 2011, 60, 79–92. [Google Scholar] [CrossRef]
- Medeiros, M.A.; Lindoso, R.M.; Mendes, I.D.; De Souza Carvalho, I. The Cretaceous (Cenomanian) continental record of the laje do coringa flagstone (Alcântara formation), northeastern South America. J South Am Earth Sci 2014, 53, 50–58. [Google Scholar] [CrossRef]
- Sousa, E.P.; Medeiros, M.A.; Bertini, R.J.; Pereira, A.A.; Toledo, C.E.V. Ocorrência inédita de picnodontiformes para a Laje do Coringa, Ilha do Cajual (Formação Alcântara), Eo-cenomaniano do Estado do Maranhão, Simpósio Brasileiro de Paleontologia de Vertebrados IV, UNESP, Rio Claro, Brazil, -16; 2004; 67–68. 12 July.
- Benedetto, J.L.; Sanchez, T. EI hallazgo de peces Pycnodontiformes (Holostei) en la Formacion Yacoraite (Cretacico superior) de la provincia de Salta (Argentina) y su importancia paleoecologica. Acta Geol Lilloana 1971, 11, 153–175. [Google Scholar]
- Benedetto, J.L.; Sanchez, T. Coelodus toncoensis nov. sp. (Pisces, Holostei, Pycnodontiformes) de la Formacion Yacoraite (Cretacico superior) de la provincia de Salta. Ameghiniana 1972, 9, 59–71. [Google Scholar]
- Cione, A.L. Algunas consideraciones sobre los Pycnodontiformes (Pisces, Holostei) procedentes de la Formacion Yacoraite, Cretacico tardio de la provincia de Salta. Ameghiniana 1977, 14, 315–316. [Google Scholar]
- Gasparini, Z.; Buffetaut, E. Dolichochampsa minima, n. gen., n. sp., a representative of a new family of eusuchian crocodiles from the late Cretaceous of northern Argentina. Neues Jahrb Geol Paläont Mh 1980, 5, 257–271. [Google Scholar]
- Cione, A.L.; Pereira, S.M.; Alonso, R.; Arias, J. Los peces bagres (Osteichthyes, Siluriformes) de la Formación Yacoraite (Cretacico tardio). Consideraciones biogeograficas y bioestratigraficas. Ameghiniana 1985, 21, 294–304. [Google Scholar]
- Marquillas, R.; Sabino, I.; Sial, A.N.; Del Papa, C.; Ferreira, V.; Matthews, S. Carbon and oxygen isotopes of Maastrichtian–Danian shallow marine carbonates: Yacoraite Formation, northwestern Argentina. J South Am Earth Sci 2007, 23, 304–320. [Google Scholar] [CrossRef]
- Deschamps, R.; Rohais, S.; Hamon, Y.; Gasparrini, M. Dynamic of a lacustrine sedimentary system during late rifting at the Cretaceous–Palaeocene transition: Example of the Yacoraite Formation, Salta Basin, Argentina. Depos Rec 2020, 6, 490–523. [Google Scholar] [CrossRef]
- Arratia, G.; Cione, A. The record of fossil fishes of southern South America. Münchner Geowiss Abh 1996, 30, 9–72. [Google Scholar]
- Gayet, M. Holostean and teleostean fishes of Bolivia. Rev Téc Yaci Petrol Fisc Bol 1991, 12, 453–494. [Google Scholar]
- Gayet, M.; Marshall, L.G.; Sempéré, T. The Mesozoic and Paleocene vertebrates of Bolivia and their stratigraphic context: a review. Rev Téc Yaci Petrol Fisc Bol 1991, 12, 393–433. [Google Scholar]
- Gayet, M.; Sempre, T.; Cappetta, H.; Jaillard, E.; Lévy, A. La présence de fossiles marins dans le Crétacé terminal des Andes centrales et ses conséquences paléogéographiques. Palaeogeogr Palaeoclimatol Palaeoecol 1993, 102, 283–319. [Google Scholar] [CrossRef]
- Gayet, M.; Marshall, L.G.; Sempere, T.; Meunier, F.J.; Cappetta, H.; Rage, J.-C. Middle Maastrichtian vertebrates (fishes, amphibians, dinosaurs and other reptiles, mammals) from Pajcha Pata (Bolivia). Biostratigraphic, palaeoecologic and palaeobiogeographic implications. Palaeogeogr. Palaeoclim. Palaeoecol. 2001, 169, 39–68. [Google Scholar] [CrossRef]
- Schultze, H.P. Pycnodont fish (Actinopterygii, Osteichthyes) from the El Molino Formation (Late Cretaceous to Early Paleocene) of Bolivia. Rev Téc Yaci Petrol Fisc Bol 1991, 12, 449–452. [Google Scholar]
- de Muizon, C.; Gayet, M.; Lavenu, A.; Marshall, L.G.; Sigé, B.; Villaroel, C. Late Cretaceous vertebrates, including mammals,from Tiupampa, Southcentral Bolivia. Geobios 1983, 16, 747–753. [Google Scholar] [CrossRef]
- Benyoucef, M.; Läng, E.; Cavin, L.; Mebarki, K.; Adaci, M.; Bensalah, M. Overabundance of piscivorous dinosaurs (Theropoda: Spinosauridae) in the mid-Cretaceous of North Africa: The Algerian dilemma. Cretac. Res. 2015, 55, 44–55. [Google Scholar] [CrossRef]
- Longbottom, A.E. New Tertiary pycnodonts from the Tilemsi valley, Republic of Mali. Bull br Mus Nat Hist 1984, 38, 1–26. [Google Scholar]
- O'Leary, M.A.; Bouaré, M.L.; Claeson, K.M.; Heilbronn, K.; Hill, R.V.; Mccartney, J.; Sessa, J.A.; Sissoko, F.; Tapanila, L.; Wheeler, E.; et al. Stratigraphy and Paleobiology of the Upper Cretaceous-Lower Paleogene Sediments from the Trans-Saharan Seaway in Mali. Bull. Am. Mus. Nat. Hist. 2019, 2019, 1–183. [Google Scholar] [CrossRef]
- Tapanila, L.; Roberts, E.; Bouaré, M.L.; Sissoko, F.; O’Leary, M.A. Phosphate taxonomy of bone and comprolite conglomerates: a case study from the Eocene of Mali, NW Africa. Palaios 2008, 23, 139–152. [Google Scholar] [CrossRef]
- Ostrowski, S.A. The teleost ichthyofauna from the Late Cretaceous of Madagascar: Systematics, distribution, and implications for Gondwanan biogeography. Unpublished PhD thesis, Michigan State University, East Lansing, 2012.
- Rogers, R.R.; Hartman, J.H.; Krause, D.W. Stratigraphic Analysis of Upper Cretaceous Rocks in the Mahajanga Basin, Northwestern Madagascar: Implications for Ancient and Modern Faunas. J. Geol. 2000, 108, 275–301. [Google Scholar] [CrossRef]
- Rogers, R.R. Fine-grained debris flows and extraordinary vertebrate burials in the Late Cretaceous of Madagascar. Geology 2005, 33, 297. [Google Scholar] [CrossRef]
- Krause, D.W.; Sertich, J.J.W.; Rogers, R.R.; Kast, S.C.; Rasoamiaramanana, A.H.; Buckley, G.A. Overview of the discovery, distribution, and geological context of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. J. Vertebr. Paléontol. 2010, 30, 4–12. [Google Scholar] [CrossRef]
- Fendley, I.M.; Sprain, C.J.; Renne, P.R.; Arenillas, I.; Arz, J.A.; Gilabert, V.; Self, S.; Vanderkluysen, L.; Pande, K.; Smit, J.; Mittal, T. No Cretaceous-Paleogene boundary in exposed Rajahmundry Traps: A refined chronology of the longest Deccan lava flows from 40Ar/39Ar dates, magnetostratigraphy, and biostratigraphy. Geochem Geophys Geosyst 2020, 21, e2020GC009149. [Google Scholar] [CrossRef]
- Bhalla, S.N. On the occurrence of Eotrigonodon in the Eocene of Rajahmundry, Andhra Pradesh. J Geol Soc India 1974, 15, 335–337. [Google Scholar]
- Prasad, G.V.R. Fossil fish remains from the intertrappean beds of Duddukuru, Andhra Pradesh. Bull Indian Geol Assoc 1987, 20, 33–39. [Google Scholar]
- Keller, G.; Adatte, T.; Gardin, S.; Bartolini, A.; Bajpai, S. Main Deccan volcanism phase ends near the K-T boundary: Evidence from the Krishna-Godavari Basin, SE India. Earth Planet Sci Lett 2008, 268, 293–311. [Google Scholar] [CrossRef]
- Ebersole, J.A.; Cicimurri, D.J.; Stringer, G.L. Taxonomy and biostratigraphy of the elasmobranchs and bonyfishes (Chondrichthyes and Osteichthyes) of the lower-to-middle Eocene (Ypresian to Bartonian) Claiborne Group in Alabama, USA,including an analysis of otoliths. Euro J Taxon 2019, 585, 1–274. [Google Scholar]
- Holman, J.A.; Case, G.R. Reptiles from the Eocene Tallahatta Formation of Alabama. J Vertebr Paleontol 1988, 8, 328–333. [Google Scholar] [CrossRef]
- Marquillas, R.A.; Del Papa, C.E.; Sabino, I.F. Sedimentary aspects and paleoenvironmental evolution of a rift basin: Salta Group (Cretaceous-Paleogene), northwestern Argentina. Int J Earth Sci 2005, 54, 94–113. [Google Scholar] [CrossRef]
- Guinot, G.; Cavin, L. Body size evolution and habitat colonization across 100 million years (Late Jurassic–Paleocene) of the actinopterygian evolutionary history. Fish Fish. 2018, 19, 577–597. [Google Scholar] [CrossRef]
- Föllmi, K. Early Cretaceous life, climate and anoxia. Cretac. Res. 2011, 35, 230–257. [Google Scholar] [CrossRef]
- Lovejoy, N.R.; Albert, J.S.; Crampton, W.G. Miocene marine incursions and marine/freshwater transitions: Evidence from Neotropical fishes. J. South Am. Earth Sci. 2006, 21, 5–13. [Google Scholar] [CrossRef]
- Kauffman, E.G. Paleobiogeography and evolutionary response dynamic in the Cretaceous Western Interior seaway of North America. In Jurassic–Cretaceous Biochronology and Paleogeography of North America; Westermann, G.E.G., Ed.; Geological Assocation of Canada: St. John’s, Canada, 1984; pp. 275–306. [Google Scholar]
- nbsp; Wang, C. S.; Scott, R.W.; Wan, X.Q.; Graham, S.A.; Huang, Y.J.; Wang, P.J.; Wu, H.C.; Dean, W.E.; Zhang, L.M. Late Cretaceous climate changes recorded in Eastern Asian lacustrine deposits and North American Epieric sea strata. Earth-Sci. Rev. 2013, 126, 275–299. [Google Scholar] [CrossRef]
- Grande, L.; Bemis, W.E. A comprehensive phylogenetic study of Amiid fishes (Amiidae) based on comparative skeleta anatomy. An empirical search for interconnected patterns of natural history. J Vertebr Palaeontol 1998, 18, 1–696. [Google Scholar]
- Clement, A.M.; Long, J.A. Air-breathing adaptation in a marine Devonian lungfish. Biol. Lett. 2010, 6, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Betancur-R, R.; Ortí, G.; Pyron, R.A. Fossil-based comparative analyses reveal ancient marine ancestry erased by extinction in ray-finned fishes. Ecol. Lett. 2015, 18, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Guinot, G.; Cavin, L. Contrasting ‘fish’ diversity dynamics between marine and freshwater environments. Curr Biol 2015, 25, 2314–2318. [Google Scholar] [CrossRef]
- Sheehan, P.M.; Fastovsky, D.E. Major extinctions of land-dwelling vertebrates at the Cretaceous-Tertiary boundary, eastern Montana. Geology 1992, 20. [Google Scholar] [CrossRef]
- Cavin, L.; Martin, M. Les actinopterygiens et la limite Cretace-Tertiaire. Geobios 1995, 28, 183–188. [Google Scholar] [CrossRef]
- Cavin, L. Effects of the Cretaceous-Tertiary event on bony fishes. In Geological and Biological Effects of Impact Events; Buffetaut, E., Koeberl, C., Eds.; Springer-Verlag: Berlin, Germany, 2002; pp. 141–158. [Google Scholar]
- D'Hondt, S.; Donaghay, P.; Zachos, J.C.; Luttenberg, D.; Lindinger, M. Organic Carbon Fluxes and Ecological Recovery from the Cretaceous-Tertiary Mass Extinction. Science 1998, 282, 276–279. [Google Scholar] [CrossRef]
- Bartholomai, A. The large aspidorhynchid fish, Richmondichthys sweeti (Etheridge Jnr and Smith Woodward, 1891) from Albian marine deposits of Queensland, Australia. Mem Queensl Mus 2004, 49, 521–536. [Google Scholar]
- Bloom, D.D.; Weir, J.T.; Piller, K.R.; Lovejoy, N.R. Do freshwater fishes diversify faster than marine fishes? A test using state-dependent diversification analyses and molecular phylogenetics of new world silversides (Atherinopsidae). Evolution 2013, 28, 2689–2690. [Google Scholar] [CrossRef] [PubMed]
- Santini, F.; Nguyen, M.T.T.; Sorenson, L.; Waltzek, T.B.; Alfaro, J.W.L.; Eastman, J.M.; Alfaro, M.E. Do habitat shifts drive diversification in teleost fishes? An example from the pufferfishes (Tetraodontidae). J. Evol. Biol. 2013, 26, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.D.; Lovejoy, N.R. On the origins of marine-derived freshwater fishes in South America. J. Biogeogr. 2017, 44, 1927–1938. [Google Scholar] [CrossRef]
- Scotese, C.R. Atlas of Jurassic Paleogeographic Maps, PALEOMAP Atlas for ArcGIS, Maps 32–42, Vol. 4, The Jurassic and Triassic,, Mollweide Projection; PALEOMAP Project: Evanston, USA, 2014. [Google Scholar]
- cotese, C.R. Atlas of Early Cretaceous Paleogeographic Maps, PALEOMAP Atlas for ArcGIS, Maps 23–31, Vol. 2, The Cretaceous, Mollweide Projection; PALEOMAP Project: Evanston, USA, 2014. [Google Scholar]
- Scotese, C.R. Atlas of Late Cretaceous Maps, PALEOMAP Atlas for ArcGIS, Maps 16–22 Vol. 2, The Cretaceous, Mollweide Projection; PALEOMAP Project, Evanston, USA, 2014; 13 pp.
- Scotese, C.R. Atlas of Paleogene Paleogeographic Maps, PALEOMAP Atlas for ArcGIS, Maps 8-15, Vol. 1, The Cenozoic, Mollweide Projection; PALEOMAP Project, Evanston, USA, 2014; 12 pp.


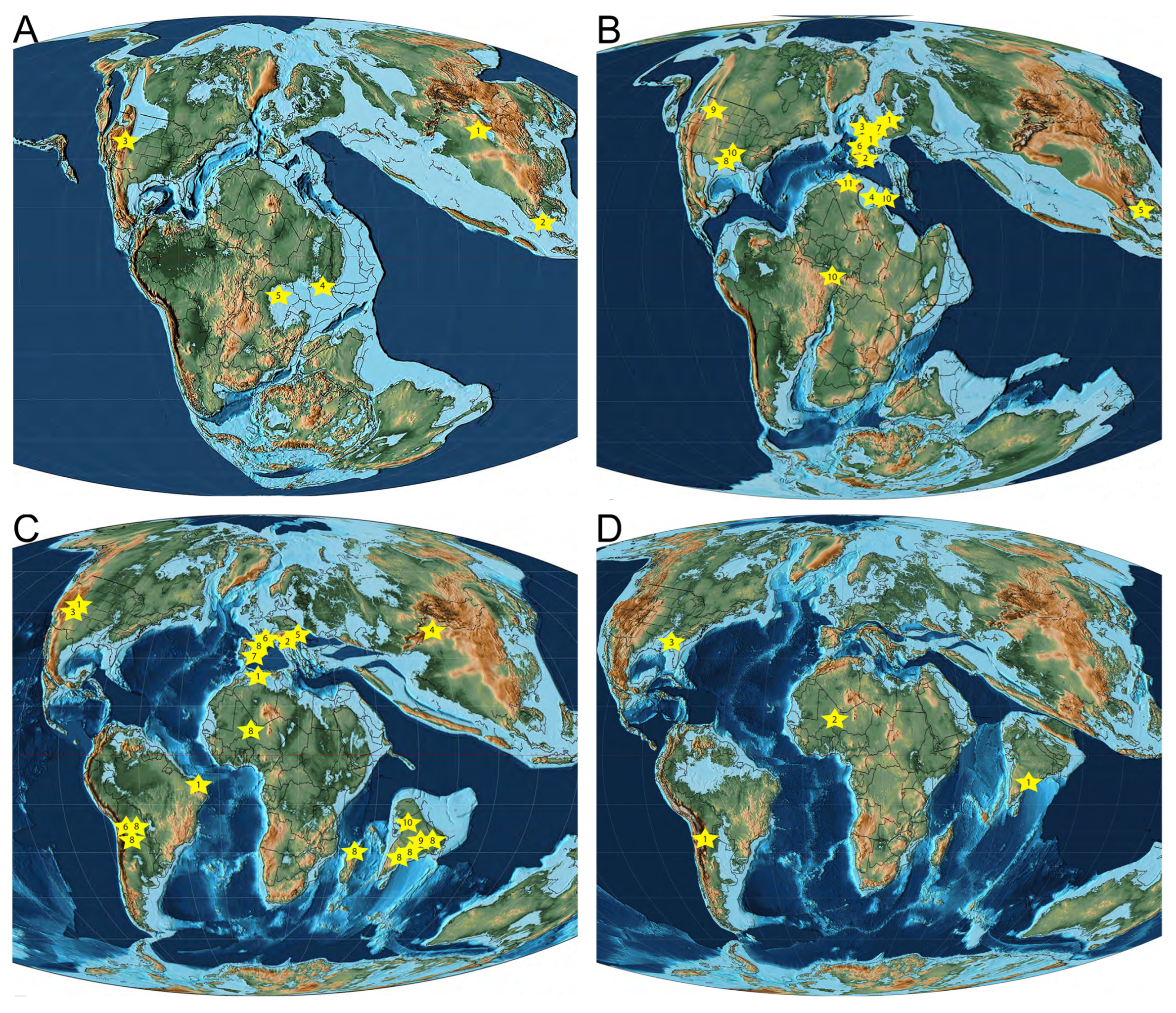
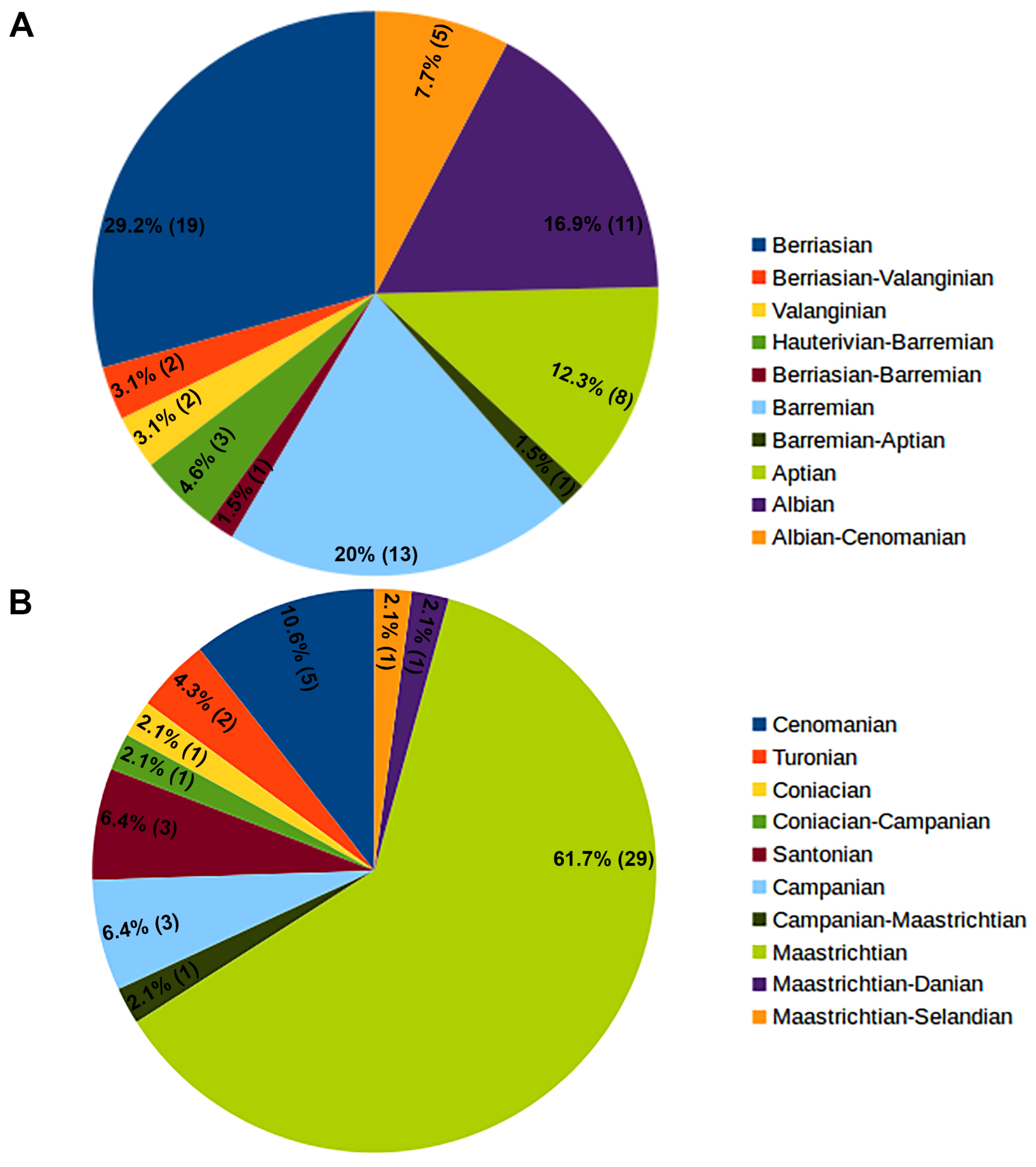
| Time Bin | Nonmarine occurrences | No. of known genera |
|---|---|---|
| Late Jurassic | 4 | 3 |
| Early Cretaceous | 61 | 15 |
| Late Cretaceous | 50 | 8 |
| Palaeocene | 9 | 2 |
| Eocene | 5 | 1 |
| Time Bin | Nonmarine occurrences | No. of known genera |
|---|---|---|
| Late Jurassic | 3 | 1 |
| Early Cretaceous | 60 | 14 |
| Late Cretaceous | 45 | 5 |
| Palaeocene | 7 | 2 |
| Eocene | 5 | 1 |
| Stage | Nonmarine occurrences | % of Cretaceous nonmarine occurrences |
|---|---|---|
|
19 | 17.3 |
|
2 | 1.8 |
|
2 | 1.8 |
|
3 | 2.7 |
|
1 | 0.9 |
|
13 | 11.8 |
|
1 | 0.9 |
|
8 | 7.3 |
|
11 | 10.0 |
|
5 | 4.5 |
|
5 | 4.5 |
|
2 | 1.8 |
|
1 | 0.9 |
|
1 | 0.9 |
|
3 | 2.7 |
|
3 | 2.7 |
|
1 | 0.9 |
|
29 | 26.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





